Abstract
Background
Intestinal fibrosis and subsequent intestinal obstruction are common complications of Crohn’s disease (CD). Current therapeutics combat inflammation, but no pharmacological therapy exists for fibrostenotic disease. Pathological persistence of activated intestinal myofibroblasts is a key driver of fibrosis in CD. In other organ systems, BH-3 mimetic drugs that affect Bcl-2 apoptotic pathways induce apoptosis in activated myofibroblasts and reduce fibrogenic gene expression, thereby reducing fibrosis.
Methods
We evaluated the proapoptotic and antifibrotic efficacy of several classes of BH-3 mimetics in 2 in vitro fibrogenesis models. The candidate molecule, ABT-263, was advanced to a 3-dimensional human intestinal organoid (HIO) model. Finally, the therapeutic efficacy of ABT-263 was evaluated in the mouse Salmonella typhimurium intestinal fibrosis model.
Results
The BH-3 mimetics induced apoptosis, repressed fibrotic protein expression, and reduced fibrogenic gene expression in normal human intestinal myofibroblasts. The BH-3 mimetics that target Bcl-2 and Bcl-xl demonstrated the greatest efficacy in vitro. The ABT-199 and ABT-263 induced apoptosis and ameliorated fibrogenesis in the in vitro myofibroblast models. In the HIO model, ABT-263 inhibited fibrogenesis and induced apoptosis. In the mouse S. typhimurium model, dose-dependent reduction in macroscopic pathology, histological inflammation, inflammatory and fibrotic gene expression, and extracellular matrix protein expression indicated ABT-263 may reduce intestinal fibrosis.
Conclusions
In vitro, the antifibrotic efficacy of BH-3 mimetics identifies the Bcl-2 pathway as a druggable target and BH-3 mimetics as putative therapeutics. Reduction of inflammation and fibrosis in the mouse intestinal fibrosis model by ABT-263 indicates BH-3 mimetics as potential, novel antifibrotic therapeutics for Crohn’s disease.
Keywords: inflammatory bowel disease, Crohn’s disease, fibrosis, myofibroblast, Bcl-2, ABT-263, navitoclax, BH-3 mimetic
Lay Summary
Intestinal fibrosis is a common complication of Crohn’s disease, yet no effective therapies exist to treat fibrostenotic disease. We report ABT-263 (navitoclax) reduces intestinal fibrosis in in vitro models and reduces inflammation and fibrosis in a mouse IBD model.
Introduction
Crohn’s disease (CD) is a chronic, progressive, and frequently debilitating intestinal disorder characterized by cycles of inflammation and tissue regeneration. Despite powerful and effective anti-inflammatory therapeutics that reduce symptoms, the natural history of CD remains unaffected. A majority of patients develop complications within 5 to 10 years after diagnosis, most commonly due to fibrostenotic or penetrating disease.1,2 Current treatments for fibrostenotic disease, specifically endoscopic intervention or surgery, are invasive and have significant risks. There are currently no medical therapies that address intestinal fibrostenosis.2
Although inflammation precedes intestinal fibrostenosis, pathological persistence of activated intestinal myofibroblasts both induces and sustains fibrosis.3,4 Myofibroblasts are the key effector cells of normal wound healing, wound edge contraction, reconstitution of the extracellular matrix (ECM), and restoration of tissue integrity.4 During the resolution phase, myofibroblasts undergo apoptosis and are cleared from the tissue.5 Dysregulation of this normal repair response, characterized by sustained myofibroblast activation and failure to undergo timely apoptosis, is responsible for the excessive synthesis, deposition, and remodeling of ECM proteins in tissue fibrosis.6
One potential approach to treating fibrosis is restoring the physiologic apoptosis of activated myofibroblasts. Bcl-2 proteins are the major regulators of the intrinsic apoptosis pathway, composed of both proapoptotic and anti-apoptotic (prosurvival) members.7,8 Proapoptotic activator and sensitizer Bcl-2 family members share a common BH-3 homology domain that functions within a complex “interactome” of proapoptotic and prosurvival Bcl-2 proteins.9 Originally developed as adjuvant chemotherapeutic drugs, BH-3 mimetics inhibit prosurvival Bcl-2 factors and restore sensitivity to apoptosis.10,11 Beyond chemotherapeutic applications, BH-3 mimetics have demonstrated antifibrotic efficacy in preclinical models including skin,12 lung,13 liver,14 and heart.15 In the intestine, Bcl-2 regulates the differentiation of intestinal myofibroblasts, but the BH-3 mimetic ABT-737 reduces intestinal fibrosis in the dextran sulfate sodium (DSS) mouse model.16 Physical properties (lack of oral bioavailability and poor aqueous solubility) of ABT-737 precluded its use as a clinical therapeutic, leading to the development of ABT-263, a second-generation BH-3 mimetic.17 ABT-263 has shown promise as an antifibrotic therapy, reducing bone marrow fibrosis in human myelofibrosis and reversing established dermal fibrosis in a preclinical mouse model.12,18 However, dose-dependent thrombocytopenia has limited the utility of ABT-263. Reverse engineering ABT-263 using an elegant structure based approach yielded ABT-199 (venetoclax), a potent, orally bioavailable, and platelet-sparing BH-3 mimetic.19 Although use of venetoclax in combination therapy with other chemotherapeutic agents represents a major advance in the treatment of hematologic malignancies, the preclinical study by Lagares et al involving ABT-263 vs ABT-199 suggests venetoclax may lack antifibrotic efficacy inasmuch as ABT-199 failed to reduce dermal fibrosis.12
Given the efficacy of various BH-3 mimetics in multiple models of organ fibrosis, we proceeded with a testing funnel initially comparing the efficacy of 4 BH-3 mimetics with different selectivities using 2 previously validated in vitro intestinal fibrosis models. The 2 best compounds were directly compared in both in vitro fibrosis models. Prior to advancing to rodent studies, the best candidate was evaluated for antifibrotic efficacy in human intestinal organoids (HIOs), a 3-dimensional, multicellular in vitro “mini-gut” model previously validated for the study of intestinal fibrosis and potential therapies. ABT-263 (navitoclax) repressed fibrosis and induced apoptosis in HIOs. Therefore, ABT-263 was advanced to a mouse model of intestinal inflammation and fibrosis.20 In the mouse Salmonella typhimurium model, ABT-263 attenuated both chronic inflammation and fibrosis in a dose-dependent manner, suggesting therapeutic potential of BH-3 mimetics for fibrostenotic Crohn’s disease.
Materials and Methods
Ethical Statement
All human intestinal organoid work was approved by the University of Michigan Human Pluripotent Stem Cell Research Oversight Committee (HPSCRO). All murine studies were performed under the approval and oversight of the Institutional Animal Care & Use Committee of the University of Michigan in accordance with the standards of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (protocol number PRO00008074).
Reagents
ABT-199 and ABT-263 were synthesized by AbbVie pharmaceuticals. Spironolactone and tofacinitib were obtained from Sigma (St. Louis, MO). Additionally, A1155463 and A120477 were generous gifts from Dr. Shaomeng Wang, University of Michigan.
Cell Culture
Human colonic fibroblast CCD-18co cells (CRL-1459) were obtained from ATCC (Bethesda, MD). Cells were cultured in α-MEM (Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum, and subcultured weekly.
TGFβ Fibrogenesis Model
Low-passage number (<10 passages) normal colonic myofibroblasts (CCD-18co cells) were seeded at 5 × 104 cells/mL on 6-well tissue culture plates. Cells were serum-starved overnight before stimulation with 0.05 ng/mL of transforming growth factor β (TGFβ) as previously described.21,22 To determine the effect of ABT-263 and ABT-199, 0.1–1 µM of inhibitors were incubated for 24 hours before RNA harvest for 48 hours before protein harvest. The media was replaced at 24 hours with fresh media containing TGFβ or TGFβ and inhibitors.
In Vitro Stiffness Model
Low-passage number (<10 passages) normal colonic myofibroblasts, CCD-18co cells, were seeded at 1 × 105 cells/mL on 6-well plates containing acrylamide gels corresponding to the matrix stiffness of normal or fibrotic tissue.23 Briefly, human colonic myofibroblasts were plated on collagen-coated acrylamide substrates corresponding to normal compliant intestinal tissue (4.3 kPa). Cells were allowed to attach to the matrix for 2 hours before the transfer of the coverslip and hydrogel to a new 6-well plate containing minimal serum (0.5%) to avoid serum stimulation and potential paracrine signaling from cells adhering to the plastic surrounding the coverslip. To determine the effect of ABT-263 and ABT-199, 0.1–1 µM concentrations of inhibitors were added to cells plated on either stiff or compliant matrices and cultured for 48 hours prior to protein harvest.
In Vitro Apoptosis Model
Low-passage number (<10 passages) normal colonic myofibroblasts (CCD-18co cells) were seeded at 1 × 105 cells/mL on 6-well tissue culture plates. Cells were treated with 100 ng/mL fas-ligand (FASL) and 0.1–1 µM of BH-3 mimetics in minimal serum (0.5%) minimum essential medium eagle-alpha modification (α-MEM) for 5 hours before being harvested for protein.
The CCD-18co cells from the in vitro models were processed for RNA or protein extraction followed by downstream analysis of fibrogenic genes (collagen I A1 [COL1A1], actin alpha 2 smooth muscle [ACTA2], fibronectin I [FN1], myosin light chain kinase [MYLK]) or protein (αSMA, collagen 1, cleaved poly ADP-ribose polymerase [c-PARP], FN1, and glutaraldehyde 3-phosphate dehydrogenase [GAPDH]) as previously described.23–25
Human Intestinal Organoid Model
Human embryonic stem cells (H9, Wicell Research Institute, Madison, WI, USA) were generously donated by Jason Spence and differentiated into human intestinal organoids as described previously.26 For the purpose of this study, organoids with high mesenchymal cell composition were chosen, as opposed to cyst-like, epithelial-high organoids. For TGFβ treatment, HIOs were embedded in growth factor-reduced Matrigel (BD Biosciences, San Jose, CA, USA) within wells of a 24-well plate and overlaid with Advanced DMEM/F12 media (Invitrogen, Carlsbad, CA, USA).
