ABSTRACT
Most measures of adherence to antiretroviral therapy require a blood sample, and none capture longitudinal daily adherence. A new noninvasive method for measuring daily adherence to antiretroviral regimens containing emtricitabine (FTC) was developed for intact hair strands using infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) mass spectrometry imaging (MSI). A directly observed therapy study of daily and intermittent (3, 1, and 0 doses/week) FTC dosing (n = 12) benchmarked adherence in hair, revealing distinct accumulation patterns and median FTC signal abundance (1,702, 495, 352, and 0, respectively) with each dosing frequency. A threshold value of FTCsignal abundance of 500 differentiated daily dosing from 3 or fewer doses/week (specificity, 100%; sensitivity, 100% over 30 days and 80% over 60 days). Using these criteria, daily FTC hair adherence was classified in young men (n = 8) who have sex with men (YMSM) engaged in or initiating preexposure prophylaxis (PrEP). Four types of adherence profiles were observed in sequential 30-day periods: consistently high, occasional missed doses, improvement following study initiation, and intermittent. Discrete days of nonadherence were identified across the 60-day window, with the average number of consecutive days classified as nonadherent increasing across the four profile types (1, 2, 19, and 58 days, respectively). Additionally, cumulative FTC response in hair (60-day average) significantly correlated with dried blood spot tenofovir diphosphate concentrations collected simultaneously (rs = 0.79, P = 0.03). Based on these data, IR-MALDESI FTC adherence classification in hair strands can better delineate short-term changes in adherence behaviors over a long retrospective window, offering great potential for noninvasive adherence monitoring and quick supportive interventions.
KEYWORDS: adherence, antiretroviral agents, emtricitabine, hair, human immunodeficiency virus, imaging, longitudinal, mass spectrometry, monitoring
INTRODUCTION
Adherence to antiretroviral medication is critical for suppressing plasma HIV RNA (1) and preventing HIV transmission (2) among individuals living with HIV and individuals at risk for HIV acquisition (3–5). The nucleoside analogue reverse transcriptase inhibitor emtricitabine (FTC) is an integrated component of many antiretroviral combinations prescribed for both treatment and prevention. However, recommended daily adherence to therapy or pre-exposure prophylaxis (PrEP) may pose a challenging burden to maintain for some individuals. Poor adherence to daily dosing can lead to viral rebound and/or drug resistance (6, 7). Therefore, noninvasive, real-time means of measuring adherence are needed to monitor for failing long-term and short-term adherence (8, 9).
Objective pharmacologic measures of adherence are more accurate than self-reporting or pill counts but are limited to either very recent or very extended time scales (10). For regimens combining FTC with the nonnucleotide reverse transcriptase inhibitor tenofovir (TFV), monitoring of recent pharmacologic adherence in clinical and research settings has been performed primarily in red blood cells preserved as dried blood spots (DBS). Phosphorylation of FTC in cells yields an active metabolite, FTC triphosphate (FTCtp), with a half-life of ∼35 h that provides a measure of recent adherence on the time scale of 2 to 7 days (11, 12). Tenofovir diphosphate (TFVdp) has a half-life of 17 days, and its measurement reflects an average of 2 to 3 months of drug exposure (12). The combined measure of FTCtp and TFVdp concentrations from DBS provides a picture of recent and cumulative adherence (12, 13) but cannot differentiate daily patterns of dosing behavior or capture time periods of either drug treatment interruptions or intermittent dosing (12).
The accumulation of antiretrovirals in hair strands, mostly incorporated into the follicle from the bloodstream during growth (14), has been used to measure average longer-term adherence (15–17). Unlike DBS, hair does not require a cold chain for sample preservation and represents a simple and relatively noninvasive monitoring method where each centimeter of hair length corresponds to approximately 1 month of hair growth (18). Traditionally, measurements of drug in hair have been performed by liquid chromatography tandem mass spectrometry (LC-MS/MS) using segmental analysis, by which hair strands are routinely cut at intervals of at least 1 cm. While the hair matrix offers a months-long record of behavior, this segmentation length limits the resolution to 1 month or greater.
Mass spectrometry imaging (MSI) provides an alternative approach to analysis of drugs in hair by using focused laser desorption to probe individual hair strands at high spatial resolution, reflecting hours of accumulation and allowing short-term fluctuations in drug response to be profiled over a long retrospective window of time (19, 20). We have developed methods for the sensitive analysis of a range of antiretrovirals in hair strands, including FTC, using infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) MSI (21, 22). Analysis is rapid, with an assessment of the prior month’s record of drug accumulation in five hair strands completed in approximately 1.5 h.
In this work, we benchmarked IR-MALDESI MSI analysis of FTC accumulation in hair strands collected under directly observed dosing with FTC/tenofovir disoproxil fumarate (TDF) using different weekly dosing schedules. We used these benchmarks to evaluate adherence from hair strands of young men using PrEP and compared these results to adherence measurements in dried blood spots.
RESULTS
IR-MALDESI MSI benchmarking of FTC in hair strands: the ENLIGHTEN study.
Twelve subjects were enrolled in the multiphase ENLIGHTEN directly observed therapy (DOT) study to assess the dose response of FTC accumulation in hair as measured by IR-MALDESI MSI. These individuals were between 21 and 63 years of age, with a median age of 26.5 years. Two participants (17%) were male and 10 participants (83%) were female, with one individual identifying as non-gender conforming. Eight individuals (67%) identified as white, 1 (8%) as Black, and 3 (25%) as Asian. Hair colors ranged from blonde to black and did not influence measurements of FTC accumulation (22). FTC was measured over an estimated 1 month of growth in samples collected at the end of daily dosing and also over an estimated 2 months of growth in samples collected at the end of the subsequent intermittent dosing period. No FTC response was detected in samples collected from one individual reporting recent hair treatment, which we have shown can significantly decrease concentrations of FTC in hair when treatment includes products for bleaching, dyeing, or relaxing (22).
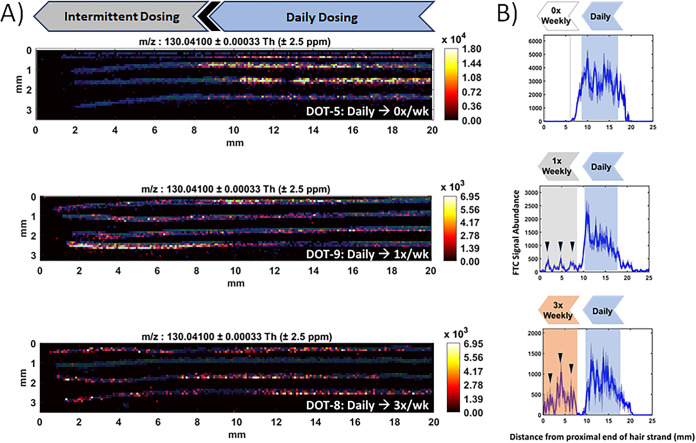
Representative examples of IR-MALDESI MSI results from the combined analysis of daily and intermittent dosing periods in single hair strands from each dosing group can be seen in Fig. 1A for samples collected at the end of intermittent dosing. Here, the position of individual hair strands is apparent based on IR-MALDESI MSI response to an endogenous ion (m/z 248.2457, unidentified lipid, in blue) that is ubiquitous throughout the strands. Measured signal abundance of FTC, determined by detection of its characteristic fragment (m/z, 130.0410), is also shown on the hair strands ranging from regions of high accumulation (orange-yellow) to low accumulation (dark red-black). A comparison of these images indicates that FTC response across all samples is highest on the right-hand, distal portion of the hair strands corresponding to hair growth during the period of daily dosing. Hair strands devoid of FTC response, for example, the single strand associated with DOT-8 in Fig. 1A showing response only to the endogenous ion, were assumed to represent a resting phase of growth common to ∼15% of scalp hair (14) and were not considered for further analysis. Additionally, the distance from the scalp at which each strand was cut can vary depending on the angle at which the scissors were held during sample collection (18), leading to small differences between strands in the position of changes in measured drug accumulation. This effect is apparent when examining proximal-to-distal changes in FTC signal abundance from the three DOT-8 strands in an active growth phase, where the position of the segment of growth associated with washout between intermittent and daily dosing periods can be seen to differ between strands.
