ABSTRACT
Klebsiella pneumoniae carbapenemases (KPC-2 and KPC-3) present a global clinical threat, as these β-lactamases confer resistance to carbapenems and oxyimino-cephalosporins. Recent clinically identified KPC variants with substitutions at Ambler position D179, located in the Ω loop, are resistant to the β-lactam/β-lactamase inhibitor combination ceftazidime-avibactam, but susceptible to meropenem-vaborbactam. To gain insights into ceftazidime-avibactam resistance conferred by D179N/Y variants of KPC-2, crystal structures of these variants were determined. The D179N KPC-2 structure revealed that the change of the carboxyl to an amide moiety at position 179 disrupted the salt bridge with R164 present in wild-type KPC-2. Additional interactions were disrupted in the Ω loop, causing a decrease in the melting temperature. Shifts originating from N179 were also transmitted toward the active site, including ∼1-Å shifts of the deacylation water and interacting residue N170. The structure of the D179Y KPC-2 β-lactamase revealed more drastic changes, as this variant exhibited disorder of the Ω loop, with other flanking regions also being disordered. We postulate that the KPC-2 variants can accommodate ceftazidime because the Ω loop is displaced in D179Y or can be more readily displaced in D179N KPC-2. To understand why the β-lactamase inhibitor vaborbactam is less affected by the D179 variants than avibactam, we determined the crystal structure of D179N KPC-2 in complex with vaborbactam, which revealed wild-type KPC-2-like vaborbactam-active site interactions. Overall, the structural results regarding KPC-2 D179 variants revealed various degrees of destabilization of the Ω loop that contribute to ceftazidime-avibactam resistance, possible substrate-assisted catalysis of ceftazidime, and meropenem and meropenem-vaborbactam susceptibility.
KEYWORDS: antibiotic resistance, beta-lactamase, protein crystallography
INTRODUCTION
Antimicrobial resistance (AMR) is an urgent worldwide public health problem and is in part mediated by β-lactamases (EC 3.5.2.6) that degrade β-lactam antibiotics. To overcome β-lactamase-mediated resistance, β-lactamase inhibitors were developed, including clavulanate, sulbactam, tazobactam, and, more recently, avibactam, relebactam, and vaborbactam (Fig. 1). When administered with a β-lactam, these inhibitors can restore susceptibility to β-lactams (1, 2). However, the emergence of inhibitor-resistant β-lactamase variants (e.g., inhibitor-resistant TEM enzymes) and β-lactamases that are difficult to inhibit (e.g., metallo-β-lactamases [MBL]) limited the utility of the β-lactam-β-lactamase inhibitor combinations (3). β-Lactamases found in Gram-negative bacteria that are presently challenging our health care system include the clinically worrisome NDM-1 (an MBL), OXA-48, and Klebsiella pneumoniae carbapenemases KPC-2 and KPC-3 (compared to KPC-2, KPC-3 has an additional H274Y substitution). Fortunately, KPC β-lactamases can be inhibited by avibactam, relebactam, and vaborbactam—novel inhibitors present in newer β-lactam-β-lactamase combinations. However, recent clinical variants of KPC-2 and KPC-3 harboring substitutions at position D179 (Ambler numbering system) are resistant to the ceftazidime-avibactam combination (i.e., D179Y found in KPC-31, -32, -33, -52, -70, and -95) (4–9). Paradoxically, these variants, when expressed in K. pneumoniae or Escherichia coli, especially D179Y, render bacteria susceptible to meropenem (10). Also worrisome is that such KPC variants can increase MICs for cefiderocol (11, 12), a recently FDA-approved β-lactam, thus causing coresistance to this antibiotic. Interestingly, the newer β-lactamase inhibitor vaborbactam combined with meropenem can serve as an empirical option for treating suspected carbapenemase-producing variants with KPC-3 D179Y (10, 13–15). Similarly, the preclinical cefpodoxime-ETX1317 combination was found to also not be as affected by the D179Y variant (16). In addition to D179Y variants, the following other residues at this position can also confer resistance to ceftazidime: D179N (found in KPC-51 [17] along with Y241H and H274N), D179V (found in KPC-57) (18), and D179A (found in KPC-78) (18). Site saturation mutagenesis of the D179 site of KPC revealed many substitutions that behave similarly (Fig. 1) (15).
FIG 1.
Structures of ceftazidime, two β-lactamase inhibitors, and KPC D179 resistance variants. (A) Chemical structures of the β-lactam ceftazidime and β-lactamase inhibitors avibactam and vaborbactam. (B) Clinically observed KPC D179 variants (73) and lab-generated variants at position 179 that show resistance to ceftazidime-avibactam (15).
D179 is located in the Ω loop; this loop is comprised of residues L162-D179, present in the active site of KPC-2 (19) (Fig. 2). This active site loop is adjacent to the catalytic S70 and plays a vital role in the catalytic activity in class A β-lactamases; the Ω loop contains residues E166 and N170 that position a catalytic water molecule needed for the deacylation reaction (20–22). The Ω loop is, in large part, stabilized by a salt bridge interaction of R164 with the buried D179 residue, and substitution of either residue in KPC or related β-lactamases causes increased ceftazidime resistance (23–25). In SHV-1 β-lactamase, substitution of R164 caused a ligand-dependent disorder in the Ω loop (26). The loop also harbors a number of buried water molecules that are conserved in related β-lactamases (27).
FIG 2.
Structure of WT KPC-2. The high-resolution KPC-2 structure (PDB accession number 5UL8) (34) is shown in cyan with the Ω loop depicted in yellow. The catalytic S70, W105, residues N170 and E166 that both position the deacylation water (W#6), H274 that is a Tyr in KPC-3, and the D179 and R164 salt bridge-forming residues are depicted. Also shown are the five water molecules buried in the Ω loop (W#1 to W#5).
