Abstract
Four Candida albicans isolates and six non-albicans Candida isolates were evaluated by time-kill methods to characterize the relationship between nystatin concentrations, the rate and extent of fungicidal activity, and the postantifungal effect (PAFE). Against Candida species, nystatin exhibits concentration-dependent fungicidal activity and a pronounced PAFE.
The incidence of superficial infections and disseminated fungal infections has steadily risen over the past decade (1). Candida now ranks as the fourth most frequently encountered microbe among nosocomial bloodstream pathogens (11). Nystatin, a polyene antifungal agent produced by Streptomyces noursei, exhibits antifungal activity against a broad spectrum of fungal pathogens. Clinical studies have reported nystatin to be effective against azole-resistant strains of Candida and, in some cases, amphotericin B-resistant strains of Candida albicans (1, 6).
Currently, knowledge of the pharmacodynamic characteristics of nystatin is limited. We have previously described the in vitro concentration-response characteristics of several antifungal agents, including fluconazole, flucytosine, amphotericin B, and the echinocandins (3–5, 9). Knowledge of the concentration-effect relationship allows for description of the rate and extent of antifungal activity and provides a more rational basis for determining optimal dosing regimens. The purpose of this study was twofold: (i) to describe the concentration-effect relationship of nystatin for a variety of Candida species, and (ii) to characterize the postantifungal effect (PAFE) of nystatin on these isolates.
Nystatin (Sigma Chemical Company, St. Louis, Mo.) was utilized for MIC determination, carryover demonstrations, and time-kill curve procedures. Dimethyl sulfoxide (DMSO) was used to aid in solubilizing nystatin. The final concentration of DMSO comprised less than 1% (vol/vol) of the total solution concentration used for each experiment in the study. Growth curves have been determined in previous studies and displayed no inhibition of fungal growth in the presence of DMSO when used at similar concentrations (2, 9). Stock solution was separated into unit-of-use vials and frozen at −70°C until needed.
Ten Candida isolates were obtained from the Division of Medical Microbiology, Department of Pathology, The University of Iowa College of Medicine, for use in this study. The strains used included four Candida albicans strains (OY31.5, 142-5609, 2733A, and ATCC 90028) and two strains each of Candida glabrata (582 and 350), Candida krusei (37-5696A, ATCC 6258), and Candida tropicalis (2697 and 3829). Test isolates were stored in sterile water at room temperature until used.
The MIC for each isolate was determined by broth microdilution techniques as outlined by the National Committee for Clinical Laboratory Standards (10). The MIC of nystatin was defined as the lowest concentration of drug that resulted in complete inhibition of visible fungal growth at 48 h.
Prior to performing time-kill studies, carryover effects were examined as previously described (7, 9). If greater than 25% reduction in CFU per milliliter versus the control was detected, carryover was assumed, and tests were repeated with a vacuum filtration system. This was performed by placing 30 μl of the test solution in 10 ml of sterile water and filtering it through a 0.22-μm-pore-size filter by using a vacuum filtration system. The filter paper was aseptically placed directly onto a potato dextrose agar (PDA) (Remel, Lenexa, Kans.) plate and incubated for colony count determination.
Time-kill experiments were conducted as previously described with RPMI 1640 buffered with MOPS (morpholine- propanesulfonic acid) as the growth medium (7, 9). Nystatin was tested over a range of concentrations: 0, (control), 0.0625, 0.125, 0.25, 0.5, 1, 2, 4, 8, and 16 times the MIC for each test isolate. One-hundred-microliter samples were removed at predetermined time points (0, 1, 2, 4, 8, and 24 h) from each of the test solutions and serially diluted, and 30 μl was plated on PDA for colony count determinations. When colony counts were expected to be less than 1,000 CFU/ml, a 30-μl sample was taken directly from the test solution and plated without dilution. Thirty-microliter test samples taken directly from tubes containing concentrations equal to 16 times the MIC were placed in 10 ml of sterile water and filtered through a 0.22-μm-pore-size filter to eliminate antifungal carryover. PDA plates were incubated at 35°C for 24 h prior to colony count determination. The lowest number of accurately and reproducibly detectable colonies obtained by using these methods is 50 CFU/ml (7). All time-kill experiments were conducted in duplicate.