FIG 1.
(A) Representative IR-MALDESI MSI FTC ion maps showing drug accumulation associated with daily dosing and each of the three intermittent dosing groups (from top: 3 times per week, once per week, and 0 times per week, respectively). FTC signal abundance is represented by a color scale increasing in concentration from regions of dark red/black to regions of orange/yellow. Also visible is the overlaid IR-MALDESI response to an endogenous ion homogeneously present in the hair strands (blue) that clearly defines the shape, orientation, and length of the individual strands to assist in seeing regions of hair growth where FTC was not measured. (B) Composite longitudinal profiles of FTC and 95% confidence intervals (CI) from individual hair strands shown in panel A. Shaded areas reflect periods of hair growth within the profile attributed to active dosing. Arrows in the intermittent-dosing region highlight repeating weekly patterns measured by IR-MALDESI.
For each sample, common features within individual strand profiles were aligned using custom software to ensure that radial averaging between strands was conducted at matched time points. Composite longitudinal profiles and 95% confidence intervals for each sample are shown in Fig. 1B, from which differences between daily dosing and intermittent dosing are more apparent. The profiles show waxing and waning FTC exposure with drug initiation and cessation. Shaded boxes in this figure denote the discrete signatures associated with each dosing frequency in the FTC profile. No response to FTC was observed following transition from daily dosing to a dosing frequency of 0 doses per week, while distinct weekly repeating dosing patterns were observed for dosing frequencies of 1 dose per week and 3 doses per week.
IR-MALDESI MSI adherence threshold for FTC.
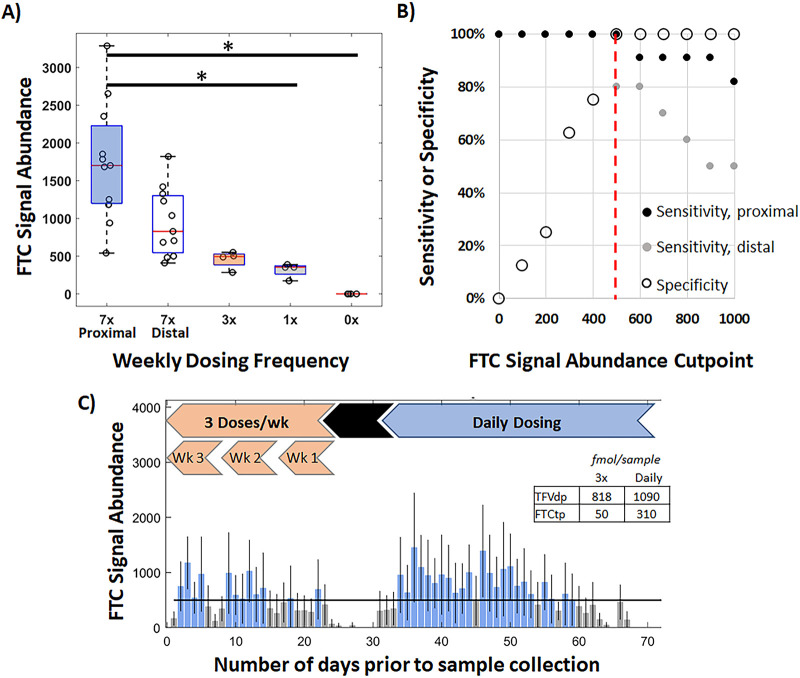
Mean FTC signal abundance evaluated from longitudinal profiles in 11 of 12 samples is summarized in Fig. 2A for regions associated with daily dosing and intermittent dosing intervals. The relative standard deviation among measurements of daily dosing (45%) exceeded median within-individual variability (35%), reflecting interindividual variability in FTC hair accumulation. When measurements of FTC within the proximal 1 cm of hair were considered to reflect growth within the past month, mean FTC responses were significantly higher for the period of daily dosing than 1 dose per week and 0 doses per week across all individuals (adjusted P value < 0.05). While median FTC signal abundance for 7 doses per week was 3.44-fold higher than 3 doses per week and individual longitudinal profiles showed consistently higher FTC signal abundance for daily dosing, differences between all measurements within these groups were not statistically significant. After the intervening month of hair growth between the end of daily dosing and intermittent dosing periods, the FTC response to daily dosing was found to decrease by 55%, which may reflect drug washout. However, a similar discrimination between daily and intermittent dosing frequencies was maintained regardless of whether the segment of hair corresponding to daily dosing was proximal or distal.
FIG 2.
(A) Mean IR-MALDESI FTC signal abundance associated with each ENLIGHTEN dosing group evaluated from composite longitudinal hair profiles. Daily dosing response was measured in the proximal 1 cm of strands collected after daily dosing and in the distal 1 cm of strands collected after intermittent dosing. (B) Cut point selection for binary classification of daily adherence based on a receiver operator characteristic curve. (C) Example bar graph of daily adherence classification of DOT-8 based on the selected cut point. Blue bars reflect days in which FTC response exceeded the adherence cut point and gray bars reflect days where FTC response did not exceed the adherence cut point. The inset shows steady-state concentrations of TFVdp and FTCtp at the end of daily and intermittent dosing.
Selection of a threshold value for FTC signal abundance for binary classification of adherence was determined from a receiver operating characteristic (ROC) curve based on all observations. The true-positive rate (sensitivity) and true-negative rate (specificity) of binary classification are shown in Fig. 2B as a function of possible FTC signal abundance threshold values. Based on clinician and patient perspectives that it was critical to minimize the likelihood of incorrectly classifying an individual as being nonadherent to medication (23), a threshold value of FTCsignal abundance of 500 was chosen (specificity, 100%; sensitivity, 100% over 30 days and 80% over 60 days) to differentiate 3 or fewer doses per week from more frequent dosing. An example of daily adherence classification by this adherence cut point is shown in Fig. 2C for the longitudinal FTC profile (daily → 3×/week) shown in Fig. 1B.
Comparison of FTC in hair to TFVdp.