Organoids were serum-starved for 24 hours, followed by 96 hours of cotreatment of various concentrations of ABT-263 with 2 ng/mL TGF-β in serum-free culture medium. Fresh media with inhibitors and TGFβ was changed every 24 hours. ABT-263 was tested at concentrations of 0.3 to 30 μM in HIOs. At 96 hours, HIOs were processed for RNA or protein extraction, followed by downstream analysis of fibrogenic genes (COL1A1, ACTA2, FN1, MYLK) or protein (αSMA, c-PARP), as previously described.22,24 For gene expression analysis, HIOs were plated using 3 to 4 organoids per well, with each treatment performed in triplicate wells. For protein analysis, 9 to 12 organoids were pooled per sample, washed, lysed, and run on SDS-PAGE, followed by western blotting to detect αSMA or c-PARP. Additionally, β-actin was used as an internal control.
Salmonella Typhimurium Mouse Model
To achieve sufficient statistical power, sample sizes for the control groups were calculated using data from numerous previous studies using the S. typhimurium model.27 In the ABT-263 therapeutic groups, we estimated a 50% reduction in pathologic and molecular endpoints (with 2-sided alpha <0.05 and a power of 0.8) for the comparison of the low-dose ABT-therapeutic arm with the fibrosis control arm for each metric including histopathology, fibrotic gene expression, and protein expression. Based on the calculated sample size of 10 per arm for this comparison, experimental groups were as follows: negative controls (uninfected, no drug, n = 5), drug control (uninfected, 100 mg/kg/day ABT-263, n = 5), positive fibrosis control (S. typhimurium infected, no drug, n = 10), low-dose drug (S. typhimurium infected, 20 mg/kg/day ABT-263, n = 10), and high-dose drug (S. typhimurium infected, 100 mg/kg/day ABT-263, n = 10).
Female CBA/J mice 8 to 12 weeks old (Jackson Laboratories, Bar Harbor, ME, USA) received 20 of mg streptomycin to eradicate the commensal microbiota 24 hours prior to infection with 1 × 106 colony-forming units (cfu) of S. typhimurium strain SL1344 by oral gavage. Control mice received streptomycin and vehicle by oral gavage. To eliminate potential effects of concurrent inflammation on fibrosis, all mice received levofloxacin intervention to eradicate S. typhimurium beginning day 8 postinfection for 5 days. As we have previously demonstrated in the S. typhimurium model, fibrosis is initiated early and progresses despite eradication of an inflammatory stimulus at day 8 postinfection.20 To avoid potential drug interactions, levofloxacin treatment was discontinued at day 12 postinfection and 1 day prior to ABT-263 treatment (day 13 postinfection.)20 Eradication of S. typhimurium post-levofloxacin treatment was confirmed by stool plating. Treatment with ABT-263 was initiated on day 13 postinfection (5 days after fibrosis is established) and continued until humane euthanization at day 22 postinfection to test the ability to reverse fibrosis. Treatment groups received ABT-263 once per day for 9 days by oral gavage with the high-dose group receiving the drug at 100 mg/kg; the low-dose group received the drug at 20 mg/kg. Negative and positive cohorts received levofloxacin and vehicle on the same schedule.
Animals were humanely euthanized 22 days after S. typhimurium infection. Stool was collected and cultured to assay potential S. typhiumurium re-infection. The cecum was harvested for macroscopic pathology, histopathology, protein, and mRNA analysis as detailed in Johnson et al.20 Quantitative real-time polymerase chain reaction (qRT-PCR) RNA was extracted from cultured cells using the RNeasy kit (Qiagen, Valencia, CA, USA). The RNAs were treated with RNase-free DNase before cDNA synthesis using the First Strand Synthesis kit (Invitrogen) according to manufacturer’s protocol. The analysis of gene expression of colonic myofibroblasts was determined by quantitative real-time PCR of collagenIA1 (COL1A1), Transforming Growth Factor Beta (TGFβ), connective tissue growth factor (CTGF), insulin-like growth factor 1 (IGF-1), Interleukin 1 Beta (IL-1β), interleukin -12B (IL-12p40), and tumor necrosis factor alpha (TNFα)genes, and GAPDH was performed with the TaqMan gene expression assays (ABI, Foster City, CA, USA). All qRT-PCR was performed using an Applied Biosystems StepOne Plus. The ddCt values were calculated from GAPDH expression.
Histology
Formalin-fixed and paraffin-embedded tissues were stained with hematoxylin and eosin (H&E, inflammatory histology) and Masson’s trichrome (fibrosis) by the University of Michigan CCGC Research Histology and Immunoperoxidase Laboratory (Ann Arbor, MI, USA). Digital photomicrographs of tissue sections were taken with a Motic MoticamX camera using Motic imaging 3.0 software. Histological scoring was performed by a veterinary pathologist (K.A.E.) in a blinded manner. Inflammation was scored on a 0 to 4 scale as follows: 0, none; 1, few inflammatory cells confined to the lamina propria; 2, moderate numbers of inflammatory cells in the lamina propria sometimes extending into the submucosa; 3, widespread inflammation throughout the lamina propria and submucosa; and 4, severe, transmural leukocyte infiltration. Fibrosis was scored on a separate 0 to 4 scale: 0, none; 1, focal mucosal collagen deposition with minimal architectural distortion; 2, widespread coalescing mucosal fibrosis with widespread architectural distortion but without obscuring of the mucosal/submucosal border; 3, diffuse mucosal and submucosal collagen deposition with marked architectural distortion obscuring the mucosal/submucosal border; 4, full thickness ulceration with transmural fibrosis. For completeness, epithelial damage was also scored as follows: 0, none; 1, dilation of individual glands +/- single cell necrosis; 2, widespread gland loss; 3, epithelial erosions; 4, ulceration. Additionally, epithelial hyperplasia was also scored as follows: 0, none; 1, mild, multifocal gland proliferation; 2, prominent widespread epithelial proliferation without dysplasia; and 3, dysplastic foci.
Western Blot Analysis
Total protein was separated by SDS-PAGE and probed for αSMA as previously described. The αSMA primary antibody (Sigma, St. Louis, MO, catalog #A5228, USA) was used at 1:500. Cleaved-PARP (c-PARP) primary antibody (Cell Signaling Technology catalog #9541) was used at 1:1000. Collagen 1 primary antibody (Abcam catalog #ab34710) was used at 1:1000. GAPDH antibody (Ambion catalog #AM4300) was used at 1:10,000. Western blot protein levels were quantitated using ImageJ (version 1.52a).28 Protein levels were normalized to either GAPDH or β-actin as a positive control.
Statistical Analysis
Statistical differences were determined by a 2-sided, unpaired Student t test. Results of P <.05 were considered statistically significant. Power calculations for the rodent study were performed using the pwr package in R version 3.6.3 (R Core Team, 2017; R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/), using a 2-sided alpha of 0.05 and power of 0.8.
Results
BH-3 Mimetics Reduce αSMA and Fibronectin Protein Expression in TGFβ-Stimulated Myofibroblasts In Vitro
In other organ systems, the proapoptotic and antifibrotic efficacy of BH-3 mimetics varies widely. To determine the efficacy of BH-3 mimetics in intestinal myofibroblasts, we first compared the BH-3 mimetics ABT-199 (venetoclax, selective Bcl-2), A1155463 (selective Bcl-xl), and A1210477 (Mcl-1 inhibitor) using 2 in vitro fibrosis models.
