Abstract
Background
There are a number of studies that suggest a relationship between decline of melatonin function and the symptoms of dementia.
Objectives
The review assessed the evidence of clinical effectiveness of melatonin in the treatment of symptoms of dementia. Relevant primary outcomes were cognition, mood, behaviour, functions of daily living, and safety of melatonin use and secondary outcomes were quality of life, morbidity, mortality and length of time to institutionalization and caregiver stress.
Search methods
The Specialized Register of the Cochrane Dementia and Cognitive Improvement Group (CDCIG), The Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS were searched on 29 June 2009 using the terms: MELATONIN and N‐ACETYL‐5‐METHOXYTRYPTAMINE. The CDCIG Specialized Register contains records from all major health care databases (The Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL, LILACS) as well as from many trials databases and grey literature sources.
The search of June 2009 retrieved several studies for consideration by the authors.
Selection criteria
All relevant, randomized controlled trials in which orally administered melatonin in any dosage was compared with a control group for the effect on managing cognitive, behavioral (excluding sleep), and mood disturbances of people with dementia of any degree of severity.
Data collection and analysis
Two to three reviewers independently assessed the retrieved articles for relevance and risk of bias, and extracted data from the selected studies. Statistically significant differences in end‐points or changes in outcomes from baseline to end of treatment between the melatonin and control groups were examined. Each study was summarized using a measure of effect (e.g. mean difference) and meta‐analyses were conducted when appropriate.
Main results
Five studies met the inclusion criteria. The pooled estimates of MMSE cognitive and ADAS‐cognitive change scores from three of these studies revealed non‐significant cognitive effects for melatonin treatment. In two of these studies, significant improvements in psychopathological behaviours (e.g., decreased mood symptoms of depression, anxiety and apathy and decreased behavior symptoms of hallucinations, delusions, agitation, irritability, and appetite disturbances), were found from meta‐analysis of the change scores from the NPI (7 weeks, 2.5 mg melatonin), and ADAS non‐cognitive (4 weeks, 3 mg melatonin) scales. Sensitivity analyses found similar results to those of the original meta‐analyses, and thus, supported the effect estimates for non‐significant cognitive outcomes. Individual study estimates for treatment effect of 2.5 mg melatonin at one year demonstrated a significant worsening of mood (e.g. decrease in positive affect) as measured by the Philadelphia Geriatric Centre Affect Rating Scale (positive). The remainder of the treatment effects for mood, behavior, and function of daily living were non‐significant. There were no reported adverse effects associated with melatonin use.
Authors' conclusions
The analyses did not support the use of melatonin for treatment of cognitive impairment associated with dementia. Meta‐analysis of psychopathologic behavior scale scores suggested that melatonin may be effective in treating these dementia‐related disturbances.
Plain language summary
Melatonin treatment may be effective for the treatment of dementia‐related behavior disturbances
There are a number of studies that suggest a relationship between decline of melatonin function and the symptoms of dementia. Meta‐analysis was conducted on data from three randomised, placebo controlled trials that were designed to evaluate melatonin for managing dementia‐related cognitive changes; data also were pooled from two of these trials that evaluated melatonin for managing mood and behavioral disturbances. Significantly improved outcomes were found from the meta‐analysis of psychopathologic behavior and mood scale scores. Melatonin treatment may be effective for the treatment of dementia‐related psychopathologic behavior disturbances. No evidence was found to support the effectiveness of melatonin for the treatment of cognitive impairment.
Background
Melatonin, a naturally‐occurring hormone secreted by the pineal gland in the centre of the brain, was discovered by Lerner and colleagues at Yale University School of Medicine in 1958 (Wurtman 1989). It is biosynthesized from tryptophan via serotonin. It has a number of effects relating to a variety of bodily functions. These include circadian rhythmicity (physiological sleep onset and sleep‐wake cycles) and cyclic hormone release (Webb 1995); regulation of the immune system (Maestroni 1993); and more recently discovered anti‐oxidant properties (Reiter 1995). In addition to the brain, there are also melatonin receptors on cells of blood vessels, ovaries and digestive system, though little is currently known about their functions.
Since melatonin is a naturally occurring substance, it is not considered a drug in most countries. However, the safety of melatonin products has not been definitely determined. Melatonin products are regulated differently in several countries. In the United States, melatonin falls under the Food and Drug Administration's Dietary Supplement Health and Education Act in the category of "other dietary supplements" and is "generally recognized as safe". In Canada, melatonin is included in the Natural Health Products Directorate of Health Canada. Melatonin is available for sale in Canada, having met the specific licensing, manufacturing, labelling, and safety standards. In the European Union, melatonin is considered a medicine or hormone and is available only by prescription. In Australia, melatonin is an unregistered product under the Therapeutic Goods administration. However, with a prescription, it can be imported for use under the Personal Import Scheme (Buscemi 2004). It should be noted that in situations where manufacture and sale of melatonin is not regulated as a drug, preparations may contain additives that have their own pharmacological actions and potential side effects (e.g. some health food store melatonin preparations are said to contain the same impurity which causes eosinophilia‐myalgia syndrome when found in tryptophan preparations) (Williamson 1998).
Dementia is an acquired, persistent global impairment of intellectual function. There are various diagnostic criteria based on demonstration of acquired defects in more than one domain of cognitive function, for example: language, memory, visuo‐spatial skills, emotion or personality, abstraction, calculation, judgment or executive function (McKhann 1984). It is a common affliction, affecting some 8% of adults aged over 65 years, rising to 35% of those older than age 85 years (CSHA 1994). Research findings have supported the use of drugs to decrease symptoms of dementia‐related depression (Thompson 2007). In addition, exercise and behavior management techniques have been associated with 'improved physical health' and a decrease in symptoms of depression in those with Alzheimer's Disease (Teri 2003). However, to date, there have been few reports of such a relationship between melatonin and cognition, mood and behavior in persons with dementia or the effectiveness of melatonin treatment for dementia‐related depression.
There are a number of factors suggesting a relationship between decline of melatonin function and the neuropathology of dementia (Wu 2003). These include:
Decline of serum melatonin levels (Ferrari 2000; Mishima 1994) (to an even greater extent than in normal aging) and the breakdown of normal circadian rhythmicity (Auger 2007; Ghali 1995; Hopkins 1992) in persons with dementia. The relationship between melatonin and circadian rhythmicity is well‐established. The suprachiasmatic nuclei (SCN) of the brain are generally accepted as the "seat" of the circadian clock in humans (Moore 1992; Swaab 1985; Yesavage 2003). Entrainment of the SCN (i.e. "setting" of the biological clock) is, in large part, due to rhythmic release of melatonin from the pineal gland (Dubocovich 1991; Wu 2007).
Disruption in sleep patterns in persons with dementia (Prinz 1982; Wu 2005), the relationship between melatonin and sleep patterns (Serfaty 2002; Webb 1995), and the relationship between sleep and cognitive function i.e. disrupted or insufficient sleep can contribute to significant difficulties with tasks requiring mental concentration and memory function (Bonnani 2005; Downey 1987). This effect is thought to be even more pronounced in people with pre‐ or co‐existing causes of cognitive impairment (Hopkins 1995).
Correlation between typical areas of cerebral atrophy in certain dementias (e.g. temporal lobes in Alzheimer's disease [AD]), and those areas containing melatonin receptors (Dubocovich 1991; Fauteck 1995).
Decrease of melatonin production associated with increasing calcification of the pineal gland in persons with dementia (Kunz 1999).
Antioxidant and antiamyloidogenic properties of melatonin (Pierrefiche 1995; Reiter 1994); and the known involvement of oxidative and amyloid‐mediated brain damage in the pathogenesis of AD (Varadarajan 2000).
Breakdown in normal function of melatonin‐related brain functions also may play a significant role in caregivers' ability to care for an individual with dementia. Specifically, problematic sleep‐related behaviours often precipitate the decision of families to institutionalize an elderly relative with dementia (Coffey 1994; Yesavage 2003).
Generally, few adverse effects have been reported in human trials in recent years (Andrade 2001; Buscemi 2006; Seabra 2000; Shamir 2000). However, because of the many organ systems containing melatonin receptors, adverse effects could be far‐reaching. Furthermore, a number of studies and animal data suggest a variety of possible side effects including:
Worsening of depression (disruption of normal circadian rhythm if not "timed with light therapy and sleep‐phase changes") leads to sleep disturbance, weight loss and an oral temperature decrease in those with depression (Carman 1976); also supported by a finding in depressed individuals, but not in controls, of a longer duration of the nocturnal period of active melatonin secretion in winter than in summer (Wehr 2001). Furthermore, because evening melatonin should produce a circadian phase advance, it may worsen early morning awakening. However, recent evidence supports the use of melatonin to improve sleep in persons with depression and the use of melatonergic receptor agonists to treat depression and seasonal affective disorder (Srinivasan 2009). Melatonin use for sleep disorders is not associated with symptoms of addiction or withdrawal, although the short half life of melatonin may be associated with equivocal sleep effects (Hardeland 2008). Evidence also supports melatonin treatment for depression (Detanico 2009).
Exogenous melatonin (or its withdrawal) may trigger or worsen manic episodes in susceptible individuals (Leibenluft 1997), although it has also been found to improve sleep and decrease severity of manic symptoms associated with treatment‐resistant insomnia (Bersani 2000; Robertson 1997).
High doses of melatonin may increase ototoxicity (Erdem 2005) and suppress insulin (Rasmussen 1999) although a lack of effect on insulin has also been found (Bizot‐Espiard 1998).
Exogenous melatonin may reduce glucose tolerance and insulin sensitivity in post‐menopausal women (Cagnacci 2001). However, recent evidence supports the use of melatonin to treat non‐insulin dependent diabetes mellitus in older adults (Kedziora‐Kornatowska 2009).
Melatonin has been found to increase retinal susceptibility to light‐induced damage (Leino 1984; Wiechmann 1992) but also to protect the retina from oxidative damage (Siu 1999). Improved opthalmic surgical outcomes have been achieved with melatonin to enhance anxiolytic and analgesic effects and to decrease intraocular pressure (Ismail 2009).
Melatonin has been reported to have both vasoconstricting (Mahle 1997; Viswanathan 1997) and vasorelaxing properties (Cagnacci 2001a; Weekley 1995): it can lower blood pressure (Chuang 1993; Tom 2001) and decrease heart rates in young adults (Yildiz 2009). In animals, melatonin can constrict cerebral and coronary arteries and reduce cerebral blood flow (Capsoni 1995). The arterial effect might account for several reports that melatonin causes headache, although it has also been reported to relieve headache (especially migraine) (Claustrat 1997; Gagnier 2001). Vasoconstriction could also, theoretically, compromise cerebral circulation in older people with atherosclerosis. However, another study suggests melatonin may diminish the risk of hypoperfusion‐induced cerebral ischaemia by shifting the lower limit of cerebral blood flow autoregulation to a lower pressure level, improving the cerebrovascular dilatatory reserve, and thus widening the security margin (Regrigny 1998). Melatonin also may be effective for use as an anti‐convulsant (Guo 2009; Munoz‐Hoyos 1998).
Melatonin appears to enhance immune function (Maestroni 1993; Reiter 2000) but may worsen such autoimmune conditions as arthritis (Maestroni 2001).
Little attention has been given to the safe and efficacious use of melatonin in populations who are diagnosed with dementia (Riemersma‐van der Lek 2008), although the adverse effects of melatonin have been investigated in relation to older adult diabetic population outcomes and cerebral vascular, opthalmic, and anticonvulsant use.
Researchers have recommended more RCTs to investigate the effectiveness of melatonin treatment (Mills 2005).
Objectives
The objective was a systematic review of evidence relating to the clinical effectiveness of melatonin in the treatment of manifestations of dementia. Relevant primary outcomes included in a prospective analyses plan were cognition, mood, behaviour, functions of daily living, and safety of melatonin use and secondary outcomes were quality of life, morbidity, mortality, length of time to institutionalization, and caregiver stress.
Methods
Criteria for considering studies for this review
Types of studies
The review included all relevant, randomized controlled trials, published or unpublished, in which individuals, the facility or residential site was randomly assigned, participant selection and treatment allocation were concealed and group assignment and assessment of outcomes were blind. The period of treatment exceeded one day. Studies were included irrespective of the language in which they were reported.
The first treatment period of crossover studies was included when data were provided. Since the conditions under evaluation may continue after withdrawal of the treatment, in order to avoid carry‐over effects, data from subsequent phases were excluded (Elbourne 2002).
Types of participants
Included studies involved persons with dementia of any severity or type of dementia. The diagnosis of dementia was based on accepted criteria such as ICD, DSM (APA 1995) and NINCDS‐ADRDA (National Institute of Neurological and Communicative Disorders and Stroke ‐ Alzheimer's Disease and Related Disorders Association (McKhann 1984). One study (Riemersma‐van der Lek 2008) included a small number of participants who did not have a diagnosis of dementia.
Types of interventions
Included trials assessed the effect of orally administered melatonin in any dosage compared with placebo or no treatment, for a minimum of one day, and with a minimum of 24 hour follow‐up.
Types of outcome measures
Relevant outcomes were cognition, mood, behavior, and function in activities of daily living.
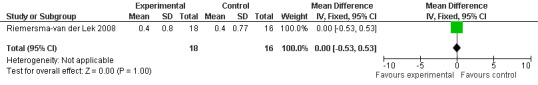
Any trial with acceptable (i.e. objective, reproducible) measures of the above was included. Sleep was not included as it is covered by a different review (Pharmacotherapies for sleep disorders in Alzheimer's disease) which is in development. The secondary outcomes of quality of life, caregiver stress, morbidity, mortality and length of time to institutionalization were not analyzed as these outcomes were not investigated in the relevant studies.
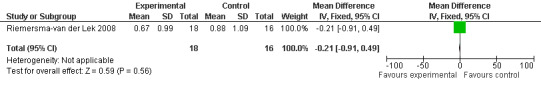
Adverse event data were collected in two studies (Riemersma‐van der Lek 2008 and Singer 2005). Meta‐analyses were not possible as data were derived from the measurement of different constructs. Single study estimates were calculated using the Riemersma‐van der Lek 2008 and Singer 2005 data.
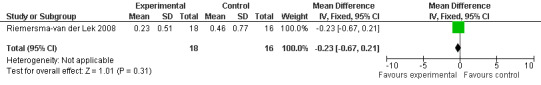
Search methods for identification of studies
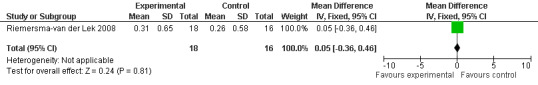
Electronic searches
We searched the Cochrane Dementia and Cognitive Improvement Group’s Specialized Register on 29 June 2009. The search terms used were: MELATONIN and N‐ACETYL‐5‐METHOXYTRYPTAMINE
The Cochrane Dementia and Cognitive Improvement Group’s Specialized Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
Monthly searches of a number of major healthcare databases: Medline, Embase, Cinahl, Psycinfo and Lilacs
Monthly searches of a number of trial registers: meta Register of Controlled Trials; IFPMA; Umin Japan Trial Register; WHO portal (which covers ClinicalTrials.gov; ISRCTN; Chinese Clinical trials Register; German Clinical trials register; Iranian Regsitry of Clinical trials and the Netherlands National Trials Regsiter, plus others)
Quarterly search of The Cochrane Library’s Central register of Controlled trials (CENTRAL)
Monthly searches of a number of grey literature sources: ISI Web of knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL and conference proceedings can be viewed in the ‘methods used in reviews’ section within the editorial information about the Cochrane Dementia and Cognitive Improvement Group.
The trials search co‐ordinator also ran additional searches in each of the sources listed above to cover the timeframe from the last searches performed for the Specialized register to 29 June 2009 to ensure that the search for the review was as up‐to‐date as possible. The search strategies used can be seen in Appendix 1.
Searching other resources
Reference lists of retrieved articles (especially literature reviews) were examined for additional trials and proceedings of relevant conferences were searched.
Data collection and analysis
Selection of trials
Titles and abstracts of citations obtained from the search were examined by three reviewers (LJ, VD and DF) and obviously irrelevant articles discarded. Articles were retrieved if there was a possibility of inclusion of a relevant randomized controlled trial.
Two authors (LJ, VD) independently assessed retrieved articles for inclusion in the review according to the criteria above. Disagreements were resolved by discussion, or if necessary referred to a third author (DF).
Assessment of methodology and quality
The trial design and risk of bias were assessed by two reviewers based on The Cochrane Handbook for Systematic Reviews of Interventions,version 5.0.1 (Higgins 2008). Appropriate randomization and blind assessment of outcomes were threshold risk of bias criteria for inclusion in the review. In addition, whether individuals or residential site was randomly assigned, whether participants were blind to their treatment allocation, and whether drop‐out was judged to be serious enough to be a potential source of bias were assessed for use in sensitivity analyses.
Concealment of allocation to treatment was rated by the following three categories:
Category A (adequate) where the report or further clarification from the original author described allocation of treatment by: (i) some form of centralized randomized scheme; e.g., randomization scheme controlled by a pharmacy; (ii) numbered or coded containers, e.g. in a pharmaceutical trial in which capsules from identical‐looking numbered bottles are administrated sequentially to enrolled participants; (iii) an on‐site or coded computer system, given that the allocations were in a locked, unreadable file that could be accessed only after inputting the characteristics of an enrolled participant; or (iv) if assignment envelopes were used, the report should at least specify that they were sequentially numbered, sealed, opaque envelopes; (v) other combinations of described elements of the process that provided assurance of adequate concealment.
Category B (intermediate) where the report or further clarification from the original author described allocation of treatment by: (i) use of a "list" or "table" to allocate assignments; (ii) use of "envelopes" or "sealed envelopes"; (iii) stating the study was "randomized" without further detail.
Category C (inadequate) where the report or further clarification from the original author described allocation of treatment by: (i) alternation; (ii) reference to case record numbers, dates of birth, day of the week, or any other such approach; (iii) any allocation procedure that was transparent before assignment, such as an open list of random numbers or assignments.
Trials were included if they conformed to categories A or B; those falling into category C were excluded.
Data extraction
Data were extracted from published reports or requested from the corresponding author when necessary. Summary statistics were required for each trial and each outcome. For continuous data, the mean change from baseline, the standard deviation of the mean change, and the number of participants for each treatment group at each assessment were extracted. Where changes in means and standard deviations from baseline to end point were not reported, the mean, standard deviation, and the number of participants for each treatment group at each end point were extracted if available.
The baseline assessment was defined as the latest available assessment prior to randomization, but no longer than two months prior.
In studies where a cross‐over design was used, only data from the first treatment phase after randomization were eligible for inclusion.
Data analysis
Continuous data were reported in all trials, therefore, these data were analysed as continuous outcomes arising from a normal distribution.
Summary statistics (sample size, mean and standard deviation) were required for each rating scale at each assessment time for each group in each trial for change from baseline. When change from baseline standard deviations were not reported, then only the end point results were used.
Meta‐analysis requires the combination of data from the trials. Inverse variance was used as the method of analysis. This method weights studies inversely according to the extent of the study's contribution to the pooled estimate of treatment effect. For example, larger weights are assigned to change scores with smaller standard deviations. The treatment difference for any outcome was the weighted mean difference when the pooled trials used the same rating scale or test to assess an outcome, and the standardised mean difference, which is the absolute mean difference divided by the standard deviation, when they used different rating scales or tests. However, meta‐analysis was not used to combine scores from different scales when the measures were derived from final endpoint and change scores (Higgins 2008).
Due to insufficient data, the following subgroup analyses were not undertaken:
Disease type:
‐ Alzheimer's disease ‐ vascular dementia ‐ mixed Alzheimer's disease and vascular dementia ‐ unclassified or other dementia ‐ cognitive impairment
Duration of treatment:
‐ < 12 weeks ‐ > = 12 weeks
Severity of dementia at baseline:
‐ mild (MMSE > 17 or similar) ‐ moderate (MMSE 10 to 17 or similar) ‐ severe (MMSE < 10 or similar)
Sensitivity analyses were performed with regard to random assignment of facilities or residential site to treatment arm:
Random assignment of facilities or residential site to treatment arm was only conducted in the Riemersma‐van der Lek 2008 study. Therefore, sensitivity analysis was undertaken by removing the Riemersma‐van der Lek 2008 6 week cognitive measurement data from the pooled estimates of the Asayama 2003, Singer 2003, and Riemersma‐van der Lek 2008 studies.
Sensitivity analyses were not conducted for blinding, participant drop‐out or imputations of missing dichotomous data.
Sensitivity analyses were not conducted for blinding, participant drop‐out or imputations of missing dichotomous data, as the three studies included in the meta‐analysis maintained double‐blind procedures and participant drop‐out was not judged to be serious enough to be a potential source of bias (see Risk of Bias Tables). As well, missing data was not imputed in the Asayama 2003 and Singer 2005 studies.
Results
Description of studies
Five randomized controlled double blind trials met the inclusion criteria (Asayama 2003; Gehrman 2009; Riemersma‐van der Lek 2008; Serfaty 2002; Singer 2003). Eleven articles were excluded: four of these studies measured sleep outcomes only (Baskett 2003; Singer 2005; Tozawa 1998; Valontonin 2005), three did not include those with dementia (Bourne 2006; Furio 2007; Peck 2004), one article was a literature review (Savaskan 2006), and three studies were unable to separate effects of combined interventions of bright light therapy and melatonin (Dowling 2008; Haffmans 2001; Riemersma‐van der Lek 2005). Three of the five included trials were excluded from the meta‐analyses of psychopathological behavior and function outcomes as the standard deviations of the change scores were not available for each time of measurement reported in the studies (Gehrman 2009; Serfaty 2002) and final endpoint data measures Riemersma‐van der Lek 2005 could not be combined with change scores that were derived from different scales Higgins 2008. Second phase crossover data from Serfaty 2002 also were excluded in accordance with the review criteria. However, cognitive outcome data, obtained from the MMSE, was pooled from three studies (Asayama 2003; Riemersma‐van der Lek 2008; Singer 2003). The first study included in the meta‐analyses was conducted in Tokyo, Japan and appeared in the literature as a translated article in English (Asayama 2003). The second study was conducted in the United States (Singer 2003). Since the publication of the original review, data from one additional study has been included in the meta‐analyses (Riemersma‐van der Lek 2008). Data from the Riemersma‐van der Lek 2008 study represented six week, one year, and two year follow up points, and includes two out of four treatment and control arms studied for the effects of bright light and melatonin on the cognitive and non‐cognitive symptoms of dementia. The study arms relevant to this review were the groups that received melatonin and a double placebo. The data pertaining to the groups that received bright light and bright light plus melatonin have not been included. The majority of the participants of the five included studies were residents of a long term care facility, nursing home or the geriatric ward of a hospital (Asayama 2003; Gehrman 2009; Riemersma‐van der Lek 2008; Serfaty 2002; Singer 2005), while five were being cared for at home (Singer 2003). Consent in all of the studies was provided by the participant's caregiver or guardian. Four of the studies also obtained the consent of the participant when possible (Asayama 2003; Gehrman 2009; Riemersma‐van der Lek 2008; Serfaty 2002). The total number of participants who were enrolled in the five studies was 334 and 323 completed the protocol (See Table 1for Description of Methodological Quality of Included Studies and indivdual Risk of Bias tables for each included study).