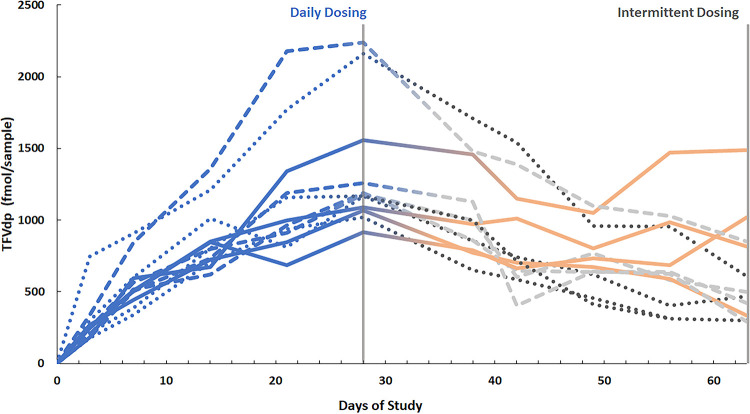
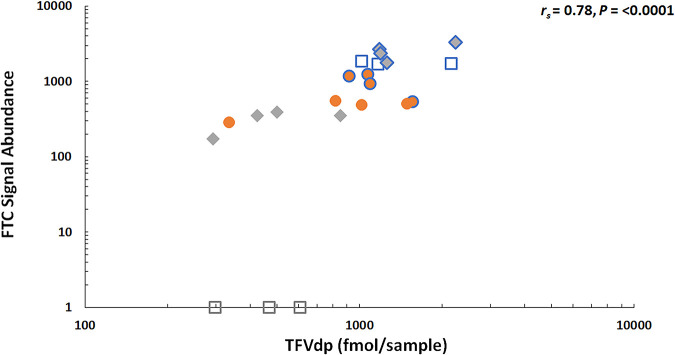
Intracellular metabolites were measured by LC-MS/MS in DBS from samples collected throughout the ENLIGHTEN study at the same time as the hair samples. Steady-state concentrations of TFVdp and FTCtp for each dosing group are summarized in Table 1. As was found for FTC in hair, concentrations of both intracellular metabolites were significantly higher in the 7-doses-per-week group than the 1-dose-per-week and 0-doses-per-week groups (adjusted P value < 0.05 for all) but not higher than in the 3-doses-per-week group. Variation of TFVdp over the course of DOT periods of daily and intermittent dosing is shown in Fig. 3, which also displays concentrations of TFVdp in each group. Due to the short half-life of FTCtp in red blood cells, FTCtp concentrations for intermittent dosing were below the limits of quantitation in 44 of 59 total samples and 10 of 12 samples under steady-state conditions. Therefore, comparison of IR-MALDESI MSI measurements of FTC in hair to DBS used TFVdp concentrations. The relationship between mean FTC signal abundance and steady-state concentrations of TFVdp is shown in Fig. 4, and the correlation was strong (rs = 0.78, P < 0.0001).
TABLE 1.
FTC hair and FTCtp and TFVdp DBS concentrations for each ENLIGHTEN DOT dosing groupa
| No. of doses/wk | FTC |
TFVdp |
FTCtp |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal abundance |
RSD (%) | Concn (fmol/sample) |
RSD (%) | Concn (fmol/sample) |
RSD (%) | |||||||
| Median | 1st quartile | 3rd quartile | Median | 1st quartile | 3rd quartile | Median | 1st quartile | 3rd quartile | ||||
| 7 | 1,702 | 1,200 | 2,228 | 45 | 1,175 | 1,080 | 1,410 | 32 | 326 | 251 | 363.5 | 28 |
| 3 | 495 | 386 | 526 | 26 | 919 | 577 | 1,255 | 52 | 133 | 50b | 243 | 78 |
| 1 | 352 | 262 | 371 | 31 | 462 | 358 | 676 | 46 | BLQ | BLQ | BLQ | NA |
| 0 | 0 | 0 | 0 | 0 | 390 | 305 | 539 | 35 | BLQ | BLQ | BLQ | NA |
RSD, relative standard deviation; NA, not applicable.
Imputed at half the limit of quantitation.
FIG 3.
TFVdp concentrations (fmol/sample) in DBS for ENLIGHTEN throughout daily dosing (blue) and intermittent dosing (orange, 3 times weekly; light gray, once weekly, dark gray, 0 times weekly). Concentrations selected for comparison to hair FTC are shown by vertical lines.
FIG 4.
Comparison of mean FTC signal abundance relative to steady-state TFVdp concentrations in DBS associated with each identified daily and differential dosing interval. Symbols reflect intermittent-dosing groups (orange circles, 3 times weekly; light gray diamonds, once weekly; white squares, 0 times weekly), with data points outlined in blue corresponding to daily dosing periods. FTC signal abundance was uniformly higher for daily dosing than intermittent dosing.
Demonstration of adherence with PrEP.
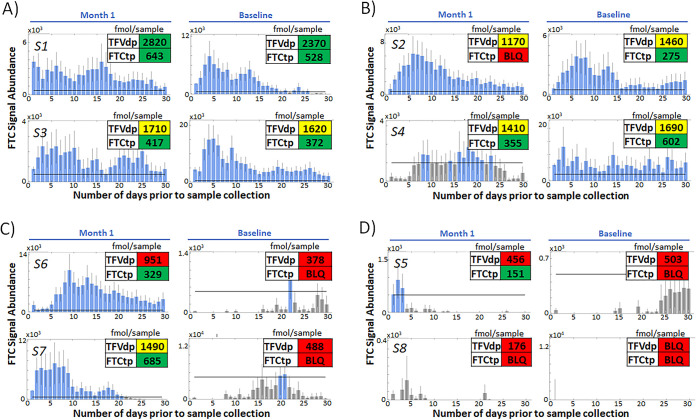
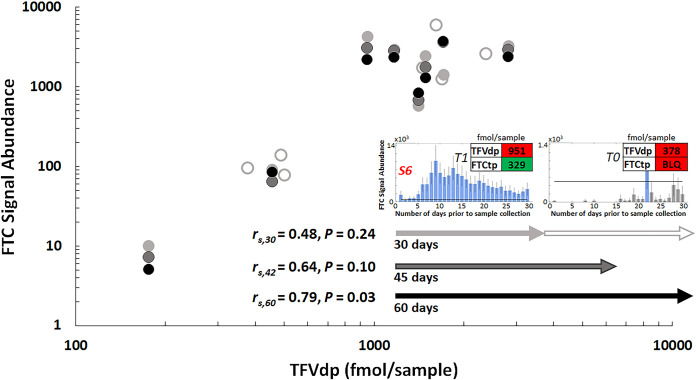
Once benchmarked, PrEP adherence was investigated using IR-MALDESI MSI of FTC in hair strands for a set of longitudinal clinical samples collected as part of field testing for the Prepared, Protected, emPowered (P3) study (24). This study is focused on use of a smartphone application (app) and is enrolling HIV-uninfected transgender women and young men who have sex with men (YMSM) who are starting PrEP or are non-adherent to PrEP. YMSM volunteers (n = 8) reporting a range of recent adherence to PrEP at enrollment (Table 2) provided hair and blood samples at study initiation (T0) and after 1 month (T1). Figure 5 shows 30-day adherence assessments to PrEP at both sampling time points based on IR-MALDESI MSI profiling of FTC. Adherence at T1 and T0 in these subjects was categorized into four groups: consistently high adherence (Fig. 5A), high adherence with occasional missed doses (Fig. 5B), dramatically improved adherence after study initiation (Fig. 5C), and intermittent adherence (Fig. 5D). Insets in this figure show matching metabolite concentrations of FTCtp and TFVdp with categorization following adherence benchmarking by Anderson et al. (12) that has been adjusted based on known bias in quantitative measurements between the two labs. Detectable FTCtp reflected dosing within the prior 7 days. Adherence based on TFVdp concentrations was differentiated into the following categories: low (<4 doses/week, BLQ [below the limit of quantification] to 980 fmol/sample), medium (4 to 6 doses/week, 980 to 1,750 fmol/sample), and high (>6 doses/week, >1,750 fmol/sample). A summary of adherence measures and classification can be seen in Table 2 along with baseline self-reported adherence. Trends in the FTC profiles agree with the metabolite data from DBS, while the hair profiles also indicate discrete patterns of adherence over days. Comparison of FTC in hair to TFVdp concentrations in DBS from P3 samples is shown in Fig. 6. We investigated the agreement between these two metrics over time scales ranging from 30 to 60 days by either evaluating the 16 paired samples at the individual time points or comparing the T1 TFVdp measurement to a longitudinal FTC profile concatenating 30-day periods of T0 and T1, as illustrated in the inset in Fig. 6. Correlation between the two measures was found to improve as the period over which FTC response was measured was extended to include a greater proportion of both T1 and T0 profiles. Ultimately, the TFVdp concentrations were most highly correlated with a 60-day FTC average, combining the entire T1 and T0 profiles (rs = 0.79, P = 0.03).
