Abstract
Cryptosporidium parvum infection of T-cell receptor alpha (TCR-α)-deficient mice results in a persistent infection. In this study, treatment with a polyamine analogue (SL-11047) prevented C. parvum infection in suckling TCR-α-deficient mice and cleared an existing infection in older mice. Treatment with putrescine, while capable of preventing infection, did not clear C. parvum from previously infected mice. These findings provide further evidence that polyamine metabolic pathways are targets for new anticryptosporidial chemotherapeutic agents.
Cryptosporidium parvum is an intracellular protozoan parasite causing enteric infection and diarrheal disease in various mammals, including cattle and humans (7). While the disease is self-limiting in immunocompetent individuals, immunocompromised patients develop chronic life-threatening infections (33). C. parvum is now the most commonly reported etiologic agent isolated from diarrheic calves in the United States. In a survey of 1,103 dairy herds, 48% of calves between 7 and 21 days of age were infected with C. parvum (9). Infected calves provide potential sources for human infection through contamination of watershed areas (22, 28, 29). In 1993, a waterborne outbreak in Milwaukee, Wis., affected approximately 400,000 people (18). Despite the high prevalence and severity of disease in immunocompromised individuals, no effective therapies are available for the prevention or treatment of cryptosporidiosis.
Many compounds have been tested for potential anticryptosporidial activity (4, 32, 39). Cell culture systems have been developed for in vitro screening of potential anticryptosporidial compounds (32, 34, 39). In addition, immunodeficient rodent models are available for in vivo testing of anticryptosporidial compounds (4, 21, 32). One of these immunodeficient strains, T-cell receptor alpha (TCR-α)-deficient mice, becomes persistently infected with C. parvum when challenged with the parasite as neonates or as adults (36). These mice lack αβ T cells, which are necessary for specific immune clearance of C. parvum. Thus, TCR-α-deficient mice are useful animals for testing anticryptosporidial compounds.
Recently, it was determined that C. parvum utilizes a unique pathway for the synthesis of polyamines (15). Mammals and most parasitic protozoa convert arginine into ornithine, which is acted upon by ornithine decarboxylase for the synthesis of putrescine, which is then converted into spermidine and spermine (2, 3, 14). However, polyamine biosynthesis in C. parvum occurs via a pathway used by plants and certain bacteria, in which arginine is converted to agmatine by the action of arginine decarboxylase (40). Agmatine then serves as the precursor for other polyamines. Neither arginine decarboxylase nor agmatine is found in other parasitic protozoa. C. parvum also has a reverse polyamine biosynthetic pathway not found in other protozoa that enables the interconversion of spermine, spermidine, and putrescine (15). These unique pathways are potential targets for anticryptosporidial chemotherapeutic agents. Indeed, compounds affecting polyamine metabolism are effective inhibitors of in vitro C. parvum growth (15).