1. Description of Methodological Quality of Included Studies.
| Control Confounders | Attrition/Compliance |
| Asayama 2003 Randomized concealed allocation. Medications stabilized, one study setting, no severe physical diseases and no disorders that could cause sleep disorders other than AD. Baseline level of cognitive impairment for placebo and intervention group in moderate range of cognitive impairment according to MMSE scores. Training was provided to those who were providing the intervention and collecting data. | 100% compliance with administration of MMSE, ADAS‐cog and ADAS non‐cog scales. |
| Serfaty 2002 Randomized concealed allocation. Medications stabilized, informal caregivers received training for intervention in two settings, training also provided for administration of Mini Mental State Examination. Study exclusion criteria reported. | 85% compliance with administration of MMSE. |
| Singer 2003 Randomized concealed allocation by blocked design. None of the potential covariates were significantly different between the groups at baseline (age, duration of AD, sex, dementia severity, and years of education). Training provided to those administering intervention and assessment scales in long term care and private home settings. Medications stabilized. Study exclusion criteria reported. | 96% compliance with administration of MMSE, ASAS‐cog, NPI, ADL, NPI |
| Riemersma‐van der Lek 2008 Randomized concealed allocation for group assignment and participant treatment with melatonin. Randomization was balanced in that none of the individual or environmental characteristics, use of medication, or pretreatment outcome variable levels differed significantly between groups. Baseline assessment and follow‐up assessments at 6 weeks post treatment and thereafter every six months. Training provided for those administering intervention and assessment scales. Study exclusion criteria reported. A post‐hoc sensitivity analysis indicated that results were not confounded due to attrition. | 100% compliance with administration of all scales in melatonin group at six weeks. 5% attrition in double placebo group at six weeks due to death, placement in nursing home and one withdrawal from the study. Over the 3.5 years of the study overall attrition rates were: MMSE 15% attrition due to inability to communicate, 1% due to absence of participant during assessment visit. CSDD 2% attrition due to absence of participant or caregiver at time of assessment. PGCARS, MOSES, NPI‐Q, CMAI, NI‐ADL 4% attrition as caregivers unable to provide a rating due to limitations of communication abilities or observability of participants and 1% due to incomplete data. |
Participants
The primary basis for selection of participants in all five studies (Asayama 2003; Gehrman 2009; Riemersma‐van der Lek 2008; Serfaty 2002; Singer 2003) was the diagnosis of some type of dementia. Two hundred and eighty‐nine participants had a diagnosis of Alzheimer disease (AD) (Asayama 2003) or a NINCDS‐ADRDA diagnosis of probable AD (Gehrman 2009; Riemersma‐van der Lek 2008; Serfaty 2002; Singer 2003), which represents 86% of the total participants in the five studies. In the fourth study, the participants had to satisfy the APA 2004 (1994) criteria for a clinical diagnosis of dementia (Serfaty 2002). Participants had to be physically able to complete the study, which excluded those who had a severe physical disease or problems. Additional study selection information was requested from the authors of the Gehrman 2009 study in an email dated 18 July, 2009. However, to date no data has been received.
The Riemersma‐van der Lek 2008 study recruited 189 residents from homes for the elderly. Forty‐six were allocated to a melatonin only treatment group while 45 were allocated to a double placebo group (no melatonin, no bright light). Participants were only excluded if they did not provide consent, or if they used monoamine oxidase inhibitors, long‐term nonsteroid anti‐inflammatory drugs, and/or had severe liver or kidney dysfunction, and aphakia. Of the 189 participants randomly assigned to the 4 treatment and control arms of the study, "120 (63%) met the NINCDS‐ADRDA criteria for probable Alzheimer disease, 20 (11%) met the DSM‐IV criteria for vascular dementia, and 24 (13%) met criteria for other types of dementia, including dementia due to multiple etiologies (9 cases), frontal‐type dementia (3 cases), Lewy body dementia (2 cases), Parkinson disease (2 cases), Wernicke‐Korsakoff (1 case), and dementia not otherwise specified (7 cases). Seventeen participants (8%) did not meet the criteria for dementia but stayed in the group care facility for various medical or psychosocial reasons. In 8 participants, data on medical history were insufficient to reach a reliable clinical diagnosis" (p. 2643).
Four of the studies required that participants be experiencing some type of sleep disturbance (Asayama 2003; Gehrman 2009; Serfaty 2002; Singer 2003). Descriptions of these sleep disturbances were provided in two studies (Serfaty 2002; Serfaty 2002). Singer 2003 included those with AD if they averaged less than 7 hours of sleep per night (as documented by wrist actigraphy), and were noted by the caregiver to experience two or more episodes per week of disturbed night‐time sleep, such as sleep latency, wandering, early wakening, and daytime somnolence. Serfaty 2002 included those with a clinical diagnosis of dementia who demonstrated at least two weekly incidents of night‐time agitated behavior as reported by the caregiver.
In all five studies, the Mini‐Mental State Examination was administered to establish the severity of dementia both at baseline and the endpoint of the study. In four of these studies (Asayama 2003;Riemersma‐van der Lek 2008; Serfaty 2002; Singer 2003) the mean MMSE scores of participants at baseline ranged from a low of 10.3 to a high of 15.3, falling into the moderate range of cognitive impairment (Tombaugh 1992). In the Gehrman 2009 study, the participants' mean MMSE score was 5.8 falling into the severe range of cognitive impairment.
Intervention
Before intervention with exogenous melatonin occurred, medications were stabilized in three of the studies (Asayama 2003; Serfaty 2002; Singer 2003). In the Asayama 2003 study, beta‐blockers were washed out for four weeks before the study, while other drugs required by participants were maintained, provided that they did not affect the symptoms of AD. In the Serfaty 2002 study, participants were either not taking hypnotic medication, or were receiving the same dose of medication for at least four weeks prior to entry into the trial. Psychotropic medication was not used during the study period. The participants in the Singer 2003 study were excluded from the study if they had been using investigational or unapproved medications within four weeks of the screening visit.The Riemersma‐van der Lek 2008 study was designed to be a 'practical clinical trial', that is, a trial that is designed to investigate the practical issues and positive outcomes of treatment in clinical settings (Tunis 2003). Hence, no restrictions on medications being started, stopped or changed during the trial period were made. Medication data was not available from the Gehrman 2009 study. This information was requested of the Gehrman 2009 authors by email on 18 July, 2009. To date, no data have been received.
Exogenous melatonin was administered to participants once a day at the participants' usual bedtime (Serfaty 2002), at one hour prior (Singer 2003;Riemersma‐van der Lek 2008), at 20:30 hours (Asayama 2003) or at 10:00 pm (Gehrman 2009). The intervention was administered by informal caregivers, researchers, physicians or registered nurses with advanced preparation. Training was provided to all those who administered the intervention. Dosage ranged from 3 to 10 mg of immediate release (IR) melatonin to 1.5 to 6 mg slow release (SR) melatonin. One study (Singer 2003) divided participants into three groups: the control group, a group which received 2.5 (SR) melatonin, and a group which received 10 mg (IR) melatonin. A 3 mg melatonin treatment was used in the Asayama 2003 study. Another study (Serfaty 2002) administered 6 mg (SR) melatonin. The Riemersma‐van der Lek 2008 study used 2.5 mg of a “medium‐fast” release, while the Gehrman 2009 used a 8.5 mg immediate release and 1.5 mg time release preparation.
Outcomes
The primary goal of four of these studies (Asayama 2003; Gehrman 2009; Serfaty 2002; Singer 2003) was to measure the effects of exogenous melatonin on sleep disorders in participants with cognitive dementia or AD. Primary outcomes in all four studies were measured objectively using wrist actigraphy. Secondary outcomes included changes in cognitive function (Asayama 2003; Gehrman 2009; Serfaty 2002; Singer 2003), non‐cognitive function, (Asayama 2003;Gehrman 2009), depressive and neuropsychiatric symptoms, and functions in activities of daily living (Singer 2003). Part of the Riemersma‐van der Lek 2008 study also focused on the effects of melatonin on the progression of cognitive symptoms of dementia, changes in psychopathologic behavior including mood, depressive and behavior symptoms, and limitations of activities of daily living.
This review primarily focused on the evaluation of the outcomes related to changes in cognition, mood, behavior, and function in activities of daily living. Other secondary outcomes of interest were quality of life, caregiver stress, morbidity, mortality and length of time to institutionalization. These secondary outcomes were not addressed as they were not examined in the included studies. Data relevant to the safe use of melatonin were investigated by observation and reporting of adverse events in Singer 2003 and adverse effects in Riemersma‐van der Lek 2008) but could not be pooled due to the use of different measurement constructs. Singer 2003 defined adverse events as "abnormal behavior, ache/pain, falls, fatigue, gastrointestinal distress, infection, respiratory/pulmonary symptom, skin/subcutaneous tissue, and urinary symptoms" (p. 898) with an additional notation of fatigue in the placebo group. Adverse effects as defined by Riemersma‐van der Lek 2008 included "dizziness, drowsiness, eye complaints, feebleness, headache, hunger, hyperactivity, inability to sleep, irritability, nausea, constipation, pins and needles, stomach ache, sweating, trembling hands, and other complaints" (p. 2653). Mean adverse effect rating data was provided by Riemersma‐van der Lek 2008, although the authors did not provide participant numbers for each of the endpoint data collection points within the melatonin treatment arm. Therefore, the mean number of placebo and melatonin group participants (cumulative number of participants over the 3.5 year study divided by the number of data collection points [n=8]) was used to calculate adverse effect estimates for the Riemersma‐van der Lek 2008 study.
Cognitive changes were measured by the Mini‐Mental State Examination (MMSE) in all studies. Additionally, the cognitive section of the Alzheimer's Disease Assessment Scale (ADAS‐Cognitive) was employed by two studies (Asayama 2003; Singer 2003), and the Clinical Dementia Rating Scale (CDR) was employed by Asayama 2003. Behavioral and mood changes in the participants were measured using: the Agitated Behavior Rating Scale (Bliwise 1983) in Gehrman 2009, the Hamilton Depression Rating Scale (Hamilton 1960) in Singer 2003, the Cornell Scale for Depression in Dementia (CSDD) (Alexopoulos 1988; Kørner 2006) in Riemersma‐van der Lek 2008, the Neuropsychiatric Inventory ) (Cummings 1994 )in Singer 2003, the Neuropsychiatric Inventory‐Questionnaire (Kaufer 2000), the Cohen‐Mansfield Agitation Index (CMAI) (Cohen‐Mansfield 1989; De Deyn 2000) in Gehrman 2009 and Riemersma‐van der Lek 2008, and the ADAS non‐cognitive scores (Rosen 1984) in Asayama 2003. The Riemersma‐van der Lek 2008 study also used the Philadelphia Geriatric Center Affect Rating Scale (PGCARS) (Lawton 1996), the Philadelphia Geriatric Center Morale Scale (PGCMS) (Lawton 1972; McDowell 1996), and the Multi Observation Scale for Elderly Subjects (MOSES) (Helmes 1987). Activities of daily living (ADL) in the participants were measured in Singer 2003 using the ADL Inventory (Galasko 1997) and in Riemersma‐van der Lek 2008 using the NI‐ADL (nurse informant activities of daily living) (Brorsson 1984). These tests and rating scales are described in Additional Tables, Table 2.
2. Description of Assessment Scale Used in Included Studies.
| Mini‐Mental State Examination (MMSE) used in Asayama 2003 and Singer 2003 study. | MMSE: Short, valid and reliable cognitive assessment tool that can evaluate the severity of dementia and chronological changes in functioning. Eleven task oriented items, key scale categories of orientation, memory and attention. Total attainable score is 30 indicating healthy cognitive status. Concurrent validity supported by correlations with the Weschler Adult Intelligence Scale [r = .776 with the Verbal Scale, p < .0001; r = .660 with Performance Scale, p < .001 (Tombaugh 1992)]. Twenty‐four hour retest (1 tester) r = .887, p < .0001; 24 hour retest (2 testers) r = .827, p < .0001, 28 day retest r = .988, p < .0001 (Folstein 1975). |
| Alzheimer Disease Assessment Scale ‐ Cognitive Scale (ADAS‐cognitive) used in Asayama 2003 and Singer 2003 studies. | ADAS‐cognitive: Short valid measure of cognitive functional decline associated with Alzheimer's Disease (AD). Cognitive Scale (11 items, task oriented, r = .989 p < .001, for interrater reliability, test‐retest reliability, r = .915, p < .001: categories include memory, language, recall, word finding difficulty, following commands). Maximum score of 70 indicates marked cognitive symptoms of AD. Significant correlation with the Sandoz Clinical Assessment Geriatric Score (r = .668, df = 16, p < .01) and total scale score (Rosen 1984). |
| Alzheimer Disease Assessment Scale Non‐Cognitive Scale (ADAS non‐cognitive) used in Asayama 2003 study. | Non‐cognitive scale (10 items, 5 point scale: Interrater reliability r=.947, p < .001, Test‐retest reliability: r = .588, p < .001: categories include depressed mood, distractibility, uncooperative to testing, delusions, hallucinations, pacing, tremors, decreased appetite) with maximum score of 50 indicating presence of construct. Non‐significant correlation with Sandoz Clinical Assessment Geriatric Score (r = .252, df = 16, p > .10) (Rosen 1984). |
| Clinical Dementia Rating Scale (CDR) used in Asayma 2003 study. | CDR: Valid and reliable measure of dementia and cognitive ability. Categories include memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. Scale rating score ranges from 0 (healthy) to 3 (severe dementia). Caregivers rate client ability from 1 to 9 (extreme debilitation). Adequate correlations support the reliability of the instrument (Hughes 1982). |
| Neuropsychiatric Inventory (NPI) used in Singer 2003 study. | NPI: Valid measure of psychopathological behavior associated with dementia. Ten dichotomous subscales: constructs of delusions, hallucinations, depression, anxiety, agitation, apathy, irritability/lability, disinhibition and euphoria. Concurrent validity supported by results of correlation with BEHAVE‐AD and the Hamilton Depression Rating Scale (HDRS). Between rater, test‐retest, and internal consistency results support reliability of the instrument. Data is obtained from caregivers. A score of 0 to 120 is obtained by multiplying the frequency of each item (rated 1 to 4) by the severity (rated 1 to 3) of each item. Higher score indicates more severe psychopathology (Cummings 1994). |
| The Activities of Daily Living Questionnaire (ADL) used in Singer 2003 study. | ADL: Valid and reliable measure of functional decline associated with Alzheimer's disease. Inventory of 27 items ranging from 2 to 5 point scales. Test‐retest reliability moderate to very good (K statistic ranged from 0.4 to 0.75, p < .01. Spearman rank order correlation coefficient between scaling levels of ADL and MMSE scores (R = 0.4 ‐ 0.7, p < .001). Higher score indicates more functional ability (Galasko 1997). Categories: self‐care, household care, employment and recreation, shopping and money, travel, and communication. Adequate reliability was supported by average correlation coefficients of 0.86. Concurrent validity was established by comparing the ADLQ with the Record of Independent Living, a previously validated measure of level of dependency in daily living activities. Negatively correlated with the Mini‐Mental State Examination and positively correlated with the Clinical Dementia Rating Scale. |
| Hamilton Depression Rating Scale (HDRS) used in Singer 2003 study. | HRDS: Clinical utility demonstrated for screening and assessment of depression. A higher score indicates a higher level of depression. Primary psychometric research reported inter‐rater correlations ranging from 0.84 to 0.90, although interviewer subjectivity may exist (Galasko 1960). Bagby 2004 reported adequate convergent and discriminant validity, but inadequate content validity. Williams 2001 supported standardization of versions of the scale and the 24 item symptom ratings to increase validity and reliability. |
| Cornell Scale for Depression in Dementia (CSDD) used in Riemersma‐van der Lek 2008 | CSSD: 19 item, 3 point scale. Categories include mood related signs, behavioral disturbance, physical signs, cyclic functions, & ideational disturbance. A higher score denotes a higher level of depression. Inter‐rater scale reliability supported by a weighted kappa of 0.67 and internal consistency (coefficient alpha = 0.84). A significant positive correlation (r=0.83) between total scale scores and Research Diagnostic Criteria for depression established convergent validity (Alexopoulos 1988; Kørner 2006) |
| Philadelphia Geriatric Center Affect Rating Scale (PGCARS) used in Riemersma‐van der Lek 2008 | PGCARS: Six item scale for use by research and clinical staff to evaluate positive affect (pleasure, interest, contentment) and negative affect (sadness, worry/anxiety and anger). Reliability supported by kappa ratings of .76 to .89. Convergent validity was supported by significant positive correlations between the constructs of positive affect and 13 external measures of well being and between the "negative state" and 9 external measures of depression, anxiety and withdrawal. Limited support exists for the two factor dimensional structure of the scale (Lawton 1996). |
| Philadelphia Geriatric Center Morale Scale (PGCMS) used in Riemersma‐van der Lek 2008 | PGCMS: Seventeen item scale representing three factors of the dimensions of morale [agitation (six items), attitude toward one’s own aging (five items), and lonely dissatisfaction (6 items)] derived from factor analysis. Reliability was supported by significant test‐retest correlations of .75 to .91. Convergent validity was supported by a positive correlation with life satisfaction index (r =.74) (Lawton 1972; McDowell 1996). |
| Psychometric Assessment of the Multidimensional Observation Scale for Elderly Subjects (MOSES) used in Riemersma‐van der Lek 2008 | MOSES: Inter‐rater reliability ranged from 0.58 (depression) to 0.97 (self care). Five scale constructs (self‐care, disorientation, depression, irritability, and withdrawal) are each represented by eight items scored on a four or five point Likert‐type scale. Lower scores indicate higher mental functioning. Validity was supported by significant correlations of 0.65 to 0.91 between a range of "theoretically similar subscales" for mental dysfunction (Helmes 1987). |
| Nurse‐informant activities of daily living adaptation of the scale by Katz (NI‐ADL) used in Riemersma‐van der Lek 2008 | The scale represents five activities and one function: bathing, dressing, toileting, transferring, continence and feeding. The scale score is derived from an index of ADL that summarizes overall scale performance. Scale scores represent grades of A,B,C, D, E, F, and G (where A is most independent and G is the most dependent). Validity was supported by a coefficient of scalability between .74 and .88. Inter‐observer variability was "low" supporting reliability. The scale is recommended as a measure of functional ability for the elderly or disabled persons in short or long term care (Brorsson 1984). |
| Cohen‐Mansfield Agitation Inventory (CMAI) used in Riemersma‐van der Lek 2008 | CMAI: The CMAI is a 29 item seven point rating scale for use by health care practitioners for the assessment of verbal agitation, non‐aggressive behavior (i.e. pacing) and disruptive behavior in the elderly. A higher score indicates a higher level of agitation. Reliability is supported by inter‐rater agreement rates between .88 and .92. Validity has been supported by significant correlations (r=.0.91) with the following scales: Nursing Home Behavior Problems and Behavioral and Emotional Problems Manifested in Dementia (Cohen‐Mansfield 1989; De Deyn 2000). |
| Agitated Behavior Rating Scale (ABRS) used in Gehrman 2009 | The ABRS is 4 point rating scale (0 = no agitation present) to 3 (high intensity agitation) used to rate 5 categories of behavior over a twenty‐four time period: manual manipulation, searching and wandering, escape behaviours, tapping and banging, and verbal agitation. The scale is based on two factors derived from the CMAI related to physical and verbal aggression. The first 4 categories are combined to create a physical agitation score (a higher score indicates a higher level of agitation). Scale reliability is supported by 85% inter‐rater agreement across scale categories and kappa coefficients at the .001 level of significance (Bliwise 1983). |
| The Neuro‐Psychiatric Inventory Questionnaire (NPI Q) used in Riemersma‐van der Lek 2008 | The NPI‐Q is a 12‐item questionnaire developed from the Neuropsychiatric Inventory, a validated clinical instrument. The NPI‐Q is used by health care practitioners for assessment of persons with neurologic disease or neuropsychiatric symptoms. The severity of the symptoms are rated on a 3‐point scale (1 = mild, 2 = moderate, 3 = severe). Neuropsychiatric symptoms are represented by the scale constructs of: Delusions, Hallucinations, Agitation, Depression, Anxiety, Euphoria, Apathy, Disinhibition, Irritability, Motor Disturbance, Nighttime behaviours, and Appetite Disturbance. The NPI‐Q demonstrates adequate test‐retest reliability (r=0.89) and convergent validity (r=0.88) with the NPI (Kaufer 2000). |
Risk of bias in included studies
Essential principles of assessing risk of bias in studies considered for inclusion in a systematic review include study design, allocation concealment, blinding of the interventions and outcome assessment and assessment of attrition (Higgins 2008; Forbes 2003). Selection bias can be addressed through a randomization process that controls for potential confounding factors and comparability of baseline states of the control and intervention groups. Performance bias refers to the systematic differences in the care provided to the participants in the comparison groups resulting from causes other than the intervention. Decreasing these types of bias can be achieved through double blinding techniques where those receiving care and those providing care are unaware of the assigned intervention, and the provision of training to those providing the intervention. Detection bias refers to systematic differences between the comparison groups in assessment of outcomes. Blinding of outcome assessors limits detection bias. The length of the study and characteristics of participants must also be considered in the examination of attrition bias as systematic differences may exist in loss of participants between the comparison groups.