TABLE 2.
Self-reported and objective adherence classifications for P3 study samples
| Patient | % adherence in last moa | Started and stopped PrEPa | Sampling time point | DBS analysis |
IR-MALDESI MSI |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FTCtp |
TFVdp |
Days above adherence threshold | Days below threshold: detectable response | Days below threshold: no response | Avg consecutive days below threshold | ||||||
| Concn (fmol/sample) | Adherence classification | Concn (fmol/sample) | Adherence classification | ||||||||
| S1 | 80 | Yes | T0 | 528 | Recent dosing | 2,370 | High | 25 | 3 | 2 | 2.5 |
| T1 | 643 | Recent dosing | 2,820 | High | 30 | 0 | 0 | 0 | |||
| S2 | 80 | No | T0 | 275 | Recent dosing | 1,460 | Medium | 30 | 0 | 0 | 0 |
| T1 | BLQ | No recent dosing | 1,170 | Medium | 30 | 0 | 0 | 0 | |||
| S3 | 70 | Yes | T0 | 372 | Recent dosing | 1,620 | Medium | 30 | 0 | 0 | 0 |
| T1 | 417 | Recent dosing | 1,710 | Medium | 30 | 0 | 0 | 0 | |||
| S4 | 80 | No | T0 | 602 | Recent dosing | 1,690 | Medium | 30 | 0 | 0 | 0 |
| T1 | 355 | Recent dosing | 1,410 | Medium | 10 | 20 | 0 | 4 | |||
| S5 | 60 | Yes | T0 | BLQ | No recent dosing | 503 | Low | 0 | 14 | 16 | 30 |
| T1 | 151 | Recent dosing | 456 | Low | 3 | 9 | 18 | 27 | |||
| S6 | 40 | No answer | T0 | BLQ | No recent dosing | 378 | Low | 1 | 17 | 12 | 14.5 |
| T1 | 329 | Recent dosing | 951 | Low | 30 | 0 | 0 | 0 | |||
| S7 | 70 | No | T0 | BLQ | No recent dosing | 488 | Low | 2 | 22 | 6 | 14 |
| T1 | 685 | Recent dosing | 1,490 | Medium | 21 | 4 | 5 | 9 | |||
| S8 | 100 | Yes | T0 | BLQ | No recent dosing | BLQ | Low | 0 | 8 | 22 | 30 |
| T1 | BLQ | No recent dosing | 176 | Low | 0 | 9 | 21 | 30 | |||
Self-reported in adherence presurvey.
FIG 5.
Longitudinal adherence profiles observed in 8 subjects (S1 to S8) enrolled in the P3 study. Profiles are grouped into four types: consistent high adherence (A), high adherence with occasional missed doses (B), dramatically improved adherence after study initiation (C) and intermittent adherence (D). Images are arranged as hair samples would be analyzed most recent information (T1 visit) closest to the hair follicle on the left and baseline information (T0 visit) on the right. Inset values are intracellular metabolite concentrations (fmol/sample) measured in paired DBS samples. These values are color coded based on adherence categorization (for FTCtp, green indicates dosing within 7 days and red indicates no recent dosing; for TFVdp, green indicates >6 doses/week, yellow indicates 4 to 6 doses/week, and red indicates <4 doses/week).
FIG 6.
Comparison of mean FTC signal abundance relative to steady-state TFVdp concentrations in DBS for P3 study samples. Light gray circles correspond to 30-day comparisons between T1 (open circles) and T0 (filled circles) sampling time points. To investigate agreement between TFVdp and longer period of adherence behavior, T0 and T1 profiles were partially or completely concatenated (inset) for 45- and 60-day FTC profile periods.
DISCUSSION
This study investigated the use of a new pharmacologic measure for classifying adherence to HIV treatment or prevention regimens containing FTC. IR-MALDESI MSI captures the changes in accumulation of FTC along individual hair strands at a high spatial resolution. This, in turn, allows us insight into longitudinal drug dosing behavior. Each discrete measurement (corresponding to 1 pixel in the MSI image) along individual hair strands represents an estimated 8 h of growth. These pixels can then be combined to represent daily drug exposure. The window over which adherence can be monitored in this way is limited only by the length of the hair and the extent to which drug may be removed from strands through hair treatments (22, 25, 26).
We characterized FTC disposition in hair strands by IR-MALDESI over approximately 2 months of known adherence behavior through directly observed dosing with FTC/TDF. TFV, which accumulates much less in hair than other antiretrovirals and typically requires pooling 50 to 100 hair strands to provide sufficient sample mass for detection by LC-MS (15, 27), could not be targeted in single-strand analysis by IR-MALDESI. This investigation revealed clearly defined patterns of FTC accumulation from which different dosing frequencies and washout can be resolved. Dose-response analysis for FTC by IR-MALDESI from DOT samples indicated discrimination between daily dosing and less frequent dosing patterns more clearly than found in the STRAND study for TFV in hair (28). While the difference in daily dosing and 3×/week dosing was not found to be statistically significant, the 25th to 75th quartiles of the respective measurements were resolvable (Fig. 2A) following the approach taken by Anderson et al. to differentiate dosing frequency based on TFVdp in DOT (12).
Although the measured FTC response to daily dosing incorporated into hair strands was found to be reduced with continued growth over time, which may be a result of personal hair care attenuating longitudinal analyte concentrations (29), daily dosing was still effectively differentiated from less frequent dosing throughout the 2-month period investigated. Selection of a threshold for classifying adherence behavior from the resulting ROC curve prioritized classification sensitivity in order to minimize the likelihood of a false-negative test result, incorrectly misclassifying an individual as nonadherent. The resulting binary classification of a longitudinal FTC profile conservatively discriminates daily drug exposure consistent with 3 or fewer doses per week from more frequent dosing. This threshold for FTC exposure in hair is likely applicable to diverse populations of individuals engaged in prevention or treatment, because FTC hair concentrations are not correlated with hair color or melanin (22) and are not expected to be dependent on patient sex or age based on findings associated with the structurally similar nucleoside reverse transcriptase inhibitor tenofovir (28, 30, 31).
In application of the benchmarked FTC adherence classification criteria to samples from YMSM engaged in PrEP, short-term fluctuations in FTC apparent in longitudinal profiles could be categorized into four general patterns across the paired longitudinal samples, which are intended solely as a descriptive means of grouping the observed patterns of behavior. In each case, the combined 30-day adherence profiling of FTC in samples collected 1 month apart provides a granularity of day-to-day behavior not offered by the information derived from FTCtp and TFVdp concentrations in DBS. For individuals exhibiting sustained adherence at >3 doses/week with signal abundance consistently above the defined cut point of 500 (S1 to S4), relative changes in FTC signal abundance within individuals can still be observed. This is most apparent in Fig. 5A, where an accumulation period is apparent in the distal portion of S1_T0 following restart of PrEP in the same month as study enrollment, in agreement with the baseline questionnaire. For samples reflecting sporadic dosing behavior (Fig. 5D), clustered patterns of dosing can be resolved either weeks prior to sampling (S5_T0) or within recent days (S5_T1). In cases where paired FTCtp and TFVdp concentrations reflected high dosing frequency (S4_T1, S7_T1), IR-MALDESI profiling of FTC was able to differentiate between instances of intermittent dosing (S4_T1 and S7_T0) either following a period of sustained adherence (S4_T0) or prior to re-engagement in PrEP in dosing akin to a prolonged drug holiday (S7_T1). In all cases, clustering of days below the adherence cut point could be evaluated from the longitudinal profiles.
