Abstract
Of 516 Staphylococcus aureus strains tested, 97.1% were susceptible to quinupristin-dalfopristin, which was bactericidal for 22 (56%) of the 39 strains tested, comparable to vancomycin. All 17 clindamycin and macrolide-resistant strains were inhibited but not killed by quinupristin-dalfopristin, whereas all 22 clindamycin-susceptible strains (5 were macrolide resistant) were killed.
Quinupristin-dalfopristin is a parenteral streptogramin composed of quinupristin (a streptogramin B antibiotic) and dalfopristin (a streptogramin A antibiotic) in a 30:70 ratio (13). This combination has been shown to exhibit synergistic in vitro antibacterial activity against staphylococci and other gram-positive bacteria (17). Greater than 90% of Staphylococcus aureus isolates have been reported to be susceptible to quinupristin-dalfopristin at ≤1.0 μg/ml (1, 3, 7, 10, 14, 17, 18), and this activity was not appreciably affected by methicillin resistance (1, 3, 17, 18) or quinolone resistance (1, 10). Although nearly all S. aureus strains are inhibited by quinupristin-dalfopristin, the bactericidal activity of this drug is much more variable (3, 6, 9). Previous studies have shown that S. aureus strains that have cross-resistance to macrolides, lincosamides, and streptogramin B antibiotics (MLSB) were not killed in vitro by quinupristin-dalfopristin, nor did endocarditis in experimental animals due to such strains respond to quinupristin-dalfopristin therapy (5). Low quinupristin MICs were demonstrated to be predictive of quinupristin-dalfopristin bactericidal activity against staphylococci, and routine testing of quinupristin MICs was suggested for this reason (5). Since adding quinupristin to routine gram-positive susceptibility test panels would be a major step, it would be of interest to know whether susceptibility to clindamycin or erythromycin might be equally predictive of quinupristin-dalfopristin bactericidal activity. Thus, the present study was designed to determine the correlation between the bactericidal activity of quinupristin-dalfopristin and the MICs of quinupristin-dalfopristin, quinupristin, dalfopristin, erythromycin, and clindamycin.
Quinupristin, dalfopristin, and quinupristin-dalfopristin were provided by Rhone-Poulenc Rorer, Collegeville, Pa. Clindamycin, erythromycin, oxacillin, and vancomycin were procured from other commercial sources.
Broth microdilution tests (15) with 516 recent clinical isolates of S. aureus compared the bacteriostatic activities of quinupristin-dalfopristin, erythromycin, and clindamycin. The results are summarized in Table 1. Nearly all S. aureus strains were susceptible to quinupristin-dalfopristin, but macrolide resistance was not uncommon, especially among methicillin-resistant S. aureus strains. All strains were susceptible to vancomycin. From that series of 516 isolates, 39 strains were selected to provide roughly equal numbers of strains susceptible and resistant to methicillin, erythromycin, and clindamycin. These 39 strains were then tested by the broth microdilution method for susceptibility to quinupristin and dalfopristin alone, as well as quinupristin-dalfopristin, erythromycin, clindamycin, vancomycin, and oxacillin (with 2% NaCl).
TABLE 1.
Susceptibility of 516 recent clinical S. aureus isolates to four antibiotics
| S. aureus strains (no.) and antimicrobial agent | MIC (μg/ml)
|
% of strains susceptiblea | ||
|---|---|---|---|---|
| Range | For 50% of strains | For 90% of strains | ||
| Methicillin resistant (199) | ||||
| Quinupristin-dalfopristin | ≤0.12–2.0 | 1.0 | 1.0 | 94.0 |
| Erythromycin | ≤0.12–>16 | >16 | >16 | 7.0 |
| Clindamycin | ≤0.12–>16 | >16 | >16 | 13.1 |
| Methicillin susceptible (317) | ||||
| Quinupristin-dalfopristin | ≤0.12–2.0 | 0.5 | 0.5 | 99.1 |
| Erythromycin | ≤0.12–>16 | 0.5 | >16 | 79.2 |
| Clindamycin | ≤0.12–>16 | ≤0.12 | 0.25 | 96.2 |
Susceptible to erythromycin and clindamycin at ≤0.5 μg/ml and quinupristin-dalfopristin at ≤1.0 μg/ml.