In a previous study, it was found that oral administration of putrescine prevents C. parvum infection of immunocompetent C57BL/6J mice (38). In the present study, we examined the efficacy of putrescine and a polyamine analogue, SL-11047, in both the prevention and treatment of C. parvum infection of TCR-α-deficient mice. Breeding pairs of TCR-α-deficient mice were purchased from Jackson Laboratories (Bar Harbor, Maine). A breeding colony was established and maintained at Iowa State University (Ames) for the generation of mice for experiments. Mice received tap water and autoclaved rodent chow (Harlan Teklad, Madison, Wis.) ad libitum. For the challenge inoculum, purified oocysts were isolated from feces collected from calves experimentally inoculated with C. parvum oocysts by a method described previously (13). Oral challenge of mice consisted of 104 oocysts in 100 μl of 0.15 M phosphate-buffered saline (PBS). Mice were challenged with C. parvum oocysts at 1 week of age by gavage using a 24-gauge animal feeding needle. To assess C. parvum colonization, fecal pellets were collected either by placing individual mice into beakers until they defecated or from the distal colon after mice were euthanatized. Fresh fecal pellets were then smeared onto glass slides, stained with carbol fuchsin, and examined microscopically (magnification, ×400) for the presence of C. parvum oocysts. Samples were scored as positive (i.e., oocysts detected) or negative (i.e., oocysts not detected). At the end of the experiment, mice were euthanatized and intestinal sections from the distal ileum and cecum were fixed in 10% formalin and embedded in paraffin. Histologic sections were cut at a thickness of 4 μm, stained with hematoxylin and eosin, and examined microscopically for C. parvum and intestinal lesions. Infectivity scores were as follows: 0, no C. parvum organisms detected; 1, few C. parvum organisms detected; and 2, many C. parvum organisms detected. Scores were determined upon examination of individual tissue sections; means were calculated for each treatment group, and data are presented as group means ± standard errors of the means. Data were analyzed by one-way analysis of variance followed by Tukey-Kramer multiple-comparison tests (mean infectivity scores), or two-by-two contingency tables were formulated and data were analyzed by Fisher's exact test (percent infected). Data were considered significant if P values of <0.05 were obtained.
Putrescine (1,4-diaminobutane) dihydrochloride was purchased from Sigma, St. Louis, Mo. SL-11047 ([1N,12N]bis (ethyl)-cis-6,7-dehydrospermine) tetrahydrochloride was prepared as described elsewhere (24). Solutions of putrescine (6.5 mg/ml) or SL-11047 (2.5 to 10 mg/ml) were prepared in PBS. Compounds were administered to mice twice daily in 100-μl volumes by gavage using a 24-gauge animal feeding needle. To determine if potential anticryptosporidial compounds were capable of preventing C. parvum infection, suckling TCR-α-deficient mice received either PBS, 0.65 mg of putrescine (130 mg/kg of body weight), or 0.25 mg of SL-11047 (50 mg/kg) twice daily from 3 through 10 days of age, and all mice were inoculated with 104 C. parvum oocysts at 7 days of age. Mice receiving either putrescine (n = 21) or SL-11047 (n = 7) had no evidence of C. parvum infection upon microscopic examination of ileal and cecal sections, whereas 18 of 21 mice receiving PBS (controls) were infected.
To determine if either putrescine or SL-11047 was effective in the treatment of an existing C. parvum infection, 1-week-old TCR-α-deficient mice were infected with C. parvum (104 oocysts) and treatment regimens were initiated 1 week later. One week after C. parvum challenge, fecal samples from each of the C. parvum-infected groups were checked to verify that mice were infected. Mice then received twice-daily treatments of either PBS for 7 days, 0.65 mg of putrescine for 7 days, 0.67 mg of SL-11047 for 7 days, 0.25 mg of SL-11047 for 10 days, or 0.67 mg of SL-11047 for 2 days. All mice were euthanatized 1 week after the final treatment. Ileal and cecal sections were examined for C. parvum and any associated histopathology. As shown in Table 1, treatment of TCR-α-deficient mice with 0.67 mg of SL-11047 for 7 days significantly decreased the level of infection. Treatment with SL-11047 at a lower dose (0.25 mg twice daily for 10 days) or for a shorter duration (0.67 mg twice daily for 2 days) did not inhibit infection (Table 1). Treatment with putrescine dihydrochloride on an equal-weight basis as the effective dose of SL-11047 (i.e., equivalent milligrams of each compound, which results in a fourfold higher molar concentration of putrescine) did not inhibit infection (Table 1). Treatment of mice with higher dosages of SL-11047 (1.0 mg twice daily for 7 days), however, was toxic. Clinical signs of SL-11047 toxicity included ataxia, inactivity, depression, wasting, and eventual death. All mice treated with 0.67 mg of SL-11047 for 7 days exhibited mild inactivity and depression, yet no ataxia, wasting, or death was observed. Effective dosage regimens, thus, were near toxic levels. Similar analogues with potentially greater efficacy and equivalent or lower toxicity are currently being tested (data not shown).
