Abstract
Background
Benign smooth muscle tumours of the uterus, known as fibroids or myomas, are often symptomless. However, about one‐third of women with fibroids will present with symptoms that are severe enough to warrant treatment. The standard treatment of symptomatic fibroids is hysterectomy (that is surgical removal of the uterus) for women who have completed childbearing, and myomectomy for women who desire future childbearing or simply want to preserve their uterus. Myomectomy, the surgical removal of myomas, can be associated with life‐threatening bleeding. Excessive bleeding can necessitate emergency blood transfusion. Knowledge of the effectiveness of the interventions to reduce bleeding during myomectomy is essential to enable evidence‐based clinical decisions. This is an update of the review published in The Cochrane Library (2011, Issue 11).
Objectives
To assess the effectiveness, safety, tolerability and costs of interventions to reduce blood loss during myomectomy.
Search methods
In June 2014, we conducted electronic searches in the Cochrane Menstrual Disorders and Subfertility Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL and PsycINFO, and trial registers for ongoing and registered trials.
Selection criteria
We selected randomised controlled trials (RCTs) that compared potential interventions to reduce blood loss during myomectomy to placebo or no treatment.
Data collection and analysis
The two authors independently selected RCTs for inclusion, assessed the risk of bias and extracted data from the included RCTs. The primary review outcomes were blood loss and need for blood transfusion. We expressed study results as mean differences (MD) for continuous data and odds ratios for dichotomous data, with 95% confidence intervals (CI). We assessed the quality of evidence using GRADE methods.
Main results
Eighteen RCTs with 1250 participants met our inclusion criteria. The studies were conducted in hospital settings in low, middle and high income countries.
Blood loss We found significant reductions in blood loss with the following interventions: vaginal misoprostol (2 RCTs, 89 women: MD ‐97.88 ml, 95% CI ‐125.52 to ‐70.24; I2 = 43%; moderate‐quality evidence); intramyometrial vasopressin (3 RCTs, 128 women: MD ‐245.87 ml, 95% CI ‐434.58 to ‐57.16; I2 = 98%; moderate‐quality evidence); intramyometrial bupivacaine plus epinephrine (1 RCT, 60 women: MD ‐68.60 ml, 95% CI ‐93.69 to ‐43.51; low‐quality evidence); intravenous tranexamic acid (1 RCT, 100 women: MD ‐243 ml, 95% CI ‐460.02 to ‐25.98; low‐quality evidence); gelatin‐thrombin matrix (1 RCT, 50 women: MD ‐545.00 ml, 95% CI ‐593.26 to ‐496.74; low‐quality evidence); intravenous ascorbic acid (1 RCT, 102 women: MD ‐411.46 ml, 95% CI ‐502.58 to ‐320.34; low‐quality evidence); vaginal dinoprostone (1 RCT, 108 women: MD ‐131.60 ml, 95% CI ‐253.42 to ‐9.78; low‐quality evidence); loop ligation of the myoma pseudocapsule (1 RCT, 70 women: MD ‐305.01 ml, 95% CI ‐354.83 to ‐255.19; low‐quality evidence); a fibrin sealant patch (1 RCT, 70 women: MD ‐26.50 ml, 95% CI ‐44.47 to ‐8.53; low‐quality evidence), a Foley catheter tied around the cervix (1 RCT, 93 women: MD ‐240.70 ml, 95% CI ‐359.61 to ‐121.79; low‐quality evidence), and a polyglactin suture round both cervix and infundibulopelvic ligament (1 RCT, 28 women: MD ‐1870.0 ml, 95% CI ‐2547.16 to 1192.84; low‐quality evidence). There was no good evidence of an effect on blood loss with oxytocin, morcellation or clipping of the uterine artery.
Need for blood transfusion We found significant reductions in the need for blood transfusion with vasopressin (2 RCTs, 90 women: OR 0.15, 95% CI 0.03 to 0.74; I2 = 0%; moderate‐quality evidence); tourniquet tied round the cervix (1 RCT, 98 women: OR 0.22, 95% CI 0.09 to 0.55; low‐quality evidence); tourniquet tied round both cervix and infundibulopelvic ligament (1 RCT, 28 women: OR 0.02, 95% CI 0.00 to 0.23; low‐quality evidence); gelatin‐thrombin matrix (1 RCT, 100 women: OR 0.01, 95% CI 0.00 to 0.10; low‐quality evidence) and dinoprostone (1 RCT, 108 women: OR 0.17, 95% CI 0.04 to 0.81; low‐quality evidence), but no evidence of effect on the need for blood transfusion with misoprostol, oxytocin, tranexamic acid, ascorbic acid, loop ligation of the myoma pseudocapsule and a fibrin sealant patch.
There were insufficient data on the adverse effects and costs of the different interventions.
Authors' conclusions
At present there is moderate‐quality evidence that misoprostol or vasopressin may reduce bleeding during myomectomy, and low‐quality evidence that bupivacaine plus epinephrine, tranexamic acid, gelatin‐thrombin matrix, ascorbic acid, dinoprostone, loop ligation, a fibrin sealant patch, a peri‐cervical tourniquet or a tourniquet tied round both cervix and infundibulopelvic ligament may reduce bleeding during myomectomy. There is no evidence that oxytocin, morcellation and temporary clipping of the uterine artery reduce blood loss. Further well designed studies are required to establish the effectiveness, safety and costs of different interventions for reducing blood loss during myomectomy.
Keywords: Female; Humans; Blood Loss, Surgical; Blood Loss, Surgical/prevention & control; Blood Transfusion; Blood Transfusion/statistics & numerical data; Hemostasis, Surgical; Hemostasis, Surgical/methods; Hemostatics; Hemostatics/therapeutic use; Leiomyoma; Leiomyoma/surgery; Randomized Controlled Trials as Topic; Tourniquets; Uterine Neoplasms; Uterine Neoplasms/surgery
Plain language summary
Interventions to reduce haemorrhage during myomectomy for treating fibroids
Background
Some women have non‐cancerous growths of the uterus, called fibroids. In a third of cases the fibroids produce symptoms, such as vaginal bleeding, that warrant treatment. The surgical removal of the fibroids, called myomectomy, is one of the treatment options for fibroids. It can be accomplished by either laparotomy (through an incision into the abdomen) or laparoscopy (keyhole surgery). The procedure is associated with heavy bleeding. Many interventions have been used by doctors to reduce bleeding during an operation for removing fibroids but it is unclear whether or not the interventions are effective.
Study characteristics
The evidence is current to June 2014. The review included 18 studies with a total of 1250 women who had myomectomy for uterine fibroids. All studies compared an intervention to reduce bleeding during myomectomy with either a placebo or no such treatment.
Key results
The data available suggest that vaginal insertion of misoprostol and infiltration of vasopressin into the uterine muscle are effective in reducing bleeding during myomectomy. Limited data available also suggest that chemical dissection (such as with mesna), vaginal insertion of dinoprostone, a gelatin‐thrombin matrix, tranexamic acid, infusion of vitamin C (ascorbic acid) during surgery, infiltration of a mixture of bupivacaine and epinephrine into the uterine muscles, the use of fibrin sealant patch (a surgical patch that improves blood clotting) or a tourniquet around the cervix or around both the cervix and the infundibulopelvic ligamentmay be effective in reducing bleeding during myomectomy. We found limited information on the harms (adverse effects) of the different interventions.
Quality of the evidence
There is moderate‐quality evidence that misoprostol reduces blood loss by between 70.24 ml and 125.52 ml; with a laparotomy vasopressin reduces blood loss by between 392.51 and 507.49 ml during myomectomy, and by between 121.73 ml and 172.17 ml during laparoscopic myomectomy. There is low‐quality evidence for the rest of the interventions (chemical dissection, dinoprostone, gelatin‐thrombin matrix, tranexamic acid, vitamin C, mixture of bupivacaine and epinephrine, a fibrin sealant patch and the two types of tourniquet).
Summary of findings
Summary of findings for the main comparison. Interventions to reduce blood loss during myomectomy for fibroids compared to placebo or no treatment.
| Interventions to reduce blood loss during myomectomy for fibroids compared to placebo or no treatment | ||||||
| Population: Women with fibroids Settings: Various settings in low income, middle income, and high income countries Intervention: Diverse interventions Comparison: Placebo or no treatment | ||||||
| Intervention | Illustrative comparative risks (95% CI) on blood loss | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Placebo or no treatment | Interventions | |||||
| Misoprostol in abdominal myomectomy | Mean blood loss with placebo was 621 ml | Mean blood loss with misoprostol was 149.00 ml lower (229.24 to 68.76 lower) | MD ‐149.00 (‐229.24 to ‐68.76) | 25 (1 study) | ⊕⊕⊕⊝ moderate | We rated down the quality of evidence (by 1) because the data were derived from one small study |
| Misoprostol in laparoscopic myomectomy | Mean blood loss with placebo was 322.39 ml | Mean blood loss with misoprostol was 91.00 ml lower (120.44 to 61.56 lower) | MD ‐91.00 (‐120.44 to ‐61.56) | 64 (1 study) | ⊕⊕⊕⊝ moderate | We rated down the quality of evidence (by 1) because the data were derived from one small study |
| Vasopressin | Mean blood loss with placebo was 483.09 ml | Mean blood loss with vasopressin was 245.87 ml lower (434.58 to 57.16 lower) | MD ‐245.87 (‐434.58 to ‐57.16) | 128 (3 studies) | ⊕⊕⊕⊝ moderate | We rated down the quality of evidence (by 1) because the data were derived from three small studies |
| Bupivicaine plus epinephrine | Mean blood loss with placebo was 212.5 ml | Mean blood loss with bupivicaine‐epinephrine was 68.6 ml lower (93.69 to 43.51 lower) | MD ‐68.60 (‐93.69, ‐43.51) | 60 (1 study) | ⊕⊕⊝⊝ low | We rated down the quality of evidence (by 2) because the data were derived from one small study, with a high risk of attrition bias (2 patients in each arm did not receive assigned intervention because of concomitant disease) |
| Intravenous injection of tranexamic acid | Mean blood loss with placebo was 1047 ml | Mean blood loss with tranexamic was 243 ml lower (460.02 to 25.98 lower) | MD ‐243.00 (‐460.02 to ‐25.980 | 100 (1 study) | ⊕⊕⊝⊝ low | We rated down the quality of evidence (by 2) because the data were derived from one small study and the pooled effect estimate was imprecise |
| Gelatin‐thrombin matrix | Mean blood loss with placebo was 625 ml | Mean blood loss with Gelatin‐thrombin was 545 ml lower (593.26 to 496.74 lower) | MD ‐545.00 (‐593.26 to ‐496.74) | 50 (1 study) | ⊕⊕⊝⊝ low | We rated down the quality of evidence (by 2) because the data were derived from one small study, and it is unclear if outcome assessors were blind |
| Ascorbic acid | Mean blood loss with no treatment was 932.9 ml | Mean blood loss with ascorbic acid was 411.46 ml lower (502.58 to 320.34 lower) |
MD ‐411.46 (‐502.58 to ‐320.34) |
102 (1 study) | ⊕⊕⊝⊝ low | We rated down the quality of evidence (by 2) because the data were derived from one small study, and it is unclear how allocation concealment was done |
| Dinoprostone (prostaglandin E2 analogue) | Mean blood loss with placebo was 485.7 ml | Mean blood loss with dinoprostone was 131.6 ml lower (253.42 to 9.78 lower) |
MD ‐131.60 (‐253.42 to ‐9.78) |
108 (1 study) | ⊕⊕⊝⊝ low | We rated down the quality of evidence (by 2) because the data were derived from one small study, and the effect estimate has wide confidence intervals |
| Loop ligation of myoma pseudocapsule plus vasopressin | Mean blood loss with no treatment was 363.68 ml | Mean blood loss with loop ligation was 305.01 lower (354.83 to 255.19 lower) |
MD ‐305.01 (‐354.83 to ‐255.19) |
70 (1 study) | ⊕⊕⊝⊝ low | We rated down the quality of evidence (by 2) because the data were derived from one small study, and it is unclear how allocation concealment was done |
| Fibrin sealant patch (collagen sponge with thrombin and fibrinogen) | Mean blood loss with no treatment was 151.1 ml | Mean blood loss with tachosil was 26.5 ml lower (44.47 to 8.53 lower) |
MD ‐26.50 (‐44.47 to ‐8.53) |
70 (1 study) | ⊕⊕⊝⊝ low | We rated down the quality of evidence (by 2) because the data were derived from one small study, and the effect estimate has wide confidence intervals |
| CI: Confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 2. Misoprostol compared to placebo to reduce blood loss during myomectomy for fibroids.
| Misoprostol compared to placebo to reduce blood loss during myomectomy for fibroids | ||||||
| Patient or population: Women with fibroids Settings: Middle and high income countries Intervention: Misoprostol Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Placebo | Misoprostol | |||||
| Blood loss (ml) Estimated blood loss during myomectomy | The mean blood loss in placebo group was 322.39 ml | The mean blood loss in misoprostol group was 97.88 ml lower (125.52 to 70.24 lower) | MD ‐97.88 (‐125.52 to ‐70.24) | 89 (2 studies) | ⊕⊕⊕⊝ moderate1 | We rated down the quality of evidence (by 1) because the data were derived from two small studies and we could not rule out the possibility of publication bias |
| Need for blood transfusion Number of participants who received blood transfusion | 87 per 1000 | 31 per 1000 (4 to 217) | OR 0.36 (0.05 to 2.5) | 89 (2 studies) | ⊕⊕⊝⊝ low1 | We rated down the quality of evidence (by 2) because (i) we could not conclusively rule out the possibility of publication bias and (ii) the pooled effect had wide confidence intervals |
| Duration of surgery (min) Operative time | The mean duration of surgery in placebo group was 69.57 min for abdominal myomectomy and 77 min for laparoscopic myomectomy | The mean duration of surgery in misoprostol group was 9.50 min lower (15.90 lower to 3.10 lower) in abdominal myomectomy and 9 min higher (1.63 lower to 19.63 higher) in laparoscopic myomectomy |
MD ‐9.50 (‐15.90 to ‐3.10) for abdominal myomectomy & MD 9.00 (‐1.63 to 19.63) for laparoscopic myomectomy |
25
(1 study) for abdominal myomectomy & 64 (1 study) for laparoscopic myomectomy |
⊕⊕⊝⊝ low1 | We rated down the quality of evidence (by 2) because (i) we could not conclusively rule out the possibility of publication bias and (ii) the pooled effect had wide confidence intervals We did not rate down the evidence due to heterogeneity because this could be explained by the type of myomectomy (laparoscopy versus laparotomy) |
| CI: Confidence interval; MD: mean difference; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1In one trial the method of allocation concealment was not reported and in the other trial, allocation concealment was achieved using sequentially numbered opaque sealed envelopes.
Summary of findings 3. Vasopressin versus placebo to reduce blood loss during myomectomy for fibroids.
| Vasopressin versus placebo to reduce blood loss during myomectomy for fibroids | ||||||
| Patient or population: Women with fibroids Settings: Middle and low income countries Intervention: Vasopressin Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Placebo | Vasopressin | |||||
| Blood loss (ml) Estimated blood loss during myomectomy | The mean blood loss in the placebo groups was 483.09 ml | The mean blood loss in the vasopressin groups was 245.87 ml lower (434.58 to 57.16 lower) | MD ‐245.87 (‐434.58 to ‐57.16) | 128 (3 studies) | ⊕⊕⊕⊝ moderate1 | We rated down the quality of evidence (by 1) because the data were derived from three small studies and we could not rule out the possibility of publication bias We did not rate down the evidence due to heterogeneity because this could be explained by the fact that in one study women had laparoscopic myomectomy and two other studies, women has open abdominal myomectomy |
| Need for blood transfusion Participants who received blood transfusion | 222 per 1000 | 33 per 1000 (7 to 164) | OR 0.15 (0.03 to 0.74) | 90 (2 studies) | ⊕⊕⊕⊝ moderate1 | We rated down the quality of evidence (by 1) because the data were derived from two small studies and we could not rule out the possibility of publication bias |
| Duration of surgery Operative time | The mean duration of surgery in the placebo groups was 111.45 min | The mean duration of surgery in the vasopressin groups was 27.72 min lower (35.82 to 19.61 lower) | MD ‐27.72 (‐35.82 to ‐19.61) | 108 (2 studies) | ⊕⊕⊕⊝ moderate1 | We rated down the quality of evidence (by 1) because the data were derived from two small studies and we could not rule out the possibility of publication bias |
| CI: Confidence interval; MD: mean difference; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1In all the trials, allocation concealment was unclear.
Summary of findings 4. Bupivicaine plus epinephrine compared to placebo to reduce blood loss during myomectomy for fibroids.
| Bupivicaine plus epinephrine compared to placebo to reduce blood loss during myomectomy for fibroids | ||||||
| Patient or population: Women with fibroids Settings: University hospital in Italy Intervention: Bupivicaine plus epinephrine Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Placebo | Bupivicaine plus epinephrine | |||||
| Blood loss (ml) Estimated blood loss during myomectomy | The mean blood loss in the placebo group was 212.5 ml | The mean blood loss in the bupivicaine‐epinephrine group was 68.6 ml lower (93.69 to 43.51 lower) | MD ‐68.60 (‐93.69 to ‐43.51) | 60 (1 study) | ⊕⊕⊝⊝ low1 | We rated down the quality of evidence (by 2) because the data were derived from one small study, with a high risk of attrition bias (2 patients in each arm did not receive assigned intervention because of concomitant disease) |
|
Need for blood transfusion Participants who received blood transfusion |
Outcome not reported by investigators | |||||
| Duration of surgery (min) Operative time | The mean duration of surgery in the placebo group was 109.2 min | The mean duration of surgery in the bupivicaine‐epinephrine group was 30.50 min lower (37.68 to 23.32 lower) | MD ‐30.50 (‐37.68 to ‐23.32) | 60 (1 study) | ⊕⊕⊝⊝ low1 | We rated down the quality of evidence (by 2) because the data were derived from one small study, with a high risk of attrition bias (2 patients in each arm did not receive assigned intervention because of concomitant disease) |
| CI: Confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1The allocation concealment was achieved by envelopes containing computer‐generated random numbers.
Summary of findings 5. Peri‐cervical tourniquet compared to no treatment to reduce blood loss during myomectomy for fibroids.
| Peri‐cervical tourniquet compared to no treatment to reduce blood loss during myomectomy for fibroids | ||||||
| Patient or population: Women with fibroids Settings: Low and high income countries Intervention: Tourniquet around the cervix only, or around both the cervix and the infundibulopelvic ligament Comparison: No treatment | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| No treatment | Peri‐cervical tourniquet | |||||
| Blood loss (ml) Estimated blood loss during myomectomy | The mean blood loss in the control groups was 756.4 ml (for cervical tourniquet) &2359.0 ml (for cervical plus infundibulopelvic ligament tourniquet) | The mean blood loss in the intervention groups was 240.70 ml lower (359.61 ml lower to 121.79 ml lower) for the cervical tourniquet study & 1870 ml lower (2547.16 ml lower to 1192.84 ml lower) for the cervical plus infundibulopelvic ligament tourniquet study | MD ‐240.70 (‐359.61 to ‐121.79) for cervical touniquet study & ‐1870 (‐2547.16 to ‐1192.84) for the cervical plus infundibulopelvic ligament tourniquet study | 121 (2 studies) | ⊕⊕⊝⊝ low1 | We rated down the quality of evidence (by 2) because the data were derived from two small studies that were not pooled together due to significant clinical and statistical heterogeneity. One study used polyglactin suture round both the cervix and infundibulopelvic ligament, while the other used a Foley catheter round the cervix. |
| Need for blood transfusion Participants who received blood transfusion | 539 per 1000 for cervical tourniquet study &786 per 1000 for cervical plus infundibulopelvic ligament study | 204 per 1000 for cervical tourniquet study &71 per 1000 for cervical plus infundibulopelvic ligament study |
OR 0.22
(0.09 to 0.55) or cervical tourniquet study & OR 0.02 (0.00 to 0.23) for cervical plus infundibulopelvic ligament study |
121 (2 studies) | ⊕⊕⊝⊝ low1 | We rated down the quality of evidence (by 2) because the data were derived from two small studies which were not pooled together because of significant heterogeneity. One study used polyglactin suture round both the cervix and infundibulopelvic ligament, while the other used a Foley catheter round the cervix. |
| Duration of surgery (min) Operative time | The mean duration of surgery in the control groups was 118 min | The mean duration of surgery in the intervention groups was 4 min lower (29.28 lower to 21.28 higher) |
MD ‐4.00 (‐29.28 to 21.28) |
28 (1 study) | ⊕⊝⊝⊝ very low | We rated down the quality of evidence (by 3) because the data were derived from one small study and the effect estimate was imprecise |
| CI: Confidence interval; MD: mean difference; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1In one trial the allocation concealment was unclear and in the other trial allocation concealment was achieved by sealed sequentially‐numbered opaque envelopes containing computer‐generated random numbers.
Summary of findings 6. Gelatin‐thrombin matrix compared to placebo or no treatment to reduce blood loss during myomectomy for fibroids.
| Gelatin‐thrombin matrix compared to placebo or no treatment to reduce blood loss during myomectomy for fibroids | ||||||
| Patient or population: Women with fibroids Settings: University teaching hospital in Spain Intervention: Gelatin‐thrombin matrix Comparison: Placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Placebo | Gelatin‐thrombin matrix | |||||
| Blood loss (ml) Estimated blood loss during myomectomy | The mean blood loss in placebo groups was 625 ml | The mean blood loss in intervention groups was 545 ml lower (593.26 to 496.74 lower) | MD ‐545.00 (‐593.26 to ‐496.74) | 50 (1 study) | ⊕⊕⊝⊝ low1 | We rated down the quality of evidence (by 2) because the data were derived from one small study, and it is unclear if outcome assessors were blind |
| Need for blood transfusion Participants who received blood transfusion | 400 per 1000 | 4 per 1000 (0 to 40) | OR 0.01 (0 to 0.1) | 50 (1 study) | ⊕⊕⊝⊝ low1 | We rated down the quality of evidence (by 2) because the data were derived from one small study, it is unclear if outcome assessors were blind, and the effect estimate was imprecise |
| Duration of surgery (min) Operative time | The mean duration of surgery in placebo group was 60 min | The mean duration of surgery in intervention group was 5.00 min higher (1.29 to 8.71 higher) |
MD 5.00 (1.29 to 8.71) |
50 (1 study) | ⊕⊕⊝⊝ low1 | We rated down the quality of evidence (by 2) because the data were derived from one small study, it is unclear if outcome assessors were blind, and the effect estimate was imprecise |
| CI: Confidence interval;MD: Mean difference; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Allocation concealment not reported.
Summary of findings 7. Ascorbic acid compared to no treatment to reduce blood loss during myomectomy for fibroids.
| Ascorbic acid compared to placebo or no treatment to reduce blood loss during myomectomy for fibroids | ||||||
| Patient or population: Women with fibroids Settings: Tertiary hospital in Iran Intervention: Ascorbic acid Comparison: No treatment | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| No treatment | Ascorbic acid | |||||
| Blood loss (ml) Estimated blood loss during myomectomy | The mean blood loss in the control group was 932.9 ml | The mean blood loss in the intervention group was 411.46 ml lower (502.58 to 320.34 lower) |
MD ‐411.46 (‐502.58 to ‐320.34) |
102 (1 study) | ⊕⊕⊝⊝ low1 | We rated down the quality of evidence (by 2) because the data were derived from one small study, and it is unclear how allocation concealment was done |
| Need for blood transfusion Participants who received blood transfusion | 180 per 1000 | 68 per 1000 (20 to 238) | OR 0.38 (0.11 to 1.32) | 104 (1 study) | ⊕⊝⊝⊝ very low1 | We rated down the quality of evidence (by 2) because the data were derived from one small study, it is unclear how allocation concealment was done, and the estimate was imprecise |
| Duration of surgery (min) Operative time | The mean duration of surgery in the control group was 68 min | The mean duration of surgery in the intervention group was 26.00 min lower (33.1 to 18.9 lower) |
MD ‐26.00 (‐33.10 to ‐18.90) |
102 (1 study) | ⊕⊕⊝⊝ low1 | We rated down the quality of evidence (by 2) because the data were derived from one small study, and it is unclear how allocation concealment was done |
| CI: Confidence interval; MD: Mean difference; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Allocation concealment not reported.
Background
Description of the condition
Uterine leiomyomas (fibroids or myomas) are benign, smooth muscle tumours of the human uterus. Most myomas are asymptomatic (symptomless) and are discovered incidentally during a routine pelvic examination or imaging studies (Vollenhoven 1990). However, about 20% to 50% of women with myomas will present with symptoms severe enough to warrant treatment (Vercellini 1993). Myomas are three times more prevalent in black women, who tend to have larger, more numerous myomas, than their white counterparts (Jacoby 2010).
