Abstract
Objective
To describe the clinical characteristics and outcomes of CVT in patients with history of recent COVID-19 infection or vaccination.
Methods
We reviewed demographic, clinical, and radiographic characteristics of non-pyrogenic, non-traumatic CVT cases at our multi-center institution between March 2020 and December 2021. Patients were grouped according to their history of recent COVID-19 infection or vaccination into group-I (+COVID-19 association) and group-II (-COVID-19 association).
Results
Fifty-one patients with CVT were included, of which 14 (27.4%) had a positive COVID-19 association: 10 with infection and 4 with mRNA-COVID-vaccine. Nine patients in group-I had COVID-19 infection or vaccine within 30 days of CVT diagnosis, including 3 patients with active infection at the time of CVT diagnosis. Half of the patients in group-I (n = 7,50.0%) and 32.4% (n = 12) of group-II were male, and mean age was 52.6 years in group-I and 51.4 years in group-II. Fever at presentation was noted in one patient who had active COVID infection (I=1 (7.1%), II= 0 (0%)). Higher rates of comorbidities were observed in group-II: hypertension (I= 2 (14.3%), II= 13 (35.1%)), deep venous thrombosis(I=1(7.1%), II= 10 (27.0%)), pulmonary emboli (I=1(7.1%), II= 8(21.6%)), or stroke(I=0(0%), II= 6(16.4%)). Three patients had thrombocytopenia at the time of CVT diagnosis (5.4%) and most patients (n = 37, 72.5%) were treated medically with anticoagulation. Complication rate during hospitalization was 17.6% (n = 6), and no mortality was noted.
Conclusion
Twenty-seven percent of CVT patients were associated with COVID-19 infection or vaccination, and the majority presented within 30 days of infection/vaccination.
Keywords: Cerebral vein thrombosis, COVID, Coronavirus, Hemorrhage, Stroke
1. Introduction
Cerebral venous thrombosis (CVT) is commonly associated with conditions such as trauma, infection, pregnancy, dehydration, leukemia, hormone replacement therapy and neoplasms [1], [2], [3], [4]. However, 30% of all CVTs are considered spontaneous [5]. Advances in diagnostic imaging techniques have led to an increased CVT incidence of 1.32–1.57/100,000 person-years [6]. Spontaneous CVT is commonly seen in young adults and may have variable clinical manifestations ranging from headaches to seizures, focal neurological deficits, and altered mental status [3], [4]. Although it may present as a benign incidental finding on imaging, severe cases can result in devastating neurological sequalae and death due to increased intracranial pressure, venous stroke, and hemorrhagic conversion [6]. Management is mainly with anticoagulant therapy with occasional need for endovascular and surgical treatment in certain cases [5], [6]. Hence, early diagnosis is paramount to minimize morbidity and mortality.
COVID-19 infection has been associated with a prothrombotic state, leading to complications such as deep venous thrombosis, pulmonary emboli, and CVT [7]. Most recently, reports of patients presenting with CVT following COVID-19 vaccination have suggested a potential association between CVT and this preventive practice [8], [9], [10]. Although multiple studies have looked at CVT in COVID-19 patients [7], [8], [9], [11], [12], [13], [14], [15], [16], the influence of COVID infection or vaccination in patients presenting with CVT and their potential manifestations is understudied. We aim to describe the clinical and radiographic characteristics, as well as management and outcomes of CVT in patients with a prior COVID infection or vaccination who were treated at our institutions during the COVID pandemic.