We initially analyzed the effect of 4 BH-3 mimetics with different selectivities in the TGFβ in vitro model. Stimulation of normal human intestinal myofibroblasts by TGFβ activates a profibrotic response characterized by induction of α-smooth muscle actin (αSMA, a key marker of activated myofibroblasts), production of extracellular matrix proteins, resistance to apoptosis, and transcriptional activation of TGFβ-responsive genes.22,25
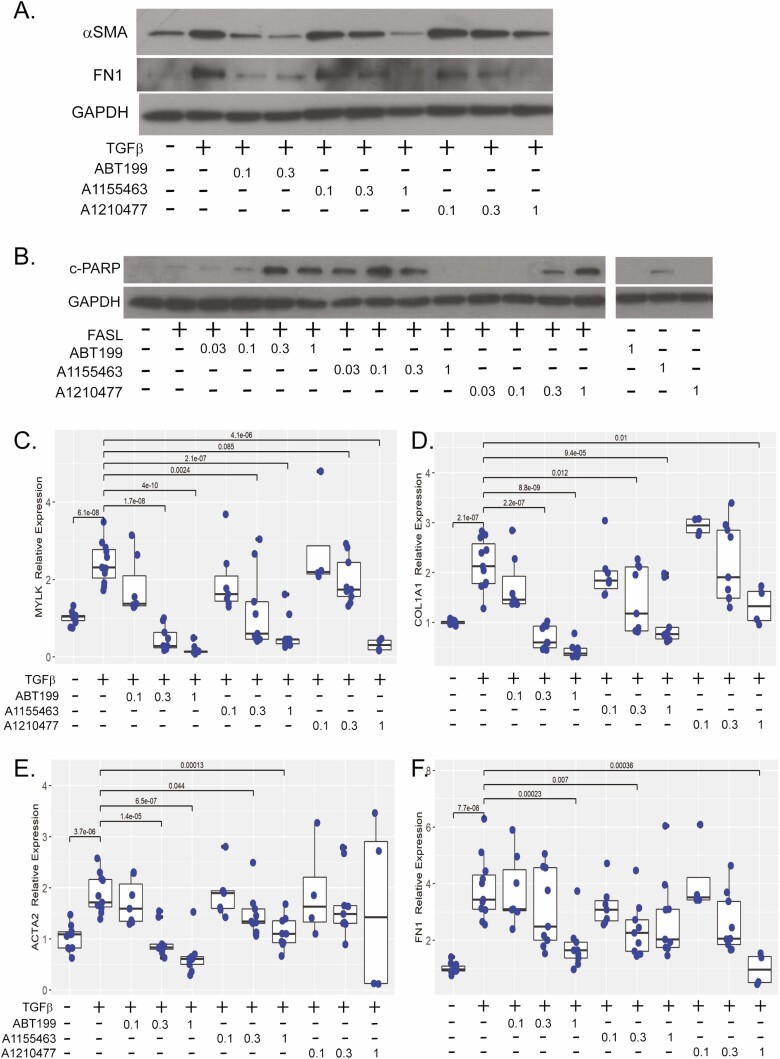
Normal human myofibroblasts (CCD-18co cells) were serum-starved, then stimulated with 0.05 ng of TGFβ and 0.1 to 1 μM of BH-3 mimetics for 48 hours and compared with unstimulated myofibroblasts. The TGFβ induced αSMA and FN1 protein expression. Both ABT-199 and A1155463 reduced αSMA protein expression to baseline levels. However, ABT-199 was approximately 10-fold more potent than A1155463 with comparable effects observed at 0.1 μM of ABT-199 vs 1 μM of A1155463 (Figure 1A). A modest reduction was observed with 1 μM of A1210477 (Figure 1A, Table S1). Similar effects were observed for FN1 protein expression (Figure 1A, Table S1).
Figure 1.
BH-3 mimetics reduce fibrotic protein expression, sensitize intestinal myofibroblasts to apoptosis, and inhibit fibrotic gene expression. A, Representative western illustrating αSMA and fibronectin (FN1) protein expression in CCD-18co cells stimulated with 0.05 ng/mL TGFβ and cotreated with 0.1 to 0.3 μM of ABT-199 or 0.1 to 1 uM of A1155463 or A1210477 for 48 hours. GAPDH protein expression was used a control for protein loading. B, Representative cleaved PARP (c-PARP) western of CCD-18co cells treated with anti-FAS activating antibody (FASL,100 ng/mL) and 0.03 to 1 μM of each BH-3 mimetic for 5 hourss compared with non-FASL sensitized cells (untreated or 1 μM of each BH-3 mimetic). The GAPDH expression was used a control for protein loading. All lanes are from the same western and exposure. Intervening lanes from a compound outside the scope of this study have been removed for clarity. Gene expression of MYLK (C), COL1A1 (D), ACTA2 (E), and FN1 (F) in CCD-18co cells treated with 0.05 ng/mL TGFβ and cotreated with 0.1 to 3 μM of ABT-199, A1155463, or A1210477 for 24 hours compared with untreated CCD-18co cells. Fibrogenic gene expression was normalized to GAPDH expression. Results are from 4 independent experiments with 2 to 3 technical replicates per experiment. Individual replicates are represented by points within boxplots denoting the median and interquartile range. Significant (P < .05) statistical comparisons are enumerated within brackets.
BH-3 Mimetics Sensitize Intestinal Myofibroblasts to FASL-Stimulated Apoptosis In Vitro
Pathological persistence of activated and apoptosis-resistant myofibroblasts contributes to tissue fibrosis, whereas inhibitors of prosurvival Bcl2 proteins sensitize myofibroblasts to undergo apoptosis.6 Therefore, we determined whether BH-3 mimetics could restore sensitivity to FASL-mediated apoptosis in intestinal myofibroblasts. The CCD-18co myofibroblasts were treated with 100 ng/mL of fas-ligand and 0.1 to 1 µM of BH-3 mimetics for 5 hours. As evidenced by increased c-PARP protein expression, A1155463 is a potent inducer of apoptosis with efficacy at 30 nM for FASL-mediated apoptosis and 1 μM in the absence of FASL (Figure 1B, Table S1). Less sensitization to FASL apoptosis was observed for ABT-199 (300 nM) and A120477 (300 nM). Overall, ABT-199 demonstrated the most consistent antifibrotic efficacy, reducing the expression of profibrogenic proteins and increasing sensitivity to apoptosis (Table S1).
BH-3 Mimetics Reduce Fibrogenic Gene Expression in TGFβ-Stimulated Myofibroblasts In Vitro
Transforming growth factor beta transcriptionally activates fibrogenic gene expression including actin stress fiber contractile genes (myosin light chain kinase, MYLK), profibrotic genes (smooth muscle actin, ACTA2 and collagen type 1, COL1A1), and extracellular matrix genes (fibronectin 1, FN1 and COL1A1).22,25 An analysis of fibrogenic gene expression in intestinal myofibroblasts treated with .05 ng/mL of TGFβ for 24 hours and cotreated with BH-3 mimentics revealed a dose-responsive reduction in MYLK and COL1A1 transcription by all 3 BH-3 mimetics over a concentration range of 0.1 to 3 μM (Figure 1C, D). Additionally, ABT-199 and A1155463 exhibited 3-fold higher potency than A1210477 (0.3 vs 1 μM) on MYLK expression. Similar results were observed for COL1A1 expression because 0.3 μM of ABT-199 or A1155463 was more effective than 3 μM of A1210477. With respect to ACTA2 expression, 0.3 μM of ABT-199 was more effective compared with A1155463 (1 μM) and A1210477 (no efficacy; Figure 1E). Fibronectin 1 expression was repressed by 1 μM of ABT-199, 0.3 μM of A1155463, and 0.3 of μM of A1210477 (Figure 1F).
Stiffness-induced Fibrogenic Gene Expression Is Reduced by BH-3 Mimetics
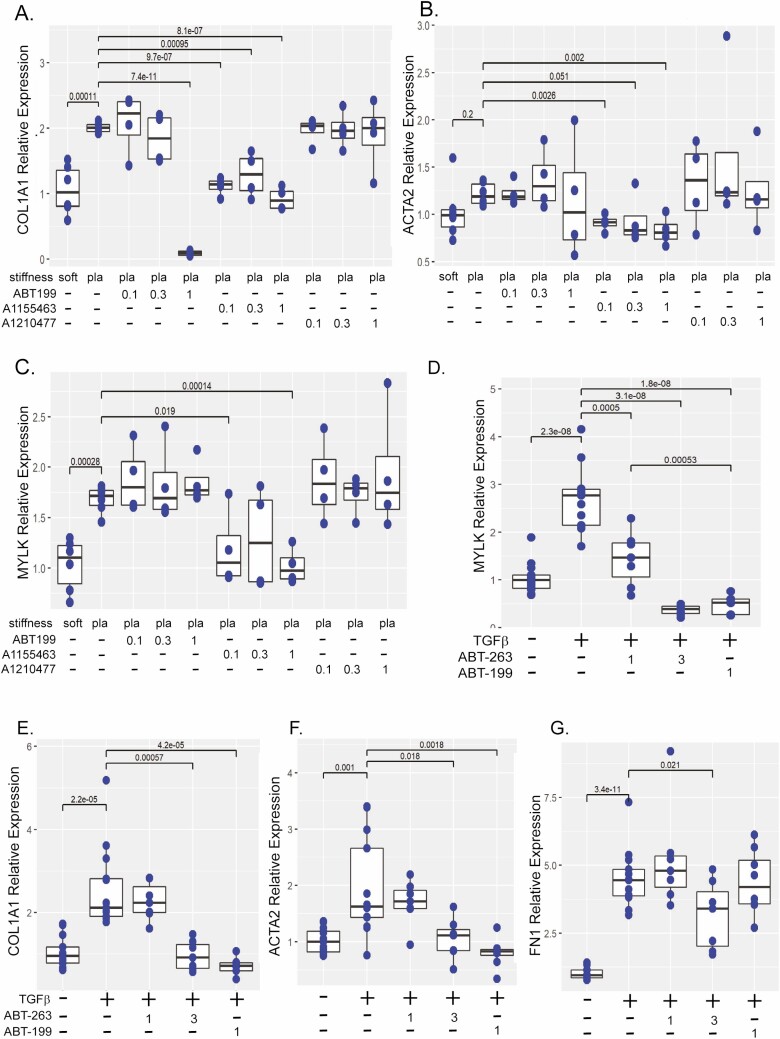
To further validate the antifibrotic efficacy of BH-3 mimetics, we analyzed ABT-199, A1155463, and A1210477 in a second in vitro model. As we have previously demonstrated, increased matrix stiffness recapitulates the physiological tissue stiffness of human Crohn’s strictures, activating myofibroblasts to express fibrogenic genes including COL1A1, ACTA2, and MYLK.23 The CCD-18co cells were cultured on physiologically soft (4.3 kPa) or pathologically stiff (plastic) collagen-coated matrices and treated with 0.1 to 1 μM of BH-3 mimetics for 48 hours. The ABT-199 and A1155463 significantly repressed COL1A1 gene expression, whereas A1210477 had no effect (Figure 2A). Though lower doses of A1155463 compared with ABT-199 were required to reduce COL1A1 gene expression (0.1 μM vs 1 μM), the largest magnitude was observed with 1 μM of ABT-199 which reduced COL1A1 23-fold (P < .001 vs plastic) in comparison with 1 μM of A1155463 (2.1-fold, P < .001 vs plastic). The A1155463 (0.1 to 1 μM) significantly reduced ACTA2 gene expression (Figure 2B). A smaller effect was observed on MYLK expression with A1155463 (0.1 and1 μM; Figure 2C). Neither ABT-199 nor A120477 had an effect on either ACTA2 or MYLK gene expression.