The standard treatment of symptomatic leiomyomas is hysterectomy for women who have completed childbearing, and myomectomy for women who wish to preserve fertility. Hysterectomy, the surgical removal of the uterus, eliminates both the symptoms and the chance of recurrence. However, many women who suffer from myomas desire future childbearing or want to preserve their uterus. For these women myomectomy, the surgical removal of the myomas with reconstruction and preservation of the uterus, is an important option. Myomectomy can be accomplished by laparotomy, laparoscopy or hysteroscopy. Myomectomy by laparotomy involves the surgical removal of the fibroids through an incision in the abdominal wall. Where there are a small number of subserous or intramural myomas and the uterine size is less than that of 16 weeks gestation, laparoscopic myomectomy may be an option (Hurst 2005). The laparoscopic approach is a minimal access technique (keyhole surgery) developed to minimize insult to the abdominal wall and to ensure quick recovery of the patient following surgery (Hasson 1992). For women with submucous myomas, transcervical hysteroscopic resection is a good option both for the gynaecologic surgeons (Derman 1991) and their patients.
Myomectomy can lead to both short and long‐term complications. Complications of hysteroscopic myomectomy include haemorrhage, uterine perforation, cervical damage and metabolic disturbances from excessive absorption of the distension medium, such as glycine (Cooper 2000). Laparoscopic myomectomy is associated with the usual risks of laparoscopy, particularly accidents during trocar (a surgical instrument) placement; and, additionally, excessive uncontrolled haemorrhage with the need to convert to a laparotomy and the risk of uterine rupture in subsequent pregnancies (Dubuisson 1997). Short‐term complications of abdominal myomectomy include bleeding, fever, infection, visceral damage and thromboembolism (LaMote 1993). A requirement for transfusion in up to 20% of cases following abdominal myomectomy has been reported in the literature (LaMote 1993). Patients undergoing myomectomy have an unusually high incidence of fever occurring in the first 48 hours following surgery (Iverson 1999). The incidence of postoperative fever following myomectomy has been reported to be as high as 36% (Celik 2003). The cause is unknown but it is believed that 'myomectomy fever' is due to the release of pyrogenic (fever‐inducing) factors during myoma dissection or to haematomas (blood clots) forming in defects left by the removed myomas. In 2% of cases there is a need for conversion of myomectomy to hysterectomy (Aubuchon 2002). Long‐term complications of abdominal myomectomy include pelvic adhesions in 59% of women after two years (Frederick 2002) and recurrent fibroids in 46.0% of women after one year (Nishiyama 2006). The risk of uterine rupture in subsequent pregnancies varies between 0% and 1% (Garnet 1964; Tulandi 1993; Fedele 1995; Somigliana 2008).
Blood loss during myomectomy can be intraoperative or postoperative and with haematoma formation. Massive blood loss associated with the dissection of huge fibroids renders myomectomy a more technically challenging procedure than hysterectomy. Sometimes myomectomy is converted to hysterectomy intraoperatively when bleeding becomes heavy and uncontrollable or when it is impossible to reconstruct the uterus because of the many defects left by the removal of multiple myomas (Iverson 1996). Excessive bleeding can necessitate emergency blood transfusion.
Description of the intervention
Many interventions have been performed to reduce bleeding during myomectomy. Four categories of interventions can be identified:
interventions on uterine arteries such as laparoscopic uterine artery dissection, uterine artery embolization, peri‐cervical mechanical tourniquet, vasopressin (natural or synthetic), a vasoconstrictive solution of bupivacaine plus epinephrine, and temporary clipping of the uterine artery;
utero‐tonics such as ergometrine, oxytocin, misoprostol, and sulprostone;
myoma dissection techniques which include myoma enucleation by morcellation and the use of laser and chemical dissectors such as sodium‐2‐mercaptoethane sulphonate (mesna);
pharmacologic manipulation of the coagulation cascade with antifibrinolytic agents such as tranexamic acid, aprotinin, aminocaproic acid, recombinant factor VIIa (Koh 2003), and gelatin‐thrombin haemostatic sealant.
In developed countries, gonadotrophin‐releasing hormone (GnRH) analogues (GnRHa) have been used prior to myomectomy. There is now clear evidence that the use of GnRH analogues reduces uterine volume and fibroid size and may reduce blood loss and operating time during myomectomy (Lethaby 2001). Although the use of preoperative GnRHa leads to less frequent vertical incisions in the case of myomectomy, a review of the cost‐effectiveness of GnRHa found that the costs outweigh its benefits (Farquhar 2002). In addition, uterine artery embolization (UAE) has been used as an alternative to myomectomy (Lumsden 2002) and to prevent haemorrhage during myomectomy (Ngeh 2004). However, there are currently no randomised trials comparing UAE with a placebo or no treatment with regard to blood loss during myomectomy. In low and middle income countries, however, the cost of using GnRH analogues and UAE may be prohibitive (especially where there is an out‐of‐pocket payment) and the necessary technology may not be available.
How the intervention might work
The mechanical tourniquet has been used during myomectomy to reduce intraoperative blood loss (Hutchins 1996). However, the pressure exerted by the tourniquet may cause damage to the uterine artery and its branches, and mask inadequate haemostasis (arrest of bleeding), which only becomes apparent once the tourniquet is removed. Uterine artery ligation can reduce both intraoperative and postoperative haemorrhage (Sapmaz 2003B). Hormonal tourniquets such as natural or synthetic vasopressin act on vasopressin V1a receptors, ubiquitously expressed in the myometrium, to reduce blood loss (Fletcher 1996; Dimitrov 1999; Kimura 2002). Hormonal tourniquets act as peri‐cervical tourniquets when administered in the cervix but act as utero‐tonics when administered in the myometrium. Preoperative UEA is a technique for the treatment of myomas which reduces uterine size and controls symptoms (Ravina 1995). It may also be a useful adjunct to surgery in women with massive fibroids (Ngeh 2004). Laparoscopic dissection of uterine vessels (LDUV) using ultrasonically activated shears for the treatment of fibroids has been reported to reduce uterine volume and symptoms (Holub 2003). Laparoscopic bipolar coagulation of the uterine blood vessels has recently been described as an alternative to UAE but a high degree of laparoscopic skill is required to isolate the uterine artery without causing damage to the ureters or blood vessels (Liu 2001).
Utero‐tonics such as ergometrine, oxytocin, misoprostol and sulprostone cause myometrial contraction and, therefore, potentially reduce blood loss during myomectomy leading to better anatomical reconstruction of the uterus (Baldoni 1995). Ergometrine may raise blood pressure while misoprostol may cause fever after its administration.
Myoma dissection techniques include the use of carbon dioxide laser and mesna for chemical dissection (McLaughin 1985). These procedures have the potential to minimize uterine defects from fibroid removal thereby facilitating uterine reconstruction, although they may be time consuming.
The coagulation cascade can be modified with the use of pharmacologic agents such as tranexamic acid, aprotinin, aminocaproic acid, recombinant factor VIIa and gelatin‐thrombin haemostatic sealant. These agents interfere with one or more stages of the coagulation cascade, from activation of coagulation to stabilisation of the fibrin clot. The end result is the formation of a stable clot that stops or prevents bleeding.
Why it is important to do this review
Excessive haemorrhage during myomectomy remains a major challenge to gynaecologic surgeons despite the many procedures that have been described to reduce intraoperative blood loss. The effects of these procedures on blood loss during myomectomy, as reported by previous non‐randomised studies, have been inconsistent. Moreover, the types of interventions are so varied that there is a need to identify the most effective procedures with minimal adverse effects to help the gynaecologic surgeon to make a correct choice.
The aim of this review was to establish which are the most effective interventions with the fewest adverse effects. The use of preoperative GnRHa was not considered in this review because their effectiveness has been examined in a separate Cochrane review (Lethaby 2001).
Objectives
To assess the effectiveness, safety, tolerability and costs of interventions to reduce blood loss during myomectomy.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomised controlled trials (RCTs) were eligible for inclusion. We excluded non‐randomised studies (for example studies with evidence of inadequate sequence generation, such as patient numbers and alternate days) as they are associated with a high risk of bias.
Types of participants
Premenopausal women undergoing myomectomy (laparotomy, laparoscopy or hysteroscopy) for uterine fibroids, for any reason, were eligible for inclusion. Women who had previously had a myomectomy were also included.
Types of interventions
Trials comparing any intervention used to reduce blood loss during myomectomy versus either placebo or no treatment were eligible for inclusion. Only interventions performed during surgery, immediately before surgery, or within the 24 hour period prior to surgery were considered for this review. Interventions that involved GnRHa were excluded because their effectiveness has been examined in a separate Cochrane review (Lethaby 2001). The interventions, compared to placebo or no treatment, that were considered in this review were:
utero‐tonics (such as ergometrine, oxytocin, misoprostol, and sulprostone);
vasopressin (natural or synthetic);
uterine artery dissection and ligation;
peri‐cervical mechanical tourniquet;
uterine artery embolization (UAE);
laser dissection;
myoma enucleation by morcellation;
chemical dissection (such as sodium‐2‐mercaptoethane sulphonate (mesna));
antifibrinolytic agents (such as tranexamic acid);
temporary clipping of the uterine artery;
use of a gelatin‐thrombin haemostatic sealant.
Types of outcome measures
Trials with at least one of the following outcomes were eligible for inclusion.
Primary outcomes
Estimated blood loss in millilitres (ml)
Need for blood transfusion (as defined by trial authors)
Secondary outcomes
1. Effectiveness outcomes:
a) postoperative haemoglobin and haematocrit;
b) postoperative recurrence of myomas;
c) pregnancy (if pregnancy desired).
2. Safety outcomes:
a) duration of operation; b) intraoperative hysterectomy; c) conversion from laparoscopy to laparotomy; d) other intraoperative complications (e.g. perforation, cervical damage); e) duration of hospital stay in days; f) postoperative morbidity (i.e. complications such as pyrexia, infection, thromboembolism, haematoma formation, and postoperative adhesions) as defined by the trial authors; g) abdominal revisions for haemoperitoneum or pelvic haematoma; h) treatment adherence (i.e. the proportion of patients who continued with the allocated treatment); i) tolerability to the intervention, as defined by trial authors.
3. Cost outcomes:
a) cost of intervention; b) total cost.
Search methods for identification of studies
We searched for all published and unpublished RCTs of myomectomy, without language restrictions and in consultation with the Menstrual Disorders and Subfertility Group (MDSG) Trials Search Co‐ordinator.
Electronic searches
The original searches were performed in March 2006 and subsequent searches were in September 2008, February 2011, October 2013 and 17 June 2014. In June 2014, we searched the following databases, trial registers and websites.
(1) The Cochrane Menstrual Disorders and Subfertility Group Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL and PsycINFO.
(2) Other electronic sources of trials included the following.
-
Trial registers for ongoing and registered trials:
http://www.clinicaltrials.gov (a service of the US National Institutes of Health);
http://www.who.int/trialssearch/Default.aspx (World Health Organization International Clinical Trials Registry Platform search portal).
PubMed (for recent trials not yet indexed in MEDLINE).
DARE (Database of Abstracts of Reviews of Effects) in The Cochrane Library at http://onlinelibrary.wiley.com/o/cochrane/cochrane_cldare_articles_fs.html (for reference lists from relevant non‐Cochrane reviews).
Searching other resources
We handsearched reference lists of identified trials and relevant review articles, specialist journals, conference abstracts. In addition, we contacted experts in the field to obtain additional data.
Data collection and analysis
Selection of studies
The Trials Search Co‐ordinator of the Cochrane Menstrual Disorders and Subfertility Group and the lead author (EJK) conducted the literature search. After an initial screen of the titles and abstracts retrieved by the search, conducted by EJK, the full texts of all potentially eligible studies were retrieved. Both authors (EJK and CSW) independently examined these full text articles for compliance with the inclusion criteria and selected studies eligible for inclusion in the review. We also contacted the study investigators, as required, to clarify study eligibility. Any disagreements as to the study eligibility were resolved by discussion and consensus. The selection process was documented in a PRISMA flow chart.
Data extraction and management
The two authors (one a topic area specialist and one a methodologist) independently extracted the data from the eligible trials using a data extraction form designed and pilot‐tested by the authors. We then compared the results and any disagreements were resolved by discussion. The following information was extracted from each of the included studies.
Characteristics of trials:
power calculation;
method of randomisation;
blinding of patients, caregivers, outcome assessors, and investigators to treatment allocation;
quality of allocation concealment;
number of patients randomised, excluded, and lost to follow up;
handling in the analysis of losses to follow up and lack of compliance with the intervention;
duration, timing, and location of the study.
Characteristics of the study participants:
age, ethnic background, and any recorded characteristics of the study participants such as size of fibroids and reason for myomectomy;
other inclusion criteria;
exclusion criteria.
Interventions:
types of interventions;
dose, timing, duration, and route of administration of the treatment;
technique of myomectomy (abdominal, laparoscopic, or hysteroscopic).
Outcomes:
types of outcomes measured or reported;
methods used to assess outcome measures.
Where studies had multiple publications, the main trial report was used as the reference and additional details were derived from secondary articles. We contacted the study investigators for further data on methods and results, as required.
Assessment of risk of bias in included studies
The two authors independently assessed the risk of bias in the included trials by addressing the seven specific domains of the Cochrane risk of bias assessment tool (www.cochrane‐handbook.org): selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective outcome reporting); and other bias, as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For risk of bias assessment we were not blinded to the names of the trial investigators, their institutions, journals of publication, nor results of the study. For each included trial we described all judgments fully and presented the conclusions in the risk of bias tables. The risk of bias was incorporated into the interpretation of the review findings by means of a sensitivity analysis. We searched for within‐trial selective reporting, such as trials failing to report obvious outcomes or reporting them in insufficient detail to allow for inclusion.
Measures of treatment effect
We performed statistical analysis using RevMan 5.2. For dichotomous data, we used numbers of events in the control and intervention groups of each study to calculate the Mantel‐Haenzel odds ratios (OR) with 95% confidence intervals (CI). For continuous data, if all studies reported exactly the same outcomes we calculated the mean difference (MD) between the groups. If they reported similar outcomes, we recorded the means and their standard deviations for each arm of the trial and expressed study results as mean difference (MD) with a 95% CI. We would have used standardised mean difference (SMD) if similar outcomes were reported on different scales across studies. If data to calculate the ORs and MDs were not available, we would have used the most detailed numerical data available that might have facilitated similar analyses of included studies (for example P values). We compared the magnitude and direction of effect reported by the studies with how they were presented in the review, taking account of legitimate differences.
Unit of analysis issues
The primary analysis was performed per woman randomised. We planned to check for 'unit‐of‐analysis error' in cluster‐randomised controlled trials. Unit‐of‐analysis error occurs if individuals in a cluster‐randomised trial are assumed to be independent and clustering is ignored during the analysis. We planned to handle these issues according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
Data were analysed on an intention‐to‐treat basis as far as possible. Where data were missing we contacted the study investigators and requested the missing data. Where only the median was reported we assumed that the mean was equal to the median and imputed the standard deviation from trials with similar values and sample sizes. Where similar trials were not available, we discussed the results of the study in a narrative format. If studies reported sufficient detail to calculate the mean differences but had no information on the associated standard deviation (SD), the outcome was assumed to have a SD equal to the highest SD from other studies within the same analysis.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included trials were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by the I2 statistic. An I2 value greater than 50% was taken to indicate substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
We planned to assess publication bias by looking for funnel plot asymmetry if there were 10 or more studies in an analysis. In view of the difficulty of detecting and correcting for publication bias and other reporting biases, attempts were made to reduce reporting bias by searching multiple sources of electronic databases and additional sources for both published and unpublished articles.
Data synthesis
If the studies were sufficiently similar, we carried out a meta‐analysis of results from trials using the fixed‐effect model (DeMets 1987). However, when significant heterogeneity was found we reported the results of the random‐effects model (DerSimonian 1986) and explored potential sources of heterogeneity. We analysed trial participants in the groups to which they were randomised, regardless of whether they actually received the assigned treatment.
Higher values of postoperative haemoglobin and haematocrit, pregnancy after myomectomy, and treatment adherence were considered a benefit rather than adverse consequences of treatment, so the presence of the effect estimates (MD, OR) and CIs to the right side of the forest plots (rather than to the left as with blood loss and other outcomes) was considered to show a benefit of treatment.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses based on the technique of myomectomy (laparotomy, laparoscopy, or hysteroscopy), and type of comparison group (placebo or no treatment).
Sensitivity analysis
We planned to perform a sensitivity analysis by considering whether the review conclusions would have differed if:
studies with high risk of bias had been excluded;
a random‐effects model had been adopted;
the summary effect measure was risk ratio rather than odds ratio (OR);
we excluded studies with imputed data.
Overall quality of the body of evidence: summary of findings table
We generated summary of findings tables using the GRADEPRO software. These tables present the overall quality of the body of evidence for main review outcomes (that is blood loss, need for blood transfusion, and duration of surgery) taking into consideration study limitations (that is risk of bias), consistency of effect, imprecision, indirectness, and publication bias. Judgments about evidence quality (high, moderate or low) was justified, documented, and incorporated into reporting of results for each outcome (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7).
Results
Description of studies
Results of the search
The search retrieved 623 records. Forty‐one studies were potentially eligible and were retrieved in full text. Eighteen studies met our inclusion criteria. Twenty‐three studies were excluded and two are ongoing.
The October 2013 search identified six additional trials (Kalogiannidis 2011; Leone Maggiore 2011; Zhao 2011; Pourmatroud 2012; Vercellino 2012; Shokeir 2013) to the 12 trials that were included in the previous version of the review (Kongnyuy 2011). See study tables: Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies. The search and selection processes for the review are shown in Figure 1.
1.
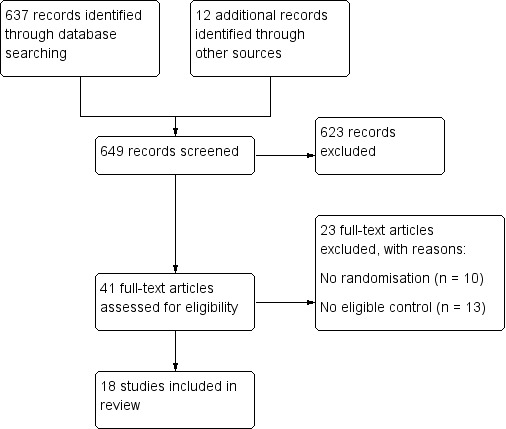
Flow diagram showing screening of search outputs and study selection.
Included studies
Study design and setting
Eighteen parallel‐design randomised controlled trials (RCTs) were included in the review. The studies were conducted in hospital settings in low, middle and high income countries. All studies were carried out in one centre except for two studies (Taylor 2005; Vercellino 2012) that were conducted in two or more centres.
Participants
The studies included 633 women in the intervention groups and 617 in the control groups. All were women of reproductive age with uterine myomas and wished to preserve their uteri. Most women had symptomatic fibroids. One trial included only infertile women (Assaf 1999). There were no significant differences between the treatment and control groups at baseline in all trials but one (Taylor 2005), in which there was a significant difference in the mean age: 39.5 years in the control group and 42.6 years in the treatment group.
Interventions
3/18 studies compared vasopressin versus placebo
1/18 studies compared bupivacaine combined with epinephrine versus placebo
1/18 studies compared peri‐cervical tourniquet versus no treatment
1/18 studies compared a tourniquet round both cervix and infundibulopelvic ligament versus no treatment
2/18 studies compared oxytocin versus placebo
2/18 studies compared misoprostol versus placebo
1/18 studies compared chemical dissection with mesna versus placebo
1/18 studies compared morcellation while attached to the uterus versus no treatment
1/18 studies compared gelatin‐thrombin matrix versus placebo
1/18 studies compared ascorbic acid versus no treatment
1/18 studies compared dinoprostone versus placebo
1/18 studies compared loop ligation of myoma pseudocapsule combined with vasopressin versus no treatment
1/18 studies compared temporary clipping of uterine artery versus no treatment
1/18 studies compared fibrin sealant patch (surgical collagen patch coated with thrombin and fibrinogen) versus no treatment
Eight of the trials included in this review used laparoscopic myomectomy (Assaf 1999; Zullo 2004; Sinha 2005; Wang 2007; Kalogiannidis 2011; Leone Maggiore 2011; Zhao 2011; Vercellino 2012), one trial included cases that had myomectomy by either the vaginal route or laparotomy (Agostini 2005) and the rest of the trials used myomectomy by laparotomy only.
Outcomes
16/18 studies reported blood loss (ml) during myomectomy
13/18 studies reported need for blood transfusion
16/18 studies reported duration of surgery
11/18 studies reported duration of hospital stay (days)
5/18 studies reported postoperative haemoglobin
5/18 studies reported postoperative haemoglobin drop
8/18 reported postoperative morbidity
1/18 reported pregnancy rate following surgery
0/18 reported cost of intervention
Excluded studies
Twenty‐three studies were excluded from the review, for the following reasons:
10/23 were not RCTs;
13/23 had ineligible control groups.
Risk of bias in included studies
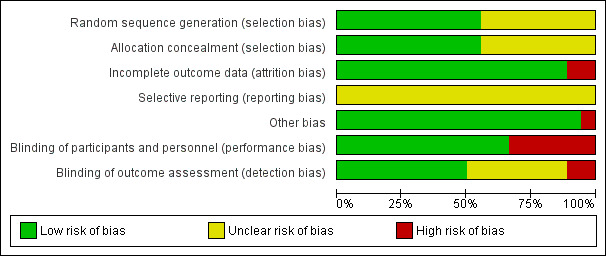
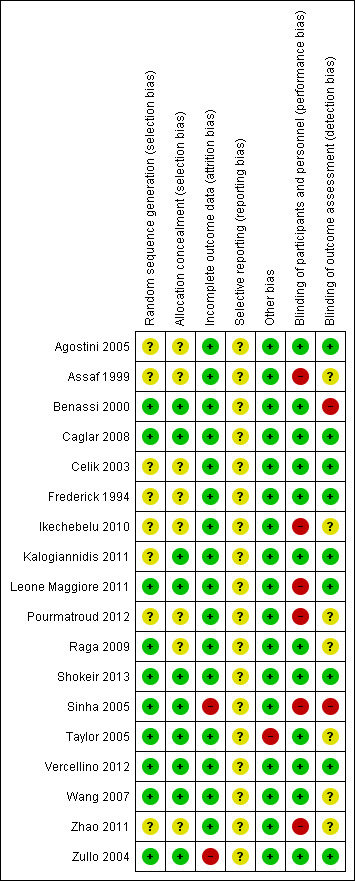
We have summarised our risk of bias assessments in Figure 2 and Figure 3.
2.
3.
Allocation
Ten studies were at low risk of selection bias related to sequence generation as they used computer randomisation or a random numbers table. The other eight studies did not describe the method used and were at unclear risk of this bias. Ten studies were at low risk of selection bias related to allocation concealment as they used adequate allocation concealment methods such as sequentially numbered opaque sealed envelopes. The other eight studies did not describe the method used and were at unclear risk of this bias.
Blinding
Blinding of participants and personnel
We did not consider that blinding was likely to influence findings for the primary review outcome (blood loss and need for blood transfusion). However, for secondary outcomes such as operative difficulties, postoperative morbidity including subjective outcomes such as severe pain, as well as tolerability and safety, blinding status could potentially affect the findings. Twelve studies described the use of a double‐dummy placebo that was identical to the intervention and were thus deemed to be at low risk of performance bias. The other six studies were considered to be at high risk of performance bias because either the care givers and patients were not blinded or because there were no placebo control groups.
Blinding of outcome assessors
Nine studies described blinding of outcome assessors and we judged them to be at low risk of detection bias. Two studies were judged to be at high risk of detection bias because the outcome assessors were not blinded. The other seven studies did not describe the blinding of assessors and were at unclear risk of this bias.
Incomplete outcome data
Sixteen studies analysed all or most (> 95%) of the women that were randomised and we judged them to be at low risk of bias. Two studies were considered to be at high risk of attrition bias: in both studies some participants (6.7% in one study and 8.3% in the other) were excluded after randomisation.
Selective reporting
We did not have access to the study protocols and therefore could not judge whether the studies reported all planned outcomes. The trial on mesna versus placebo did not report blood loss. The trials on bupivacaine plus epinephrine, chemical dissection with mesna, myoma morcellation, temporary clipping of the uterine artery and fibrin sealant patch did not report the need for blood transfusion. Trials on bupivacaine plus epinephrine, mesna, myoma morcellation, temporary clipping of uterine artery and fibrin sealant patch did not report on the adverse effects.
