Abstract
Background
The range of the ornate dog tick Dermacentor reticulatus is rapidly expanding in Europe. This tick species is the vector of canine babesiosis, caused by Babesia canis, and also plays a role in the transmission of Theileria equi and Babesia caballi in equids.
Methods
The geographic range of D. reticulatus in the Czech Republic was re-assessed, and an up-to-date distribution map is presented based on material and data obtained during a nationwide citizen science campaign. Received and flagged individuals of D. reticulatus were also analysed for the presence of B. canis DNA.
Results
In striking contrast to historical records, D. reticulatus was found in all regions of the Czech Republic, with most reports coming from the southeast and northwest of the country. Between February 2018 and June 2021, the project team received 558 photo reports of ticks and 250 packages containing ticks. Of the former, 71.1% were identified as Dermacentor sp. with the remainder identified as Ixodes sp., Haemaphysalis sp., Argas sp. or Hyalomma sp. The majority of specimens in the subset of ticks that were received (N = 610) were D. reticulatus (N = 568, 93.7%), followed by Ixodes ricinus and Hyalomma spp. A total of 783 adult D. reticulatus, either received (568) or collected by flagging (215), were tested for the presence of B. canis DNA using species-specific nested PCR targeting part of the 18S rRNA gene; B. canis DNA was demonstrated in 22 samples (2.81%).
Conclusions
The continuous spread of D. reticulatus in the Czech Republic was documented in this study. In addition, DNA of B. canis was also detected in a number of ticks, suggesting the establishment of B. canis in the Czech Republic. These results suggest that veterinarians need to consider the possibility of canine babesiosis even in dogs without a history of travel.
Graphical Abstract
Keywords: Dermacentorreticulatus, Babesiacanis, Citizen science, Czech Republic, Geographic distribution, Europe
Background
Environmental and societal changes attributable to the climate change have a significant impact on the spread of vector-borne diseases and their arthropod vectors [1]. There are also species in the tick fauna whose ranges are changing rapidly. Rhipicephalus microplus is a prominent example of a tick associated with cattle that has invaded both tropical and subtropical areas [2, 3]. In general, changes in the geographic ranges of ticks occur in two ways, although these may overlap. The first is long-distance dispersal followed by local dispersal. Range expansion in this way is usually associated with transboundary animal movements. An example of such range expansion is the Asian tick Haemaphysalis longicornis and its recent spread into the northeastern and southeastern regions of the USA [4, 5]. The second form of range expansion is a gradual expansion of geographic range (usually to higher altitudes or latitudes) due to environmental and climate changes associated with host migration. The ticks Amblyomma americanum and A. maculatum have shown this type of expansion in North America [6, 7].
The most prominent example of a tick showing a rapidly expanding distribution in Europe is the ornate dog tick Dermacentor reticulatus [8], one of two members of the genus Dermacentor distributed in Europe [9]. Its geographic range is highly focal and discontinuous, and consists of two main macroregions [10, 11]. The first is the western European macroregion, which extends from northern Spain to western Poland, France, with isolated foci in the Netherlands, Belgium, Hungary, the Czech Republic, Slovakia and Germany. The second is the eastern European macroregion, which extends from eastern Poland to the Baltic States and Russia [9, 12]. The ornate dog tick occurs much further north than its congener Dermacentor marginatus, reaching the British Isles [13], northern Germany [14], Poland [15] and the Baltic States [16–18], and it may be also spreading southward [19, 20].
Dermacentor reticulatus is a three-host tick that circulates among rodents (larvae and nymphs) and larger carnivores and herbivores (adults). Consequently, it is associated with the transmission of a number of tick-borne pathogens throughout its range, including tick-borne encephalitis virus Rickettsia raoultii and Rickettsia slovaca [8, 21]. Dermacentor reticulatus is the only vector of canine babesiosis, which is caused by Babesia canis. In temperate Europe this tick may also play a role in the transmission cycle of Theileria equi and Babesia caballi, the two piroplasmids found in equids [8, 22].
The occurrence of D. reticulatus in the Czech Republic was described as early as 1952 by Rosický [23] in the southeastern corner of the country in Tvrdonice (Břeclav district), and it presence was confirmed in the 1970s in the same region [24, 25] (Fig. 1). In a recent comprehensive study carried out in 2009–2010 [26], the tick was found in 46 out of 100 surveyed sites in the South Moravia region, more or less defining the distribution limits of D. reticulatus in the Czech Republic (Fig. 2d). Until recently, babesiosis caused by B. canis was considered to be an imported disease in the Czech Republic. The presence of B. canis DNA was detected for the first time in the Czech Republic in 2017 in shelter dogs in the core area of D. reticulatus distribution in the South Moravia region, and the first clinical autochthonous case of B. canis was diagnosed 1 year later in a non-travelling dog in the South Moravia region [27, 28].
Fig. 1.

Typical habitat of Dermacentor reticulatus along the Morava River at the border with Slovakia (a) and close-up picture of questing male in the same locality (b)
Fig. 2.
Georeferenced findings of D. reticulatus and Babesia canis in the Czech Republic. a Morphologically confirmed records of D. reticulatus based on the received ticks, b findings based on photo reports (tentatively identified as D. reticulatus), c finding of B. canis DNA as revealed by PCR targeting partial 18S rDNA, d previous reports of D. reticulatus extracted from Široký et al. [26]. Green colour in a–c indicates reported travel history (of dogs) in the 2 weeks prior to the observation
Lewis et al. [29] reviewed the role of citizen science in tick research and presented an analysis of the advantages and disadvantages of this strategy. Recent nationwide mapping of ticks in the Netherlands [30] and Germany [14] are examples of different citizen science approaches that have been used in European projects. While the Dutch study used trained volunteers to collect ticks in defined areas, the latter study collected Dermacentor spp. ticks sent in by mail by citizens.