The resistance of D179 KPC variants is unique to ceftazidime. Other tested β-lactams or monobactams become more susceptible to such variants (except for the D179N variant) (9, 15, 28). This D179N variant has increased resistance to ceftazidime and ceftazidime-avibactam when expressed in E. coli laboratory strains and maintains wild-type (WT) KPC-2-like resistance to other β-lactams, including imipenem and meropenem (15). The resistance of D179 KPC variants is postulated to be a consequence of the decrease in Km for ceftazidime, allowing the enzyme to operate closer to its maximal efficiency despite a concomitant reduction in kcat (14, 16, 29). As alluded to above, other substitutions in the Ω loop can also yield a similar ceftazidime and ceftazidime-avibactam resistance phenotype (11, 30, 31), including a deletion in the Ω loop that encompasses E166 (11). These Ω loop alterations indicate that displacement/disorder/deletion of (part of) the Ω loop without the need for the deacylation machinery residue E166 are sufficient for this phenotype (10, 11). The likely needed displacement or disorder of the Ω loop agrees with a crystallographic study that showed that soaking ceftazidime into crystals of KPC-2 and KPC-4 with a substitution that renders them deacylation deficient, E166Q, the Ω loop becomes disordered in KPC-2 and shifts in KPC-4 (32). These observed changes were needed to accommodate the aminothiazole ring of the covalently bound ceftazidime (32). An additional potential compounding factor regarding resistance to ceftazidime-avibactam is that the D179Y variant was also shown to be significantly less (or more slowly) inhibited by avibactam (10, 14, 29), although another study found no significant difference (16), but this inconsistency could be due to assay differences because of the mutant’s lower rate of hydrolysis of the reporter substrate nitrocefin. These studies did show that avibactam restored ceftazidime efficacy to a significant degree but not to the susceptibility range. In contrast to avibactam, the β-lactamase inhibitors vaborbactam and ETX1317 are not as affected by the D179Y KPC variants (14, 16).
To investigate and compare the molecular basis of the D179Y and D179N substitutions in KPC-2 on ceftazidime-avibactam resistance, we determined the crystal structures of both variants. Although clinically less prevalent, D179N is the only KPC variant that confers ceftazidime-avibactam resistance and maintains carbapenemase activity (15) and warrants further study. To further probe the ability of vaborbactam to inhibit a D179 KPC-2 variant, we determined the crystal structure of KPC-2 D179N in complex with vaborbactam.
RESULTS AND DISCUSSION
We present here biophysical and biochemical findings regarding the thermal stability of the D179N KPC-2 variant, the crystallographic analysis of this variant, the crystallographic analysis of the D179Y variant, the mode of vaborbactam inhibition of the D179N variant, and an in-depth discussion explaining our results regarding this observed phenotype of ceftazidime resistance and specific effects of β-lactamase inhibitors.
Thermal stability analysis of KPC-2 D179N.
The thermal stability of the D179N KPC-2 β-lactamase was assessed using differential scanning fluorimetry (DSF). The melting temperature (Tm) of D179N KPC-2 is 43.8 ± 0.0°C, which is significantly lower than the Tm of 54.6 ± 0.28°C for WT KPC-2, indicating a significant destabilizing effect by the D179N substitution (Fig. 3).
FIG 3.
Differential scanning fluorimetry/thermal shift assay probing protein stabilization of KPC-2 and D179N KPC-2 by vaborbactam. Experiments are carried out in duplicates.
Crystal structure of KPC-2 D179N.
We next determined the crystal structure of apo D179N KPC-2 β-lactamase at 0.99 Å resolution. One molecule of citrate buffer was observed bound in the active site; a citrate molecule has been previously observed in the active site of KPC-2 (33).
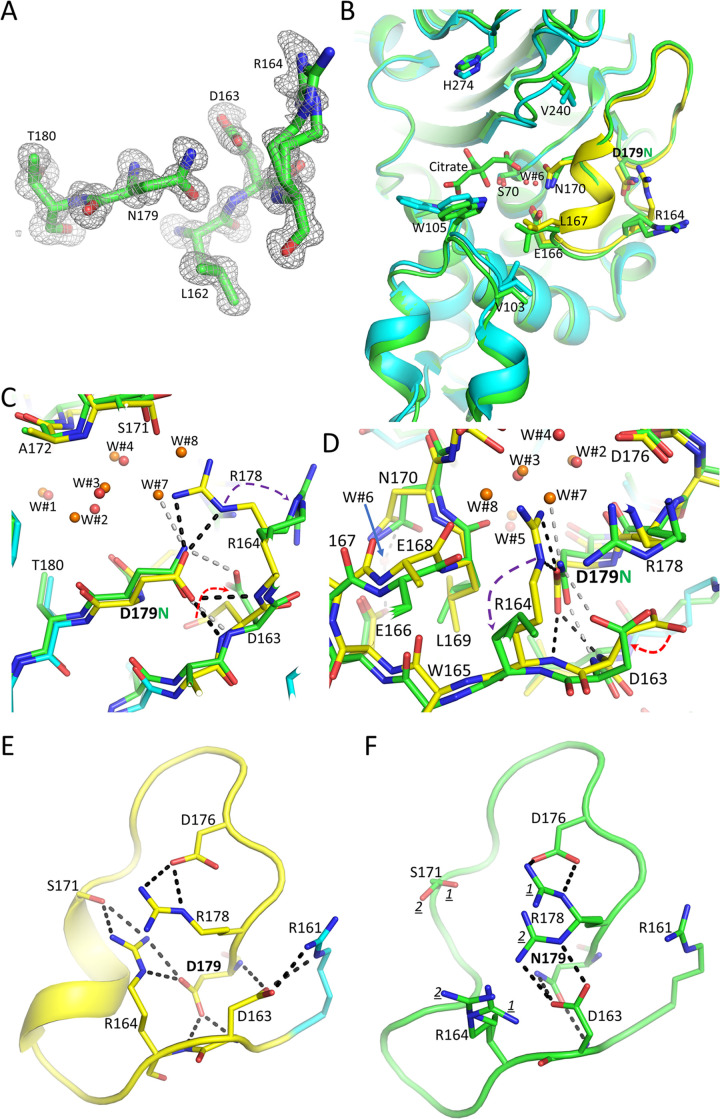
Residue N179 is well ordered in the electron density map, as are the Ω loop main chain atoms (Fig. 4A). Upon initial inspection, the overall structure of D179N KPC-2 is similar to WT KPC-2 (PDB accession number 5UL8) (34); their superimposition yields a root mean square deviation (RMSD) of 0.57 Å for 264 Cα atoms, but there are some significant localized conformational changes (Fig. 4B to D). The loss of the negatively charged carboxyl group of residue 179 upon the D179N substitution disrupted the D179-R164 salt bridge, causing R164 to reorient itself (Fig. 4). As a result, the R164 side chain is moved away toward the solvent and occupies two alternate conformations (Fig. 4A). The loss of the interactions between residues 164 and 179 in the D179N variant was previously anticipated based on molecular dynamics simulations (15). Also, the change from an oxygen atom to a nitrogen atom upon the D179N substitution allowed the side chain of D163 to rotate toward N179 to form an H-bond with this nitrogen side chain atom (Fig. 4). Compared to WT KPC-2, the N179 side chain maintains the H-bond interaction with the backbone nitrogen of D163, but the second backbone interaction, with the nitrogen of R164, has been lost, as the latter distance is now 3.8 Å. The loss of this interaction in the D179N variant is due to a 0.7-Å shift of the R164 backbone nitrogen (Fig. 4).
FIG 4.