Other potential sources of bias
In one study (Taylor 2005) there was a substantial baseline difference in age between the two groups and the risk of bias was deemed high. We found no potential sources of within‐study bias in the other 17 studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Table 1 presents the effects of different interventions on blood loss during myomectomy.
1. Comparision of misoprostol versus placebo
Primary outcomes
1.1. Blood loss
Misoprostol was associated with significant reduction in blood loss compared to placebo (2 RCTs, 89 women: MD ‐97.88 ml, 95% CI ‐125.52 to ‐70.24; I2 = 43%; moderate‐quality evidence; Analysis 1.1; Table 2).
1.1. Analysis.
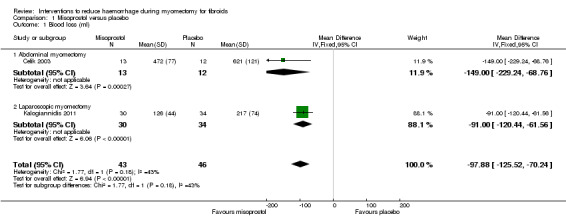
Comparison 1 Misoprostol versus placebo, Outcome 1 Blood loss (ml).
A subgroup analysis showed a significant reduction in blood loss in both the abdominal myomectomy group (1 RCT, 25 women: MD ‐149.00 ml, 95% CI ‐229.24 to ‐ 68.76) and the laparoscopic myomectomy group (1 RCT, 64 women: MD ‐91.00 ml, 95% CI ‐120.44 to ‐61.56).
1.2. Need for blood transfusion
One trial involving 25 women found no evidence of effect of misoprostol on the need for blood transfusion (OR 0.36, 95% CI 0.05 to 2.50), and no woman needed a blood transfusion in the second trial involving 64 women (Analysis 1.2). We judged the quality of the evidence on the effect of misoprostol on the need for blood transfusion as low (Table 2).
1.2. Analysis.
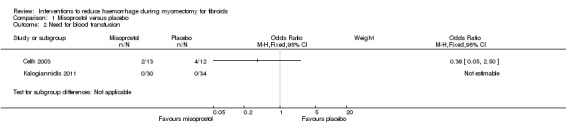
Comparison 1 Misoprostol versus placebo, Outcome 2 Need for blood transfusion.
Secondary outcomes
1.3. Postoperative haemoglobin
Misoprostol was associated with higher postoperative haemoglobin compared to placebo (2 RCTs, 89 women: MD 0.69 g/dl, 95 CI 0.37 to 1.02; I2 = 0%; Analysis 1.4).
1.4. Analysis.
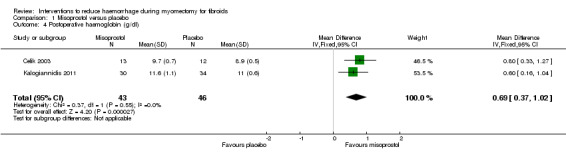
Comparison 1 Misoprostol versus placebo, Outcome 4 Postoperative haemoglobin (g/dl).
1.4. Duration of surgery
There was no evidence that the use of misoprostol changed the duration of surgery (Table 2). The results were not pooled as pooling led to substantial heterogeneity (I2 = 88%), which could be explained by the fact that in one study myomectomy was achieved by laparotomy and in the other study myomectomy was performed by laparoscopy.
A subgroup analysis showed a significant reduction of blood loss in the abdominal myomectomy group (1 RCT, 25 women: OR ‐9.50, 95% CI ‐15.90 to ‐3.10) but not in the laparoscopic myomectomy group (1 RCT, 64 women: OR 9.00 min, ‐1.63 to 19.63).
1.5. Duration of hospital stay
There was no evidence of effect on the duration of hospital stay (1 RCT, 25 women: MD 0.00 days, 95% CI ‐0.82 to 0.82; Analysis 1.5).
1.5. Analysis.
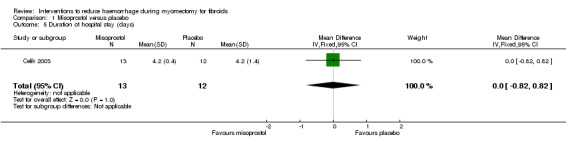
Comparison 1 Misoprostol versus placebo, Outcome 5 Duration of hospital stay (days).
1.6. Postoperative morbidity
There was no evidence of effect on febrile morbidity compared to placebo (2 RCTs, 89 women: OR 0.94, 95% CI 0.23 to 3.88; I2 = 0%; Analysis 1.7).
1.7. Analysis.
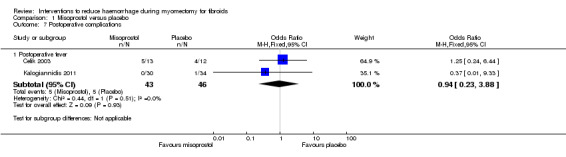
Comparison 1 Misoprostol versus placebo, Outcome 7 Postoperative complications.
2. Vasopressin versus placebo
Primary outcomes
2.1. Blood loss
There was significant heterogeneity between the studies that assessed the effect of vasopressin. The heterogeneity between studies could be explained by the fact that in one study women had laparoscopic myomectomy and in two other studies the women had open abdominal myomectomy.
A subgroup analysis revealed that when compared to placebo, vasopressin was associated with significant reduction in blood loss during abdominal myomectomy (1 RCT, 20 women: MD ‐450 ml, 95% CI ‐507.49 to ‐392.51) as well as during laparoscopic myomectomy (2 RCTs, 108 women: MD ‐147.95 ml, 95% CI ‐174.17 to ‐121.73; I2 = 0%; Analysis 2.1).
2.1. Analysis.
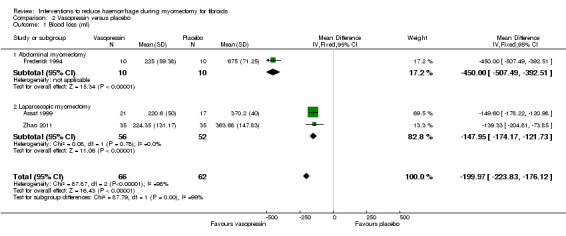
Comparison 2 Vasopressin versus placebo, Outcome 1 Blood loss (ml).
2.2. Need for blood transfusion
Vasopressin was associated with a reduction in the need for blood transfusion compared to placebo (2 RCTs, 90 women: OR 0.15, 95% CI 0.03 to 0.74; I2 = 0%; moderate‐quality evidence; Analysis 2.2) (Table 3).
2.2. Analysis.
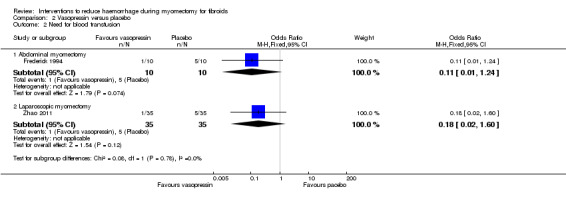
Comparison 2 Vasopressin versus placebo, Outcome 2 Need for blood transfusion.
A subgroup analysis showed no significant reduction in the need for blood transfusion in both the abdominal myomectomy group (1 RCT, 20 women: OR 0.11, 95% CI 0.01 to 1.24) and laparoscopic myomectomy group (1 RCT, 70 women: OR 0.18, 95% CI 0.02 to 1.60).
Secondary outcomes
2.3. Pregnancy (if desired)
There was no evidence of a difference in pregnancy one year after myomectomy between vasopressin and placebo (1 RCT, 38 women: OR 0.64, 95% CI 0.18 to 2.31; Analysis 2.6).
2.6. Analysis.
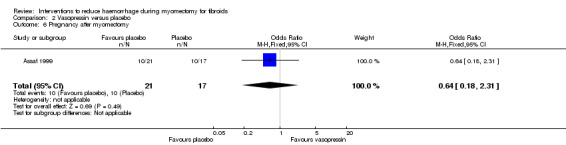
Comparison 2 Vasopressin versus placebo, Outcome 6 Pregnancy after myomectomy.
2.4. Duration of surgery
Vasopressin was associated with a reduction in the operating time compared to placebo (2 RCTs, 108 women: MD ‐27.72 min, 95% CI ‐35.82 to ‐19.61; I2 = 0%; moderate‐quality evidence; Analysis 2.3) (Table 3).
2.3. Analysis.
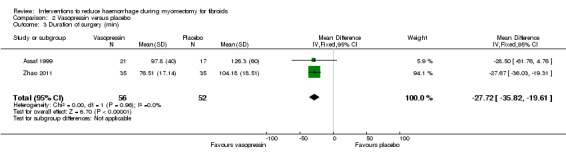
Comparison 2 Vasopressin versus placebo, Outcome 3 Duration of surgery (min).
2.5. Length of hospital stay
There was no evidence of a difference in the length of hospital stay between vasopressin and placebo (2 RCTs, 108 women: MD 0.11 days, 95% CI ‐0.69 to 0.91; P = 0.96; I2 = 75%; Analysis 2.4).
2.4. Analysis.
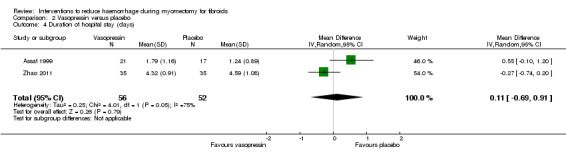
Comparison 2 Vasopressin versus placebo, Outcome 4 Duration of hospital stay (days).
2.6. Conversion of laparoscopy to laparotomy
Compared to placebo, there was no evidence of an effect of vasopressin on conversion of laparoscopy to laparotomy (1 RCT, 70 women: OR 7.65, 95% CI 0.38 to 153.75; Analysis 2.7).
2.7. Analysis.
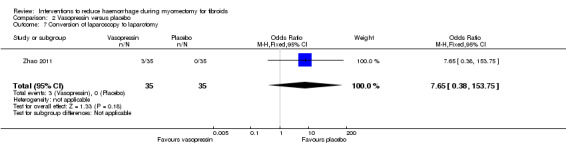
Comparison 2 Vasopressin versus placebo, Outcome 7 Conversion of laparoscopy to laparotomy.
2.7. Postoperative adhesions
Compared to placebo, there was no evidence of an effect of vasopressin on postoperative adhesions (1 RCT, 38 women: OR 2.02, 95% CI 0.54 to 7.49; Analysis 2.5).
2.5. Analysis.
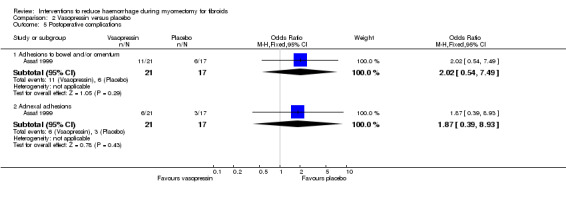
Comparison 2 Vasopressin versus placebo, Outcome 5 Postoperative complications.
3. Bupivacaine plus epinephrine versus placebo
Primary outcomes
3.1. Blood loss
Compared to placebo, bupivacaine plus epinephrine significantly reduced blood loss (1 RCT, 60 women: MD ‐68.6 ml, 95% CI ‐93.69 to ‐43.51; low‐quality evidence; Analysis 3.1) (Table 4).
3.1. Analysis.
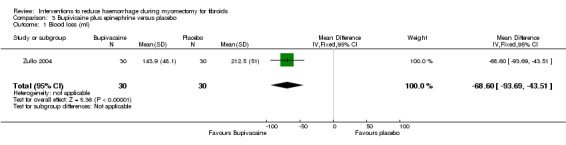
Comparison 3 Bupivicaine plus epinephrine versus placebo, Outcome 1 Blood loss (ml).
3.2. Need for blood transfusion
The need for blood transfusion was not reported by investigators.
Secondary outcomes
3.3. Duration of surgery
Bupivicaine plus epinephrine was associated with a reduction in the operating time compared to placebo (1 RCT, 60 women: MD ‐30.50 min, 95% CI ‐37.68 to ‐23.32; low‐quality evidence; Analysis 3.2) (Table 4).
3.2. Analysis.
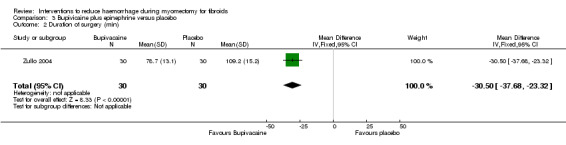
Comparison 3 Bupivicaine plus epinephrine versus placebo, Outcome 2 Duration of surgery (min).
3.4. Other secondary outcomes
Other secondary outcomes were not reported by investigators.
4. Peri‐cervical tourniquet versus no treatment
Primary outcomes
4.1. Blood loss
There was significant heterogeneity between studies that evaluated the effect of a peri‐cervical tourniquet (I2 = 95%; Analysis 4.1). Due to the significant heterogeneity between the studies, the studies were not combined. We attributed the heterogeneity between studies to the different methods used for the peri‐cervical tourniquet. One study (Taylor 2005) used a polyglactin suture tied around the cervix to occlude the uterine artery and left there after surgery, plus a polythene tourniquet tied round the infundibulopelvic ligament and removed after the operation. This study found a significant effect in blood loss, favouring the use of the tourniquet (1 RCT, 28 women: MD ‐1870.0, 95% CI ‐2547.16 to ‐1192.84; low‐quality evidence ). The other study (Ikechebelu 2010) used a Foley catheter that was tied round the base of the uterus and was released intermittently during surgery; and also revealed evidence that a peri‐cervical tourniquet reduced blood loss compared to no treatment (1 RCT, 93 women: MD ‐240.70, 95% CI ‐359.61 to ‐121.79; low‐quality evidence).
4.1. Analysis.
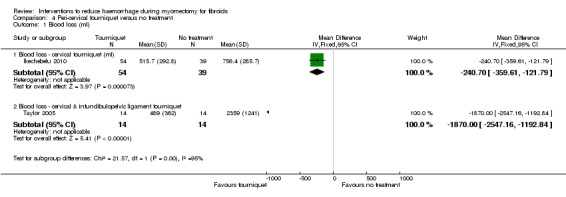
Comparison 4 Peri‐cervical tourniquet versus no treatment, Outcome 1 Blood loss (ml).
4.2. Need for blood transfusion
Peri‐cervical tourniquet was associated with a significantly reduced need for blood transfusion compared to placebo (1 RCT, 98 women: OR 0.22, 95% CI 0.09 to 0.55; low‐quality evidence). The use of a tourniquet around both the cervix and the infundibulopelvic ligament also significantly reduced blood loss (1 RCT, 28 women: OR 0.02, 95% CI 0.00 to 0.23; low‐quality evidence).
Secondary outcomes
4.3. Duration of surgery
There was no evidence of an effect on operating time with a peri‐cervical tourniquet compared to no treatment (1 RCT, 28 women: MD ‐4.00 min, 95% CI ‐29.28 to 21.28; low quality evidence; Analysis 4.3) (Table 5).
4.3. Analysis.
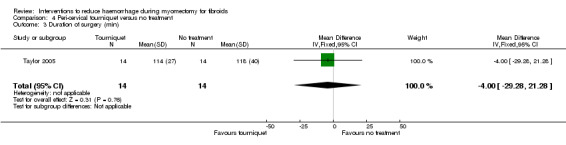
Comparison 4 Peri‐cervical tourniquet versus no treatment, Outcome 3 Duration of surgery (min).
4.4. Postoperative morbidity
Peri‐cervical tourniquet had no evidence of an effect on postoperative morbidity (Analysis 4.4): fever (1 RCT, 93 women: OR 1.09, 95% CI 0.46 to 2.59; Analysis 4.4), anaemia (1RCT, 93 women: OR 1.09, 95% CI 0.46 to 2.59), urinary tract infection (1 RCT, 93 women: OR 0.71, 95% CI 0.13 to 3.70), prolonged vaginal bleeding (1 RCT, 93 women: OR 2.21, 95% CI 0.09 to 55.82), pelvic abscess (1 RCT, 93 women: OR 2.21, 95% CI 0.09 to 55.82), and intestinal obstructions (1 RCT, 93 women: OR 2.21, 95% CI 0.09 to 55.82).
4.4. Analysis.
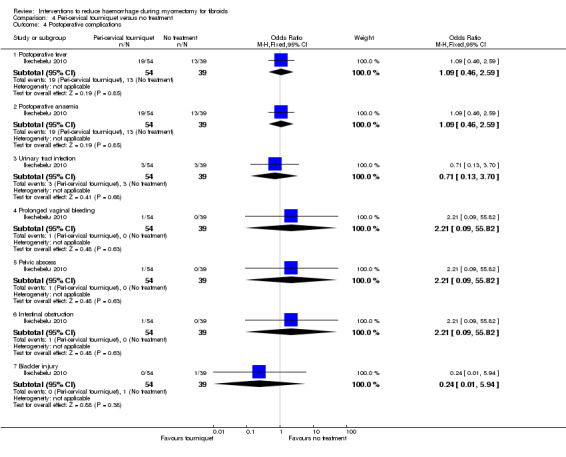
Comparison 4 Peri‐cervical tourniquet versus no treatment, Outcome 4 Postoperative complications.
5. Oxytocin versus placebo
Primary outcomes
5.1. Blood loss
The effect of oxytocin on blood loss compared to placebo was inconsistent with significant heterogeneity across studies. There was no obvious explanation for the heterogeneity. A subgroup analysis revealed a reduction in blood loss when oxytocin was used in laparoscopic myomectomy (1 RCT, 60 women: MD ‐175.50 ml, 95% CI ‐301.01 to ‐49.93) but not open abdominal myomectomy (1 RCT, 94 women: MD 57.00 ml, 95% CI ‐129.22 to 243.22; Analysis 5.1).
5.1. Analysis.
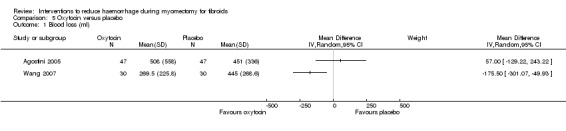
Comparison 5 Oxytocin versus placebo, Outcome 1 Blood loss (ml).
5.2. Need for blood transfusion
Oxytocin did not appear to have an effect on the need for blood transfusion (2 RCTs, 154 women: OR 0.54, 95% CI 0.03 to 8.51; I2 = 89%; Analysis 5.2).
5.2. Analysis.
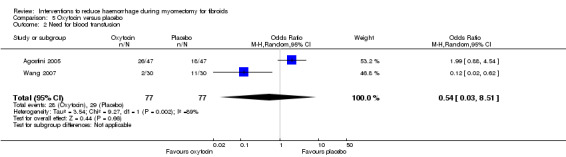
Comparison 5 Oxytocin versus placebo, Outcome 2 Need for blood transfusion.
Secondary outcomes
5.3. Duration of surgery
Oxytocin had no significant effect on the operating time (2 RCTs, 154 women: MD 3.5 min, 95% CI ‐1.88 to 8.88; I2 = 0%; Analysis 5.3).
5.3. Analysis.
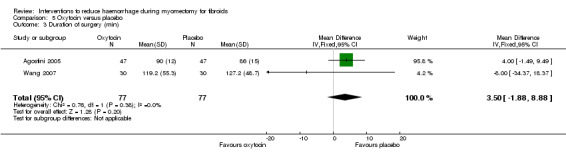
Comparison 5 Oxytocin versus placebo, Outcome 3 Duration of surgery (min).
5.4. Postoperative hospital stay
Compared to placebo, oxytocin significantly reduced the duration of postoperative hospital stay (1 RCT, 60 women: MD ‐0.60 days, 95% CI ‐1.19 to ‐0.01; Analysis 5.5).
5.5. Analysis.
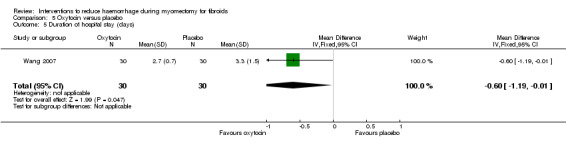
Comparison 5 Oxytocin versus placebo, Outcome 5 Duration of hospital stay (days).
5.5. Postoperative morbidity
There was no evidence of an effect on postoperative morbidity by oxytocin compared to placebo (1 RCT, 60 women: OR 1.00, 95% CI 0.06 to 16.76; Analysis 5.5).
6. Mesna versus placebo
Primary outcomes
6.1. Blood loss
Blood loss was not reported by the investigators (Benassi 2000).
6.2. Need for blood transfusion
The need for blood transfusion was not reported by the investigators (Benassi 2000).
Secondary outcomes
6.3. Postoperative haemoglobin and haematocrit
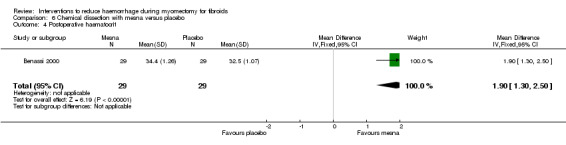
Postoperative haemoglobin (1 RCT, 58 women: MD 0.50 g/dl, 95% CI 0.42 to 0.58; Analysis 6.3) and haematocrit (1 RCT, 58 women: MD 1.90%, 95% CI 1.30 to 2.50; Analysis 6.4) were significantly higher with mesna compared to placebo.
6.3. Analysis.
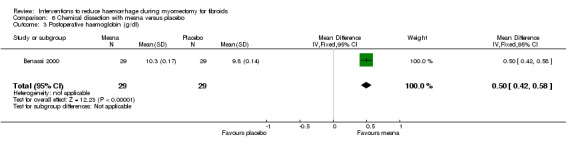
Comparison 6 Chemical dissection with mesna versus placebo, Outcome 3 Postoperative haemoglobin (g/dl).
6.4. Analysis.
Comparison 6 Chemical dissection with mesna versus placebo, Outcome 4 Postoperative haematocrit.
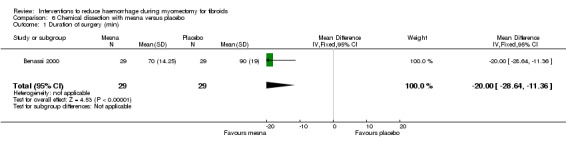
6.4. Duration of surgery
Chemical dissection with mesna was associated with a reduction in the operating time compared to placebo (1 RCT, 58 women: MD ‐20 min, 95% CI ‐28.64 to ‐11.36; Analysis 6.1).
6.1. Analysis.
Comparison 6 Chemical dissection with mesna versus placebo, Outcome 1 Duration of surgery (min).
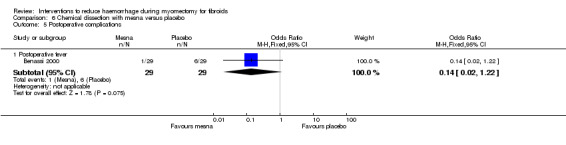
6.4. Postoperative morbidity
There was no evidence of an effect on the incidence of postoperative fever by mesna compared to placebo (1 RCT, 58 women: OR 0.14, 95% CI 0.02 to 1.22; Analysis 6.5).
6.5. Analysis.
Comparison 6 Chemical dissection with mesna versus placebo, Outcome 5 Postoperative complications.
6.5. Length of hospital stay
Mesna was associated with a reduction in length of hospital stay compared to placebo (1 RCT, 58 women: MD ‐ 1.00 day, 95% CI ‐1.12 to ‐0.88; Analysis 6.2).
6.2. Analysis.
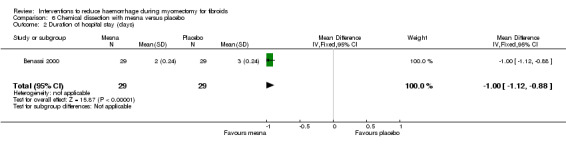
Comparison 6 Chemical dissection with mesna versus placebo, Outcome 2 Duration of hospital stay (days).
7. Myoma enucleation by morcellation versus no treatment
Primary outcomes
7.1. Blood loss
Myoma enucleation by morcellation did not have a significant effect on blood loss during laparoscopic myomectomy (1 RCT, 48 women: MD 65.40 ml, 95% CI ‐36.47 to 167.27; Analysis 7.1).
7.1. Analysis.
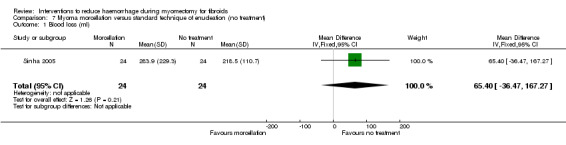
Comparison 7 Myoma morcellation versus standard technique of enucleation (no treatment), Outcome 1 Blood loss (ml).
7.2. Need for blood transfusion
The need for blood transfusion was not reported by the investigators (Sinha 2005).
Secondary outcomes
7.3. Duration of surgery
Myoma morcellation was associated with a reduction in the operating time compared to placebo (1 RCT, 48 women: MD ‐25.30 min, 95% CI ‐44.23 to ‐6.37; Analysis 8.3).