Authors of four of the included research studies (Asayama 2003; Gehrman 2009; Serfaty 2002; Singer 2003) were contacted to obtain details of the random allocation and concealment process referred to in the published articles. To date, random allocation and concealment information has not been received from the Gehrman 2009 authors. The key codes for the double blind allocation sequence in the remaining three studies were not opened until after the data analyses were completed. Pharmaceutical staff in one study labelled the placebo and melatonin medication through a random number treatment order allocation sequence (Asayama 2003). Two studies (Riemersma‐van der Lek 2008; Serfaty 2002) used a computer generated numbering system to achieve randomised allocation to treatment or control group. Serfaty 2002 also described the evaluation process for the double blind technique employed to address performance bias. Researchers, participants and care providers reported they were unaware of the nature of the drug (melatonin or a placebo) administered during the intervention phase of the research. In the Riemersma‐van der Lek 2008 study, a research assistant external to the research study used a computer random number function to randomly assign six facilities to light treatment, six facilities to placebo light exposure and participants to double blind daily intake of melatonin. Singer 2003 reported that randomization and code development were done at the Alzheimer's Disease Cooperative Study Unit (ADCS) at the University of California San Diego. Sealed code breakers were delivered to all sites and collected following study completion. A block randomization process was applied to all ADCS study protocols. Four of the studies were rated as adequate for design and allocation concealment to intervention or control group; those who assessed outcomes were also blind to allocation to the intervention or control group (Asayama 2003; Riemersma‐van der Lek 2008; Serfaty 2002; Singer 2003). All authors provided information in the publications or as requested by the reviewers detailing the procedures used to train those administering the intervention and cognitive and non‐cognitive assessment instruments (Asayama 2003; Gehrman 2009; Riemersma‐van der Lek 2008; Serfaty 2002; Singer 2003). The review authors’ assessments related to each risk of bias item are presented as percentages across all included trials in Figure 1 and for each included study as a risk of bias summary in Figure 2.
1.
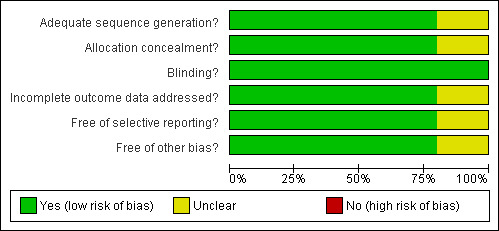
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
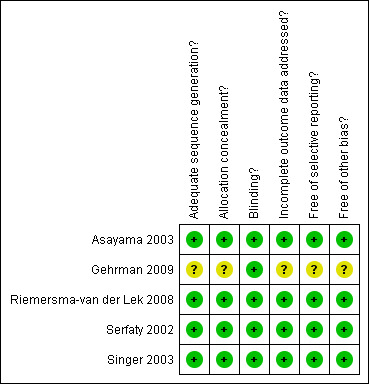
Risk of Bias summary: review authors' judgements about each risk of bias item for each included study.
Attrition rates were calculated without the Gehrman 2009 and Serfaty 2002 data as the standard deviations from the change scores were not available for each time reported in these studies. A 4% attrition rate occurred in the three included studies (Asayama 2003; Gehrman 2009; Singer 2003; Riemersma‐van der Lek 2008) with 268 recruited and 257 completing the trials. One hundred percent compliance (n = 20 at baseline and endpoint) with the Mini Mental State Examination was achieved in Asayama 2003. Singer 2003 provided unpublished data indicating a 96% participant compliance rate (4% attrition equal between intervention and control group) (n = 157 at baseline, n = 151 at endpoint) with the administration of the cognitive and non‐cognitive assessment instruments used in the study. Attrition was low at the initial six week follow‐up stage of the Riemersma‐van der Lek 2008 study (5% attrition rate, n = 91 at baseline, n = 86 at endpoint) and remained equal between the treatment and placebo groups during the three and one half years of the study. However, significant attrition did occur during each six month follow‐up due to death and transfer to long term care (54% participation rate at 1 year, n= 49 at endpoint; 21% participation rate at 2 years, n = 19 at endpoint). None of the treatment attrition effect estimates reached significance within the post‐hoc sensitivity analyses (Riemersma‐van der Lek 2008). In addition, missing data due to death or nursing home placement and inability of the participant to communicate were dummy coded to investigate their possible effects in a 'pattern mixture model'. Missing data was not imputed in the Singer 2003 study.
Selective reporting was addressed through the availability of study intervention protocols in all included studies; all of the studies' pre‐specified outcomes were reported in the published papers.
Other sources of study bias were examined. These criteria included potential confounding factors such as unstable physical disease (Asayama 2003; Riemersma‐van der Lek 2008; Serfaty 2002; Singer 2003), depression (Serfaty 2002; Singer 2003) and acute sleep disturbance (Asayama 2003; Gehrman 2009; Singer 2003). Singer 2003 reported that none of the potential covariates were significantly different between the groups at baseline (age, duration of AD, sex, dementia severity, and years of education). Riemersma‐van der Lek 2008 reported that there were no significant differences between the control and experimental groups in age, gender, use of medication and environmental setting. The MMSE mean baseline scores ranged from 10.3 to 15.3, a moderate degree of dementia, as supported by data obtained from the publications of Asayama 2003, Singer 2003, Riemersma‐van der Lek 2008, and upon request from Serfaty 2002. Medications were stabilized in three of the studies (Asayama 2003; Serfaty 2002; Singer 2003). One study reported that essential drug therapy was maintained, however, drugs such as beta‐blockers that may affect AD were eliminated four weeks prior to the study (Asayama 2003). Singer 2003 also identified that participants were excluded from this study if: (1) they received investigational or unapproved medications within four weeks of the screening visit prior to the study, (2) psychotropic sleep medication was discontinued within two weeks of the screening visit prior to the trial, or (3) melatonin was administered within two weeks of the screening visit. Medications were not altered in the Riemersma‐van der Lek 2008 study in accordance with the design of a practical clinical trial. Potential confounding factor information such as multi‐morbidity and covariates were requested from the Gehrman 2009 authors. However, to date, no reply has been received.
Effects of interventions
Data were pooled from three studies (Asayama 2003; Riemersma‐van der Lek 2008; Singer 2003) based on combination of similar doses (2.5 to 3 mg), duration (4 weeks to 7 weeks) of melatonin in each study, and similarity of the measured constructs for cognition. As the same measurement scale, that is, the MMSE was used to obtain change score (as measured from endpoint to baseline) and final endpoint measures, the data were analyzed using the unstandardized mean (Higgins 2008). The Riemersma‐van der Lek 2008 psychopathologic and functional endpoint data could not be pooled with the Asayama 2003 and Singer 2003 change score data, as the measures were obtained with different scales. Although no consensus appeared to exist in the literature on melatonin dosage, support was found for the efficacy, safety and tolerance of melatonin across a pharmacologic dosage range of 1 ‐ 10 mg in populations without dementia (Krinsky 2004). Singer 2003 also reported that therapeutic blood levels were attained with administration of 2.5 mg (SR) and 10 mg (IR) of melatonin in pharmacokinetic studies conducted in elderly healthy subjects and elderly subjects with AD. Single study estimates were provided for a significantly larger melatonin dosage (e.g. 10 mg). Additional single study data analyses were reported from the Riemersma‐van der Lek 2008 research at six weeks, one year, and two years as this was the only study that provided longitudinal data. Adverse event estimates were presented from Singer 2003 (2.5 and 10 mg of melatonin at 7 weeks from baseline) and for adverse effect estimates from Riemersma‐van der Lek 2008 (2.5 mg of melatonin at each data collection point up to 3.5 years).
Study outcomes are presented under the following headings:
Cognition
Meta‐analysis of MMSE scores from Asayama 2003 (melatonin 3 mg, 4 weeks at endpoint from baseline), Riemersma‐van der Lek 2008 (melatonin 2.5 mg, 6 weeks at final endpoint measure), and Singer 2003 (melatonin mg (SR), 7 weeks at endpoint from baseline) revealed a non‐significant effect for changing cognition (WMD 0.29, 95% CI ‐ 0.63, 1.22) (Figure 3). Non‐significant results for melatonin treatment effect were also obtained from the single study estimates of: Singer 2003 including the second pharmacologic treatment dose of 10 mg melatonin at the seven week change score from baseline (WMD ‐0.54, 95% CI ‐1.76, 0.68) (Figure 4), and the Riemersma‐van der Lek 2008 study results for final endpoint scores in the MMSE score at one year (WMD 2.00. 95% CI ‐1.36, 5.36) (Figure 5) and two years (WMD 2.80, 95% CI ‐2.87, 8.47) (Figure 6). The ADAS cognitive subscale was used to measure the effect of melatonin 3 mg (Asayama 2003) and melatonin 2.5 mg (SR) (Singer 2003) at 4 and 7 weeks respectively from baseline (WMD ‐ 2.64, 95% CI ‐ 5.98, 0.71) (Figure 7). As the I2 was 68% indicating substantial heterogeneity associated with clinical and methodological differences in the studies (Higgins 2008), a random effects model was used in the meta‐analysis of the combined ADAS‐cognitive scores; no significant effect was found. Similar non‐significant results were found in the ADAS cognitive subscale scores (Singer 2003) using melatonin 10 mg at 7 weeks endpoint from baseline (WMD ‐0.43, 95% CI ‐2.50, 1.64) (Figure 8).
3.
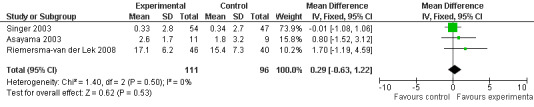
Forest plot of comparison: 1 Cognition: Melatonin vs Placebo, outcome: 1.1 MMSE Cognition Score at endpoint from baseline (change scores at 4 weeks, 3 mg MLT; 7 weeks, 2.5 mg MLT) and at final endpoint measure (6 weeks, 2.5 mg MLT).
4.
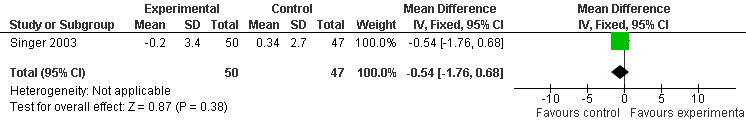
Forest plot of comparison: 1 Cognition: Melatonin vs Placebo, outcome: 1.2 MMSE Cognition Score at endpoint from baseline (7 weeks, 10 mg MLT).
5.
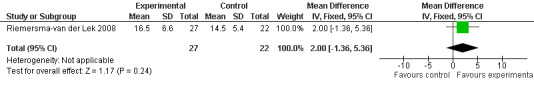
Forest plot of comparison: 1 Cognition: Melatonin vs Placebo, outcome: 1.3 MMSE Cognition Score at final endpoint measure (1 year, 2.5 mg MLT).
6.
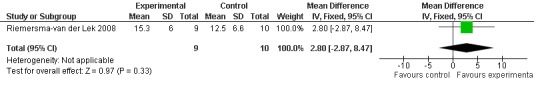
Forest plot of comparison: 1 Cognition: Melatonin vs Placebo, outcome: 1.4 MMSE Cognition Score at final endpoint measure (2 years, 2.5 mg MLT).
7.
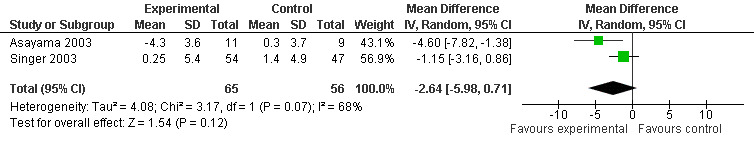
Forest plot of comparison: 1 Cognition: Melatonin vs Placebo, outcome: 1.5 ADAS Cognitive score at endpoint from baseline (4 weeks, 3 mg MLT; 7 weeks, 2.5 mg MLT).
8.
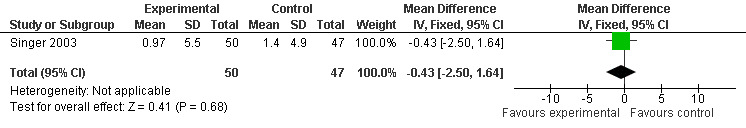
Forest plot of comparison: 1 Cognition: Melatonin vs Placebo, outcome: 1.6 ADAS Cognitive Score at endpoint from baseline (7 weeks, 10.0 mg MLT).
Behavior and Mood
A significant improvement in psychopathological behaviours was revealed from the combined data analysis of the ADAS non‐cognitive scale (3 mg melatonin, 4 week change score from baseline) and the NPI (7 week change score at endpoint from baseline, 2.5 mg melatonin) (WMD ‐3.48, 95% CI ‐ 4.89, ‐ 2.07) (Figure 9).
9.
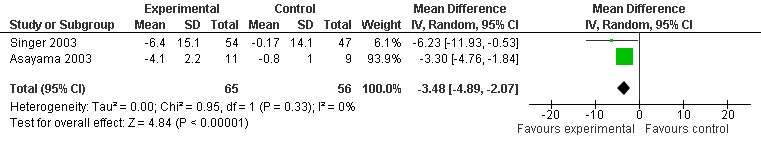
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.1 Mood and Behavioral Score at endpoint from baseline (NPI, 7 weeks, 2.5 mg; ADAS non‐cognitive, 4 weeks, 3 mg MLT)
Non‐significant effects for melatonin treatment effect were found from the following single study estimates of psychopathological behaviours change: NPI score with 10 mg melatonin at 7 weeks from baseline (Singer 2003) (WMD 0.63, 95% CI ‐4.58, 5.84) (Figure 10), NPI‐Q at final endpoint measure with 2.5 mg melatonin at 6 weeks (WMD ‐1.60 95% CI ‐3.63, 0.43) Figure 11 (one year (WMD ‐0.70, CI ‐3.00, 1.60) (Figure 12), and two years (WMD ‐2.70, 95% CI ‐7.70, 2.30) (Figure 13), Cohen Mansfield Agitation Inventory with 2.5 mg melatonin at six weeks (WMD ‐1.00, CI ‐8.06, 6.06) (Figure 14) (one year (WMD 0.00, 95% CI ‐9.64, 9.64) (Figure 15), and two year final endpoint measures (WMD ‐14.00, 95% CI ‐29.89, 1.89) (Figure 16), and the Multi Observation Scale for Elderly subjects with 2.5 mg melatonin at six weeks (WMD 1.70, 95% CI, ‐0.85, 4.25) (Figure 17), one year (WMD 2.20 95% CI ‐0.82, 5.22) (Figure 18) and two year (WMD ‐2.90 95% CI ‐7.80, 2.00) (Figure 19) final endpoint measures. However, the longitudinal mixed effect regression analyses conducted by Riemersma‐van der Lek 2008 revealed a significant melatonin effect for "aggravated withdrawn behavior (1.02, 95% CI 0.18, 1.86)" (p. 2649), on the Multi‐Observational Scale for Elderly subjects.
10.
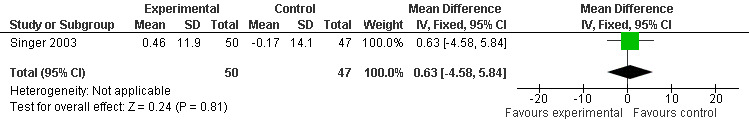
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.2 Mood and Behavioral score at endpoint from baseline (NPI, 7 weeks, 10 mg MLT).
11.
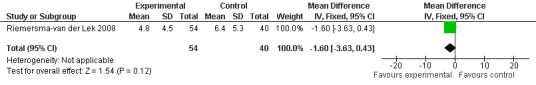
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.3 NPI‐Q Severity Score at final endpoint measure (6 weeks, 2.5 mg MLT)
12.
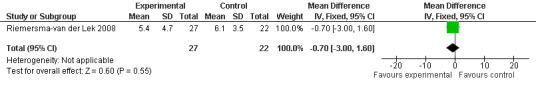
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.4 NPI‐Q Severity Score at final endpoint measure (1 year, 2.5 mg MLT).
13.
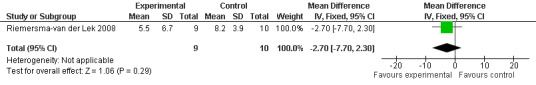
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.5 NPI‐Q severity Score at final endpoint measure (2 years, 2.5 mg MLT).
14.
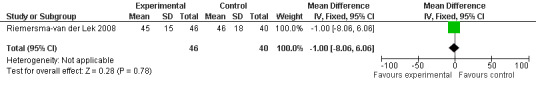
Forest plot of comparison: 2 Behavior and/or Mood: Melatonin vs Placebo, outcome: 2.6 Cohen‐Mansfield Agitation Inventory Score at final endpoint measure (6 weeks, 2.5 mg MLT).
15.
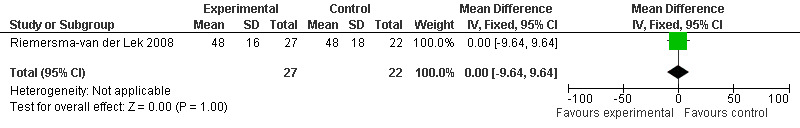
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.7 Cohen‐Mansfield Agitation Inventory Score at final endpoint measure (1 year, 2.5 mg MLT).
16.
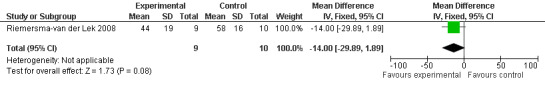
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.8 Cohen‐Mansfield Agitation Inventory Score at final endpoint measure (2 years, 2.5 mg MLT).
17.
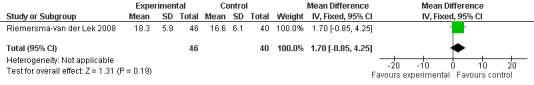
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.9 Mood and behavior score in Multi Observation Scale for Elderly Subjects at final endpoint measure (6 weeks, 2.5 mg MLT).
18.
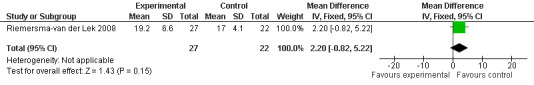
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.10 Mood and behavior score in Multi Observation Scale for Elderly Subjects at final endpoint measure (1 year, 2.5 mg MLT).
19.
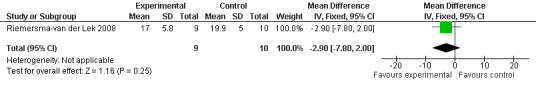
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.11 Mood and behavior score in Multi Observation Scale for Elderly Subjects at final endpoint measure (2 years, 2.5 mg MLT).
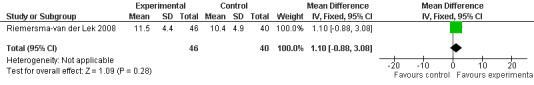
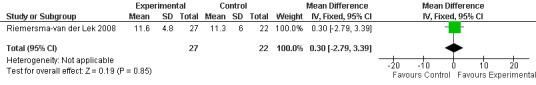
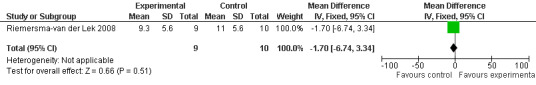
Single study final endpoint measures of the effect of 2.5 mg melatonin (Cornell Scale for Depression in Dementia) from Riemersma‐van der Lek 2008 at six weeks (WMD ‐0.30, 95% CI ‐2.71, 2.11) (Figure 20), one year (WMD ‐1.70, 95% CI ‐5.99, 2.59) (Figure 21) and two years (WMD ‐5.00, 95% CI ‐12.47, 2.47) (Figure 22) were also non‐significant. The final endpoint measure at one year of 2.5 mg melatonin (Philadelphia Geriatric Center Affect Rating Scale positive) demonstrated a significant effect (WMD ‐1.60 95% CI ‐3.14, ‐0.06) (Figure 23) for a worsening of mood, that is, a decrease in positive affect. However, non‐significant results were obtained at 6 weeks (Figure 24) and at 2 years (WMD ‐0.20 95% CI ‐2.57, 2.17) (Figure 25). Riemersma‐van der Lek 2008 in the longitudinal mixed effect regression analyses found significant adverse melatonin effects for "lowering positive mood ratings on the Philadelphia Geriatric Center Affect Rating Scale (positive) (‐0.55, 95% CI, ‐1.00, ‐0.10) and increasing negative mood ratings (0.8, 95% CI, 0.20, ‐1.44) on the Philadelphia Geriatric Centre Affect Rating Scale (negative)" (p. 2649). The remaining Riemersma‐van der Lek 2008 longitudinal study data were non‐significant: Philadelphia Geriatric Centre Affect Rating Scale (negative) at 6 weeks (WMD ‐0.50 95% CI ‐1.65, 0.65) (Figure 26), one year (WMD 1.30 95% CI ‐0.05, 2.65) (Figure 27); and two years (WMD ‐2.30 95% CI ‐4.96, 0.36) (Figure 28), and the Philadelphia Geriatric Center Morale Scale at six weeks (WMD 1.10, 95% CI ‐0.88, 3.08) (Figure 29); one year (WMD .30 95% CI ‐2.79. 3.39) (Figure 30); and two years (WMD ‐1.70 95% CI ‐6.74, 3.34) (Figure 31).
20.
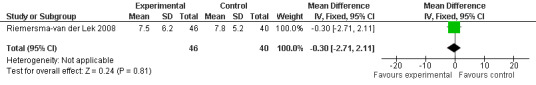
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.12 Cornell Depression Rating Scale Score for Dementia at final endpoint measure (6 weeks, 2.5 mg MLT).
21.
