Abstract
Background
The need for preventive therapies that interrupt the progression of Alzheimer’s disease (AD) before the onset of symptoms or when symptoms are emerging is urgent and has spurred the ongoing development of disease-modifying therapies (DMTs) in preclinical and early AD (mild cognitive impairment [MCI] to mild dementia). Assessing the meaningfulness of what are likely small initial treatment effects in these earlier stages of the AD patho-clinical disease continuum is a major challenge and warrants further consideration.
Body
To accommodate a shift towards earlier intervention in AD, we propose meaningful benefits as a new umbrella concept that encapsulates the spectrum of potentially desirable outcomes that may be demonstrated in clinical trials and other studies across the AD continuum, with an emphasis on preclinical AD and early AD (i.e., MCI due to AD and mild AD dementia). The meaningful benefits framework applies to data collection, assessment, and communication across three dimensions: (1) multidimensional clinical outcome assessments (COAs) including not only core disease outcomes related to cognition and function but also patient- and caregiver-reported outcomes, health and economic outcomes, and neuropsychiatric symptoms; (2) complementary analyses that help contextualize and expand the understanding of COA-based assessments, such as number-needed-to-treat or time-to-event analyses; and (3) assessment of both cumulative benefit and predictive benefit, where early changes on cognitive, functional, or biomarker assessments predict longer-term clinical benefit.
Conclusion
The concept of meaningful benefits emphasizes the importance of multidimensional reporting of clinical trial data while, conceptually, it advances our understanding of treatment effects in preclinical AD and mild cognitive impairment due to AD. We propose that such an approach will help bridge the gap between the emergence of DMTs and their clinical use, particularly now that a DMT is available for patients diagnosed with MCI due to AD and mild AD dementia.
Keywords: Alzheimer’s disease, Clinical trials, Clinical meaningfulness, Meaningful benefit, Biomarkers, Preclinical, Mild cognitive impairment due to Alzheimer’s disease
Background: the need for a multidimensional approach to assess meaningful benefits in clinical trials in earlier stages of the Alzheimer’s disease continuum
Alzheimer’s disease (AD) is a public health crisis [1–3] that is expected to worsen in the years ahead as the world’s population ages [4, 5]. However, the availability in the USA of the first AD therapy targeting the fundamental pathophysiology of the disease, along with the likelihood of other novel and potentially disease-modifying therapies (DMTs) to follow, are crucial steps toward addressing this substantial unmet need. In this context, it is imperative that we continue to expand our understanding of how best to measure and communicate the potential benefits of these AD medications as they start to enter clinical practice. Greater clarity on the relationships between biological effects (e.g., removal of amyloid plaques; effects on downstream biomarkers) and the benefit/risk profile of these medications, informed by biomarkers, will be transformative to AD care.
Interventions for AD must be associated with demonstrable benefit to patients, and these benefits must outweigh potential harms. Historically, drugs indicated for mild, moderate, or severe AD dementia have been approved based upon trials with co-primary endpoints that assess cognition as well as either functional or global clinical status; the two latter measures are intended to ensure treatment effects on cognition are clinically meaningful for patients and their caregivers [6]. More specifically, these medications are used for treatment of patients in the dementia phase of the illness with the intent of cognitive and functional improvement (or stabilization). Despite this explicit framework, translating the outcomes from clinical studies of these well-established symptomatic therapies to clinical practice and demonstrating the meaningfulness of changes has been difficult.
Challenges in assessing and communicating the meaningfulness of a treatment effect in AD are magnified for drugs designed to treat earlier, asymptomatic, or minimally symptomatic AD stages: (1) the available DMT and others currently under development are not expected to improve symptoms, but rather, to slow disease progression and mitigate clinical decline; (2) by definition, patients with preclinical AD are not experiencing symptoms and, therefore, would not be expected to demonstrate clinical improvement; (3) individuals in preclinical and early symptomatic phases of AD have limited or no clinical changes and drug-placebo differences are difficult to demonstrate; and (4) statistically significant differences between treatment and placebo arms are not necessarily clinically significant or meaningful without a persuasive definition of meaningful change on the selected trial endpoints. Pragmatically, the motivation for healthcare professionals, patients and their families, and payers to initiate and maintain therapy could be diminished if clear expectations for emerging DMTs are not established and if indicators of successful treatment are not communicated in a manner tailored to relevant AD stakeholders.
We propose meaningful benefits as a new umbrella term that describes the spectrum of potentially desirable outcomes that may be demonstrated in clinical trials across the AD continuum, with an emphasis on preclinical AD and early AD (i.e., mild cognitive impairment [MCI] due to AD and mild AD dementia). Our conceptualization of meaningful benefits extends the idea of clinical meaningfulness into populations without clinical symptoms. In addition, meaningful benefits may emerge downstream from intervention, in longer-term follow-up, and may be evident in clinical trials only via proxy measures or surrogate biomarkers and endpoints. Further, we base our recommendations about meaningful benefits on the reality that different stakeholders may desire different data outputs to interpret and contextualize the results of clinical trials, and ultimately, make informed decisions.
Thus, this proposal leverages the foundational components of clinical trials in AD (i.e., clinical outcome assessments [COAs]) while advocating for broader use and reporting of expanded outcomes beyond the “core” dementia phenomena of declining cognition and function [7], and encouraging the consistent application of complementary analyses. The former set of expanded outcomes encompasses measures of neuropsychiatric symptoms and socioeconomic burden, as well as patient- and caregiver-reported outcomes. The second component in this framework, complementary analyses, includes established and standardized statistical measures (e.g., number needed to treat [NNT]) that can be used to contextualize clinical trial results for a broader audience. Finally, meaningful benefits incorporate two additional, novel concepts believed to reflect key outcomes differentiating DMTs from symptomatic interventions: predictive benefit and cumulative benefits. Predictive benefit may be demonstrated if changes captured on a disease-relevant biomarker or on a core disease domain, such as cognition, predict longer-term clinical benefit, such as reduced clinical decline [8]. Cumulative benefits [9] reflect an accrual of effects over long-term therapy, such that the difference in outcome between those treated with placebo and those treated with drug increases over time. The recent approval of aducanumab under the accelerated pathway (i.e., that β-amyloid plaque removal is reasonably likely to predict clinical benefit) makes the concept of predictive benefit unquestionably relevant in AD drug development and clinical practice. Consistent reporting of AD clinical trial results in a manner that is mindful of the proposed meaningful benefits approach will serve to capture and communicate the anticipated benefits seen with DMTs in early phases of AD.
The AD continuum, clinical staging, and regulatory guidelines
The concept of meaningful benefit takes on special relevance in relation to clinical development and regulatory pathways in preclinical and early AD. A brief overview of the staging criteria proposed by the US Food and Drug Administration (FDA) in their draft guidance for drug development in Early Alzheimer’s Disease: Developing Drugs for Treatment [10] and the AD continuum staging criteria suggested by the National Institute on Aging and Alzheimer’s Association (NIA-AA) [11] (Table 1) provide the context for a discussion of meaningful benefit in the early stages of AD.
Table 1.
FDA-proposed stages for drug development vis-à-vis the preclinical AD, MCI due to AD, and AD dementia phases of AD proposed by NIA-AA
| AD stage | Clinical/biomarker presentation | Cognitive and functional assessment | AD clinical continuum correlate |
|---|---|---|---|
| 1 | No cognitive impairment, including no subjective complaints, but AD pathology is present | No detectable abnormalities with sensitive neuropsychological measures | Preclinical |
| 2 |
Transitional cognitive change from individual baseline, with cognition remaining within normal bounds and no functional impairment AD pathology is present |
Detectable change on sensitive neuropsychological measures or subjective report of change | Preclinical |
| 3 |
Subtle or more apparent objective cognitive impairment Impairment in ability to perform instrumental activities of daily living No loss of independence AD pathology is present |
Detectable abnormalities on sensitive neuropsychological measures; mild but detectable functional impairments on sensitive measures | MCI due to AD |
| 4 |
From 4 to 6, gradual progression on levels of cognitive impairment; impact on ability to perform basic activities of daily living and loss of independence AD pathology is present |
Detectable abnormalities on COAs of cognition and function | Mild AD dementia |
| 5 | Moderate AD dementia | ||
| 6 | Severe AD dementia |
Abbreviations: AD Alzheimer’s disease, COA clinical outcome assessment, MCI mild cognitive impairment
In its draft guidance, FDA noted that it is “highly desirable to intervene as early as possible in AD,” [10] making individuals in stages 1 and 2 (preclinical AD) and 3 (MCI due to AD) high-priority candidates for DMT trials. Congruent with the perspective outlined in this paper, the agency recognized the inherent difficulty in establishing any clinically significant impact of an intervention in trials including individuals in stages 1 and 2 because (1) by definition, these individuals have no clinical impairment to rescue; and (2) trials are likely to take several years to detect transition from asymptomatic to symptomatic disease.
Biomarkers of response to treatment in early AD are in a nascent period of development. At present, amyloid plaque reduction on amyloid positron emission tomography (PET) is accepted by the FDA as reasonably likely to predict clinical benefit and the basis for accelerated approval. There are many additional biomarkers in development (discussed below) that may ultimately be used to reflect successful therapeutic intervention and might be considered as surrogates for drug approval. The optimal biomarkers of different brain pathologies may differ across the AD continuum. Biomarkers may change without a corresponding clinical change and the value of a biomarker change may be better defined by what it predicts for later in the disease course. More data on the use of biomarkers across early stages of AD are being developed in trials and longitudinal cohorts.