The aim of our study was to redefine the geographic range of D. reticulatus in the Czech Republic and to present an up-to-date distribution map based on georeferenced material obtained during a nationwide citizen participatory science campaign. We also present data on the prevalence of B. canis DNA in received and flagged ticks.
Methods
Citizen science
The citizen science project “Najdi pijáka” (literally translated from Czech as: “Find the ornate tick”) was launched by the project team in 2018. An informative website (www.najdipijaka.cz) for the public was created in the Czech language and launched in late February 2018 with a contact email address and subsequent Facebook account (FB) for receiving photo reports and communicating with individuals who provide tick records. The project website included three parts: (i) a brief description of the project aims and research team; (ii) the basic biology of D. reticulatus and key morphological features that allow easy differentiation between ticks of the genera Ixodes and Dermacentor in Central Europe; and (iii) information on how to report observations of target ticks and how to safely turn in collected specimens. The final section included simple instructions on how to take photographs, the accompanying data that were necessary (date of find/collection, location, host), tips on how to send the ticks and records by mail, email or via a social media account and contact information. A key component of the website was a map showing current distribution data. To maximise public awareness, the project was announced through various media sources during its duration (February 2018–June 2021). The releases always included images comparing D. reticulatus with Ixodes ricinus and a link to the project website. The importance of D. reticulatus as a vector of canine babesiosis was emphasised in the press releases, which were primarily directed to dog owners. The research team informed the participants about the identification of the received ticks (both physical and photo reports) and shared the results of molecular detection of B. canis in the received ticks. Through the email account and FB, the research team communicated with participants and answered various questions related to D. reticulatus and B. canis infection. All participants either provided or were asked to provide additional information for the report to be added to the data collection. These consisted of: (i) site of discovery (either GPS coordinates or address); (ii) date of discovery; (iii) host association; and (iv) travel history of the associated/perceived host in the past 14 days.
Tick collection and identification
To expand the dataset of ticks used for B. canis detection, material collected within the framework of the citizen science project was supplemented with ticks collected by the project team using a white cotton flag (79 × 94 cm) attached to a 150-cm wooden pole [31]. The collection sites were based on data obtained from the citizen science part of the project and from published reports on the distribution of D. reticulatus [26]. A total of seven collections were made at five sites in the southeastern part of the Czech Republic (South Moravia region) in April (5 collections) and September (2 collections) 2019. Only adult ticks were collected.
Tick reports consisting of photographs (photo reports) were identified to the genus level based on general appearance, body and leg shape, and pattern of scutum. All ticks received and collected were identified to the species level based on their morphology. The identification of the Dermacentor ticks received/collected was performed using the key to the species of genus Dermacentor in Europe and Northern Africa [18] by microscopic observation (model SZ51; Olympus Corp., Tokyo, Japan). A tick was placed dorsal side up on a microscopic slide with forceps, and the species was determined by the presence of a distinct, posteriorly directed spur on the dorsal palp article II [18].
DNA isolation and B. canis detection
Ticks were stored in individual tubes containing DNase-free water in the freezer before DNA isolation. Each tick was cut in half lengthwise. DNA was extracted from one half using the Exgene Cell SV Mini 250p Kit (GeneAll, Seoul, Korea) according to the standard protocol for animal tissues, with 50 µl of elution buffer added in the final step. The unused tick half was stored under the above conditions for future experiments or re-analysis.
For detection of B. canis in isolated DNA, part of the small subunit of the 18S ribosomal RNA (rRNA) gene was targeted using nested PCR. The 376-bp fragment of 18S rDNA was amplified using primers Bc_F1, GR2 and Bc_F2, Bc_R1 [32, 33]. The first round of PCR was prepared in a total volume of 15 µl, which included 7.5 µl of 2× PCRBIO Taq Mix Red (PCR Biosystems, London, UK), 7.5 pmol of each primer (Bc_F1 and GR2) and 2 µl of template DNA. PCR conditions for the first round consisted of an initial denaturation at 95 °C for 1 min, followed by 35 cycles of denaturation at 95 °C for 15 s, annealing at 50 °C for 15 s and extension at 72 °C for 5 s, with a final extension at 72 °C for 5 min. The second round of PCR was performed in a total volume of 25 µl, which included 12.5 µl of 2× PCRBIO Taq Mix Red, 10 pmol of each primer (Bc_F2 and Bc_R1) and 1 µl of the PCR product from the first round). The PCR conditions for the second round consisted of an initial denaturation at 95 °C for 1 min, followed by 35 cycles of denaturation at 95 °C for 15 s, annealing at 53 °C for 15 s and extension at 72 °C for 15 s, with a final extension at 72 °C for 5 min. The PCR products were visualised in a 2% agarose gel stained with Midori Green Advance DNA Stain (Nippon Genetics Europe, Düren, Germany). All samples that yielded an amplicon of the appropriate size were excised and purified using the Gel PCR DNA Fragments Extraction Kit (Geneaid, New Taipei City, Taiwan). The purified PCR products were sent for commercial Sanger sequencing (Macrogen Europe, Amsterdam, The Netherlands). Geneious Prime software (Biomatters, Auckland, New Zealand) was used to assemble and analyse the sequences obtained.