Structure of D179N KPC-2. (A) Omit electron density map of the D179N Ω loop region (contoured at the 3-σ level). To generate the density map, residues 179 to 180 and 162 to 164 were omitted from crystallographic refinement and subsequent electron density map calculations. Residue R164 is modeled in two conformations. (B) Superimposition of the D179N KPC-2 structure (green) onto the WT KPC-2 structure (cyan with the Ω loop in yellow as in Fig. 2). Key active site residues are shown, including the D179N and R164 residues. The citrate buffer molecule in the active site near S70 is shown. (C) Closeup view of the superimposition of panel B. The water molecules in the WT KPC-2 and D179N KPC-2 structures are shown as red and orange spheres, respectively (labeled W#1 to W#8). Hydrogen bonds of N179 in the D179N structure are shown as gray dashed lines; hydrogen bonds of the side chain of D179 in the WT KPC-2 structure are shown as black dashed lines. The movements of the R164 and D163 side chains in the D179N structure, compared to WT KPC-2, are indicated by the purple and red arrows, respectively. (D) Same as panel C but review rotated about 90°. (E) Hydrogen bonding network involving Ω loop Arg and Asp residues in the WT KPC-2. (F) Hydrogen bonding network involving Ω loop Arg and Asp residues in D179N KPC-2.
WT KPC-2 harbors an extensive hydrogen bonding network in the Ω loop that extends beyond D179:R164 and plays an important role in stabilizing this motif. Additional interactions in this network are significantly altered in the D179N KPC-2 structure (Fig. 4E and F). R164 no longer forms hydrogen bonds with S171, and both residues are more disordered, each occupying two conformations. Also, the two hydrogen bonds of D163 with R161 (salt bridge) and with the main chain nitrogen of residue 179 are disrupted. Residue D163 instead forms hydrogen bonds with one of the conformations of R178 and with the nitrogen of the N179 side chain (as discussed above). Last, R178 is no longer in a single conformation, making two hydrogen bonds with D176 (salt bridge). Instead, R178 is more disordered, occupying two conformations, of which one of them is interacting with D176 (Fig. 4E and F). Overall, the Ω loop hydrogen bonding network involving charged residues, including multiple salt bridge interactions, is substantially weakened in the D179N KPC-2 variant.
In addition to the changes in the hydrogen bonding network detailed above, additional conformational changes are observed in the Ω loop in the D179N KPC-2 structure. Although the five water molecules buried in the Ω loop region are in similar positions in the WT KPC-2 and D179N KPC-2 structures (W#1 to W#5), the space vacated by the R164 movement allowed two additional water molecules to be bound in this region (W#7 and W#8) (Fig. 4C and D). Also, the repositioning of R164 permits the backbone carbonyl oxygen of E168 to shift 1.4 Å toward this vacated pocket (Fig. 4D). Concomitant with the changes in main chain atom positions of E168 and R164 are movements in the adjacent L167 and W165 main chain atoms, respectively (Fig. 4D). The repositioned L167 also likely caused a 0.8 Å shift in V103 (Fig. 4B).
Notably, there are also changes closer to the active site that may bear upon the mechanism of hydrolysis. The oxygen of the N170 side chain has shifted by 1.1 Å; this could be due to the rotation of the adjacent L169 or the presence of the bound citrate molecule (Fig. 4B). The deacylation water (W#6) held in place by E166 and N170 is shifted by 0.9 Å compared to WT KPC-2, likely due to the shift in N170 (Fig. 4). The shifts in the 165 to 170 region in the D179N KPC-2 variant disrupt their backbone hydrogen bonds such that this region is no longer a helix (Fig. 4E and F). Overall, the drastic reduction of interactions in the Ω loop in the D179N KPC-2 variant supports the DSF data demonstrating a significant decrease in thermal stability.
The observed conformational differences and similarities of the D179N variant compared to WT KPC-2 could, in part, explain the observed phenotype of hydrolyzing ceftazidime significantly slower yet having a higher affinity for ceftazidime (15). The D179N variant is also the only D179 variant whose resistance phenotype is most similar to WT KPC-2: the D179N variant maintains resistance to 17 other tested antibiotics or antibiotic-β-lactamase inhibitor combinations, except meropenem, while acquiring an increased resistance to ceftazidime, a trait that WT KPC-2 partially lacks (15). The crystallographic results are supported by kinetic and microbiological observations of the D179N variant. This structure is relatively similar to WT KPC-2 as a result of both (i) Asn’s close resemblance to Asp, and (ii) the uniqueness of the Asn side chain. The N179 side chain can maintain, via its remaining oxygen atom, one of the two H-bonds with nitrogens of main chain atoms of the Ω loop, whereas the introduced nitrogen atom of the N179 side chain facilitates a new interaction with the D163 side chain (Fig. 4).
The impact of the observed subtle conformational changes of residues closer to the catalytic S70 on the affinity and the acylation and deacylation rates of the D179N KPC-2 variant is more nuanced. These changes include N170, the deacylation water, and L167. Although we observed a modest change in the position of residue N170, this minor shift is not enough to accommodate acylated ceftazidime bound in the active site. Superimposing the deacylation deficient E166Q variant of KPC-2 with ceftazidime bound (32) onto the D179N KPC-2 structure revealed a significant steric clash of the aminothiazole ring of ceftazidime with N170 in the D179N structure (Fig. 5); this clash is not present in the E166Q KPC-2 ceftazidime complex structure, as the N170-containing section of the Ω loop has been displaced upon ceftazidime binding (32).
FIG 5.

Superimposition of D179N KPC-2 onto KPC-2 E166Q complexed with covalently bound ceftazidime. The D179N KPC-2 (green) and the KPC-2 E166Q ceftazidime complex (brown; PDB accession number 6Z24) (32) are superimposed (RMSD, 0.87 Å for 258 Cα atoms). The KPC-2 E166Q ceftazidime structure has several disordered loops that are not modeled; the endpoints of these loops are indicated by brown spheres, and the corresponding residue numbers are labeled. Ceftazidime is shown in the stick model with the aminothiazole (a), the dimethyl-carboxyl containing moiety (b), dihydrothiazine ring (c), carbon from which the pyridine ring has been eliminated upon acylation (d), and the amide moiety (e) labeled.