2. Methods
2.1. Patient selection
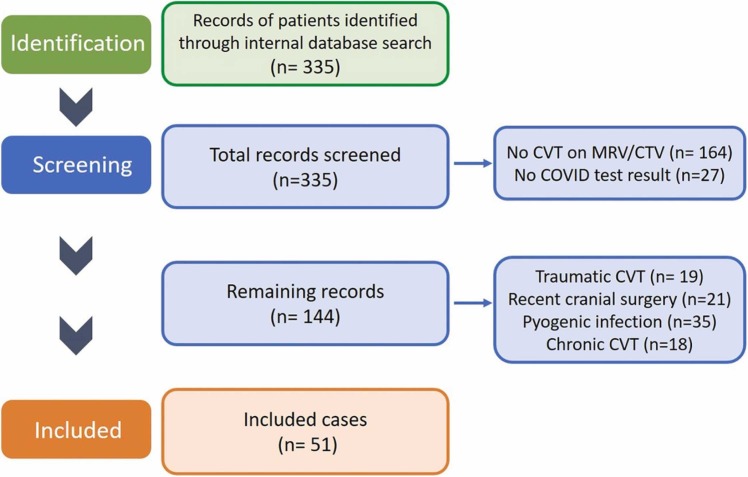
Electronic medical records of 335 consecutive adult patients with a presumed diagnosis of nonpyrogenic thrombosis of the intracranial venous system treated at our 3 main centers between March 1, 2020, and December 1, 2021 were reviewed. Inclusion criteria were as follows: 1) International Classification of Diseases (ICD) code I67.6, 163.6; 2) confirmed diagnosis of CVT by Magnetic Resonance Venography (MRV) or Computed Tomography Venography (CTV), 3) Available COVID-19 PCR test results and vaccination history. Patients with CVT due to recent trauma, cranial surgery, bacterial sinus infection, and chronic CVT were excluded ( Fig. 1). This study was approved by the Institutional Review Board #20–004849, waiving patient consents in view of its retrospective nature.
Fig. 1.
Flow-chart describing the inclusion and exclusion criteria used in our methodology.
2.2. Patient demographic and clinical characteristics
Demographic and clinical data were extracted from the electronic medical records at the time of presentation with CVT. The following variables were obtained: age, sex, past medical history, vital signs at presentation (systolic blood pressure (SBP), diastolic blood pressure (DBP), temperature, heart rate, and respiration rate), presenting symptoms, Glasgow coma scale (GCS), platelet count, hypercoagulable state (included Factor V Leiden, Prothrombin gene mutation, pro-thrombotic protein deficiencies, homocystinuria, active systemic cancer, supplemental estrogen use, and antiphospholipid antibody syndrome), and use of anticoagulation or antiplatelet medication. Data on past medical history included hypertension (HTN), diabetes mellitus (DM), deep vein thrombosis (DVT), pulmonary embolus (PE), and prior stroke. Presenting symptoms included headaches, seizures, focal neurologic deficit(s), altered mental status, or asymptomatic (incidental finding on imaging). Fever was considered an oral, transtympanic, axillary or rectal temperature of 38.0 ͦC or higher recorded at least once during patient’s hospitalization.
2.3. Radiographic characteristics
Radiographic variables were collected from review of available imaging of the brain vasculature (MRV or CTV). The following variables were collected: location (cortical veins, venous sinus involvement, or both), extensive thrombosis (defined as 3 or more sinuses involved), and radiographic presentation (thrombosis with or without venous stroke and hemorrhagic conversion).
2.4. Hospital course
Management and treatment details were collected from the progress and procedural notes including medical management with anticoagulation, endotracheal intubation, intravenous thrombolysis with tissue plasminogen activator (tPA), and mechanical thrombectomy.
2.5. Clinical outcomes
Functional outcomes were assessed at discharge and last follow-up using the modified Rankin Scale (mRS), which ranges from 0 (no symptoms) to 6 (death). Other variables included length of stay, complications during hospitalization, hemorrhagic conversion, ischemic stroke, mortality, and follow-up time.
2.6. Data arrangement and statistical analysis
Patients were classified according to their COVID-19 association prior to diagnosis of CVT into group I (positive COVID-19 association defined as a positive COVID-19 infection that was confirmed by testing or a recent COVID-19 vaccination) or group II (negative COVID-19 association defined as no history of COVID-19 infection or vaccination); recent vaccination was considered as < 30 days prior to CVT diagnosis and the date of the second dose was used to calculate the time. The extracted data from the medical records were summarized using descriptive statistics, including percentages and counts for categorical data. Fisher exact and Mann-Whitney U test were used where appropriate. Significance was considered at α ≤ 0.05 (two-sided). R (version 3.6.0) was utilized to analyze patient data.
3. Results
Fifty-one patients with confirmed diagnosis of non-pyogenic, non-traumatic CVT were included in this study. Demographic and clinical characteristics at presentation are summarized in Table 1, while management and outcomes are summarized in Table 2, and Table 3, respectively.