The PAFE of nystatin was determined by previously described methods (12). Briefly, 1 ml of standardized fungal suspension was added to tubes containing 9 ml of medium or 9 ml of medium and nystatin. The concentrations of nystatin tested were 0.25, 0.5, and 1 times the MIC, and a control was used for each isolate. Samples were exposed to nystatin for a half-hour. Following the exposure period, drug was removed via centrifugation for 10 min at 1,400 × g. The supernatant was removed, and two sequential washings were done following resuspension of the cell pellet in 9 ml of drug-free sterile normal saline. Similar procedures have been shown to decrease the concentration of the drug by as much as 10,000-fold, eliminating any carryover effect (2). After the third washing, the fungal pellet was resuspended in sterile normal saline and readjusted to a 0.5 McFarland turbidity standard. One milliliter of the suspension was placed into 9 ml of drug-free, warm RPMI with MOPS medium to create a final starting fungal concentration of 1 × 105 to 5 × 105 CFU/ml. Samples for colony count determinations were removed at predetermined time points (0, 8, 9, 10, 12, 14, 16, 18, and 22 h) and diluted by 10-fold serial dilutions, and 30 μl was streaked on PDA plates. All experiments were performed in duplicate.
Antifungal carryover study analysis was performed by comparing the means from quintuplicate runs for controls and test cultures. Carryover was defined as a >25% reduction in the mean number of CFU per milliliter when compared to the control in the test samples (9).
Colony count (log10 CFU per milliliter) data from duplicate runs were averaged and plotted versus time for each isolate. The rate and extent of antifungal activity were visually compared for each nystatin concentration. Fungicidal activity was defined as ≥99.9% (≥3-log10) reduction in CFU/ml from the starting fungal concentration. Composite concentration graphs were constructed by plotting the change in log10 CFU per milliliter from the starting inoculum at each time point for all isolates versus nystatin concentrations standardized to multiples of the MIC. Mean data for all Candida isolates at each time point were fitted with a sigmoidal hill 4-parameter model with SigmaPlot (Jandel Scientific, San Rafael, Calif.). The 50% effective concentration (EC50), EC90, and maximal effect (Emax) were determined for each time point.
The PAFE was calculated by taking the difference in time required for control and test isolates to grow 1 log10 following drug removal.
Median nystatin MICs for isolates ranged from 4 to 8 μg/ml. The median MICs for Candida albicans 2733A and Candida krusei 37-5696A were 8 μg/ml, whereas the nystatin MIC for all other isolates was 4 μg/ml.
Significant carryover (>25% reduction in mean colony counts from control) was noted for all isolates at 16 times the MIC with a sampling volume of 30 μl. Residual inhibitory effects of nystatin were completely eliminated following filtration of samples. Colony counts resulting from filtered non-drug-exposed control samples were consistently within 25% of the unfiltered control colony count.
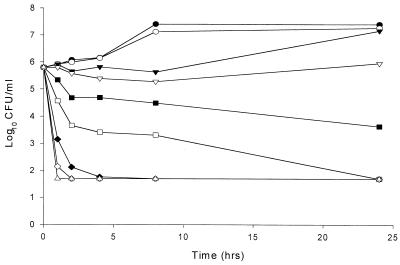
Plots of the log10 CFU per milliliter versus time for all test isolates demonstrate concentration-dependent fungicidal activity. Increases in nystatin concentration consistently resulted in an increase in the rate and/or extent of fungicidal activity with all isolates tested. Figure 1 displays a time-kill plot representative of those noted in this study. Fungistatic activity was generally observed between 0.5 and 2 times the MIC for all isolates. Rapid fungicidal activity was observed with concentrations ≥2 times the MIC for all strains, except for both C. krusei isolates, which did not reach fungicidal endpoints until ≥4 times the MIC.