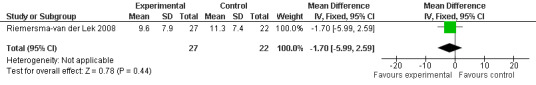
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.13 Cornell Depression Rating Scale Score for Dementia at final endpoint measure (1 year, 2.5 mg MLT).
22.
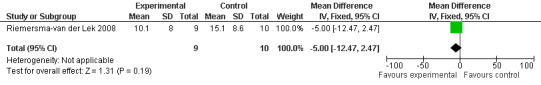
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.14 Cornell Depression Rating Scale Score for Dementia at final endpoint measure (2 years, 2.5 mg MLT).
23.
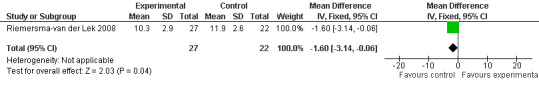
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.16 Mood score in Philadelphia Geriatric Centre Affect Rating Scale (positive) at final endpoint measure (1 year, 2.5 mg MLT).
24.
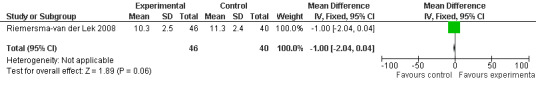
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.15 Mood Score in Philadelphia Geriatric Centre Rating Scale (positive) at final endpoint measure (6 weeks, 2.5 mg MLT).
25.
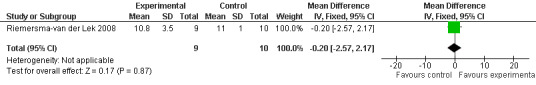
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.17 Mood score in Philadelphia Geriatric Centre Affect Rating Scale (positive) at final endpoint measure (2 years, 2.5 mg MLT).
26.
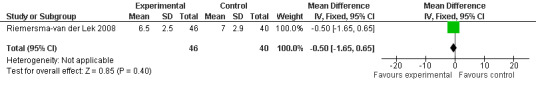
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.18 Mood score in Philadelphia Geriatric Centre Affect Rating Scale (negative) at final endpoint measure (6 weeks, 2.5 mg MLT).
27.
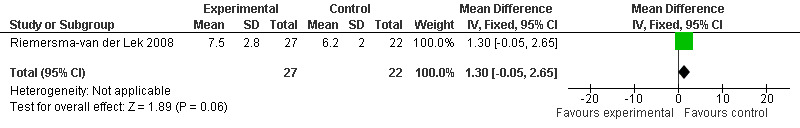
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.19 Mood score in Philadelphia Geriatric Centre Affect Rating Scale (negative) at final endpoint measure (1 year, 2.5 mg MLT).
28.
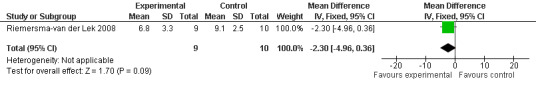
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.20 Mood score in Philadelphia Geriatric Centre Affect Rating Scale (negative) at final endpoint measure (2 years, 2.5 mg MLT).
29.
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.21 Mood score in Philadelphia Geriatric Centre Morale Scale at final endpoint measure (6 weeks, 2.5 mg MLT).
30.
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.22 Mood score in Philadelphia Geriatric Centre Morale Scale at final endpoint measure (1 year, 2.5 mg MLT).
31.
Forest plot of comparison: 2 Behavior and Mood: Melatonin vs Placebo, outcome: 2.23 Mood score in Philadelphia Geriatric Centre Morale Scale at final endpoint measure (2 years, 2.5 mg MLT).
Functions of Daily Living
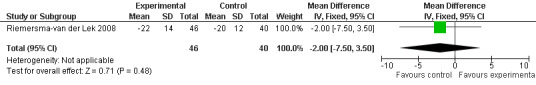
The Riemersma‐van der Lek 2008 study data obtained from the NI‐ADL scale for 2.5 mg at six weeks (WMD ‐2.00, 95% CI ‐7.50, 3.50) (Figure 32), one year (WMD 5.00, 95% CI ‐2.00, 12.00) (Figure 33) and two years (WMD ‐1.00 95% CI ‐14.09, 12.09) (Figure 34) were also non‐significant.
32.
Forest plot of comparison: 3 Functions of Daily Living: Melatonin vs Placebo, outcome: 3.1 ADL score at final endpoint measure (NI‐ADL, 6 weeks, 2.5 mg MLT).
33.
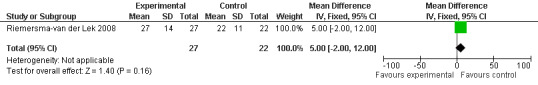
Forest plot of comparison: 3 Functions of Daily Living: Melatonin vs Placebo, outcome: 3.2 NI‐ADL Score at final endpoint measure (1 year, 2.5 mg).
34.
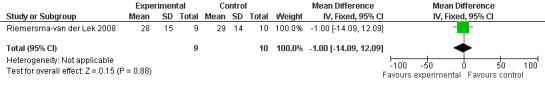
Forest plot of comparison: Functions of Daily Living: Melatonin vs Placebo, outcome: 3.3 NI‐ADL Score at final endpoint measure (ADL, 2 years, 2.5 mg MLT).
Sensitivity Analyses
Random assignment of facilities or residential site to treatment arm was only conducted in the Riemersma‐van der Lek 2008 study. Therefore, sensitivity analyses was undertaken by removing the Riemersma‐van der Lek 2008 6 week measurement data from the cognitive outcome pooled estimates of the Asayama 2003, Singer 2003, and Riemersma‐van der Lek 2008 studies. Similar to the meta‐analyses that included the Riemersma‐van der Lek 2008 study (Figure 3), non‐significant effects were revealed for the combined MMSE scores from the Asayama 2003 (melatonin 3 mg, 4 weeks at endpoint from baseline) and Singer 2003 data (melatonin 2.5 mg (SR), 7 weeks at endpoint from baseline) (WMD 0.13 95% CI ) (Figure 35).
35.
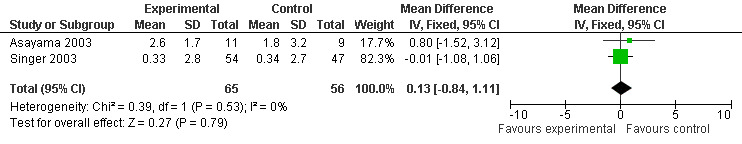
Forest plot of comparison: 4 Sensitivity Analysis: MMSE Cognition Score, outcome: 4.1 MMSE Cognition Score at endpoint from baseline (change scores at 4 weeks, 3 mg MLT; 7 weeks, 2.5 mg MLT).
Adverse Events
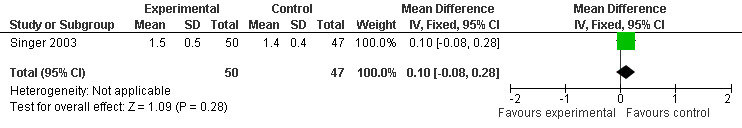
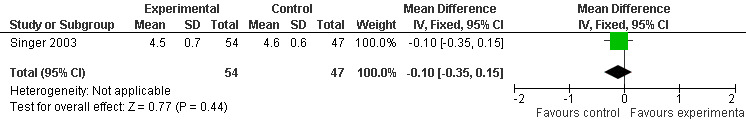
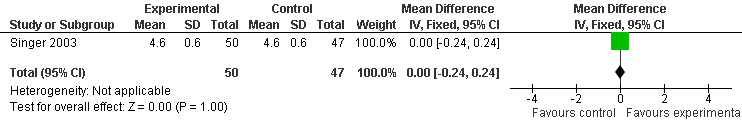
Two studies investigated adverse events associated with melatonin use (Singer 2003; Riemersma‐van der Lek 2008). Different adverse event constructs were used in each study and Riemersma‐van der Lek 2008 did not provide adverse event participant number data at each data collection point. Single study estimates, calculated from the Singer 2003 data, revealed a significant decrease in the mean seriousness of adverse events in the treatment group with 2.5 mg (WMD ‐0.10 95% CI ‐0.18, ‐0.02) (Figure 36) and 10 mg melatonin (WMD ‐0.10 95% CI ‐0.16, ‐0.04) (Figure 37) at the 7 week endpoint to baseline measure. All other estimates were non‐significant: mean number of adverse events per person with 2.5 mg (WMD 1.00 95% CI ‐0.19, 2.19) (Figure 38) and 10 mg (WMD ‐0.40 95 % CI ‐1.33, 0.53) (Figure 39); mean severity with 2.5 mg (WMD 0.10 95% CI ‐0.10, 0.30) (Figure 40) and 10 mg (WMD 0.10 95% CI ‐0.08, 0.28) (Figure 41) and relatedness to melatonin use with 2.5 mg (WMD ‐0.10 95% CI ‐0.35, 0.15) (Figure 42) and 10 mg (WMD 0.00 95% CI ‐0.24, 0.24) (Figure 43) .
36.
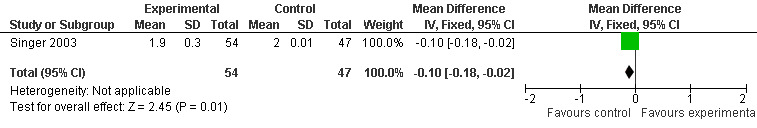
Forest plot of comparison: 6 Adverse Events, outcome: 5.3 Mean AE Seriousness (Melatonin 2.5 mg at 7 weeks).
37.
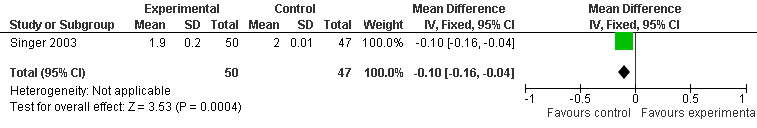
Forest plot of comparison: 6 Adverse Events (AE), outcome: 5.7 Mean AE Seriousness (Melatonin 10 mg at 7 weeks).
38.
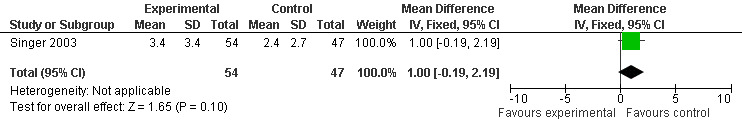
Forest plot of comparison: 6 Adverse Events: outcome 5.1 Mean Number of AE Reports per Person (Melatonin 2.5 mg at 7 weeks).
39.
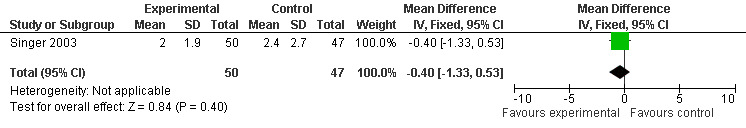
Forest plot of comparison: 6 Adverse Events, outcome: 5.5 Mean Number of AE Reports per Person (Melatonin 10 mg at 7 weeks).
40.
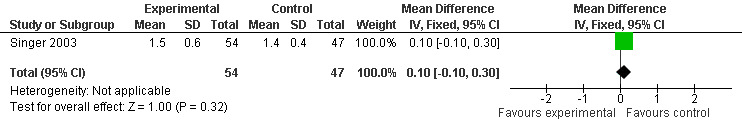
Forest plot of comparison: 6 Adverse Events, outcome: 5.2 Mean AE Severity (Melatonin 2.5 mg at 7 weeks).
41.
Forest plot of comparison: 6 Adverse Events, outcome: 5.6 Mean AE Severity (Melatonin 10 mg at 7 weeks).
42.
Forest plot of comparison: 6 Adverse Events, outcome: 5.4 Mean AE Relatedness to Melatonin (Melatonin 2.5 mg at 7 weeks).
43.
Forest plot of comparison: 6 Adverse Events (AE), outcome: 5.8 Mean AE Relatedness (Melatonin 10 mg at 7 weeks).
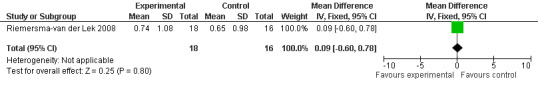
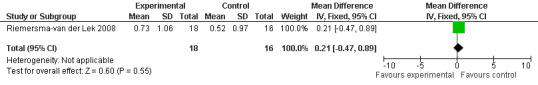
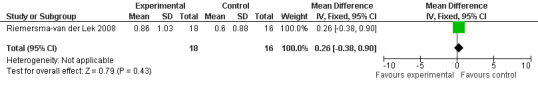
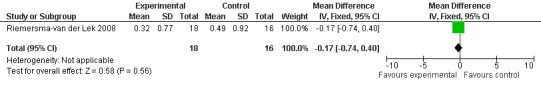
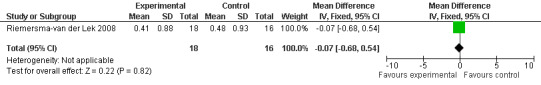
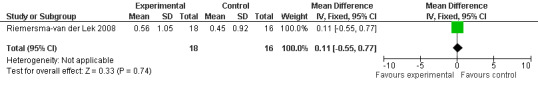
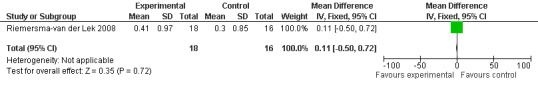
All single study estimates calculated from mean adverse effect data (2.5 mg melatonin at each of the endpoint intervals) (Riemersma‐van der Lek 2008) were non‐significant for melatonin use: dizziness (WMD ‐0.16 95% CI ‐0.89, 0.57) (Figure 44); drowsiness (WMD 0.15 95% CI ‐0.68, 0.98) (Figure 45); eye complaints (WMD 0.09 95% CI ‐0.60, 0.78) (Figure 46); feebleness (WMD 0.21 95% CI ‐0.47, 0.89) (Figure 47); headache (WMD 0.26 95% CI ‐0.38, 0.90) (Figure 48); hunger (WMD ‐0.17 95% CI ‐0.74, 0.40) (Figure 49); hyperactivity (WMD ‐0.16 95% CI ‐0.77, 0.45) (Figure 50); inability to sleep (WMD ‐0.19 95% CI ‐0.88, 0.50) (Figure 51); irritability (WMD ‐0.29 95% CI ‐1.09, 0.51) (Figure 52); nausea (WMD 0.00 95% CI ‐0.53, 0.53) (Figure 53); constipation (WMD ‐0.21 95% CI ‐0.91, 0.49) (Figure 54); pins and needles (WMD ‐0.23 95% CI ‐0.67, 0.21) (Figure 55); stomach ache (WMD 0.05 95% CI ‐0.36, 0.46) (Figure 56); sweating (WMD ‐0.07 95% CI ‐0.68, 0.54) (Figure 57); trembling hands (WMD 0.11 95% CI ‐0.55, 0.77) (Figure 58); and other complications (WMD 0.11 95% CI ‐0.50, 0.72) (Figure 59).
44.
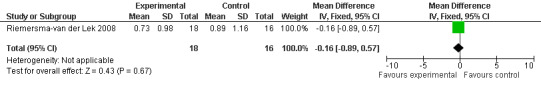
Forest plot of comparison: 7 Adverse Effects: Melatonin vs Placebo, outcome: 6.1 Mean Adverse Effect Ratings for Dizziness (Melatonin 2.5 mg over 3.5 years follow‐up).
45.
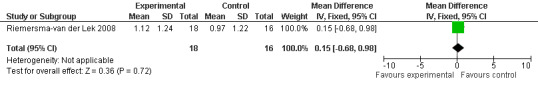
Forest plot of comparison: 7 Adverse Effects, outcome: 6.2 Mean Adverse Effect Ratings for Drowsiness (Melatonin 2.5 mg over 3.5 yeas follow‐up).
46.
Forest plot of comparison: 7 Adverse Effects: Melatonin vs Placebo, outcome: 6.3 Mean Adverse Effect Ratings for Eye Complaints (Melatonin 2.5 mg over 3.5 years follow‐up).
47.
Forest plot of comparison: 7 Adverse Effects: Melatonin vs Placebo, outcome: 6.4 Mean Adverse Effect Ratings for Feebleness (Melatonin 2.5 mg over 3.5 years follow‐up).
48.
Forest plot of comparison: 7 Adverse Effects: Melatonin vs Placebo, outcome: 6.5 Mean Adverse Effect Ratings for Headache (Melatonin 2.5 mg over 3.5 years follow‐up).
49.
Forest plot of comparison: 7 Adverse Effects: Melatonin vs Placebo, outcome: 6.6 Mean Adverse Effect Ratings for Hunger (Melatonin 2.5 mg over 3.5 years follow‐up).
50.
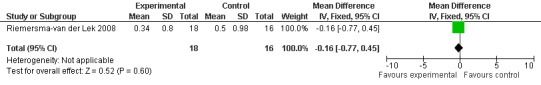
Forest plot of comparison: 7 Adverse Effects: Melatonin vs Placebo, outcome: 6.7 Mean Adverse Effects Ratings for Hyperactivity (Melatonin 2.5 mg over 3.5 years follow‐up).
51.
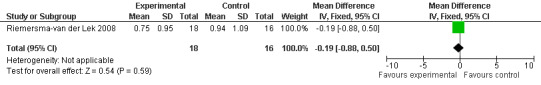
Forest plot of comparison: 7 Adverse Effects: Melatonin vs Placebo, outcome: 6.8 Mean Adverse Effect Ratings for Inability to Sleep (Melatonin 2.5 mg over 3.5 years follow‐up).
52.
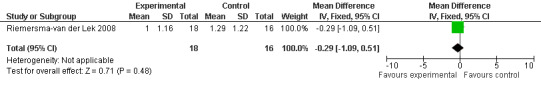
Forest plot of comparison: 7 Adverse Effects: Melatonin vs Placebo, outcome: 6.9 Mean Adverse Effect Ratings for Irritability (Melatonin 2.5 mg over 3.5 years follow‐up).
53.
Forest plot of comparison: 7 Adverse Effects: Melatonin vs Placebo, outcome: 6.10 Mean Adverse Effect Ratings for Nausea (Melatonin 2.5 mg over 3.5 years follow‐up).
54.
Forest plot of comparison: 7 Adverse Effects: Melatonin vs Placebo, outcome: 6.11 Mean Adverse Effect Ratings for Constipation (Melatonin 2.5 mg over 3.5 years follow‐up).
55.
Forest plot of comparison: 7 Adverse Effects: Melatonin vs Placebo, outcome: 6.12 Mean Adverse Effect Ratings for Pins and Needles (Melatonin 2.5 mg over 3.5 years follow‐up).
56.
Forest plot of comparison: 7 Adverse Effects: Melatonin vs Placebo, outcome: 6.13 Mean Adverse Effect Ratings for Stomach Ache (Melatonin 2.5 mg over 3.5 years follow‐up).
57.
Forest plot of comparison: 7 Adverse Effects: Melatonin vs Placebo, outcome: 6.14 Mean Adverse Effect Ratings for Sweating (Melatonin 2.5 mg over 3.5 years follow‐up).
58.
Forest plot of comparison: 7 Adverse Effects: Melatonin vs Placebo, outcome: 6.15 Mean Adverse Effect Ratings for Trembling Hands (Melatonin 2.5 mg over 3.5 years follow‐up).
59.
Forest plot of comparison: 7 Adverse Effects: Melatonin vs Placebo, outcome: 6.16 Mean Adverse Effect Ratings for Other Complications (Melatonin 2.5 mg over 3.5 years follow‐up).
Discussion
No significant evidence was revealed in this review for the effect of melatonin administration on cognitive impairment associated with dementia and specifically AD. Study estimates for the effect of melatonin on cognition were non‐significant for the meta‐analysis of MMSE change scores (melatonin 3 mg, 4 weeks at endpoint from baseline; melatonin 2.5 mg, 6 weeks at final endpoint measure; melatonin 2.5 mg (SR), 7 weeks at endpoint from baseline) (Figure 3). The treatment effect was also non‐significant for the combined ADAS‐cognitive scores for melatonin 3 mg and melatonin 2.5 mg (SR), measured at 4 and 7 weeks respectively from baseline (Figure 7).
Significant effects were revealed for an improvement in psychopathologic behavior from data analysis of the ADAS non‐cognitive scale (3 mg melatonin, 4 week change score from baseline) and the NPI (7 weeks at endpoint from baseline, 2.5 mg melatonin). The only significant findings from analyses of the longitudinal data (Riemersma‐van der Lek 2008) was for a deterioration in mood, an undesirable outcome, at the 1 year final endpoint measure of 2.5 mg (SR) melatonin (Philadelphia Geriatric Center Affect Rating Scale positive) (Figure 23). The remainder of the single study estimates and combined treatment effects for mood, behavior and activities of daily living were non‐significant.
Several factors must be examined when considering the strength of the conclusions.
Strength of the conclusions was supported by homogeneity of the overall study sample. Eighty‐one percent of the participants in the studies included in the meta‐analyses were diagnosed with dementia and were experiencing a moderate degree of this disease. However, the findings may not be applicable to milder or more severe forms of cognitive impairment. The length of time diagnosed with dementia at the time of enrolment (only Singer 2003 reported average duration of Alzheimer's Disease (AD) at time of enrolment as 4.9 years, SD 3.0 years) also may have influenced the outcomes.
Other explanations for the non‐significant treatment effects may be related to the short time interval of the studies. Since the conditions under evaluation may continue after withdrawal of the treatment, in order to avoid carry‐over effects (Elbourne 2002), the second three week trial data from the Serfaty 2002 crossover study were excluded from the review. In addition, differences between treatment and control groups from baseline to end of treatment may be difficult due to small increments of change and may require longer periods of time. The Riemersma‐van der Lek 2008 study was the only trial which included mixed‐effect regression analysis for use with long‐term data sets collected over 3.5 years. Several of the Riemersma‐van der Lek 2008 findings contrasted to the meta‐analysis findings of significant melatonin effect for improved psychopathologic behavior and the single estimates of non‐significant melatonin effect for a worsening of mood (Philadelphia Geriatric Center Affect Rating Scale negative scores at 6 weeks, 1 year, and 2 years). Riemersma‐van der Lek 2008 found a significant effect for increased withdrawn behavior, an undesirable outcome, from score analyses of the Multi‐Observational Scale for Elderly subjects and a significant melatonin effect for a worsening of mood, and an increase in negative mood ratings from analyses of the Philadelphia Geriatric Centre Affect Rating Scale negative scores.Therefore, the meta‐analysis did not derive the same mood and behavior data findings as the analyses conducted by Riemersma‐van der Lek 2008. Revman software may not capture the findings of a repeated measures design, specifically, the 'interaction effects' that are part of the mixed effect regression analysis . Regression analysis "allowed for inclusion of linear changes over time, and for modification of level (3‐level nested structure of the data set), time course, and treatment effect by missing data patterns" (Riemersma‐van der Lek 2008, p. 2648). Missing data due to death or nursing home placement and inability of the participant to communicate were dummy coded in this study to investigate their possible effects in a 'pattern mixture model'. Post‐hoc sensitivity analysis verified that treatment effects were not affected by the dropout pattern (Riemersma‐van der Lek 2008).