Identification of short-term patterns within long-term dosing behavior has important implications for the care of individuals using antiretrovirals for treatment and prevention. Perfect adherence with modern, long-half-life antiretrovirals may not be required to maintain full viral suppression or protection from infection, but frequently missed doses may have deleterious consequences. Treatment interruptions longer than 48 h can result in HIV viral load rebound (6) and may lead to enhanced inflammation and immune activation (32, 33) even in the absence of virologic failure. Capturing short-term changes in patterns of adherence may become increasingly beneficial as on-demand and time-based nondaily dosing schemes for PrEP become more popular (34, 35). It may also be useful to understand the role of missed doses in the last few months in breakthrough infections (36).
Long-term adherence behavior from longitudinal hair profiles, assessed by averaging FTC signal abundance measured in hair strands over a designated growth period, was compared to intracellular metabolite concentrations based on their prevalence in adherence monitoring. Through combination of the IR-MALDESI hair profiles for T0 and T1 time points, adherence behavior could be assessed over a period of 1 to 2 months, corresponding to 1 to 2 cm of hair growth. Correlation between hair FTC and TFVdp measurements was greatest when evaluating the 2-month cumulative response in hair strands, which is consistent with the 2 to 3 months of dosing behavior that the TFVdp concentrations reflect. Agreement between cumulative measurements of FTC in hair and TFVdp was similar to those from the iPreX OLE study evaluating 806 paired samples (rs = 0.742, P < 0.001), which used the segmental analysis of the proximal 1.5 cm of hair strands (15).
The long-term, daily adherence classification provided by IR-MALDESI FTC hair profiles may allow new and easy to interpret capabilities in adherence monitoring. These data have the potential to be directly correlated with self-reported daily adherence (including through use of the medication tracking feature within the P3 app) or nonpharmacologic adherence monitoring measures such as Wisepill data. The retrospective daily adherence information offered by IR-MALDESI FTC hair profiles, which can also be represented in calendar format, was found to be readily interpretable by both patients and providers. Its short analysis time is compatible with the time scale of a clinic visit. As part of care, it has the potential to facilitate discussions and shared decision-making about adherence behavior and may represent a useful new approach for targeting interventions to individuals at greatest risk for transmission or viral rebound. We have demonstrated sensitivity of IR-MALDESI MSI to physiologically relevant concentrations of other antiretrovirals (ARVs) in hair (21, 22), and the MSI approach offers flexibility for assessment of multiple analytes simultaneously from the same analysis such that the adherence classification approach implemented for FTC can be expanded to other ARVs alone or in combination.
Primary limitations of this study relate to sample size. Benchmarking of FTC in hair strands by IR-MALDESI MSI from the ENLIGHTEN study included a total of 12 participants. With only 4 individuals in each of the three intermittent dosing groups, we did not observe statistically significant differences between daily dosing and 3 doses per week. The resulting adherence threshold can distinguish daily hair responses consistent with dosing of 3 or fewer doses per week from more frequent dosing behavior, but additional benchmarking would be required to further refine detection of dosing frequency. Additionally, agreement between FTCtp and the proximal 5 to 7 days of FTC adherence classification was not always consistent. We attribute this incongruence to the sample collection approach for the hair strands. It can take 3 to 5 days for hair to emerge above the scalp line, and cutting techniques likely add additional uncertainty to how recently in the past accumulation of drug into the proximal end of a cut strand occurred. We adjusted hair sampling to plucking. Including the hair root ensures that profiling reflects the most recent incorporation of drug. With only 5 strands of hair needed to account for variability in hair growth phases, this represents minimal participant burden. While acceptability of hair collection is among patients is generally high (27, 37), this may differ in some communities (38). Given that some hair treatments can remove FTC from hair or modulate FTC in hair, cultural differences in hair care may also need to be considered for diverse populations. Benchmarking has focused on scalp hair, but grooming habits or lack of hair growth may preclude sampling from this location for some individuals. Longitudinal profiling can also be performed using body hair from other sampling locations, though variability in growth rates and possible external contamination from sweat would need to be considered through further benchmarking.
We have presented a sensitive new approach for measuring FTC in hair strands at clinically relevant concentrations that preserves the record of drug accumulation associated with different dosing behaviors through a unique approach to mass spectrometry imaging, IR-MALDESI. This approach is a noninvasive, long-term measure of daily adherence for patients and clinicians, applicable to both treatment and prevention, that can differentiate patterns of adherence. Feasibility and acceptability of implementing this approach in a clinical setting are being evaluated as part of the ENLIGHTEN study (NCT04232540), and scalability will be a focus of future work.
MATERIALS AND METHODS
Reagents.
Methanol (high-performance liquid chromatography [HPLC] grade), acetonitrile (HPLC grade), water (HPLC grade), acetic acid (80% [wt/wt]), and formic acid (Optima) were obtained from Fisher Scientific (Hampton, NH). The electrospray solvent was a 50/50 (vol/vol) mixture of methanol and water with 0.2% formic acid. Blank (drug-free) human hair samples were obtained from BioreclamationIVT (Westbury, NY).
Study design.
Samples were evaluated from two clinical trials:
(i) Benchmarking: ENLIGHTEN.
As part of the ENLIGHTEN study (NCT03218592), conducted at the North Carolina Translational and Clinical Sciences (NC TraCS) Institute Clinical and Translational Research Center (CTRC), HIV-uninfected healthy volunteers (n = 12) were administered TDF 300 mg plus FTC 200 mg by directly observed therapy. All study volunteers (DOT-1 to DOT-12) participated in a 28-day period of daily dosing after which they were randomized (n = 4) for a subsequent 28-day period to one of three differentiated dosing frequencies: 0 doses per week, 1 dose per week, or 3 doses per week. An interval of 7 days separated each dosing period. This enabled characterization of FTC accumulation in hair strands by IR-MALDESI MSI under different dosing patterns.
Hair and whole blood samples were collected during study visits on days 3, 7, 14, 21, and 28 of each dosing period. Benchmarking of IR-MALDESI MSI response to FTC accumulation in ENLIGHTEN hair strands in daily and intermittent dosing periods was conducted using samples collected at the end of daily and intermittent dosing phases. FTC was evaluated in the proximal 1 cm of each strand collected following daily dosing and the proximal 2 cm of each strand collected following intermittent dosing. The segment lengths associated with these longitudinal samples reflect 1 month and 2 months of hair growth, respectively, and correspond to accumulation of FTC in each of the two dosing periods. FTCtp and TFVdp were measured in DBS collected at the same time as hair samples.
(ii) Application: P3.
P3 (Prepared, Protected, emPowered) (NCT03320512) is an ongoing clinical study investigating the 6-month use of an electronic social networking, gamification, and adherence support application to promote PrEP adherence and persistence in PrEP care among HIV-uninfected young men who have sex with men and young trans women aged 16 to 24 years. Eligible individuals are currently on PrEP or are planning to start PrEP soon. As part of a randomized-controlled trial across 11 study locations, longitudinal analysis of TFVdp and FTCtp in dried blood spots and FTC in hair is being performed as objective measures of adherence. Prior to commencement of the trial, a brief field test was undertaken to validate study protocols. Participants eligible for participation in the field trial included those who were not currently on PrEP but were planning to initiate in the next 7 days and those who had an active PrEP prescription or were on PrEP and had an active PrEP prescription. In the current work, we evaluated hair and blood samples (n = 16) collected from 8 YMSM field testing participants in the field test at two time points: at enrollment (T0) and 1 month after enrollment (T1). These measures were compared to self-reported adherence administered through an online survey at baseline and study completion (1 month).