Time-kill tests were performed with quinupristin-dalfopristin and vancomycin (as the control drug) following the principles outlined by the National Committee for Clinical Laboratory Standards (16). The drug concentrations used in these studies were 10 μg/ml for quinupristin-dalfopristin and 20 μg/ml for vancomycin. For both drugs, these concentrations were 10 to 40 times their MICs for the organisms tested but were equivalent to readily achievable blood levels with standard dosing (2, 4, 8). The initial inocula were targeted to be 1.5 × 106 CFU/ml. Colony counts were performed on the control suspension (no antibiotic) at time zero and on the control and both antibiotic suspensions at 1, 3, 6, 8, and 12 h. A drug was considered bactericidal if it produced a ≥3-log10 reduction in colony counts during this incubation period (≥99.9% killing).
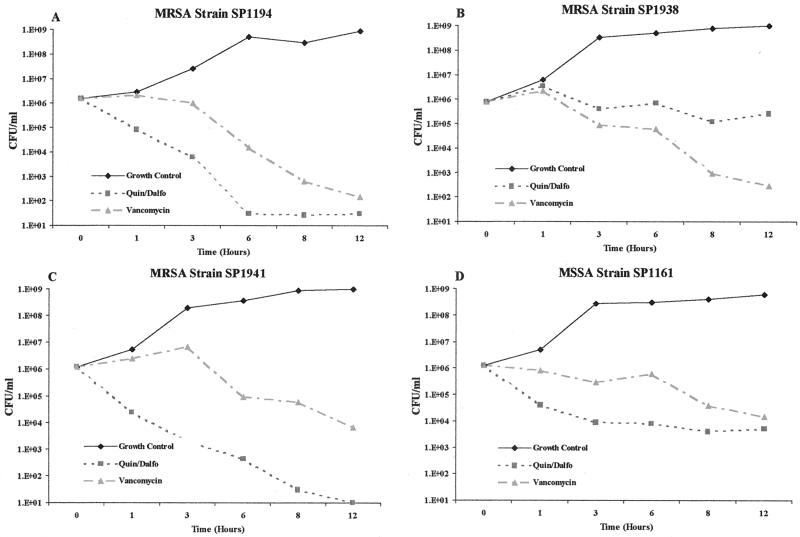
Figure 1 provides examples of typical time-kill curves achieved with quinupristin-dalfopristin and vancomycin. When both drugs were bactericidal (Fig. 1A), the time required to achieve ≥99.9% killing was generally 2 to 6 h shorter for quinupristin-dalfopristin than for vancomycin. That is consistent with the findings of Hoban et al. (9). Overall, quinupristin-dalfopristin was bactericidal for 56% of our 39 selected strains and vancomycin was bactericidal for 64% of these isolates.
FIG. 1.
Kill curves for four strains of methicillin-resistant S. aureus (MRSA) for which both drugs were bactericidal (A) vancomycin was bactericidal but quinupristin-dalfopristin (Quin/Dalfo) was not (B); quinupristin-dalfopristin was bactericidal but vancomycin was not (C); and neither drug was bactericidal (D).
The MICs of dalfopristin ranged from 2.0 to 16 μg/ml, with 35 (90%) of the dalfopristin MICs being 4.0 or 8.0 μg/ml. MICs of dalfopristin could not predict the bactericidal activity of quinupristin-dalfopristin. However, quinupristin MICs did correlate well with clindamycin MICs and with the bactericidal activity of quinupristin-dalfopristin (Table 2). Quinupristin-dalfopristin was bactericidal for all clindamycin-susceptible isolates, including the five that were erythromycin resistant. Furthermore, none of the clindamycin-resistant strains were killed by quinupristin-dalfopristin. With one exception, isolates for which the quinupristin MICs were ≤16 μg/ml were clindamycin susceptible and were killed by quinupristin-dalfopristin and those for which the quinupristin MICs were ≥32 μg/ml were not. The one exception was a clindamycin-resistant isolate for which the quinupristin MIC was 8.0 μg/ml that was not killed by quinupristin-dalfopristin. Although the quinupristin MICs were ≤16 μg/ml for all clindamycin-susceptible strains, the geometric mean quinupristin MIC for those strains that were erythromycin resistant was higher (8.0 μg/ml) than for those that were erythromycin susceptible (3.0 μg/ml). However, quinupristin-dalfopristin was bactericidal for both phenotypes.