Results
Identification of ticks received
In total, the project team received 558 photo reports and 250 packages containing ticks (Table 1); 71.1% of the photo reports were identified as Dermacentor sp. and the remaining ticks on the photo reports were identified as Ixodes sp., Hyalomma sp., Haemaphysalis sp. and Argas sp. In the subgroup of identified received ticks (N = 610), the majority of ticks were identified as D. reticulatus (N = 568, 93.1%), followed by Ixodes ricinus and Hyalomma spp. (Table 1). The number of received ticks ranged from 1 to 102 ticks per package.
Table 1.
Records of ticks received as a result of the citizen science campaign either as photographs (photo reports) or as ticks delivered by post (received ticks)
| Type of report | Dermacentor | Ixodes | Hyalomma | Haemaphysalis | Argas |
|---|---|---|---|---|---|
| Photo reporta | 397 | 140 | 3 | 9 | 9 |
| Ticks receivedb | 568 (212) | 30 (26) | 12 (12) | 0 | 0 |
aNumbers indicate the record numbers; it was not possible to evaluate the real number of observed ticks
bNumbers in brackets indicate the number of reports
Geographic distribution of D. reticulatus
The site of discovery was indicated in all 609 reports of Dermacentor ticks (see Fig. 2a–c, with the location of tick discovery indicated in green together with reported travel history (of dogs). As the determination of the ticks to species level was possible only in physically received specimens (568 ticks in 212 records), distribution data for physically received ticks and photo records are shown separately in the maps shown in Fig. 2 and 2b, respectively. Dermacentor ticks were reported in all 14 regions of the Czech Republic. The majority of findings (N = 429, 70.4% of reports) were from the South Moravia region; this part of the distribution is termed the core area hereafter. The second most represented region was the Ústí nad Labem region in Northern Bohemia (N = 57, 9.4% of reports). However, individuals of D. reticulatus were reported from the whole Czech Republic (Fig. 2a, b).
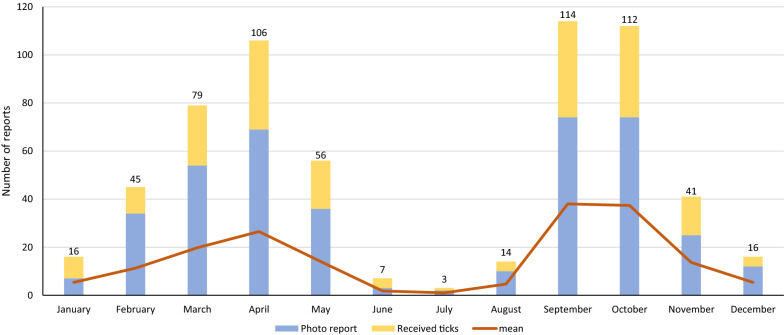
Seasonality of D. reticulatus findings and host association
The date of collection/observation was available for all 609 Dermacentor observations reported through this citizen science project. Analysis of the monthly tick occurrence data (Fig. 3) reveals an obvious seasonality, with peaks in the spring and early fall. Host association information for the reported Dermacentor ticks was available for 463 of the 609 records (Table 2). Most of these finds (80.6%) were reported from dogs, followed by finds from humans. A minority of reported D. reticuatus ticks were observed on other animals or in the environment. The number of ticks observed per host ranged from 1 to 40 individuals, with several ticks occurring almost exclusively on dogs. Of the 373 reports of ticks on dogs, 29 indicated recent travel of the dog within the Czech Republic (24), Slovakia (4) and Germany (1).
Fig. 3.
Seasonal activity of D. reticulatus. Cumulative number of Dermacentor findings reported through the citizen science project in each month (February 2018–June 2021) shows bimodal seasonal distribution and occurrence in winter months. As not all the months were equally represented “Mean” refers to the number of reports in that month divided by the number of that specific month
Table 2.
Reported host association of Dermacentor findings
| Hosts | Findings |
|---|---|
| Dog | 373 |
| Human | 68 |
| Horse | 5 |
| Cat | 2 |
| Lama | 1 |
| Environment | 18 |
| Data not provided | 149 |
Numbers indicate the number of photo record/parkages; for photo records, the real number of ticks could not be evaluated
Occurrence of B. canis in examined ticks
All physically received (568) or flagged (215) D. reticulatus individuals were tested for the presence of B. canis DNA. The amplicon of the expected size (376 bp) was detected in 22 samples (2.81%). Nineteen positive ticks were sent in by citizen scientists, and three positive ticks (2 females, 1 male) were collected by flagging. Of the 19 ticks sent in, 17 were found on dogs (11 males, 6 females) and two were found on a person (both females). Based on only ticks from the core area, there were 18 positive results out of 566 tests (3.18%). Sequencing resulted in high-quality, 100% identical sequences of 320–357 bp (GenBank acc. no. OK135945, this study). The BLAST analysis confirmed 100% identity and query cover of the sequence to more than 50 different B. canis sequences from different geographic regions and/or hosts, including sequences from the Czech Republic and surrounding countries (e.g. MK024714 from a domestic dog, Poland; KY693669 from a red fox, Austria; KY021188 from a domestic dog, Czech Republic).
Discussion
Citizen science is a research concept based on the concept of using interested citizens to investigate various scientific topics. Field biology and wildlife monitoring are one of the areas where citizen science has gained prominence in recent decades [34]. Dermacentor spp. are highly recognisable ticks, so we were able to use both photographic reports and physically received ticks in our geographic survey. The ability to participate in this study by submitting only a photograph facilitated the participation of a large number of interested individuals and increased the reliability of the data collected. Although tick identification can be difficult for untrained volunteers, participants in our study were able to identify ticks in most cases and contributed immensely to mapping the distribution of D. reticulatus (Table 1).