Sample collection for both clinical trials. (i) Hair.
Hair sampling was conducted by cutting approximately 10 hair strands from the occipital region close to the scalp using scissors. Strands were adhered to aluminum foil at their distal end to preserve orientation before being sealed and stored with a desiccant gel pack at 4°C until analysis.
(ii) Dried blood spots.
Whole blood was collected in one 3-mL K2 EDTA tube (Becton, Dickinson & Company, Franklin Lakes, NJ, USA) and then spotted (40 to 60 μL) within 1 h of collection onto Whatman 903 Protein Saver cards that were subsequently dried at room temperature for at least 2 h but no more than 24 h before being placed in the −80°C freezer.
IR-MALDESI MSI analysis of FTC in hair.
Hair strands (n = 4 to 5) were adhered to glass microscope slides with VHB double-sided tape (3M, St. Paul, MN) and oriented horizontally with proximal strand ends positioned to the left side of the slides. Sample slides were then transferred to an IR-MALDESI MSI source enclosure, described in detail elsewhere (39). Briefly, samples were placed on a temperature-controlled stage and cooled to −9°C under dry nitrogen gas flow to reduce humidity. After temperature stabilization, the enclosure was opened to the ambient atmosphere, allowing growth of an ice layer promoting absorption of energy from single IR laser pulses (λ = 2,940 nm; IR Opolette; Opotek, Carlsbad, CA) for sample desorption (40). An orthogonal electrospray plume entrained and ionized volatilized sample material, with resulting ions measured by the mass spectrometer coupled to the IR-MALDESI MSI source (Q Exactive Plus; Thermo Fisher, Bremen, Germany). During experiments, the source was closed and humidity was controlled to ∼14% by a low flow of nitrogen gas to maintain ice thickness. The sample stage was translated in 100-μm increments such that each sampling location (100- by 100-μm pixel size) along the hair strands corresponds to approximately 7 to 8 h of growth based on the average growth rate (∼1 cm/month) in the occipital region (18). Interrogated regions of interest on hair strands were at least 1 cm in length. For analysis of FTC, the mass spectrometer was operated in a selected reaction monitoring tandem mass spectrometry mode in positive polarity (m/z 248.1 ± 4 → m/z 130.041; normalized collision energy (NCE) = 10 with charge z set at +2; resolving power, 140,000 at m/z 200; s-lens RF level, 50) (22).
Data were processed using MSiReader (41) and custom MATLAB software (MathWorks, Inc., Natick, MA). Raw mass spectrometry files were converted into the imZML file structure using MSconvert (42) and imzMLconverter (43) serially before applying MSiReader to extract the ion heat maps delineating ions of interest along the hair strands with a mass window of 5 ppm. A custom MATLAB interface (44) evaluated longitudinal profiles of FTC response in individual strands within a sample, which were then aligned to a common reference and averaged radially to create a composite sample profile. For daily adherence classification, responses from adjacent sampling locations (n = 3) were binned to provide an average FTC ion abundance reflecting a period of 24 h.
LC-MS/MS analysis of DBS.
Analysis of TFVdp and FTCtp in DBS was performed by a validated LC-MS/MS assay (45). Extraction of metabolites from 3-mm DBS punches was performed using a combination of protein precipitation and a liquid-liquid extraction. Separation was performed by anion exchange (Thermo Scientific BioBasic AX column) in a Shimadzu Prominence HPLC system using 750 mM ammonium acetate (mobile phase A) and 75:25 5 mM ammonium acetate-acetonitrile, pH 10.1 (mobile phase B). Detection of analytes was performed using an API-5000 triple quadruple mass spectrometer (SCIEX, Foster City, CA, USA) operated in positive ion TurboIonspray mode by selected reaction monitoring to detect the analyte transitions (precursor m/z/product m/z) as follows: TFVdp, 448/270; FTCtp, 488/130. Limits of detection were 100 fmol/sample.
Statistical analyses. (i) Determination of adherence cut point.
A receiver operating characteristic curve was evaluated to guide selection of an adherence cut point for IR-MALDESI MSI daily adherence classification profiles. This analysis compared DOT FTC signal abundance measurements associated with daily dosing to the pooled measurements from all intermittent dosing frequencies to discriminate between daily dosing and 3 or fewer doses per week. True-positive and false-positive rates for IR-MALDESI MSI FTC response on the hair strands were determined based on known adherence behavior. Specificity and sensitivity were then used as criteria to delineate a cut point threshold for classifying FTC signal abundance as either adherent or nonadherent in an approach similar to that used in a prior assessment of FTC dose ranging (46).
(ii) Comparing FTC signal abundance in hair with TFVdp concentration in DBS.
Comparisons between FTC signal abundance in hair associated with each of the investigated dosing frequencies were analyzed by the Kruskal-Wallis one-way analysis of variance (ANOVA) followed by Dunn-Šidák P value corrections for multiple comparisons with significance at a P value of <0.05. The Spearman’s rank order correlation coefficient (Spearman’s rho [rs]) was determined to characterize the relationship between response of FTC in hair measured by IR-MALDESI MSI and concentrations of TFVdp measured in DBS by LC-MS.
ACKNOWLEDGMENT
We gratefully acknowledge the tireless contribution of Heather Prince to this study.
REFERENCES
- 1.Genberg BL, Wilson IB, Bangsberg DR, Arnsten J, Goggin K, Remien RH, Simoni J, Gross R, Reynolds N, Rosen M, Liu HH, MACH14 Investigators. 2012. Patterns of antiretroviral therapy adherence and impact on HIV RNA among patients in North America. AIDS 26:1415–1423. 10.1097/QAD.0b013e328354bed6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, Corbelli GM, Estrada V, Geretti AM, Beloukas A, Asboe D, Viciana P, Gutierrez F, Clotet B, Pradier C, Gerstoft J, Weber R, Westling K, Wandeler G, Prins JM, Rieger A, Stoeckle M, Kummerle T, Bini T, Ammassari A, Gilson R, Krznaric I, Ristola M, Zangerle R, Handberg P, Antela A, Allan S, Phillips AN, Lundgren J, Grp PS, PARTNER Study Group. 2016. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 316:171–181. 10.1001/jama.2016.5148. [DOI] [PubMed] [Google Scholar]