TABLE 2.
Susceptibility patterns of 39 S. aureus strains when divided by clindamycin and erythromycin susceptibility phenotype
| Phenotypea | No. of strains | No. with quinupristin MICs (μg/ml) of:
|
No. (%) Oxarb | Q-D MICsc | No. (%) of strains with ≥99.9% killing by:
|
||
|---|---|---|---|---|---|---|---|
| ≤16 | ≥32 | Quinupristin-dalfopristin | Vancomycin | ||||
| Clins Erys | 17 | 17 | 0 | 4 (24) | 0.25–0.5 | 17 (100) | 12 (71) |
| Clins Eryr | 5 | 5 | 0 | 3 (60) | 0.25–0.5 | 5 (100) | 3 (60) |
| Clinr Eryr | 17 | 1 | 16 | 14 (82) | 0.5–1.0 | 0 | 10 (59) |
Clins and Clinr, clindamycin susceptible and resistant, respectively; Erys and Eryr, erythromycin susceptible and resistant, respectively.
Oxar, oxacillin resistant.
Q-D MICs; quinupristin-dalfopristin MIC range (μg/ml).
These data confirm the observation that MLSB-resistant strains of S. aureus are not killed by quinupristin-dalfopristin (5). Furthermore, the data strongly suggest that clindamycin susceptibility is a good surrogate indicator of quinupristin-dalfopristin in vitro bactericidal activity; MICs of quinupristin alone may also serve as a useful surrogate. Since clindamycin is a common component of gram-positive susceptibility test panels, it may provide useful information for the clinical laboratory in this regard. It should be noted, however, that there are multiple mechanisms of MLSB resistance among staphylococci (11), and these were not determined for the isolates studied here. The ermA gene is by far the most prevalent determinant of MLSB resistance in S. aureus (12), and it is reasonable to assume that the majority, if not all, of our MLSB-resistant strains resulted from this determinant. Whether other determinants of MLSB resistance would yield similar results remains to be determined.
Acknowledgments
This study was supported by a financial grant from Rhone-Poulenc Rorer, Collegeville, Pa.
REFERENCES
- 1.Archer G L, Auger P, Doern G V, Ferraro M J, Fuchs P C, Jorgensen J H, Low D E, Murray P R, Reller L B, Stratton C W, Wennersten C B, Moellering R C., Jr RP-59500, a new streptogramin, highly active against recent isolates of North American staphylococci. Diagn Microbiol Infect Dis. 1993;16:223–226. doi: 10.1016/0732-8893(93)90113-l. [DOI] [PubMed] [Google Scholar]
- 2.Bergeron M, Montay G. The pharmacokinetics of quinupristin/dalfopristin in laboratory animals and in humans. J Antimicrob Chemother. 1997;39(Suppl. A):129–138. doi: 10.1093/jac/39.suppl_1.129. [DOI] [PubMed] [Google Scholar]
- 3.Brumfitt W, Hamilton-Miller J M T, Shah S. In vitro activity of RP 59500, a new semisynthetic streptogramin antibiotic, against Gram-positive bacteria. J Antimicrob Chemother. 1992;30(Suppl. A):29–37. doi: 10.1093/jac/30.suppl_a.29. [DOI] [PubMed] [Google Scholar]
- 4.Etienne S D, Montay G, Le Liboux A, Frydman A, Garaud J J. A phase I, double-blind, placebo-controlled study of the tolerance and pharmacokinetic behaviour of RP 59500. J Antimicrob Chemother. 1992;30(Suppl. A):123–131. doi: 10.1093/jac/30.suppl_a.123. [DOI] [PubMed] [Google Scholar]