These data lead us to hypothesize that although the Ω loop is in a primarily WT KPC-2 position, the reason that the D179N KPC-2 variant binds ceftazidime better (i.e., lower Km) yet hydrolyzes it slower (i.e., lower kcat) is by permitting more readily a ligand-induced displacement of the Ω loop. Such ligand-induced Ω loop shifts were also observed for KPC-2 and KPC-4 deacylation-deficient E166Q variants when complexed with ceftazidime (32) as well as ceftazidime-resistant SHV-1 R164 variants with an inhibitor bound (26). D179N KPC-2 would permit such a ceftazidime-induced Ω loop conformational change more readily due to loss of the stabilizing WT D179-R164 salt bridge, decrease in the number of hydrogen bonds that residue 179 makes (4 in WT KPC-2 and 2 in D179N KPC-2), and additional loss of stabilizing interactions in the Ω loop (Fig. 4C to F). This would explain the kinetic “trapping” observed for the D179N variant (15) by improving the binding of a single ceftazidime molecule per β-lactamase, yet without fast turnover/hydrolysis. This phenomenon could lead to quickly decreasing periplasmic ceftazidime concentrations potentially sufficient to display the resistance phenotype. For β-lactam substrates that can be accommodated without a need for Ω loop displacement, the D179N variant likely maintains its Ω loop in a mostly WT KPC-2 position, thus supporting the relatively WT-like MICs for such substrates (15). It is still important to note that although the carboxyl moiety of E166 itself has not substantially shifted (only 0.3 Å), the observed changes around residue E166 could still contribute to changes in β-lactam hydrolysis, as was previously observed when the environment around E166 was altered (22).
Crystal structure of D179Y KPC-2.
While purifying the D179Y KPC-2 β-lactamase for structural studies, we observed that this variant behaves differently from D179N and WT KPC-2. First, the D179Y variant did not bind to the phenyl-boronic acid resin that allowed affinity-based purification of WT KPC-2 (35) and D179N KPC-2. As a result, we developed a novel purification strategy for D179Y KPC-2 that involved blue Sepharose fast flow beads, for which the D179Y was found to have a weak affinity. Secondly, when comparing size exclusion chromatography elution times, the D179Y variant eluted 0.32 mL earlier than D179N KPC-2 and WT KPC-2 (Fig. S1 in the supplemental material). The elution time difference suggests that the D179Y variant behaves like a “larger protein,” likely a consequence of a region being disordered; this is consistent with nuclear magnetic resonance (NMR) studies of D179Y KPC-2 (10). Thirdly, the D179Y KPC-2 variant is known to trap β-lactams via a slower deacylation reaction, which allowed us to track the purification of the D179Y KPC-2 variant using the fluorescent β-lactam reporter Bocillin SDS-PAGE assay; this assay is commonly used to detect penicillin-binding proteins (PBPs) since they do not deacylate β-lactams rapidly (36, 37).
The D179Y KPC-2 β-lactamase shows a significantly greater Bocillin fluorescence binding than D179N KPC-2 (Fig. S2), which, in turn, has a more intense signal than WT KPC-2 (WT KPC-2 turns over Bocillin more rapidly, thus having less covalently bound Bocillin). Finally, D179Y KPC-2 was very recalcitrant to crystallization, even when testing known KPC-2 crystallization conditions. Only when WT KPC-2 microcrystal seeds were added to freshly set up D179Y KPC-2 crystallization trays using Midwest Center for Structural Genomics (MCSG) crystallization screens (Anatrace, Inc.) did this KPC-2 variant crystallize.
Processing the 3.15-Å resolution diffraction data collected at National Synchrotron Light Source II (NSLS II) revealed that D179Y KPC-2 crystallized in a space group, C2, not observed for previous crystallized KPC β-lactamases or variants or complexes thereof. Here, we observed that there are two molecules in the asymmetric unit. Inspection of the electron density maps indicated a lack of apparent density for the Ω loop residues 163 to 178 in both monomers, indicating a disordered Ω loop (Fig. 6A and B); these residues are not included in the model. The two molecules in the asymmetric unit do not have any neighboring crystallographically related molecules near the Ω loop, thus allowing the flexible Ω loop to be accommodated in the crystal lattice. The two D179Y KPC-2 noncrystallographic monomers are overall very similar; the RMSD after superimpositioning is 0.25 Å for 219 Cα atoms (Fig. 6C and D). The monomers are also similar to WT KPC-2; the RMSDs for superimposing D179Y KPC-2 molecules A and B onto WT KPC-2 are 0.77 and 0.65 Å, respectively, for 213 aligned Cα atoms (Fig. 6C and D).
FIG 6.
Crystal structure of D179Y KPC-2. (A) Omit electron density of Ω loop region of molecule A. Ω Loop residues 163 to 178 could not be observed in the electron density and are not modeled; residues 159 to 162 and 179 to 182 were omitted from crystallographic refinement and subsequent electron density map calculations. Density around residues 159 to 182 (also extending to where WT KPC-2 Ω loop coordinates would be located) is contoured at the 2.6-σ level. (B) Same as panel A, but now includes superimposed WT KPC-2 showing residues 159 to 182 (cyan, but with Ω loop residues in yellow). (C) Superimposition of D179Y KPC-2 molecules A (brown) and B (magenta) and WT KPC-2 (colored as in Fig. 2). Endpoints of missing loops in D179Y KPC-2 molecules A and B are indicated by spheres, and the corresponding residue numbers are labeled. The missing loop regions in D179Y KPC-2 are labeled as follows (bold in parentheses): 163 to 178 (Ω loop, 1), 239 to 241 (2, 239 to 242 in molecule B), 266 to 274 (3), and 96 to 116 (4, missing only in molecule B). (D) Same as panel C but rotated about 90°.
In addition to the disordered Ω loop, two adjacent regions are also disordered. These include residues 239 to 241/242 and 266 to 274. Monomer B has a fourth disordered section, residues 96 to 116 adjacent to the Ω loop (this region contains W105) (Fig. 6C and D); monomer A has this region ordered, likely due to a crystal contact providing stability to this region.
The observed additional regions of disorder observed in the D179Y KPC-2 structure are interesting and warrant further investigation. The concomitant disorder in one of the monomers of the W105-containing region upon the disorder of the Ω loop is likely due to the loss of the hydrophobic interactions of Ω loop residue L167 with V103 and P104. Such a conformational “domino effect” was also observed in D179N KPC-2, with the more modest conformation changes in the D179N region affecting the position of residue V103 (Fig. 4). Similarly, the disorder of residues 239 to 241/242 is likely due to disruption of its van der Waals interaction with the Ω loop (i.e., V240, Y241, and T243 interact with Ω loop residues 170 to 174). The disordered 239 to 241/242 region likely impacted the position and conformation of residues 266 to 274 due to the loss of hydrogen bonds and van der Waals interactions between these two regions (involving residues 269 and 272).
These crystallographic results show that the D179Y substitution disrupts the WT Ω loop structure. The inability of Y179 to form any of the stabilizing D179-mediated hydrogen bonds compounded by the likely steric clashes that the larger Tyr residue generates is responsible for this. The disordered Ω loop and additional disordered regions are consistent with the size exclusion data indicating a larger apparent size (Fig. S1) and that the D179Y variant has a slow deacylation rate for Bocillin (Fig. S2) due to the displacement of the Ω loop residues E166 and N170 needed to position the deacylation water.