Investigating meaningful benefits across the AD continuum
One of the earliest attempts to define the tangible benefits, or “clinical meaningfulness,” of a drug for treatment of symptomatic AD was the 1990 FDA draft document by Leber [7]. This guideline stipulated dual-outcome measures for clinical trials: the use of a cognitive assessment in a clinical trial, such as the Alzheimer’s Disease Assessment Scale, Cognitive Subscale (ADAS-Cog) [12], ensured that an intervention had an effect on the “core phenomena of dementia”; drug–placebo differences on an additional global measure or functional measure established that treatment effects were clinically meaningful. These early efforts by FDA were important foundational concepts and remain influential, forming the core of clinical trial outcomes in stages 4, 5, and 6 (mild, moderate, and severe) AD dementia.
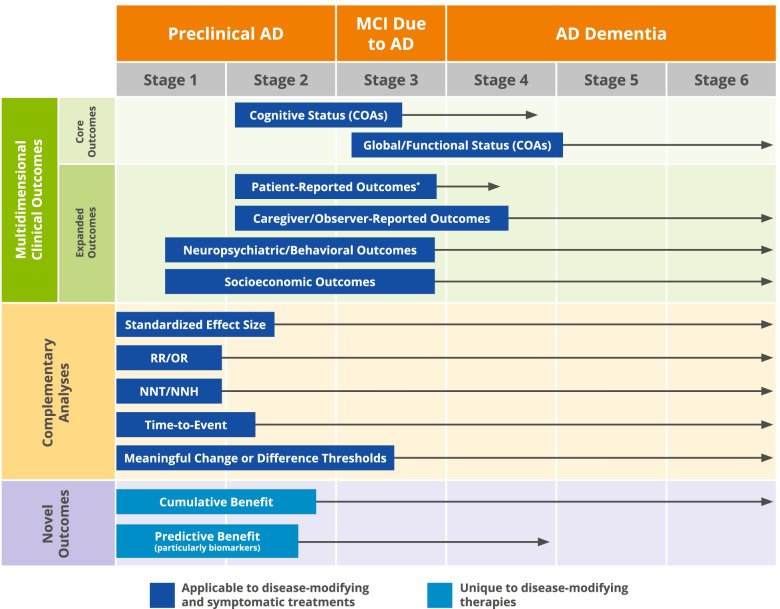
The expanded concept of meaningful benefits (Fig. 1) can advance drug development in the AD continuum as (1) it encourages a broad collection and comprehensive presentation of clinical trial data, which would ensure that a wide range of potential therapeutic dimensions of a DMT are considered, including some that have not been systematically investigated before; and (2) it conveys meaningfulness in multiple ways to resonate with various stakeholders, and to more clearly communicate the potential benefits of a given intervention.
Fig. 1.
Meaningful benefits: a comprehensive spectrum of approaches across Alzheimer’s disease. Each assessment is introduced when it first becomes relevant in the disease continuum (boxes) and each arrow shows the length of time for which the assessment strategy remains relevant. The numbered stages refer to stage definitions introduced by the Food and Drug Administration. *The reliability of patient-reported outcomes may be compromised early in the expression of AD dementia; however, a number of different concepts can best be assessed with PROs, particularly when the concept being measured is best known to the patient or best measured from the patient’s perspective, such as subjective cognitive decline. Abbreviations: AD, Alzheimer’s disease; COA, clinical outcome assessment; DMT, disease-modifying therapy; MCI, mild cognitive impairment; NNH, number needed to harm; NNT, number needed to treat; OR, odds ratio; RR, relative risk
Multidimensional clinical outcomes assessed in Alzheimer’s disease trials
The clinical course of AD is characterized by a progressive deterioration in cognition that leads to impairment in daily functioning, loss of independence and, consequently, increased caregiver burden. Preserving cognitive and functional domains—or slowing their deterioration—are central goals in AD clinical trials and treatment. However, a valid assessment of the benefit of any intervention for AD must reflect and encompass a diverse set of outcomes. A drug’s effect on AD can be assessed using a variety of COAs; for example, a recent review by Webster and colleagues [13] identified 81 different COAs of cognition, activities of daily living, neuropsychiatric symptoms, quality of life, global well-being, and biomarkers used in trials of mild to moderate AD dementia. Beginning with core outcomes related to cognition and function, we review how an assessment of meaningful benefits should address outcomes across a full range of domains and measures.
Core outcomes
As studies in AD have moved toward assessing patients in earlier stages along the disease continuum, established COAs to measure cognition or functioning have proven to be less sensitive in detecting changes over time in asymptomatic or minimally symptomatic populations [14, 15], demonstrating, for example, ceiling effects in MCI and mild AD dementia [6] or little change over the course of short trials in preclinical or early AD [16]. Recognizing the limitations of existing AD assessments, researchers and clinicians have modified and refined existing tools and have developed novel instruments that are sufficiently sensitive to detect more subtle changes over time in preclinical AD and MCI due to AD (Table 2) [12, 16–42].
Table 2.
Outcome measures in early phases of the AD continuuma
| Domain | Preclinical ADb | MCI due to AD |
|---|---|---|
| Cognition |
Preclinical Alzheimer Cognitive Composite (PACC) • Composite cognitive measure that assesses episodic memory, timed executive function, and global cognition • In several studies, has reliably identified cognitive decline in individuals with preclinical AD over a 2- to 3-year observation period [17] • Primary endpoint in first interventional trial in cognitively normal individuals identified as “at-risk” for progression to AD dementia—the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s (A4) study [18] Alzheimer’s Prevention Initiative Composite Cognitive Test (APCC) • Composite instrument of word recall, naming, praxis, orientation, and abstract reasoning [19] • Primary outcome measure in Alzheimer’s Prevention Initiative (API) [19] • Sensitivity has been independently confirmed in a cohort of cognitively normal older adults who progressed to late-onset AD [20] |
Alzheimer’s Disease Assessment Scale, Cognitive Subscale (ADAS-Cog) • 11 subtests related to memory, praxis, and language [12] • Standard tool in pivotal clinical trials to detect therapeutic efficacy in cognition [21] • Best for patients with symptomatic AD; although often used in earlier AD, ceiling effects may limit its utility unless the assessment is modified (e.g., adding one or more tests) [21, 22] Neuropsychological Test Battery (NTB) • Uses widely available tests with known reliability and validity to overcome some of the limitations of the ADAS-Cog • Assesses 5 cognitive domains: attention, language, memory, spatial, and executive function • High degree of reliability for characterizing individuals with mild to moderate AD [23–25] • More sensitive to change in mild AD dementia than the ADAS-Cog [23–25] |
| Global/composite | None |
Clinical Dementia Rating (CDR) – GS or SB • Clinician rating scale widely used in clinical trials in MCI due to AD and mild AD dementia • Can yield global score (CDR-GS) or sum of boxes (CDR-SB) • CDR-GS supports trial eligibility and staging: score of 0.5 corresponds to MCI; score of 1 corresponds to mild dementia [26] • CDR-SB is often the primary outcome; has both cognitive (e.g., memory, orientation, judgment, and problem solving) and functional (community affairs, home and hobbies, personal care) aspects and serves as a composite measure Alzheimer’s Disease Composite Score (ADCOMS) • Composite scoring approach designed as outcome measure for trials in patients with MCI due to AD and mild AD dementia [27] • Based on weighted scores from 4 ADAS-Cog subscale items, 2 Mini-Mental State Examination (MMSE) items, and all 6 CDR items • Demonstrated improved sensitivity over individual scales to detect clinical decline in people with amnestic MCI and those individuals with mild AD dementia • Also detected treatment effects associated with the use of cholinesterase inhibitors in these populations Integrated Alzheimer’s Disease Rating Scale (iADRS) • Combines scores from the ADAS-Cog and the Alzheimer’s Disease Cooperative Study—Instrumental Activities of Daily Living (ADCS-iADL) • Across all phase 3 trials of the anti-amyloid treatment, solanezumab, in mild AD dementia, the iADRS differentiated between active treatment and placebo [28] |
| Function |
Emerging options • Performance-based assessment of functional capacity as demonstrated by: o A performance-based assessment of everyday function [29] like the University of California San Diego Performance-Based Skills Assessment (UPSA), or o Navigating an interactive voice response system, as in the Harvard Automated Phone Task, [30] or o Demonstrating skills in a virtual setting, such as the Virtual Reality Functional Capacity Assessment Tool (VRFCAT) [31] • Brief performance measure of financial skills, such as with the Financial Capacity Instrument [32] • Informant- and/or patient-reported ratings of everyday function, such as o The Everyday Cognition (ECog) scale [33] |
ADCS-ADL-MCI Scale • Widely used endpoint in clinical trials [38] • Inventory of ADL elements rated based on the extent of assistance the individual needs with each activity (e.g., going shopping or keeping appointments or meetings) [38] • Successfully distinguishes those with MCI from those with unimpaired function [39] Amsterdam IADL Questionnaire • Study partner report about the individual’s ability to perform a range of everyday activities, including cooking, finances, and everyday technology use [40] • Correlates longitudinally with cognitive decline [41] • Detects difficulties with IADLs in preclinical AD compared to healthy controls [42] |
Abbreviations: AD Alzheimer’s disease, MCI mild cognitive impairment
aA more comprehensive review of a range of composite batteries developed for secondary prevention trials in AD, as well as their strengths and weaknesses, was recently provided by Schneider and Goldberg [16]
bFDA has suggested an integrated scale that adequately and meaningfully assesses both daily function and cognitive effects. This scale may be acceptable as a single primary efficacy outcome in trials of preclinical AD
FDA guidance on the development of drugs in early AD has been an important catalyst to the development, validation, and application of novel COAs in preclinical and early AD. For example, FDA has noted that in stage 3 AD, a composite scale that adequately assesses daily function and cognitive effects may be acceptable as a single primary efficacy outcome measure [10]. The Clinical Dementia Rating sum of boxes (CDR-SB) is the most commonly used primary endpoint in phase 3 clinical studies in this population; some phase 2 studies, however, have used other composite analyses and tools such as the Alzheimer’s Disease Composite Score (ADCOMS) and Integrated Alzheimer’s Disease Rating Scale (iADRS).