Table 1.
Demographic and Clinical Characteristics at Presentation.
| Characteristics | All Patients | Group I (+ COVID association) | Group II (- COVID association) | P-Value |
|---|---|---|---|---|
| Number | 51 | 14 | 37 | |
| Age (mean (SD)) | 51.8 (22.2) | 52.6 (33.4) | 51.4 (17.3) | 0.87 |
| Men (%) | 19 (37.2) | 7 (50.0) | 12 (32.4) | 0.41 |
| Hypertension (%) | 15 (29.4) | 2 (14.3) | 13 (35.1) | 0.14 |
| DM (%) | 3 (5.8) | 1 (7.1) | 2 (5.4) | 0.82 |
| DVT (%) | 11 (21.6) | 1 (7.1) | 10 (27.0) | 0.13 |
| PE (%) | 9 (17.6) | 1 (7.1) | 8 (21.6) | 0.23 |
| Hypercoagulable State (%) | 14 (25.4) | 4 (28.5) | 10 (27.0) | 0.91 |
| Prior Stroke (%) | 6 (11.8) | 0 (0.0) | 6 (16.2) | 0.11 |
| SBP > 140 mmHg | 16 (31.1) | 5 (35.7) | 11 (29.7) | 0.69 |
| DBP > 90 mmHg | 18 (35.3) | 5 (35.7) | 13 (35.1) | 0.97 |
| T > 38.0 C | 1 (2.0) | 1 (7.1) | 0 (0.0) | 0.11 |
| Tachycardia | 10 (19.6) | 4 (28.6) | 6 (16.2) | 0.33 |
| Tachypnea | 36 (70.6) | 9 (64.3) | 27 (73.0) | 0.55 |
| Presentation (%) | 0.51 | |||
| Headache | 26 (50.9) | 4 (28.6) | 22 (59.4) | |
| Incidental | 7 (13.7) | 3 (21.4) | 4 (10.8) | |
| Seizure | 3 (5.9) | 1 (7.1) | 2 (5.4) | |
| Focal Neurological Deficit | 13 (25.4) | 4 (28.6) | 9 (24.3) | |
| Syncope | 2 (3.9) | 2 (4.3) | 0 (0) | |
| GCS (mean (SD)) | 14.3 (2.0) | 14.5 (0.7) | 14.2 (2.4) | 0.64 |
| Platelet Count (mean (SD)) | 268.5 (118.3) | 222.8 (93.3) | 286.7 (123.4) | 0.09 |
| Thrombocytopenia (%) | 3 (5.9) | 1 (7.1) | 2 (5.4) | 0.81 |
| Location | 0.40 | |||
| Venous Sinus | 43 (84.3) | 11 (78.6) | 32 (86.4) | |
| Cortical Veins | 3 (5.9) | 1 (7.1) | 2 (5.4) | |
| Both | 5 (9.8) | 2 (14.2) | 3 (8.1) | |
| Radiographic Presentation | 0.30 | |||
| Thrombosis w/o stroke (%) | 38 (74.5) | 12 (85.7) | 26 (70.2) | |
| Venous Stroke w/ hemorrhagic conversion (%) | 9 (17.6) | 2 (14.2) | 7 (18.9) | |
| Venous Stroke w/o hemorrhagic conversion (%) | 4 (7.8) | 0 (0.0) | 4 (10.8) | |
| Medicines at Diagnosis | 0.23 | |||
| Anticoagulation Medicine (%) | 6 (11.7) | 0 (0.0) | 6 (16.2) | |
| Antiplatelet Medicine (%) | 3 (5.9) | 1 (7.1) | 2 (5.4) | |
| None | 42 (82.3) | 13 (92.9) | 29 (78.3) |
SD: standard deviation. IQR: interquartile range. HTN: hypertension. DM: diabetes mellitus. DVT: deep vein thrombosis. PE: pulmonary emboli. SBP: systolic blood pressure. DBP: diastolic blood pressure. BPM: beats per minute.
Table 2.