Since only the sent in and flagged ticks could be determined to species level, all photo reports were considered to be Dermacentor sp. While the genus Dermacentor is represented in Europe by D. reticulatus and D. marginatus [9], there are currently no records of D. marginatus in the Czech Republic. The nearest known populations to the Czech Republic are those in Germany, in the Rhine and Main valleys [35], and in Slovakia [25, 36]. Since no significant spread of D. marginatus has been observed in the German population [14] and there are no recent data indicating dispersal in Slovakia, it is very likely that all photo reports of Dermacentor from the Czech Republic are D. reticulatus.
The most recent data published by Široký et al. in 2011 [26] demonstrated the occurrence of D. reticulatus only in the South Moravia region. The majority of the records collected in our study are also from this region. However, according to our data, the population of D. reticulatus has expanded to an area between 16.2–17.5 °E and 48.7–49.25 °N, making it the core area of distribution in the Czech Republic (the core area). Participants in our citizen science campaign recorded D. reticulatus ticks in all regions of the Czech Republic, with a clustering of reports in the northwestern part of the country, indicating that a subpopulation has been established in this region. Most other reports outside the core area were presented as sporadic findings. A recent travel history of dogs was reported for 29 of these findings, and 15 dog owners reported travelling to the core area of D. reticulatus in the Czech Republic prior to tick observation (Fig. 2a–c). It would appear that the movement of dogs is a route by which D. reticulatus spreads in the Czech Republic. According to our observations, most Dermacentor ticks observed on dogs were found as freely moving adult individuals. As such, these ticks are not affected by oral ectoparasiticides based on isoxazolines and avermectins and can spontaneously leave the host to infest new areas. In the same way, detached, engorged females can establish new populations. Therefore, a more thorough survey of areas where the presence of D. reticulatus has been repeatedly reported is needed to assess the distribution of this tick species in the Czech Republic and the resulting risk of canine babesiosis transmission.
Currently, D. reticulatus is present in all neighbouring countries of the Czech Republic [9]. In Slovakia it is a common tick [37, 38], and from Austria there are records of observations in the northeastern part of the country and around Vienna [39, 40]. The core population of D. reticulatus in the Czech Republic is likely associated with the distribution area in these two countries. Similarly, the distribution of D. reticulatus in Poland [15, 41] and Germany [14, 42] is well documented. In particular, the German foci in Saxony are located in close proximity to the Czech border. It is therefore quite possible that some (if not all) observations of the ornate dog tick in the northwestern part of the Czech Republic are due to dispersal from Germany (and possibly Poland) rather than to dispersal of the core population in the southeastern part of the Czech Republic.
The spread of D. reticulatus is commonly attributed to climatic and socioeconomic changes as suitable habitats for the ticks or their hosts emerge due to a warmer climate, changes in agricultural use of the landscape and/or increased migration and colonisation of vacant territories by vertebrate hosts [42, 43]. The ecology of ticks and their preferred hosts may play a critical role in range expansion. Dermacentor reticulatus is a three-host tick with a shorter life-cycle and higher cold tolerance compared to I. ricinus [18, 44]. Larvae and nymphs usually feed on smaller mammals and occasionally birds, while adult ticks use larger herbivores and carnivores as hosts [45, 46]. Our data show that dogs are the most important host species of the ornate dog tick (Table 2); however, these findings are heavily impacted by the citizen science campaign. Dogs play an important role in the life-cycle of D. reticulatus, especially in urban areas [8], but other large mammals, such as roe deer Capreolus capreolus, red deer Cervus elaphus and wild boar Sus scrofa are also involved in the life-cycle of this tick [47]. The grey wolf Canis lupus, Eurasian golden jackal Canis aureus and the elk Alces alces can all harbour D. reticulatus, and all three of these species also have great migratory potential [48–51]. In the last decade, the wolf population has become established in the northern parts of the Czech Republic [52]. Genetic analyses show that these wolves belong to the so-called lowland population that is spreading into the Czech Republic from Germany and Poland [53]. During necropsies of wolf carcasses found in the northern part of the Czech Republic (data not shown; University of Veterinary Sciences Brno), we found high numbers of D. reticulatus in three cases, suggesting that these predators may play a role in the spread of this tick. The spread of the D. reticulatus population in the Czech Republic is likely due to a combination of several factors: (i) direct links to nearby populations in Germany and Poland [14, 41]; (ii) migration of host species, especially large carnivores; (iii) movement of resident dogs with their owners across the country.
Dermacentor reticulatus is the only confirmed definitive host of B. canis [22]. Canine babesiosis caused by B. canis is becoming more common in all neighbouring countries of the Czech Republic [54–56]; however, cases in the Czech Republic are still very rare. The only documented autochthonous clinical case of canine babesiosis was recently reported from the core area of D. reticulatus in the Czech Republic [28]. All ticks positive for B. canis DNA were reported from the southeastern part of the Czech Republic, mainly from the core population; however, this may be due to the relatively small number of samples from other parts of the country. Although 17 of 22 positive D. reticulatus were found on dogs in our study, none of the owners reported clinical signs attributable to canine babesiosis. One explanation is that none of the positive female ticks were engorged. In addition, most of these ticks were found crawling on the host shortly after a walk, indicating that there was insufficient time for transmission. Since none of these dogs were tested for the presence of B. canis, they could be asymptomatic carriers of the disease [57]. Babesia canis is also known to be a parasite in the Eurasian golden jackal [27] and grey wolf [58], and a recent study from Poland has also demonstrated the presence of B. canis DNA in the red fox Vulpes vulpes [59]. Because all of these canids co-occur in the Czech Republic, it is possible that they play a role in maintaining B. canis foci, as the number of infected dogs is minimal. Transovarial transmission of B. canis must also be considered. Dermacentor reticulatus could also be a vector of B. caballi and T. equi [8, 60, 61], both of which were recently detected in the Czech Republic [62]. Although the autochthonous nature of equine piroplasmosis remains to be confirmed, the growing population of D. reticulatus may play a role in creation of an environment suitable for the persistence of endemic transmission cycles of these piroplasmids.