Figure 2.
BH-3 mimetics reduce fibrogenic gene expression in the in vitro matrix stiffness and TGF-β models. Gene expression of COL1A1 (A), ACTA2 (B), and MYLK (C) in CCD-18co cells cultured on physiologically soft (4.3 kPa) or pathologically stiff (plastic) matrices and treated with 0.1 to 1 μM of ABT-199, A1155463, or A1210477 for 48 hours compared with untreated CCD-18co cells cultured on soft or stiff matrices. Results are from 2 independent experiments with 3 biological replicates per experiment. (D–G) Comparison of ABT-263 and ABT-199 effects on gene expression of MYLK (D), COL1A1 (E), ACTA2 (F), and FN1 (G) in CCD-18co cells treated with 0.05 ng/mL TGFβ and cotreated with 1 μM or 3 μM of ABT-263 or 1 μM of ABT-199 for 24 hours compared with untreated CCD-18co cells. Results are from 4 independent experiments with 2 to 3 biological replicates per experiment. In both in vitro models, fibrogenic gene expression was normalized to GAPDH expression. Individual replicates are represented by points within boxplots denoting the median and interquartile range. Significant (P < .05) statistical comparisons are enumerated within brackets.
Overall, ABT-199 (Bcl-2 inhibitor) and A1155463 (Bcl-xl inhibitor) had the greatest antifibrotic efficacy, repressing fibrogenic gene expression, reducing ECM protein expression, and increasing sensitivity to apoptosis. These results identify Bcl-2 and Bcl-xl as potential antifibrotic targets in intestinal myofibroblasts. In other organ systems, in vivo studies have demonstrated targeting Bcl-2/Bcl-xl/Bcl-w reduces fibrosis.12,13,15
ABT-263 and ABT-199 Represses TGFβ-stimulated Fibrogenic Gene and Protein Expression in Intestinal Myofibroblasts
These initial in vitro results identified ABT-199 as our lead antifibrotic compound and suggested the Bcl-2 pathway as the lead target. However, several factors contributed to our consideration of ABT-263, another Bcl-2/Bcl-xl/Bcl-w inhibitor from the same chemical series of reversed established fibrosis12 as an alternative lead compound. Other studies have demonstrated that ABT-199 failed to reduce dermal fibrosis in vivo. Given the reported lack of in vivo antifibrotic efficacy with ABT-199, the success of ABT-263 therapy in human fibrotic disease, and a communication demonstrating superior oral bioavailability of ABT-263 over ABT-199 (AbbVie personal communication, communication was from Lisa Hazelwood, Abbvie employee, verbally, at the Keystone Fibrosis conference sometime during the meeting from March 26–30, 2017), we postulated ABT-263 may be a better candidate drug in vivo. Therefore, we directly compared ABT-263 and ABT-199 in our in vitro intestinal fibrosis models.
We performed a dose-response experiment evaluating the effect of ABT-263 and ABT-199 on profibrogenic gene transcription in the TGFβ in vitro model. The CCD-18co cells were cotreated with TGFβ and 10 nM to 3 μM of ABT-263 or 100 nM to 1 μM of ABT-199. Concentrations <1 μM of ABT-263 failed to repress transcription of MYLK, COL1A1, ACTA2, or FN1 (Figure S1). Cell loss was observed with 3 μM of ABT-199. In contrast, no cell loss was observed with 3 μM of ABT-263 after 24 hours. Therefore, we proceeded to compare in vitro efficacy of 1 and 3 μM of ABT-263 and 1 μM of ABT-199. In the TGFβ model, 1 μM of ABT-263 repressed MYLK transcription by 2.5-fold (P < .001 vs TGFβ; Figure 2D). Additionally, 1 μM of ABT-263 was less effective than either 3 μM of ABT-263 (7-fold, P < .001 vs TGFβ) or 1uM of ABT-199 (6-fold, P < .001 vs TGFβ). However, 1 μM of ABT-263 had no effect on COL1A1, ACTA2, or FN1 transcription (Figure 2D–G). However, 3 μM of ABT-263 repressed COL1A1 2.5-fold (P < .001 vs TGFβ) and ACTA2 roughly 2-fold (P = .018 vs TGFβ) to levels undistinguishable from untreated cells (Figure 2E, F). A lesser but statistically significant reduction in FN1 transcription was observed with 3 μM of ABT-263 (40% reduction, P = .021 vs TGFβ; Figure 2G).
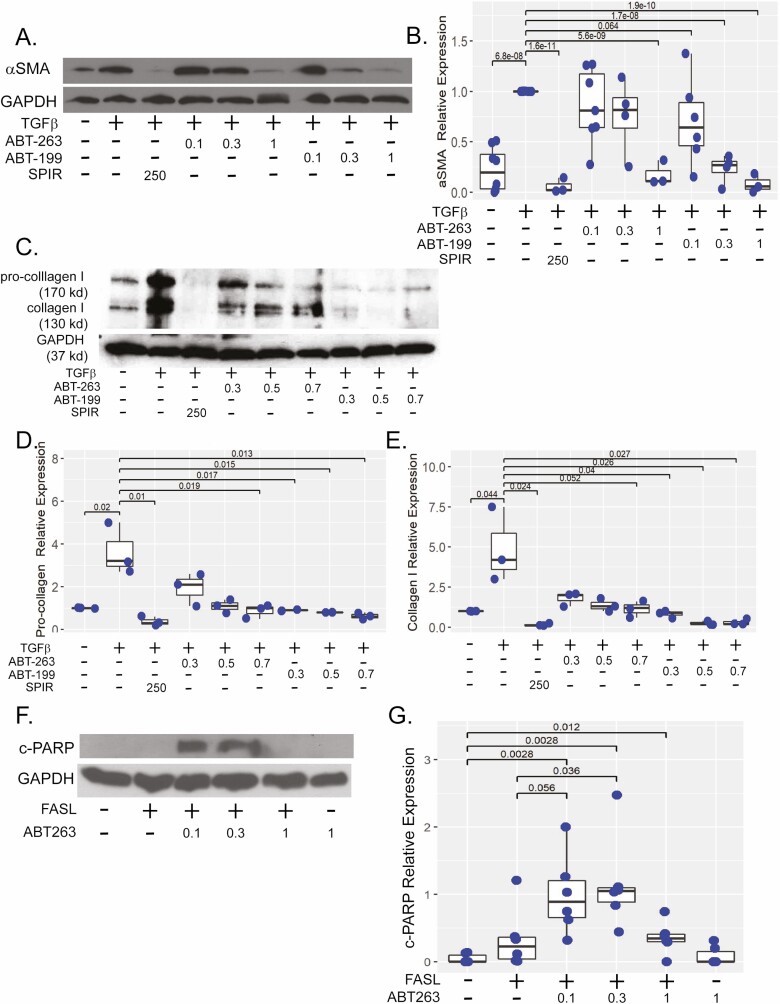
ABT-263 and ABT-199 Reduce TGFβ-induced αSMA and Collagen 1 Protein Expression
In intestinal myofibroblasts treated with TGFβ, αSMA and collagen 1 protein expression are strongly induced after 48 hours of TGFβ exposure. Previous in vitro studies have demonstrated cotreatment with TGFβ and putative antifibrotics, specifically spironolactone, represses fibrogenic protein expression in vitro in intestinal myofibroblasts.29 Therefore, we compared the efficacy of ABT-263 and ABT-199 using 250 μM spironolactone as an antifibrotic drug control. The CCD-18co cells were cotreated for 48 hours with TGFβ and 0.1 to 3 μM of ABT-263 or ABT-199.
Similar to our initial BH-3 mimetic screening, both 0.3 and 1 μM of ABT-199 significantly repressed TGFβ induction of αSMA protein expression, whereas 1 μM of ABT-263 repressed TGFβ induction of αSMA protein expression by 5-fold (P < .001 vs TGFβ) to levels indistinguishable to either the negative or antifibrotic controls (Figure 3A). Though our initial screening indicated 0.1 μM of ABT-199 may repress αSMA protein expression (Figure 1A), multiple independent experiments determined this observation was not statistically significant (P = .064 vs TGFβ; Figure 3B).
Figure 3.
ABT-263 reduces αSMA protein expression and sensitizes intestinal myofibroblasts to apoptosis. A, Representative western illustrating αSMA protein expression in CCD-18co cells stimulated with 0.05 ng/mL TGFβ and cotreated with 0.1 to 1 μM of ABT-263 or ABT-199 48 hours compared with untreated cells or cells treated with 250 μM spironolactone, a previously described antifibrotic compound. B, Quantification of αSMA protein as normalized to GAPDH expression. Results are from 7 independent experiments. C, Representative western illustrating procollagen 1 and collagen 1 protein expression in CCD-18co cells stimulated with 0.05 ng/mL TGFβ and cotreated with 0.3 to 0.7 μM of ABT-263 or ABT-199 for 48 hours compared with untreated cells or cells treated with 250 μM of spironolactone. D, E, Quantification of procollagen and collagen 1 (normalized to GAPDH) expression. Results are from 3 independent experiments. F, Representative c-PARP western of CCD-18co cells treated with anti-FAS activating antibody (FASL, 100 ng/mL) and 0.1 to 1 μM of ABT-263 or ABT-199 for 5 hourss compared with non-FASL sensitized cells. G, Quantification of c-PARP as normalized to GAPDH protein expression. Results are from 3 independent experiments. In all experiments, GAPDH protein expression was used a control for protein loading. Individual replicates are represented by points within boxplots denoting the median and interquartile range. Significant (P < .05) statistical comparisons are enumerated within brackets.