Management of CVT patients.
| Hospital Course | All Patients | Group I (+ COVID association) | Group II (- COVID association) | P-Value |
|---|---|---|---|---|
| Endotracheal Intubation (%) | 8 (15.6) | 2 (14.3) | 6 (16.2) | 0.87 |
| tPA (%) | 1 (2.0) | 0 (0.0) | 1 (2.7) | 0.54 |
| MT (%) | 1 (2.0) | 0 (0.0) | 1 (2.7) | 0.54 |
| Anti-coagulation medicine (%) | 37 (72.5) | 11 (78.6) | 26 (91.9) | 0.55 |
tPA: tissue plasminogen activator. MT: mechanical thrombectomy.
Table 3.
Outcomes of CVT patients.
| Outcomes | All Patients | Group I (+ COVID association) | Group II (- COVID association) | P-Value |
|---|---|---|---|---|
| LOS (mean (SD)) | 6.12 (9.9) | 9.14 (17.1) | 4.97 (5.3) | 0.19 |
| mRS at discharge (median (IQR)) | 1.31 (1.5) | 1.1 (1.44) | 1.41 (1.54) | 0.48 |
| F/U (days) (mean (SD)) | 134.8 (106.97) | 78.9 (63.4) | 155.9 (113.0) | 0.02 |
| mRS at last F/U (median (IQR)) | 1.14 (1.4) | 0.93 (1.5) | 1.22 (1.4) | 0.53 |
| Hospitalization Complications (%) | 9 (17.6) | 2 (14.2) | 7 (18.9) | 0.70 |
| Mortality (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
LOS: length of stay. mRS: modified Rankin scale. F/U: follow-up. SD: standard deviation. IQR: interquartile range.
3.1. Group I: positive COVID-19 association
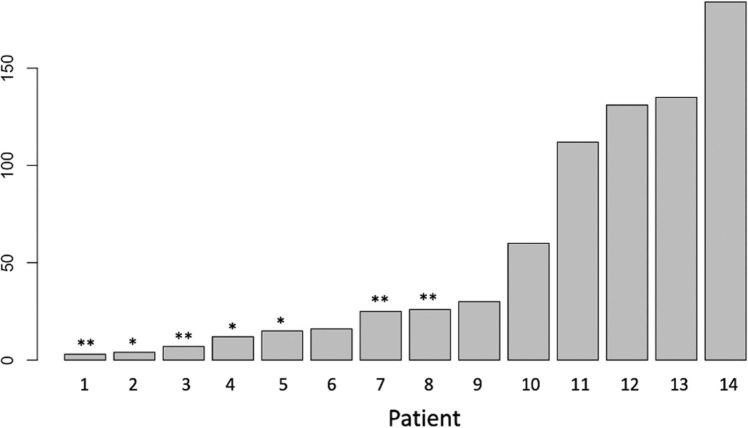
Fourteen patients (27.4%) had a positive COVID-19 association: 10 (71.4%) COVID-19 infections and 4 (28.6%) recent vaccinations against COVID-19 (mRNA vaccine, Pfizer-BioNTech). Half of the patients were male (n = 7, 50.0%), and average age was 52.6 years. Medical comorbidities included HTN (n = 2, 14.3%), DM (n = 1, 7.1%), PE (n = 1, 7.1%), DVT (n = 1, 7.1%), and hypercoagulable state (n = 4, 28.5%), while no patients had history of prior stroke. Eleven (78.6%) patients in group I had venous sinus thrombosis without evidence of cortical vein thrombosis. Twelve patients (85.7%) had venous stroke with CVT, including two patients with COVID-19 association (14.2%) with hemorrhagic conversion. Management consisted of anticoagulation in all but 3 patients (n = 11, 78.6%) who were treated with supportive measures only. Two patients (14.3%) required mechanical ventilation. Complications during hospitalization were reported in 2 cases (14.2%) corresponding to a case of heparin induced thrombocytopenia and a case of asystole with spontaneous resolution; no mortality was reported. Mean mRS at discharge and last follow-up was 1.1 and 0.93, respectively. Length of stay was 9.14 ( ± 17.1) days and mean follow-up time was 78.9 days. Nine patients (60%) had a positive COVID-19 test or were vaccinated within 30 days prior to CVT diagnosis, including three patients (20%) with active infection at the time of CVT diagnosis ( Fig. 2).
Fig. 2.