All studies incorporated random allocation to intervention, and blind assessment of outcomes, thus meeting the risk of bias criteria for adequate design, allocation to intervention, performance, detection, and attrition bias. Only one study (Riemersma‐van der Lek 2008) conducted random group assignment to treatment and placebo. However, results of the sensitivity analyses (Asayama 2003; Singer 2003) supported the findings of the original meta‐analyses (Asayama 2003; Riemersma‐van der Lek 2008; Singer 2003). Further efforts were made in all three studies to control for potential confounding variables through exclusion criteria for clinically significant co‐morbidity.
It should be noted that the primary goal of two of the studies (Asayama 2003;Singer 2003) was to measure the effects of exogenous melatonin on sleep disorders in participants with dementia or specifically AD. Since all participants in these two studies had a sleep disorder, this may have influenced the outcome scores examined in this review thus limiting the generalizability of the review. Asayama 2003 proposed that melatonin may indirectly affect cognitive and non‐cognitive function through an improved sleep wake rhythm. Singer 2003 discussed the possible hypnotic effect of melatonin.
Several outcomes of interest were not addressed by the included studies. Longer term studies are needed to examine outcomes such as morbidity, mortality, length of time to institutionalization, and caregiver outcomes. Data was collected on adverse events (Singer 2003) and adverse effects associated with the use of melatonin (Riemersma‐van der Lek 2008). Data within these two studies were not combined due to the difference in measurement constructs. Single study estimates were not significant for seriousness of adverse events (Singer 2003) or adverse effects in the treatment groups (Riemersma‐van der Lek 2008). These findings suggest that there was not an increase in occurrence of adverse events and effects in the treatment group and thus, may support the safety of melatonin use. However, meta‐analysis of RCT data is required to further investigate the safety of melatonin for the treatment of dementia.
Authors' conclusions
Implications for practice.
Meta‐analysis did not support the use of melatonin for treatment of cognitive impairment associated with dementia. Meta‐analysis of data from psychopathologic behavior scales revealed significantly improved outcomes. These results suggest that melatonin treatment may be effective for the treatment of these dementia‐related disturbances. Therefore, further research is recommended to investigate the effectiveness of melatonin for the treatment of dementia‐related mood symptoms.
Implications for research.
Results may be strengthened by additional longitudinal studies that examine the influence of melatonin over an extended time span. In addition, when cross‐over designs are used, it is recommended that appropriate paired analysis be conducted and that the data be reported to allow for meta‐analysis. Additional single interventions should be tested so that the effects can be clearly attributed to one intervention. Several articles could not be included in the review because of the inability to separate the effects of bright light therapy from melatonin and the non‐availability of data for meta‐analysis.
What's new
| Date | Event | Description |
|---|---|---|
| 3 August 2009 | New search has been performed | An update search was performed for this review in which new studies were retrieved for either inclusion or exclusion within the review. Two new studies have been included in this update. |
History
Protocol first published: Issue 3, 2002 Review first published: Issue 1, 2006
| Date | Event | Description |
|---|---|---|
| 2 June 2008 | New search has been performed | An update search in January 2008 retrieved some new studies for consideration by the authors. |
| 2 June 2008 | Amended | Converted to new review format. |
| 15 November 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors gratefully acknowledge the contributions of the Cochrane Dementia and Cognitive Improvement Group, who provided invaluable editorial and statistical advice and assisted with the searches; Nikki Jahnke who translated the Savaskan 2006 paper; and Dr. A Rusak‐Maguire who was the lead author for the original protocol.
Appendices
Appendix 1. Sources searched and search strategies used
| Source searched | Search strategy |
| Medline (Ovid SP) | 1. Melatonin/ 2. melatonin.mp. 3. (melatonin or N‐acetyl‐5‐methoxytryptamine).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 4. 1 or 2 or 3 5. Delirium, Dementia, Amnestic, Cognitive Disorders/ or Dementia, Vascular/ or Dementia, Multi‐Infarct/ or Dementia/ 6. Alzheimer Disease/ 7. Lewy Bodies/ 8. Arteriosclerosis/ 9. Huntington Disease/ 10. Kluver‐Bucy Syndrome/ 11. "Pick Disease of the Brain"/ 12. Delirium/ 13. Cerebrovascular Disorders/ 14. Wernicke Encephalopathy/ 15. Korsakoff Syndrome/ 16. dement*.mp. 17. alzheimer*.mp. 18. lewy* bod*.mp. 19. arteriosclerosis.mp. 20. huntington* disease.mp. 21. Kluver Bucy.mp. 22. Pick* disease.mp. 23. cerebrovascular disorder*.mp. 24. wernicke* encephalopathy.mp. 25. Korsakoff psychosis.mp. 26. ((cognit$ or memory$ or mental$) and (declin$ or impair$ or los$ or deteriorat$)).mp. 27. cerebr$ deteriorat$.mp. 28. cerebr$ insufficien$.mp. 29. memory complain*.mp. 30..mp. 31. 11 or 21 or 7 or 26 or 17 or 22 or 18 or 30 or 23 or 16 or 13 or 29 or 27 or 25 or 6 or 28 or 9 or 14 or 15 or 20 or 8 or 4 or 24 or 10 or 19 or 5 32. 4 and 31 33. 2008*.ed. 34. 2009*.ed. 35. 33 or 34 36. 35 and 32 37. randomized controlled trial.pt. 38. controlled clinical trial.pt. 39. randomi?ed.ab. 40. placebo.ab. 41. drug therapy.fs. 42. randomly.ab. 43. trial.ab. 44. groups.ab. 45. "double‐blind method".mp. 46. clinical trial.pt. 47. "randomi?ed controlled trial".mp. 48. 39 or 40 or 41 or 47 or 38 or 42 or 46 or 45 or 37 or 43 or 44 49. 36 and 48 50. (animals not (humans and animals)).sh. 51. 49 not "59".mp. [mp=title, original title, abstract, name of substance word, subject heading word] 52. (rat or rats).ti. 53. 51 not 52 54. "ADHD".ti. 55. 53 not 54 56. child*.ti. 57. 55 not 56 58. (mice or mouse).ti. 59. 57 not 58 60. embryo*.ti. 61. 59 not 60 62. 61 |
| Embase (Ovid SP) | 1. Melatonin/ 2. melatonin.mp. 3. N‐acetyl‐5‐methoxytryptamine.mp. 4. 1 or 2 or 3 5. Cognitive Defect/ 6. Senile Dementia/ or Frontotemporal Dementia/ or Pick Presenile Dementia/ or Dementia/ or Multiinfarct Dementia/ or "Mixed Depression and Dementia"/ or Presenile Dementia/ 7. Alzheimer Disease/ 8. Lewy Bodies/ 9. Arteriosclerosis/ 10. Huntington Chorea/ 11. Kluver Bucy Syndrome/ 12. Pick Presenile Dementia/ 13. Delirium/ 14. Cerebrovascular Disease/ 15. Wernicke Encephalopathy/ 16. Korsakoff Psychosis/ 17. dement*.mp. 18. alzheimer*.mp. 19. lewy* bod*.mp. 20. arteriosclerosis.mp. 21. huntington* disease.mp. 22. Kluver Bucy.mp. 23. Pick* disease.mp. 24. cerebrovascular disorder*.mp. 25. wernicke* encephalopathy.mp. 26. Korsakoff psychosis.mp. 27. ((cognit$ or memory$ or mental$) and (declin$ or impair$ or los$ or deteriorat$)).mp. 28. cerebr$ deteriorat$.mp. 29. memory complain*.mp. 30. CADASIL.mp. 31. 11 or 21 or 7 or 26 or 17 or 22 or 18 or 30 or 23 or 16 or 13 or 29 or 27 or 25 or 6 or 28 or 9 or 12 or 14 or 15 or 20 or 8 or 24 or 10 or 19 or 5 32. 4 and 31 33. randomi?ed.ab. 34. Randomized Controlled Trial/ 35. Drug Therapy/ or controlled clinical trial.mp. or Controlled Clinical Trial/ or Placebo/ 36. "double blind".ab. 37. trial.ab. 38. placebo.ab. 39. 38 or 35 or 33 or 34 or 36 or 37 40. 32 and 39 |
| PsycINFO (Ovid SP) | 1. exp Melatonin/ 2. melatonin.mp. 3. N‐acetyl‐5‐methoxytryptamine.mp. 4. 1 or 2 or 3 5. exp Memory Disorders/ or exp Cognitive Impairment/ or exp Amnesia/ or exp Delirium/ or exp Mental Disorders/ or exp Dementia/ 6. Alzheimer Disease/ 7. exp Dementia with Lewy Bodies/ 8. exp Cerebral Arteriosclerosis/ or exp Arteriosclerosis/ 9. exp Huntingtons Disease/ 10. exp Kluver Bucy Syndrome/ 11. exp Picks Disease/ 12. exp Delirium/ 13. exp Cerebrovascular Disorders/ 14. exp Wernickes Syndrome/ or exp Encephalopathies/ or exp Korsakoffs Psychosis/ 15. dement*.mp. 16. alzheimer*.mp. 17. lewy* bod*.mp. 18. arteriosclerosis.mp. 19. huntington* disease.mp. 20. Kluver Bucy.mp. 21. Pick* disease.mp. 22. cerebrovascular disorder*.mp. 23. wernicke* encephalopathy.mp. 24. Korsakoff psychosis.mp. 25. ((cognit$ or memory$ or mental$) and (declin$ or impair$ or los$ or deteriorat$)).mp. 26. cerebr$ deteriorat$.mp. 27. cerebr$ insufficien$.mp. 28. memory complain*.mp. 29. CADASIL.mp. 30. 11 or 21 or 7 or 26 or 17 or 22 or 18 or 23 or 16 or 13 or 29 or 27 or 25 or 6 or 28 or 9 or 12 or 14 or 15 or 20 or 8 or 24 or 10 or 19 or 5 31. 4 and 30 32. random*.ab. 33. exp Clinical Trials/ 34. trial.ab. 35. placebo.ab. 36. "double blind".ab. 37. 35 or 33 or 32 or 34 or 36 38. 37 and 31 |
| CINAHL (Ovid SP) | S35 S31 and S34 S34 S32 or S33 S33 em 2009 S32 em 2008 S31 S4 and S30 S30 S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 S29 CADASIL S28 "memory complain*" S27 "cerebr* insufficien*" S26 ((cognit$ or memory$ or mental$) and (declin$ or impair$ or los$ or deteriorat$)) S25 Korsakoff psychosis S24 wernicke* encephalopathy S23 cerebrovascular disorder* S22 Pick* disease S21 Kluver Bucy S20 huntington* disease S19 arteriosclerosis S18 alzheimer* S17 dement* S16 (MH "Wernicke's Encephalopathy") S15 (MH "Cerebrovascular Disorders") S14 (MH "Pick Disease of the Brain") S13 Pick Disease of the Brain S12 Kluver Bucy S11 (MH "Huntington's Disease") S10 (MH "Arteriosclerosis") or (MH "Cerebral Ischemia, Transient") S9 Arteriosclerosis S8 Lewy* Bod* S7 (MH "Alzheimer's Disease") S6 (MH "Dementia") or (MH "Dementia, Vascular") or (MH "Dementia, Multi‐Infarct") or (MH "Dementia, Presenile") or (MH "Dementia, Senile") S5 (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") or (MH "Cognition Disorders") S4 S1 or S2 or S3 S3 N‐acetyl‐5‐methoxytryptamine S2 melatonin S1 (MH "Melatonin") |
| LILACs | (Melatonin OR “N‐acetyl‐5‐methoxytryptamine”) AND (2008 OR 2009) |
| CDCIG SR | (Melatonin OR “N‐acetyl‐5‐methoxytryptamine”) AND (2008 OR 2009) |
| mRCT (metaRegister of Controlled Trials) http://www.controlled‐trials.com/mrct/ [searches: ISRCTN Register; Action Medical Research; Medical Research Council (UK); National Health Service Research and Development Health Technology Assessment Programme (HTA); National Institutes of Health (NIH) ‐ randomized trial records held on NIH ClinicalTrials.gov website.; The Wellcome Trust; UK Clinical Trials Gateway] |
melatonin AND (2008 OR 2009) AND (cognit% OR dement%) |
| IFPMA | ((Melatonin AND dementia) AND (2008 OR 2009))And NOT children |
| UMIN Japan Trial Register | ((Melatonin AND dementia) AND (2008 OR 2009))And NOT children |
| WHO Portal [searches: Australian New Zealand Clinical Trials Registry; ClinicalTrials.gov; ISRCTN; Chinese Clinical Trial Register; Clinical Trials Registry ‐ India; German Clinical Trials Register; Iranian Registry of Clinical Trials; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] |
melatonin OR N‐acetyl‐5‐methoxytryptamine AND dementia |
| Australasian Digital Theses | Melatonin AND (2008 OR 2009) |
| Index to Theses | (Melatonin& OR melatonin) AND (2008 OR 2009) |
| ISI Web of Knowledge | #1 Topic=(melatonin) #2 Topic=(“ N‐acetyl‐5‐methoxytryptamine”) #3 #2 OR #1 #4 Topic=(dement*) #5 #3 AND #4 #6 #5 Timespan=2008‐2009 |
Data and analyses
Comparison 1. Cognition: Melatonin vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 MMSE Cognition Scores at endpoint from baseline (change scores at 4 weeks, 3 mg MLT; 7 weeks, 2.5 mg MLT) and at final endpoint measure (6 weeks, 2.5 mg MLT) | 3 | 207 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.63, 1.22] |
| 2 MMSE Cognition Scores at endpoint from baseline (7 weeks, 10 mg MLT) | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐1.76, 0.68] |
| 3 MMSE Cognition Score at final endpoint measure (1 year, 2.5 mg MLT) | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐1.36, 5.36] |
| 4 MMSE Cognition Score at final endpoint measure (2 years, 2.5 mg MLT) | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 2.80 [‐2.87, 8.47] |
| 5 ADAS Cognitive Score at final endpoint measure (4 weeks, 3 mg MLT; 7 weeks, 2.5 mg MLT) | 2 | 121 | Mean Difference (IV, Random, 95% CI) | ‐2.64 [‐5.98, 0.71] |
| 6 ADAS Cognitive Score at final endpoint meaure (7 weeks, 10 mg MLT) | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐2.50, 1.64] |
1.1. Analysis.
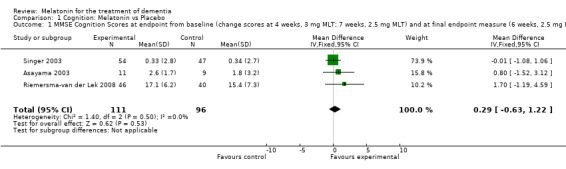
Comparison 1 Cognition: Melatonin vs Placebo, Outcome 1 MMSE Cognition Scores at endpoint from baseline (change scores at 4 weeks, 3 mg MLT; 7 weeks, 2.5 mg MLT) and at final endpoint measure (6 weeks, 2.5 mg MLT).
1.2. Analysis.
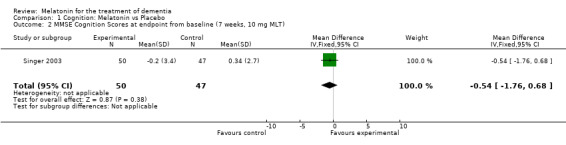
Comparison 1 Cognition: Melatonin vs Placebo, Outcome 2 MMSE Cognition Scores at endpoint from baseline (7 weeks, 10 mg MLT).
1.3. Analysis.
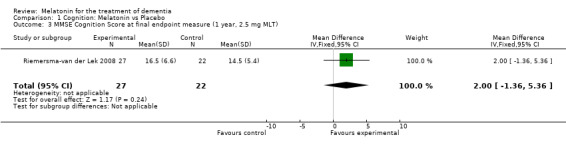
Comparison 1 Cognition: Melatonin vs Placebo, Outcome 3 MMSE Cognition Score at final endpoint measure (1 year, 2.5 mg MLT).
1.4. Analysis.
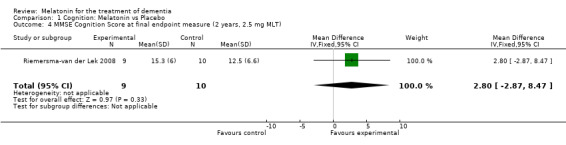
Comparison 1 Cognition: Melatonin vs Placebo, Outcome 4 MMSE Cognition Score at final endpoint measure (2 years, 2.5 mg MLT).
1.5. Analysis.
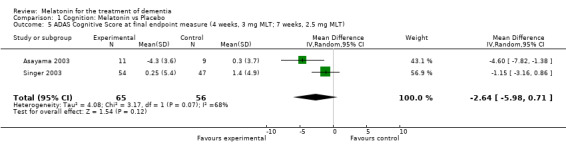
Comparison 1 Cognition: Melatonin vs Placebo, Outcome 5 ADAS Cognitive Score at final endpoint measure (4 weeks, 3 mg MLT; 7 weeks, 2.5 mg MLT).
1.6. Analysis.
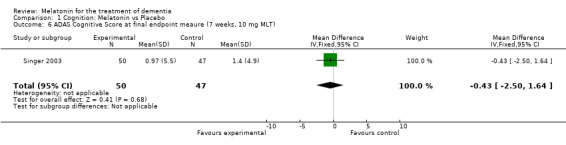
Comparison 1 Cognition: Melatonin vs Placebo, Outcome 6 ADAS Cognitive Score at final endpoint meaure (7 weeks, 10 mg MLT).
Comparison 2. Behavior and/or Mood: Melatonin vs Placebo.
2.1. Analysis.
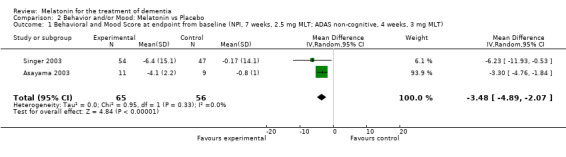
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 1 Behavioral and Mood Score at endpoint from baseline (NPI, 7 weeks, 2.5 mg MLT; ADAS non‐cognitive, 4 weeks, 3 mg MLT).
2.2. Analysis.
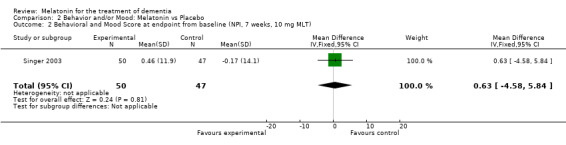
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 2 Behavioral and Mood Score at endpoint from baseline (NPI, 7 weeks, 10 mg MLT).
2.3. Analysis.
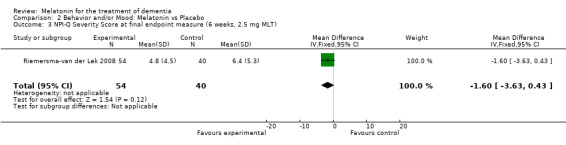
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 3 NPI‐Q Severity Score at final endpoint measure (6 weeks, 2.5 mg MLT).
2.4. Analysis.
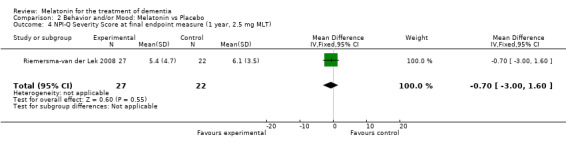
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 4 NPI‐Q Severity Score at final endpoint measure (1 year, 2.5 mg MLT).
2.5. Analysis.
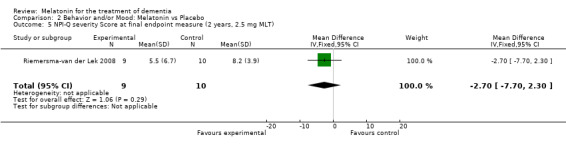
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 5 NPI‐Q severity Score at final endpoint measure (2 years, 2.5 mg MLT).
2.6. Analysis.
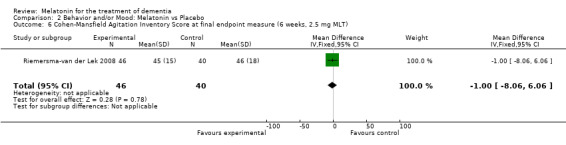
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 6 Cohen‐Mansfield Agitation Inventory Score at final endpoint measure (6 weeks, 2.5 mg MLT).
2.7. Analysis.
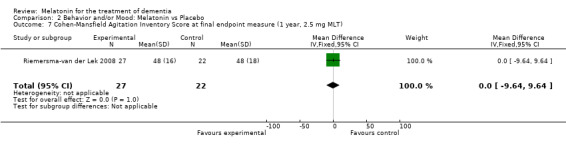
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 7 Cohen‐Mansfield Agitation Inventory Score at final endpoint measure (1 year, 2.5 mg MLT).
2.8. Analysis.
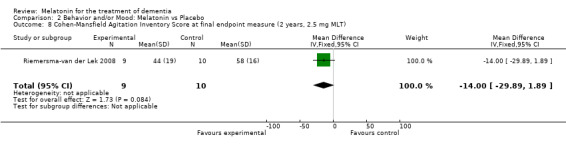
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 8 Cohen‐Mansfield Agitation Inventory Score at final endpoint measure (2 years, 2.5 mg MLT).