The disorder of the D179Y KPC-2 variant is also in agreement with the NMR results indicating a significant increase in disorder upon the D179Y substitution (10). For comparison, the Tm of D179Y KPC-2 was 51.2 ± 0.6°C (10), which is less than WT KPC-2, yet much higher than for the D179N KPC-2 variant. The difference could be due to both enthalpic contributions via fewer hydrogen bonds and salt bridge interactions in D179N KPC-2 and entropic contributions involving an ordered yet destabilized Ω loop. In contrast, the D179Y KPC-2 variant has its Ω loop already disordered with thus increased local entropy for these residues.
Explaining the mechanism whereby D179Y KPC-2 hydrolyzes ceftazidime, we maintain that the observed conformational changes and disorder in the Ω loop observed in the D179Y structure permit ceftazidime to be accommodated without steric hindrance since residue N170, and the rest of the Ω loop, is displaced. This allows ceftazidime in the acyl-enzyme-bound state, and likely also in the Michaelis-Menten preacylation binding mode, to bind unhindered, thus contributing to the decreased Km for the D179Y variant compared to WT KPC-2. The observed reduced kcat for D179Y KPC-2 is likely a direct consequence of the displacement of the E166/N170 residues that position the deacylation water; this deacylation water is needed for fast deacylation. Despite not hydrolyzing ceftazidime as quickly as WT KPC-2 at steady-state levels, D179Y KPC-2 more readily “captures” ceftazidime, which likely is responsible for the observed burst of decreasing the ceftazidime concentration (15).
In addition to the prominent role of D179, the residue it forms a salt bridge with in WT KPC-2 (i.e., Ω loop residue R164) is also important for ceftazidime resistance (24, 38). The clinically observed substitution R164S in KPC-2 (i.e., KPC-49) (39) results in ceftazidime resistance (24). This substitution is also present in related β-lactamases such as SHV-1; the Ω loop in this variant becomes disordered upon binding the inhibitor SA2-13 (26), an Ω loop displacement not observed when SA2-13 binds to WT SHV-1 (40).
When comparing the conformational changes related to ceftazidime resistance described in the manuscript, the following observations are evident (Fig. 7). In the WT KPC-2 structure, the D179 side chain is making four hydrogen bonds in the Ω loop, two with the R164 side chain (i.e., a salt bridge) and two with main chain nitrogens (Fig. 4 and Fig. 7A). Ceftazidime acylation to KPC-2 requires a displacement of a substantial part of the Ω loop in KPC-2 (observed when the deacylation reaction is slowed down via the E166Q substitution) (32). Notably, ceftazidime binding in the active site causes disorder of Ω loop residues 166 to 172. The D179-R164 salt bridge interaction remains, however, intact, thus likely contributing to keeping the remaining residues of the Ω loop ordered (Fig. 7B). The R164S substitution in the related SHV-1 β-lactamase weakened the interactions in the Ω loop such that this loop transitioned from ordered to disordered upon binding the SA2-13 inhibitor (Fig. 7C; residues 161 to 166 became disordered). These weakened interactions are because D179 in the R164S structure only makes three hydrogen bonds (one with the S164 side chain and two with the backbone) and no longer can make the salt bridge interaction.
FIG 7.
Ω Loop position and disorder in β-lactamases related to ceftazidime resistance. (A) WT KPC-2 (PDB 5UL8) (34). (B) KPC-2 E166Q with ceftazidime bound (PDB accession number 6Z24) (32). (C) R164S SHV-1 in complex with SA2-13 (PDB accession number 3OPP) (26). (D) D179N KPC-2. (E) D179Y KPC-2.
We anticipate that the ceftazidime resistance phenotype observed for R164S variants of KPC-2, SHV-1, and other β-lactamases is due to an analogous mechanism in which the (destabilized) Ω loop can more readily swing out to now accommodate ceftazidime binding. The D179N KPC-2 structure showed that residue N179 also makes fewer interactions in the Ω loop than WT KPC-2 (i.e., only two hydrogen bonds and no salt bridge) (Fig. 7D). Similar to apo R164S SHV-1, the Ω loop in D179N KPC-2 is ordered, yet some significant conformational changes occurred compared to WT KPC-2, such as movements of R164 and D163 and main chain shifts. We hypothesize that, as for the R164S variant, the destabilized Ω loop in D179N KPC-2 can also more readily be displaced to promote ceftazidime binding. Finally, the D179Y KPC-2 variant structure reveals that its entire Ω loop is disordered (Fig. 7E). This disorder would thus allow ceftazidime to bind to this KPC-2 variant. This improved binding is apparent in the lower Km for this KPC-2 variant. However, a disordered Ω loop will not allow fast turnover of ceftazidime since this loop contains residue E166 and N170 needed to position the deacylation water involved in deacylation. This is evidenced by the significantly decreased kcat for ceftazidime hydrolysis by the D179Y KPC-2 variant.
The observed progression of disorder originating from the Ω loop to the adjacent 239 to 241/242 loop is interesting considering other substitutions that can give rise to resistance to ceftazidime or ceftazidime-avibactam. These include T243M (can be found together with D179Y) (9, 16, 28, 41, 42), V240G (9, 16, 42), and L169P (14, 43, 44). Residue T243 is at the boundary of this disordered 239 to 241/242 region, and V240 is in this disordered region. Both V240 and T243 are more distant from S70 and are not anticipated to interact with ceftazidime (32). However, both residues are making van der Waals interactions with the Ω loop. A possible explanation for the V240G and T243M variants to contribute to resistance could be by aiding in destabilizing the Ω loop, analogous to the D179 variants and L169P variant. A destabilized Ω loop could more readily accommodate ceftazidime binding and/or affect kcat.
D179N and D179Y KPC-2 inhibition by β-lactamase inhibitors.
In addition to the KPC-2/-3 D179 variants’ acquired phenotypic differences regarding the β-lactam of the ceftazidime-avibactam combination, the changes at position 179 also affect the β-lactamase inhibitor, with D179Y KPC-2 being less inhibited by avibactam (29). Avibactam also took longer to form an adduct with the D179Y KPC-2 and D179Y KPC-3 than the WT KPC-2 and KPC-3, respectively (10). Inspection of the previously determined KPC-2 avibactam complex revealed that the amide moiety of avibactam is making 3.4- to 3.5-Å van der Waals interactions with both E166 and N170 of the Ω loop (45). Also, the oxygen atom of the amide moiety of avibactam makes a 2.9-Å hydrogen bond with the deacylation water that is held in place by E166 and N170 (45). These interactions of avibactam in the acyl-enzyme-bound state will be compromised in the D179Y KPC-2 variant due to the disorder of the E166- and N170-containing Ω loop. The loss of these interactions with avibactam could be the basis of the decreased inhibition of D179Y KPC-2 by avibactam (note that avibactam does not use the deacylation water for the reversible nature of binding to serine β-lactamases) (46). In contrast to D179Y, D179N KPC-2 has a higher affinity for avibactam, as it has a 3-fold lower apparent Ki (Ki app) than WT KPC-2 (15). This suggests that the more modest conformational changes observed in the D179N KPC-2 variant, including changes near N170, could contribute to the improved binding of avibactam. Despite the higher affinity for avibactam, the D179N KPC-2 variant is resistant to ceftazidime-avibactam, as the MICs only decreased from 512 to 16 μg/mL upon the addition of avibactam to ceftazidime (15).