In stage 2 AD (i.e., preclinical with transitional cognitive change), FDA has suggested that a persuasive effect on one or preferably more sensitive measures of neuropsychological function along with effects on characteristic pathophysiologic changes of AD may suffice for drug approval. In this context, most trials in this stage include continuous measures as the primary endpoint, such as the Preclinical Alzheimer Cognitive Composite (PACC) or the Alzheimer’s Prevention Initiative Composite Cognitive Test (APCC) (Table 2) [12, 16–42]. Recent studies suggest that early change on the PACC is associated with subtle functional decline and is predictive of future functional impairment [43]. FDA indicated that the use of a time-to-event (TTE) survival analysis—such as time to progress from stage 2 to stage 3—can be an acceptable efficacy measure in early AD trials, and such TTE endpoints have been included in some ongoing trials as either primary or secondary measures [44]. One point of discussion regarding TTE is the lack of broad consensus on how to define a meaningful event in the earlier stages of the AD continuum—especially one that is considered consistently meaningful, despite heterogeneity in the population.
In describing trials in stage 1 AD, FDA has stated that it may suffice to demonstrate an effect on pathophysiological changes of AD, as shown by an effect on one or more biomarkers—assuming that the biomarker effect is reasonably likely to predict clinical benefit in the future. Designing trials for preclinical AD intervention is in its relative infancy; neither change on a specific biomarker nor change on any of the above-mentioned cognitive composite batteries is yet to be linked to meaningful outcomes such as a delay in onset of MCI or dementia or a delay in functional decline [15]. FDA’s approval of aducanumab for use in patients with early AD may motivate the use of surrogate markers in preclinical AD studies.
Expanded outcomes
Meaningful benefits in preclinical and early AD should include patient- and caregiver-reported outcomes, health and economic outcomes, and neuropsychiatric symptoms.
Patient- and caregiver-reported outcomes
The benefits reported by patients or individuals at risk of developing symptoms as a result of receiving an intervention reflect an important dimension of intervention effectiveness [45]. Perceived patient benefits may be systematically collected via patient-reported outcome (PRO) measures; PROs are defined as any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else [46]. Preservation of everyday functioning, maintaining relationships and social connections, enjoying life, preserving a sense of identity, and alleviating symptoms are the qualities consistently identified by cognitively normal older individuals as desirable outcomes of a hypothetical DMT and are target outcomes to be captured by PROs [46, 47]. A recent review by the Alzheimer’s Disease Patient and Caregiver Engagement initiative demonstrated that some of the most consequential symptoms and effects of AD as identified by patients and their care partners are not adequately captured by widely used COAs, indicating the need for companion tools to fully capture concepts of interest for patients and care partners [48].
Validated PROs are needed in AD trials and some are under development [46, 49, 50]. The Patient-Reported Outcome Consortium’s Cognition Working Group had led efforts to develop a novel, self-reported outcome measure in persons with MCI due to AD, emphasizing two functional domains: (1) complex activities of daily living (e.g., handling personal finances and meal preparation) and (2) interpersonal functioning (e.g., conversational skill or comprehension of written material) [49]. However, concerns that the characteristic loss of insight among patients with MCI due to AD could impact the reliability of a PRO in this target population led the working group to refocus on qualification of a performance-based measure assessing the ability to perform instrumental activities of daily living [51].
Although PROs have limited use in AD dementia due to patients’ loss of insight and/or memory loss, they could be particularly important in the preclinical and early AD stages (stages 1–3), when insight is preserved and changes may be too subtle to be reliably observed by others (e.g., clinicians, friends, or family members). More studies are needed to determine the utility of such measures in trials in stages 1–3.
Finally, regulatory agencies encourage PRO assessments in clinical studies [52]. For the collection of patient experience data, FDA recommends direct reports from patients, unless they are unable to report reliably on the concept of interest [53]. When collection of direct patient experience is limited, valuable but distinct information may still be obtained from informants, such as family members and/or caregivers [53].
Health and economic outcomes
Health and economic outcomes represent another expanded means of assessing meaningful benefit. AD is one of the costliest diseases to society; expenses include direct costs (e.g., medical and residential care payments), indirect costs (e.g., the unpaid work of informal caregivers), and intangible costs (e.g., diminished quality of life for patients and caregivers; caregiver burden [54]. Not only are these impacts substantial, accruing in greater amount over the course of symptomatic AD, but most of these costs and burdens begin even in the years before the onset of clinical manifestations—that is, when a person is experiencing MCI or early symptomatic dementia [54]. Different stakeholders—and indeed, even different payers (e.g., private payers, employers, Medicare)—may value different health and economic outcomes (e.g., placing more or less value on retained worker productivity). Significant data must still be accrued to enable dialogue around the benefit captured by different assessments. Major health and economic goals associated with treatment in early AD—some of which may not be fully evident until later stages of the disease continuum—include reduced formal and informal resource utilization (Table 3) [55–58] as well as retained patient autonomy. These outcomes may translate into reduced caregiver burden and, ultimately, reduced institutionalization or prolonged time to nursing home placement [59].
Table 3.
Health and economic outcomes and neuropsychiatric symptom measures for early AD
| Domain | Potential Measure |
|---|---|
| Health and economic outcomes |
Resource Utilization in Dementia (RUD) Questionnaire • Structured interview with study partner to obtain information about patient and caregiver, including [55] o Healthcare resource utilization o Work status o Living accommodations o Level of formal and informal care attributable to AD, including caregiving time spent assisting patient’s instrumental ADLs or basic ADLs • Emerging evidence leveraging the RUD indicates that early AD, including MCI, poses a financial burden to the patient, caregiver, and society [56] |
| Neuropsychiatric symptoms |
Neuropsychiatric Inventory [57] • 12-item informant-based interview about delusions, hallucinations, anxiety, depression, agitation/aggression, euphoria, disinhibition, irritability/lability, apathy, aberrant motor activity, night-time behavioral disturbances, and appetite/eating abnormalities • Widely accepted measure of neuropsychiatric symptoms in dementia Mild Cognitive Behavioral Impairment Checklist (MBI-C) [58] • Specifically developed as an MBI case ascertainment instrument, which also allows for the monitoring of MBI symptoms over time • 34-item instrument for completion by patient, close informant, or clinician • Assesses 5 domains of (1) decreased motivation; (2) emotional dysregulation; (3) impulse dyscontrol; (4) social inappropriateness; and (5) abnormal perception or thought content |
Neuropsychiatric symptoms in AD
Neuropsychiatric symptoms (e.g., depression, agitation, psychosis, apathy, anxiety, irritability, and social withdrawal) are well-known manifestations of AD [60]. The emergence of these symptoms late in life may reflect the development of mild behavioral impairment (MBI), an “at-risk state” for cognitive decline and dementia that may arise ahead of or in parallel with MCI [60, 61]. The effect of neuropsychiatric symptoms in the AD continuum can be severe, as they are associated with reduced quality of life, earlier institutionalization, faster disease progression, increased caregiver stress, and greater overall costs of care [60, 62]. Thus, neuropsychiatric symptoms in AD may be present and detected early, and both their initial expression and their long-term course may serve as important markers of meaningful therapeutic benefits. Understanding of MBI and early manifestations of these symptoms is less robust earlier in the AD continuum compared with understanding of these same phenomena in later disease stages. However, existing tools and emerging ones (Table 3) [55–58] may help guide our current assessment of incident and prevalent neuropsychiatric symptoms in early AD [63]. Reduction in the severity or emergence of neuropsychiatric symptoms is a key measure of meaningful benefit.
Complementary analyses: expanding assessments and reframing clinical trial data to communicate the full spectrum of potential benefit from DMTs
Certain elements of clinical trials, such as co-primary endpoints, may be of particular relevance only to select audiences (e.g., regulators and clinical trialists). However, a diverse set of stakeholders is involved in the care of individuals with AD and the payment for care, and each stakeholder may require analyses that differ from outcomes used in trials. Table 4 summarizes complementary analyses that may further contextualize trial data [10, 17, 42, 53, 64–72]. With exception of effect size, the other analyses require the definition of an event of interest. AD stakeholders may assign different values to certain events, and some events may be more reliably assessed than others. Furthermore, defining “an event” that is relevant for all stakeholders is a challenge in a disease as heterogenous as AD. Thus, research is ongoing to understand how to best define and assess discrete events in preclinical and early AD trials.
Table 4.