Dermacentor reticulatus is known for its seasonal activity [63]. The data we obtained show year-round activity with peaks in the spring and early fall. Interestingly, we received more reports from the winter months (December to February) than from the summer months (June to August), showing a similar pattern as described by other authors [64]. These results have striking practical implications for protecting dogs from this tick as they suggest that preventive measures in the form of repellents or acaricides should be applied at least from early spring to late fall and ideally, particularly in lowland regions, throughout the year. Although we detected the presence of B. canis only in ticks from the core population, veterinarians should be aware of the presence of B. canis in the Czech Republic and consider this disease regardless of the travel history of their canine patient, as a continuous spread of B. canis and its vector in the Czech Republic is expected.
Conclusions
The continuous spread of D. reticulatus in the Czech Republic was documented in this study by a citizen science project. The core population of this tick, described in a previous study [26], has significantly expanded its range since the publication of the study. In addition, D. reticulatus has been observed in all regions of the Czech Republic, with most reports coming from the southeast and northwest of the country. Babesia canis DNA was also detected in a number of ticks (2.81%), mainly in the core population of D. reticulatus, the only known vector of this piroplasm. These findings indicate that B. canis has become established in the Czech Republic, necessitating extensive research on potential reservoir hosts in the region. They also indicate that the number of endemic cases of canine babesiosis may be increasing and that veterinarians should not consider this disease to be only as an imported one. Also, based on seasonality data on D. reticulatus activity, dog owners should be advised to protect their dogs with acaricides at least from early spring to late fall but ideally throughout the year.
Acknowledgements
We would like to thank Martin Modrý for designing the Najdi pijáka website, maintaining our databases and providing technical advice related to the citizen science project. We would like to thank all the participants of the Najdi pijáka citizen science project for their invaluable contribution in data collection.
Authors' contributions
DM and KH designed the study. DK and OD coordinated the citizen science project and communication with public as well as laboratory work and molecular analysis, with KH supervising their laboratory work. DJ analysed geographic data and prepared the maps. OD, DM and KH worked on the manuscript. All authors read and approved the final manuscript.
Funding
This study is part of the project QK1920258, financed by the Ministry of Agriculture of the Czech Republic and is further supported by the Ministry of Education, Youth and Sports of the Czech Republic under the project CEITEC 2020 (LQ1601). KH was supported by the project Nr. CZ.02.1.01/0.0/0.0/16_019/0000787 ‘Fighting Infectious Diseases' provided by the Ministry of Education Youth and Sports of the Czech Republic.
Availability of data and materials
The nucleotide sequence generated in the present study has been deposited in GenBank (https://www.ncbi.nlm.nih.gov/) under accession number OK135945. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ondřej Daněk, Email: danek.skvrnity@seznam.cz.
Kristýna Hrazdilová, Email: kristyna@hrazdilova.cz.
Dominika Kozderková, Email: dominika.mazgutova20@gmail.com.
Daria Jirků, Email: daska229@gmail.com.
David Modrý, Email: modrydav@gmail.com.
References
- 1.Medlock JM, Leach SA. Effect of climate change on vector-borne disease risk in the UK. Lancet Infect Dis. 2015;15:721–730. doi: 10.1016/S1473-3099(15)70091-5. [DOI] [PubMed] [Google Scholar]
- 2.Madder M, Thys E, Achi L, Touré A, De Deken R. Rhipicephalus(Boophilus)microplus: a most successful invasive tick species in West-Africa. Exp Appl Acarol. 2011;53:139–145. doi: 10.1007/s10493-010-9390-8. [DOI] [PubMed] [Google Scholar]
- 3.Adakal H, Biguezoton A, Zoungrana S, Courtin F, de Clercq EM, Madder M. Alarming spread of the Asian cattle tick Rhipicephalusmicroplus in West Africa-another three countries are affected: Burkina Faso, Mali and Togo. Exp Appl Acarol. 2013;61:383–386. doi: 10.1007/s10493-013-9706-6. [DOI] [PubMed] [Google Scholar]
- 4.Egizi A, Bulaga-Seraphin L, Alt E, Bajwa WI, Bernick J, Bickerton M, et al. First glimpse into the origin and spread of the Asian longhorned tick, Haemaphysalislongicornis, in the United States. Zoonoses Public Health. 2020;67:637–650. doi: 10.1111/zph.12743. [DOI] [PubMed] [Google Scholar]
- 5.Ronai I, Tufts DM, Diuk-Wasser MA. Aversion of the invasive Asian longhorned tick to the white-footed mouse, the dominant reservoir of tick-borne pathogens in the USA. Med Vet Entomol. 2020;34:369–373. doi: 10.1111/mve.12441. [DOI] [PubMed] [Google Scholar]