2.9. Analysis.
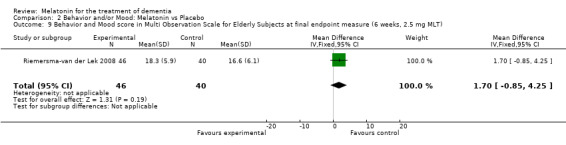
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 9 Behavior and Mood score in Multi Observation Scale for Elderly Subjects at final endpoint measure (6 weeks, 2.5 mg MLT).
2.10. Analysis.
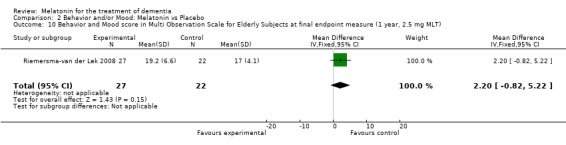
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 10 Behavior and Mood score in Multi Observation Scale for Elderly Subjects at final endpoint measure (1 year, 2.5 mg MLT).
2.11. Analysis.
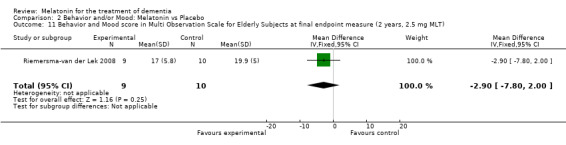
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 11 Behavior and Mood score in Multi Observation Scale for Elderly Subjects at final endpoint measure (2 years, 2.5 mg MLT).
2.12. Analysis.
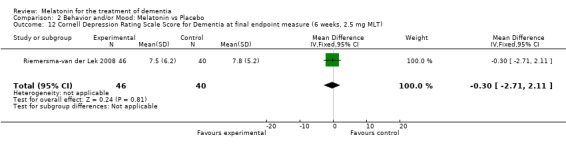
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 12 Cornell Depression Rating Scale Score for Dementia at final endpoint measure (6 weeks, 2.5 mg MLT).
2.13. Analysis.
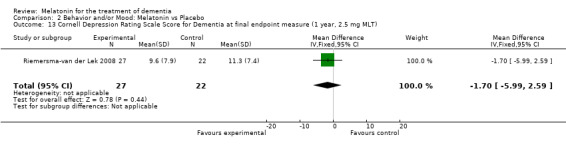
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 13 Cornell Depression Rating Scale Score for Dementia at final endpoint measure (1 year, 2.5 mg MLT).
2.14. Analysis.
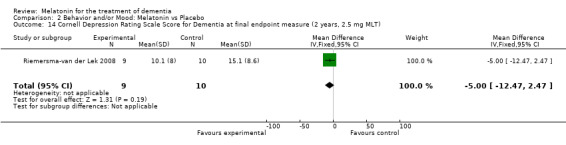
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 14 Cornell Depression Rating Scale Score for Dementia at final endpoint measure (2 years, 2.5 mg MLT).
2.15. Analysis.
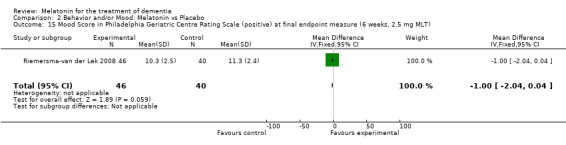
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 15 Mood Score in Philadelphia Geriatric Centre Rating Scale (positive) at final endpoint measure (6 weeks, 2.5 mg MLT).
2.16. Analysis.
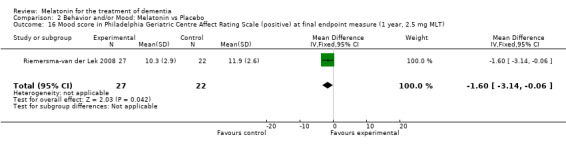
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 16 Mood score in Philadelphia Geriatric Centre Affect Rating Scale (positive) at final endpoint measure (1 year, 2.5 mg MLT).
2.17. Analysis.
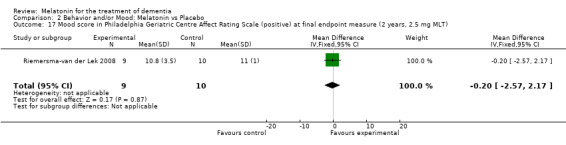
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 17 Mood score in Philadelphia Geriatric Centre Affect Rating Scale (positive) at final endpoint measure (2 years, 2.5 mg MLT).
2.18. Analysis.
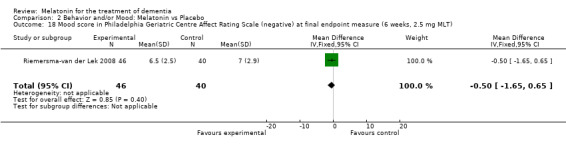
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 18 Mood score in Philadelphia Geriatric Centre Affect Rating Scale (negative) at final endpoint measure (6 weeks, 2.5 mg MLT).
2.19. Analysis.
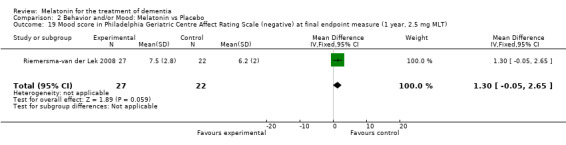
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 19 Mood score in Philadelphia Geriatric Centre Affect Rating Scale (negative) at final endpoint measure (1 year, 2.5 mg MLT).
2.20. Analysis.
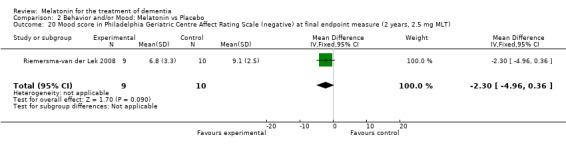
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 20 Mood score in Philadelphia Geriatric Centre Affect Rating Scale (negative) at final endpoint measure (2 years, 2.5 mg MLT).
2.21. Analysis.
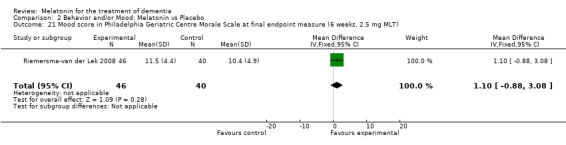
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 21 Mood score in Philadelphia Geriatric Centre Morale Scale at final endpoint measure (6 weeks, 2.5 mg MLT).
2.22. Analysis.
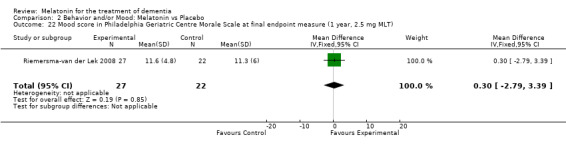
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 22 Mood score in Philadelphia Geriatric Centre Morale Scale at final endpoint measure (1 year, 2.5 mg MLT).
2.23. Analysis.
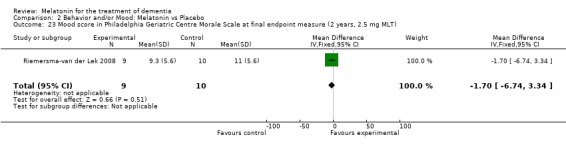
Comparison 2 Behavior and/or Mood: Melatonin vs Placebo, Outcome 23 Mood score in Philadelphia Geriatric Centre Morale Scale at final endpoint measure (2 years, 2.5 mg MLT).
Comparison 3. Functions of Daily Living: Melatonin vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADL score at final endpoint measure (6 weeks, 2.5 mg MLT) | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐7.50, 3.50] |
| 2 ADL score at final endpoint measure (1 year, 2.5 mg MLT) | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [‐2.00, 12.00] |
| 3 ADL score at final endpoint measure (2 years, 2.5 mg MLT) | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐14.09, 12.09] |
3.1. Analysis.
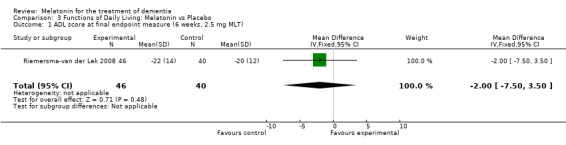
Comparison 3 Functions of Daily Living: Melatonin vs Placebo, Outcome 1 ADL score at final endpoint measure (6 weeks, 2.5 mg MLT).
3.2. Analysis.
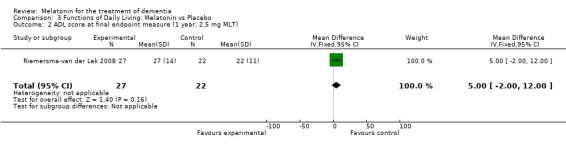
Comparison 3 Functions of Daily Living: Melatonin vs Placebo, Outcome 2 ADL score at final endpoint measure (1 year, 2.5 mg MLT).
3.3. Analysis.
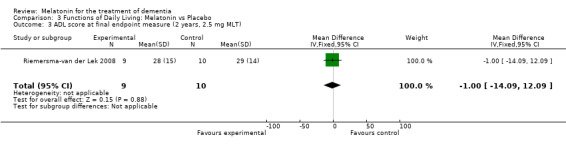
Comparison 3 Functions of Daily Living: Melatonin vs Placebo, Outcome 3 ADL score at final endpoint measure (2 years, 2.5 mg MLT).
Comparison 4. Sensitivity Analysis: MMSE Cognition Score.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 MMSE Cognition Score at endpoint from baseline (change scores at 4 weeks, 3 mg MLT; 7 weeks, 2.5 mg MLT) | 2 | 121 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.84, 1.11] |
4.1. Analysis.
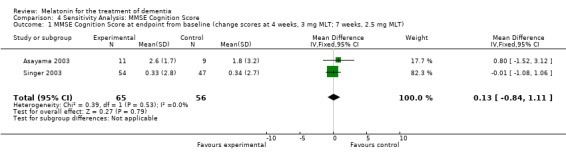
Comparison 4 Sensitivity Analysis: MMSE Cognition Score, Outcome 1 MMSE Cognition Score at endpoint from baseline (change scores at 4 weeks, 3 mg MLT; 7 weeks, 2.5 mg MLT).
Comparison 5. Adverse Events (AE):Melatonin vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean Number of AE Reports per Person (Melatonin 2.5 mg at 7 weeks) | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.19, 2.19] |
| 2 Mean AE Severity (Melatonin 2.5 mg at 7 weeks) | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.10, 0.30] |
| 3 Mean AE Seriousness (Melatonin 2.5 mg at 7 weeks) | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.18, ‐0.02] |
| 4 Mean AE Relatedness to Melatonin (Melatonin 2.5 mg at 7 weeks) | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.35, 0.15] |
| 5 Mean Number of AE Reports per Person (Melatonin 10 mg at 7 weeks) | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.33, 0.53] |
| 6 Mean AE Severity (Melatonin 10 mg at 7 weeks) | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.08, 0.28] |
| 7 Mean AE Seriousness (Melatonin 10 mg at 7 weeks) | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.16, ‐0.04] |
| 8 Mean AE Relatedness (Melatonin 10 mg at 7 weeks) | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.24, 0.24] |
5.1. Analysis.
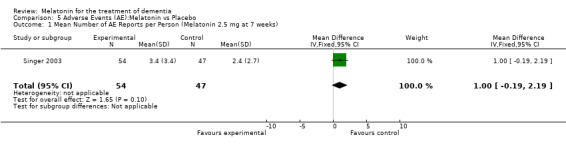
Comparison 5 Adverse Events (AE):Melatonin vs Placebo, Outcome 1 Mean Number of AE Reports per Person (Melatonin 2.5 mg at 7 weeks).
5.2. Analysis.
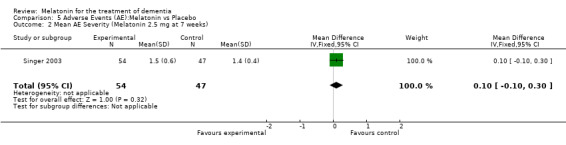
Comparison 5 Adverse Events (AE):Melatonin vs Placebo, Outcome 2 Mean AE Severity (Melatonin 2.5 mg at 7 weeks).
5.3. Analysis.
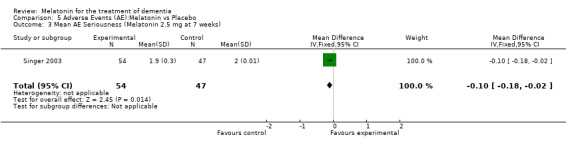
Comparison 5 Adverse Events (AE):Melatonin vs Placebo, Outcome 3 Mean AE Seriousness (Melatonin 2.5 mg at 7 weeks).
5.4. Analysis.
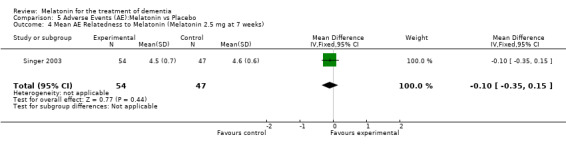
Comparison 5 Adverse Events (AE):Melatonin vs Placebo, Outcome 4 Mean AE Relatedness to Melatonin (Melatonin 2.5 mg at 7 weeks).
5.5. Analysis.
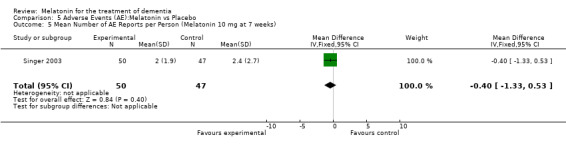
Comparison 5 Adverse Events (AE):Melatonin vs Placebo, Outcome 5 Mean Number of AE Reports per Person (Melatonin 10 mg at 7 weeks).
5.6. Analysis.
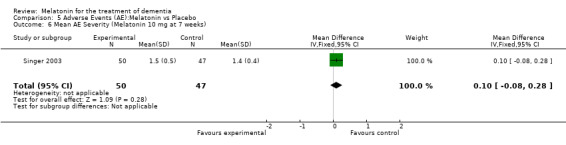
Comparison 5 Adverse Events (AE):Melatonin vs Placebo, Outcome 6 Mean AE Severity (Melatonin 10 mg at 7 weeks).
5.7. Analysis.
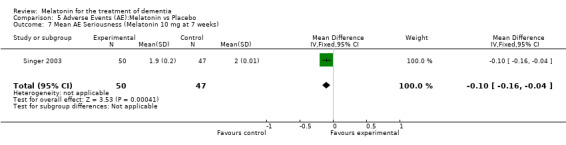
Comparison 5 Adverse Events (AE):Melatonin vs Placebo, Outcome 7 Mean AE Seriousness (Melatonin 10 mg at 7 weeks).
5.8. Analysis.
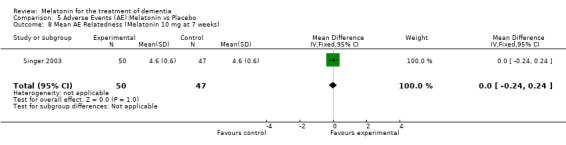
Comparison 5 Adverse Events (AE):Melatonin vs Placebo, Outcome 8 Mean AE Relatedness (Melatonin 10 mg at 7 weeks).
Comparison 6. Adverse Effects: Melatonin vs Placebo.
6.1. Analysis.
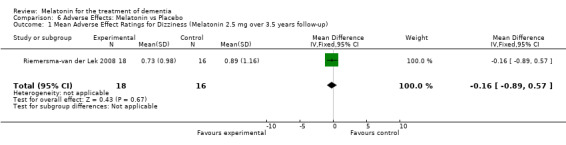
Comparison 6 Adverse Effects: Melatonin vs Placebo, Outcome 1 Mean Adverse Effect Ratings for Dizziness (Melatonin 2.5 mg over 3.5 years follow‐up).
6.2. Analysis.
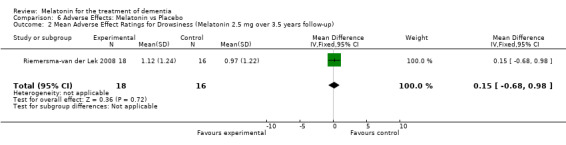
Comparison 6 Adverse Effects: Melatonin vs Placebo, Outcome 2 Mean Adverse Effect Ratings for Drowsiness (Melatonin 2.5 mg over 3.5 years follow‐up).
6.3. Analysis.
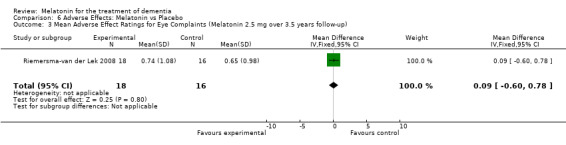
Comparison 6 Adverse Effects: Melatonin vs Placebo, Outcome 3 Mean Adverse Effect Ratings for Eye Complaints (Melatonin 2.5 mg over 3.5 years follow‐up).
6.4. Analysis.
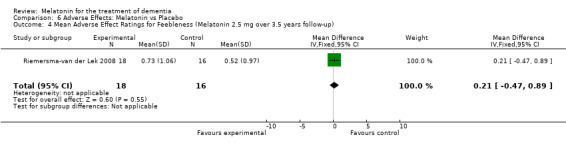
Comparison 6 Adverse Effects: Melatonin vs Placebo, Outcome 4 Mean Adverse Effect Ratings for Feebleness (Melatonin 2.5 mg over 3.5 years follow‐up).
6.5. Analysis.
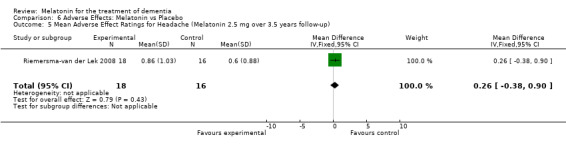
Comparison 6 Adverse Effects: Melatonin vs Placebo, Outcome 5 Mean Adverse Effect Ratings for Headache (Melatonin 2.5 mg over 3.5 years follow‐up).
6.6. Analysis.
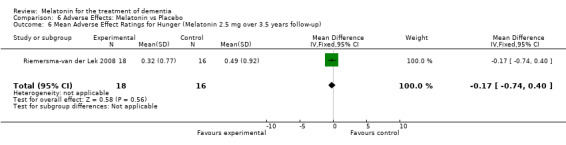
Comparison 6 Adverse Effects: Melatonin vs Placebo, Outcome 6 Mean Adverse Effect Ratings for Hunger (Melatonin 2.5 mg over 3.5 years follow‐up).
6.7. Analysis.
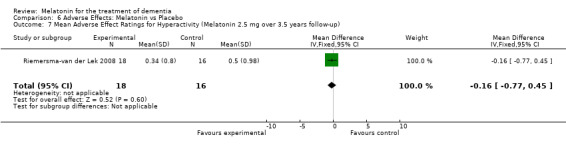
Comparison 6 Adverse Effects: Melatonin vs Placebo, Outcome 7 Mean Adverse Effect Ratings for Hyperactivity (Melatonin 2.5 mg over 3.5 years follow‐up).
6.8. Analysis.
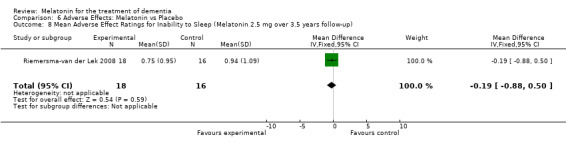
Comparison 6 Adverse Effects: Melatonin vs Placebo, Outcome 8 Mean Adverse Effect Ratings for Inability to Sleep (Melatonin 2.5 mg over 3.5 years follow‐up).
6.9. Analysis.
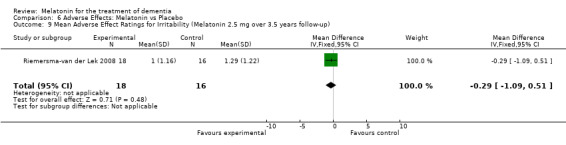
Comparison 6 Adverse Effects: Melatonin vs Placebo, Outcome 9 Mean Adverse Effect Ratings for Irritability (Melatonin 2.5 mg over 3.5 years follow‐up).
6.10. Analysis.
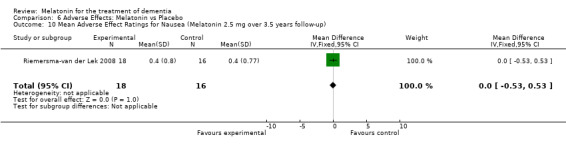
Comparison 6 Adverse Effects: Melatonin vs Placebo, Outcome 10 Mean Adverse Effect Ratings for Nausea (Melatonin 2.5 mg over 3.5 years follow‐up).
6.11. Analysis.
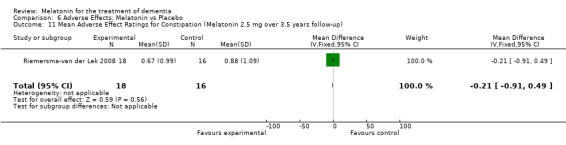
Comparison 6 Adverse Effects: Melatonin vs Placebo, Outcome 11 Mean Adverse Effect Ratings for Constipation (Melatonin 2.5 mg over 3.5 years follow‐up).
6.12. Analysis.
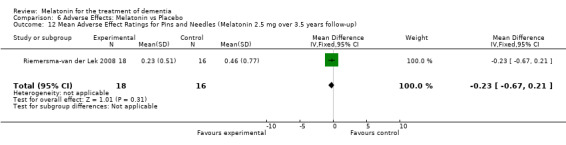
Comparison 6 Adverse Effects: Melatonin vs Placebo, Outcome 12 Mean Adverse Effect Ratings for Pins and Needles (Melatonin 2.5 mg over 3.5 years follow‐up).
6.13. Analysis.
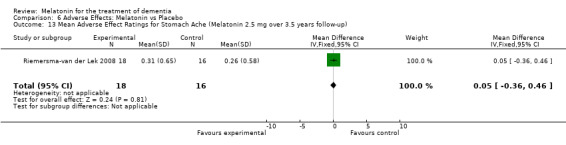
Comparison 6 Adverse Effects: Melatonin vs Placebo, Outcome 13 Mean Adverse Effect Ratings for Stomach Ache (Melatonin 2.5 mg over 3.5 years follow‐up).