Analyses to convey clinical trials results in non-trial settings
| Analysis | Interpretation | Potential utility/application |
|---|---|---|
| Standardized effect size (e.g., Cohen’s d) |
• For a comparison of group means, Cohen’s d large effect size: ≥0.8; medium effect size 0.5–0.8; small effect size: 0.2–0.5 • Cohen’s d can be applied to any continuous measure, including those used in preclinical AD and MCI due to AD [42, 64] |
• Effect sizes can provide an interpretable index of the direction and magnitude of the effect of an intervention [65–67] • Because effect size estimates enable some control of variability, they also allow for some level of comparison across similar studies [65–68] |
| Relative risk (RR)/odds ratio (OR) |
• Complement standard effect sizes such as Cohen’s d [65–67] • Useful for estimating effect sizes from categorical measures, such as “improved” versus “not improved” or “converted to MCI” or “did not convert to MCI” [66, 69] |
• Both RR and OR are ways in which clinicians often generally consider treatment effects [66] |
| Number needed to treat (NNT)/number needed to harm (NNH) |
• A high NNT indicates a less effective treatment [70]: interventions with an NNT in the single or low double digits are generally considered effective, although an NNT in the lower hundreds may also be considered useful, depending on the significance of the outcome, such as preventing death [71] • NNH provides similar index for the occurrence of one or more specified adverse events |
• NNT, which is related to absolute risk reduction, may be the effect size estimate that best reflects clinical significance for binary outcomes such as success or failure [66] |
| Time-to-event (TTE) |
• Versatile analytical method also known as survival analysis [17] • Refers to a set of methods for analyzing the length of time until the occurrence of a well-defined endpoint of interest [17] • In preclinical and early AD trials, an outcome of great interest is conversion from one stage (e.g., preclinical AD) to the next (MCI due to AD) on the AD continuum [17] |
• Determining what is considered a meaningful event can be challenging, especially in early AD. In addition, operationalizing transitions may be burdensome given the subtle differences between AD stages; nonetheless, TTE analyses may provide useful information as part of a broader evaluation of effect • Recognized as endpoint option by both FDA and EMA [10] |
| Meaningful change or difference threshold |
• Reflects the level of score change(s) on a COA that is perceived to be meaningful in the target patient population • There are two main approaches: o Clinically meaningful change thresholds for individual patients (within-patient approach, recommended by the FDA) [53] o Clinically important difference thresholds applied at the group level (between-groups approach) [72] • The aim is to ensure that the observed treatment benefit as measured by the COA is meaningful to a patient |
• Responder analyses convey the proportion of patients who meaningfully respond to treatment (i.e., achieve or exceed the within-patient meaningful change threshold) • In the context of a progressive disease like AD, a progressor analysis may be more appropriate (i.e., define meaningful progression on the COA), to demonstrate the proportion of patients who meaningfully progress on treatment vs placebo • Exceeding a threshold for MCID between groups can support the meaningfulness of a statistically significant treatment effect at the group level |
The contents of this table are representative of complementary analyses, and do not reflect a comprehensive list
Abbreviations: AD Alzheimer’s disease, COA clinical outcome assessment, EMA European Medicines Agency, FDA US Food and Drug Administration, MCI mild cognitive impairment, MCID minimum clinically important difference
Effects sizes, a means of assessing benefit and comparing interventions, have been widely used in studying treatment but have had limited application in AD. For example, cholinesterase inhibitors are known to produce small to moderate effect sizes in clinical trials on both continuous and ordinal measures of cognition and global well-being [73]. Small to medium effects on cognition and global scales have been documented in moderate to severe AD dementia treated by memantine [74]. Effect sizes for long-term real-world treatment of AD dementia with cholinesterase inhibitors with and without add-on therapy with memantine have also been reported [68, 75]. “Effect sizes” for categorical measures may be best expressed in terms of relative risk (RR) and odds ratio (OR): when comparing a treatment with placebo, an RR or OR of 1 indicates that outcomes did not differ between the two groups, whereas an RR or OR > 1 indicates an increased probability of the event in the treatment group. For example, an RR of 5 indicates that the treatment group had a fivefold greater probability of showing improvement than the placebo group. Fields such as oncology have embraced RR and OR for estimating the ability of a treatment to prevent or delay progression or mortality.
The NNT with a cholinesterase inhibitor has been calculated as ranging from 4 to 14, whereas the number needed to harm (NNH) for the most common adverse reactions with this class of agents is between 6 and 20 [64], indicating a relatively favorable benefit/harm ratio. NNT/NNH ratios will be important for DMTs, especially those that may produce amyloid-related imaging abnormalities, and particularly as NNT and NNH calculations are often used to compare interventions and to guide reimbursement decisions [76]. NNT can be combined with TTE analyses to determine the NNT to prevent one patient from progressing from one stage to the next.
Meaningful change or difference thresholds are often used to aid interpretation of COAs [77] by defining a score change (or range of score changes) beyond which a patient or group of patients may be considered to have “responded” meaningfully to treatment [78]. Thresholds should be established a priori using gold-standard methodology and informed by patient need [79]; they can be applied to clinical trial data to support the interpretation of results at the individual within-patient level (FDA-recommended approach; e.g., responder analyses) or at the group level (e.g., minimal clinically important difference) [80].
Ultimately, these complementary analyses can be applied to clinical trial data and may help convey the results of ongoing DMT trials in AD to different audiences and support cross-study comparison (see Keefe et al. [65] for a more comprehensive assessment of the role and utility of different measures for different audiences).
Cumulative and predictive benefits with DMTs
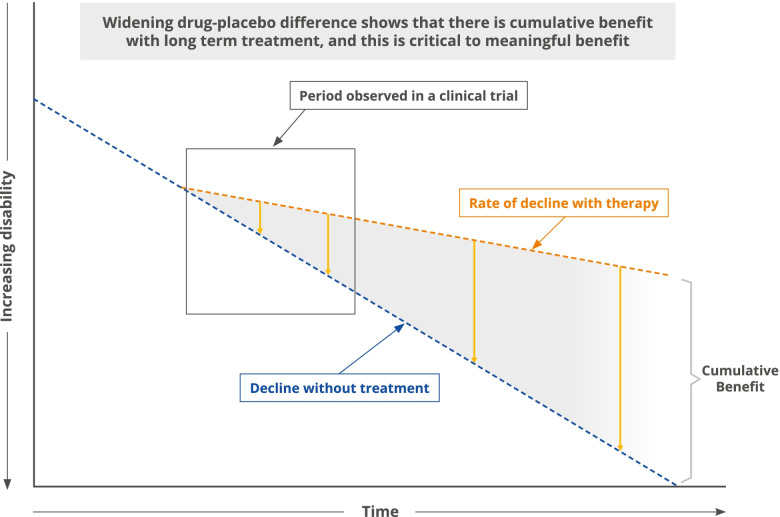
Finally, we anticipate both cumulative and predictive benefits seen with DMTs to be essential components of the meaningful benefits associated with these drugs in the earliest stages of AD. A unique feature of DMTs compared with symptomatic AD treatments is that DMTs slow cognitive and functional decline, manifested by a change in the slope of decline and an increasing drug–placebo difference over time (Fig. 2) [81]. The benefit of DMTs is time-dependent and the value of intervention with these therapies is anticipated to increase as the patient’s duration of therapy increases [6]. As a result, included within the meaningful benefits of DMTs is the cumulative benefit of long-term therapy, a unique aspect to DMTs when compared with symptomatic therapies [82–84]. For example, studies have demonstrated that disease-modifying immunotherapies for multiple sclerosis reduce disability accrual over the short term of 1 to 3 years [85], and more recent real-world evidence has demonstrated long-term cumulative benefit over years to decades [83, 86]. Studies of DMTs in patients with multiple sclerosis have shown benefits of starting DMTs earlier in the disease course compared with later, including improvement in mortality and reduced disability over the longer term [87, 88]. Understanding how to measure the cumulative effects of AD DMTs is crucial to characterizing their full benefit; as more AD DMTs become available, data from observations beyond the trial period will allow greater insight into long-term outcomes.
Fig. 2.
Theoretical rate of decline with DMTs. Model showing the theoretical rates of decline with disease-modifying treatment and without. There is an increasing drug–placebo difference over time with cumulative benefit of long-term therapy. Adapted from Cummings & Zhong [81]
From a regulatory perspective, COAs continue to be the gold standard for demonstrating efficacy of a medication. In several disease areas, however, certain biomarkers qualify as surrogate endpoints because treatment effects on the biomarkers predict clinical benefit. This advancement has contributed to the more expeditious development of new therapies [89, 90]. The importance of these biomarkers is observed in clinical practice, where they are used to inform the need for and success of treatment, including both well-validated biomarkers (e.g., HbA1c as a predictor of diabetic microvascular complications, or blood pressure as a predictor of primary and secondary cardiovascular events) as well as ones that are less well validated but still provide important information (e.g., RNA copy number for clinical monitoring of antiviral therapy in patients with HIV) [91, 92].
Thus, the second novel concept to be integrated into the meaningful benefit framework is predictive benefit. In AD, this concept may be indexed by early changes on cognitive or functional assessments that predict long-term outcomes [6], although it is more commonly expected that a change or an impact on an underlying biomarker might serve as a surrogate that predicts eventual clinical benefits of an AD DMT. As biomarker knowledge has grown, many drugs in different therapeutic areas have been approved—including aducanumab for the treatment of AD—on the basis of changes in surrogate biomarkers considered “reasonably likely” to predict clinical benefit [93, 94]. Biomarkers used in this way are not fully validated surrogates for clinical benefit, and approval using this approach is coupled with the requirement to confirm beneficial clinical effects with additional studies. Knowledge about AD is growing rapidly and surrogate markers or more biomarkers “reasonably likely” to predict benefit are anticipated to emerge. Many of these biomarkers (Table 5) have been included in long-term trials [95] embedded in large collaborations, such as the Alzheimer’s Disease Neuroimaging Initiative [95, 96], or collected in industry-sponsored clinical trials.
Table 5.