In contrast to avibactam, D179Y KPC-2/KPC-3 is efficiently inhibited by vaborbactam (13, 14). The 50% inhibitory concentration (IC50) for vaborbactam increased only ∼2-fold for the D179Y KPC-2 variant (1.9 μM) compared to WT KPC-2 (0.94 μM) whereas the IC50 for avibactam increased ∼20-fold (8.9 μM for D179Y KPC-2 versus 0.47 μM for WT KPC-2) (14). A possible explanation is that the amide oxygen of vaborbactam is more distant from E166/N170 (i.e., 3.8 Å from N170 and 4.4 Å from E166) (47) compared to how avibactam is complexed to WT KPC-2 (see above). Therefore, destabilization of the Ω loop in the D179Y KPC-2 variant might thus not affect vaborbactam binding as much. To gain insights into vaborbactam inhibition of a D179 KPC-2 variant, we measured vaborbactam induced Tm changes and determined the crystal structure of vaborbactam complexed to D179N KPC-2.
Vaborbactam binding at 0.5 mM concentration increased the Tm of D179N KPC-2 by 15.8°C to 59.6 ± 0.0°C (Fig. 3). For comparison, vaborbactam binding to WT KPC-2 increased the Tm by 23.8°C to 78.4 ± 0.0°C (Fig. 3). These thermal shift measurements indicate that both WT and D179N KPC-2 are significantly stabilized upon vaborbactam binding.
Upon determining the crystal structure of D179N KPC-2 soaked with vaborbactam at 1.17 Å resolution, the electron density in the active site revealed a bound vaborbactam molecule (Fig. 8A and B). Vaborbactam is covalently bound to S70 via its boron atom. The boron hydroxyl interacts with the backbone nitrogen of S70, the backbone oxygen of T237, and the deacylation water molecule (W#8) (Fig. 8C and D). The carboxyl moiety of vaborbactam hydrogen bonds with S130, T235, and T237, and the amide moiety interacts with N132 and the backbone oxygen of T237. The vaborbactam thiophene ring is observed in two conformations and makes van der Waals interactions with the side chains of T237 and W105; the thiophene ring also makes hydrophobic intramolecular interactions with carbon atoms of the boronate ring of vaborbactam. Two of the carbon atoms of the boronate ring of vaborbactam also make hydrophobic interactions with W105; this residue in KPC-2 was found to have an important role for β-lactam and β-lactamase inhibitor discrimination (48).
FIG 8.
Crystal structure of D179N KPC-2 in complex with vaborbactam. (A) Unbiased electron density omit map after 10 cycles refinement with vaborbactam removed prior to refinement and map calculation (contoured at the 3-σ level). The two conformations of the thiophene of vaborbactam are labeled 1 and 2. (B) Same as panel A but rotated about 90°. (C) Interactions of vaborbactam in the active site. Hydrogen bonds are shown as dashed lines. (D) Same as panel C but rotated about 90°. (E) Arg-Asp interactions in the Ω loop region near N179 of the D179N KPC-2 vaborbactam complex.
The Ω loop hydrogen bonding network involving Arg/Asp residues in the vaborbactam-bound D179N KPC-2 structure is similar to that in the apo D179N KPC-2 structure except that the residues R164 and R178 are more ordered, having only a single conformation (Fig. 8E). Superimpositioning of the vaborbactam-bound structures of D179N KPC-2 and WT KPC-2 revealed a similar active site conformation and vaborbactam binding mode (Fig. 9; RMSD 0.54 Å for 265 Cα atoms). There are some observed changes in the backbone conformation of G239 (Fig. 9), but that conformation has also been observed among different apo KPC-2 structures and is due to crystal packing. Compared to the apo D179N KPC-2 structure, the binding of vaborbactam induces a more WT KPC-2 conformation of the Ω loop region, although the R164 side chain still is oriented toward solvent, and D163 is interacting with N179, as observed in the apo D179N KPC-2 structure.
FIG 9.
Superimposition of the vaborbactam-bound structures of D179N KPC-2 and WT KPC-2. (A) Superimposition showing vaborbactam-bound active sites of WT KPC-2 (gray carbon atoms; PDB accession number 6TD0) (47) and D179N KPC-2 (colored as in Fig. 8). (B) Ω Loop region of the superimposition.
Overall, the D179N change in KPC-2 does not perturb the binding mode of vaborbactam significantly. This, combined with the observation that the vaborbactam moiety closest to the Ω loop, its amide moiety, is 0.4 to 0.9 Å more distant from E166/N170 than avibactam’s amide moiety, is likely the reason why vaborbactam is less affected by the D179Y substitution than avibactam (14). Vaborbactam could thus be useful in combination with meropenem when treating infections harboring D179N KPC variants since these still confer intermediate meropenem susceptibility (MICs around 2 μg/mL) (15, 17). In contrast, D179Y KPC variants are not resistant to meropenem (MIC is 0.08 μg/mL) (15), and combining meropenem with vaborbactam would therefore not have an added benefit.
Why is ceftazidime unique regarding D179Y/N KPC resistance?
Ceftazidime is unique compared to 16 other β-lactams or monobactams in that it is the only one that KPC D179 variants increase their resistance to (4, 5, 7, 9, 14–16, 28, 29, 31, 42). β-Lactam trapping by D179 variants was proposed as a mechanism for ceftazidime resistance, but other β-lactams and monobactams are trapped as well, which did not lead to resistance against these latter antibiotics (15, 30). Ceftazidime was, however, still hydrolyzed to some degree. In contrast, other β-lactams were hydrolyzed significantly more slowly, indicating that a careful balance of lowering Km, allowing trapping, and decreased yet sufficient subsequent hydrolysis is needed for resistance (15, 16, 29). How the balance of the antagonistic shifts in Km and kcat leads to only ceftazidime resistance suggests that other aspects could play a key role as well. Such factors include periplasmic β-lactam concentrations affected by influx and efflux, β-lactamase concentration, the β-lactamase Km and kcat, which and how many PBPs are inhibited by the β-lactam, the expression levels of these PBP(s), the affinity of the β-lactam for the PBP(s), and to which degree the PBP(s) need to be inhibited to elicit a bactericidal effect. Ceftazidime has many of these potential contributing factors working against itself, which will be discussed next.