Time from positive COVID test or vaccine to diagnosis of CVT. *Patients COVID positive test at time of CVT diagnosis. * *Patients with COVID vaccine.
3.2. IA: COVID-19 infection
Ten patients had recent history of COVID-19 infection, including 3 patients with active infection at the time of CVT diagnosis. The most common presenting symptom was headache (n = 3, 30%), followed by focal neurological deficit (n = 3, 30%), syncope (n = 2, 20%), and seizures (n = 1, 10%). One patient presented with fever corresponding to a case of active COVID-19 infection. Another patient (10%) with active COVID-19 infection had PE at presentation. No patients were on anticoagulation prior to diagnosis of CVT, and one patient (10%) was on daily aspirin. Two patients with active COVID-19 infection required mechanical ventilation and were admitted to the ICU, including one patient with thrombocytopenia (30,000 per microliter of blood), complicated by pneumonia and sepsis. The other reported complication in this group corresponded to encephalopathy associated with COVID-19. All patients were treated with anticoagulation.
3.3. IB: vaccination against COVID-19
Four patients had full vaccination against COVID-19 with mRNA-vaccines (Pfizer-BioNTech) < 30 days prior to CVT diagnosis. Two (50%) cases were diagnosed with CVT during work-up for other conditions: The first case had a focal, non-occlusive CVT of the transverse-sigmoid junction diagnosed during outpatient evaluation for dural arteriovenous fistula and did not require treatment. The second case had an extensive non-occlusive CVT involving the superior sagittal, transverse, and sigmoid sinuses found incidentally during evaluation for cochlear implants and was treated with anticoagulation. The third case corresponded to a patient in their 40 s without significant past medical history other than COVID-19 vaccination 4 days prior to presentation. The patient was diagnosed with intracranial hemorrhage involving the left parietal lobe secondary to CVT. The fourth case was diagnosed with CVT after presenting with progressive cognitive decline 30 days after receiving the second COVID-19 vaccine dose. The patient was subsequently diagnosed with severe obstructive sleep apnea.
3.4. Group II: negative COVID-19 association
Thirty-seven patients had a negative history of COVID-19 infection or vaccination, of which 12 (32.4%) were male, and the average age was 51.4 years. The number of patients with associated co-morbidities was as following: thirteen (35.1%) had HTN, 2 (5.4%) had DM, 10 (27.0%) had prior DVT, 8 (21.6%) had prior PE, 10 patients were considered in a hypercoagulable state (27.0%) and 6 (16.2%) had prior stroke ( Table 1 ). Furthermore, 2 patients were found with PE and 1 patient was found with DVT at presentation.
Headache was the most common presenting symptom in 22 patients (59.4%) corresponding to 18 patients with worsening of chronic headaches and 4 patients with acute onset. Other presenting symptoms included focal neurological deficit (n = 9, 24.3%) and seizures (n = 2, 5.4%). CVT was found incidentally on imaging in 4 cases. Two patients (5.4%) had thrombocytopenia at presentation with CVT. Six patients (16.2%) were on anticoagulation medication at the time of diagnosis, while 2 patients (5.4%) were on aspirin.
Most patients had venous sinus thrombosis without evidence of cortical vein thrombosis (n = 32, 86.4%); 3 patients (8.1%) had sinus thrombosis with cortical vein thrombosis; and 2 patient (5.4%) had isolated cortical vein thrombosis. Twenty-six patients (70.3%) presented with CVT without stroke and 11 (29.7%) had radiographic evidence of venous stroke and 7 patients (18.9%) had hemorrhagic conversion. The most common therapeutic approach was medical management with anticoagulation in 26 patients. Four patients were managed conservatively and did not receive medical therapy as they had a non-occlusive stenosis. Six patients (16.2%) required endotracheal intubation. One patient had no COVID-19 association and was found with extensive and occlusive CVT that was complicated by hemorrhagic transformation. This patient experienced neurological decline despite medical management and received intravenous thrombolysis followed by mechanical thrombectomy.
Complications during the hospital course were reported in 7 patients (18.9%): heparin-induced thrombocytopenia and increased intracranial pressure requiring CSF flow diversion. There was no in-hospital mortality. Mean mRS at discharge and last follow-up was 1.41 and 1.22, respectively. Mean length of stay was 4.9 days, and mean follow-up time was 155.9 days.