AD biomarkers used in assessing disease state or prognosis: potential surrogate markers
| Source | Biomarkers |
|---|---|
| Cerebrospinal fluid | Aβ42 (alone or when measured as a ratio with Aβ40, total tau, or p-tau) |
| Total tau | |
| Phosphorylated-tau (p-tau; alone or when measured as a ratio with Aβ42) | |
| β-site amyloid precursor protein cleaving enzyme 1 (BACE1) | |
| Triggering receptor expressed on myeloid cells 2 (TREM2) | |
| YKL-40 | |
| Neurogranin | |
| Synaptosome-associated protein 25 (SNAP-25) | |
| Synaptotagmin | |
| Visinin-like protein 1 (VILIP-1) | |
| Neurofilament light (NfL) | |
| Blood | p-tau 181; p-tau 217 |
| Aβ42/Aβ40 ratio | |
| Neurofilament light (NfL) | |
| Imaging | Amyloid PET |
| Tau PET | |
| MRI atrophy | |
| FDG PET hypometabolism |
Abbreviations: AD Alzheimer’s disease, FDG PET fluorodeoxyglucose positron emission tomography, MRI magnetic resonance imaging, PET positron emission tomography
Studies have shown that biomarkers, including those accessible in plasma, can predict cognitive decline and dementia with high accuracy [97, 98]. As more trials include these biomarkers and more data accrue, it will be possible to understand the trajectory of biomarker change, the relationship of treatment response to baseline levels, and the magnitude of change in biomarkers that correlates with meaningful benefit on clinical outcomes. These data may guide the identification of additional, validated surrogate biomarker endpoints for future AD trials and eventual clinical monitoring [99]. New information regarding the relationship of specific biomarkers to drug mechanism of action will be key to choosing the most appropriate biomarkers for trials and using them to interpret treatment effects. It is anticipated that some biomarker effects will be predominantly class-based (e.g., anti-amyloid monoclonal antibodies will best be assessed by their ability to lower amyloid plaque levels, which may be less important for a future anti-inflammatory DMT), while others may be more mechanism-independent (e.g., neurofilament light chain holds promise as a marker of downstream neurodegeneration) regardless of the cause of the neuronal death [100]. In addition, a profile, ratio, or composite of biomarkers may be more informative than single biomarker measures.
Next steps and gaps: moving meaningful benefits forward
The AD field is undergoing a period of dramatic and rapid transition. The emergence of disease-modifying therapies in AD is reasonably associated with more questions than answers at present. Accordingly, we offer our proposal as a first step in a necessary discussion the field must entertain as pivotal data about the utility of DMTs continues to emerge. Much is not yet known: it is anticipated that there will soon be answers as to whether the removal of amyloid plaque is truly an acceptable surrogate marker; this, in turn, may enable the registration of multiple drugs. Relationships between amyloid removal and shorter-term cognitive/functional benefit will also be better understood.
Other questions will require creativity and additional data sets to address. For example, how long do we need to follow up with participants from pivotal clinical trials to confirm predictive benefits from measures demonstrated in these trials? The answer to that question must consider issues of replicability in other trials, as well as pragmatics (e.g., some placebo-controlled studies cannot ethically be extended for many years), and how much real-world and open-label data can be reasonably compiled and analyzed to provide answers both cross-sectionally and longitudinally.
The challenges the field of AD faces are not unique to this disease area—for example, there are currently many FDA-approved drugs for multiple sclerosis with different mechanisms of action that target distinct immune-mediated disease processes [101]. A framework similar to biomarker-guided treatment development and use similar to that of multiple sclerosis may guide drug development efforts in AD. Personalized care and treatment of AD based upon a precision medicine approach that takes into account individual differences in biology, lifestyle, and environment and aims to optimize the effectiveness of disease prevention and treatment is the goal of AD drug development and clinical care. Implementation of the meaningful benefit approach will facilitate accomplishing this goal by looking at the data through different lenses.
Conclusions
DMTs have the potential to transform AD treatment paradigms. Translating clinical trial results into clinical practice will be crucial in demonstrating the anticipated meaningful benefits of DMTs to the diverse stakeholders of the AD community. Healthcare providers, patients and their caregivers, regulators, and payers apply different metrics and thresholds when considering the efficacy of a drug and the meaningfulness of its effects. Demonstrating meaningful benefits in AD in a robust, multidimensional way may enable physicians to communicate the potential outcomes of therapy to their patients and caregivers and track progress on an individual level. The meaningful benefits framework discussed in this paper may serve to facilitate such a goal. This novel approach is particularly important as drug development in AD moves into the earlier stages of disease and can help bridge the gap between the emergence of a DMT and understanding its clinical application. A more comprehensive understanding of the data, communicated consistently using this proposed multidimensional model, will facilitate decision-making among AD stakeholders and establish reasonable expectations regarding the use of DMTs in the future.
Acknowledgements
The authors wish to thank Clare Sonntag, MA, Steven F Candela, PhD, and Sally Farrand of Health & Wellness Partners, LLC, Upper Saddle River, NJ, USA, for providing support with medical editing and medical writing. The authors would also like to thank Fiona McDougall, PhD, for constructive criticism of earlier drafts of this manuscript. The authors also wish to thank the anonymous reviewers of the initial draft of this manuscript, not only for their enthusiastic reviews, but for their shaping of key sections of this manuscript, and in particular the penultimate section on next steps.
Abbreviations
- A4
Anti-Amyloid Treatment in Asymptomatic Alzheimer’s study
- AD
Alzheimer’s disease
- ADAS-Cog
Alzheimer’s Disease Assessment Scale, Cognitive Subscale
- ADCOMS
Alzheimer’s Disease Composite Score
- ADCS ADL-PI
Alzheimer’s Disease Cooperative Study—Activities of Daily Living Prevention Instrument
- ADCS-iADL
Alzheimer's Disease Cooperative Study—Instrumental Activities of Daily Living
- APCC
Alzheimer’s Prevention Initiative Composite Cognitive Test
- API
Alzheimer’s Prevention Initiative
- CDR-GS
Clinical Dementia Rating – Global Score
- CDR-SB
Clinical Dementia Rating – Sum of Boxes
- CFI
Cognitive Function Index
- COA
Clinical outcomes assessment
- DMT
Disease-modifying therapy
- ECog
Everyday Cognition scale
- EMA
European Medicines Agency
- FDA
US Food and Drug Administration
- FDG PET
Fluorodeoxyglucose positron emission tomography
- iADRS
Integrated Alzheimer’s Disease Rating Scale
- MBI
Mild behavioral impairment
- MCI
Mild cognitive impairment
- MCID
Minimum clinically important difference
- MMSE
Mini-Mental State Examination
- MRI
Magnetic resonance imaging
- NIA-AA
National Institute on Aging and Alzheimer’s Association
- NNH
Number needed to harm
- NNT
Number needed to treat
- NTB
Neuropsychological Test Battery
- OR
Odds ratio
- PACC
Preclinical Alzheimer Cognitive Composite
- PET
Positron emission tomography
- PRO
Patient-reported outcome
- RR
Relative risk
- TTE
Time to event
- UPSA
University of California San Diego Performance-Based Skills Assessment
- VRFCAT
Virtual Reality Functional Capacity Assessment Tool
Authors’ contributions
All authors have made substantial contributions to the conception of the work. All authors have approved the submitted version and have agreed both to be personally accountable for their own contributions and will ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated, resolved, and the resolution documented in the literature.
Funding
Medical editing and medical writing support was funded by Genentech, A Member of the Roche Group, South San Francisco, CA, USA, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
SSA is a full-time employee of Genentech and receives salary and bonuses, and owns company stock.
RAS reports grants from NIH, research funding from Alzheimer’s Association, Janssen, Eli Lilly, and Eisai. She reports receiving personal fees from AC Immune, Acumen, Alnylam, Cytox, Genentech, Janssen, JOMDD, Nervgen, Neuraly, Neurocentria, Oligomerix, Prothena, Renew, Shionogi, and Vigil Neuroscience.
CR has been a paid consultant for several companies developing treatments for Alzheimer’s disease over the last 5 years including Biogen, Eli Lilly, Merck, Roche, Janssen, Abbvie, Kyowa Kirin, Actinogen, and Eisai. He was the UK Chief Investigator for the ENGAGE Trial and Academic Lead on the EPAD (European Prevention of Alzheimer’s Dementia) Programme, which was a public:private partnership between the EU and several companies with an interest in developing treatments for AD www.ep-ad.org. His unit at the University of Edinburgh (Edinburgh Dementia Prevention) has received grant funding from Biogen, Janssen, AC Immune, and Actinogen. He is the unpaid chairperson of the Brain Health Clinic Consortium established in the UK by Biogen.
DRK has nothing to disclose.
PSA reports research agreements with Janssen, Lilly and Eisai; grants from NIA, the Alzheimer’s Association, and FNIH and consulting fees from Biogen, Roche, Merck, Abbvie, Immunobrain Checkpoint, Rainbow Medical, and Shionogi.
CL is a full-time employee and shareholder of F. Hoffmann-La Roche Ltd.
AA reports receiving grants to his institution for clinical trials and research from NIA/NIH, Alzheimer’s Clinical Trials Consortium, Alzheimer's Disease Cooperative Study, Alzheimer’s Therapeutics Research Institute, American College of Radiology, Arizona Alzheimer’s Research Consortium, Alzheimer's Prevention Initiative, Biohaven, Global Alzheimer’s Platform, Johns Hopkins, Lilly, NIH/NIA, University of Indiana, University of Southern California, Athira, Alzheon, and Gates Ventures. He reports having received personal fees from AbbVie, Acadia, Allergan, Axovant, AZTherapies, Biogen, Eisai, Grifols, the Japanese Organization for Medical Device Development, Medical Care Corporation, Lundbeck, Merck, Novo Nordisk, Roche/Genentech, Sunovion, Suven, and Synexus. Dr. Atri receives book royalties from Oxford University Press.