To elucidate effects on procollagen 1 and collagen 1 in TGFβ-stimulated myofibroblasts, a narrower concentration range of 0.3 to 0.7 μM of ABT-263 or ABT-199 was used compared with the antifibrotic control (250 μM of spironolactone; Figure 3C). Higher concentrations of ABT-263 were required to repress procollagen and collagen 1 protein expression; 0.5 μM and 0.7 μM of ABT-263 significantly repressed procollagen protein expression by 4- to 5-fold (P = .024, P = .019 vs TGFβ, respectively) and 0.7 μM of ABT-263 repressed collagen 1 by 8-fold (P = .05 vs TGFβ). In comparison, ABT-199 repressed both procollagen 1 and collagen 1 at concentrations of 0.3 μM to 0.7 μM (Figure 3D, E).
ABT-263 Sensitizes Intestinal Myofibroblasts to Apoptosis in the FASL-treated Myofibroblasts In Vitro
To determine whether ABT-263 sensitizes intestinal myofibroblasts to FASL-mediated apoptosis, c-PARP protein expression was quantified in CCD-18co cells sensitized with FASL and treated with 0.1, 0.3, or 1 μM of ABT-263. Although both 0.1 μM and 0.3 μM of ABT-263 significantly induced c-PARP protein >20-fold compared with nonsensitized cells and approximately 3-fold compared with FASL alone, only 0.3 μM of ABT-263 achieved statistical significance (P = .036 vs FASL; Figure 3F, G).
ABT-263 and ABT-199 Represses Matrix Stiffness-induced Fibrosis In Vitro
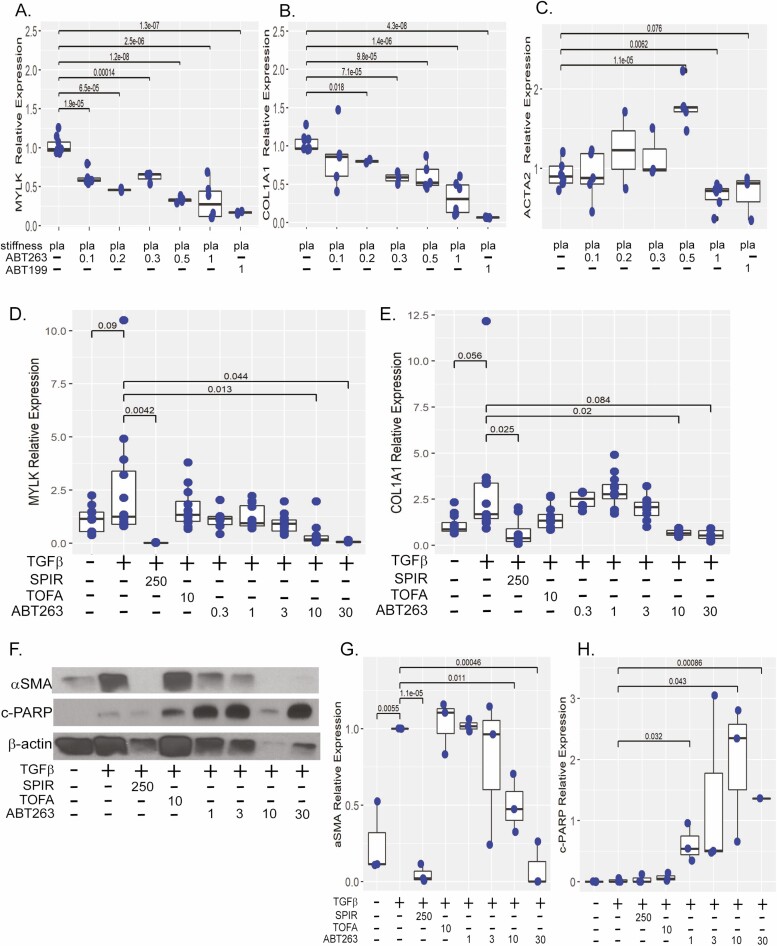
To confirm in vitro efficacy of ABT-263, we performed dose-response experiments in a second in vitro model. The CCD-18co cells were plated on collagen-coated stiff matrices and treated with 0.1 μM to 1 μM of ABT-263 or 1 μM of ABT-199 for 48 hours. As we have demonstrated, transcription of mechanosensitive fibrotic genes COL1A1, ACTA2, and MYLK are induced by high matrix stiffness (Figure 2).23 Treatment with 0.1 μM to 1 μM of ABT-263 demonstrated a dose-dependent repression of MYLK transcription with a 3-fold reduction observed at 0.5 μM and 1 μM, respectively (P < .001, P < .001 vs plastic; Figure 4A). A similar dose-response effect was observed with 0.2 to 1 μM of ABT-263 which repressed COL1A1 by 3-fold (P < .001 vs plastic; Figure 4B). A modest but statistically significant reduction in ACTA2 gene expression was observed at the highest concentration of 1 μM of ABT-263 (45% reduction, P = .006 vs plastic; Figure 4C). A similar trend was observed with 1 μM of ABT-199, but results did not reach statistical significance.
Figure 4.
ABT-263 represses matrix stiffness-induced fibrosis in myofibroblasts and HIOs. Gene expression of MYLK (A), COL1A1 (B), and ACTA (C) in CCD-18co cells cultured on pathologically stiff (plastic) matrix and treated with 0.1 to 1 μM of ABT-263 for 48 hours compared with 1 μM of ABT-199 treated or untreated CCD-18co cells. Fibrogenic gene expression was normalized to GAPDH expression. Results are from 3 independent experiments with 3 biological replicates per experiment. D–H, Human intestinal organoids HIOs were stimulated with 2 ng/mL TGFβ and cotreated with 0.3 to 30 μM of ABT-263 for 96 hours. Antifibrogenic response was compared with TGF-β-stimulated HIOs cotreated with 250 μM of spironolactone (antifibrotic, positive drug control) or 10 μM of tofacitinib (anti-inflammatory, negative drug control) and untreated HIOs (no drug, negative control). Gene expression of MYLK (D) and COL1A1 (E), normalized to GAPDH expression. Results are from 4 independent experiments with 3 biological replicates per experiment. F, Representative western demonstrating αSMA and c-PARP protein expression in HIOs stimulated with 2 ng/mL TGFβ and cotreated with 1 to 30 μM of ABT-263 for 96 hours. Quantification of αSMA (G) and c-PARP (H) as normalized to β-actin expression. Results are from 3 independent experiments. Individual replicates are represented by points within boxplots denoting the median and interquartile range. Significant (P < .05) statistical comparisons are enumerated within brackets.
ABT-263 Represses Fibrosis and Induces Apoptosis in Human Intestinal Organoids
Given the in vitro antifibrotic efficacy of ABT-263 in fibroblast monocultures, we advanced ABT-263 to a multicellular model. As previously described, human intestinal organoids recapitulate the 3-dimensional architecture and multiple cell types characteristic of human intestine, thereby bridging the gap between in vitro and in vivo drug discovery models.24,26 These HIOs are composed of epithelial and mesenchymal layers, the latter containing a subpopulation of fibroblasts. To evaluate the antifibrotic efficacy of ABT-263, mesenchymal-rich HIOs were stimulated with TGFβ and cotreated with 0.3 to 30 μM of ABT-263 for 96 hours. Antifibrotic efficacy, as determined by repression of MYLK, COL1A1, FN1, and ACTA2 transcription, was compared with untreated HIOs (no drug, negative control), 250 μM of spironolactone (antifibrotic, positive drug control), and 10 μM of tofacitinib (anti-inflammatory, negative drug control).22,29 Ten μM of ABT-263 significantly repressed MYLK gene expression by 7-fold (P = .013 vs TGFβ) and COL1A1 by 4-fold (P = .02 vs TGFβ; Figure 4D, E). Despite cell loss, similar efficacy was observed with 30 μM of ABT-263, although COL1A1 did not reach statistical significance. No effect was observed on FN1 or ACTA2 gene expression or for concentrations of <10 μM of ABT-263.
Both 10 μM and 30 μM of ABT-263 reduced TGFβ-induced αSMA protein expression by 2-fold and 11-fold, respectively (P = .01 and P < .001 vs TGFβ; Figure 4F, G). At the 30 μM concentration, profound cell loss and reduction of β-actin protein expression were observed. Marked apoptosis as evidenced by increased cleaved PARP expression was observed. There was a 30-fold increase in c-PARP expression at 1 μM of ABT-263 (P = .032 vs TGFβ) and approximately 100-fold at 10 μM (P < .001; Figure 4H). Even though high c-PARP expression was observed with 30 μM of ABT-263, insufficient β-actin protein expression due to increased cell loss observed in some experimental replicates precluded quantification and statistical analysis.
ABT-263 Reduces Inflammation In Vivo
Because ABT-263 demonstrated antifibrotic efficacy in multiple in vitro models, including a “mini-gut” HIO model, we investigated whether ABT-263 could ameliorate intestinal fibrosis in vivo. Mice chronically infected with S. typhimurium infection developed an intestinal fibrosis phenotype similar to human Crohn’s disease.27,30 Previous pharmacokinetic studies in multiple species including mice have demonstrated high oral bioavailability of ABT-263.17 We therefore treated mice with either 20 or 100 mg/kg/day by oral gavage daily beginning on day 13 postinfection daily for 9 days until the termination of the study at day 22 postinfection.