Ceftazidime targets a single PBP primarily, whereas other β-lactams, for the most part, inhibit multiple PBPs (49–52). Ceftazidime inhibits Klebsiella pneumoniae PBP3 and also inhibits PBP1a/1b, albeit more weakly (53); this study did not distinguish whether PBP1a or PBP1b or both are inhibited. PBP1a and PBP1b are redundant, as deletion of either is not lethal, yet deletion of both is bactericidal; inhibition of either PBP2 or PBP3 leads to bacterial killing (49–52). Regarding expression levels of the targeted PBPs, the relative expression levels in K. pneumoniae and E. coli are PBP1a > PBP3 > PBP2 > PBP1b (52, 54). Keeping the uncertainty regarding inhibition of PBP1a and/or PBP1b in mind, ceftazidime thus mainly targets a single essential PBP, PBP3, the one that has the highest expression level, which would therefore take larger amounts of β-lactam than an inhibitor targeting only PBP2. Regarding the uncertainty of inhibition of PBP1a and/or PBP1b, if only PBP1a is inhibited by ceftazidime, then this (nonbactericidal) PBP1a inhibition could act as a sink, thus also decreasing periplasmic ceftazidime concentration levels. Although the absolute cellular concentration levels of PBPs have not been quantified to our knowledge, the β-lactamase concentrations are likely higher than PBPs since their expression localization is not limited to a membrane; β-lactamase concentrations are in the low-to-high micromolar range (55). Enhanced trapping of a single β-lactam molecule by each β-lactamase could have a considerable effect on periplasmic β-lactam concentrations when the Km for D179 KPC-2 variants shifts closer to or below the periplasmic concentration levels for ceftazidime. However, considering trapping as the main mechanism for ceftazidime resistance for D179Y KPC variants requires knowledge of the periplasmic ceftazidime, β-lactamase, and PBP concentrations, which are currently not known. Furthermore, D179Y KPC variants do have ceftazidime hydrolysis capabilities, which likely contribute to their resistance phenotype and will be discussed later.
Regarding PBP inhibition potency, the IC50s for ceftazidime inhibition of PBP3s from E. coli and K. pneumoniae are 0.2 and 0.06 to 0.25 μg/mL, respectively (53, 54). For comparison, meropenem and imipenem have a much higher affinity for their target PBP, PBP2 (IC50s are 0.0075 μg/mL for both) (53, 54). Regarding cell entry to replenish β-lactams to the periplasm after trapping and hydrolysis by β-lactamases, the permeability coefficient for ceftazidime was low for K. pneumoniae (<3 nm/s), which was worse than imipenem, meropenem, and cefepime and similar to or worse than aztreonam (56). In E. coli and Enterobacter cloacae, ceftazidime also has the worst permeation rate compared to the five other β-lactams tested, including cefepime and cefotaxime (57). Unlike the other aspects, efflux pumps do not seem to be a ceftazidime-specific issue contributing to resistance (58). In summary, ceftazidime is a relatively unique β-lactam that has several efficacy-hampering factors aligned against itself that could further counteract its efficacy once the D179 KPC-2 variant arises that increases affinity for ceftazidime and maintains enough catalytic efficiency. Note that regarding D179 KPC-2 variants’ resistance to the combination, i.e., ceftazidime-avibactam, the decreased inhibitory efficacy of avibactam also needs to be considered (29).
Perhaps most importantly, ceftazidime’s unusual characteristics could be related to its chemical structure. Ceftazidime has relatively bulky R1 substituents, the dimethylcarboxyl moiety and the aminothiazole ring. In contrast, the R2 group of ceftazidime, the pyridine moiety, is relatively small compared to other β-lactams. The bulky features of ceftazidime permit WT KPC-2 to only accommodate the acylated ceftazidime if the N170-containing region of the Ω loop has been displaced/disordered (32). For comparison, cefotaxime, which lacks the dimethylcarboxyl moiety, does not require the Ω loop to be displaced when acylated to KPC-2 (32). The structure of ceftazidime acylated to E166Q KPC-2 reveals a ceftazidime conformation observed in many other acylated ceftazidime complexes with β-lactamases and PBPs. The scaffold ring of ceftazidime, the 6-membered, mostly hydrophobic, dihydrothiazine ring, interacts with the dimethyl moiety of the R1 group via hydrophobic interactions. This interaction, therefore, forces the other bulky end of the trifurcated R1 group, the aminothiazole ring, to be pointing away. The intramolecular hydrophobic interactions between the dimethyl and dihydrothiazine moieties potentially increase the rigidity of the bulky ceftazidime. This likely intrinsic rigidity is compounded by an amide moiety at the third trifurcated end of the R1 that is often involved in hydrogen bonding interactions across the active sites of β-lactamases and PBPs (this amide moiety mimics part of the terminal tripeptide portion of the peptidoglycan PBP substrate). This intrinsic rigidity of ceftazidime is consistent with that the same conformation is also observed in the following ceftazidime complexes (PDB accession numbers listed in parenthesis): KPC-2 E166Q (6Z24), CTX-M-14 (5U53), AmpC (1IEL), ADC-7 (6PWM), OXA-160 V130D (4X56), OXA-225 K82D (4X55), and Pseudomonas aeruginosa PBP3 (3OCN and 3PBO).
Another contributing factor that might make ceftazidime more rigid could involve the pyridine R2 group. This group is usually eliminated upon acylation (e.g., ceftazidime binding to P. aeruginosa PBP3) (59, 60), yet prior to acylation, the hydrophobic portions of this pyridine ring could also contribute to hydrophobic interactions with the dimethyl moiety, thus further rigidifying ceftazidime. Alternatively, the positive charge on the pyridine ring could allow the R1 dimethylcarboxyl group to reorient such that its carboxyl moiety can form an electrostatic interaction with the positively charged nitrogen in the pyridine ring (as docked by Shapiro et al.) (16). This ceftazidime conformation would still have the aminothiazole ring pointing away from the rest of the molecule. Overall, a more rigid bulky ceftazidime would thus have decreased structural plasticity for protein binding, as, for example, observed for KPC-2 acylation needing to displace part of the Ω loop (32).