- 6.Childs JE, Paddock CD. The ascendancy of Amblyommaamericanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol. 2003;48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- 7.Pascoe EL, Marcantonio M, Caminade C, Foley JE. Modeling potential habitat for Amblyomma tick species in California. Insects. 2019;10:201. 10.3390/insects10070201. [DOI] [PMC free article] [PubMed]
- 8.Földvári G, Široký P, Szekeres S, Majoros G, Sprong H. Dermacentor reticulatus: a vector on the rise. Parasit Vectors. 2016;9:314. 10.1186/s13071-016-1599-x.. [DOI] [PMC free article] [PubMed]
- 9.Rubel F, Brugger K, Pfeffer M, Chitimia-Dobler L, Didyk YM, Leverenz S, et al. Geographical distribution of Dermacentormarginatus and Dermacentorreticulatus in Europe. Ticks Tick Borne Dis. 2016;7:224–233. doi: 10.1016/j.ttbdis.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Kloch A, Mierzejewska EJ, Karbowiak G, Slivinska K, Alsarraf M, Rodo A, et al. Origins of recently emerged foci of the tick Dermacentorreticulatus in central Europe inferred from molecular markers. Vet Parasitol. 2017;237:63–69. doi: 10.1016/j.vetpar.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Paulauskas A, Galdikas M, Galdikaitė-Brazienė E, Stanko M, Kahl O, Karbowiak G, et al. Microsatellite-based genetic diversity of Dermacentorreticulatus in Europe. Infect Genet Evol. 2018;66:200–209. doi: 10.1016/j.meegid.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 12.Karbowiak G. The occurrence of the Dermacentorreticulatus tick—its expansion to new areas and possible causes. Ann Parasitol. 2014;60:37–47. [PubMed] [Google Scholar]
- 13.Medlock JM, Jameson LJ, Phipps LP. Status of Dermacentorreticulatus in the UK. Vet Rec. 2011;168:386–387. doi: 10.1136/vr.d2186. [DOI] [PubMed] [Google Scholar]
- 14.Drehmann M, Springer A, Lindau A, Fachet K, Mai S, Thoma D, et al. The spatial distribution of Dermacentor ticks (Ixodidae) in Germany—evidence of a continuing spread of Dermacentorreticulatus. Front Vet Sci. 2020;7:578220. 10.3389/fvets.2020.578220. [DOI] [PMC free article] [PubMed]
- 15.Mierzejewska EJ, Estrada-Peña A, Alsarraf M, Kowalec M, Bajer A. Mapping of Dermacentorreticulatus expansion in Poland in 2012–2014. Ticks Tick Borne Dis. 2016;7:94–106. doi: 10.1016/j.ttbdis.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Sidorenko M, Radzijevskaja J, Mickevičius S, Bratčikovienė N, Paulauskas A. Prevalence of tick-borne encephalitis virus in questing Dermacentorreticulatus and Ixodesricinus ticks in Lithuania. Ticks Tick Borne Dis. 2021;12:101594. 10.1016/j.ttbdis.2020.101594. [DOI] [PubMed]
- 17.Paulauskas A, Radzijevskaja J, Mardosaite-Busaitiene D, Aleksandravičiene A, Galdikas M, Krikštolaitis R. New localities of Dermacentorreticulatus ticks in the Baltic countries. Ticks Tick Borne Dis. 2015;6:630–635. doi: 10.1016/j.ttbdis.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Hornok S. Dermacentorreticulatus (Fabricius, 1794) In: Estrada-Peña A, Mihalca ADPT, editors. Ticks of Europe and North Africa. A guide to species identification. Springer: Cham; 2017. pp. 287–291. [Google Scholar]
- 19.Olivieri E, Zanzani SA, Latrofa MS, Lia RP, Dantas-Torres F, Otranto D, et al. The southernmost foci of Dermacentorreticulatus in Italy and associated Babesiacanis infection in dogs. Parasit Vectors. 2016 doi: 10.1186/s13071-016-1502-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Vozmediano A, Giglio G, Ramassa E, Nobili F, Rossi L, Tomassone L. Dermacentormarginatus and Dermacentorreticulatus, and their infection by sfg rickettsiae and Francisella-like endosymbionts, in mountain and periurban habitats of Northwestern Italy. Vet Sci. 2020;7:1–15. doi: 10.3390/vetsci7040157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wójcik-Fatla A, Cisak E, Zajac V, Sroka J, Sawczyn A, Dutkiewicz J. Study on tick-borne rickettsiae in eastern Poland. I. prevalence in Dermacentor reticulatus (Acari: Amblyommidae) Ann Agric Environ Med. 2013;20:276–279. [PubMed] [Google Scholar]
- 22.Solano-Gallego L, Sainz Á, Roura X, Estrada-Peña A, Miró G. A review of canine babesiosis: the European perspective. Parasit Vectors. 2016;9:336. 10.1186/s13071-016-1596-0. [DOI] [PMC free article] [PubMed]
- 23.Rosický B. Důležitá klíšťata rodu Dermacentor v ČSR. Folia Zool Ent Brno. 1952;1:85–89. [Google Scholar]
- 24.Cerný V. The tick fauna of Czechoslovakia. Folia Parasitol (Praha) 1972;19:87–92. [PubMed] [Google Scholar]
- 25.Nosek J. The ecology and public health importance of Dermacentormarginatus and D.reticulatus ticks in Central Europe. Folia Parasitol (Praha). 1972;19:93–102. [PubMed] [Google Scholar]
- 26.Siroký P, Kubelová M, Bednar M, Modry D, Hubálek Z, Tkadlec E. The distribution and spreading pattern of Dermacentorreticulatus over its threshold area in the Czech Republic—How much is range of this vector expanding? Vet Parasitol. 2011;183:130–135. doi: 10.1016/j.vetpar.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Mitková B, Hrazdilová K, D’Amico G, Duscher GG, Suchentrunk F, Forejtek P, et al. Eurasian golden jackal as host of canine vector-borne protists. Parasit Vectors. 2017;10:1–11. doi: 10.1186/s13071-017-2110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Křivánková J, Lásková K, Sitařová B, Hrazdilová K, Modrý D, Hanzlíček D. Autochthonous babesiosis in a dog—a description of clinical case. Veterinarstvi. 2018;763–6.