Macroscopic pathology revealed the typical disease phenotype in S. typhimurium-infected mice, characterized by decreased cecal area, qualitatively thick stiff tissue, and increased tissue weight compared with uninfected controls (Figure 5A). A dose-response reduction in tissue weight was observed in infected mice treated with 20 or 100 mg/kg of ABT-263 (Figure 5B). At the highest ABT-263 dose (100 mg/kg), tissue weight was significantly lower than in the infected, untreated mice (0.46 vs 0.60 g, P < .001) and did not differ from uninfected controls (0.47 vs 0.46 g, P = .73 (Figure 5B). The tissue weight of the 20 mg/kg/day ABT-263 cohort was lower than that of infected controls but did not reach statistical significance (0.56 vs 0.60 g, P = .058). No effect of ABT-263 was observed on either colon length or cecal area.
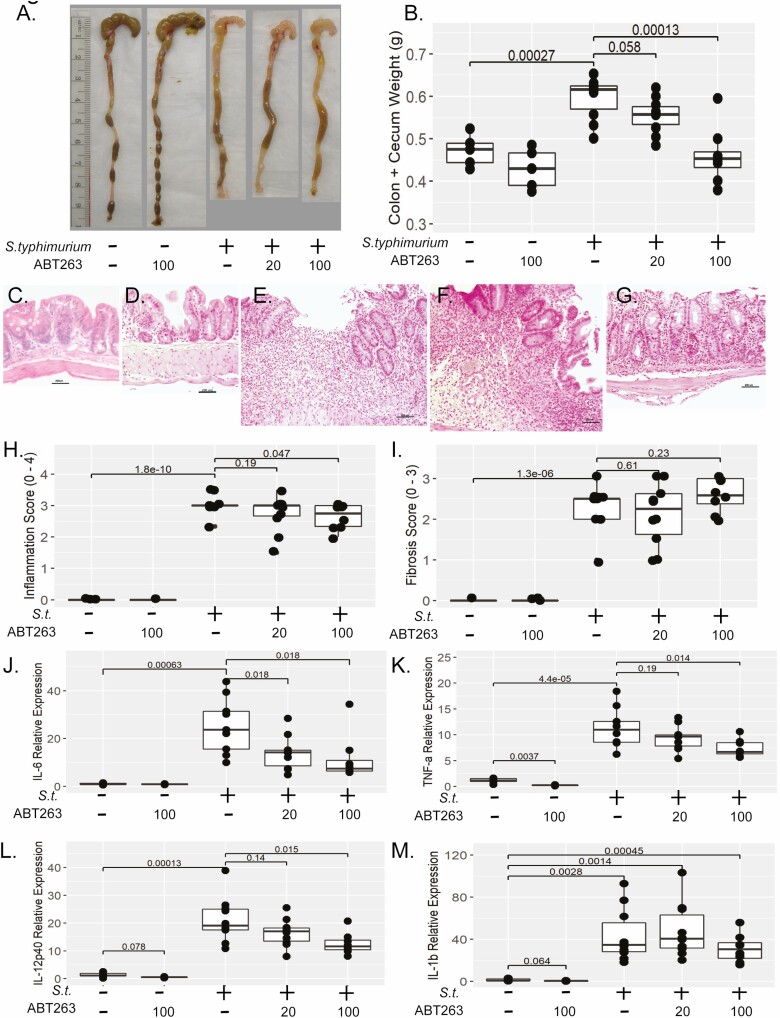
Figure 5.
ABT-263 reduces bowel inflammation in vivo. A, Representative macroscopic pathology of mouse cecum and distal colon weight in mice infected with S. typhimurium and treated daily with 20 or 100 mg/kg/day ABT-263 compared with S. typhimurium-infected and uninfected controls (no drug or 100 mg/kg/day ABT-263). B, Dose-dependent reduction in cecal and colon tissue weight. C–G, Photomicrographs (100x magnification) of H&E stained sections of mouse cecum of (C) uninfected, no drug. No lesions are present. D, Uninfected, drug control (100 mg/kg/day ABT-263). No lesions are present. E, S. typhimurium infected. There is a large chronic ulcer containing a dense accumulation of neutrophils extending through the lamina propria and submucosa. Adjacent cecal glands are hyperplastic. F, S. typhimurium infected treated with 20 mg/kg/day of ABT-263, similar to (E); there is epithelial erosion and hyperplasia accompanied by marked neutrophilic infiltration that extends into the submucosa. G, S. typhimurium infected treated with 100 mg/kg/day ABT-263. Inflammation and hyperplasia are moderate, and inflammation is limited to the lamina propria The scale bar denotes 200 μm. H, Summary of histological inflammation of H&E stained sections (blinded scoring, 0–4 point scale). I, Summary of histological fibrosis of Masson’s trichrome stained sections (blinded scoring, 3-point scale). Inflammatory gene expression of IL-6 (J), TNFα (K), IL-12p40 (L), and IL-1β (M) in cecal tissue from mice infected with S. typhimurium and treated daily with 20 or 100 mg/kg/day of ABT-263 compared with S. typhimurium-infected and uninfected controls (no drug or 100 mg/kg/day of ABT-263). Inflammatory gene expression was normalized to GAPDH gene expression. Individual animals are represented by points within boxplots denoting the median and interquartile range. Statistical comparisons are enumerated within brackets with P values denoted.
As both edema (inflammation) and scarring (fibrosis) contribute to increased tissue weight, assessment of histologic inflammation and fibrosis was performed by a veterinary pathologist (K.A.E.) blinded to the experimental groups. Characteristic epithelial damage, hyperplasia, inflammation, and transmural fibrosis was observed in the cecum of S. typhimurium-infected mice compared with the infected controls (Figure 5C–G). Despite treatment with levofloxacin to minimize the inflammatory effects of chronic S. typhimurium infection, marked histological inflammation was observed in the S. typhimurim-infected group. Stool plating revealed all mice in all S. typhimurium-infected groups were recolonized with S. typhimurium at the termination of the experiment at day 22 despite initial eradication of S. typhimurium at day 8 postinfection. Irrespective of the confounding effects with chronic S. typhimurium infection, a reduction in histological inflammation score was observed with in S. typhimurium-infected mice in the group treated with 100 mg/kg/day of ABT-263 compared with the S. typhimurium-infected group (2.6 vs 3.0, P = .047; Figure 5H). However, treatment with ABT-263 did not reduce histological fibrosis (Figure 5I).
Because histologic findings suggested a possible anti-inflammatory efficacy of ABT-263 despite chronic S. typhiumirum infection, we performed a more sensitive biochemical analysis of pro-inflammatory gene expression. The S. typhimurium infection profoundly induced expression of pro-inflammatory cytokines IL-6, TNFα, and IL-12b and IL-1β (26-, 10-, 16-, and 33-fold, respectively vs uninfected; Figure 5J–M). The ABT-263 reduced pro-inflammatory gene expression of IL-6, TNFα, and IL-12b. The largest effect of ABT-263 treatment was observed for IL-6 gene expression. Treatment with either 20 or 100 mg/kg/day of ABT-263 repressed IL-6 by approximately 2-fold compared with the S.typhimurium group (20 mg/kg/day, 2.1-fold, P = .019; 100 mg/kg/day, 2.6-fold, P = .02; Figure 5J). Additionally, 100 mg/kg/day of ABT-263 repressed S.typhimurium induction of TNFα and IL-12b expression by approximately 35% and 40%, respectively (P = .014, P = .015 vs S.typhimurium-infected) but had no effect on IL-1β (Figure 5K–M).
Interestingly in the uninfected drug control group, 100 mg/kg/day of ABT-263 significantly reduced endogenous TNFα gene expression by 5-fold compared with uninfected control mice (TNFα, P = .0037; Figure 5K). Although the effect of ABT-263 on other pro-inflammatory cytokines was not significant, a similar trend was observed with a 4-fold and 2.6-fold decrease in IL-1β and IL-12b expression, respectively (P = .064 and P = .078, respectively), suggesting ABT-263 has modest anti-inflammatory properties.