- 3.Haberer JE, Baeten JM, Campbell J, Wangisi J, Katabira E, Ronald A, Tumwesigye E, Psaros C, Safren SA, Ware NC, Thomas KK, Donnell D, Krows M, Kidoguchi L, Celum C, Bangsberg DR. 2013. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med 10:e1001511. 10.1371/journal.pmed.1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapía M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernández T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker L-G, Mayer KH, Kallás EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng J-H, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 363:2587–2599. 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, Hosek S, Mosquera C, Casapia M, Montoya O, Buchbinder S, Veloso VG, Mayer K, Chariyalertsak S, Bekker LG, Kallas EG, Schechter M, Guanira J, Bushman L, Burns DN, Rooney JF, Glidden DV. 2014. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 14:820–829. 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haberer JE, Musinguzi N, Boum Y, Siedner MJ, Mocello AR, Hunt PW, Martin JN, Bangsberg DR. 2015. Duration of antiretroviral therapy adherence interruption is associated with risk of virologic rebound as determined by real-time adherence monitoring in rural Uganda. J Acquir Immune Defic Syndr 70:386–392. 10.1097/QAI.0000000000000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parienti JJ, Das-Douglas M, Massari V, Guzman D, Deeks SG, Verdon R, Bangsberg DR. 2008. Not all missed doses are the same: sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS One 3:e2783. 10.1371/journal.pone.0002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanters S, Park JJH, Chan K, Socias ME, Ford N, Forrest JI, Thorlund K, Nachega JB, Mills EJ. 2017. Interventions to improve adherence to antiretroviral therapy: a systematic review and network meta-analysis. Lancet HIV 4:e31–e40. 10.1016/S2352-3018(16)30206-5. [DOI] [PubMed] [Google Scholar]
- 9.Hill LM, Golin CE, Pack A, Carda-Auten J, Wallace DD, Cherkur S, Farel CE, Rosen EP, Gandhi M, Prince HMA, Kashuba ADM. 2020. Using real-time adherence feedback to enhance communication about adherence to antiretroviral therapy: patient and clinician perspectives. J Assoc Nurses AIDS Care 31:25–34. 10.1097/JNC.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spinelli MA, Haberer JE, Chai PR, Castillo-Mancilla J, Anderson PL, Gandhi M. 2020. Approaches to objectively measure antiretroviral medication adherence and drive adherence interventions. Curr HIV/AIDS Rep 17:301–314. 10.1007/s11904-020-00502-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spinelli MA, Glidden DV, Anderson PL, Gandhi M, Cohen S, Vittinghoff E, Coleman ME, Scott H, Bacon O, Elion R, Kolber MA, Buchbinder SP, Liu AY. 2019. Short-term adherence marker to PrEP predicts future nonretention in a large PrEP demo project: implications for point-of-care adherence testing. J Acquir Immune Defic Syndr 81:158–162. 10.1097/QAI.0000000000002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson PL, Liu AY, Castillo-Mancilla JR, Gardner EM, Seifert SM, McHugh C, Wagner T, Campbell K, Morrow M, Ibrahim M, Buchbinder S, Bushman LR, Kiser JJ, MaWhinney S. 2018. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother 62:e01710-17. 10.1128/AAC.01710-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velloza J, Bacchetti P, Hendrix CW, Murnane P, Hughes JP, Li M, Curlin ME, Holtz TH, Mannheimer S, Marzinke MA, Amico KR, Liu A, Piwowar-Manning E, Eshleman SH, Dye BJ, Gandhi M, Grant RM, Team HAS, HPTN 067/ADAPT Study Team. 2019. Short- and long-term pharmacologic measures of HIV pre-exposure prophylaxis use among high-risk men who have sex with men in HPTN 067/ADAPT. J Acquir Immune Defic Syndr 82:149–158. 10.1097/QAI.0000000000002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pragst F, Balikova MA. 2006. State of the art in hair analysis for detection of drug and alcohol abuse. Clin Chim Acta 370:17–49. 10.1016/j.cca.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi M, Glidden DV, Liu A, Anderson PL, Horng H, Defechereux P, Guanira JV, Grinsztejn B, Chariyalertsak S, Bekker L-G, Grant RM, Lama J, Schechter M, Kallas EG, Veloso V, Mayer K, Buchbinder S, Montoya O, Amico R, Koester K, Mulligan K, Casapia M, Benet LZ, Huang Y, Yee S, Kuncz K, Louie A, Francisco S, Greenblatt R, Tien P, Aouizerat B, iPrEx Study Team. 2015. Strong correlation between concentrations of tenofovir (TFV) emtricitabine (FTC) in hair and TFV diphosphate and FTC triphosphate in dried blood spots in the iPrEx Open Label Extension: implications for pre-exposure prophylaxis adherence monitoring. J Infect Dis 212:1402–1406. 10.1093/infdis/jiv239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koss CA, Natureeba P, Mwesigwa J, Cohan D, Nzarubara B, Bacchetti P, Horng H, Clark TD, Plenty A, Ruel TD, Achan J, Charlebois ED, Kamya MR, Havlir DV, Gandhi M. 2015. Hair concentrations of antiretrovirals predict viral suppression in HIV-infected pregnant and breastfeeding Ugandan women. AIDS 29:825–830. 10.1097/QAD.0000000000000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hickey MD, Salmen CR, Tessler RA, Omollo D, Bacchetti P, Magerenge R, Mattah B, Salmen MR, Zoughbie D, Fiorella KJ, Geng E, Njoroge B, Jin CS, Huang Y, Bukusi EA, Cohen CR, Gandhi M. 2014. Antiretroviral concentrations in small hair samples as a feasible marker of adherence in rural Kenya. J Acquir Immune Defic Syndr 66:311–315. 10.1097/QAI.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeBeau MA, Montgomery MA, Brewer JD. 2011. The role of variations in growth rate and sample collection on interpreting results of segmental analyses of hair. Forensic Sci Int 210:110–116. 10.1016/j.forsciint.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Poetzsch M, Steuer AE, Roemmelt AT, Baumgartner MR, Kraemer T. 2014. Single hair analysis of small molecules using MALDI-triple quadrupole MS imaging and LC-MS/MS: investigations on opportunities and pitfalls. Anal Chem 86:11758–11765. 10.1021/ac503193w. [DOI] [PubMed] [Google Scholar]
- 20.Kamata T, Shima N, Sasaki K, Matsuta S, Takei S, Katagi M, Miki A, Zaitsu K, Nakanishi T, Sato T, Suzuki K, Tsuchihashi H. 2015. Time-course mass spectrometry imaging for depicting drug incorporation into hair. Anal Chem 87:5476–5481. 10.1021/acs.analchem.5b00971. [DOI] [PubMed] [Google Scholar]
- 21.Rosen EP, Thompson CG, Bokhart MT, Prince HMA, Sykes C, Muddiman DC, Kashuba ADM. 2016. Analysis of antiretrovirals in single hair strands for evaluation of drug adherence with infrared-matrix-assisted laser desorption electrospray ionization mass spectrometry imaging. Anal Chem 88:1336–1344. 10.1021/acs.analchem.5b03794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilliland WM, White NR, Yam BH, Mwangi JN, Prince HMA, Weideman AM, Kashuba ADM, Rosen EP. 2020. Influence of hair treatments on detection of antiretrovirals by mass spectrometry imaging. Analyst 145:4540–4550. 10.1039/d0an00478b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pack AP, Golin CE, Hill LM, Carda-Auten J, Wallace DD, Cherkur S, Farel CE, Rosen EP, Gandhi M, Asher Prince HM, Kashuba ADM. 2019. Patient and clinician perspectives on optimizing graphical displays of longitudinal medication adherence data. Patient Educ Couns 102:1090–1097. 10.1016/j.pec.2018.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeGrand S, Knudtson K, Benkeser D, Muessig K, McGee A, Sullivan PS, Hightow-Weidman L. 2018. Testing the efficacy of a social networking gamification app to improve pre-exposure prophylaxis adherence (P3: Prepared, Protected, emPowered): protocol for a randomized controlled trial. JMIR Res Protoc 7:e10448. 10.2196/10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuypers E, Flinders B, Bosman IJ, Lusthof KJ, Van Asten AC, Tytgat J, Heeren RMA. 2014. Hydrogen peroxide reactions on cocaine in hair using imaging mass spectrometry. Forensic Sci Int 242:103–110. 10.1016/j.forsciint.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 26.Jurado C, Kintz P, Menendez M, Repetto M. 1997. Influence of the cosmetic treatment of hair on drug testing. Int J Legal Med 110:159–163. 10.1007/s004140050056. [DOI] [PubMed] [Google Scholar]