6.14. Analysis.
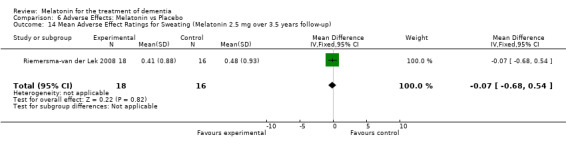
Comparison 6 Adverse Effects: Melatonin vs Placebo, Outcome 14 Mean Adverse Effect Ratings for Sweating (Melatonin 2.5 mg over 3.5 years follow‐up).
6.15. Analysis.
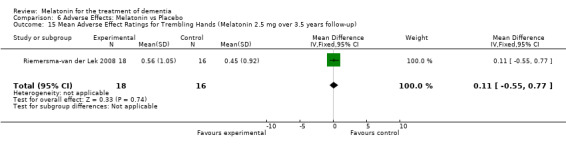
Comparison 6 Adverse Effects: Melatonin vs Placebo, Outcome 15 Mean Adverse Effect Ratings for Trembling Hands (Melatonin 2.5 mg over 3.5 years follow‐up).
6.16. Analysis.
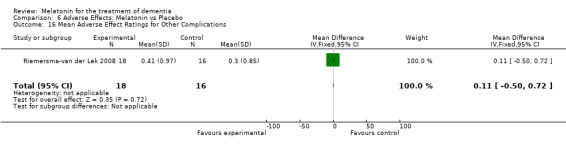
Comparison 6 Adverse Effects: Melatonin vs Placebo, Outcome 16 Mean Adverse Effect Ratings for Other Complications.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Asayama 2003.
| Methods | Randomized controlled, double blind trial 4 weeks duration | |
| Participants | 3 males 17 females Mean age: 79.2 (SD: 6.4). Placebo group; n=9 [Mean age: 79.4 (SD: 5.3); 2 males, 7 females]. Melatonin treatment group: n=11 [Mean age: 78.9 (SD: 7.3); 1 male, 10 females] All diagnosed with AD. Setting: geriatric ward of a hospital from 2000‐2002. Diagnosed with AD with brain CT or brain MRI and EEG and DSM‐1V and NINCDS‐ARDRA. Baseline moderate MMSE rating for both groups. | |
| Interventions | 1. Melatonin 3 mg administered at 20:30 hours 2. Placebo | |
| Outcomes | Cognitive and non‐cognitive changes in CDR, MMSE, and ADAS‐cognitive and non‐cognitive (behavioral and affective scores). Outcome measured at 4 weeks. | |
| Notes | PI: Dr. Kentaro Asayama, Department of Neuropsychiatry, Nippon Medical School. E‐mail: asayama@nms.ac.jp | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A ‐ Adequate Placebo and melatonin medication labelled through a random number treatment order allocation sequence. |
| Allocation concealment? | Low risk | A ‐ Adequate Key codes for the double blind allocation sequence were not opened until after the data analyses were completed |
| Blinding? All outcomes | Low risk | A ‐ Adequate. Those who administered melatonin and assessed outcomes were blind to allocation to the intervention or control group. |
| Incomplete outcome data addressed? All outcomes | Low risk | A ‐ Adequate. 100% compliance with study protocol. |
| Free of selective reporting? | Low risk | A ‐ Adequate. Zero percent attrition rate. The study protocol is available and all of the study's pre‐specified outcomes have been reported in the pre‐specified way. |
| Free of other bias? | Low risk | A ‐ Adequate. Potential confounding factors addressed in exclusion criteria. |
Gehrman 2009.
| Methods | Randomized controlled, double blind trial | |
| Participants | 41 participants [mean age of 82.9 (SD 7.0), 68.3% female] who had resided on average 18.9 months in long term care settings. Mean education of 14.2 years with SD of 2.5 years. All were diagnosed were Alzheimer Dementia through the use of National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association Diagnostic Critieria. MMSE rating: severe. |
|
| Interventions | 3 days baseline (no treatment), 10 days treatment (melatonin combined dose of 8.5 mg immediate release and 1.5 mg time release, or placebo), and 5 days follow‐up (no treatment). | |
| Outcomes | Cognitive and non‐cognitive changes in MMSE, Agitated Behavior Rating Scale, and Cohen‐Mansfield Agitated Inventory at 10 days post intervention. | |
| Notes | Corresponding author: Dr. Sonia Ancoli‐Israel Department of Psychiatry, University of California, San Diego. Email: sancoliisrael@ucsd.edu |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Although the authors identify the study as a double blind trial, information has not been received to date regarding details of the sequence generation. |
| Allocation concealment? | Unclear risk | To date, It is not known how allocation concealment was undertaken in the study. |
| Blinding? All outcomes | Low risk | Adequate. Those who administered melatonin and assessed outcomes were blind to allocation to the intervention or control group. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Although all participants appear to have completed the randomized control trial, to date, information has not been received from the author to verify 100% completion of the study. |
| Free of selective reporting? | Unclear risk | The intervention protocol is available and all of the study's pre‐specified outcomes have been reported in the pre‐specified way. However, it is unclear whether 100% of the study participants completed the study. |
| Free of other bias? | Unclear risk | Information regarding potential confounding factors addressed in exclusion criteria or controlled for in the study have not been received from the author. |
Riemersma‐van der Lek 2008.
| Methods | Randomized double blind placebo controlled trial of 3.5 years duration. | |
| Participants | Placebo group; n=45; mean age: 85 (SD: 5); 5 males, 40 females. Melatonin treatment group: n=46; mean age: 86 (SD: 5); 8 male, 38 females. Of the 189 participants randomly assigned to the 4 treatment and control arms, "120 (63%) met the NINCDS‐ADRDA criteria for probable Alzheimer disease, 20 (11%) met the DSM‐IV criteria for vascular dementia, and 24 (13%) met criteria for other types of dementia, including dementia due to multiple etiologies (9 cases), frontal‐type dementia (3 cases), Lewy body dementia (2 cases), Parkinson disease (2 cases), Wernicke‐Korsakoff (1 case), and dementia not otherwise specified (7 cases). Seventeen participants (8%) did not meet the criteria for dementia but stayed in the group care facility for various medical or psychosocial reasons. In 8 participants, data on medical history were insufficient to reach a reliable clinical diagnosis" (p. 2643). Setting:12 group care facilities in the Netherlands. To determine the clinical diagnosis for probable AD, criteria were used from the National Institute of Neurological and Communicative Disorders and Stroke (NINCDSD) and the Alzheimer's Disease and Related Disorders Assciation (ADRDA). Baseline moderate MMSE rating for treatment and intervention groups. | |
| Interventions | Nursing staff administered 2.5 mg MLT or placebo once a day at bedtime. | |
| Outcomes | Main outcomes were assessed at 6 weeks and then every 6 months by administration of standardized scales(cognitive: MMSE; mood: CSDD, PGCARS positive, PGCARS negative, PGCMS; behavioral: MOSES; NPI‐Q severity; NPI‐Q, distress; CMAI; functional limitations: NI‐ADL). | |
| Notes | Eus JW Van Someren, PhD Head Dept. Sleep & Cognition, Netherlands Institute for Neuroscience and VU Medical Center Meibergdreef 1105, BA Amsterdam, The Netherlands tel: +31 20 5665500 fax:+31 20 6961006 e.van.someren@nin.knaw.nl |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A ‐ Adequate. Randomized allocation to treatment and placebo occurred through microsoft excel random number function. |
| Allocation concealment? | Low risk | A ‐ Adequate. MLT was administered in a double blind placebo controlled trial. |
| Blinding? All outcomes | Low risk | A ‐ Adequate. Double blind daily intake for MLT. |
| Incomplete outcome data addressed? All outcomes | Low risk | A ‐ Adequate. 95% compliance rate within control group and 100% compliance rate within experimental group at 6 weeks. Attrition due to logistical limitations such as death and transfer to long term care. Rate of attrition between groups equal. None of the treatment attrition effect estimates reached significance in the overall analyses. Verified by post‐hoc sensitivity analysis that treatment effects were not affected by dropout pattern. 100% included in intention to treat analysis. |
| Free of selective reporting? | Low risk | A ‐ Adequate. Treatment effects were not a result of confounding by selective data and were of equal size for participants with and without missing data. |
| Free of other bias? | Low risk | A ‐ Adequate. The study appears to be free of other sources of bias. The study protocol is available and all of the study's pre‐specified outcomes have been reported in the pre‐specified way. There were no significant differences in the treatment and placebo group regarding individual or environmental characteristics, medication use or pre‐treatment outcome variables. |
Serfaty 2002.
| Methods | Randomized, controlled, double blind placebo‐controlled two period crossover design (2 weeks + 2 weeks) | |
| Participants | 16 males 9 females Mean age: 84.2 (SD 7.6). Diagnosed with DSM‐1V . Clinical Diagnosis: AD (18); MultiInfarct Dementia (4); Mixed Dementia (3). Carers: multiple carers (20), single carer (5). Setting: nursing home room (16), home setting (5), hospital setting (4). Mean MMSE at baseline = 13.4 (SD 8.5). Demographic description of four remaining participants was not published. | |
| Interventions | 1. Melatonin 6 mg (SR) given at participants' usual bedtime. 2. placebo | |
| Outcomes | Cognitive change in MMSE. Outcome measured at 2 weeks. | |
| Notes | PI: Dr. Marc Serfaty, Department of Psychiatry and Behavioral Sciences, Royal Free and University College Medical School, London E‐mail: mserfaty@rfc.ucl.ac.uk. Requested mean change score and MMSE demographics. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A ‐Adequate. Computer generated numbering system to achieve randomized allocation to treatment or control group. |
| Allocation concealment? | Low risk | A ‐ Adequate. Key codes for the double blind allocation sequence were not opened until after the data analyses were completed |
| Blinding? All outcomes | Low risk | A ‐ Adequate. Those who administered melatonin and assessed outcomes were blind to allocation to the intervention or control group. |
| Incomplete outcome data addressed? All outcomes | Low risk | A ‐ Adequate. 85% compliance rate. |
| Free of selective reporting? | Low risk | A ‐ Attrition rate reported. |
| Free of other bias? | Low risk | A ‐ Potential confounding factors addressed in exclusion criteria. |
Singer 2003.
| Methods | Randomized, controlled, double blind study, 7 weeks duration | |
| Participants | 157 in total 88 females 69 males Mean age: 77.4 (SD 8.9) NINCDS‐ADRD diagnosis of probable AD. Setting: Long Term care facility and private homes. Extensive baseline data for all 3 groups on data abstraction tool. Baseline moderate MMSE for all groups. | |
| Interventions | 1. Melatonin 2.5 mg (SR) or 10 mg (IR) given once a day 1 hour prior to usual bedtime. 2. Placebo | |
| Outcomes | Cogitive and non‐cognitive changes in MMSE, CDR, ADAS‐cognitive and ADAS non‐cognitive, NPI behavioral and affective. Outcome measured at 7 weeks. | |
| Notes | PI: Dr. Clifford Singer, Sleep and Mood Disorders Laboratory, Oregon Health and Science University. E‐mail: singer@ohsu.edu | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A ‐ Adequate. Randomization and code development was done at the Alzheimer's Disease Cooperative Study Unit (ADCS) at the University of California San Diego. |
| Allocation concealment? | Low risk | A ‐ Adequate. Key codes for the double blind allocation sequence were not opened until after the data analyses were complete. |
| Blinding? All outcomes | Low risk | A ‐ Adequate. Those who administered melatonin and assessed outcomes were blind to allocation to the intervention or control group. |
| Incomplete outcome data addressed? All outcomes | Low risk | A ‐ Adequate. Four percent attrition equal between intervention and control group. |
| Free of selective reporting? | Low risk | A ‐ Adequate. Attrition rate reported. The study protocol is available and all of the study's pre‐specified outcomes have been reported in the pre‐specified way. |
| Free of other bias? | Low risk | A ‐ Adequate. Potential confounding factors addressed in exclusion criteria. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baskett 2003 | Study exclusion criteria included cognitive impairment as measured by score below 26 on Mini‐Mental State Examination. Sleep quality was only outcome measured by the study. |
| Bourne 2006 | Participants were not diagnosed with dementia or cognitive impairment. |
| Dowling 2008 | Unable to separate effects of combined interventions of bright light therapy and melatonin. |
| Eeles 2003 | No record of study completion. |
| Furio 2007 | This melatonin for cognitive impairment study does not include those with dementia (only a 12% risk of developing dementia). |
| Haffmans 2001 | Unable to separate effects of combined interventions of bright light therapy and melatonin. |
| Haworth 2001 | Reply from author on 20 February 2005 indicated that study did not proceed due to lack of funding. |
| Peck 2004 | Participants were not diagnosed with dementia or cognitive impairment. |
| Riemersma 2004 | Unable to separate effects of combined interventions of melatonin and bright light therapy. |
| Riemersma‐van der Lek 2005 | Unable to separate effects of combined interventions of bright light therapy and melatonin. |
| Savaskan 2006 | Literature review. The article does mention two placebo controlled trials, however they are not named in the text. |
| Singer 2005 | Sleep quality was only outcome measured by the study. |
| Tozawa 1998 | Sleep waking and activity levels were measured concomitantly in the study. |
| Valontonin 2005 | Sleep outcomes were the only outcome measured in the first study. The second study measured sleep quality and activity levels for fairly healthy older adults living in rest homes. |
Contributions of authors
All correspondence: DF Search for trials: VD Obtaining copies of trial reports: VD Selection of trials for inclusion/exclusion: LJ, VD, DF
Extraction of data: LJ, VD Entry of data: LJ, VD Interpretation of data analysis: DF, LJ, VD, DM Drafting review: LJ, DF, VD, DM
‐Contact editor: Rupert McShane ‐Consumer editor: Toby Scott
This review was peer reviewed in January 2006
Declarations of interest
None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Asayama 2003 {published and unpublished data}
- Asayama K, Yamadera H, Ito T, Suzuki H, Kudo Y, Endo S. Double blind study of melatonin effects on the sleep‐wake rhythm, cognitive and non‐cognitive functions in Alzheimer type dementia. Journal of Nippon Medical School; Nihon Ika Daigaku zasshi 2003;70(4):334‐41. [DOI] [PubMed] [Google Scholar]
Gehrman 2009 {published data only}
- Gehrman PR, Connor DJ, Martin JL, Shochat T, Corey‐Bloom J, Ancoli‐Israel S. Melatonin Fials to improve sleep or agitation in double‐blind randomized trial of institutionalized patients with Alzheimer Disease. American Journal of Geriatric Psychiatry 2009;17(2):166‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Riemersma‐van der Lek 2008 {published and unpublished data}
- Riemersma‐van der Lek, RF, Swaab, DF, Twisk, J, Hol, EM, Hoogendijik, WJG, Van Someren, EJW. Effect of Bright Light and Melatonin on Cognitive and Noncognitive Function in Elderly Residents of Group Care Facilities. Journal of the American Medical Association 2008;299:2642‐2655. [DOI] [PubMed] [Google Scholar]
Serfaty 2002 {published and unpublished data}
- Serfaty M, Kennell Webb S, Warner J, Blizard R, Raven P. Double blind randomised placebo controlled trial of low dose melatonin for sleep disorders in dementia. International Journal of Geriatric Psychiatry 2002;17(12):1120‐7. [DOI] [PubMed] [Google Scholar]
Singer 2003 {published and unpublished data}
- Singer C, Tractenberg RE, Kaye J, Schafer K, Gamst A, Grundman M, Thomas R, Thal LJ, Alzheimer's Disease Cooperative Study. A multicenter, placebo‐controlled trial of melatonin for sleep disturbance in Alzheimer's disease. Sleep 2003;26(7):893‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Baskett 2003 {published data only}
- Baskett JJ, Broad JB, Wood PC, Duncan JR, Pledger MJ, English J, et al. Does melatonin improve sleep in older people? A randomised crossover trial. Age and Ageing 2003;32(2):164‐70. [DOI] [PubMed] [Google Scholar]
Bourne 2006 {unpublished data only}
- Bourne R. Evaluation of melatonin therapy on sleep and delirium in intensive care patients. ISRCTN Register: ISRCTN47578325 2006. [ISCTRN Register 2006]
Dowling 2008 {published data only}
- Dowling GA, Burr RL, Someren EJ, Hubbard EM, Luxenberg JS, Mastick J, et al. Melatonin and bright‐light therapy for rest activity disruption in institutionalized patients with Alzheimer's disease. Journal of the American Geriatrics Society 2008;56:239‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eeles 2003 {unpublished data only}
- Eeles. The effect of melatonin on sleep pattern and levels of agitation in patients with dementia. National Research Register 2003. [Google Scholar]
Furio 2007 {published data only}
- Furio AM, Brusco AI, Cardinali DP. Possible therpeutic value of melatonin in mild cognitive impairment. A retrospective study. Journal of Pineal Research 2007;43(4):404‐9. [DOI] [PubMed] [Google Scholar]
Haffmans 2001 {published data only}
- Haffmans PM, Sival RC, Lucius SA, Cats Q, Gelder L. Bright light therapy and melatonin in motor restless behaviour in dementia: A placebo‐controlled study. International Journal of Geriatric Psychiatry 2001;16(1):106‐10. [DOI] [PubMed] [Google Scholar]
- Sival RC, Haffmans PMJ, Cats Q, et al. The effect of bright light and melatonin on motorrestless behavior in dementia. Neurobiology of Aging 1998;19(Suppl 4):S213. [Google Scholar]
Haworth 2001 {unpublished data only}
- Haworth J. A pilot, double‐blind, placebo controlled, parellel group study of the effect of melatonin treatment in patients with Alzheimer's disease and sleep. National Research Register 2001a.
Peck 2004 {published data only}
- Peck JS, LeGoff DB, Ahmed I, Goebert D. Cognitive effects of exogenous melatonin administration in elderly persons. A pilot study. American Journal of Geriatric Psychiatry 2004;12(4):432‐6. [DOI] [PubMed] [Google Scholar]
Riemersma 2004 {published data only}
- Riemersma RF, Netherlands Institute for Brain Research, Amsterdam, Netherlands. Light and Melatonin: Effect on sleep, mood and cognition in demented elderly. NeuroBiology of Aging 2004;25(S2):194. [Google Scholar]
Riemersma‐van der Lek 2005 {unpublished data only}
- Riemersma van der Lek. The effect of light and/or melatonin on sleep, mood, cognition and behavior in demented elderly. ISRCTN Register 2005. [ISRCTN Register 2005]
Savaskan 2006 {published data only}
- Savaskan E. Melatonin in the elderly and Alzheimer's disease. Schweiz Rundsch Med Prax 2006;95(47):1837‐9. [DOI] [PubMed] [Google Scholar]
Singer 2005 {unpublished data only}
- Singer C. Quetiapine for the treatment of insomnia associated with Alzheimer's disease. Clinical Trials.Gov 2005.
Tozawa 1998 {published and unpublished data}
- Tozawa T, Mishima K, Satoh K, Matsumoto Y, Echizenya M, Sasaki M, et al. Melatonin replacement therapy for rest‐activity rhythm disorders in patients with senile dementia of alzheimer's type. Proceedings of the 21st Collegium Internationale Neuropsychopharmacologium (CINP) Congress; 1998 Jul 12‐16 Glasgow, Scotland. 1998.