8.3. Analysis.
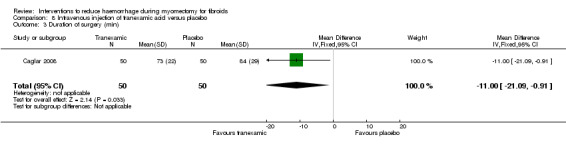
Comparison 8 Intravenous injection of tranexamic acid versus placebo, Outcome 3 Duration of surgery (min).
7.4. Length of hospital stay
Myoma morcellation did not show a significant effect on the length of hospital stay (1 RCT, 48 women: MD ‐0.07 days, 95% CI ‐0.18 to 0.04; Analysis 7.3).
7.3. Analysis.
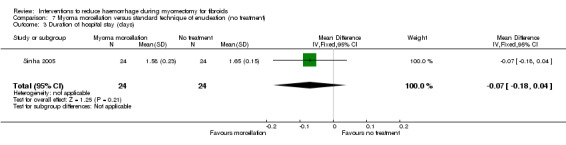
Comparison 7 Myoma morcellation versus standard technique of enucleation (no treatment), Outcome 3 Duration of hospital stay (days).
8. Tranexamic acid versus placebo
Primary outcomes
8.1. Blood loss
Intravenous tranexamic acid reduced blood loss during myomectomy compared to placebo (1 RCT, 100 women: MD ‐243 ml, 95% CI ‐460.02 to ‐25.98; Analysis 8.1).
8.1. Analysis.
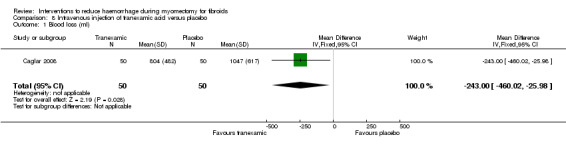
Comparison 8 Intravenous injection of tranexamic acid versus placebo, Outcome 1 Blood loss (ml).
8.2. Need for blood transfusion
Tranexamic acid did not have a significant effect on the need for blood transfusion (1 RCT, 100 women: OR 1.71, 95% CI 0.63 to 4.30; Analysis 8.2).
8.2. Analysis.
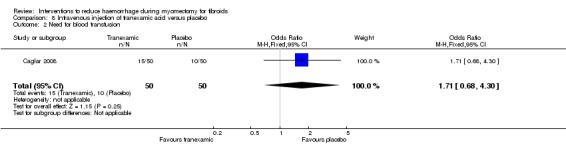
Comparison 8 Intravenous injection of tranexamic acid versus placebo, Outcome 2 Need for blood transfusion.
Secondary outcomes
8.3. Postoperative haemoglobin and haematocrit
There was no evidence of effect by tranexamic acid compared to placebo on postoperative haemoglobin (1 RCT, 100 women: MD 0.21 g/dl, 95% CI ‐0.36 to 0.78; Analysis 8.4) and haematocrit (1 RCT, 100 women: MD 1.00%, 95% CI ‐0.43 to 2.43; Analysis 8.5).
8.4. Analysis.
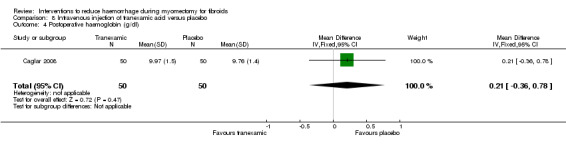
Comparison 8 Intravenous injection of tranexamic acid versus placebo, Outcome 4 Postoperative haemoglobin (g/dl).
8.5. Analysis.
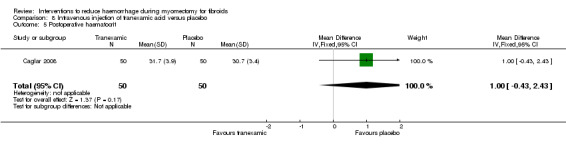
Comparison 8 Intravenous injection of tranexamic acid versus placebo, Outcome 5 Postoperative haematocrit.
8.4 Duration of surgery
Tranexamic acid was associated with a reduction in the operating time compared to placebo (1 RCT, 100 women: MD ‐11 min, 95% CI ‐21.09 to ‐0.91; Analysis 8.3).
9. Gelatin‐thrombin matrix versus placebo or no treatment
Primary outcomes
9.1. Blood loss
Compared to no treatment, the application of a gelatin‐thrombin matrix on the uterine incision reduced blood loss during myomectomy (1 RCT, 50 women: MD ‐545.00 ml, 95% CI ‐593.26 to ‐496.74; low‐quality evidence; Analysis 9.1) (Table 6) and postoperative vaginal blood loss (1 RCT, 50 women: MD ‐225.00 ml, 95% CI ‐254.46 to ‐195.54; Analysis 9.3).
9.1. Analysis.
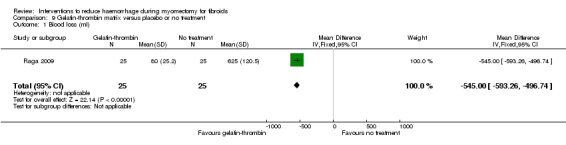
Comparison 9 Gelatin‐thrombin matrix versus placebo or no treatment, Outcome 1 Blood loss (ml).
9.3. Analysis.
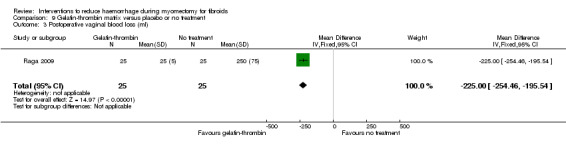
Comparison 9 Gelatin‐thrombin matrix versus placebo or no treatment, Outcome 3 Postoperative vaginal blood loss (ml).
9.2. Need for blood transfusion
Gelatin‐thrombin matrix reduced the need for blood transfusion compared to no treatment (1 RCT, 100 women: OR 0.01, 95% CI 0.00 to 0.10; low‐quality evidence; Analysis 9.2) (Table 6).
9.2. Analysis.
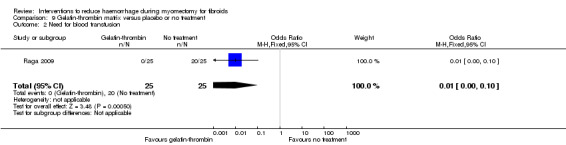
Comparison 9 Gelatin‐thrombin matrix versus placebo or no treatment, Outcome 2 Need for blood transfusion.
Secondary outcomes
9.3. Postoperative haemoglobin and haematocrit
Gelatin‐thrombin matrix reduced the drop in haemoglobin after surgery compared to no treatment (1 RCT, 50 women: MD ‐2.30 g/dl, 95% CI ‐2.66 to ‐1.94; Analysis 9.5).
9.5. Analysis.
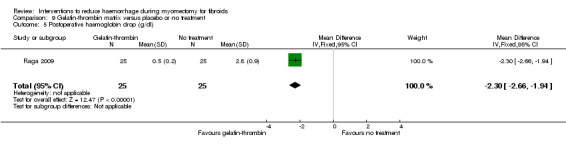
Comparison 9 Gelatin‐thrombin matrix versus placebo or no treatment, Outcome 5 Postoperative haemoglobin drop (g/dl).
9.4. Duration of surgery
Gelatin‐thrombin was associated with an increase in the operating time compared to no treatment (1 RCT, 50 women: MD 5.00 min, 95% CI 1.29 to 8.71; low‐quality evidence; Analysis 9.4) (Table 6).
9.4. Analysis.
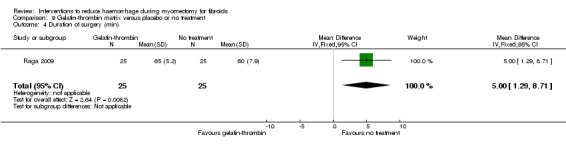
Comparison 9 Gelatin‐thrombin matrix versus placebo or no treatment, Outcome 4 Duration of surgery (min).
9.4. Length of hospital stay
Gelatin‐thrombin matrix reduced the duration of hospital stay compared to no treatment (1 RCT, 50 women: MD ‐2.00 days, 95% CI ‐2.69 to ‐1.31; Analysis 9.6).
9.6. Analysis.
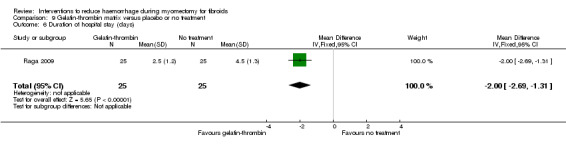
Comparison 9 Gelatin‐thrombin matrix versus placebo or no treatment, Outcome 6 Duration of hospital stay (days).
9.5. Postoperative morbidity
There was no evidence of an effect on postoperative fever by gelatin‐thrombin matrix compared to no treatment (1 RCT, 50 women: OR 0.32, 95% CI 0.01 to 8.25; Analysis 9.7).
9.7. Analysis.
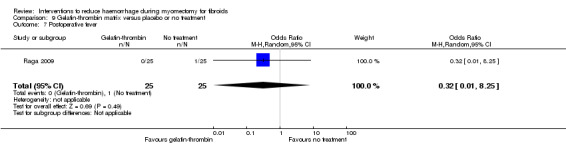
Comparison 9 Gelatin‐thrombin matrix versus placebo or no treatment, Outcome 7 Postoperative fever.
10. Ascorbic acid versus no treatment
Primary outcomes
10.1. Blood loss
Compared to no treatment, the administration of ascorbic acid during myomectomy reduced blood loss (1 RCT, 102 women: MD ‐411.46 ml, 95% CI ‐502.58 to ‐320.34; P < 0.00001; low‐quality evidence; Analysis 10.1) (Table 7).
10.1. Analysis.
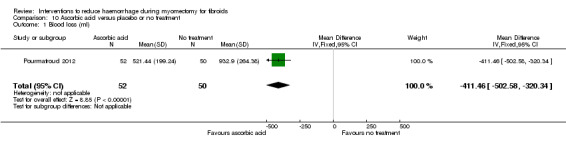
Comparison 10 Ascorbic acid versus placebo or no treatment, Outcome 1 Blood loss (ml).
10.2. Need for blood transfusion
There was no evidence that ascorbic acid had an effect on the need for blood transfusion compared to no treatment (1 RCT, 102 women: OR 0.38, 95% CI 0.11 to 1.32; P = 0.13; very low‐quality evidence).
Secondary outcomes
10.3. Postoperative haemoglobin and haematocrit
There was evidence that, compared to no treatment, ascorbic acid reduced the drop in postoperative haematocrit (1 RCT, 102 women: MD ‐0.70%, 95% CI ‐1.34 to ‐0.06; P = 0.03) but not the drop in postoperative haemoglobin (1 RCT, 102 women: MD 0.14 g/dl, 95% CI ‐0.22 to 0.50; P = 0.44).
10.4. Duration of surgery
Ascorbic acid reduced the operating time compared to no treatment (1 RCT, 102 women: MD ‐26.00 min, 95% CI ‐33.10 to ‐18.90; P < 0.00001; low‐quality evidence).
10.5. Duration of hospital stay
Compared to no treatment, there was evidence that ascorbic acid reduced the duration of hospital stay (1 RCT, 102 women: MD ‐0.4 days, 95% CI ‐0.65 to ‐0.15; P = 0.002).
10.6. Postoperative morbidity
Overall, there was no evidence of an effect on postoperative morbidity by ascorbic acid compared to no treatment (1 RCT, 102 women: OR 1.64, 95% CI 0.71 to 3.82; P = 0.25), which included postoperative fever (OR 1.88, 95% CI 0.58 to 6.07; P = 0.29), vomiting (OR 1.96, 95% CI 0.17 to 22.32; P = 0.59), constipation (OR 0.31, 95% CI 0.01 to 7.90; P = 0.48) and severe pain (OR 2.00, 95 CI 0.36 to 11.44; P = 0.44).
11. Dinoprostone versus placebo or no treatment
Primary outcomes
11.1. Blood loss
Compared to placebo, a dinoprostone vaginal suppository administered before myomectomy reduced blood loss (1 RCT, 108 women: MD ‐131.60 ml, 95% CI ‐253.42 to ‐9.78; P = 0.03; Analysis 11.1).
11.1. Analysis.
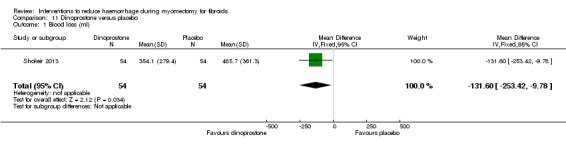
Comparison 11 Dinoprostone versus placebo, Outcome 1 Blood loss (ml).
11.2. Need for blood transfusion
Diniprostone reduced the need for blood transfusion compared to placebo (1 RCT, 108 women: OR 0.17, 95% CI 0.04 to 0.81; P = 0.61; low‐quality evidence).
Secondary outcomes
11.3. Postoperative haemoglobin and haematocrit
Compared to a placebo, dinoprostone reduced the drop in postoperative haemoglobin (1 RCT, 108 women: MD ‐0.50, 95% CI ‐0.88 to ‐0.12; P = 0.009), but had no effect on postoperative haematocrit (1 RCT, 108 women: MD 0.10, 95% CI ‐0.39 to 0.59; P = 0.69).
11.4. Duration of surgery
Compared to placebo, dinoprostone had no effect on the operating time (1 RCT, 108 women: MD ‐2.60 min, 95% CI ‐12.55 to 7.35; P = 0.25).
11.5. Length of hospital stay
There was no evidence of an effect on the duration of hospital stay by dinoprostone compared to no treatment (1 RCT, 108 women: MD 0.30 days, 95% CI ‐0.22 to 0.82; P = 0.25).
11.6. Postoperative morbidity
There was no evidence of an effect on postoperative fever by dinoprostone compared to no treatment (OR 1.53, 95% CI 0.25 to 9.54; P = 0.65).
12. Loop ligation of myoma pseudocapsule combined with vasopressin versus no treatment
12.1. Blood loss
Compared to no treatment, loop ligation of the myoma pseudocapsule during myomectomy reduced blood loss (1 RCT, 70 women: MD ‐305.01 ml, 95% CI ‐354.83 to ‐255.19; P < 0.00001; Analysis 12.1).
12.1. Analysis.
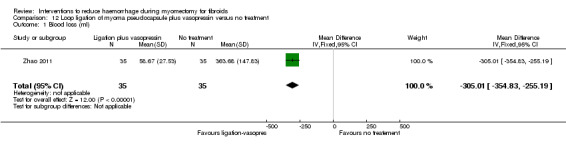
Comparison 12 Loop ligation of myoma pseudocapsule plus vasopressin versus no treatment, Outcome 1 Blood loss (ml).
12.2. Need for blood transfusion
Compared to no treatment, loop ligation of the myoma pseudocapsule had no effect on the need for blood transfusion (1 RCT, 70 women: OR 0.08, 95% CI 0.00 to 1.47; P = 0.09).
Secondary outcomes
12.3. Duration of surgery
Loop ligation of the myoma pseudocapsule significantly reduced the operating time (1 RCT, 70 women: MD ‐32.76 min, 95% CI ‐40.81 to ‐24.71; P < 0.00001).
12.4. Length of hospital stay
Compared to no treatment, loop ligation of the myoma pseudocapsule significantly reduced the duration of hospital stay (1 RCT, 70 women: MD ‐1.46 days, 95% CI ‐1.85 to ‐1.07; P < 0.00001).
13. Temporary clipping of uterine artery versus no treatment
13.1. Blood loss
This outcome was not reported by the investigators.
13.2. Need for blood transfusion
No patient required blood transfusion.
Secondary outcomes
13.3. Postoperative haemoglobin and haematocrit
We found no evidence of an effect on postoperative haemoglobin by the temporary clipping of the uterine artery compared to no treatment (1 RCT, 166 women: MD ‐2.25 g/dl, 95% CI ‐0.61 to 0.11; P = 0.17).
13.4. Duration of surgery
The temporary clipping of the uterine artery significantly increased the operating time (1 RCT, 166 women: MD 40.00 min, 95% CI 30.01 to 49.99; P < 0.00001).
13.5. Length of hospital stay
We found no evidence of an effect on the duration of hospital stay by the temporary clipping of the uterine artery compared to no treatment (1 RCT, 70 women: MD 0.00 days, 95% CI ‐0.15 to 0.15; P = 1.00).
13.6. Postoperative morbidity
We found no evidence of an effect on postoperative morbidity by the temporary clipping of the uterine artery compared to no treatment (1 RCT, 166 women: OR 1.95, 95% CI 0.79 to 4.79; P = 0.14).
14. Fibrin sealant patch versus no treatment
14.1. Blood loss
Compared to no treatment, the application of a fibrin sealant patch (surgical collagen patch or sponge coated with thrombin and fibrinogen to stop local bleeding) on the uterus reduced blood loss during myomectomy (1 RCT, 70 women: MD ‐26.50 ml, 95% CI ‐44.46 to ‐8.53; P = 0.004; Analysis 14.1) and postoperative blood loss in the drainage bag (1 RCT, 70 women: MD ‐44.60 ml, 95% CI ‐65.06 to ‐24.14; P < 0.0001).
14.1. Analysis.
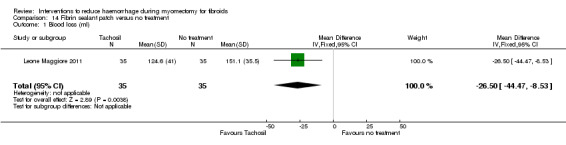
Comparison 14 Fibrin sealant patch versus no treatment, Outcome 1 Blood loss (ml).
14.2. Need for blood transfusion
No patient required blood transfusion.
Secondary outcomes
14.3. Pregnancy (if desired)
We found no evidence of an effect on conception after myomectomy by the fibrin sealant patch compared to no treatment (1 RCT, 70 women: OR 3.16, 95% CI 0.76 to 13.11; P = 0.11).
14.4. Duration of surgery
We found no evidence of an effect on the operating time by fibrin sealant patch compared to no treatment (1 RCT, 70 women: MD ‐0.4 min, 95% CI ‐4.47 to 3.67; P = 0.85).
14.5. Length of hospital stay
We found no effect of the fibrin sealant patch on the duration of hospital stay (1 RCT, 70 women: MD 0.00 days, 95% CI ‐0.09 to 0.09; P = 1.00).
15. Other analysis
Where data were available, we performed subgroup analyses based on the technique of myomectomy (Analysis 1.1; Analysis 1.3; Analysis 2.1; Analysis 2.2). The use of a random‐effects model compared to a fixed‐effect model made no difference. All other planned subgroup and sensitivity analyses were not conducted due to insufficient data.
1.3. Analysis.
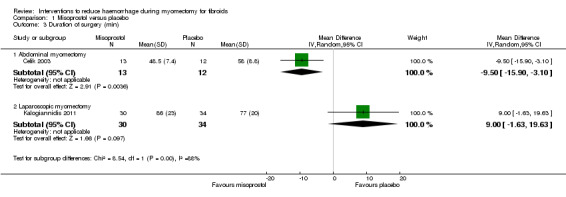
Comparison 1 Misoprostol versus placebo, Outcome 3 Duration of surgery (min).
Discussion
Summary of main results
This review has evaluated the effect of different interventions on blood loss during myomectomy for uterine fibroids. There are a few well designed randomised trials that have assessed the effect of each intervention on blood loss. Compared to placebo or no treatment, misoprostol, vasopressin, bupivacaine plus epinephrine, tranexamic acid, gelatin‐thrombin matrix, peri‐cervical tourniquet, tourniquet round both cervix and infundibulopelvic ligament, mesna, ascorbic acid, dinoprostone, loop ligation of the myoma pseudocapsule and a fibrin sealant patch (collagen sponge with thrombin and fibrinogen) were found to significantly reduce bleeding during myomectomy. In contrast, oxytocin, myoma morcellation and temporary clipping of the uterine artery did not significantly reduce blood loss when compared to placebo or no treatment.
Utero‐tonics
Some interventions have shown promising effects on reducing blood loss during myomectomy. Both misoprostol (MD ‐97.88 ml, 95% CI ‐125.52 to ‐70.24) and dinoprostone (MD ‐131.60 ml, 95% CI ‐253.42 to ‐9.78), prostaglandin E2 analogues, were shown to significantly reduce blood loss, probably by causing uterine contraction and reducing uterine blood flow. The trials on oxytocin, a known utero‐tonic agent, showed no statistically significant effect on blood loss during myomectomy.
Pharmacologic manipulation of the coagulation cascade
Similarly to prostaglandin E2 analogues, tranexamic acid (MD ‐243 ml, 95% CI ‐460.02 to ‐25.98) was found to significantly reduce blood loss during myomectomy. Tranexamic acid is an antifibrinolytic agent that acts by blocking the lysine‐binding site on plasmin thereby inhibiting fibrinolysis, the process wherein a fibrin clot, the product of coagulation, is broken down. Tranexamic acid has been used in clinical settings since the 1960s and has been found to reduce blood loss and the need for blood transfusion in cardiac surgery, liver transplantation and orthopaedic surgery (Dunn 1999).
The application of gelatin‐thrombin haemostatic sealant (MD ‐545.00 ml, 95% CI ‐593.26 to ‐496.74), a matrix of cross‐linked bovine‐derived gelatin granules and a bovine‐derived thrombin component, on the site of uterine incision significantly reduced blood loss during surgery and the need for blood transfusion. The gelatin matrix is hydrophilic and therefore adheres very well to wet tissue. The matrix activates the coagulation cascade and causes haemostasis.
The application of a fibrin sealant patch (MD ‐26.50 ml, 95% CI ‐44.47 to ‐8.53), which is a surgical patch coated with human coagulation factors fibrinogen and thrombin, significantly reduced blood loss during myomectomy.
Ascorbic acid (MD ‐411.46 ml, 95% CI ‐502.58 to ‐320.34) significantly reduced blood loss during myomectomy. Ascorbic acid (vitamin C) has important functions in platelets. During the first phase of haemostasis platelets are activated and aggregate to form a haemostasis plug. Ascorbic acid is water‐soluble and is not stored in the body, and when depleted the bleeding tendency increases due to dysfunctional connective tissue production in the blood vessel wall.
Interventions on the uterine artery
We found a significant reduction in intraoperative blood loss when vasopressin (MD ‐245.87 ml, 95% CI ‐434.58 to ‐57.16) is injected into the uterine muscles overlying the myoma during myomectomy. The injection of bupivacaine plus epinephrine into the myometrium overlying the myoma was evaluated in one study and the result showed evidence of a reduction in blood loss, although this might not be clinically significant (68.6 ml). Both vasopressin and bupivacaine plus epinephrine are known local vasoconstrictors and may reduce local blood flow when injected around the myoma.
A peri‐cervical tourniquet and use of a tourniquet round both the cervix and the infundibulopelvic ligament also significantly reduced blood loss during myomectomy, as anticipated. The uterus receives its blood supply primarily from the uterine artery and the occlusion of the uterine artery is expected to reduce blood loss by reducing blood flow to the uterus.However, there is no evidence that temporary clipping of the uterine artery reduce blood loss.
Chemical dissection of myoma
The study on the effect of chemical dissection of the myoma with mesna did not directly evaluate blood loss but showed a significant gain in postoperative haemoglobin levels. Mesna is a lytic agent that can disrupt connections between tissue layers (Denaro 2001) and may thus facilitate myoma enucleation. Similarly, we found that loop ligation of the myoma pseudocapsule during laparoscopic myomectomy significantly reduced blood loss.
Other outcomes
One way of evaluating the difficulties encountered during myomectomy was by measuring the operating time. Trials on vasopressin, bupivacaine plus epinephrine, mesna, tranexamic acid, ascorbic acid, myoma enucleation by morcellation, and loop ligation of the myoma pseudocapsule recorded a significant reduction in operating time. The use of misoprostol, oxytocin, peri‐cervical tourniquet, dinoprostone and a fibrin sealant patch showed no evidence of effect on the duration of surgery. The use of a gelatin‐thrombin matrix and the temporary clipping of the uterine artery increased the operating time.
Postoperative outcome was assessed by the duration of hospitalisation. A few trials included the duration of hospital stay in their evaluations. The trials on mesna, gelatin‐thrombin matrix, ascorbic acid and loop ligation of the myoma pseudocapsule recorded a significant decrease in the duration of hospital stay. No other trials found a significant effect on the duration of hospitalisation.
Overall completeness and applicability of evidence
Most comparisons included few trials with small sample sizes and insufficient power to detect moderate differences in blood loss. This review assessed only the effects of interventions versus placebo or no treatment. Head‐to‐head comparisons were not assessed by the review.
There are insufficient data on the adverse effects and costs of the different interventions. The trials on the gelatin‐thrombin matrix and ascorbic acid showed no significant difference in adverse effects between the intervention and the control groups. Most trials that commented on the adverse effects simply stated that there were no such effects recorded in the trial. Knowledge of these adverse events and the tolerability of the interventions is important because we have to make a trade off between the estimated benefits and the harms and costs before making any appropriate decisions about use or non‐use of any intervention. Nevertheless, evidence from clinical practice has shown that mesna is well tolerated and can be taken orally (Burkert 1983).