- 29.Lewis J, Boudreau C, Patterson J, Bradet-Legris J, Lloyd V. Citizen science and community engagement in tick surveillance—a Canadian case study. Healthcare. 2018;6:22. doi: 10.3390/healthcare6010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Martí I, Zurita-Milla R, van Vliet AJH, Takken W. Modelling and mapping tick dynamics using volunteered observations. Int J Health Geogr. 2017;16:1. doi: 10.1186/s12942-017-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dantas-Torres F, Lia RP, Capelli G, Otranto D. Efficiency of flagging and dragging for tick collection. Exp Appl Acarol. 2013;61:119–127. doi: 10.1007/s10493-013-9671-0. [DOI] [PubMed] [Google Scholar]
- 32.Zintl A, Finnerty EJ, Murphy TM, de Waal T, Gray JS. Babesias of red deer (Cervuselaphus) in Ireland. Vet Res. 2011;42:7. doi: 10.1186/1297-9716-42-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sgroi G, Iatta R, Veneziano V, Bezerra-Santos MA, Lesiczka P, Hrazdilová K, et al. Molecular survey on tick-borne pathogens and Leishmaniainfantum in red foxes (Vulpesvulpes) from southern Italy. Ticks Tick Borne Dis. 2021;12:3. doi: 10.1016/j.ttbdis.2021.101669. [DOI] [PubMed] [Google Scholar]
- 34.Frigerio D, Pipek P, Kimmig S, Winter S, Melzheimer J, Diblíková L, et al. Citizen science and wildlife biology: synergies and challenges. Ethology. 2018;124:365–377. doi: 10.1111/eth.12746. [DOI] [Google Scholar]
- 35.Liebisch A, Rahman MS. Prevalence of the ticks Dermacentormarginatus (Sulzer, 1776) and Dermacentorreticulatus (Fabricius, 1794) and their importance as vectors of diseases in Germany. Tropenmed Parasitol Germany. 1976;27:393–404. [PubMed] [Google Scholar]
- 36.Zhang YK, Yu ZJ, Wang D, Bronislava V, Branislav P, Liu JZ. The bacterial microbiome of field-collected Dermacentormarginatus and Dermacentorreticulatus from Slovakia. Parasit Vectors. 2019;12:1. doi: 10.1186/s13071-019-3582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bullová E, Lukán M, Stanko M, Peťko B. Spatial distribution of DermacentorreticulatustickinSlovakia in the beginning of the 21st century. Vet Parasitol. 2009;165:357–360. doi: 10.1016/j.vetpar.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 38.Kubelová M, Tkadlec E, Bednář M, Roubalová E, Široký P. West-to-east differences of Babesiacaniscanis prevalence in Dermacentorreticulatus ticks in Slovakia. Vet Parasitol. 2011;180:191–196. doi: 10.1016/j.vetpar.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 39.Duscher GG, Feiler A, Leschnik M, Joachim A. Seasonal and spatial distribution of ixodid tick species feeding on naturally infested dogs from Eastern Austria and the influence of acaricides/repellents on these parameters. Parasit Vectors. 2013;6:1. doi: 10.1186/1756-3305-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodžić A, Zörer J, Duscher GG. Dermacentor reticulatus, a putative vector of Babesiacf.microti (syn. Theileria annae) piroplasm. Parasitol Res. 2017;116:1075–1077. doi: 10.1007/s00436-017-5379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dwużnik-Szarek D, Mierzejewska EJ, Rodo A, Goździk K, Behnke-Borowczyk J, Kiewra D, et al. Monitoring the expansion of Dermacentorreticulatus and occurrence of canine babesiosis in Poland in 2016–2018. Parasit Vectors. 2021;14:1. doi: 10.1186/s13071-021-04758-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dautel H, Dippel C, Oehme R, Hartelt K, Schettler E. Evidence for an increased geographical distribution of Dermacentorreticulatus in Germany and detection of Rickettsiasp. RpA4. Int J Med Microbiol. 2006;296:149–156. doi: 10.1016/j.ijmm.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Gray JS, Dautel H, Estrada-Peña A, Kahl O, Lindgren E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip Perspect Infect Dis. 2009;2009:1–12. doi: 10.1155/2009/593232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arthur DR. Ticks: a monograph of the Ixodoidea part V. CambridgeK: Cambridge University Press; 1960. [Google Scholar]
- 45.Akimov IA, Nebogatkin IV. Distribution of ticks from of the genus Dermacentor (Acari, Ixodidae) in Ukraine. Vestnik Zool. 2011;45:1. doi: 10.2478/v10058-011-0001-x. [DOI] [Google Scholar]
- 46.Pfäffle M, Littwin N, Petney T. Host preferences of immature Dermacentorreticulatus (Acari: Ixodidae) in a forest habitat in Germany. Ticks Tick Borne Dis. 2015;6:508–515. doi: 10.1016/j.ttbdis.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Karbowiak G. Changes in the occurrence range of hosts cause the expansion of the ornate dog tick Dermacentorreticulatus (Fabricius, 1794) in Poland. Biologia (Bratisl). 2021 doi: 10.1007/s11756-021-00945-0. [DOI] [Google Scholar]
- 48.Nosek J. Overwintering cycles in Dermacentor ticks. Angew Parasitol. 1979;20:34–37. [PubMed] [Google Scholar]