FIG. 1.
Representative time-kill plot for Candida species following exposure to nystatin. Average data points from duplicate time-kill assays for Candida glabrata 582 are shown. ●, control; ○, 0.0625 times the MIC; ▾, 0.125 times the MIC; ▿, 0.25 times the MIC; ■, 0.5 times the MIC; □, 1.0 times the MIC; ⧫, 2.0 times the MIC; ◊, 4.0 times the MIC; ▴, 8.0 times the MIC; ▵, 16 times the MIC.
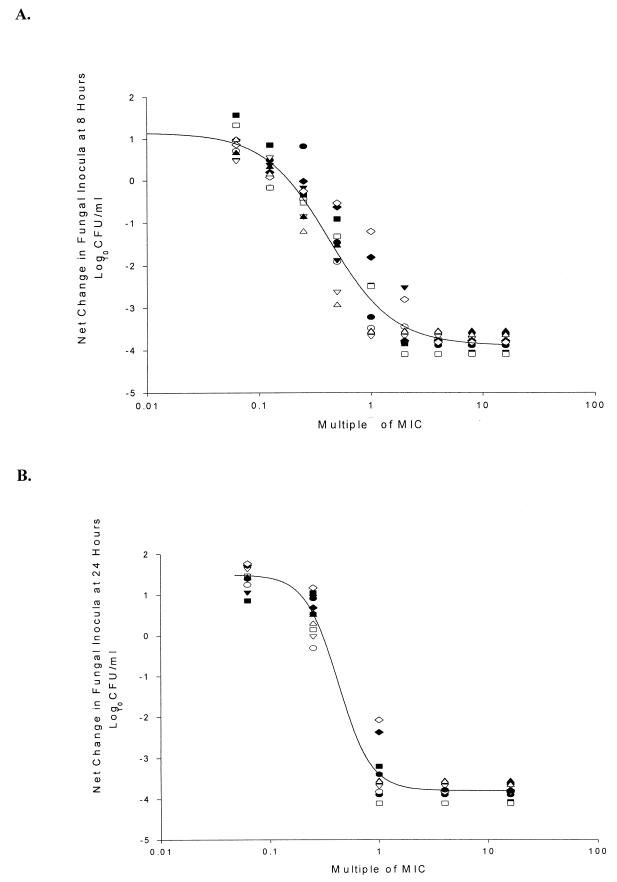
Calculated EC50, EC90 and Emax values at each composite time point are summarized in Table 1. Concentration-effect plots for 8- and 24-h data are shown in Fig. 2A and B, respectively. The EC50 decreased from 1.4 times the MIC to 0.4 times the MIC from h 1 to h 24. Over the same time frame, the EC90 decreased from 5.3 times the MIC at 1 h to 0.9 times the MIC at 24 h. These findings suggest a link between nystatin concentrations and the rate of fungicidal activity.
TABLE 1.
Average Emax, EC50, and EC90 of all Candida isolates at various time points
| Time point (h) | Emax (change in CFU/ml) | EC50 (multiple of MIC) | EC90 (multiple of MIC) |
|---|---|---|---|
| 1 | −3.2336 | 1.4498 | 5.2706 |
| 2 | −3.7740 | 0.9017 | 2.8825 |
| 4 | −4.2186 | 0.6536 | 2.7502 |
| 8 | −5.0506 | 0.4201 | 1.8339 |
| 24 | −5.2858 | 0.4223 | 0.9056 |
FIG. 2.
Average sigmoidal dose-response curve of change in log10 CFU per milliliter compared to starting inocula at 8 h (A) and 24 h (B) for Candida isolates. The line is fitted for all isolates. ●, C. albicans 142-5609a; ○, C. albicans 2733a; ▾, C. albicans 90028; ▿, C. albicans OY31.5; ■, C. glabrata 350; □, C. glabrata 582; ⧫, C. krusei 37.5696a; ◊, C. krusei 6258; ▴, C. tropicalis 2697; ▵, C. tropicalis 3829.