TABLE 1.
Treatment of C. parvum-infected TCR-α-deficient mice with putrescine or SL-11047a
| Treatment | No. infected/no. inoculated | Mean infectivity score ± SEM |
|---|---|---|
| PBS (control) | 25/25 | 1.32 ± 0.10 |
| Putrescine (0.65 mg BID, 7 days) (130 mg/kg) | 17/17 | 1.12 ± 0.08 |
| SL-11047 (0.67 mg BID, 7 days) (134 mg/kg) | 2/14* | 0.15 ± 0.10* |
| SL-11047 (0.67 mg BID, 2 days) (134 mg/kg) | 13/13 | 1.46 ± 0.14 |
| SL-11047 (0.25 mg BID, 10 days) (50 mg/kg) | 3/3 | 1.00 ± 0.0 |
Infectivity scores were determined as described in the text. BID, twice a day; *, significantly different from controls (P < 0.05).
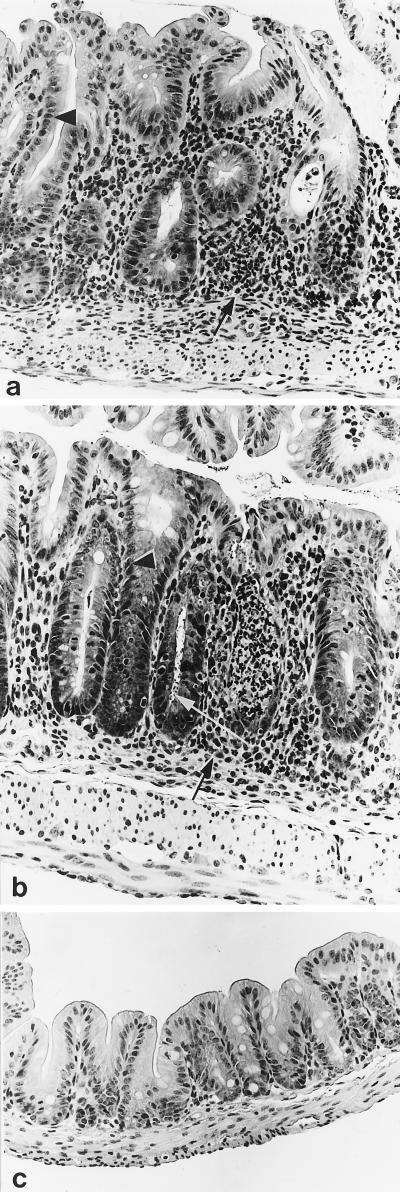
Mice receiving the effective regimen of SL-11047 treatment (0.67 mg twice daily for 7 days), despite clearance of the parasite, still developed hyperplastic and inflammatory cecal lesions (Fig. 1a). These lesions are typically seen in C. parvum infection of TCR-α-deficient mice, and this system has been previously presented as a model for inflammatory bowel disease (37). The most prominent aspects of these lesions were inflammatory cell infiltrates in the lamina propria, epithelial cell hyperplasia, and gland microabscesses. Lesions were detected in all C. parvum-challenged mice in this study (Fig. 1a and b). In addition to hyperplastic and inflammatory cecal lesions, mice treated with PBS, putrescine, or ineffective regimens of SL-11047 also had numerous C. parvum organisms within cecal tissues (Fig. 1b and Table 1). Only 2 of 14 intestinal sections from mice receiving the effective regimen of SL-11047 treatment had detectable C. parvum organisms upon microscopic evaluation (Table 1). No C. parvum organisms or hyperplastic or inflammatory intestinal lesions were detected in the nonchallenged, nontreated control mice (Fig. 1c).
FIG. 1.