JC has provided consultation to AB Science, Acadia, Alkahest, AlphaCognition, ALZPath, Annovis, AriBio, Artery, Avanir, Biogen, Biosplice, Cassava, Cerevel, Clinilabs, Cortexyme, Diadem, EIP Pharma, Eisai, GatehouseBio, GemVax, Genentech, Green Valley, Grifols, Janssen, Karuna, Lexeo, Lilly, Lundbeck, LSP, Merck, NervGen, Novo Nordisk, Oligomerix, Otsuka, PharmacotrophiX, PRODEO, Prothena, ReMYND, Renew, Resverlogix, Roche, Signant Health, Suven, Unlearn AI, Vaxxinity, VigilNeuro, Zai Laboratories pharmaceutical, assessment, and investment companies. Dr Cummings is supported by NIGMS grant P20GM109025; NINDS grant U01NS093334; NIA grant R01AG053798; NIA grant P20AG068053; NIA grant R35AG71476; the Alzheimer’s Disease Drug Discovery Foundation (ADDF); and the Joy Chambers-Grundy Endowment. Dr. Cummings has copyright on the Neuropsychiatric Inventory (NPI).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alzheimer’s Association. A public health approach to Alzheimer’s and other dementias. Alzheimer’s Association; 2016. Module 1. https://www.alz.org/media/documents/public-health-approach-alzheimers-faculty-guide-1.pdf. Accessed 15 June 2021.
- 2.Carmona R, Elders J, Novello A, Satchher D. U.S. surgeons general: dementia is our top public health crisis. Commentary. Us Against Alzheimer’s. October 10, 2019. https://www.usagainstalzheimers.org/blog/us-surgeons-general-dementia-our-top-public-health-crisis-commentary. Accessed 15 June 2021.
- 3.Resolution recognizing Alzheimer’s disease as a public health crisis impacting the nation’s health care infrastructure. National Lieutenant Governors Association; 2018. https://nlga.us/wp-content/uploads/Resolution-Recognizing-Alzheimer%E2%80%99s-Disease-as-a-Public-Health-Crisis-Impacting-the-Nation%E2%80%99s-Health-Care-Infrastructure.pdf. Accessed 15 June 2021.
- 4.Alzheimer's disease facts and figures [published online ahead of print, 2020 Mar 10]. Alzheimers Dement. 2020;2020. 10.1002/alz.12068.
- 5.Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. Alzheimer's disease. Lancet. 2021;397:1577–1590. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siemers E, Holdridge KC, Sundell KL, Liu-Seifert H. Function and clinical meaningfulness of treatments for mild Alzheimer’s disease. Alzheimers Dement (Amst) 2016;2:105–112. doi: 10.1016/j.dadm.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leber P. Guidelines for the clinical evaluation of antidementia drugs (1990 draft). Rockville, MD: US Department of Health and Human Services. Food Drug Adm. 1990. 10.13140/2.1.4164.9445.
- 8.Blennow K, Zetterberg H. Biomarkers for Alzheimer’s disease: current status and prospects for the future. J Intern Med. 2018;284:643–663. doi: 10.1111/joim.12816. [DOI] [PubMed] [Google Scholar]
- 9.Cummings J, Fox N. Defining disease modifying therapy for Alzheimer’s disease. J Prev Alzheimers Dis. 2017;4:109–115. doi: 10.14283/jpad.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidance for Industry: Early Alzheimer’s Disease: Developing Drugs for Treatment. US Department of Health and Human Services, Food and Drug Administration, Washington, DC; 2018. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/alzheimers-disease-developing-drugs-treatment-guidance-industy. Accessed 19 Oct 2020.
- 11.Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 13.Webster L, Groskreutz D, Grinbergs-Saull A, Howard R, O'Brien JT, Mountain G, et al. Core outcome measures for interventions to prevent or slow the progress of dementia for people living with mild to moderate dementia: systematic review and consensus recommendations. PLoS One. 2017;12:e0179521. doi: 10.1371/journal.pone.0179521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverberg NB, Ryan LM, Carrillo MC, Sperling R, Petersen RC, Posner HB, et al. Assessment of cognition in early dementia. Alzheimers Dement. 2011;7:e60–e76. doi: 10.1016/j.jalz.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weintraub S, Carrillo MC, Farias ST, Goldberg TE, Hendrix JA, Jaeger J, et al. Measuring cognition and function in the preclinical stage of Alzheimer’s disease. Alzheimers Dement (N Y) 2018;4:64–75. doi: 10.1016/j.trci.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider LS, Goldberg TE. Composite cognitive and functional measures for early stage Alzheimer’s disease trials. Alzheimers Dement (Amst) 2020;12:e12017. doi: 10.1002/dad2.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donohue MC, Sperling RA, Petersen P, Sun KC, Weiner MW, Aisen PS. Alzheimer’s Disease Neuroimaging Initiative. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA. 2017;317:2305–2316. doi: 10.1001/jama.2017.6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sperling RA, Donohue MC, Raman R, Sun CK, Yaari R, Holdridge K, et al. Association of factors with elevated amyloid burden in clinically normal older individuals. JAMA Neurol. 2020;77:735–745. doi: 10.1001/jamaneurol.2020.0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tariot PN, Lopera F, Langbaum JB, Thomas RG, Hendrix S, Schneider LS, et al. The Alzheimer’s Prevention Initiative Autosomal-Dominant Alzheimer’s Disease Trial: a study of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers to evaluate efficacy and safety in the treatment of autosomal-dominant Alzheimer’s disease, including a placebo-treated noncarrier cohort. Alzheimers Dement (N Y) 2018;4:150–160. doi: 10.1016/j.trci.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayutyanont N, Langbaum JB, Hendrix SB, Chen K, Fleisher AS, Friesenhahn M, et al. The Alzheimer's prevention initiative composite cognitive test score: sample size estimates for the evaluation of preclinical Alzheimer’s disease treatments in presenilin 1 E280A mutation carriers. J Clin Psychiatry. 2014;75:652–660. doi: 10.4088/JCP.13m08927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robert P, Ferris S, Gauthier S, Ihl R, Winblad B, Tennigkeit F. Review of Alzheimer’s disease scales: is there a need for a new multi-domain scale for therapy evaluation in medical practice? Alzheimers Res Ther. 2010;2:24. doi: 10.1186/alzrt48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kueper JK, Speechley M, Montero-Odasso M. The Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog): modifications and responsiveness in pre-dementia populations. a narrative review. J Alzheimers Dis. 2018;63:423–444. doi: 10.3233/JAD-170991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison J, Minassian SL, Jenkins L, Black RS, Koller M, Grundman M. A neuropsychological test battery for use in Alzheimer disease clinical trials. Arch Neurol. 2007;64:1323–1329. doi: 10.1001/archneur.64.9.1323. [DOI] [PubMed] [Google Scholar]
- 24.Harrison J, Rentz DM, McLaughlin T, Niecko T, Gregg KM, Black RS, et al. Cognition in MCI and Alzheimer’s disease: baseline data from a longitudinal study of the NTB. Clin Neuropsychol. 2014;28:252–268. doi: 10.1080/13854046.2013.875595. [DOI] [PubMed] [Google Scholar]
- 25.Harrison JE, Rentz DM, Brashear HR, Arrighi HM, Ropacki MT, Liu E. Psychometric evaluation of the Neuropsychological Test Battery in individuals with normal cognition, mild cognitive impairment, or mild to moderate Alzheimer’s disease: results from a longitudinal study. J Prev Alzheimers Dis. 2018;5:236–244. doi: 10.14283/jpad.2018.31. [DOI] [PubMed] [Google Scholar]
- 26.O'Bryant SE, Lacritz LH, Hall J, Waring SC, Chan W, Khodr ZG, et al. Validation of the new interpretive guidelines for the Clinical Dementia Rating Scale Sum of Boxes Score in the national Alzheimer's coordinating center database. Arch Neurol. 2010;67:746–749. doi: 10.1001/archneurol.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Logovinsky V, Hendrix SB, Stanworth SH, Perdomo C, Xu L, et al. ADCOMS: A composite clinical outcome for prodromal Alzheimer’s disease trials. J Neurol Neurosurg Psychiatry. 2016;87:993–999. doi: 10.1136/jnnp-2015-312383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wessels AM, Andersen SW, Dowsett SA, Siemers ER. The integrated Alzheimer’s Disease Rating Scale (iADRS) findings from the EXPEDITION3 Trial. J Prev Alzheimers Dis. 2018;5:134–136. doi: 10.14283/jpad.2018.10. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg TE, Koppel J, Keehlisen L, Christen E, Dreses-Werringloer U, Conejero-Goldberg C, et al. Performance-based measures of everyday function in mild cognitive impairment. Am J Psychiatry. 2010;167:845–853. doi: 10.1176/appi.ajp.2010.09050692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall GA, Dekhtyar M, Bruno JM, Jethwani K, Amariglio RE, Johnson KA, et al. The Harvard Automated Phone Task: new performance-based activities of daily living tests for early Alzheimer's disease. J Prev Alzheimers Dis. 2015;2:242–253. doi: 10.14283/jpad.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atkins AS, Khan A, Ulshen D, Vaughan A, Balentin D, Dickerson H, et al. Assessment of instrumental activities of daily living in older adults with subjective cognitive decline using the Virtual Reality Functional Capacity Assessment Tool (VRFCAT) J Prev Alzheimers Dis. 2018;5:216–234. doi: 10.14283/jpad.2018.28. [DOI] [PubMed] [Google Scholar]
- 32.Marson DC, Gerstenecker A, Triebel K, Martin R, Christianson R, Swenson-Dravis D, et al. Detecting functional impairment in preclinical Alzheimer’s disease using a brief performance measure of financial skills. Alzheimers Dement. 2016;12:373. doi: 10.1016/j.jalz.2016.06.701. [DOI] [Google Scholar]