4. Discussion
Recent studies have found a significant association between COVID-19 and CVT [8], [9], [12], [13], [14]. In this study, we reviewed our multi-center experience with CVT during the current COVID-19 pandemic with a primary focus on the patients’ presentation, management, and outcomes. We found that 27% of the CVT cases treated at our institution had a positive COVID-19 association corresponding to 10 COVID-19 infections and 4 recent vaccinations against COVID-19 prior to CVT diagnosis. Most patients were treated with anticoagulation and clinical outcomes were favorable.
Multiple factors have been identified as predictors of poor outcomes in patients diagnosed with CVT such as age, male sex, coma at presentation, seizures, deep CVT, hemorrhage, and concomitant diseases [1], [2]. In our cohort, 50% of patients in group I were males, whereas group II had 34.5% males. This should be taken into consideration as male patients with CVT are considered to have a worse prognosis than females based on prior studies of CVT patients [5], [6]. Nevertheless, we did not observe a difference in the outcomes according to sex (P = 0.41). Recent studies on COVID-19 patients suggested that CVT presents at a younger age in this population when compared to the general population, with multiple reports of CVT in COVID-19 patients younger than 40 years [17], [18]. Although no statistical significance was achieved on comparative analysis, various trends were observed: the mean age was similar between the 2 groups and patients without COVID-19 association had higher rates of comorbidities including HTN, DM, DVT, PE, and prior stroke. Based on this observation, COVID-19-associated CVT may present in a healthier population, with COVID-19 as the main predisposing factor for CVT. Fever was a presenting sign noted only in 1 patient with CVT and active COVID-19 infection. Therefore, concerns about active COVID-19 infection may be raised in patients with CVT and fever.
Prior studies on COVID-19-associated CVT have considered thrombocytopenia as a characteristic factor of this prothrombotic phenomenon, especially in patients with recent vaccination against COVID-19 [8], [9], [10]. In our cohort, only three patients had thrombocytopenia, corresponding to 1 patient in group I with active COVID-19 infection complicated by pneumonia and sepsis, and 2 patients in group II patient with heparin induced thrombocytopenia. No patient with recent vaccination against COVID-19 presented with thrombocytopenia. Furthermore, no patient in group I was on anticoagulation at the time of CVT diagnosis, while 20% of patients without COVID-19 association were anticoagulated prior to CVT diagnosis. The role of prophylactic anticoagulation to prevent thromboembolic complications in COVID-19 patients has been a topic of discussion and no clear practice guidelines exist.
CVT commonly occurs at the junction between cortical veins and venous sinuses (the vein of Trolard and the superior sagittal sinus or the vein of Labbe and the transverse sinus) and at the cavernous sinuses [3], [4], [6]. In our cohort, CVT was commonly focal and involved the transverse-sigmoid junction. Most patients in group I had thrombosis of the venous sinuses without evidence of cortical vein thrombosis and only 1 patient had cortical vein thrombosis without venous sinus thrombosis. On the other hand, 2 patients in group II had thrombosis of the cortical veins, including 1 with no noted thrombosis of the venous sinuses. Furthermore, 2 patients with a positive COVID-19 association had venous stroke with hemorrhagic conversion (1 case of active COVID infection and 1 case with recent COVID-19 vaccination) and the rest of group I patients had CVT without stroke. In contrast, 11 out of 37 (29.7%) of patients in group II had venous stroke at presentation, including 7 patients with hemorrhagic conversion. Although COVID-19 may predispose to CVT, these findings suggest that patients without COVID-19 association have a more severe and extensive form of CVT and this could certainly be related to the higher rates of comorbidities observed in this group.
The influence of the COVID-19 pandemic on the care of CVT patients is unknown. Recent studies reported on the changes that were made to the healthcare workflow and how each department adapted to the new demands that were mandated by this unforeseen disease [19], [20]. In our experience, management and outcomes of CVT patients was similar in patients with and without COVID-19 association. Medical therapy with anticoagulation and proper hydration was the mainstay of treatment. Of note, 6 patients were taking anticoagulants and 3 were taking antiplatelets at the time of CVT diagnosis.