Quality of the evidence
The methodological quality of the included studies was generally good. The trials had small sample sizes (and, therefore, the effect sizes had large confidence intervals) and there was heterogeneity of effect between the included trials with several of the interventions. Despite the small sample sizes and heterogeneity of effect, there was a significant reduction in blood loss during myomectomy with 10 interventions when compared to placebo or no treatment.
Misoprostol and vasopressin: we rated each as moderate quality evidence because with both interventions the data were derived from two small studies and we could not rule out the possibility of publication bias.
Tranexamic acid, fibrin sealant patch, dinoprostone,peri‐cervical tourniquet and use of a tourniquet round both the cervix and the infundibulopelvic ligament : we rated each as low quality evidence because the data from each of the interventions were derived from one or two small studies and the pooled effect estimate was imprecise.
Ascorbic acid and loop ligation of myoma pseudocapsule: we rated each as low quality of evidence because with both interventions the data were derived from one small study and it was unclear how allocation concealment was done.
Bupivacaine plus epinephrine: we rated this as low quality evidence because the data were derived from one small study with a high risk of attrition bias (two patients in each arm did not receive the assigned intervention because of concomitant disease).
Gelatin‐thrombin matrix: we rated this as low quality evidence because the data were derived from one small study and it was unclear if the outcome assessors were blind.
Potential biases in the review process
Publication and selection biases are potential threats to all systematic reviews. We are confident that we have identified the existing clinical trials relevant to our question but cannot rule out the possibility that there are additional trials that are unpublished or published in sources not accessible to our search.
Agreements and disagreements with other studies or reviews
We are not aware of any reviews that have assessed the effectiveness of interventions to reduce haemorrhage during myomectomy. Evidence from this review supports findings previously reported in non‐randomised studies (McLaughin 1985; Baldoni 1995; Hutchins 1996; Dimitrov 1999).
The use of oxytocin showed no evidence of an effect on blood loss during myomectomy. This is consistent with other evidence that the myometrial concentration of oxytocin receptors is very low in non‐pregnant uteri (Fuchs 1984).
Authors' conclusions
Implications for practice.
Currently, there is moderate‐quality evidence that misoprostol (MD ‐97.88 ml, 95% CI ‐125.52 to ‐70.24) and vasopressin (MD ‐245.87 ml, 95% CI ‐434.58 to ‐57.16), and low‐quality evidence that bupivacaine plus epinephrine (MD ‐68.60 ml, 95% CI ‐93.69 to ‐43.51), gelatin‐thrombin matrix (MD ‐545.00 ml, 95% CI ‐593.26 to ‐496.74), tranexamic acid (MD ‐243 ml, 95% CI ‐460.02 to ‐25.98), peri‐cervical tourniquet (MD ‐240.70 ml. 95% CI ‐359.61 to 121.7),tourniquet round both the cervix and the infundibulopelvic ligament (MD 1870.00 ml, 95% CI ‐2547.16, to ‐1192.84), ascorbic acid (MD ‐411.46 ml, 95% CI ‐502.58 to ‐320.34), dinoprostone (MD ‐131.60 ml, 95% CI ‐253.42 to ‐9.78), loop ligation of the myoma pseudocapsule (MD ‐305.01 ml, 95% CI ‐354.83 to ‐255) and a fibrin sealant patch (MD ‐26.50, 95%CI ‐44.47 to ‐8.53) may reduce blood loss during myomectomy. However, since we did not include trials with head‐to‐head comparisons, we cannot draw any conclusions about the superiority of one intervention over the other. Due to the small reduction in blood loss (< 70 ml), the use of bupivacaine plus epinephrine and a fibrin sealant patch have limited clinical importance. At present there is no evidence that oxytocin, myoma enucleation by morcellation, and the temporary clipping of uterine arteries during myomectomy have an effect on intraoperative blood loss.
Implications for research.
There is a need for well designed randomised controlled trials to shed more light on the effectiveness of different interventions in reducing blood loss during myomectomy. To date, a few randomised trials, often will small sample sizes, have addressed this issue and so there is a need to fill the current gap of knowledge which has very important practical implications. Apart from the effectiveness, data on the cost‐effectiveness, future pregnancy (if desired), and adverse effects of different interventions are lacking. This is important for clinical decision making since such decisions are based on trade offs between benefits on the one hand and costs and adverse events on the other.
Feedback
Pericervical tourniquet data
Summary
Feedback received from Adam Magos on 29th June 2015:
I believe that the analysis of data regarding the use of tourniquets included in this review is misleading. Two studies are included, of which I was a co‐author of one (Taylor et al 2005): 1. The tourniquet in both studies was not placed only around the cervix as stated in the review. In our study, additional suture tourniquets were placed around the infundibulo‐pelvic ligaments to occlude the ovarian arteries. In the study by Ikechebelu, a single Foley catheter was used around the cervix and infundiblo‐pelvic ligaments, a technique which is very different and unlikely to achieve total occlusion of the uterine and ovarian vessels as does triple tourniquets (which is why we use sutures). 2. We did not release the tourniquets during the surgery, whereas Ikechebelu did so every 30 minutes. Of course, when a tourniquet is released, the patient bleeds. These are two fundamental differences which I fear were not appreciated by the authors of this review. In my view, as the two techniques are so dissimilar, it is not valid to combine the data and then conclude that "there was no difference in blood loss between the peri‐cervical tourniquet and no treatment". I hope you agree and I would be grateful if you would revisit this analysis! PS The title of the paper by Ikechebelu was misquoted in the review ‐ they used the word "torniquet" in the title, not "tourniquet".
Reply
The first author of the review comments as follows: The author (Adam Magos) is right. We were aware of the differences (which we considered minor) between the two studies and that is included in the description of interventions. Given the email from the authors, I agree that we should not aggregate the data from the two studies.
The review authors have now unpooled these two studies and reported their findings separately throughout the review.
Contributors
Adam Magos, Consultant Gynaecologist, The Royal Free Hospital, London
Review authors
What's new
| Date | Event | Description |
|---|---|---|
| 23 July 2015 | Feedback has been incorporated | Data from two studies on peri‐cervical tourniquet were unpooled in response to feedback from one of the trial authors |
History
Protocol first published: Issue 3, 2005 Review first published: Issue 1, 2007
| Date | Event | Description |
|---|---|---|
| 5 July 2014 | New search has been performed | Six additional trials have been included in the current update (Kalogiannidis 2011; Leone Maggiore 2011; Zhao 2011; Pourmatroud 2012; Vercellino 2012; Shokeir 2013). Five new interventions have been added to the review: ascorbic acid, dinoprostone, loop ligation of myoma pseudocapsule, temporary clipping of uterine artery and fibrin sealant patch (surgical patch coated with fibronogen and thrombin to stop bleeding of internal organs). |
| 5 July 2014 | New citation required and conclusions have changed | The conclusions of the review have changed. |
| 18 July 2011 | New citation required and conclusions have changed | Two additional trials have been included in this update. One of the new trials compared peri‐cervical tourniquet with no treatment (Ikechebelu 2010). With this new trial, there are now two trials on peri‐cervical tourniquet included in this review. The inclusion of the new trial to this review has changed the conclusions; unlike the previous versions of the review where we concluded that there is limited evidence that peri‐cervical tourniquet reduce blood loss during myomectomy, the conclusion in the current updated version is that there is no evidence that peri‐cervical tourniquet has an effect on blood loss. The other new trial compared the application of gelatin‐thrombin matrix to the uterine incision with normal saline (Raga 2009). There is evidence from this second trial that gelatin thrombin matrix reduced uterine bleeding.The previous versions of this review did not include any trial on gelatin thrombin matrix. |
| 20 September 2010 | Amended | Contact details updated. |
| 13 February 2009 | New citation required but conclusions have not changed | Two additional trials have been included in this update: one of the new trials compared intravenous oxytocin with a placebo (Wang 2007) and the other trial compared intravenous infusion of tranexamic acid with a placebo (Caglar 2008). In addition to the limited evidence from the original version of this review that misoprostol, vasopressin, bupivacaine plus epinephrine, tourniquet, and mesna may reduce bleeding during myomectomy, we found limited evidence in this updated version that tranexamic acid may also reduce bleeding. No major changes were made to the protocol. |
| 14 November 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We wish to thank Professor Justus Hofmeyr (Effective Care Research Unit, East London, South Africa) for the training course he organised in July 2004 on randomised controlled trials, systematic reviews, and the use of Revman. The course, attended by the lead author of this review, has been instrumental in developing this systematic review. We also wish to thank Professor Cindy Farquhar, Anne Lyddiatt, Anne Lethaby, Michael Mara, and Roger Hart whose comments helped in the completion of the original version of this review as well as the update. Last but not the least, we wish to acknowledge Josie Rishworth for translating one article included in the review from Italian to English.
Appendices
Appendix 1. Cochrane Menstrual Disorders and Subfertility Group search strategy for this review
MDSG SS for EJK1071 17.06.14
Search string
Keywords CONTAINS "myomectomy" or Title CONTAINS "myomectomy"
AND
Keywords CONTAINS "misoprostol" or "sulprostone" or "tourniquet" or "uterine artery embolization" or "uterine artery ligation" or "laser" or "haemorrhages" or "haemostasis" or "haemostatic factors" or "hemostasis" or "uterotonics" or "oxytocin" or "vasopressin" or "morcellation" or "morcellator" or "bupivacaine" or "Epinephrine" or "blood loss" or "Blood Loss, Surgical" or dinoprostone or "prostaglandins" or "Bleeding" or Title CONTAINS "misoprostol" or "sulprostone" or "tourniquet" or "uterine artery embolization" or "uterine artery ligation" or "laser" or "haemorrhages" or "haemostasis" or "haemostatic factors" or "hemostasis" or "uterotonics" or "oxytocin" or "vasopressin" or "morcellation" or "morcellator" or "bupivacaine" or "Epinephrine" or "blood loss" or "Blood Loss, Surgical" or dinoprostone or "prostaglandins" or "Bleeding""
PsycINFO <1801 to February week 03 2011> search strategy
1 exp Gynecological Disorders/ (1252) 2 myoma$.tw. (17) 3 fibroid$.tw. (29) 4 Leiomyoma$.tw. (9) 5 or/1‐4 (1290) 6 myomectom$.tw. (5) 7 Laparotom$.tw. (94) 8 laparoscop$.tw. (185) 9 hysteroscop$.tw. (7) 10 or/6‐9 (278) 11 Misoprostol.tw. (33) 12 misoprostol.tw. (33) 13 sulprostone.tw. (4) 14 tourniquet.tw. (99) 15 uterine artery ligation$.tw. (6) 16 uterine artery emboli$.tw. (0) 17 UAE.tw. (117) 18 mesna.tw. (4) 19 (laser adj3 dissection).tw. (5) 20 hemorrhag$.tw. (2585) 21 haemorrhag$.tw. (605) 22 uterotonic$.tw. (2) 23 ergometrin$.tw. (21) 24 Ergonovine.tw. (9) 25 oxytocin$.tw. (1434) 26 vasopressin$.tw. (1976) 27 terlipressin$.tw. (0) 28 uterine artery dissection.tw. (0) 29 morcellation.tw. (1) 30 (chemical$ adj3 dissection).tw. (0) 31 h?emosta$.tw. (116) 32 bupivacaine.tw. (149) 33 epinephrine.tw. (1525) 34 or/11‐33 (7930) 35 5 and 10 and 34 (0)
Appendix 2. MEDLINE
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp Myoma/ (2048) 2 myoma$.tw. (4499) 3 fibroid$.tw. (4144) 4 exp Leiomyoma/ (16821) 5 leiomyoma$.tw. (10538) 6 or/1‐5 (24210) 7 myomectom$.tw. (2224) 8 exp Uterine Myomectomy/ (111) 9 exp Laparotomy/ (15546) 10 laparotom$.tw. (38261) 11 laparoscop$.tw. (82709) 12 exp Laparoscopy/ or exp Hand‐Assisted Laparoscopy/ (68805) 13 exp Hysteroscopy/ (3534) 14 hysteroscop$.tw. (4718) 15 or/7‐14 (135540) 16 exp Misoprostol/ (3321) 17 misoprostol.tw. (3711) 18 sulprostone.tw. (608) 19 tourniquet.tw. (4107) 20 uterine artery ligation$.tw. (223) 21 uterine artery emboli$.tw. (1113) 22 UAE.tw. (2160) 23 mesna.tw. (1154) 24 (laser adj3 dissection).tw. (275) 25 hemorrhag$.tw. (145643) 26 haemorrhag$.tw. (40098) 27 uterotonic$.tw. (792) 28 ergometrin$.tw. (534) 29 exp Ergonovine/ (1579) 30 Ergonovine.tw. (887) 31 oxytocin$.tw. (17953) 32 exp Oxytocin/ (16608) 33 vasopressin$.tw. (29732) 34 exp Vasopressins/ (33429) 35 terlipressin$.tw. (554) 36 uterine artery dissection.tw. (6) 37 morcellation.tw. (356) 38 (chemical$ adj3 dissection).tw. (104) 39 h?emosta$.tw. (38396) 40 bupivacaine.tw. (10102) 41 epinephrine.tw. (28702) 42 Dinoprostone.tw. (321) 43 exp Dinoprostone/ (25030) 44 or/16‐43 (344668) 45 6 and 15 and 44 (665) 46 randomised controlled trial.pt. (376290) 47 controlled clinical trial.pt. (88554) 48 randomized.ab. (296481) 49 placebo.tw. (159171) 50 clinical trials as topic.sh. (170414) 51 randomly.ab. (214512) 52 trial.ti. (127660) 53 (crossover or cross‐over or cross over).tw. (60985) 54 or/46‐53 (929704) 55 exp animals/ not humans.sh. (3951934) 56 54 not 55 (856541) 57 45 and 56 (95) 58 (201310$ or 201311$ or 201312$).ed. (235970) 59 2014$.ed. (458535) 60 2014$.dp. (449300) 61 58 or 59 or 60 (1042058) 62 57 and 61 (5)
Appendix 3. EMBASE
Database: Embase <1980 to 2014 Week 24> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp UTERUS MYOMA/ (9594) 2 myoma$.tw. (5831) 3 fibroid$.tw. (6109) 4 exp LEIOMYOMA/ (13409) 5 leiomyoma$.tw. (12331) 6 or/1‐5 (29054) 7 myomectomy.tw. (3369) 8 exp MYOMECTOMY/ (3469) 9 exp LAPAROTOMY/ (51818) 10 laparotom$.tw. (45529) 11 laparoscop$.tw. (117162) 12 exp LAPAROSCOPY/ (99372) 13 hysteroscop$.tw. (7419) 14 exp HYSTEROSCOPY/ (7632) 15 or/7‐14 (203184) 16 misoprostol.tw. (4826) 17 sulprostone.tw. (639) 18 tourniquet.tw. (4505) 19 uterine artery ligation.tw. (332) 20 uterine artery emboli$.tw. (1654) 21 exp uterine artery embolization/ (2021) 22 mesna.tw. (1429) 23 (laser adj3 dissection).tw. (424) 24 hemorrhag$.tw. (171912) 25 haemorrhag$.tw. (48032) 26 uterotonic$.tw. (1009) 27 ergometrin$.tw. (530) 28 oxytocin$.tw. (18561) 29 hormon$ tourniquet$.tw. (1) 30 vasopressin$.tw. (30846) 31 terlipressin$.tw. (809) 32 uterine artery dissection.tw. (8) 33 morcellation.tw. (713) 34 (chemical$ adj3 dissection).tw. (100) 35 h?emosta$.tw. (50607) 36 bupivacaine.tw. (12861) 37 epinephrine.tw. (31082) 38 exp prostaglandin E2/ (40681) 39 Dinoprostone.tw. (532) 40 or/16‐39 (395204) 41 6 and 15 and 40 (1343) 42 Clinical Trial/ (831601) 43 Randomized Controlled Trial/ (343448) 44 exp randomization/ (62313) 45 Single Blind Procedure/ (18367) 46 Double Blind Procedure/ (113645) 47 Crossover Procedure/ (39147) 48 Placebo/ (240637) 49 Randomi?ed controlled trial$.tw. (98971) 50 Rct.tw. (13930) 51 random allocation.tw. (1308) 52 randomly allocated.tw. (20183) 53 allocated randomly.tw. (1921) 54 (allocated adj2 random).tw. (712) 55 Single blind$.tw. (14252) 56 Double blind$.tw. (140404) 57 ((treble or triple) adj blind$).tw. (370) 58 placebo$.tw. (197255) 59 prospective study/ (252453) 60 or/42‐59 (1358985) 61 case study/ (26347) 62 case report.tw. (258214) 63 abstract report/ or letter/ (891787) 64 or/61‐63 (1170729) 65 60 not 64 (1321392) 66 41 and 65 (240) 67 (201310$ or 201311$ or 201312$).em. (67086) 68 2014$.em. (812650) 69 2014$.dp. (52878) 70 67 or 68 or 69 (881905) 71 66 and 70 (15)
Appendix 4. CINAHL
EJK1071 CINAHL 17.06.14
| # | Query | Results |
| S56 | S42 AND S54 date limited from 01.01.13 to 17.06.14 | 6 |
| S55 | S42 AND S54 | 69 |
| S54 | S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 | 895,110 |
| S53 | TX allocat* random* | 3,921 |
| S52 | (MH "Quantitative Studies") | 12,109 |
| S51 | (MH "Placebos") | 8,760 |
| S50 | TX placebo* | 31,698 |
| S49 | TX random* allocat* | 3,921 |
| S48 | (MH "Random Assignment") | 37,387 |
| S47 | TX randomi* control* trial* | 73,723 |
| S46 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 718,901 |
| S45 | TX clinic* n1 trial* | 163,832 |
| S44 | PT Clinical trial | 76,084 |
| S43 | (MH "Clinical Trials+") | 175,691 |
| S42 | S6 AND S15 AND S41 | 291 |
| S41 | S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 | 47,390 |
| S40 | TX epinephrine | 4,072 |
| S39 | TX bupivacaine | 2,850 |
| S38 | TX haemosta* | 726 |
| S37 | TX hemosta* | 5,008 |
| S36 | TX (chemical* N3 dissection*) | 14 |
| S35 | "(chemical$ N3 dissection)" | 0 |
| S34 | TX morcellation* | 41 |
| S33 | TX uterine artery dissection* | 0 |
| S32 | TX terlipressin* | 99 |
| S31 | (MH "Vasopressins") | 1,306 |
| S30 | TX vasopressin* | 1,650 |
| S29 | (MH "Oxytocin") OR "oxytocin" | 1,561 |
| S28 | (MM "Ergonovine") OR "Ergonovine" | 96 |
| S27 | TX Eergometrin* | 0 |
| S26 | TX uterotonic* | 108 |
| S25 | TX haemorrhag* | 3,146 |
| S24 | TX hemorrhag* | 31,367 |
| S23 | TX (laser N3 dissection) | 14 |
| S22 | TX mesna | 58 |
| S21 | TX UAE | 550 |
| S20 | TX uterine artery emboli* | 442 |
| S19 | (MM "Uterine Artery Embolization") OR "uterine artery ligation" | 157 |
| S18 | (MM "Tourniquets") OR "tourniquet" | 672 |
| S17 | "sulprostone" | 13 |
| S16 | (MM "Misoprostol") | 678 |
| S15 | S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 | 25,624 |
| S14 | (MH "Surgery, Gynecologic+") OR "gynaecologial surgery" | 8,498 |
| S13 | TX Hysteroscop* | 803 |
| S12 | (MH "Hysteroscopy") | 632 |
| S11 | (MH "Laparoscopy") OR (MM "Surgery, Laparoscopic") | 10,835 |
| S10 | TX laparoscop* | 15,437 |
| S9 | TX laparotom* | 3,348 |
| S8 | (MM "Laparotomy") | 668 |
| S7 | TX myomectom* | 302 |
| S6 | S1 OR S2 OR S3 OR S4 OR S5 | 2,409 |
| S5 | TX fibroid* | 832 |
| S4 | TX leiomyoma* | 2,044 |
| S3 | (MM "Leiomyoma") OR "Leiomyoma" | 1,970 |
| S2 | TX myoma* | 282 |
| S1 | (MH "Myoma+") OR "Myoma" | 258 |
Appendix 5. CENTRAL
Database: EBM Reviews ‐ Cochrane Central Register of Controlled Trials <May 2014> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp Myoma/ (16) 2 myoma$.tw. (230) 3 fibroid$.tw. (261) 4 exp Leiomyoma/ (372) 5 leiomyoma$.tw. (209) 6 or/1‐5 (649) 7 myomectom$.tw. (221) 8 exp Uterine Myomectomy/ (7) 9 exp Laparotomy/ (558) 10 laparotom$.tw. (1203) 11 laparoscop$.tw. (5936) 12 exp Laparoscopy/ or exp Hand‐Assisted Laparoscopy/ (3569) 13 exp Hysteroscopy/ (265) 14 hysteroscop$.tw. (482) 15 or/7‐14 (7682) 16 exp Misoprostol/ (1003) 17 misoprostol.tw. (1659) 18 sulprostone.tw. (60) 19 tourniquet.tw. (815) 20 uterine artery ligation$.tw. (10) 21 uterine artery emboli$.tw. (75) 22 UAE.tw. (250) 23 mesna.tw. (153) 24 (laser adj3 dissection).tw. (18) 25 hemorrhag$.tw. (5607) 26 haemorrhag$.tw. (1950) 27 uterotonic$.tw. (109) 28 ergometrin$.tw. (73) 29 exp Ergonovine/ (106) 30 Ergonovine.tw. (36) 31 oxytocin$.tw. (1709) 32 exp Oxytocin/ (860) 33 vasopressin$.tw. (1169) 34 exp Vasopressins/ (1036) 35 terlipressin$.tw. (156) 36 uterine artery dissection.tw. (0) 37 morcellation.tw. (17) 38 (chemical$ adj3 dissection).tw. (3) 39 h?emosta$.tw. (3106) 40 bupivacaine.tw. (5715) 41 epinephrine.tw. (3448) 42 Dinoprostone.tw. (221) 43 exp Dinoprostone/ (926) 44 or/16‐43 (24627) 45 6 and 15 and 44 (64) 46 limit 45 to yr="2013 ‐Current" (4)
Data and analyses
Comparison 1. Misoprostol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss (ml) | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐97.88 [‐125.52, ‐70.24] |
| 1.1 Abdominal myomectomy | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | ‐149.0 [‐229.24, ‐68.76] |
| 1.2 Laparoscopic myomectomy | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐91.0 [‐120.44, ‐61.56] |
| 2 Need for blood transfusion | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Duration of surgery (min) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Abdominal myomectomy | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐9.5 [‐15.90, ‐3.10] |
| 3.2 Laparoscopic myomectomy | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 9.0 [‐1.63, 19.63] |
| 4 Postoperative haemoglobin (g/dl) | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | 0.69 [0.37, 1.02] |
| 5 Duration of hospital stay (days) | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.82, 0.82] |
| 6 Postoperative haemoglobin drop (g/dl) | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐0.78, ‐0.42] |
| 7 Postoperative complications | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Postoperative fever | 2 | 89 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.23, 3.88] |
1.6. Analysis.
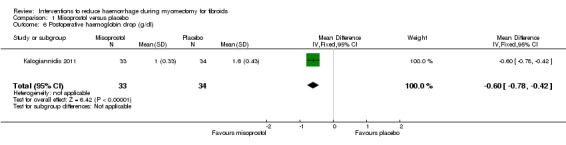
Comparison 1 Misoprostol versus placebo, Outcome 6 Postoperative haemoglobin drop (g/dl).