ABT-263 Reduces Fibrotic Gene and Protein Expression In Vivo
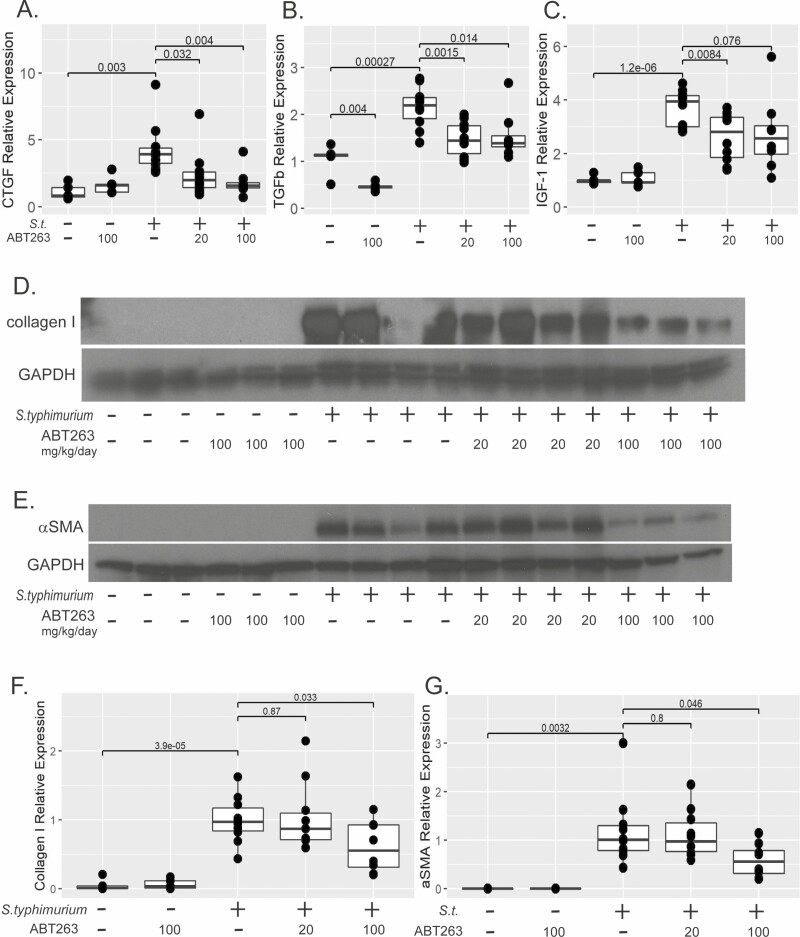
As we have observed, inflammation precedes fibrosis characterized by increased tissue collagen, induction of profibrotic genes, myofibroblast activation, and induction of ECM proteins.20,27 In this study, S.typhimurium infection induced expression of profibrotic genes such as CTGF (4-fold, P = .003), TGFβ (2.2-fold, P = .0003), and IGF-1 (3.7-fold, P < .0001) compared with uninfected controls (Figure 6A–C). Despite a lack of reduction in histological fibrosis (Figure 5I), a more sensitive gene expression analysis revealed a dose-response decrease in CTGF gene expression was observed in S.typhimurium-infected animals treated with ABT-263, specifically a 44% reduction in CTGF gene expression in the group treated with 20 mg/kg/day (P = .032 vs S.typhimurium) and 58% in the group treated with 100 mg/kg/day (P = .004 vs S.typhimurium). The CTGF gene expression did not statistically differ from the uninfected or drug control groups (P = .19, P = .76; Figure 6A). Both 20 and 100 mg/kg/day of ABT-263 significantly repressed TGFβ gene expression by approximately 30% (P = .0015, P = .014 vs S. typhimurium-infected; Figure 6B). In the uninfected drug control group, 100 mg/kg/day of ABT-263 repressed endogenous TGFβ gene expression by 56% compared with the uninfected control group (P = .004). Treatment of S. typhimurium-infected mice with ABT-263 at 20 mg/kg/day significantly repressed IGF-1 gene expression vs S. typhimurium-infected mice (44%, P = .008; Figure 6C). Though reduced IGF-1 gene expression was observed in the group treated with 100 mg/kg/day, this did not reach statistical significance (P = .076 vs S. typhimurium-infected).
Figure 6.
ABT-263 Reduces fibrotic gene and protein expression in vivo. Expression of fibrogenic genes CTGF (A), TGFβ (B), and IGF-1 (C) in mice infected with S. typhimurium and treated daily with 20 or 100 mg/kg/day of ABT-263 compared with S. typhimurium-infected and uninfected controls (no drug or 100 mg/kg/day of ABT-263) as normalized to GAPDH gene expression. Representative westerns blots of collagen 1 (D) and αSMA (E). GAPDH expression was used a control for protein loading. E, Quantification of collagen 1 (F) and αSMA (G) normalized to GAPDH expression. Individual animals are represented by points within boxplots denoting the median and interquartile range. Statistical comparisons are enumerated within brackets with P values denoted.
Salmonella typhimurium strongly induced collagen 1 protein by 20-fold (P < .0001 vs uninfected mice). Although 20 mg/kg/day of ABT-263 had no effect on collagen 1 protein expression, 100 mg/kg/day significantly reduced collagen 1 protein expression by 39% (P = .033; Figure 6D, F). Collagen 1 synthesis is promoted by activated myofibroblasts. Because αSMA is a hallmark of myofibroblast activation, we determined the effects of ABT-263 on αSMA protein expression. Typical of this mouse model, S. typhimurium profoundly induces αSMA protein expression (Figure 6E). Treatment of S. typhimurium infected mice with100 mg/kg/day of ABT-263 reduced αSMA protein expression by half (P = .046 vs S. typhimurium), but 20 mg/kg/day had no effect (Figure 6E, G).
Thus, ABT-263 demonstrated a dose-dependent reduction in macroscopic pathology, histological inflammation, inflammatory and fibrotic gene expression, and fibrogenic proteins, indicating ABT-263 may reduce intestinal inflammation and fibrosis.
Discussion
Effective therapeutics for intestinal fibrosis are a critical unmet need in management of Crohn’s disease as a majority of patients progress to fibrostenotic disease and intestinal failure.31 In other organ systems, the advent of antifibrotic therapeutics has countered the original paradigm of inexorable progression and irreversibility of fibrosis.2 Over the last decade, preclinical animal studies and human trials have identified multiple druggable targets in organ fibrosis, including the Bcl-2 apoptotic pathway.32,33
Similar to other organ systems, intestinal fibrosis is a function of dysregulated wound healing inasmuch as the key effector cells, subepithelial myofibroblasts, fail to undergo apoptosis. Pathological persistence of these activated myofibroblasts generates an autopropagative environment of excessive ECM protein synthesis, deposition, and subsequent tissue stiffness, which further stimulates myofibroblast activation and survival.6 The BH-3 mimetics, small molecule compounds, inhibit prosurvival Bcl-2 factors and restore myofibroblast sensitivity to apoptosis.10,11
Here, we demonstrate that multiple BH-3 mimetic compounds that pharmacologically target Bcl-2 and Bcl-xl also repress fibrogenesis in normal human intestinal myofibroblast cell line. In 2 complimentary in vitro models, TGFβ (cytokine) and matrix stiffness (mechanical), BH-3 mimetics ABT-199, A1155463, A1210477, and ABT-263 sensitized intestinal myofibroblasts to apoptosis, decreased ECM protein expression, and inhibited fibrogenic gene expression. These in vitro studies support the hypothesis that targeting Bcl-2 and Bcl-xl is an effective strategy to abrogate fibrogenesis. Further, we demonstrate that ABT-199, a Bcl-2-specific inhibitor, had the highest in vitro efficacy.
However, concerns were raised about the in vivo antifibrotic efficacy of ABT-199 as Lagares demonstrated ABT-199 lacked antifibrotic efficacy, whereas ABT-263 reduced dermal fibrosis,12 In addition, a communication from AbbVie indicated ABT-263 may be superior to ABT-199 (AbbVie personal communication).
Due to uncertainty that ABT-199 in vitro efficacy would translate to in vivo efficacy, we directly compared antifibrotic efficacy of ABT-199 against ABT-263 in our intestinal in vitro fibrosis models. In both the TGFβ and matrix stiffness models, ABT-263 sensitized intestinal myofibroblasts to apoptosis, decreased ECM protein expression, and inhibited fibrogenic gene expression. Given that ABT-263 had roughly 3-fold lower potency than ABT-199 prior to initiating rodent therapeutic studies, we determined ABT-263 efficacy in the “mini-gut” organoid model. As we and others have demonstrated, human intestinal organoids accurately represent in vivo bowel physiology, including complex, 3-dimensional architecture, composed of multilineage cells, an epithelial and mesenchymal layer, and a defined lumen that are an important resource for drug discovery.26 In contrast to the typical myofibroblast monolayer, HIO myofibroblasts reside within the mesenchyme, adjacent to the epithelium, and are sequestered from both the lumen and surrounding environment. The limited drug diffusion across the epithelial layer in concert with multiple cell-cell interactions recapitulates some of the physiology of drug uptake in the human bowel.34 In HIOs stimulated with TGFβ, ABT-263 induced apoptosis and decreased αSMA protein expression and fibrogeneic gene expression in a dose-responsive manner. A 10-fold higher concentration of ABT-263 was required in HIOs compared with myofibroblast monolayer cultures; however, this is consistent with our previous observations, reflecting the limited diffusion across the multicellular organoid, and is consistent with the effective dose of ABT-263 in pancreatic HIOs.24,35
As ABT-263 demonstrated antifibrotic efficacy in multiple in vitro models, including a “mini-gut” model, we hypothesized that ABT-263 would abrogate intestinal fibrosis in the mouse S. typhimurium model. The S. typhimurium model is particularly advantageous because it is the only preclinical intestinal fibrosis model in which inflammation can be manipulated to reduce the confounding effects of inflammation on fibrosis.20 While unintended recolonization by S. typhimurium limited our efforts to reduce the effects of concurrent inflammation, this produced an unexpected and potentially important finding. Our study demonstrated that ABT-263 unexpectedly reduced inflammation as measured by macroscopic pathology, histopathology, and inflammatory gene expression in contrast to the mouse bleomycin dermal fibrosis study, which showed ABT-263 did not impact inflammation.12 This is also in contrast with our previous study using spironolactone in mouse and rodent models of intestinal inflammation, in which the antifibrotic efficacy was associated with a secondary increase in intestinal inflammation and mortality. Similar increased mortality with spironolactone use in the context of intestinal inflammation due to Clostridium difficile infection was observed in human patients.29 Despite chronic stimulation by S. typhimurium infection, we observed a dose-dependent decrease in inflammatory gene expression with a concurrent dose-dependent decrease in fibrotic gene and protein expression.
Though ABT-263 did not completely abrogate intestinal fibrosis as determined by histopathology, a dose-dependent reduction in macroscopic pathology, histological inflammation, inflammatory and fibrotic gene expression, and ECM proteins indicate ABT-263 may have antifibrotic efficacy. Even though we did not directly interrogate the interaction of inflammation and fibrosis in the mouse model, we postulate that the unexpected anti-inflammatory in vivo efficacy of ABT-263 may reduce tissue fibrosis through a mechanism complementary to the myofibroblast apoptosis observed in vitro.