The role of endovascular treatment of CVT is not well established and this technology is considered an adjunct to medical management in certain cases with progressive clinical worsening. When treatment with anticoagulation fails, the prognosis is poor and endovascular treatment has been used with increased frequency. In out series, only 1 patient in group II underwent MT after failing thrombolysis for extensive thrombosis of the superior sagittal sinus, straight sinus, and right transverse sinus with hemorrhagic conversion and progressive neurological decline. No difference in the rate of endotracheal intubation was noted between the 2 groups. The length of stay was longer for group I and this might be related to the need for prolonged medical care in patients with active COVID-19 infection. Clinical outcomes at discharge or last follow-up were similar between the 2 groups and no mortality was noted in this study. Although this may suggest that CVT tends to have favorable outcomes if treated in a timely manner, it is important to note that some of the included patients may have been lost to follow-up, especially during the pandemic as the healthcare system was overwhelmed and social distancing guidelines were in effect [6]. Moreover, we only included cases with CVT confirmed on vascular imaging, thus resulting in underdiagnosis of severe cases of CVT in which vascular imaging was not feasible. We did not observe any mortality within our cohort and outcomes were generally favorable. In contrast, pooled mortality from available studies in the literature was 36.8%. This difference in the results between the literature and our study could be related to the difference in studies population. Although the overall outcomes seem better in group I and this may lead to the perception that CVT has a milder presentation in this group, this difference might be related to the associated comorbidities in group II. In terms of management of CVT during the COVID pandemic, there is no significant difference in the treatment modalities and anticoagulation remains the mainstay of medical management with endovascular treatment considered for patients who fail medical management.
4.1. Limitations
This study has inherent limitations due to its retrospective nature. There is a potential for referral bias, which prevents measuring true prevalence of this disease at a time when the COVID pandemic has changed the patterns or referrals and transfers significantly. Data may be incomplete with non-standardized treatment, follow-up, and outcomes measures. Furthermore, the small cohort size limited the power of our comparative analyses. Although we accounted for known risk factors of CVT, the influence of other potential unknown cofounders cannot be ruled out as well.
5. Conclusion
Twenty-seven percent of CVT patients who presented during the COVID-19 pandemic were associated with COVID-19. In patients with CVT and fever, COVID-19 infection should be considered. Patients with CVT without recent COVID-19 infection or vaccination have more co-morbidities and might have slightly worse outcomes compared to patients with no history of COVID infection or vaccine. Our findings suggest that patients with active COVID-19 infection and CVT have an extended and complicated hospital course likely related to COVID-19-associated complications. Further studies are needed to shed more light on clinical outcomes and mortality in patients with CVT and associated COVID-19 infection and vaccine.
Disclosure of Funding
None.
CRediT authorship contribution statement
Ricardo A. Domingo: Conceptualization, Data curation, Methodology, Resources, Writing – original draft. Andres Ramos-Fresnedo: Data curation, Resources, Writing – review & editing. Carlos Perez-Vega: Data curation, Writing – review & editing. Shashwat Tripathi: Statistical analysis, Methodology, Software, Writing – original draft. Michael W. Pullen: Data curation, Writing – review & editing. Jaime L. Martinez: Writing – review & editing. Young M. Erben: Writing – review & editing. James Meschia: Conceptualization, Writing – review & editing. Rabih G. Tawk: Conceptualization, Supervision, Writing – review & editing.
Declaration of Competing Interest
None.