Comparison 2. Vasopressin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss (ml) | 3 | 128 | Mean Difference (IV, Fixed, 95% CI) | ‐199.97 [‐223.83, ‐176.12] |
| 1.1 Abdominal myomectomy | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐450.00 [‐507.49, ‐392.51] |
| 1.2 Laparoscopic myomectomy | 2 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐147.95 [‐174.17, ‐121.73] |
| 2 Need for blood transfusion | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Abdominal myomectomy | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.24] |
| 2.2 Laparoscopic myomectomy | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.02, 1.60] |
| 3 Duration of surgery (min) | 2 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐27.72 [‐35.82, ‐19.61] |
| 4 Duration of hospital stay (days) | 2 | 108 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.69, 0.91] |
| 5 Postoperative complications | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Adhesions to bowel and/or omentum | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.54, 7.49] |
| 5.2 Adnexal adhesions | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.39, 8.93] |
| 6 Pregnancy after myomectomy | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.18, 2.31] |
| 7 Conversion of laparoscopy to laparotomy | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.65 [0.38, 153.75] |
Comparison 3. Bupivicaine plus epinephrine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss (ml) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐68.6 [‐93.69, ‐43.51] |
| 2 Duration of surgery (min) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐30.5 [‐37.68, ‐23.32] |
Comparison 4. Peri‐cervical tourniquet versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss (ml) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Blood loss ‐ cervical tourniquet (ml) | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐240.70 [‐359.61, ‐121.79] |
| 1.2 Blood loss ‐ cervical & infundibulopelvic ligament tourniquet | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐1870.0 [‐2547.16, ‐1192.84] |
| 2 Need for blood transfusion | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Need for blood transfusion ‐ cervical tourniquet | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.09, 0.55] |
| 2.2 Need for blood transfusion ‐ cervical & infundibulopelvic ligament tourniquet | 1 | 28 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.23] |
| 3 Duration of surgery (min) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐29.28, 21.28] |
| 4 Postoperative complications | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Postoperative fever | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.46, 2.59] |
| 4.2 Postoperative anaemia | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.46, 2.59] |
| 4.3 Urinary tract infection | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.13, 3.70] |
| 4.4 Prolonged vaginal bleeding | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.09, 55.82] |
| 4.5 Pelvic abscess | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.09, 55.82] |
| 4.6 Intestinal obstruction | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.09, 55.82] |
| 4.7 Bladder injury | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 5.94] |
4.2. Analysis.
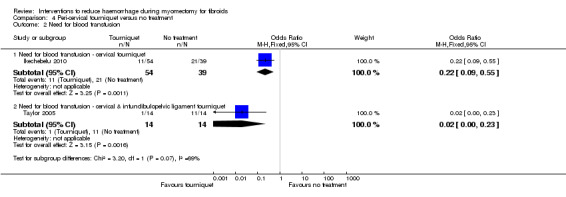
Comparison 4 Peri‐cervical tourniquet versus no treatment, Outcome 2 Need for blood transfusion.
Comparison 5. Oxytocin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss (ml) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Need for blood transfusion | 2 | 154 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.03, 8.51] |
| 3 Duration of surgery (min) | 2 | 154 | Mean Difference (IV, Fixed, 95% CI) | 3.50 [‐1.88, 8.88] |
| 4 Postoperative complications | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 16.76] |
| 5 Duration of hospital stay (days) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.19, ‐0.01] |
5.4. Analysis.
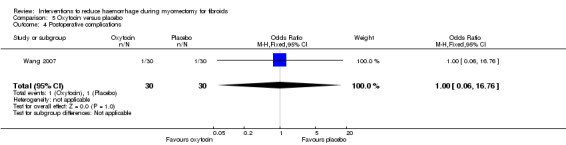
Comparison 5 Oxytocin versus placebo, Outcome 4 Postoperative complications.
Comparison 6. Chemical dissection with mesna versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of surgery (min) | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐20.0 [‐28.64, ‐11.36] |
| 2 Duration of hospital stay (days) | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐1.12, ‐0.88] |
| 3 Postoperative haemoglobin (g/dl) | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [0.42, 0.58] |
| 4 Postoperative haematocrit | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [1.30, 2.50] |
| 5 Postoperative complications | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Postoperative fever | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.22] |
Comparison 7. Myoma morcellation versus standard technique of enucleation (no treatment).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss (ml) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 65.40 [‐36.47, 167.27] |
| 2 Duration of surgery (min) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐25.30 [‐44.23, ‐6.37] |
| 3 Duration of hospital stay (days) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.18, 0.04] |
7.2. Analysis.
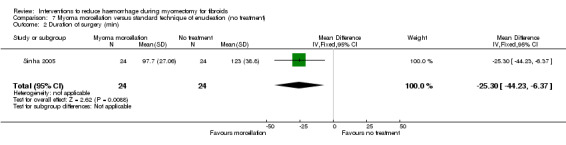
Comparison 7 Myoma morcellation versus standard technique of enucleation (no treatment), Outcome 2 Duration of surgery (min).
Comparison 8. Intravenous injection of tranexamic acid versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss (ml) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐243.0 [‐460.02, ‐25.98] |
| 2 Need for blood transfusion | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.68, 4.30] |
| 3 Duration of surgery (min) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐11.0 [‐21.09, ‐0.91] |
| 4 Postoperative haemoglobin (g/dl) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.36, 0.78] |
| 5 Postoperative haematocrit | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.43, 2.43] |
Comparison 9. Gelatin‐thrombin matrix versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss (ml) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐545.0 [‐593.26, ‐496.74] |
| 2 Need for blood transfusion | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.01 [0.00, 0.10] |
| 3 Postoperative vaginal blood loss (ml) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐225.0 [‐254.46, ‐195.54] |
| 4 Duration of surgery (min) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [1.29, 8.71] |
| 5 Postoperative haemoglobin drop (g/dl) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐2.3 [‐2.66, ‐1.94] |
| 6 Duration of hospital stay (days) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐2.69, ‐1.31] |
| 7 Postoperative fever | 1 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.25] |
Comparison 10. Ascorbic acid versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss (ml) | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | ‐411.46 [‐502.58, ‐320.34] |
| 2 Need for blood transfusion | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.11, 1.32] |
| 3 Duration of surgery (min) | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | ‐26.0 [‐33.10, ‐18.90] |
| 4 Duration of hospital stay (days) | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.65, ‐0.15] |
| 5 Posoperative haemoglobin drop (g/dl) | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.22, 0.50] |
| 6 Pospoerative hematocrit drop (%) | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.34, ‐0.06] |
| 7 Posoperative complications | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Postoperative fever | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Postoperative vomiting | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Postoperative constipation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Severe pain | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
10.2. Analysis.
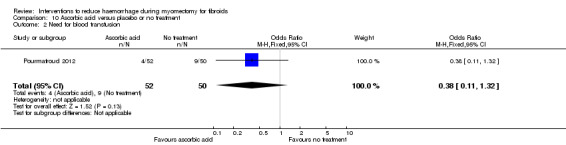
Comparison 10 Ascorbic acid versus placebo or no treatment, Outcome 2 Need for blood transfusion.
10.3. Analysis.
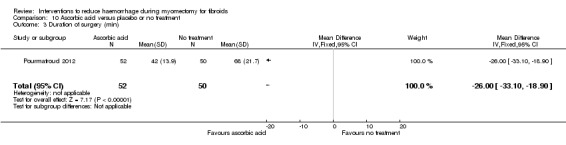
Comparison 10 Ascorbic acid versus placebo or no treatment, Outcome 3 Duration of surgery (min).
10.4. Analysis.
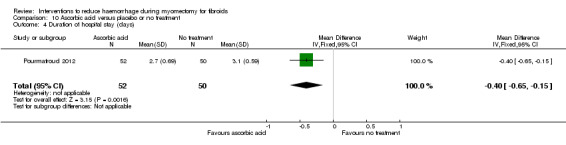
Comparison 10 Ascorbic acid versus placebo or no treatment, Outcome 4 Duration of hospital stay (days).
10.5. Analysis.
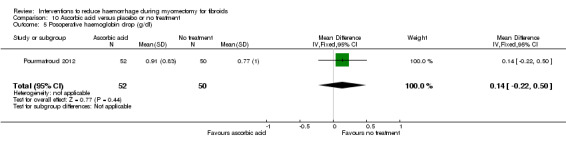
Comparison 10 Ascorbic acid versus placebo or no treatment, Outcome 5 Posoperative haemoglobin drop (g/dl).
10.6. Analysis.
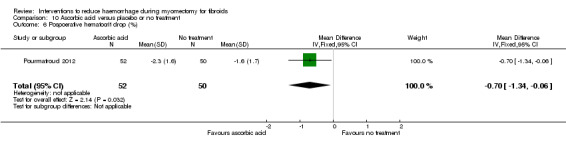
Comparison 10 Ascorbic acid versus placebo or no treatment, Outcome 6 Pospoerative hematocrit drop (%).
10.7. Analysis.
Comparison 10 Ascorbic acid versus placebo or no treatment, Outcome 7 Posoperative complications.
Comparison 11. Dinoprostone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss (ml) | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐131.60 [‐253.42, ‐9.78] |
| 2 Need for blood transfusion | 1 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.04, 0.81] |
| 3 Duration of surgery (min) | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐12.55, 7.35] |
| 4 Duration of hospital stay (days) | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.22, 0.82] |
| 5 Postoperative haemoglobin (g/dl) | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.39, 0.59] |
| 6 Postoperative haemoglobin drop (g/dl) | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐0.88, ‐0.12] |
| 7 Postoperative complications | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Postoperative fever | 1 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.25, 9.54] |
11.2. Analysis.
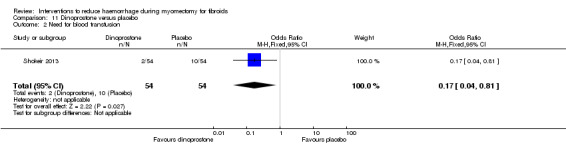
Comparison 11 Dinoprostone versus placebo, Outcome 2 Need for blood transfusion.
11.3. Analysis.
Comparison 11 Dinoprostone versus placebo, Outcome 3 Duration of surgery (min).
11.4. Analysis.
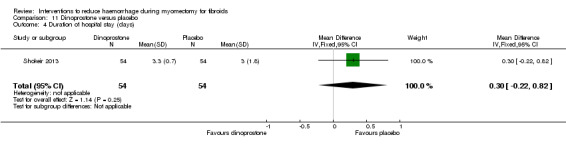
Comparison 11 Dinoprostone versus placebo, Outcome 4 Duration of hospital stay (days).
11.5. Analysis.
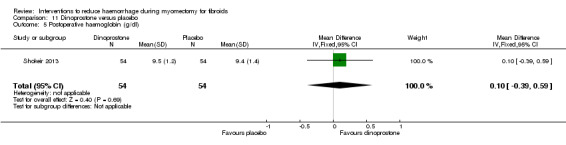
Comparison 11 Dinoprostone versus placebo, Outcome 5 Postoperative haemoglobin (g/dl).
11.6. Analysis.
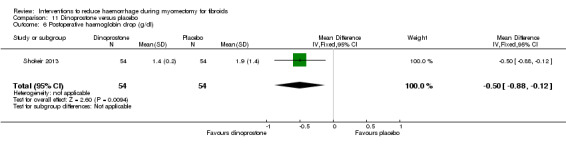
Comparison 11 Dinoprostone versus placebo, Outcome 6 Postoperative haemoglobin drop (g/dl).
11.7. Analysis.
Comparison 11 Dinoprostone versus placebo, Outcome 7 Postoperative complications.
Comparison 12. Loop ligation of myoma pseudocapsule plus vasopressin versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss (ml) | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐305.01 [‐354.83, ‐255.19] |
| 2 Need for blood transfusion | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.47] |
| 3 Duration of surgery (min) | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐32.76 [‐40.81, ‐24.71] |
| 4 Duration of hospital stay (days) | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐1.46 [‐1.85, ‐1.07] |
12.2. Analysis.
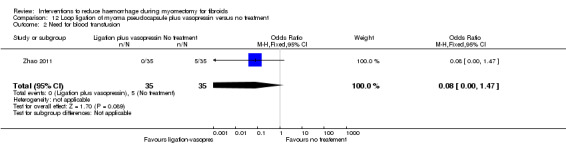
Comparison 12 Loop ligation of myoma pseudocapsule plus vasopressin versus no treatment, Outcome 2 Need for blood transfusion.
12.3. Analysis.
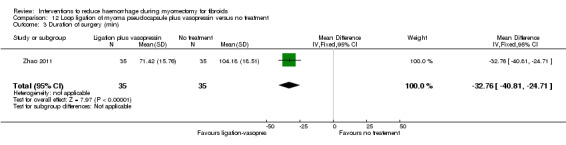
Comparison 12 Loop ligation of myoma pseudocapsule plus vasopressin versus no treatment, Outcome 3 Duration of surgery (min).
12.4. Analysis.
Comparison 12 Loop ligation of myoma pseudocapsule plus vasopressin versus no treatment, Outcome 4 Duration of hospital stay (days).
Comparison 13. Temporary clipping of uterine artery versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Need for blood transfusion | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Duration of surgery (min) | 1 | 166 | Mean Difference (IV, Fixed, 95% CI) | 40.0 [30.01, 49.99] |
| 3 Postoperative haemoglobin drop (g/dl) | 1 | 166 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.61, 0.11] |
| 4 Duration of hospital stay (days) | 1 | 166 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.15, 0.15] |
| 5 Postoperative complications | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Postoperative bleeding | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.19, 24.51] |
| 5.2 Uterine hematoma | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.31 [0.34, 32.51] |
| 5.3 Postoperative sepsis | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.13, 81.29] |
| 5.4 Bleeding from point of trocar insertion | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.13, 81.29] |
| 5.5 Postoperative urinary tract infection | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.13, 81.29] |
| 5.6 Trocar site hernia | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.13, 81.29] |
| 5.7 Sinus venous thrombosis | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.13, 81.29] |
| 5.8 Lung artery embolism | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.82] |
| 5.9 Cardiac arrhythmia | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.82] |
13.1. Analysis.
Comparison 13 Temporary clipping of uterine artery versus no treatment, Outcome 1 Need for blood transfusion.
13.2. Analysis.
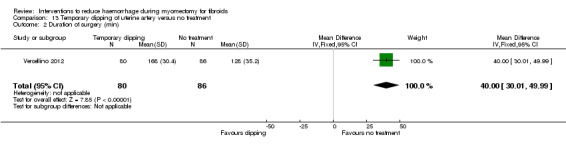
Comparison 13 Temporary clipping of uterine artery versus no treatment, Outcome 2 Duration of surgery (min).
13.3. Analysis.
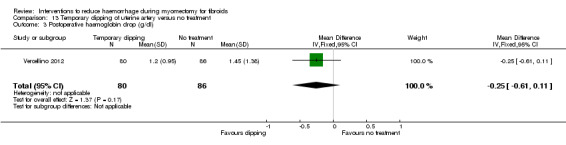
Comparison 13 Temporary clipping of uterine artery versus no treatment, Outcome 3 Postoperative haemoglobin drop (g/dl).
13.4. Analysis.
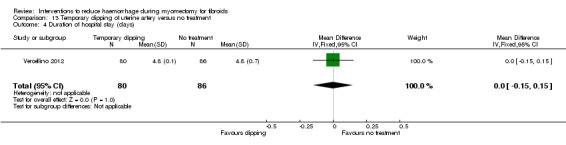
Comparison 13 Temporary clipping of uterine artery versus no treatment, Outcome 4 Duration of hospital stay (days).
13.5. Analysis.
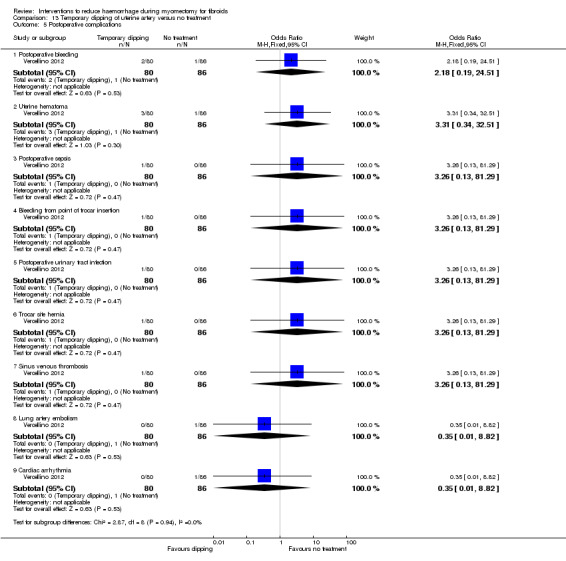
Comparison 13 Temporary clipping of uterine artery versus no treatment, Outcome 5 Postoperative complications.
Comparison 14. Fibrin sealant patch versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss (ml) | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐26.5 [‐44.47, ‐8.53] |
| 2 Postoperative blood loss in drainage bag | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐44.60 [‐65.06, ‐24.14] |
| 3 Need for blood transfusion | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Duration of surgery (min) | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐4.47, 3.67] |
| 5 Duration of hospital stay (days) | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.09, 0.09] |
| 6 Conception after surgery | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.76, 13.11] |
14.2. Analysis.
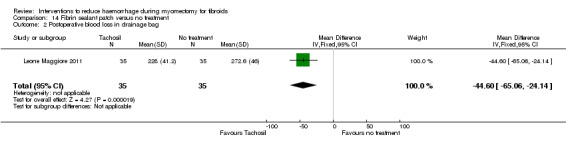
Comparison 14 Fibrin sealant patch versus no treatment, Outcome 2 Postoperative blood loss in drainage bag.
14.3. Analysis.
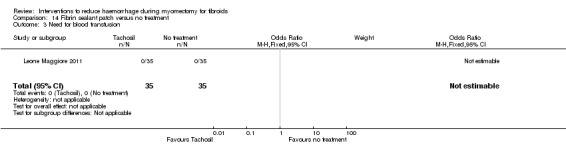
Comparison 14 Fibrin sealant patch versus no treatment, Outcome 3 Need for blood transfusion.
14.4. Analysis.
Comparison 14 Fibrin sealant patch versus no treatment, Outcome 4 Duration of surgery (min).
14.5. Analysis.
Comparison 14 Fibrin sealant patch versus no treatment, Outcome 5 Duration of hospital stay (days).
14.6. Analysis.
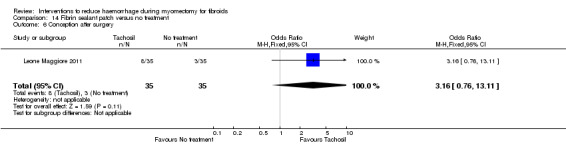
Comparison 14 Fibrin sealant patch versus no treatment, Outcome 6 Conception after surgery.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agostini 2005.
| Methods | Single centre study. Described as randomised, but no details on how patients were allocated to trial interventions Patients, care providers, and outcome assessors were blinded to treatment allocation Power calculation performed No losses to follow up, and all patients were analysed in groups to which they were assigned Source of funding not mentioned | |
| Participants | Setting: university hospital in Marseille, France Inclusion criteria: patients with uterine myoma who required abdominal or vaginal myomectomy Exclusion criteria: preoperative embolisation and preoperative administration of any GnRh analogue n = 94 Mean age: 40 years (SD 5.2) in the treatment group and 39 years (SD 4.3) in the placebo group Ethnicity: not described Sizes of the fibroids: no significant difference in the weight and number of myomas between the treatment and control groups | |
| Interventions | Treatment arm (n = 47): 15 IU oxytocin administered by an IV infusion of 125cm3 of physiological serum over 30 min, beginning at the start of uterine incision
Control group (n = 47): physiological serum (placebo) administered in place of oxytocin Type of operation: laparotomy or vaginal route |
|
| Outcomes | Perioperative blood loss, blood transfusion, and duration of surgery Blood loss was estimated using both the blood aspirated into the canisters, minus the irrigant, and the blood absorbed in the lap sponges | |
| Notes | Indications for myomectomy: bleeding, pelvic pain, and fertility Authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no details on how patients were allocated to trial interventions |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the study protocol |
| Other bias | Low risk | No other sources of bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participants and care providers were blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to treatment allocation |
Assaf 1999.
| Methods | Single centre study. Patients allocated to trial interventions using a random numbers table (but no description is given of how the table was generated) Neither patients nor care providers were blinded Power calculation: not done No losses to follow up, and all patients were analysed in groups to which they were assigned Source of funding: not mentioned | |
| Participants | Setting: university hospital in Cairo, Egypt Inclusion criteria: infertile patients with fewer than 3 myomas, the largest of which was less than 7 cm, myoma on the anterior wall, subserous‐interstitial myoma, and no procedure other than myomectomy required Exclusion criteria: posterior myoma, subserous myoma only, myoma smaller than 3 cm, operative procedures in addition to myomectomy n = 38 Mean age: 33.4 years (SD 2.2) in both groups Ethnicity: not described Myoma sizes: 30‐70 mm | |
| Interventions | Treatment arm (n = 21): ornipressin injection (5IU) diluted in 100 ml saline was injected to form 2 to 3 weals in the base of the myomas, immediately prior to incision of the uterine capsule
Control group (n = 17): no treatment offered All patients had second‐look laparoscopy 1 month after the original procedure and were then followed up for 1 year Type of operation: laparotomy |
|
| Outcomes | Perioperative blood loss, operation time, hospital stay, adhesions, and pregnancy outcome | |
| Notes | Indications for myomectomy: infertility after excluding all other causes except fibroid Authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients allocated to trial interventions using a random numbers table (but no description is given of how the table was generated) |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the study protocol |
| Other bias | Low risk | No other sources of bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither patients nor care providers were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unblinded |
Benassi 2000.
| Methods | Single centre study Allocation to interventions using computer‐generated random numbers Patients blinded, but assessors unblinded Power calculation: not done No losses to follow up, and all patients were analysed in groups to which they were assigned Source of funding: not mentioned | |
| Participants | Setting: university hospital, Parma, Italy Inclusion criteria: patients with symptomatic fibroids (menorrhagia, pelvic pain, and compression) Exclusion criteria: use of hormonal substances within 6 months proceeding myomectomy, previous uterine surgery, pelvic inflammatory diseases n = 58 Age range: 25‐45 years Ethnicity not described Size of myomas: 2‐17 cm | |
| Interventions | Treatment group (n = 29): use of sodium‐2‐mercaptoethane sulfonate (mesna) for chemical dissection (removal of fibroids with the aid of a chemical substance that breaks down tissues) of myoma during myomectomy Control group (n = 29): use of saline for dissection of myomas Type of operation: laparotomy | |
| Outcomes | Postoperative haemoglobin and haematocrit, duration of operation, hospital stay, and postoperative complications | |
| Notes | Indications for myomectomy: menorrhagia, pelvic pain, and compression Authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation to interventions using computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the study protocol |
| Other bias | Low risk | No other sources of bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors unblinded |
Caglar 2008.
| Methods | Single centre study Patients were randomised according to a computer‐generated sequence Participants allocated into groups by sequentially numbered drug containers of identical appearance Patients, surgeons and anaesthesiologists were blinded Power calculation: performed No losses to follow up and all patients were analysed in the group in which they were allocated No details given about the source of funding |
|
| Participants | Setting: Ankara Etlik Maternity and Women's Health Teaching Research Hospital, Ankara, Turkey Inclusion criteria: participants were women scheduled for myomectomy due to myoma uteri Exclusion criteria: patients with malignancy, history of thromboembolic disease, ischaemic heart disease, subarachnoidal bleeding, hematuria, and body mass index >30 were excluded n = 100 Age: mean age of the patients was 35.3 ± 5 years (range 23 to 40) Ethnicity: not described Total volume of myomas: 457cm3 (SD = 669) in the intervention group and 286cm3 (SD = 259) in the control group |
|
| Interventions | Treatment arm (n = 50): a bolus intravenous injection of tranexamic acid (a medication that prevents bleeding by inhibiting the breakdown of blood clots) 10mg/kg (maximum 1g) 15 min before incision followed by continuous infusion of 1mg/kg/h dissolved in 1 L of saline for 10 h (maximum 1 g/10 h) Control arm (n = 50): a bolus injection of placebo (saline of similar volume to tranexamic acid) 15 min before incision followed by continuous infusion of 1 L of saline for 10 h Type of operation: laparotomy |
|
| Outcomes | Perioperative blood loss, postoperative blood loss, total blood loss, duration of surgery, postoperative haemoglobin, postoperative haematocrit, blood transfusion requirements on ward | |
| Notes | Authors contacted No significant difference in the baseline characteristics such as age, body mass index, bleeding time, coagulation time, prothrombin time, the number and volume of myomas removed between the intervention and control groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised according to a computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Participants allocated into groups by sequentially numbered drug containers of identical appearance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the study protocol |
| Other bias | Low risk | No other sources of bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded |
Celik 2003.
| Methods | Single centre study Described as randomised, but no details on how patients were allocated to trial interventions Both patients, care providers, and outcome assessors were blinded to treatment allocation Power calculation: performed No losses to follow up, and all patients were analysed in groups to which they were assigned Source of funding: not mentioned |
|
| Participants | Setting: university hospital, Elazig, Turkey Inclusion criteria: symptomatic uterine fibroids scheduled for myomectomy n = 25 Mean age: 31.7 years (SD 4.4) in the treatment arm and 32.2 years (SD 2.9) in the placebo group Ethnicity: not mentioned | |
| Interventions | Treatment arm (n = 13): 400 uG misoprostol administered vaginally 1 hour before surgery Control arm (n = 12): placebo of identical shape and colour Sizes of fibroids: mean diameter of myomas 150.7 mm (SD 28.6) in the treatment arm and 154.2 mm (SD 24.7) in the control arm Type of operation: laparotomy | |
| Outcomes | Perioperative blood loss, postoperative haemoglobin, operation time, blood transfusion, hospital stay, and postoperative morbidity Blood loss determined by aspiration equipment during the operation |
|
| Notes | Indication for myomectomy: symptomatic uterine fibroid Authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no details on how patients were allocated to trial interventions |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the study protocol |
| Other bias | Low risk | No other sources of bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participants and personnel were blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to treatment allocation |
Frederick 1994.