Significant PAFEs were induced by nystatin against each of the test isolates. PAFE was consistently lengthened with higher nystatin exposure concentrations (Table 2). All of the multiples of the MIC caused a notable PAFE, except with both isolates of C. tropicalis, against which no observable PAFE was noted at 0.25 times the MIC. A reduction in starting inoculum was seen with drug exposure, especially at 1 times the MIC, even with only a half-hour exposure time. Restandardization of the washed starting inoculum eliminated any differences between experimental and control fungal concentrations.
TABLE 2.
PAFE induced by nystatin in experimental isolates
| Candida strain | Avg PAFE (h) at:
|
||
|---|---|---|---|
| 0.25× MIC | 0.5× MIC | 1× MIC | |
| C. albicans | |||
| ATCC 90028 | 5 | 7 | 9 |
| 142-5609A | 6 | 4 | 6 |
| OY31.5 | 4 | 4 | 4 |
| 2733A | 5 | 5 | 7 |
| C. tropicalis | |||
| 3829 | 0 | 2 | 4 |
| 2697 | 0 | 2 | 6 |
| C. glabrata | |||
| 350 | 1 | 2 | 4 |
| 582 | 6 | 10 | 10 |
| C. krusei | |||
| ATCC 6259 | 3 | 5 | 7 |
| 37-5696A | 1 | 4 | 6 |
| Avg of all Candida isolates | 3.1 | 4.5 | 6.3 |
Nystatin exhibits concentration-dependent activity against a variety of Candida species. Against Candida species, examination of the concentration-response profile of nystatin over time reveals a curve with a changing slope. This finding suggests that the rate of activity produced by nystatin is concentration dependent. Furthermore, examination of time-kill plots reveals that the extent of fungicidal activity is also dependent on concentration.
Sequential EC50, EC90, and Emax data are useful parameters for describing concentration-response relationships. By examining how the EC50 and EC90 change relative to each other over time, one is able to get a feel for the slope of the concentration-response curve at each time point. If the slope of the curve does not change over time, i.e., the difference between EC50 and EC90 remains relatively constant, then the rate of killing would not appear to be dependent on concentration. This premise is exemplified by the azole antifungal agents (8). In contrast, if the difference between EC50 and EC90 becomes smaller over time, as noted with nystatin in this study, this suggests that the slope of the concentration-response curve becomes steeper with time. This indicates that at earlier time points, higher concentrations are required to produce the maximal effect. Lower concentrations, however, also will produce the maximal effect, only it takes longer secondary to a slower rate of kill. Examination of the EC50 and EC90 at a single time point may provide information regarding the increase in drug concentration required to produce a 40% improvement in kill; however, no data are gathered regarding the dependency of rate of kill on concentration. If one were to look at a time-kill plot of the fungicidal activity of the polyenes and focus on just data at 24 h, all concentrations >1 to 2 times the MIC would result in basically the same amount of kill. However, the rate to get to that endpoint is more rapid with the higher concentrations. Therefore, looking at EC50 and EC90 data from just one time point would be similar to looking at colony count data at just one time point. For the polyenes in vitro, the rate of kill is more dependent on concentration than is the extent of kill because the killing is so efficient. Therefore, if the data from this study were to have been analyzed at only one late time point, false conclusions regarding concentration-dependent and concentration-independent activity may be drawn.
Nystatin concentrations of 0.25 to 1 times the MIC with a 30-min drug exposure produced significant PAFEs. The 30-min drug exposure period and the subinhibitory concentration were selected because longer exposure and concentrations above the MIC exhibited rapid fungicidal activity. Our findings are in agreement with those of Ellepola and colleagues, who reported PAFEs ranging between 6 and 12 h following a 1-h exposure to nystatin (2). The slightly prolonged PAFEs noted by these investigators may be the result of their use of a longer exposure time (1 h) or may occur because they used optical density of cultures to describe fungal growth. Optical density may not be a precise tool for measuring PAFE because the fungal suspension may become turbid with cellular debris.