References
- 1.Bushnell C., Saposnik G. Evaluation and management of cerebral venous thrombosis. CONTINUUM: Lifelong Learn. Neurol. 2014;20(2):335–351. doi: 10.1212/01.CON.0000446105.67173.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gessler F., Bruder M., Duetzmann S., et al. Risk factors governing the development of cerebral vein and dural sinus thrombosis after craniotomy in patients with intracranial tumors. J. Neurosurg. 2017;128(2):373–379. doi: 10.3171/2016.11.JNS161871. [DOI] [PubMed] [Google Scholar]
- 3.Behrouzi R., Punter M. Diagnosis and management of cerebral venous thrombosis. Clin. Med. 2018;18(1):75. doi: 10.7861/clinmedicine.18-1-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo Y., Tian X., Wang X. Diagnosis and treatment of cerebral venous thrombosis: a review. Front. Aging Neurosci. 2018;10:2. doi: 10.3389/fnagi.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee D.J., Ahmadpour A., Binyamin T., Dahlin B.C., Shahlaie K., Waldau B. Management and outcome of spontaneous cerebral venous sinus thrombosis in a 5-year consecutive single-institution cohort. J. neurointerventional Surg. 2017;9(1):34–38. doi: 10.1136/neurintsurg-2015-012237. [DOI] [PubMed] [Google Scholar]
- 6.Saposnik G., Barinagarrementeria F., Brown R.D., Jr., et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158–1192. doi: 10.1161/STR.0b013e31820a8364. [DOI] [PubMed] [Google Scholar]
- 7.Hanff T.C., Mohareb A.M., Giri J., Cohen J.B., Chirinos J.A. Thrombosis in COVID‐19. Am. J. Hematol. 2020;95(12):1578–1589. doi: 10.1002/ajh.25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taquet M., Husain M., Geddes JR, Luciano S., Harrison PJ. Cerebral venous thrombosis: a retrospective cohort study of 513,284 confirmed COVID-19 cases and a comparison with 489,871 people receiving a COVID-19 mRNA vaccine. Center for Open Science Preprint. 2021.
- 9.Thakur K.T., Tamborska A., Wood G.K., et al. Clinical review of cerebral venous thrombosis in the context of COVID-19 vaccinations: evaluation, management, and scientific questions. J. Neurol. Sci. 2021 doi: 10.1016/j.jns.2021.117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greinacher A., Thiele T., Warkentin TE, Weisser K., Kyrle P., Eichinger S. A prothrombotic thrombocytopenic disorder resembling heparin-induced thrombocytopenia following coronavirus-19 vaccination. 2021.
- 11.Cavalcanti D.D., Raz E., Shapiro M., et al. Cerebral venous thrombosis associated with COVID-19. Am. J. Neuroradiol. 2020;41(8):1370–1376. doi: 10.3174/ajnr.A6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh R., Roy D., Mandal A., et al. Cerebral venous thrombosis in COVID-19. Diabetes Metab. Syndr.: Clin. Res. Rev. 2021;15(3):1039–1045. doi: 10.1016/j.dsx.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdalkader M., Shaikh S.P., Siegler J.E., et al. Cerebral venous sinus thrombosis in COVID-19 patients: a multicenter study and review of literature. J. Stroke Cerebrovasc. Dis. 2021 doi: 10.1016/j.jstrokecerebrovasdis.2021.105733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zicarelli C., Martins J., Doni W. Cerebral venous thrombosis and Covid 19: literature review. J. Neurol. Stroke. 2021;11(2):57–68. [Google Scholar]
- 15.Al-Mufti F., Amuluru K., Sahni R., et al. Cerebral venous thrombosis in COVID-19: a New York metropolitan cohort study. Am. J. Neuroradiol. 2021 doi: 10.3174/ajnr.A7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benny R., Singh R.K., Venkitachalam A., et al. Characteristics and outcomes of 100 consecutive patients with acute stroke and COVID-19. J. Neurol. Sci. 2021 doi: 10.1016/j.jns.2021.117348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavalcanti D.D., Raz E., Shapiro M., et al. Cerebral venous thrombosis associated with COVID-19. AJNR Am. J. Neuroradiol. 2020;41(8):1370–1376. doi: 10.3174/ajnr.A6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein D.E., Libman R., Kirsch C., Arora R. Cerebral venous thrombosis: a typical presentation of COVID-19 in the young. J. Stroke Cereb. Dis. 2020;29(8) doi: 10.1016/j.jstrokecerebrovasdis.2020.104989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Biase G., Freeman W., Elder B., et al. Path to reopening surgery in the COVID-19 pandemic: neurosurgery experience. Mayo Clin. Proc.: Innov., Qual. Outcomes. 2020;4(5):557–564. doi: 10.1016/j.mayocpiqo.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khoshsirat NA, Qorbani M., Farivar AM, Mohammadpoor Nami S., Mohammadian Khonsari N. Effects of the covid‐19 pandemic on neurological diseases. Brain and Behavior. 2021. [DOI] [PMC free article] [PubMed]