| Methods | Single centre study Randomisation by drawing numbers from a box. The numbers ranged from 1 to 20: when an odd number was drawn the patient received vasopressin, whereas an even number resulted in placebo being given Patients, care providers, and outcome assessors were blinded Power calculation: performed but study stopped half‐way and the code broken because of heavy bleeding in 10 patients No losses to follow up, and all patients were analysed in groups to which they were assigned Source of funding: not mentioned | |
| Participants | Setting: university hospital, Kingston, Jamaica
Inclusion criteria: symptomatic uterine myoma at least size of 14 weeks gestation Exclusion criteria: contraindication to vasopressin (notably cardiovascular and respiratory diseases) n = 20 Median age: 32 years (range 24‐40) Ethnicity: not described Sizes of fibroids: 5.3‐14.0 cm |
|
| Interventions | Treatment (n = 10): injection into the broad ligaments of 20 units/ml of vasopressin (i.e. 1ml diluted in 19 ml saline) made up at the time of surgery Control: n = 10, Injection of placebo (20 ml normal saline) Type of operation: laparotomy | |
| Outcomes | Perioperative blood loss, blood transfusion | |
| Notes | Indications for myomectomy: menorrhagia, recurrent miscarriage, subfertility Authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by drawing numbers from a box |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the study protocol |
| Other bias | Low risk | No other sources of bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded |
Ikechebelu 2010.
| Methods | Single study centre. Patients were randomly allocated to either the control group (n = 39), or the intervention group (n = 54). The method of randomisation is not mentioned. Informed consent was obtained in all cases before the procedure Power calculation: Not done Loss to follow up: There was no loss to follow up and all patients were analysed in the group in which they were allocated |
|
| Participants | Setting: Tertiary University Teaching hospital in Nigeria Inclusion criteria: Not stated Exclusion criteria: Not stated Mean age: The mean age of patients was 35 years (standard deviation 6.1 years). The mean age was similar in the two groups Ethnicity: Not mentioned Myoma: 83.9% were intramural, 53.8% were subserous and 33.0% were submucous. There was no significant difference with regards to fibroid location |
|
| Interventions | Treatment group (tourniquet): Foley catheter was used as an improvised tourniquet applied to the base of the uterus close to the insertion of the uterosacral ligaments. The Fallopian tubes and the ovaries were carefully excluded from the line of the tourniquet to avoid direct compression and necrosis. The tourniquet was released intermittently (at about 30 minutes interval) during the surgery and finally removed after the repair of the uterus Control group (no tourniquet): Myomectomy was performed without the use of any uterine clamp or improvised tourniquet Type of operation: Laparotomy |
|
| Outcomes | Blood loss, transfusion rate, and postoperative complications | |
| Notes | Authors not contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is unclear how the authors generated the random sequence |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment is not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the study protocol |
| Other bias | Low risk | No other sources of bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The control group receive no treatment, rather than placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome assessors were blinded |
Kalogiannidis 2011.
| Methods | Randomised controlled trial with 30 participants assigned to group I (misoprostol group) and 34 participants assigned to group II (placebo group) Prospective randomised trial performed among women scheduled from February 2007 and February 2009 Setting: Hospital in Greece Power calculation performed No loss to follow up |
|
| Participants | Inclusion criteria: menstruating women aged ≤ 45 years, with three or less myomas of diameter ranging between 30mm and 90mm. Patients with cervical or endometrial pathology as well as with adnexal masses were excluded Intervention group: mean age of 37.2 years (SD = 6.5) and control group: mean age of 34.8 years (SD = 4.6) Ethnicity not reported Intervention group: n = 30 Control group: n = 34 |
|
| Interventions | Intervention group received 400mg intravaginal misoprostol 1hr before surgery and the control group received intravaginal placebo 1hr before surgery. The intravaginal placebo was ‘similar’ to the misoprostol. All patients had laparoscopic myomectomy | |
| Outcomes | Outcomes measured included postoperative Hb, postoperative anaemia, blood transfusion, duration of the operation, side‐effects, and blood loss. Blood loss was estimated as the difference between the irrigated and aspirated liquid from the peritoneal cavity during the operation (no gauze was used) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants described as ‘randomly’ allocated to the intervention and control groups, but no details given |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was achieved using sequentially numbered opaque sealed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data reported |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the study protocol |
| Other bias | Low risk | No other sources of bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participants and health personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded |
Leone Maggiore 2011.
| Methods | Randomised controlled trial with 70 participants randomised: 35 participants to Group A (Tachosil group) and 35 participants to Group B (no treatment group) Setting: Italy |
|
| Participants | Patients of reproductive age with intramural uterine fibroids Inclusion criteria: only patients with diagnosis of intramural fibroids via ultrasound where the distance between the deepest part of the fibroid and the endometrium is ≥0.5cm Exclusion criteria: women with >3 fibroids, subserosal or submucosal fibroids, fibroid diameter >10cm, use of hormonal treatments in the 6 months before surgery, previous uterine surgery, any pathology requiring further surgical treatment, BMI ≥ 29, women with contraindications to general anaesthesia, or any women with a psychiatric condition that prevents informed consent Ethinicity not reported |
|
| Interventions | All myomectomies were done laparoscopically Participants of the intervention group were administered fibrin sealant patch called Tachosil i.e. collagen sponge with thrombin and fibrinogen used to stop local bleeding on internal organs (n=35) The control group received no additional treatment (n=35) Time required to apply the Tachosil was measured The median number of Tachosil used on each patient was 1 with a range of 1‐3 |
|
| Outcomes | The outcomes measured included the postoperative complications, volume of blood collected intraoperatively, volume of blood in drainage bag, No. of days with drainage bag and No. of days of hospitalisation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by a “computer‐generated list” |
| Allocation concealment (selection bias) | Low risk | A statistician with no role in the study assigned the groups into sealed, opaque envelopes in sequential order |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow up |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the study protocol |
| Other bias | Low risk | No other sources of bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control group had no treatment rather than a placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A researcher who did not know to which group the patients were randomised, recorded the post‐op complications, volume of blood in the drainage bag, and the no. of days the drainage bag was used |
Pourmatroud 2012.
| Methods | Randomised controlled trial involving 102 participants: 52 participants in received ascorbic acid and 50 participants received no treatment Setting: Tertiary hospital in Ahvaz, Iran From October 2009 to August 2010, women (n = 102) of reproductive age who were admitted for an abdominal myomectomy were randomly assigned to receive either ascorbic acid to no treatment No power calculation performed |
|
| Participants | At least one non‐cavitary fibroma ≥ 40 mm2 in size; a normal coagulation profile; non‐smoker; no history of chronic illness (renal failure, drug abuse, haemoglobinopathy or respiratory, liver or heart disease); no history of recent hospitalization; regular consumption of fresh fruit or vegetables at least once a day and no history of glucose 6‐phophatase dehydrogenase deficiency. Baseline characteristics were similar in both groups Ethnicity not described |
|
| Interventions | Intervention group received ascorbic acid 500mg in 15ml normal saline at a rate of 50ug/min, first ampoule of 500mg ascorbic acid given after reaching the uterus and before myomectomy, the second administered during myoma enucleation and the third during wound closure. After the operation, two additional ascorbic acid ampoules were infused at a rate of 20ug/min over the first 12h. Control group received no treatment. In both groups, myomectomy was performed by laparotomy | |
| Outcomes | The outcomes measured were the size and number of myoma, duration of the operation, blood loss, haemoglobin and haematocrit 6h after the operation, blood transfusion, and other complications. Blood loss was measured by adding the blood collected via suction with the number of soaked surgical 4X4 gauze (each one assigned a value of 5 ml blood) and towels (each one was assigned a value of 50 ml blood) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants described as ‘randomly assigned’ to the intervention and control groups. No details given on the method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No details given on the method of allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the study protocol |
| Other bias | Low risk | No other sources of bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is described that the surgical team was blinded, but no information given on the blinding of study participants. However the control group did not receive an identical placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
Raga 2009.
| Methods | Single study centre. Patients were randomly allocated to either the control group (n = 25), or the intervention group (n = 25) according to a computer‐generated sequence. Informed consent was obtained in all cases according to the local ethics committee Power calculation: not done Loss of follow up: there was no loss to follow up and all patients were analysed in the group in which they were allocated |
|
| Participants | Setting: Tertiary University Teaching Hospital in Spain Inclusion criteria: symptomatic fibroids with uterine size greater or equal to 16 weeks gestation, and a request to retain their uterus Exclusion criteria: history of bleeding disorder, concurrent anticoagulant therapy, a haemoglobin level less than 10g/dl at the time of surgery, and pre‐malignant endometrial histologic findings Mean age: the mean age of patients was 32 years (range 24‐35 years) Ethnicity: not mentioned Myoma: mean number of fibroids per patient was 3.1 in the control and 3.2 in the intervention group. The mean uterine size was 17.9cm in the control group and 18.2cm in the intervention group |
|
| Interventions | Treatment group: gelatin‐thrombin matrix (Floseal Matrix, Baxter: a substance that activates the clotting process and stops bleeding) was delivered to the site of the uterine bleeding (wound) via a single‐barrel syringe and a special applicator tip according to the manufacturers instruction, before closure Control group: isotonic sodium chloride solution was placed in the uterine bleeding site before closure Diluted vasopressin (1:60) was injected into the myometrium around the myoma and into the myoma tissue in both groups Type of operation: Laparotomy |
|
| Outcomes | Operative time, blood loss, intraoperative and postoperative complications, and duration of hospital stay | |
| Notes | Authors not contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated according to a computer generated sequence |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment is not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the study protocol |
| Other bias | Low risk | No other sources of bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Isotonic sodium chloride solution as placebo was placed in the uterine bleeding site before closure |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information on blinding of outcome assessment not provided |
Shokeir 2013.
| Methods | Randomised controlled trial with 54 participants assigned to receive a single dose of intravaginal 20mg dinoprostone and 54 participants received a placebo Setting: Mansoura University Hospital in Egypt Power calculation performed |
|
| Participants | Women with uterine leiomyomas. Exclusion criteria were any contraindication to dinoprostone, a known history of pelvic/ovarian endometriosis, a known history or active medical disease, a known history of previous myomectomy, women who had preoperative mifepristone, GnRH analogue or oral contraceptive pills, women with mental impairment or incompetent in giving consent and women who did not wish to participate in the study Ethnicity not reported |
|
| Interventions | The intervention group received 20mg dinoprostone (prostaglandin E2 analogue, medications that arrest bleeding by causing contraction of muscles of the uterus) vaginal suppository before the operation. The control group received intravaginal placebo tablet of the same size and shape as dinoprostone. All patients had open abdominal myomectomy | |
| Outcomes | The primary outcome was blood loss and the secondary outcome measures were blood transfusion, change in Hb level, and incidence of side effects. Blood loss was estimated by measuring the amount of blood accumulated in the aspiration equipment and the amount of blood on the surgical gauze using alkaline hematin technique | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were assigned serial numbers and were allocated through a stratified randomisation according to the surgeons. Random sequence was generated by computer in blocks of four |
| Allocation concealment (selection bias) | Low risk | Group assignment was put into sealed, opaque envelopes. After recruitment the envelope was opened by the research nurse not involved in the project |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete data; No loss of follow up |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the study protocol |
| Other bias | Low risk | No other sources of bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and the personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded |
Sinha 2005.
| Methods | Single centre study Allocation by computer‐generated random numbers Unblinded Power calculation: not performed 8.3% (4/48) patients excluded because of difficulties which led to them receiving the treatment of the intervention arm and not the control arm as randomised Intention‐to‐treat analysis: not performed Source of funding: not mentioned | |
| Participants | Setting: private endoscopy centre, Mumbai, India
Inclusion criteria: uterus larger than 12 weeks on bimanual examination, ultrasound confirmation of presence of at least one myoma 7 cm or greater and/or presence of three or more myomas greater than 5 cm in size Exclusion criteria: submucosal myoma, associated ovarian lesions or other pathology discovered by ultrasound exam, a history of surgery n = 48 Mean age: 32.95 years (SD 4.65) in treatment arm and 33.8 years (SD 6.35) in control arm Ethnicity: not described Sizes of myomas: the mean size of the myoma was 7.6 cm (SD 4.2) in treatment arm and 7.6 cm (SD 4. 5) in the control arm |
|
| Interventions | Treatment arm (n = 24): enucleation of myoma by morcellation while still attached to uterus Control arm (n = 20): conventional technique of complete enucleation followed by morcellation Type of operation: laparoscopy | |
| Outcomes | Perioperative blood loss, hospital stay, and length of surgery Perioperative blood loss estimated by consistently sucking the blood into a suction bottle without any irrigation until the intracorporeal suturing was completed and the haemostasis confirmed | |
| Notes | Indications for myomectomy: symptomatic myomas Authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation by computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8.3% (4/48) patients excluded because of difficulties which led to them receiving the treatment of the intervention arm and not the control arm as randomised. |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the study protocol |
| Other bias | Low risk | No other sources of bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
Taylor 2005.
| Methods | Two centre study Allocation by sealed sequentially numbered opaque envelopes containing computer‐generated random numbers Patients blinded but care providers not blinded Power calculation: performed No losses to follow up, and all patients were analysed in groups to which they were assigned Source of funding: not mentioned | |
| Participants | Setting: two university hospitals, London, UK Inclusion criteria: symptomatic fibroids, uterine size at least 14 weeks, and patient request for myomectomy Exclusion criteria: history of bleeding disorder, concurrent anti‐coagulant therapy, haemoglobin less than 10.5g/dl at the time of surgery, pre‐malignant endometrial histology n = 28 Mean age: 39.5 years (SD 4.7) in control arm and 42.6 years (SD 6.7) in treatment arm Ethnicity: not described Number of fibroids: range 1‐34 | |
| Interventions | Treatment arm (n = 14): a number one polyglactin suture tied around the cervix to occlude uterine artery and left there after surgery, plus a polythene tourniquet tied round the infundibulopelvic ligament and removed after the operation Control arm (n = 14): no treatment added to the normal myomectomy procedure Type of operation: laparotomy | |
| Outcomes | Perioperative blood loss, need for blood transfusion, operative morbidity Blood loss was assessed by weighing swabs and measuring blood collected by suction | |
| Notes | Indications for myomectomy: symptomatic uterine fibroids Preoperative GnRH agonists prescribed to anaemic patients to increase preoperative haemoglobin to more than 10.5g/dl Authors contacted July 2015 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation by sealed sequentially‐numbered opaque envelopes containing computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the study protocol |
| Other bias | High risk | Statistically significant difference in mean age of the two groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients blinded but care providers not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome assessment blinded |
Vercellino 2012.
| Methods | Randomised controlled trial with 80 participants assigned to receive laparoscopic uterine artery clipping and myomectomy, while 86 participants received laparoscopic myomectomy only Study conducted in three centres in Germany from January 2007 to December 2009 Power calculation performed |
|
| Participants | Women between the ages of 18 and 50 years with the diagnosis symptomatic uterine myomas and who had a combined diameter of ≥ 4cm were included into the study. Patients with severe accompanying medical problems, or psychiatric illnesses, which jeopardize participation, or undergoing treatment affecting coagulation and/or hematopoiesis, and patients with suspected malignancy were excluded Ethnicity not described |
|
| Interventions | All patients had laparoscopic myomectomy. The intervention group (Group A) had temporary bilateral clipping of uterine arteries during myomectomy using Yasargil vascular clips and the control group (Group B) received no additional treatment | |
| Outcomes | Outcomes measured were haemoglobin drop, duration of surgery, duration of hospital stay, need for blood transfusion and complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation by computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Patients allocated to study group temporary clipping of bilateral uterine arteries, Group A) and control (Group B) by means of sealed sequentially numbered brown envelopes containing computer‐generated random numbers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the study protocol |
| Other bias | Low risk | No other sources of bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomisation was done the day before the surgery and patients were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information |
Wang 2007.
| Methods | Single centre study Participants were randomly allocated to either intervention or control group according to a computer‐generated sequence Allocation using preprinted slips on sealed envelopes that had been prepared before the started of the study with a computer‐generated sequence Information not given about blinding Power calculation: performed No losses to follow up and all patients were analysed in the group in which they were allocated Funding from the Medical Research Project, Chang Gung Memorial Hospital |
|
| Participants | Setting: Chang Gung Memorial Hospital, Taiwan Inclusion criteria: 60 women who had clinical diagnosis of symptomatic leiomyoma (at least an intramural myoma that measured 5cm or more). Diagnosis of leiomyomata was made by pelvic examination and ultrasound scanning in all patients Exclusion criteria: patients with intrauterine lesions were excluded n = 60 Age: mean age of women in the intervention group was 38.1 years (SD = 6.8) and of the control group was 35.8 years (SD =6.1) Ethnicity: not described Mean fibroid size: 7.6 cm (SD = 2.2) in the treatment group and 7.5cm (SD = 1.9) in the control group |
|
| Interventions | Treatment arm (n = 30): oxytocin (10 u/mL/amp) in 1000 ml normal saline and run at a rate of 120 mL/h. The administration of oxytocin was started after the anaesthesia was completed. Intravenous oxytocin was stopped immediately after the end of the surgery Control arm (n = 30): normal saline without oxytocin run at a rate of 120 mL/h All patients had bowel preparation the morning of surgery and intravenous cephalosporin prophylaxis was given just before surgery commenced Type of operation: laparoscopy |
|
| Outcomes | Number of fibroids removed, operation time, blood loss, hospital day (days), blood transfusion, and complications | |
| Notes | Authors contacted Fibroid size and number removed were similar in both groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated to either intervention or control group according to a computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Adequate (allocation using preprinted slips on sealed envelopes that had been prepared before the start of the study with a computer‐generated sequence) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the study protocol |
| Other bias | Low risk | No other sources of bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Normal saline without oxytocin was administered to control group as placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not given on blinding |
Zhao 2011.
| Methods | Single centre randomised controlled trial in which 35 participants received loop ligation combined with vasopressin, 35 participants received vasopressin alone and 35 participants received neither loop ligation nor vasopressin From January 2006 to January 2008 No power calculation performed |
|
| Participants | Setting: Sheng Jing Hospital, China Medical University Women with symptomatic myomas (diameter ≥ 6cm) needing surgical treatment Ethnicity not reported Age ranges from 22 to 49 years |
|
| Interventions | Group A: loop ligation of larger myoma pseudocapsules combined with vasopressin; group B: vasopressin injection; and group C: neither loop ligation nor vasopressin injection. Vasopressin injection consisted of 6U diluted with 20ml of normal saline and injected into the myometrium and myoma. All patients had laparoscopic myomectomy. Blood loss was measured by suction irrigator through subtracting the fluid used from the total measured loss | |
| Outcomes | The outcomes measured were blood loss, operating time, postoperative stay, blood transfusions, conversion to laparotomy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is stated that patients were randomly divided into three groups by ‘simple random method’. No details are given to explain the simple random method |
| Allocation concealment (selection bias) | Unclear risk | The randomisation was concealed from the surgeon until before the start of the operation. No further details are given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the study protocol |
| Other bias | Low risk | No other sources of bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The control group receive no treatment rather than an identical placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
Zullo 2004.
| Methods | Single centre study Allocation by envelopes containing computer‐generated random numbers Patients and care providers were blinded Power calculation: performed 4 (6.7%) patients (2 in each arm ) did not receive the assigned intervention because of concomitant disease (endometriosis) Withdrawals were excluded from the analysis | |
| Participants | Setting: university hospital in Naples, Italy Inclusion criteria: symptomatic fibroids Exclusion criteria: liver disease, angina or ischaemic heart disease, chronic obstructive pulmonary disease, dyslipidaemia, diabetes, acute or recent vascular thrombosis, malignancy, intramural largest myoma less than 3 cm, or more than 5 cm, more than 2 myomas, calcification or hypoechoic leiomyoma, submucosal fibroids, cytologic evidence of endometrial atypia, abnormal Pap smear, or positive urine pregnancy test n = 60 Mean age: 28.2 years (SD 3.1) for treatment arm and 27.1 years (SD 2.9) for control arm Ethnicity: not described Number of myomas: mean 1.3 (SD 0.4) in treatment arm and 1.2 (SD 0.3) in control arm | |
| Interventions | Treatment arm (n = 30): 50 ml of bupivacaine cloridrate 0.25% + 0.5 ml of epinephrine infiltrated into the serosa or myometrium overlying and just around the myoma before incision Control (n = 30): infiltration of normal saline Type of operation: laparoscopy | |
| Outcomes | Perioperative blood loss, and operation time Blood loss was estimated from the balance between the aspirated and the irrigated liquid | |
| Notes | Indications for myomectomy: history of infertility for more than 3 years, recurrent abortions during first trimester, increased vaginal bleeding, pelvic pressure and pain, urinary frequency, and constipation Author not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation by envelopes containing computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 (6.7%) patients (2 in each arm ) did not receive the assigned intervention because of concomitant disease (endometriosis) |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the study protocol |
| Other bias | Low risk | No other sources of bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients and care providers were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Shabibi 2010 | No randomisation was performed |
| Alborzi 2009 | No randomisation was performed |
| Alessandri 2006 | The control group did not receive a placebo or not treatment. The trial compared two different techniques |
| Alessandri 2010 | The control group did not receive a placebo or not treatment. The trial compared two different techniques |
| Chang 2009 | No randomisation was performed |
| Chang 2011 | No randomisation was performed |
| Chang 2012 | No randomisation was performed |
| Fletcher 1996 | The control group was not a placebo or no treatment group but rather another intervention |
| Frederick 2013 | The control group was not a placebo or no treatment |
| Ginsburg 1993 | The control group was not a placebo or no treatment group but rather another intervention |
| Kathiresan 2011 | The control group was not a placebo or no treatment group but rather another intervention |
| Kimura 2002 | The study compared blood loss when vertical and transverse incisions were performed during laparoscopic myomectomy. Thus there was no distinction between the treatment and control groups |
| Lee 2009 | No randomisation was performed |
| Lin 2008 | The control group was not a placebo or no treatment |
| Liu 2004 | No randomisation was performed |
| Morita 2004 | No randomisation was performed |
| Mousa 2012 | Control group received another intervention rather than a placebo or no treatment |
| Narin 2007 | Control group received another intervention rather than a placebo or no treatment |
| Ngeh 2004 | No randomisation was performed |
| Rossetti 1999 | No randomisation was performed |
| Sapmaz 2003B | The control group was not a placebo or no treatment group but rather another intervention |
| Sapmaz 2003C | The control group was not a placebo or no treatment group, but rather another intervention |
| Wang 2011 | The control group did not receive a placebo or not treatment. The trial compared two different techniques |
Characteristics of ongoing studies [ordered by study ID]
Atashkhoei 2012.
| Trial name or title | Effect of oxytocin infusion on reducing operative blood loss during abdominal myomectomy |
| Methods | Randomised placebo controlled clinical trial |
| Participants | Inclusion criteria: Including criteria: Women undergoing abdominal myomectomy; over 35 years of age Excluding criteria: Patients candidate for hysteroscopic myomectomy; Patients with preoperative GnRH agonist consumption; Patients with history of cardiopulmonary disease |
| Interventions | Intervention 1: In the study group (n=40) oxytocin 30 u into 1000 ml normal saline will be infused during myomectomy. Intervention 2: In the placebo group (n=40) normal saline alone 1000 ml will be infused during myomectomy |
| Outcomes | Primary Outcome(s): The amount of bleeding. Timepoint: During myomectomy. Method of measurement: Count sponges and the amount of blood collected in the suction device ‐ according to ml Secondary Outcome(s): Hb and HcT values. Timepoint: Prior to operation and 24 h after operation. Method of measurement: Elyza method‐CBC ‐ Hb(g/dl), HcT(%) The amount of blood transfusion. Timepoint: Intra and postoperative. Method of measurement: Observation, Physical examination‐ Blood unit according to ml |
| Starting date | November 2012 |
| Contact information | Dr Simin Atashkhoei, Al Zahra hospital, Artesh Jonoubi Ave, Tabriz, Iran |
| Notes |
Behdad 2013.
| Trial name or title | The effect of infusion of vitamin C during myomectomy on the blood loss during the procedure and postoperative pain |
| Methods | Randomised placebo controlled clinical trial |
| Participants | Inclusion criteria: women with American Society of Anesthesia (ASA) physical status I or II who are candidate for elective abdominal myomectomy, age 25‐40 years old, BMI <35 Exclusion criteria: patients with mental impairment, chronic pain, BMI>35, a history of coagulopathy, psychiatric disease, addiction were excluded |
| Interventions | Intervention 1: Infusion of 3 gr vitamin C in 500 ml normal saline during the surgery Intervention 2: Infusion of 500 ml normal saline without vitamin C during the surgery |
| Outcomes | Primary Outcome(s): uterine bleeding. Timepoint: during the surgery. Method of measurement: ml blood loss Secondary Outcome(s): postoperative pain. Timepoint: 1, 6, 12 and 24 hours after the operation. Method of measurement: VAS Score |
| Starting date | October 2012 |
| Contact information | Dr Shekoufeh Behdad, Shahid Sadoughi Hospital Yazd, Iran |
| Notes |
Differences between protocol and review
There is no significant difference between this review and the original protocol.