We determined the pharmacologic efficacy of BH-3 mimetics with different selectivities, but our focus on preclinical models precluded detailed mechanistic or drug discovery studies. In phase 2 studies of ABT-263 for lung cancer, thrombocytopenia is recognized as a serious potential adverse effect of ABT-263.36,37 This can be particularly problematic when treating patients with malignancy during chemotherapy, which can dramatically reduce platelet counts. Although our in vivo study did not examine the potential effects of ABT-263 on platelet function, thrombocytopenia is not a common effect of the underlying disease state in IBD.
Intestinal fibrosis is a major unsolved problem in Crohn’s disease, resulting in significant costs, morbidity, and mortality. To date, no antifibrotic therapeutics have been identified for the treatment of fibrostenotic CD. Fibrosis was once considered irreversible, but advances in preclinical models and therapeutic advances in other organ systems have identified legitimate drug targets, leading to effective pharmaceutical therapeutic candidates. Overall, this work identifies BH-3 mimetics as candidates for antifibrotic therapeutics and suggests ABT-263 may have anti-inflammatory and antifibrotic efficacy in a preclinical mouse model of intestinal fibrosis.
Supplementary Material
Acknowledgments
The authors thank Shaomeng Wang (University of Michigan) for the generous gifts of A1155463 and A120477. Portions of this manuscript have been presented in abstract form at Digestive Disease Week (DDW).
Glossary
Abbreviations
- CD
Crohn’s disease
- HIO
human intestinal organoid
- ECM
extracellular matrix
- FASL
fas-ligand
- kPa
kilopascal
- COL1A1
collagen 1 A1
- ACTA2
actin alpha 2, smooth muscle
- FN1
fibronectin 1
- MYLK
myosin light chain kinase
- c-PARP
cleaved poly (ADP-ribose) polymerase
- GAPDH
glutaraldehyde 3-phosphate dehydrogenase, β-actin, beta-actin
- α-SMA
alpha smooth muscle actin
- IL-1β
interleukin 1 beta
- TGF-β
transforming growth factor beta
- H&E
hematoxylin and eosin
Author Contribution
P.D.R.H., L.A.H., and E.S.R. conceptualized and designed the studies; E.S.R., B.M., S.G.C., and A.T. performed the experiments; S.H. and J.R.S. provided material and experimental support. E.S.R., L.A.J., B.M., S.G.C., A.T., K.A.E., and P.D.R.H. analyzed and interpreted the data; L.A.J., C.A.S., and P.D.R.H. drafted the manuscript. All authors had access to the study data and have reviewed and approved the final manuscript.
Funding
P.D.R.H. received funding from AbbVie for the organoid and mouse experiments with ABT-263. Research time of P.D.R.H was protected by NIH R01 DK118154, NIH R01 DK 125687, and NIH R01 DK 109032.
Conflicts of Interest
P.D.R.H. has received consulting fees from AbbVie, Amgen, Genentech, JBR Pharma and Lycera. None of the authors are employed by Abbvie. Supplies and technician time were funded by Abbvie in response to an investigator-initiated funding request from P.D.R.H.; P.D.R.H. has previously been local site PI for Abbvie clinical trials with Upadacitinib and Risankizumab. Abbvie currently has no anti-fibrotic products approved for use in humans, or in ongoing clinical trials in Crohn’s disease. The data were analyzed and the manuscript written without any input from Abbvie employees. All other authors report no conflicts of interest.
References
- 1. Loftus CG, Loftus EV Jr, Harmsen WS, et al. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis. 2007;13:254–261. [DOI] [PubMed] [Google Scholar]
- 2. Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:340–350.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li C, Kuemmerle JF. The fate of myofibroblasts during the development of fibrosis in Crohn’s disease. J Dig Dis. 2020;21:326–331. [DOI] [PubMed] [Google Scholar]
- 4. Hinz B. The role of myofibroblasts in wound healing. Curr Res Transl Med. 2016;64:171–177. [DOI] [PubMed] [Google Scholar]
- 5. Horowitz JC, Thannickal VJ. Mechanisms for the resolution of organ fibrosis. Physiology (Bethesda). 2019;34:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hinz B, Lagares D. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat Rev Rheumatol. 2020;16:11–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20:175–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuehl T, Lagares D. BH3 mimetics as anti-fibrotic therapy: unleashing the mitochondrial pathway of apoptosis in myofibroblasts. Matrix Biol. 2018;68-69:94–105. [DOI] [PubMed] [Google Scholar]
- 9. Kale J, Osterlund EJ, Andrews DW. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 2018;25:65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Delbridge AR, Strasser A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015;22:1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garner TP, Lopez A, Reyna DE, et al. Progress in targeting the BCL-2 family of proteins. Curr Opin Chem Biol. 2017;39:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lagares D, Santos A, Grasberger PE, et al. Targeted apoptosis of myofibroblasts with the BH3 mimetic ABT-263 reverses established fibrosis. Sci Transl Med. 2017;9(420). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pan J, Li D, Xu Y, et al. Inhibition of Bcl-2/xl with ABT-263 selectively kills senescent type ii pneumocytes and reverses persistent pulmonary fibrosis induced by ionizing radiation in mice. Int J Radiat Oncol Biol Phys. 2017;99:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moncsek A, Al-Suraih MS, Trussoni CE, et al. Targeting senescent cholangiocytes and activated fibroblasts with B-cell lymphoma-extra large inhibitors ameliorates fibrosis in multidrug resistance 2 gene knockout (Mdr2-/-) mice. Hepatology. 2018;67:247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jia K, Dai Y, Liu A, et al. The senolytic agent navitoclax inhibits angiotensin II-induced heart failure in mice Navitoclax inhibits heart failure. J Cardiovasc Pharmacol. 2020;76(4):452–460.. [DOI] [PubMed] [Google Scholar]
- 16. Weder B, Mamie C, Rogler G, et al. BCL2 regulates differentiation of intestinal fibroblasts. Inflamm Bowel Dis. 2018;24:1953–1966. [DOI] [PubMed] [Google Scholar]
- 17. Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. [DOI] [PubMed] [Google Scholar]
- 18. Chifotides HT, Verstovsek S. New Therapies in development for myelofibrosis. Clin Lymphoma Myeloma Leuk. 2020;20:S69–S71. [DOI] [PubMed] [Google Scholar]
- 19. Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. [DOI] [PubMed] [Google Scholar]
- 20. Johnson LA, Luke A, Sauder K, et al. Intestinal fibrosis is reduced by early elimination of inflammation in a mouse model of IBD: impact of a “top-down” approach to intestinal fibrosis in mice. Inflamm Bowel Dis. 2012;18(3):460–471.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson LA, Sauder KL, Rodansky ES, et al. CARD-024, a vitamin D analog, attenuates the pro-fibrotic response to substrate stiffness in colonic myofibroblasts. Exp Mol Pathol. 2012;93:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steiner CA, Rodansky ES, Johnson LA, et al. AXL Is a potential target for the treatment of intestinal fibrosis. Inflamm Bowel Dis. 2021;27(3):303–316.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson LA, Rodansky ES, Sauder KL, et al. Matrix stiffness corresponding to strictured bowel induces a fibrogenic response in human colonic fibroblasts. Inflamm Bowel Dis. 2013;19:891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodansky ES, Johnson LA, Huang S, et al. Intestinal organoids: a model of intestinal fibrosis for evaluating antifibrotic drugs. Exp Mol Pathol. 2015;98:346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johnson LA, Rodansky ES, Haak AJ, et al. Novel Rho/MRTF/SRF inhibitors block matrix-stiffness and TGF-β-induced fibrogenesis in human colonic myofibroblasts. Inflamm Bowel Dis. 2014;20:154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spence JR, Mayhew CN, Rankin SA, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson LA, Rodansky ES, Moons DS, et al. Optimisation of intestinal fibrosis and survival in the mouse S. Typhimurium model for anti-fibrotic drug discovery and preclinical applications. J Crohns Colitis. 2017;11:724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson LA, Govani SM, Joyce JC, et al. Spironolactone and colitis: increased mortality in rodents and in humans. Inflamm Bowel Dis. 2012;18:1315–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grassl GA, Valdez Y, Bergstrom KS, et al. Chronic enteric salmonella infection in mice leads to severe and persistent intestinal fibrosis. Gastroenterology. 2008;134:768–780. [DOI] [PubMed] [Google Scholar]
- 31. D’Haens G, Rieder F, Feagan BG, et al. ; I. F. W. Group . Challenges in the pathophysiology, diagnosis and management of intestinal fibrosis in inflammatory bowel disease. Gastroenterology. 2019;S0016-5085(19)41035-4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosenbloom J, Mendoza FA, Jimenez SA. Strategies for anti-fibrotic therapies. Biochim Biophys Acta. 2013;1832:1088–1103. [DOI] [PubMed] [Google Scholar]
- 33. Ma C, Battat R, Khanna R, et al. What is the role of C-reactive protein and fecal calprotectin in evaluating Crohn’s disease activity? Best Pract Res Clin Gastroenterol. 2019;38–39:101603. [DOI] [PubMed] [Google Scholar]
- 34. Almeqdadi M, Mana MD, Roper J, Yilmaz ÖH. Gut organoids: mini-tissues in culture to study intestinal physiology and disease. Am J Physiol Cell Physiol. 2019;317:C405–C419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Duan Z, Chinn D, Tu MJ, et al. Novel synergistic combination of mitotic arrest and promotion of apoptosis for treatment of pancreatic adenocarcinoma. Transl Oncol. 2019;12:683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rudin CM, Hann CL, Garon EB, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res. 2012;18:3163–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kipps TJ, Eradat H, Grosicki S, et al. A phase 2 study of the BH3 mimetic BCL2 inhibitor navitoclax (ABT-263) with or without rituximab, in previously untreated B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2015;56:2826–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.