- Tozawa T, Mishima K, Satoh K, et al. Melatonin replacement therapy for rest‐activity rhythm disorder in patients with senile dementia of Alzheimer's Type. Neurobiology of Aging 1998;19(Suppl 4):S182. [Google Scholar]
Valontonin 2005 {published data only}
- Valtonin M, Niskanen L, Kangas AP, Koskinen T. Effect of melatonin‐rich night‐time milk on sleep and activity in institutionalized elderly institutionalized subjects. Nordic Journal of Psychiatry 2005;59(3):217‐21. [DOI] [PubMed] [Google Scholar]
Additional references
Alexopoulos 1988
- Alexopoulos GS, Abrams RC, Young RC, Shamoian RA. Cornell Scale for Depression in Dementia. Biological Psychiatry 1988;23(3):271‐284. [DOI] [PubMed] [Google Scholar]
Andrade 2001
- Andrade C, Srihari BS, Reddy KP, Chandramma L. Melatonin in medically ill patients with insomnia: a double‐blind, placebo‐controlled study. Journal of Clinical Psychiatry 2001;62(1):41‐5. [DOI] [PubMed] [Google Scholar]
APA 1995
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: American Psychiatric Association, 1995. [Google Scholar]
APA 2004
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
Auger 2007
- Auger R, Boeve B. Sleep and neurodegenerative disorders. Sleep Disorders 2007;13 (3):201‐224. [Google Scholar]
Bagby 2004
- Bagby RM, Ryder AG, Shuller DR, Marshall MB. The Hamilton Depression Rating Scale: has the gold standard become a lead weight. American Journal of Psychiatry 2004;161:2163‐77. [DOI] [PubMed] [Google Scholar]
Bersani 2000
- Bersani G, Garavini A. Melatonin add‐on in manic patients with treatment resistant insomnia. Progress in Neuropsychopharmacology and Biological Psychiatry 2000;24(2):185‐91. [DOI] [PubMed] [Google Scholar]
Bizot‐Espiard 1998
- Bizot‐Espiard JG, Double A, Cousin B, Lesieur D, Guardiola‐Lemaitre B, Delagrange P, Ktorza A, Penicaud L. Lack of melatonin effects on insulin action in normal rats. Hormone and Metabolic Research 1998;30(12):711‐6. [DOI] [PubMed] [Google Scholar]
Bliwise 1983
- Bliwise DL, Lee KA. Development of an Agitated Behavior Rating Scale for discrete temporal observations. Journal of Nursing Measurement 1983;1:115‐124. [PubMed] [Google Scholar]
Bonnani 2005
- Bonanni E, Maestri M, Tognoni G, Fabbrini M, Nucciarone B, Manca ML, Gori S, Iudice A, Murri L. Daytime sleepiness in mild and moderate Alzheimer’s disease and its relationship with cognitive impairment. Journal of Sleep Research 2005;14:311‐317. [DOI] [PubMed] [Google Scholar]
Brorsson 1984
- Brorsson B, HAsberg K. Katz index of independence in ADL. Reliability and validity in short‐term care. Scandinavian Journal of Rehabilitation Medicine 1984;16(3):125‐32. [PubMed] [Google Scholar]
Buscemi 2004
- Buscemi N, Vandermeer B, Pandya R, Hooton N, Tjosvold L, Hartling L, Baker G, Vohra S, Klassin T. Melatonin for treatment of sleep disorders. Evidence Report/Technology Assessment No. 108. Prepared by the University of Alberta Evidence‐based Practice Centre, Contract No. 290‐02‐0023. AHRQ Publication No. 05‐E002‐2.. Rockville, MD: Agency for Healthcare Research and Quality, November 2004. [DOI] [PMC free article] [PubMed]
Buscemi 2006
- Buscemie N, Vandermaeer B, Hooton, N, Pandya R, Tjosvold L, Hartling L, Vohra S, Klassen TP, Baker G. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta‐analysis. British Medical Journal 2006;332:385‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cagnacci 2001
- Cagnacci A, Arangino S, Renzi A, Paoletti AM, Melis GB, Cagnacci P, Volpe A. Influence of melatonin administration on glucose tolerance and insulin sensitivity of postmenopausal women. Clinical Endocrinology 2001;54(3):339‐46. [DOI] [PubMed] [Google Scholar]
Cagnacci 2001a
- Cagnacci A, Arangino S, Angiolucci M, Melis GB, Facchinetti F, Malmusi S, Volpe A. Effect of exogenous melatonin on vascular reactivity and nitric oxide in postmenopausal women: role of hormone replacement therapy. Clinical Endocrinology 2001;54(2):261‐6. [DOI] [PubMed] [Google Scholar]
Capsoni 1995
- Capsoni S, Stankov BM, Fraschini F. Reduction of regional cerebral blood flow by melatonin in young rats. Neuroreport 1995;6(9):1346‐8. [DOI] [PubMed] [Google Scholar]
Carman 1976
- Carman JS, Post RM, Buswell R, Goodwin FK. Negative effects of melatonin on depression. American Journal of Psychiatry 1976;133(10):1181‐6. [DOI] [PubMed] [Google Scholar]
Chuang 1993
- Chuang JI, Chen SS, Lin MT. Melatonin decreases brain serotonin release, arterial pressure and heart rate in rats. Pharmacology 1993;47(2):91‐7. [DOI] [PubMed] [Google Scholar]
Claustrat 1997
- Claustrat B, Brun J, Geoffriau M, Zaidan R, Mallo C, Chazot G. Nocturnal plasma melatonin profile and melatonin kinetics during infusion in status migrainosus. Cephalalgia 1997;17(4):511‐7. [DOI] [PubMed] [Google Scholar]
Coffey 1994
- Coffey CE, Cummings JL, editors. Textbook of Geriatric Neuropsychiatry. Washington, DC: American Psychiatric Press, 1994. [Google Scholar]
Cohen‐Mansfield 1989
- Cohen‐Mansfield J, Marx M, Rosenthal A. A description of agitation in a nursing home. Journal of Gerontology 1989;44:M77‐M84. [DOI] [PubMed] [Google Scholar]
CSHA 1994
- Canadian Study of Health and Aging Working Group. Canadian study of health and aging: Study methods and prevalence of dementia. Canadian Medical Association Journal 1994;150(6):899‐913. [PMC free article] [PubMed] [Google Scholar]
Cummings 1994
- Cummings JL, Mega M, Gray M, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308‐14. [DOI] [PubMed] [Google Scholar]
De Deyn 2000
- Deyn P, Wirshing WC. Scales to assess efficacy and safety of pharmacologic agents in the treatment of behavioral and psychological symptoms of dementia. The Journal of Clinical Psychiatry 2001;62(Suppl 21):19‐22. [PubMed] [Google Scholar]
Detanico 2009
- Detainico BC, Piato AL, Freitas JJ, Lhullier FL, Hidalgo MP, Caumo W, Elisabetsky E. Antidepressant‐like effects of melatonin in the mouse chronic mild stress model. European Journal of Pharmacology 2009;607(1‐3):121‐125. [DOI] [PubMed] [Google Scholar]
Downey 1987
- Downey R, Bonnet MH. Performance during frequent sleep disruption. Sleep 1987;10:354‐63. [DOI] [PubMed] [Google Scholar]
Dubocovich 1991
- Dubocovich ML. Melatonin receptors in the central nervous system. Advances in Experimental Medicine and Biology 1991;294:255‐65. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins J, Curtin F, Worthington H, Vail A. Meta‐analyses involving cross‐over trials: Methodological issues. International Journal of Epidemiology 2002;31:140‐149. [DOI] [PubMed] [Google Scholar]
Erdem 2005
- Erdem T, Ozturan O, Iraz M, Miman MC, Olmez E. Dose‐dependent dual effect of melatonin on ototoxicity induced by amikacin in adult rats. European Archives of Otorhinolaryngology 2005;262(4):314‐321. [DOI] [PubMed] [Google Scholar]
Fauteck 1995
- Fauteck JD, Bockmann J, Böckers TM, Wittkowski W, Köhling R, Lücke A, et al. Melatonin reduces low‐Mg2+ epileptiform activity in human temporal slices. Experimental Brain Research 1995;107:321‐5. [DOI] [PubMed] [Google Scholar]
Ferrari 2000
- Ferrari E, Arcaini A, Gornati R, Pelanconi L, Cravello L, Fioravanti M, Solerte SB, Magri F. Pineal and pituitary‐adrenocortical function in physiological aging and in senile dementia. Experiemental Gerontology 2000;35(9‐10):1239‐1250. [DOI] [PubMed] [Google Scholar]
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189‐98. [DOI] [PubMed] [Google Scholar]
Forbes 2003
- Forbes DA. An example of the use of systematic reviews to answer effectiveness questions. Western Journal of Nursing Research 2003;25(2):179‐92. [DOI] [PubMed] [Google Scholar]
Gagnier 2001
- Gagnier JJ. The therapeutic potential of melatonin in migraines and other headache types. Alternative Medicine Review 2001;6(4):383‐9. [PubMed] [Google Scholar]
Galasko 1997
- Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, et al. An inventory to assess activities of daily living for clinical trails in Alzheimer's disease: The Alzheimer's Disease Cooperative Study. Alzheimer's Disease and Associated Disorders 1997;11(Suppl 2):S33‐9. [PubMed] [Google Scholar]
Ghali 1995
- Ghali L, Hopkins RW, Rindlisbacher P. The fragmentation of the rest/activity cycles in Alzheimer's disease. International Journal of Geriatric Psychiatry 1995;10:299‐304. [Google Scholar]
Guo 2009
- Guo 2009. Serum melatonin levels in children with epilepsy or febrile seizures. Chinese Journal of Contemporary Pediatrics 2009;11(4):288‐290. [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hardeland 2008
- Hardeland R, Poeggeler B, Srinivasan V, Trakt I, Pandi‐Perumal SR, Cardinali DP. Melatonergic drugs in clinical practice. Arzneimittelforschung 2008;58(1):1‐10. [DOI] [PubMed] [Google Scholar]
Helmes 1987
- Helmes EK, Csapo G, Short JA. Standardization and validation of the Multidimensional Observation Scale for Elderly Subjects (MOSES). The Journals of Gerontology 1987;42(4):395‐405. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org. Interscience Wiley.
Hopkins 1992
- Hopkins RW, Rindlisbacher P. Fragmentation of activity periods in Alzheimer's disease. International Journal of Geriatric Psychiatry 1992;7:805‐12. [Google Scholar]
Hopkins 1995
- Hopkins RW, Rindlisbacher P. Some clinical consequences of the rest and activity disturbance in Alzheimer's disease. American Journal of Alzheimer's Care and Related Disorders Research 1995;10:16‐25. [Google Scholar]
Hughes 1982
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for staging of dementia. British Journal of Psychiatry 1982;140:566‐72. [DOI] [PubMed] [Google Scholar]
Ismail 2009
- Ismail S, Mowafi H. Melatonin provides anxiolysis, enhances analgesia, decreases intraocular pressure, and promotes better operating conditions during cataract surgery under topical anesthesia. Anesthesia and Analgesia 2009;108(4):1058‐1061. [DOI] [PubMed] [Google Scholar]
Kaufer 2000
- Kaufer DL, Jeffrey L, Cummings MD, Ketchel P, Smith V, MacMillan, A, Shelley T, Lopez O. Validation of the NPI‐Q, a Brief Clinical Form of theNeuropsychiatric Inventory. The Journal of Neuropsychiatry and Clinical Neurosciences 2000;12:233‐239. [DOI] [PubMed] [Google Scholar]
Kedziora‐Kornatowska 2009
- Kedziora‐Kornatowska K, Szewczyk‐Golec K, Kozakiewicz M, Pawluk H, Czuczejko J, Kornatowski T, Bartosz G, Kedziora J. Melatonin improves oxidative stress parameters in the blood of elderly type 2 diabetic patients. Journal of Pineal Reseach 2009;46(3):333‐337. [DOI] [PubMed] [Google Scholar]
Krinsky 2004
- Krinsky DL, LaValle J, Hawkins E, Pelton R, Ashbrook Willis N. Lexi‐Natural Products Online TM, Melatonin. Hudson, Ohio: lexi‐Comp, Inc. January 29, 2004.
Kunz 1999
- Kunz D, Schmitz S, Mahlberg R, Mohr A, Stöter C, Wolf K, Herrmann W. A new concept for melatonin deficit: On pineal calcification and melatonin excretion. Neuropsychopharmacology 1999;21:765‐772. [DOI] [PubMed] [Google Scholar]
Kørner 2006
- Kørner A, Lauritzen L, Abelskov K, Gulmann N, Marie Brodersen A, Wedervang‐Jensen T, et al. The Geriatric Depression Scale and the Cornell Scale for Depression in Dementia. A validity study. Nordic Journal Of Psychiatry 2006;60(5):360‐364. [DOI] [PubMed] [Google Scholar]
Lawton 1972
- Lawton MP. The Philadelphia Geriatric Center morale scale. In: Kent DP, Kastenbaum R, Sherwood S editor(s). Research planning and action for the elderly: the power and potential of social science. Behavioral Publications, 1972:144‐165. [Google Scholar]
Lawton 1996
- Lawton MP, Haitsma K, Klapper J . Observed affect in nursing home residents with Alzheimer's disease. The Journals of Gerontology 1996;51:P3. [DOI] [PubMed] [Google Scholar]
Leibenluft 1997
- Leibenluft E, Feldman‐Naim S, Turner EH, Wehr TA, Rosenthal NE. Effects of exogenous melatonin administration and withdrawal in five patients with rapid‐cycling bipolar disorder. Journal of Clinical Psychiatry 1997;58(9):383‐8. [DOI] [PubMed] [Google Scholar]
Leino 1984
- Leino M, Aho IM, Kari E, Gynther J, Markkanen S. Effects of melatonin and 6‐methoxy‐tetrahydro‐beta‐carboline in light induced retinal damage: a computerized morphometric method. Life Sci 1984;35(20):1997‐2001. [DOI] [PubMed] [Google Scholar]
Maestroni 1993
- Maestroni GJ. The immunoneuroendocrine role of melatonin. Journal of Pineal Research 1993;14(1):1‐10. [DOI] [PubMed] [Google Scholar]
Maestroni 2001
- Maestroni GJ. The immunotherapeutic potential of melatonin. Expert Opinion Investigational Drugs 2001;10(3):467‐76. [DOI] [PubMed] [Google Scholar]
Mahle 1997
- Mahle CD, Goggins GD, Agarwal P, Ryan E, Watson AJ. Melatonin modulates vascular smooth muscle tone. Journal of Biological Rhythms 1997;12(6):690‐6. [DOI] [PubMed] [Google Scholar]
McDowell 1996
- McDowell I. Measuring Health: A Guide to Rating Scales and Questionnaires. Oxford University Press, 2006. [Google Scholar]
McKhann 1984
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 1984;34:939‐944. [DOI] [PubMed] [Google Scholar]
Mills 2005
- Mills E, Wu P, Seely D, Guyatt G. Melatonin in the treatment of cancer: a systematic review of randomized controlled trials and meta‐analysis. Journal of Pineal Research 2005;39(4):360‐388. [DOI] [PubMed] [Google Scholar]
Mishima 1994
- Mishima K, Okawa M, Hishikawa Y, Hozumi S, et al. Morning bright light therapy for sleep and behavior disorders in elderly patients with dementia. Acta Psychiatrica Scandinavica 1994;89(1):1‐7. [DOI] [PubMed] [Google Scholar]
Moore 1992
- Moore RY. The organization of the human circadian timing system. Progress in Brain Research 1992;93:101‐17. [PubMed] [Google Scholar]
Munoz‐Hoyos 1998
- Munoz‐Hoyos A, Sanchez‐Forte M, Molina‐Carballo A, Escames G, Martin‐Medina E, Reiter RJ, Molina‐Font JA, Acuna‐Castroviejo D. Melatonin's role as an anticonvulsant and neuronal protector: experimental and clinical evidence. Journal of Child Neurology 1998;13(10):501‐9. [DOI] [PubMed] [Google Scholar]
Pierrefiche 1995
- Pierrefiche G, Laborit H. Oxygen free radicals, melatonin, and aging. Experimental Gerontology 1995;30(3‐4):213‐27. [DOI] [PubMed] [Google Scholar]
Prinz 1982
- Prinz PN, Vitaliano PP, Vitiello MV, Bokan J, Raskind M, Peskind E, Gerber C. Sleep, EEG and mental function changes in senile dementia of the Alzheimer's type. Neurobiology of Aging 1982;3:361‐370. [DOI] [PubMed] [Google Scholar]
Rasmussen 1999
- Rasmussen DD, Boldt BM, Wilkinson CW, Yellon SM, Matsumoto AM. Daily melatonin administration at middle age suppresses male rat visceral fat, plasma leptin, and plasma insulin to youthful levels. Endocrinology 1999;140(2):1009‐12. [DOI] [PubMed] [Google Scholar]
Regrigny 1998
- Regrigny O, Delagrange P, Scalbert E, Atkinson J, Lartaud‐Idjouadiene I. Melatonin improves cerebral circulation security margin in rats. American Journal of Physiology 1998;275(1 Pt 2):H139‐44. [DOI] [PubMed] [Google Scholar]
Reiter 1994
- Reiter RJ, Tan DX, Poeggeler B, et al. Melatonin as a free radical scavenger: implications for aging and age‐related diseases. Annals of the New York Academy of Sciences 1994;719:1‐12. [DOI] [PubMed] [Google Scholar]
Reiter 1995
- Reiter RJ, Melchiorri D, Sewerynek E, Poeggeler B, Barlow‐Walden L, Chuang J, Ortiz GG, Acuna‐Castroviejo D. A review of the evidence supporting melatonin's role as an antioxidant. Journal of Pineal Research 1995;18(1):1‐11. [DOI] [PubMed] [Google Scholar]
Reiter 2000
- Reiter RJ, Calvo JR, Karbownik M, Qi W, Tan DX. Melatonin and its relation to the immune system and inflammation. Annals of the New York Academy of Sciences 2000;917:376‐86. [DOI] [PubMed] [Google Scholar]
Robertson 1997
- Robertson JM, Tanguay PE. Case study: the use of melatonin in a boy with refractory bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(6):822‐5. [DOI] [PubMed] [Google Scholar]
Rosen 1984
- Rosen WG, Richard CM, Davis KL. A new scale for Alzheimer's Disease. American Journal of Psychiatry 1984;141(11):1356‐64. [DOI] [PubMed] [Google Scholar]
Seabra 2000
- Seabra ML, Bignotto M, Pinto LR Jr, Tufik S. Randomized, double‐blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. Journal of Pineal Research 2000;29(4):193‐200. [DOI] [PubMed] [Google Scholar]
Shamir 2000
- Shamir E, Barak Y, Plopsky I, Zisapel N, Elizur A, Weizman A. Is melatonin treatment effective for tardive dyskinesia?. Journal of Clinical Psychiatry 2000;61(8):556‐8. [DOI] [PubMed] [Google Scholar]
Siu 1999
- Siu AW, Reiter RJ, To CH. Pineal indoleamines and vitamin E reduce nitric oxide‐induced lipid peroxidation in rat retinal homogenates. Journal of Pineal Research 1999;27(2):122‐8. [DOI] [PubMed] [Google Scholar]
Srinivasan 2009
- Srinivasan V, Pandi‐Perumal SR, Trakht I, Spence DW, Hardeland R, Poeggeler B, Cardinali DP. Pathophysiology of depression: role of sleep and the melatonergic system. Psychiatry Research 2009;165(3):201‐214. [DOI] [PubMed] [Google Scholar]
Swaab 1985
- Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res 1985;342:37‐44. [DOI] [PubMed] [Google Scholar]
Teri 2003
- Teri L, Logsdon R, Gibbons L, McCurry, S, Logsdon R, Buchner D, Barlow W, Kukull W, LaCroix A, McCormick W, Larson E. Exercise plus behavioral management in patients with Alzheimer Disease. JAMA 2003;290:2015‐2022. [DOI] [PubMed] [Google Scholar]
Thompson 2007
- Thompson S, Hermann N, Rapoport MJ, Lanctot KL. Efficacy and safety of antidepressants for treatment of depression in Alzheimer's disease: a meta‐analysis. Canadian Journal of Psychiatry: Revue Canadienne De Psychiatrie 2007;52(4):248‐255. [DOI] [PubMed] [Google Scholar]
Tom 2001
- Tom B, Vries P, Heiligers JP, Willems EW, Scalbert E, Delagrange P, Saxena PR. The lack of vasoconstrictor effect of the pineal hormone melatonin in an animal model predictive of antimigraine activity. Cephalalgia 2001;21(6):656‐63. [DOI] [PubMed] [Google Scholar]
Tombaugh 1992
- Tombaugh TN, McIntyre NJ. The Mini‐Mental State Examination: A comprehensive review. American Geriatrics Society 1992;40:922‐35. [DOI] [PubMed] [Google Scholar]
Tunis 2003
- Tunis Sr, Stryer DB, Clancy CM. Practical clinical trials: Increasing the value of clinical research for decision making in clinical and health policy. Journal of the Americal Medical Association 2003, (290 (12)):1624‐1632. [DOI] [PubMed] [Google Scholar]
Varadarajan 2000
- Varadarajan S, Yatin S, Aksenova M, Butterfield DA. Review: Alzheimer's amyloid beta‐peptide‐associated free radical oxidative stress and neurotoxicity. Journal of Structural Biology 2000;130(2‐3):184‐208. [DOI] [PubMed] [Google Scholar]
Viswanathan 1997
- Viswanathan M, Scalbert E, Delagrange P, Guardiola‐Lemaitre B, Saavedra JM. Melatonin receptors mediate contraction of a rat cerebral artery. Neuroreport 1997;8(18):3847‐9. [DOI] [PubMed] [Google Scholar]
Webb 1995
- Webb SM, Puig‐Domingo M. Role of melatonin in health and disease. Clinical Endocrinology (Oxf) 1995;42(3):221‐34. [DOI] [PubMed] [Google Scholar]
Weekley 1995
- Weekley LB. Pharmacologic studies on the mechanism of melatonin‐induced vasorelaxation in rat aorta. Journal of Pineal Research 1995;19(3):133‐8. [DOI] [PubMed] [Google Scholar]
Wehr 2001
- Wehr TA, Duncan WC Jr, Sher L, Aeschbach D, Schwartz PJ, Turner EH, et al. A circadian signal of change of season in patients with seasonal affective disorder. Archives of General Psychiatry 2001;58(12):1108‐14. [DOI] [PubMed] [Google Scholar]
Wiechmann 1992
- Wiechmann AF, O'Steen WK. Melatonin increases photoreceptor susceptibility to light‐induced damage. Investigative Ophthalmology and Visual Science 1992;33(6):1894‐902. [PubMed] [Google Scholar]
Williams 2001
- Williams JBW. Standardizing the Hamilton Depression Rating Scale: past, present, and future. European Archives of Psychiatry and Clinical Neuroscience 2001;251:Supplement 2. [DOI] [PubMed] [Google Scholar]
Williamson 1998
- Williamson BL, Mishra PK, Gleich GJ, Naylor S. Structural characterization of contaminants found in commercial preparations of melatonin: similarities to case‐related compounds from L‐tryptophan associated with eosinophilia‐myalgia syndrome. Chemical Research in Toxicology 1998;11(3):234‐240. [DOI] [PubMed] [Google Scholar]
Wu 2003
- Wu YH, Feenstra, MG, Shou, et al. Molecular changes underlying reduced pineal melatonin levels in Alzheimer disease: Alterations in preclinical and clinical stages.. Journal of Clinical Endocrinological Metabolism 2003;88:5898‐5906. [DOI] [PubMed] [Google Scholar]
Wu 2005
- Wu Y, Swaab D. The human pineal gland and melatonin in aging and Alzheimer's disease. Journal of Pineal Research 2005;38(3):145‐152. [DOI] [PubMed] [Google Scholar]
Wu 2007
- Wu Y, Swaab D. The human pineal gland and melatonin in aging and Alzheimer's disease. Journal of Pineal Research 2007;38(3):145‐152. [DOI] [PubMed] [Google Scholar]
Wurtman 1989
- Wurtman RJ, Wurtman JJ. Carbohydrates and Depression. Scientific American 1989;260(1):68‐75. [DOI] [PubMed] [Google Scholar]
Yesavage 2003
- Yesavage J, Friedman L, Ancoli‐Israel S, Bliwise D, Singer C, Vitiello M, Monjan A, Lebowitz B. Development of diagnostic criteria for defining sleep disturbance in Alzheimer's disease. Journal of Geriatric Psychiatry and Neurology 2003;16:131‐139. [DOI] [PubMed] [Google Scholar]
Yildiz 2009
- Yildiz M, Osman A. Assessment of the effects of physiological release of melatonin on arterial distensibility and blood pressure. Cardiology in the Young 2009;19:198‐203. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Jansen 2006
- Jansen SL, Forbes DA, Duncan V, Morgan DG. Melatonin for cognitive impairment. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD003802.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