Histopathology of ceca from TCR-α-deficient mice infected with C. parvum. (a) Cecum from a TCR-α-deficient mouse treated with the effective SL-11047 treatment regimen (0.67 mg twice daily for 7 days). Note the absence of C. parvum organisms despite the presence of hyperplastic epithelium (arrowhead) and inflammatory cell infiltrates within the lamina propria (arrow). (b) Cecum from a TCR-α-deficient mouse treated with PBS (infected control). Note the presence of C. parvum organisms (white arrow), hyperplastic epithelium (arrowhead), and inflammatory cell infiltrates within the lamina propria (black arrow). Similar numbers of C. parvum organisms and associated hyperplastic or inflammatory changes were detected in cecal sections from mice treated with either putrescine or ineffective SL-11047 treatment regimens. (c) Cecum from an age-matched (28-day-old), noninfected, nontreated TCR-α-deficient mouse.
Numerous compounds have been examined for anticryptosporidial activity (4, 39). Many of these compounds are effective against other protozoa yet are not effective against C. parvum (16). A few compounds, however, have shown marginal anticryptosporidial efficacy, including benzimidazoles, paromomycin, azithromycin, clarithromycin, roxithromycin, minocycline, and pyrimethamine (8, 10, 19). Other therapies have also been examined for treatment of C. parvum infection, including orally administered colostrum-derived bovine immunoglobulin, vaccination with lyophilized C. parvum oocysts, probiotics, and monoclonal antibodies to C. parvum exoantigens, and parenteral injection of recombinant gamma interferon, recombinant interleukin-12, or dehydroepiandrosterone (1, 6, 11, 12, 23, 25, 27, 35). Despite extensive research, no safe and effective therapies have emerged for the treatment of cryptosporidiosis.
Unlike in Eimeria, Toxoplasma, and Plasmodium spp., polyamine biosynthesis in C. parvum occurs by a pathway used by plants and some bacteria (15). This pathway is initiated by the conversion of arginine to agmatine by the action of arginine decarboxylase. In Escherichia coli, arginine decarboxylase is secreted into the periplasmic space for conversion of arginine to agmatine, thus providing a close proximity to rich stores of arginine within the intestinal lumen and preventing toxic intracellular accumulations of putrescine (5, 30). This location of polyamine biosynthesis within E. coli demonstrates the potential for cytotoxic interactions of polyamine metabolites within organisms using this biosynthetic pathway. Interestingly, C. parvum is uniquely situated in an intracellular yet extracytoplasmic location on the apical side of enterocytes. As with E. coli, this location allows maximal access to intestinal lumen-derived polyamines. In addition, this location provides ease in delivery of high concentrations of putrescine or SL-11047 by oral administration. Thus, oral administration of polyamines may inhibit C. parvum development through cytotoxic accumulations of polyamine biosynthesis metabolites within the parasite.
In our study, we found that oral administration of putrescine or SL-11047 (a spermine analogue) to suckling TCR-α-deficient mice prevented C. parvum infection. In addition, SL-11047 treatment of C. parvum-infected TCR-α-deficient mice cleared an ongoing C. parvum infection. The mechanism of in vivo inhibition of C. parvum by these compounds is unclear. They may act by affecting the parasite as well as the host (15, 20, 31). Evidence for action against the parasite includes (i) inhibition of in vitro C. parvum development by compounds affecting polyamine metabolism (15) and (ii) the ability of the compound to clear C. parvum in immunodeficient mice (e.g., those lacking αβ T cells, which are necessary for the specific immune clearance of the parasite). Alternatively, these compounds may enhance host resistance to the parasite by affecting intestinal cell proliferation and differentiation. Polyamines, including putrescine, induce proliferative changes (e.g., crypt cell proliferation, elongation of crypts, lengthening of villi, and increases in the numbers of intestinal cells) as well as maturational changes (e.g., expression of disaccharidases and immune cell development) within the intestine (17, 31). Proliferative changes typical of the onset of inflammatory bowel disease were detected in all C. parvum-infected TCR-α-deficient mice in this study, including mice which were successfully treated with SL-11047. Thus, it is unlikely that enhanced proliferation induced by polyamine treatment is a mechanism involved in clearance of C. parvum infection of TCR-α-deficient mice. Other effects of SL-11047 administration on enterocyte and/or intestinal immune cell maturation, however, may be involved in C. parvum clearance.