Concentration-response curves give an approximation of the concentration at which maximal activity is experienced, which in turn can be used to maximize the pharmacokinetic and pharmacodynamic properties of the drug. A drug displaying concentration-dependent activity and a long PAFE, like nystatin, may allow for formulation of an infrequent administration schedule, thus allowing for a prolonged drug-free interval and potentially decreasing drug toxicity without compromising efficacy.
REFERENCES
- 1.Alvarez Alvarez M E, Sanchez-Sousa A, Baquero F. A reevaluation of nystatin in prophylaxis and treatment of oropharyngeal candidiasis. Rev Esp Quimioter. 1998;11:295–315. [PubMed] [Google Scholar]
- 2.Ellepola A N, Samaranayake L P. The in vitro post-antifungal effect of nystatin on Candida species of oral origin. J Oral Pathol Med. 1999;28:112–116. doi: 10.1111/j.1600-0714.1999.tb02007.x. [DOI] [PubMed] [Google Scholar]
- 3.Ernst E J, Klepser M E, Ernst M E, Messer S A, Pfaller M A. In vitro pharmacodynamic properties of MK-0991 determined by time-kill methods. Diagn Microbiol Infect Dis. 1999;33:75–80. doi: 10.1016/s0732-8893(98)00130-8. [DOI] [PubMed] [Google Scholar]
- 4.Ernst E J, Klepser M E, Pfaller M A. In vitro interaction of fluconazole and amphotericin B administered sequentially against Candida albicans: effect of concentration and exposure time. Diagn Microbiol Infect Dis. 1998;32:205–210. doi: 10.1016/s0732-8893(98)00099-6. [DOI] [PubMed] [Google Scholar]
- 5.Ernst M E, Klepser M E, Wolfe E J, Pfaller M A. Antifungal dynamics of LY 303366, an investigational echinocandin B analog, against Candida ssp. Diagn Microbiol Infect Dis. 1996;26:125–131. doi: 10.1016/s0732-8893(96)00202-7. [DOI] [PubMed] [Google Scholar]
- 6.Johnson E M, Ojwang J O, Szekely A, Wallace T L, Warnock D W. Comparison of in vitro antifungal activities of free and liposome-encapsulated nystatin with those of four amphotericin B formulations. Antimicrob Agents Chemother. 1998;42:1412–1416. doi: 10.1128/aac.42.6.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klepser M E, Ernst E J, Lewis R E, Ernst M E, Pfaller M A. Influence of test conditions on antifungal time-kill curve results: proposal for standardized methods. Antimicrob Agents Chemother. 1998;42:1207–1212. doi: 10.1128/aac.42.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klepser M E, Malone D, Lewis R E, Ernst E J, Pfaller M A. Evaluation of voriconazole pharmacodynamics using time-kill methodology. Antimicrob Agents Chemother. 2000;44:1917–1920. doi: 10.1128/aac.44.7.1917-1920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klepser M E, Wolfe E J, Jones R N, Nightingale C H, Pfaller M A. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested against Candida albicans. Antimicrob Agents Chemother. 1997;41:1392–1395. doi: 10.1128/aac.41.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 11.Pfaller M A, Jones R N, Messer S A, Edmond M B, Wenzel R P. National surveillance of nosocomial bloodstream infection due to species of Candida other than Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. SCOPE Participant Group. Surveillance and Control of Pathogens of Epidemiologic. Diagn Microbiol Infect Dis. 1998;30:121–129. doi: 10.1016/s0732-8893(97)00192-2. [DOI] [PubMed] [Google Scholar]
- 12.Turnidge J D, Gudmundsson S, Vogelman B, Craig W A. The postantibiotic effect of antifungal agents against common pathogenic yeasts. J Antimicrob Chemother. 1994;34:83–92. doi: 10.1093/jac/34.1.83. [DOI] [PubMed] [Google Scholar]