- 5.Fantin B, Leclercq R, Merle Y, Saint-Julien L, Veyrat C, Duval J, Carbon C. Critical influence of resistance to streptogramin B-type antibiotics on activity of RP 59500 (quinupristin-dalfopristin) in experimental endocarditis due to Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:400–405. doi: 10.1128/aac.39.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fass R J. In vitro activity of RP 59500, a semisynthetic injectable pristinamycin, against staphylococci, streptococci, and enterococci. Antimicrob Agents Chemother. 1991;35:553–559. doi: 10.1128/aac.35.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goto S, Miyazaki S, Kaneko Y. The in vitro activity of RP 59500 against Gram-positive cocci. J Antimicrob Chemother. 1992;30(Suppl. A):25–28. doi: 10.1093/jac/30.suppl_a.25. [DOI] [PubMed] [Google Scholar]
- 8.Griswold M W, Lomaestro B M, Briceland L L. Quinupristin-dalfopristin (RP 59500): an injectable streptogramin combination. Am J Health Syst Pharm. 1996;53:2045–2053. doi: 10.1093/ajhp/53.17.2045. [DOI] [PubMed] [Google Scholar]
- 9.Hoban D J, Weshnoweski B, Palatnick L, Zhanel C G, Davidson R J. In vitro activity of streptogramin RP 59500 against staphylococci including bacterial kinetic studies. J Antimicrob Chemother. 1992;30(Suppl. A):59–65. doi: 10.1093/jac/30.suppl_a.59. [DOI] [PubMed] [Google Scholar]
- 10.Jones M E, Visser M R, Klootwijk M, Heisig P, Verhoef J, Schmitz F-J. Comparative activities of clinafloxacin, grepafloxacin, levofloxacin, moxifloxacin, ofloxacin, sparfloxacin, and trovafloxacin and nonquinolones linozelid, quinupristin-dalfopristin, gentamicin, and vancomycin against clinical isolates of ciprofloxacin-resistant and -susceptible Staphylococcus aureus strains. Antimicrob Agents Chemother. 1999;43:421–423. doi: 10.1128/aac.43.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leclercq R, Nantas L, Soussy C J, Duval J. Activity of RP 59500, a new parenteral semisynthetic streptogramin, against staphylococci with various mechanisms of resistance to macrolide-lincosamide-streptogramin antibiotics. J Antimicrob Chemother. 1992;30(Suppl. A):67–75. doi: 10.1093/jac/30.suppl_a.67. [DOI] [PubMed] [Google Scholar]
- 12.Lina G, Quaglia A, Reverdy M, Leclercq R, Vandenesch F, Etienne J. Distribution of genes encoding resistance to macrolides, lincosamides, and streptogramins among staphylococci. Antimicrob Agents Chemother. 1999;43:1062–1066. doi: 10.1128/aac.43.5.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Low D E. Quinupristin/dalfopristin: spectrum of activity, pharmacokinetics, and initial clinical experience. Microb Drug Resist. 1995;1:223–234. doi: 10.1089/mdr.1995.1.223. [DOI] [PubMed] [Google Scholar]
- 14.Low D E, Nadler H L. A review of in-vitro antibacterial activity of quinupristin/dalfopristin against methicillin-susceptible and -resistant Staphylococcus aureus. J Antimicrob Chemother. 1997;39(Suppl. A):53–58. doi: 10.1093/jac/39.suppl_1.53. [DOI] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Methods for determining bactericidal activity of antimicrobial agents. Proposed guideline M26-P. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1987. [Google Scholar]
- 17.Neu H C, Chin N, Gu J. The in vitro activity of new streptogramins, RP 59500, RP57669, and RP 54476, alone and in combination. J Antimicrob Chemother. 1992;30(Suppl. A):83–94. doi: 10.1093/jac/30.suppl_a.83. [DOI] [PubMed] [Google Scholar]
- 18.Pechère J C. In vitro activity of RP 59500, a semisynthetic streptogramin, against staphylococci and streptococci. J Antimicrob Chemother. 1992;30(Suppl. A):15–18. doi: 10.1093/jac/30.suppl_a.15. [DOI] [PubMed] [Google Scholar]