In conclusion, we have shown that both putrescine and a spermine analogue (SL-11047) prevent C. parvum infection when administered to suckling TCR-α-deficient mice. In addition, SL-11047 treatment of C. parvum-infected TCR-α-deficient mice cleared an otherwise persistent infection.
Acknowledgments
We thank Diane McDonald and Eldon Whitaker for excellent animal care and Mitchell Palmer for assistance with histopathological analysis.
This research was supported by Public Health Service grants AI43931 (M.J.W.), AI45739-01 (W.R.W.), and AI45739-01 (B.F.) from the National Institute of Allergy and Infectious Diseases, as well as funding provided by the Iowa Livestock Health Advisory Council (W.R.W.).
REFERENCES
- 1.Alak J I B, Wolf B W, Mdurvwa E G, Pimentel-Smith G E, Adeyemo O. Effect of Lactobacillus reuteri on intestinal resistance to Cryptosporidium parvum infection in a murine model of acquired immunodeficiency syndrome. J Infect Dis. 1997;175:218–221. doi: 10.1093/infdis/175.1.218. [DOI] [PubMed] [Google Scholar]
- 2.Bacchi C J, Yarlett N. Polyamine metabolism. In: Marr J J, Muller M, editors. Biochemistry and molecular biology of parasites. New York, N.Y: Academic Press; 1995. pp. 119–131. [Google Scholar]
- 3.Bacchi C J, McCann P P. Parasitic protozoa and polyamines. In: McCann P P, Pegg A E, Sjoerdsma A, editors. Inhibition of polyamine metabolism. New York, N.Y: Academic Press; 1987. pp. 317–344. [Google Scholar]
- 4.Blagburn B L, Soave R. Prophylaxis and chemotherapy: human and animal. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press; 1997. pp. 111–128. [Google Scholar]
- 5.Buch J K, Boyle S M. Biosynthetic arginine decarboxylase in Escherichia coli is synthesized as a precursor and located in the cell envelope. J Bacteriol. 1985;163:522–527. doi: 10.1128/jb.163.2.522-527.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Simone C, Famularo G, Harp J A, Tzantzoglou S, Chen W. Effect of lactobacilli on Cryptosporidium parvum infection in man and animals. Microecol Ther. 1995;25:32–36. [Google Scholar]
- 7.Fayer R, Speer C A, Dubey J P. The general biology of Cryptosporidium. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press; 1997. pp. 1–42. [Google Scholar]
- 8.Fayer R, Fetterer R. Activity of benzimidazoles against cryptosporidiosis in neonatal BALB/c mice. J Parasitol. 1995;81:794–795. [PubMed] [Google Scholar]
- 9.Garber L P, Salman M D, Hurd H S, Keefe T, Schlater J L. Potential risk factors for Cryptosporidium infection in dairy calves. J Am Vet Med Assoc. 1994;205:86–91. [PubMed] [Google Scholar]
- 10.Giacometti A, Cirioni O, Scalise G. In-vitro activity of macrolides alone and in combination with artemisin, atovaquone, dapsone, minocycline, or pyrimethamine against Cryptosporidium parvum. J Antimicrob Chemother. 1996;38:399–408. doi: 10.1093/jac/38.3.399. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg P D, Cello J P. Treatment of severe diarrhea caused by Cryptosporidium parvum with oral bovine immunoglobulin concentrate in patients with AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:348–354. doi: 10.1097/00042560-199612010-00008. [DOI] [PubMed] [Google Scholar]