- 49.Hornok S, Fuente J, Horváth G, Fernández De Mera I, Wijnveld M, Tánczos B, et al. Molecular evidence of Ehrlichiacanis and Rickettsiamassiliae in ixodid ticks of carnivores from South Hungary. Acta Vet Hung. 2013;61:42–50. doi: 10.1556/avet.2012.050. [DOI] [PubMed] [Google Scholar]
- 50.Klitgaard K, Chriél M, Isbrand A, Jensen TK, Bødker R. Identification of Dermacentorreticulatus ticks carrying Rickettsiaraoultii on Migrating Jackal. Denmark Emerg Infect Dis. 2017;23:2072–2074. doi: 10.3201/eid2312.170919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zygner W, Górski P, Wçdrychowicz H. New localities of Dermacentorreticulatus tick (vector of Babesiacaniscanis) in central and eastern Poland. Pol J Vet Sci. 2009;12:549–555. [PubMed] [Google Scholar]
- 52.Kutal M, Belotti E, Volfová J, Mináriková T, Bufka L, Poledník L, et al. Occurrence of large carnivores—Lynxlynx, Canislupus, and Ursusarctos—and of Felissilvestris in the Czech Republic and western Slovakia in 2012–2016 (Carnivora) Lynx, New Ser. 2019;48:93–107. doi: 10.2478/lynx-2017-0006. [DOI] [Google Scholar]
- 53.Hulva P, Černá Bolfíková B, Woznicová V, Jindřichová M, Benešová M, Mysłajek RW, et al. Wolves at the crossroad: fission–fusion range biogeography in the Western Carpathians and Central Europe. Divers Distrib. 2018;24:179–192. doi: 10.1111/ddi.12676. [DOI] [Google Scholar]
- 54.Pantchev N, Pluta S, Huisinga E, Nather S, Scheufelen M, Vrhovec MG, et al. Tick-borne diseases (borreliosis, snaplasmosis, babesiosis) in German and Austrian dogs: Status quo and review of distribution, transmission, clinical findings, diagnostics and prophylaxis. Parasitol Res. 2015;114:19–54. doi: 10.1007/s00436-015-4513-0. [DOI] [PubMed] [Google Scholar]
- 55.Víchová B, Miterpáková M, Iglódyová A. Molecular detection of co-infections with Anaplasmaphagocytophilum and/or Babesiacaniscanis in Dirofilaria-positive dogs from Slovakia. Vet Parasitol. 2014;203:167–172. doi: 10.1016/j.vetpar.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 56.Król N, Kiewra D, Lonc E, Janaczyk B, Chodorowska-Skubiszewska A, Dzięcioł M, et al. Dermacentorreticulatus (Fabricius, 1794) and Babesiacanis (Piana et Galli-Valerio, 1895) as the parasites of companion animals (dogs and cats) in the Wrocław area, south-western Poland. Ann Parasitol. 2016;62:125–130. doi: 10.17420/ap6202.44. [DOI] [PubMed] [Google Scholar]
- 57.Beck R, Vojta L, Mrljak V, Marinculić A, Beck A, Živičnjak T, et al. Diversity of Babesia and Theileria species in symptomatic and asymptomatic dogs in Croatia. Int J Parasitol. 2009;39:843–848. doi: 10.1016/j.ijpara.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 58.Beck A, Huber D, Polkinghorne A, Kurilj AG, Benko V, Mrljak V, et al. The prevalence and impact of Babesiacanis and Theileria sp. in free-ranging grey wolf (Canislupus) populations in Croatia. Parasit Vectors. 2017;10:1–9. doi: 10.1186/s13071-017-2106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mierzejewska EJ, Dwużnik D, Koczwarska J, Stańczak Ł, Opalińska P, Krokowska-Paluszak M, et al. The red fox (Vulpesvulpes), a possible reservoir of Babesiavulpes, B.canis and Hepatozooncanis and its association with the tick Dermacentorreticulatus occurrence. Ticks Tick Borne Dis. 2021;12:101551. doi: 10.1016/j.ttbdis.2020.101551. [DOI] [PubMed] [Google Scholar]
- 60.Scoles GA, Ueti MW. Vector ecology of equine piroplasmosis. Annu Rev Entomol. 2015;60:561–580. doi: 10.1146/annurev-ento-010814-021110. [DOI] [PubMed] [Google Scholar]
- 61.Bajer A, Dwużnik-Szarek D. The specificity of Babesia-tick vector interactions: recent advances and pitfalls in molecular and field studies. Parasit Vectors. 2021;14:507. 10.1186/s13071-021-05019-3. [DOI] [PMC free article] [PubMed]
- 62.Bělková T, Bártová E, Řičařová D, Jahn P, Jandová V, Modrý D, et al. Theileriaequi and Babesiacaballi in horses in the Czech Republic. Acta Trop. 2021;221:1–4. doi: 10.1016/j.actatropica.2021.105993. [DOI] [PubMed] [Google Scholar]
- 63.Daniel M, Szymański S, Cerný V, Dusbábek F, Honzáková E, Olejnícek J. A comparison of developmental dynamics of Dermacentorreticulatus (Fabr) of different geographic origins and their affection by different microclimate. Folia Parasitol (Praha). 1980;27:63–69. [PubMed] [Google Scholar]
- 64.Mierzejewska EJ, Welc-Faleciak R, Karbowiak G, Kowalec M, Behnke JM, Bajer A. Dominance of Dermacentorreticulatus over Ixodesricinus (Ixodidae) on livestock, companion animals and wild ruminants in eastern and central Poland. Exp Appl Acarol. 2015;66:83–101. doi: 10.1007/s10493-015-9889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The nucleotide sequence generated in the present study has been deposited in GenBank (https://www.ncbi.nlm.nih.gov/) under accession number OK135945. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.