- 33.Farias ST, Mungas D, Reed BR, Cahn-Weiner D, Jagust W, Baynes K, et al. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22:531–544. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C, Neugroschl J, Luo X, Zhu C, Aisen P, Ferris S, et al. The utility of the Cognitive Function Instrument (CFI) to detect cognitive decline in non-demented older adults. J Alzheimers Dis. 2019;60:427–437. doi: 10.3233/JAD-161294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amariglio RE, Sikkes SAM, Marshall GA, Buckley RF, Gatchel JR, Johnson KA, et al. Item-level investigation of participant and study partner report on the cognitive function index from the A4 study screening data. J Prev Alzheimers Dis. 2021;8:257–262. doi: 10.14283/jpad.2021.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galasko D, Bennett DA, Sano M, Marson D, Kaye J, Edland SD. ADCS Prevention Instrument Project: assessment of instrumental activities of daily living for community-dwelling elderly individuals in dementia prevention clinical trials. Alzheimer Dis Assoc Disord. 2006;20(4 Suppl 3):S152–S169. doi: 10.1097/01.wad.0000213873.25053.2b. [DOI] [PubMed] [Google Scholar]
- 37.Marshall GA, Sikkes SAM, Amariglio RE, Gatchel JR, Rentz DM, Johnson KA, et al. Instrumental activities of daily living, amyloid, and cognition in cognitively normal older adults screening for the A4 Study. Alzheimers Dement (Amst) 2020;12:e12118. doi: 10.1002/dad2.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frank L, Lenderking WR, Howard K, Cantillon M. Patient self-report for evaluating mild cognitive impairment and prodromal Alzheimer's disease. Alzheimers Res Ther. 2011;3:35. doi: 10.1186/alzrt97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pedrosa H, De Sa A, Guerreiro M, Maroco J, Simoes MR, Galasko D, et al. Functional evaluation distinguishes MCI patients from healthy elderly people--the ADCS/MCI/ADL scale. J Nutr Health Aging. 2010;14:703–709. doi: 10.1007/s12603-010-0102-1. [DOI] [PubMed] [Google Scholar]
- 40.Sikkes SA, De Lange-de Klerk ES, Pijnenburg YA, Gillissen F, Romkes R, Knol DL, et al. A new informant-based questionnaire for instrumental activities of daily living in dementia. Alzheimers Dement. 2012;8:536–543. doi: 10.1016/j.jalz.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Koster N, Knol DL, Uitdehaag BM, Scheltens P, Sikkes SA. The sensitivity to change over time of the Amsterdam IADL Questionnaire(©) Alzheimers Dement. 2015;11:1231–1240. doi: 10.1016/j.jalz.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Jutten RJ, Peeters CFW, Leijdesdorff SMJ, Visser PJ, Maier AB, Terwee CB, et al. Detecting functional decline from normal aging to dementia: Development and validation of a short version of the Amsterdam IADL Questionnaire. Alzheimers Dement (Amst) 2017;8:26–35. doi: 10.1016/j.dadm.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papp KV, Buckley R, Mormino E, Maruff P, Villemagne VL, Masters CL, et al. Clinical meaningfulness of subtle cognitive decline on longitudinal testing in preclinical AD. Alzheimers Dement. 2020;16:552–560. doi: 10.1016/j.jalz.2019.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hung A, Schneider M, Lopez MH, McClellan M. Preclinical Alzheimer disease drug development: early considerations based on phase 3 clinical trials. J Manag Care Spec Pharm. 2020;26(7):888–900. doi: 10.18553/jmcp.2020.26.7.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weldring T, Smith SM. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs) Health Serv Insights. 2013;6:61–68. doi: 10.4137/HSI.S11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watson J, Saunders S, Muniz Terrera G, Ritchie C, Evans A, Luz S, et al. What matters to people with memory problems, healthy volunteers and health and social care professionals in the context of developing treatment to prevent Alzheimer's dementia? A qualitative study. Health Expect. 2019;22:504–517. doi: 10.1111/hex.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hauber B, Paulsen R, Callahan L, Potashman M, Lee D, Hartry A, et al. Quantifying what matters most to patients and care partners in Alzheimer’s disease. Poster presented at: 2020 Alzheimer’s Association Virtual International Conference (AAIC) 2020. [Google Scholar]
- 48.Hartry A, Menne H, Wronski S, Paulsen R, Callahan L, Potashman M, et al. Evaluation of what matters most in existing clinical outcomes assessments in Alzheimer’s disease. Poster presented at: 2020 Alzheimer’s Association Virtual International Conference (AAIC) 2020. [Google Scholar]
- 49.Gordon MF, Lenderking WR, Duhig A, Chandler J, Lundy JJ, Miller DS, et al. Development of a patient-reported outcome instrument to assess complex activities of daily living and interpersonal functioning in persons with mild cognitive impairment: The qualitative research phase. Alzheimers Dement. 2016;12:75–84. doi: 10.1016/j.jalz.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 50.Saunders S, Muniz-Terrera G, Sheehan S, Ritchie CW, Luz S. A UK-wide study employing natural language processing to determine what matters to people about brain health to improve drug development: The Electronic Person-Specific Outcome Measure (ePSOM) Programme. J Prev Alzheimers Dis. 2021;8:448–456. doi: 10.14283/jpad.2021.30. [DOI] [PubMed] [Google Scholar]
- 51.Cognition Working Group. Prepared for: 11th Annual PRO Consortium workshop; April 22-23, 2020. PRO Consortium Critical Path Institute. https://c-path.org/wp-content/uploads/2020/11/2020_Cognition_WG_Poster.pdf Accessed 20 Oct 2021.
- 52.Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas. 2018;9:353–367. doi: 10.2147/PROM.S156279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patient-Focused Drug Development: Collecting Comprehensive and Representative Input Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. US Department of Health and Human Services, Food and Drug Administration, Washington, DC; 2018. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-collecting-comprehensive-and-representative-input. Accessed 28 Sept 2021.
- 54.El-Hayek YH, Wiley RE, Khoury CP, Daya RP, Ballard C, Evans AR, et al. Tip of the iceberg: assessing the global socioeconomic costs of Alzheimer's disease and related dementias and strategic implications for stakeholders. J Alzheimers Dis. 2019;70(2):323–341. doi: 10.3233/JAD-190426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wimo A, Gustavsson A, Jönsson L, Winblad B, Hsu MA, Gannon B. Application of resource utilization in dementia (RUD) instrument in a global setting. Alzheimers Dement. 2013;9(4):429–435. doi: 10.1016/j.jalz.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 56.Robinson RL, Rentz DM, Andrews JS, Zagar A, Kim Y, Bruemmer V, et al. Costs of early stage Alzheimer's disease in the United States: cross-sectional analysis of a prospective cohort study (GERAS-US)1. J Alzheimers Dis. 2020;75(2):437–450. doi: 10.3233/JAD-191212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48:S10–S16. doi: 10.1212/WNL.48.5_Suppl_6.10S. [DOI] [PubMed] [Google Scholar]
- 58.Ismail Z, Agüera-Ortiz L, Brodaty H, Cieslak A, Cummings J, Fischer CE, et al. The Mild Behavioral Impairment Checklist (MBI-C): a rating scale for neuropsychiatric symptoms in pre-dementia populations. J Alzheimers Dis. 2017;56(3):929–938. doi: 10.3233/JAD-160979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jessen F. What are we trying to prevent in Alzheimer disease? Dialogues Clin Neurosci. 2019;21(1):27–34. doi: 10.31887/DCNS.2019.21.1/fjessen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lanctôt KL, Amatniek J, Ancoli-Israel S, et al. Neuropsychiatric signs and symptoms of Alzheimer's disease: New treatment paradigms. Alzheimers Dement (N Y) 2017;3(3):440–449. doi: 10.1016/j.trci.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ismail Z, Smith EE, Geda Y, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12(2):195–202. doi: 10.1016/j.jalz.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liew TM. Neuropsychiatric symptoms in early stage of Alzheimer's and non-Alzheimer's dementia, and the risk of progression to severe dementia. Age Ageing. 2021;50(5):1709–1718. doi: 10.1093/ageing/afab044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eikelboom WS, van den Berg E, Singleton EH, Baart SJ, Coesmans M, Leeuwis AE, et al. Neuropsychiatric and cognitive symptoms across the Alzheimer Disease clinical spectrum: cross-sectional and longitudinal associations. Neurology. 2021;97(13):e1276–e1287. doi: 10.1212/WNL.0000000000012598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peters KR. Utility of an effect size analysis for communicating treatment effectiveness: a case study of cholinesterase inhibitors for Alzheimer's disease. J Am Geriatr Soc. 2013;61:1170–1174. doi: 10.1111/jgs.12308. [DOI] [PubMed] [Google Scholar]
- 65.Keefe RS, Kraemer HC, Epstein RS, Frank E, Haynes G, Laughren TP, et al. Defining a clinically meaningful effect for the design and interpretation of randomized controlled trials. Innov Clin Neurosci. 2013;10(5-6 Suppl A):4S–19S. [PMC free article] [PubMed] [Google Scholar]
- 66.McGough JJ, Faraone SV. Estimating the size of treatment effects: moving beyond p values. Psychiatry (Edgmont) 2009;6:21–29. [PMC free article] [PubMed] [Google Scholar]
- 67.Schuele CM, Justice LM. The importance of effect sizes in the interpretation of research. Primer on Research: Part 3. ASHA Leader. 2006;11(10) https://leader.pubs.asha.org/doi/full/10.1044/leader.FTR4.11102006.14. Accessed 15 June 2021.