- 12.Harp J A, Jardon P, Atwill E R, Zylstra M, Checel S, Goff J P, De Simone C. Field testing of prophylactic measures against Cryptosporidium parvum infection in calves in a California dairy herd. Am J Vet Res. 1996;57:1586–1588. [PubMed] [Google Scholar]
- 13.Harp J A, Chen W, Harmsen A G. Resistance of severe combined immunodeficient mice to infection with Cryptosporidium parvum: the importance of intestinal microflora. Infect Immun. 1992;60:3509–3512. doi: 10.1128/iai.60.9.3509-3512.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keithly J S, Fairlamb A H. Inhibition of Leishmania species by α-difluoro-methylornithine. In: Hart D T, editor. Leishmaniasis: current status and new strategies for control. New York, N.Y: Plenum Publishers; 1989. pp. 746–756. [Google Scholar]
- 15.Keithly J S, Zhu G, Upton S J, Woods K M, Martinez M P, Yarlett N. Polyamine biosynthesis in Cryptosporidium parvum and its implications for chemotherapy. Mol Biochem Parasitol. 1997;88:35–42. doi: 10.1016/s0166-6851(97)00063-7. [DOI] [PubMed] [Google Scholar]
- 16.Koudela B, Bokova A. The effect of cotrimoxazole on experimental Cryptosporidium parvum infection in kids. Vet Res. 1997;28:405–412. [PubMed] [Google Scholar]
- 17.Luk G D. Polyamines in normal and adaptive gastrointestinal growth. In: Dowling R H, Dowling U R, Folsch C, Loser C, editors. Polyamines in the gastrointestinal tract. London, United Kingdom: Kluwer Academic Publishers; 1992. pp. 205–216. [Google Scholar]
- 18.MacKenzie W R, Hoxie N J, Proctor M E, Gradus M S, Blair K A, Peterson D E, Kazmierczak J J, Addiss D G, Fox K R, Rose J B, et al. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N Engl J Med. 1994;331:161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- 19.Mancassola R, Reperant J-M, Naciri M, Chartier C. Chemoprophylaxis of Cryptosporidium parvum infection with paromomycin in kids and immunological study. Antimicrob Agents Chemother. 1995;39:75–78. doi: 10.1128/aac.39.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCormack S A, Johnson L R. Role of polyamines in gastrointestinal growth. Am J Physiol. 1991;23:G795–G806. doi: 10.1152/ajpgi.1991.260.6.G795. [DOI] [PubMed] [Google Scholar]
- 21.Mead J R, Arrowood M J, Sidwell R W, Healey M C. Chronic Cryptosporidium parvum infections in congenitally immunodeficient SCID and nude mice. J Infect Dis. 1991;163:1297–1304. doi: 10.1093/infdis/163.6.1297. [DOI] [PubMed] [Google Scholar]
- 22.Pell A N. Manure and microbes: public and animal health problem? J Dairy Sci. 1997;80:2673–2681. doi: 10.3168/jds.S0022-0302(97)76227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen K R, Healey M C, Cheng L, Yang S. Effects of dehydroepiandrosterone in immunosuppressed adult mice infected with Cryptosporidium parvum. J Parasitol. 1995;81:429–433. [PubMed] [Google Scholar]
- 24.Reddy V K, Valasinas A, Sarkar A, Baser H S, Marton L J, Frydman B. Conformationally restricted analogues of 1N,12N-bisethylspermine: synthesis and growth inhibitory effects on human tumor cell lines. J Med Chem. 1998;41:4723–4732. doi: 10.1021/jm980172v. [DOI] [PubMed] [Google Scholar]
- 25.Rehg J E. Effect of interferon-gamma in experimental Cryptosporidium parvum infection. J Infect Dis. 1996;174:229–232. doi: 10.1093/infdis/174.1.229. [DOI] [PubMed] [Google Scholar]