- 27.Koss CA, Hosek SG, Bacchetti P, Anderson PL, Liu AY, Horng H, Benet LZ, Kuncze K, Louie A, Saberi P, Wilson CM, Gandhi M. 2018. Comparison of measures of adherence to human immunodeficiency virus preexposure prophylaxis among adolescent and young men who have sex with men in the United States. Clin Infect Dis 66:213–219. 10.1093/cid/cix755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu AY, Yang QY, Huang Y, Bacchetti P, Anderson PL, Jin CS, Goggin K, Stojanovski K, Grant R, Buchbinder SP, Greenblatt RM, Gandhi M. 2014. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP). PLoS One 9:e83736. 10.1371/journal.pone.0083736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamel AF, Meyer JS, Henchey E, Dettmer AM, Suomi SJ, Novak MA. 2011. Effects of shampoo and water washing on hair cortisol concentrations. Clin Chim Acta 412:382–385. 10.1016/j.cca.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koss CA, Liu AY, Castillo-Mancilla J, Bacchetti P, McHugh C, Kuncze K, Morrow M, Louie A, Seifert S, Okochi H, MaWhinney S, Gandhi M, Anderson PL. 2018. Similar tenofovir hair concentrations in men and women after directly observed dosing of tenofovir disoproxil fumarate/emtricitabine: implications for preexposure prophylaxis adherence monitoring. AIDS 32:2189–2194. 10.1097/QAD.0000000000001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seifert SM, Castillo-Mancilla JR, Erlandson K, Morrow M, Gandhi M, Kuncze K, Horng H, Zheng JH, Bushman LR, Kiser JJ, MaWhinney S, Anderson PL. 2018. Brief report: Adherence biomarker measurements in older and younger HIV-infected adults receiving tenofovir-based therapy. J Acquir Immune Defic Syndr 77:295–298. 10.1097/QAI.0000000000001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castillo-Mancilla JR, Phillips AN, Neaton JD, Neuhaus J, Sharma S, Baker JV, Collins S, Mannheimer S, Pett S, Touzeau-Romer V, Polizzotto MN, Lundgren JD, Gardner EM, the INSIGHT START Study Group. 2019. Incomplete ART adherence is associated with higher inflammation in individuals who achieved virologic suppression in the START study. J Intern Aids Soc 22:e25297. 10.1002/jia2.25297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castillo-Mancilla JR, Brown TT, Erlandson KM, Palella FJ, Gardner EM, Macatangay BJC, Breen EC, Jacobson LP, Anderson PL, Wada NI. 2016. Suboptimal adherence to combination antiretroviral therapy is associated with higher levels of inflammation despite HIV suppression. Clin Infect Dis 63:1661–1667. 10.1093/cid/ciw650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grant RM, Mannheimer S, Hughes JP, Hirsch-Moverman Y, Loquere A, Chitwarakorn A, Curlin ME, Li M, Amico KR, Hendrix CW, Anderson PL, Dye BJ, Marzinke MA, Piwowar-Manning E, McKinstry L, Elharrar V, Stirratt M, Rooney JF, Eshleman SH, McNicholl JM, van Griensven F, Holtz TH. 2018. Daily and nondaily oral preexposure prophylaxis in men and yransgender women who have sex with men: the Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT Study. Clin Infect Dis 66:1712–1721. 10.1093/cid/cix1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, Tremblay C, Le Gall JM, Cua E, Pasquet A, Raffi F, Pintado C, Chidiac C, Chas J, Charbonneau P, Delaugerre C, Suzan-Monti M, Loze B, Fonsart J, Peytavin G, Cheret A, Timsit J, Girard G, Lorente N, Préau M, Rooney JF, Wainberg MA, Thompson D, Rozenbaum W, Doré V, Marchand L, Simon MC, Etien N, Aboulker JP, Meyer L, Delfraissy JF, ANRS IPERGAY Study Group. 2015. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 373:2237–2246. 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- 36.Thaden JT, Gandhi M, Okochi H, Hurt CB, McKellar MS. 2018. Seroconversion on preexposure prophylaxis: a case report with segmental hair analysis for timed adherence determination. AIDS 32:F1–F4. 10.1097/QAD.0000000000001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herbertson EC, Lahiri CD, Nwogu JN, Soremekun RO, Olugbake OA, Ezechi OC, Akanmu AS, Gandhi M. 2021. High acceptability of donating hair and other biological samples for research among people living with HIV in an outpatient clinic in Lagos, Nigeria. AIDS Res Hum Retroviruses 37:676–682. 10.1089/AID.2020.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nwogu JN, Babalola CP, Ngene SO, Taiwo BO, Berzins B, Gandhi M. 2019. Willingness to donate hair samples for research among people living with HIV/AIDS attending a tertiary health facility in Ibadan, Nigeria. AIDS Res Hum Retroviruses 35:642–648. 10.1089/AID.2018.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robichaud G, Barry JA, Garrard KP, Muddiman DC. 2013. Infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) imaging source coupled to a FT-ICR mass spectrometer. J Am Soc Mass Spectrom 24:92–100. 10.1007/s13361-012-0505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robichaud G, Barry J, Muddiman D. 2014. IR-MALDESI mass spectrometry imaging of biological tissue sections using ice as a matrix. J Am Soc Mass Spectrom 25:319–310. 10.1007/s13361-013-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bokhart MT, Nazari M, Garrard KP, Muddiman DC. 2018. MSiReader v1.0: evolving open-source mass spectrometry imaging software for targeted and untargeted analyses. J Am Soc Mass Spectrom 29:8–16. 10.1007/s13361-017-1809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chambers MC, Maclean B, Burke R, Amodei D, Ruderman DL, Neumann S, Gatto L, Fischer B, Pratt B, Egertson J, Hoff K, Kessner D, Tasman N, Shulman N, Frewen B, Baker TA, Brusniak M-Y, Paulse C, Creasy D, Flashner L, Kani K, Moulding C, Seymour SL, Nuwaysir LM, Lefebvre B, Kuhlmann F, Roark J, Rainer P, Detlev S, Hemenway T, Huhmer A, Langridge J, Connolly B, Chadick T, Holly K, Eckels J, Deutsch EW, Moritz RL, Katz JE, Agus DB, MacCoss M, Tabb DL, Mallick P. 2012. A cross-platform toolkit for mass spectrometry and proteomics. Nat Biotechnol 30:918–920. 10.1038/nbt.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Race AM, Styles IB, Bunch J. 2012. Inclusive sharing of mass spectrometry imaging data requires a converter for all. J Proteomics 75:5111–5112. 10.1016/j.jprot.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 44.Gilliland WM, Prince HMA, Poliseno A, Kashuba ADM, Rosen EP. 2019. Infrared matrix-assisted laser desorption electrospray ionization mass spectrometry imaging of human hair to characterize longitudinal profiles of the antiretroviral maraviroc for adherence monitoring. Anal Chem 91:10816–10822. 10.1021/acs.analchem.9b02464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schauer AP, Sykes C, Cottrell ML, Prince H, Kashuba ADM. 2018. Validation of an LC–MS/MS assay to simultaneously monitor the intracellular active metabolites of tenofovir, emtricitabine, and lamivudine in dried blood spots. J Pharm Biomed Anal 149:40–45. 10.1016/j.jpba.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hendrix CW, Andrade A, Bumpus NN, Kashuba AD, Marzinke MA, Moore A, Anderson PL, Bushman LR, Fuchs EJ, Wiggins I, Radebaugh C, Prince HA, Bakshi RP, Wang R, Richardson P, Shieh E, McKinstry L, Li X, Donnell D, Elharrar V, Mayer KH, Patterson KB. 2016. Dose frequency ranging pharmacokinetic study of tenofovir-emtricitabine after directly observed dosing in healthy volunteers to establish adherence benchmarks (HPTN 066). Aids Res Hum Retroviruses 32:32–43. 10.1089/AID.2015.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]