- 68.Atri A, Shaughnessy LW, Locascio JJ, Growdon JH. Long-term course and effectiveness of combination therapy in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22:209–221. doi: 10.1097/WAD.0b013e31816653bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nordahl-Hansen A, Øien RA, Volkmar F, Shic F, Cicchetti DV. Enhancing the understanding of clinically meaningful results: A clinical research perspective. Psychiatry Res. 2018;270:801–806. doi: 10.1016/j.psychres.2018.10.069. [DOI] [PubMed] [Google Scholar]
- 70.Page P. Beyond statistical significance: clinical interpretation of rehabilitation research literature. Int J Sports Phys Ther. 2014;9:726–736. [PMC free article] [PubMed] [Google Scholar]
- 71.Siwek J, Newman DH. Introducing medicine by the numbers: a collaboration of the NNT Group and AFP. Am Fam Physician. 2015;91:434–435. [PubMed] [Google Scholar]
- 72.Edgar CJ, Vradenburg G, Hassenstab J. The 2018 revised FDA guidance for early Alzheimer's disease: establishing the meaningfulness of treatment effects. J Prev Alzheimers Dis. 2019;6:223–227. doi: 10.14283/jpad.2019.30. [DOI] [PubMed] [Google Scholar]
- 73.Rockwood K. Size of the treatment effect on cognition of cholinesterase inhibition in Alzheimer’s disease [published correction appears in J Neurol Neurosurg Psychiatry. 2004;75(7):1086]. J Neurol Neurosurg Psychiatry. 2004;75:677–85. [DOI] [PMC free article] [PubMed]
- 74.Smith M, Wells J, Borrie M. Treatment effect size of memantine therapy in Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord. 2006;20:133–137. doi: 10.1097/00002093-200607000-00002. [DOI] [PubMed] [Google Scholar]
- 75.Atri A, Rountree SD, Lopez OL, Doody RS. Validity, significance, strengths, limitations, and evidentiary value of real-world clinical data for combination therapy in Alzheimer’s disease: comparison of efficacy and effectiveness studies. Neurodegener Dis. 2012;10(1-4):170–174. doi: 10.1159/000335156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gensini GF, Conti AA. Evidence-based evaluation of benefits in therapeutic interventions: methodologically controlled and non-randomly assigned reflections on the number needed to treat. Ital Heart J. 2003;4:80–83. [PubMed] [Google Scholar]
- 77.Watt JA, Veroniki AA, Tricco AC, Straus SE. Using a distribution-based approach and systematic review methods to derive minimum clinically important differences. BMC Med Res Methodol. 2021;21:41. doi: 10.1186/s12874-021-01228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McMichael AJ, Rolison JJ, Boeri M, Kane JPM, O'Neill FA, Kee F. How do psychiatrists apply the minimum clinically important difference to assess patient responses to treatment? MDM Policy Pract. 2016;1:2381468316678855. doi: 10.1177/2381468316678855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patient-Focused Drug Development Guidance Public Workshop: Incorporating Clinical Outcome Assessments into Endpoints for Regulatory Decision-Making. US Department of Health and Human Services, Food and Drug Administration, Washington, DC; 2019. https://www.fda.gov/media/132505/download. Accessed 21 Oct 2021.
- 80.Liu KY, Schneider LS, Howard R. The need to show minimum clinically important differences in Alzheimer’s disease trials. Lancet Psychiatry. 2021;8(11):1013–6. doi: 10.1016/S2215-0366(21)00197-8. [DOI] [PubMed] [Google Scholar]
- 81.Cummings JL, Zhong K. Clinical trials and drug development in neurodegenerative diseases: unifying principles. In: Cummings JL, Pillai JA, editors. Neurodegenerative Diseases Unifying Principles. Oxford: Oxford University Press; 2017. pp. 323–336. [Google Scholar]
- 82.Halpin DM, Tashkin DP. Defining disease modification in chronic obstructive pulmonary disease. COPD. 2009;6:211–225. doi: 10.1080/15412550902918402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amato MP, Fonderico M, Portaccio E, Pastò L, Razzolini L, Prestipino E, et al. Disease-modifying drugs can reduce disability progression in relapsing multiple sclerosis. Brain. 2020;143:3013–3024. doi: 10.1093/brain/awaa251. [DOI] [PubMed] [Google Scholar]
- 84.Cummings JL. Challenges to demonstrating disease-modifying effects in Alzheimer's disease clinical trials. Alzheimers Dement. 2006;2:263–271. doi: 10.1016/j.jalz.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Claflin SB, Broadley S, Taylor BV. The effect of disease modifying therapies on disability progression in multiple sclerosis: a systematic overview of meta-analyses. Front Neurol. 2019;9:1150. doi: 10.3389/fneur.2018.01150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kalincik T, Diouf I, Sharmin S, Malpas C, Spelman T, Horakova D, et al. Effect of disease-modifying therapy on disability in relapsing-remitting multiple sclerosis over 15 years. Neurology. 2021;96:e783–e797. doi: 10.1212/WNL.0000000000011242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harding K, Williams O, Willis M, Hrastelj J, Rimmer A, Joseph F, et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol. 2019;76:536–541. doi: 10.1001/jamaneurol.2018.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cerqueira JJ, Compston DAS, Geraldes R, Rosa MM, Schmierer K, Thompson A, et al. Time matters in multiple sclerosis: can early treatment and long-term follow-up ensure everyone benefits from the latest advances in multiple sclerosis? J Neurol Neurosurg Psychiatry. 2018;89:844–850. doi: 10.1136/jnnp-2017-317509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beach TG. A review of biomarkers for neurodegenerative disease: will they swing us across the valley? Neurol Ther. 2017;6(Suppl 1):5–13. doi: 10.1007/s40120-017-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McGhee DJ, Ritchie CW, Thompson PA, Wright DE, Zajicek JP, Counsell CE. A systematic review of biomarkers for disease progression in Alzheimer’s disease [published correction appears in PLoS One. 2014;9(5):e97960] PLoS One. 2014;9:e88854. doi: 10.1371/journal.pone.0088854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Twaddell S. Surrogate outcome markers in research and clinical practice. Aust Prescri. 2009;32:47–50. doi: 10.18773/austprescr.2009.023. [DOI] [Google Scholar]
- 92.Martin JH, Fay MF. Surrogate end-points in clinical practice: are we providing worse care? Intern Med J. 2010;40:395–398. doi: 10.1111/j.1445-5994.2010.02248.x. [DOI] [PubMed] [Google Scholar]
- 93. Surrogate endpoint resources for drug and biologic development. US Department of Health and Human Services, Food and Drug Administration, Washington, DC; updated July 24, 2018. https://www.fda.gov/drugs/development-resources/surrogate-endpoint-resources-drug-and-biologic-development. Accessed 15 June 2021.
- 94.Cummings J, Aisen P, Lemere C, Atri A, Sabbagh M, Salloway S. Aducanumab produced a clinically meaningful benefit in association with amyloid lowering. Alzheimers Res Ther. 2021;13:98. doi: 10.1186/s13195-021-00838-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lawrence E, Vegvari C, Ower A, Hadjichrysanthou C, De Wolf F, Anderson RM. A systematic review of longitudinal studies which measure Alzheimer’s disease biomarkers. J Alzheimers Dis. 2017;59:1359–1379. doi: 10.3233/JAD-170261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, et al. Recent publications from the Alzheimer’s Disease Neuroimaging Initiative: reviewing progress toward improved AD clinical trials. Alzheimers Dement. 2017;13:e1–e85. doi: 10.1016/j.jalz.2016.07.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cullen NC, Leuzy A, Janelidze S, Palmqvist S, Svenningsson AL, Stomrud E, et al. Plasma biomarkers of Alzheimer's disease improve prediction of cognitive decline in cognitively unimpaired elderly populations. Nat Commun. 2021;12(1):3555. doi: 10.1038/s41467-021-23746-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hansson O, Cullen N, Zetterberg H. Alzheimer’s Disease Neuroimaging Initiative, Blennow K, Mattsson-Carlgren N. Plasma phosphorylated tau181 and neurodegeneration in Alzheimer’s disease. Ann Clin Transl Neurol. 2021;8(1):259–265. doi: 10.1002/acn3.51253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cash DM, Rohrer JD, Ryan NS, Ourselin S, Fox NC. Imaging endpoints for clinical trials in Alzheimer's disease. Alzheimers Res Ther. 2014;6:87. doi: 10.1186/s13195-014-0087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gaetani L, Parnetti L, Calabresi P, Di Filippo M. Tracing neurological diseases in the presymptomatic phase: insights from neurofilament light chain. Front Neurosci. 2021;15:672954. doi: 10.3389/fnins.2021.672954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Robertson D, Moreo N. Disease-modifying therapies in multiple sclerosis: overview and treatment considerations. Fed Pract. 2016;33(6):28–34. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.