- 26.Reinemeyer C R. Parasitisms of dairy and beef cattle in the United States. J Am Vet Med Assoc. 1994;205:670–680. [PubMed] [Google Scholar]
- 27.Riggs M W, Stone A L, Yount P A, Langer R C, Arrowood M J, Bentley D L. Protective monoclonal antibody defines a circumsporozoite-like glycoprotein exoantigen of Cryptosporidium parvum sporozoites and merozoites. J Immunol. 1997;158:1787–1795. [PubMed] [Google Scholar]
- 28.Rose J B. Environmental ecology of Cryptosporidium and public health implications. Annu Rev Public Health. 1997;18:135–161. doi: 10.1146/annurev.publhealth.18.1.135. [DOI] [PubMed] [Google Scholar]
- 29.Scott C A, Smith H V, Mtambo M M, Gibbs H A. An epidemiological study of Cryptosporidium parvum in two herds of adult beef cattle. Vet Parasitol. 1995;57:277–288. doi: 10.1016/0304-4017(94)00694-8. [DOI] [PubMed] [Google Scholar]
- 30.Tabor C W, Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985;49:81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ter steege J C, Buurman W A, Forget P P. Spermine induces maturation of the intestinal immune system in neonatal mice. J Pediatr Gastroenterol Nutr. 1997;25:332–340. doi: 10.1097/00005176-199709000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Tzipori S. Cryptosporidiosis: laboratory investigations and chemotherapy. Adv Parasitol. 1998;40:187–221. doi: 10.1016/s0065-308x(08)60121-9. [DOI] [PubMed] [Google Scholar]
- 33.Ungar B L P. Cryptosporidiosis in humans (Homo sapiens) In: Dubey J P, Speer C A, Fayer R, editors. Cryptosporidiosis of man and animals. Boca Raton, Fla: CRC Press; 1990. pp. 59–82. [Google Scholar]
- 34.Upton S J, Tilley M, Nesterenko M V, Brillhart D B. A simple and reliable method for producing in vitro infections of Cryptosporidium parvum (Apicomplexa) FEMS Microbiol Lett. 1994;118:45–50. doi: 10.1111/j.1574-6968.1994.tb06801.x. [DOI] [PubMed] [Google Scholar]
- 35.Urban J F, Fayer R, Chen S J, Gause W C, Gately M K, Finkelman F D. IL-12 protects immunocompetent and immunodeficient neonatal mice against infection with Cryptosporidium parvum. J Immunol. 1996;156:263–268. [PubMed] [Google Scholar]
- 36.Waters W R, Harp J A. Cryptosporidium parvum infection in T-cell receptor (TCR)-α- and TCR-δ-deficient mice. Infect Immun. 1996;64:1854–1857. doi: 10.1128/iai.64.5.1854-1857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waters W R, Palmer M V, Ackermann M R, Harp J A. Accelerated inflammatory bowel disease of TCR-α-deficient mice persistently infected with Cryptosporidium parvum. J Parasitol. 1997;83:460–464. [PubMed] [Google Scholar]
- 38.Waters W R, Reinhardt T A, Harp J A. Oral administration of putrescine inhibits Cryptosporidium parvum infection of neonatal C57BL-6 mice and is independent of nitric oxide synthesis. J Parasitol. 1997;83:746–750. [PubMed] [Google Scholar]
- 39.Woods K M, Nesterenko M V, Upton S J. Efficacy of 101 antimicrobials and other agents on the development of Cryptosporidium parvum in vitro. Ann Trop Med Parasitol. 1996;90:603–615. doi: 10.1080/00034983.1996.11813090. [DOI] [PubMed] [Google Scholar]
- 40.Yarlett N, Martinez M P, Zhu G, Keithly J S, Woods K, Upton S J. Cryptosporidium parvum: polyamine biosynthesis from agmatine. J Eukaryot Microbiol. 1996;43:73S. doi: 10.1111/j.1550-7408.1996.tb05004.x. [DOI] [PubMed] [Google Scholar]