Five new interventions have been added to the review: ascorbic acid, dinoprostone, loop ligation of myoma pseudocapsule, temporary clipping of uterine artery, and fibrin sealant patch (surgical patch coated with fibronogen and thrombin to stop bleeding of internal organs).
A proposed subgroup analysis on the basis of ethnic background has been deleted.
Contributions of authors
EJK conceived the systematic review and conducted the literature search.
EJK and CSW developed the protocol, selected studies for inclusion in the review, extracted the data, performed statistical analysis and interpreted the data, and wrote the systematic review.
EJK is guarantor of the systematic review.
Sources of support
Internal sources
-
Stellenbosch University (CSW), South Africa.
Professor CS Wiysonge was employed by the Centre for Evidence‐based Health Care, Department of Interdisciplinary Health Sciences, Stellenbosch University, during the current update of the review.
Braun School of Public Health, Hebrew University (EJK), Israel.
University of Yaounde I (EJK), Cameroon.
Liverpool School of Tropical Medicine (EJK), UK.
External sources
Effective Care Research Unit, East London (EJK), South Africa.
-
Wellcome Trust (CSW), UK.
CS Wiysonge receives funding from the Wellcome Trust, through the Clinical Infectious Diseases Research Initiative, University of Cape Town, South Africa
Declarations of interest
None known
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Agostini 2005 {published data only}
- Agostini A, Ronda I, Franchi F, Bretelle F, Roger V, Cravello L, et al. Oxytocin during myomectomy: a randomized study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2005;118:235‐8. [DOI] [PubMed] [Google Scholar]
Assaf 1999 {published data only}
- Assaf A. Adhesions after laparoscopic myomectomy: effect of the technique used. Gynaecological Endoscopy 1999;8:225‐9. [Google Scholar]
Benassi 2000 {published data only}
- Benassi L, Lopopolo G, Pazzoni F, Ricci L, Kaihura C, Piazza F, et al. Chemically assisted dissection of tissues: an interesting support in abdominal myomectomy. Journal of the American College of Surgeons 2000;191:65‐9. [DOI] [PubMed] [Google Scholar]
Caglar 2008 {published data only}
- Caglar GS, Tasci Y, Kayikcioglu F, Haberal A. Intravenous tranexamic acid use in myomectomy: a prospective randomized double‐blind placebo controlled study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2008;137(2):227‐31. [DOI] [PubMed] [Google Scholar]
Celik 2003 {published data only}
- Celik H, Sapmaz E. Use of a single preoperative dose of misoprostol is efficacious for patients who undergo abdominal myomectomy. Fertility and Sterility 2003;79:1207‐10. [DOI] [PubMed] [Google Scholar]
Frederick 1994 {published data only}
- Frederick J, Fletcher H, Simeon D, Mullings A, Hardie M. Intramyometrial vasopressin as a haemostatic agent during myomectomy. British Journal of Obstetrics and Gynaecology 1994;101:435‐7. [DOI] [PubMed] [Google Scholar]
Ikechebelu 2010 {published data only}
- Ikechebelu JI, Ezeama CO, Obiechina NJ. The use of torniquet to reduce blood loss at myomectomy. Nigerian Journal of Clinical Practice 2010;13(2):154‐8. [PubMed] [Google Scholar]
Kalogiannidis 2011 {published data only}
- Kalogiannidis I, Xiromeritis P, Prapas N, Prapas Y. Intravaginal misoprostol reduces intraoperative blood loss in minimally invasive myomectomy: a randomized clinical trial. Clinical and Experimental Obstetrics and Gynecology 2011;38(1):46‐9. [PubMed] [Google Scholar]
Leone Maggiore 2011 {published data only}
- Maggiore Leone Roberti U, Alessandri F, Ferrero S. Tachosil application after laparoscopic myomectomy: A prospective randomized trial [Applicazione di Tachosil dopo miomectomia laparoscopica: studio prospettico randomizzato]. Italian Journal of Gynaecology and Obstetrics 2011;23(4):147‐54. [Google Scholar]
Pourmatroud 2012 {published data only}
- Pourmatroud E, Hormozi L, Masoud H, Golshahi R. Intravenous ascobi acid (vitamin C) administration in myomectomy: a prospective, randomized, clinical trial. Archives of Gynecology and Obstetrics 2012;285:111‐5. [DOI] [PubMed] [Google Scholar]
Raga 2009 {published data only}
- Raga F, Sanz‐Cortes M, Bonilla F, Casan EM, Bonilla‐Musoles F. Reducing blood loss at myomectomy with use of a gelatin‐thrombin matrix hemostatic sealant. Fertility and Sterility 2009;92(1):356‐60. [DOI] [PubMed] [Google Scholar]
Shokeir 2013 {published data only}
- Shokeir T, Shalaby H, Nabil H, Barakat R. Reducing blood loss at abdominal myomectomy with preoperative use of dinoprostone intravaginal suppository: a randomized placebo‐controlled pilot study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2013;166:61‐4. [DOI] [PubMed] [Google Scholar]
Sinha 2005 {published data only}
- Sinha R, Hedge A, Warty N, Mahajan C. Laparoscopic myomectomy: Enucleation of the myoma by morcellation while attached to the uterus. Journal of Minimally Invasive Gynecology 2005;12:284‐9. [DOI] [PubMed] [Google Scholar]
Taylor 2005 {published data only}
- Taylor A, Sharma M, Tsirkas P, Spiezio Sardo A, Setchell M, Magos A. Reducing blood loss at open myomectomy using triple tourniquets: a randomized controlled trial. International Journal of Obstetrics and Gynecology 2005;112:340‐5. [DOI] [PubMed] [Google Scholar]
Vercellino 2012 {published data only}
- Vercellino G, Erdemoglu E, Joe A, Hopfenmueller W, Holthaus B, Kohler C, Schneider A, Hasenbein K, Chiantera V. Laparoscopic temporary clipping of uterine artery during laparoscopic myomectomy. Arch Gynecol Obstet 2012;286(5):1181‐1186. [DOI] [PubMed] [Google Scholar]
Wang 2007 {published data only}
- Wang CJ, Lee CL, Yuen LT, Kay N, Han CM, Soong YK. Oxytocin infusion in laparoscopic myomectomy may decrease operative blood loss. Journal of Minimally Invasive Gynecology 2007;14(2):184‐8. [DOI] [PubMed] [Google Scholar]
Zhao 2011 {published data only}
- Zhao F, Jiao Y, Guo Z, Hou R, Wang M. Evaluation of loop ligation of larger myoma pseudocapsule combined with vasopressin on laparoscopic myomectomy. Fertility and Sterility 2011;95(2):762‐6. [DOI] [PubMed] [Google Scholar]
Zullo 2004 {published data only}
- Zullo F, Palomba S, Corea D, Pellicano M, Russo T, Falbo A, et al. Bupivacaine plus epinephrine for laparoscopic myomectomy: a randomized placebo‐controlled trial. Obstetrics and Gynecology 2004;104:243‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alborzi 2009 {published data only (unpublished sought but not used)}
- Alborzi S, Ghannadan E, Alborzi S, Alborzi M. A comparison of combined laparoscopic uterine artery ligation and myomectomy versus laparoscopic myomectomy in treatment of symptomatic myoma. Fertility and Sterility 2009;92(2):742‐7. [DOI] [PubMed] [Google Scholar]
Alessandri 2006 {published data only}
- Alessandri F, Lijoi D, Mistrangelo E, Ferrero S, Ragni N. Randomized study of laparoscopic versus minilaparotomic myomectomy for uterine myomas. Journal of Minimally Invasive Gynecology 2006;13(2):92‐7. [DOI] [PubMed] [Google Scholar]
Alessandri 2010 {published data only}
- Alessandri F, Remorgida V, Venturini PL, Ferrero S. Unidirectional barbed suture versus continuous suture with intracorporeal knots in laparoscopic myomectomy: a randomized study. Journal of Minimally Invasive Gynecology 2010;17(6):725‐9. [DOI] [PubMed] [Google Scholar]
Al‐Shabibi 2010 {published data only}
- Al‐Shabibi N, Korkontzelos I, Gkioulekas N, Stamatopoulos C, Tsibanakos I, Magos A. Cable ties as tourniquets at open myomectomy. International Journal of Gynecology and Obstetrics 2010;110(3):265‐6. [DOI] [PubMed] [Google Scholar]
Chang 2009 {published data only}
- Chang WC, Huang SC, Sheu BC, Shih JC, Hsu WC, Chen SY, Chang DY. Changes in uterine blood flow following laparoscopic myomectomy with or without uterine artery ligation on two‐ and three‐dimensional power Doppler ultrasound. Ultrasound in Obstetrics and Gynaecology 2009;33(2):221‐7. [DOI] [PubMed] [Google Scholar]
Chang 2011 {published data only}
- Chang WC, Chou LY, Chang DY, Huang PS, Huang SC, Chen SY, Sheu BC. Simultaneous laparoscopic uterine artery ligation and laparoscopic myomectomy for symptomatic uterine myomas with and without in situ morcellation. Human Reproduction 2011;26(7):1735‐40. [DOI] [PubMed] [Google Scholar]
Chang 2012 {published data only}
- Chang WC, Huang PS, Wang PH, Chang DY, Huang SC, Chen SY, et al. Comparison of laparoscopic myomectomy using in situ morcellation with and without uterine artery ligation for treatment of symptomatic myomas. Journal of Minimally Invasive Surgery 2012;19(6):715‐21. [DOI] [PubMed] [Google Scholar]
Fletcher 1996 {published data only}
- Fletcher H, Frederick J, Hardie M, Simeon D. A randomized comparison of vasopressin and tourniquet as hemostatic agents during myomectomy. Obstetrics and Gynecology 1996;87:1014‐8. [DOI] [PubMed] [Google Scholar]
Frederick 2013 {published data only}
- Frederick S, Frederick J, Fletcher H, Reid M, Hardie M, Gardner W. A trial comparing the use of rectal misoprostol plus perivascular vasopressin with perivascular vasopressin alone to decrease myometrial bleeding at the time of abdominal myomectomy. Fertility and Sterility 2013;100(4):1044‐9. [DOI] [PubMed] [Google Scholar]
Ginsburg 1993 {published data only}
- Ginsburg ES, Benson CB, Garfield JM, Gleason RE, Friedman AJ. The effect of operative technique and uterine size on blood loss during myomectomy: a prospective randomized study. Fertility and Sterility 1993;60:956‐62. [PubMed] [Google Scholar]
Kathiresan 2011 {published data only}
- Kathiresan AS, Brookfield KF, Gonzalez‐quintero VH, Verma U. Vasopressin versus a combination of vasopressin and tourniquets: a comparison of blood loss in patients undergoing abdominal myomectomies. Australian & New Zealand Journal of Obstetrics & Gynaecology 2011;51(1):79‐83. [DOI] [PubMed] [Google Scholar]
Kimura 2002 {published data only}
- Kimura T, Kusui, Matsumura Y, Ogita K, Isaka S, Nakajima A, et al. Effectiveness of hormonal tourniquet by vasopressin during myomectomy through vasopressin V1a receptor ubiquitously expressed in the myometrium. Gynecologic and Obstetric Investigation 2002;54:125‐31. [DOI] [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee KH, Park LH. Rapid and secure laparoscopic myomectomy: laparoscopic myomectomy with morcellation and suture by ultraminilaparotomy – a prospective study. Molecular Human Reproduction: 25th Annual Meeting of the European Society of Human Reproduction and Embryology, ESHRE Amsterdam Netherlands. Oxford: Oxford University Press, June 2009:i235.
Lin 2008 {published data only}
- Lin XN, Zhang SY, Fang SH, Wang MZ, Lou HY. Assessment of different homeostatic methods used in laparoscopic intramural myomectomy. Zhonghua Yi Xue Za Zhi 2008;88(13):905‐8. [PubMed] [Google Scholar]
Liu 2004 {published data only}
- Liu W, Tang W, Wang I, Tzeng C. Combined laparoscopic uterine artery occlusion and mini‐laparotomy in treating recurrent uterine myomas. 34th Global Congress on Minimally Invasive Gynecology. Chicago, USA, 9‐12 November 2005.
- Liu W, Tzeng C, Yi‐Jen C, Wang P. Combining the uterine depletion procedure and myomectomy may be useful for treating symptomatic fibroids. Fertility and Sterility 2004;82:205‐10. [DOI] [PubMed] [Google Scholar]
Morita 2004 {published data only}
- Morita M, Asakawa Y, Uchiide I, Nakakuma M, Kubo H. Surgery results using different uterine wall incision directions in laparoscopic myomectomy of the intramural myoma. Reproductive Medicine and Biology 2004;3:33‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mousa 2012 {published data only}
- Mousa SA, Yassen AM, Alhadary HS, Sadek EES, Abdel‐Hady E‐S. Hematological profile and transfusion requirement during hysteroscopic myomectomy: A comparative study between oxytocin and tranexamic acid infusion. Egyptian Journal of Anaesthesia 2012;28(2):125‐32. [Google Scholar]
Narin 2007 {published data only}
- Narin MA, Caliskan E, Kayikcioglu F, Haberal A, Meydanli MM. Blood loss and power Doppler ultrasound characteristics of uterine artery blood flow after 2 levels of bilateral internal iliac artery ligation before extensive myomectomies. The Journal of Reproductive Medicine 2007;52(8):696‐702. [PubMed] [Google Scholar]
Ngeh 2004 {published data only}
- Ngeh N, Belli A, Morgan R, Manyonda I. Pre‐myomectomy uterine embolization minimizes operative blood loss. British Journal of Obstetrics and Gynaecology 2004;111:1139‐40. [DOI] [PubMed] [Google Scholar]
Rossetti 1999 {published data only}
- Rossetti A, Paccosi M, Sizzi O, Zulli S, Mancuso S, Lanzone A. Dilute ornitin vasopressin and a myoma drill for laparoscopic myomectomy. The Journal of the American Association of Gynecologic Laparoscopists 1999;6:189‐93. [DOI] [PubMed] [Google Scholar]
Sapmaz 2003B {published data only}
- Sapmaz E, Celik H, Altungil A. Bilateral ascending uterine artery ligation vs tourniquet use for hemostasis in cesarian myomectomy. A comparison. The Journal of Reproductive Medicine 2003;48:950‐4. [PubMed] [Google Scholar]
Sapmaz 2003C {published data only}
- Sapmaz E, Celik H. Comparison of the effects of the ligation of ascending branches of bilateral arteria uteria with tourniquet method on the intra‐operative and post‐operative hemorrhage in abdominal myomectomy cases. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2003;111:74‐7. [DOI] [PubMed] [Google Scholar]
Wang 2011 {published data only}
- Wang JJ, Yang F, Gao T, Li L, Xia H, Li HF. Gasless laparoscopy versus conventional laparoscopy in uterine myomectomy: a single‐centre randomized trial. The Journal of International Medical Research 2011;39:172‐8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Atashkhoei 2012 {unpublished data only}
Behdad 2013 {unpublished data only}
- Behdad S. The effect of infusion of vitamin C during myomectomy on the blood loss during the procedure and postoperative pain. WHO International Trials Registry Platform 2013.
Additional references
Aubuchon 2002
- Aubuchon M, Pinto AB, Williams DB. Treatment of uterine fibroids. Primary Care Update for Ob/Gyns 2002;9:231‐7. [Google Scholar]
Baldoni 1995
- Baldoni A, Moscioni P, Colonnelli M, Affronti G, Garardi G. The possibility of using sulprostone during laparoscopic myomectomy in uterine fibromyomatosis. Preminary studies. Minerva Ginecologica 1995;47:341‐6. [PubMed] [Google Scholar]
Burkert 1983
- Burkert H. Clinical overview of mesna. Cancer Treatment Review 1983;10 Suppl A:175‐87. [DOI] [PubMed] [Google Scholar]
Cooper 2000
- Cooper JM, Brady RM. Intraoperative and early postoperative complications of operative hysteroscopy. Obstetrics and Gynecology Clinics of North America 2000;27:347‐66. [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Practical aspects in monitoring: a brief review. Statistics in Medicine 1987;6:753‐60. [DOI] [PubMed] [Google Scholar]
Denaro 2001
- Denaro V, Forriol F, Martino A, Denaro L, Papalia R, Caione G. Effect of a mucolytic agent on collagen fibres. An optical and polarized light histology study. European Journal of Orthopaedic Surgery & Traumatology 2001;11:209‐12. [Google Scholar]
Derman 1991
- Derman G, Rehnstrm J, Neuwirth S. The long‐term effectiveness of hysteroscopic treatment of menorrhagia and leiomyomas. Obstetrics and Gynecology 1991;77:591‐4. [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Dimitrov 1999
- Dimitrov A. The use of the vasoconstrictor hemostatic Remestyp in surgical obstetrics. Akusherstvo i Ginekologiia (Sofia) 1999;38:58‐60. [PubMed] [Google Scholar]
Dubuisson 1997
- Dubuisson JB, Chapron C, Fauconnier A, Kreiker G. Laparoscopic myomectomy and myolysys. Current Opinion in Obstetrics & Gynecology 1997;9:233‐8. [PubMed] [Google Scholar]
Dunn 1999
- Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs 1999;57(6):1005‐32. [DOI] [PubMed] [Google Scholar]
Farquhar 2002
- Farquhar CM, Brown PM, Furness S. Cost‐effectiveness of preoperative gonadotrophin releasing analogues for women with uterine fibroids undergoing hysterectomy or myomectomy. British Journal of Obstetrics and Gynaecology 2002;109:1273‐80. [DOI] [PubMed] [Google Scholar]
Fedele 1995
- Fedele L, Parazzini F, Luchini L, Mezzopane R, Tozzi L, Villa L. Recurrence of fibroids after myomectomy: a transvaginal ultrasonographic study. Human Reproduction 1995;10:1795‐6. [DOI] [PubMed] [Google Scholar]
Frederick 2002
- Frederick J, Hardie M, Reid M, Fletcher H, Wynter S, Frederick C. Operative morbidity and reproductive outcomes in secondary myomectomy: a prospective cohort. Human Reproduction 2002;17:2967‐71. [DOI] [PubMed] [Google Scholar]
Fuchs 1984
- Fuchs AR, Fuchs F, Husslein P, Soloff MS. Oxytocin receptors in the human uterus during pregnancy and parturition. American Journal of Obstetrics and Gynecology 1984;150:734‐41. [DOI] [PubMed] [Google Scholar]
Garnet 1964
- Garnet JD. Uterine rupture during pregnancy. Obstetrics and Gynecology 1964;23:898‐905. [PubMed] [Google Scholar]
Hasson 1992
- Hasson HM, Rotman C, Rana N. Laparoscopic myomectomy. Obstetrics and Gynecology 1992;80:884‐8. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. http://www.cochrane.org/training/cochrane‐handbook/ (accessed 30th March 2011).
Holub 2003
- Hulob Z, Lukac J, Kliment L, Urbanet S. Short‐term results from laparoscopic dissection of uterine vessels in women with symptomatic fibroids. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2003;110(1):94‐8. [DOI] [PubMed] [Google Scholar]
Hurst 2005
- Hurst BS, Mathews ML, Marshburn PB. Laparoscopic myomectomy for symptomatic uterine myomas. Fertility and Sterility 2005;83(1):1‐23. [DOI] [PubMed] [Google Scholar]
Hutchins 1996
- Hutchins FL Jr. A randomized comparison of vasopressin and tourniquet as hemostatic agents during myomectomy. Obstetrics and Gynecology 1996;88:639‐40. [PubMed] [Google Scholar]
Iverson 1996
- Iverson RE Jr, Chelmow D, Strohbehn K, Waldman L, Evanstash EG. Relative morbidity of abdominal hysterectomy and myomectomy for the management of uterine leiomyomas. Obstetrics and Gynecology 1996;88:415‐9. [DOI] [PubMed] [Google Scholar]
Iverson 1999
- Iverson RE Jr, Chelmow D, Strohbehn K. Myomectomy fever: testing the dogma. Fertility and Sterility 1999;72:104‐8. [DOI] [PubMed] [Google Scholar]
Jacoby 2010
- Jacoby VL, Fujimoto VY, Giudice LC, Kuppermann M, Washington E. Racial and ethnic disparities in benign gynecologic conditions and associated surgeries. American Journal of Obstetrics and Gynecology 2010;202(6):514‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koh 2003
- Koh MBC, Hunt BJ. The management of perioperative bleeding. Blood Reviews 2003;17:179‐85. [DOI] [PubMed] [Google Scholar]
LaMote 1993
- LaMote AI, Lalwani S, Diamond MP. Morbidity associated with abdominal myomectomy. Obstetrics and Gynecology 1993;82:897‐900. [PubMed] [Google Scholar]
Lethaby 2001
- Lethaby A, Vollenhoven B, Sowter M. Pre‐operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD000547] [DOI] [PubMed] [Google Scholar]
Liu 2001
- Liu WM, Ng HT, Wu YC, Yen YK, Yuan CC. Laparoscopic bipolar coagulation of uterine vessels: a new method for treating symptomatic fibroids. Fertility and Sterility 2001;75:417‐22. [DOI] [PubMed] [Google Scholar]
Lumsden 2002
- Lumsden MA. Embolization versus myomectomy versus hysterectomy: which is best, when?. Human Reproduction 2002;17:253‐9. [DOI] [PubMed] [Google Scholar]
McLaughin 1985
- McLaughin DS. Metroplasty and myomectomy with CO2 laser for maximizing the preservation of normal tissue and minimizing blood loss. The Journal of Reproductive Medicine 1985;30:1‐9. [PubMed] [Google Scholar]
Nishiyama 2006
- Nishiyama S, Saito M, Sato K, Kurishito M, Itasaka T, Shioda K. High recurrence rate of uterine fibroids on transvaginal ultrasound after abdominal myomectomy in Japanese women. Gynecologic and Obstetric Investigation 2006;61:155‐9. [DOI] [PubMed] [Google Scholar]
Ravina 1995
- Ravina JH, Herbreteau D, Ciraru‐Vigneron N, et al. Arterial embolisation to treat uterine myomata. Lancet 1995;346:671‐72. [DOI] [PubMed] [Google Scholar]
Somigliana 2008
- Somigliana E, Vercellini P, Benaglia L, Abbiati A, Barbara G, Fedele L. The role of myomectomy in fertility enhancement. Current Opinion in Obstetrics & Gynecology 2008;20(4):379‐85. [DOI] [PubMed] [Google Scholar]
Tulandi 1993
- Tulandi T, Murray C, Gualnick M. Adhesion formation and reproductive outcome after myomectomy and second‐look laparoscopy. Obstetrics and Gynecology 1993;82:213‐5. [PubMed] [Google Scholar]
Vercellini 1993
- Vercellini P, Vendola N, Ragni G, Trespidi L, Oldani S, Crosignani PG. Abnormal uterine bleeding associated with iron‐deficiency anaemia: Etiology and role of hysteroscopy. The Journal of Reproductive Medicine 1993;38:502‐4. [PubMed] [Google Scholar]
Vollenhoven 1990
- Vollenhoven BJ, Lawrence AS, Healey DL. Uterine fibroids. A clinical review. British Journal of Obstetrics and Gynaecology 1990;97:285‐98. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kongnyuy 2007
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005355.pub3] [DOI] [PubMed] [Google Scholar]
Kongnyuy 2008
- Kongnyuy EJ, Broek N, Wiysonge CS. A systematic review of randomised controlled trials to reduce hemorrhage during myomectomy for uterine fibroids. International Journal of Gynaecology and Obstetrics 2008;100(1):4‐9. [DOI] [PubMed] [Google Scholar]
Kongnyuy 2009
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD005355.pub3] [DOI] [PubMed] [Google Scholar]
Kongnyuy 2011
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD005355.pub4] [DOI] [Google Scholar]