The likely more rigid conformation of the bulky ceftazidime with the aminothiazole ring pointing away might have an additional benefit contributing to ceftazidime resistance for D179 KPC variants. When ceftazidime is bound to E166Q KPC-2, the deacylation water is still in the same WT KPC-2 position despite that the Ω loop residues E166 and N170 are disordered (32). This deacylation water is held in place via a 3.4-Å hydrogen bond with the ring nitrogen of the aminothiazole moiety (32). This nitrogen is a hydrogen bond acceptor and could act as a base since the reported pKa for aminothiazole is 5.36 (https://organicchemistrydata.org/). The aminothiazole ring positioning the deacylation water molecule and possibly abstracting a proton from this water molecule to activate it for the deacylation reaction could explain why ceftazidime is hydrolyzed more rapidly than other tested β-lactams by D179 variants of KPC-2 (15, 16, 29). The aminothiazole ring of ceftazidime could thus aid deacylation in a substrate-assisted manner to compensate for the displaced deacylation-assisting residues E166 and N170 in the D179Y KPC variants. In contrast, meropenem has a much smaller R1 group (i.e., 6α-hydroxyethyl moiety) that likely cannot assist deacylation in a substrate-assisted manner; meropenem deacylation is also dependent on a well-positioned deacylation water held in place by E166 and N170 (22). The absence of these two deacylation mechanisms for meropenem in D179Y KPC variants explains why meropenem is more slowly hydrolyzed (i.e., inhibits) and that these variants are susceptible to meropenem (15, 29).
Finally, another unique aspect of ceftazidime is that its acylation to either a β-lactamase or a PBP will result in the release of the R2 group pyridine. Pyridine is known to destabilize phosho-histine bonds, and such bonds are present in transport proteins and proteins that are part of the bacterial two-component system (61, 62). The two-component system has an important role in antibiotic resistance (63), which thus raises the possibility of ceftazidime acylation and concomitant release of pyridine having a contributing effect on antibiotic resistance.
Conclusion.
We crystallographically characterized the D179N and D179Y variants of KPC-2 to gain insights into their ceftazidime-avibactam resistance phenotype. Compared to WT KPC-2, the D179N crystal structure revealed that Ω loop residue 179 loses two hydrogen bonds, including the salt bridge interaction with R164. Additional hydrogen bonds and salt bridge interactions in the Ω loop are also disrupted. These conformational disruptions cause a strong protein destabilizing effect evident in our measured Tm of D179N KPC-2. The structure of the D179Y KPC-2 variant revealed that the entire Ω loop is disordered. Structural comparisons with the previously reported ceftazidime-bound complex of the deacylation deficient E166Q KPC-2 suggest that the destabilized Ω loop in D179N KPC-2 can more readily be displaced to promote ceftazidime binding or is already displaced in D179Y KPC-2, allowing unhindered ceftazidime binding. These observations provide a possible explanation for the observed decrease in Km for ceftazidime binding to these D179 KPC variants. We further speculate that a reason why ceftazidime is the only β-lactam for which resistance is observed for D179 KPC variants is because of ceftazidime’s bulky nature with potentially rigidifying intramolecular hydrophobic interactions between its dihydrothiazine ring and dimethylcarboxyl moiety. This conformation is observed in virtually all ceftazidime protein complexes and positions the aminothiazole ring distant from the rest of ceftazidime. It is this aminothiazole ring that sterically clashes with the WT KPC-2 Ω loop conformation such that binding is promoted in KPC variants where this loop is destabilized or disordered. Furthermore, this aminothiazole ring could also aid substrate-assisted deacylation via positioning (and possibly activating) the deacylation water molecule for D179 KPC variants where E166 in the Ω loop is displaced. The D179N and D179Y KPC-2 structures also yield insights into why avibactam is a less potent inhibitor of D179Y KPC-2 than WT KPC-2. Finally, we determined the crystal structure of the D179N KPC-2 complexed with vaborbactam, which revealed inhibitor interactions that are very similar as observed when vaborbactam is bound to WT KPC-2. This observation is consistent with that vaborbactam inhibition is not as affected by KPC variants at position 179. These studies shed important insight not only on the evolution of substrate specificity, perhaps as a consequence of overuse of ceftazidime, but also on the importance of drug design and interactions in the active site. These observations serve to inform the future design of novel antibiotics.
MATERIALS AND METHODS
Mutagenesis.
Single-base-pair substitutions at position 179 of the blaKPC-2 gene in the pET-28a expression plasmid were generated using the Q5 site-directed mutagenesis kit (New England Biolabs); the open reading frame contains WT KPC-2 with four residues truncated from the C terminus to facilitate improved crystallization (33, 35). The D179N and D179Y mutations were confirmed by standard nucleotide sequencing.
Protein expression and purification.
The D179N KPC-2 containing the pET-28a plasmid was transformed into BL21 Star (DE3) competent cells (Thermo Fisher). A 4-L bacterial culture in terrific broth medium (TB) with 50 μg/mL kanamycin was grown and induced to an optical density at 600 nm (OD600) between 0.6 to 0.8 using 50 μM final concentration of isopropyl-β-d-thiogalactopyranoside (IPTG). The flasks were continued to shake for 18 h at 18°C as described previously (35). After collecting the pellet using centrifugation, the cells were suspended in a lysis buffer (20 mM Tris, pH 8, 300 mM NaCl). One c0mplete EDTA-free protease inhibitor tablet (Sigma-Aldrich), 1,000 units of Benzonase (AcroBiosystems), and 3 mM MgCl2 were added to the lysis buffer. Cells were lysed using Avestin Emulsiflex B15 apparatus at 4°C twice, and the cell suspension was centrifuged at 15,000 rpm for 35 min. To the supernatant, 0.1% polyethyleneimine (PEI) from a 6.5% stock was added to precipitate contaminating DNA for a 20-minute incubation at room temperature. The lysate was subsequently spun down at 8,000 rpm for 15 min. The protein was then affinity purified using washed and equilibrated phenyl-boronic acid agarose beads (Sigma-Aldrich) as previously described (35). The supernatant was incubated with the beads for 16 h at 4°C before the phenyl-boronic acid beads were washed with 20 mM Tris, pH 7.9, and 150 mM NaCl2 buffer. The D179N KPC-2 protein was then eluted with 0.85 M Tris, pH 7.9, with 0.2 M sorbitol and 0.2 M NaCl. The protein was further purified using a Superdex 75 size exclusion column (Cytiva LifeSciences) with running buffer (10 mM Tris, pH 8, 60 mM NaCl, and 10 mM sorbitol). The fractions containing the desired protein were collected and concentrated. WT KPC-2 for comparative DSF experiments was purified in a similar manner.
D179Y KPC-2 was expressed as above. The pellet was dissolved in 50 mM glycine, pH 9.9, 0.2 M NaCl, and 1 mM MgCl2; Benzonase and a protease inhibitor tablet were added as described above. The pellet was resuspended and passed through a French press twice and then spun down at 16,000 rpm for 30 min. To the supernatant, PEI was added to a final concentration of 0.3% and incubated and centrifuged as described above. The D179Y protein did not bind to phenyl-boronic acid agarose, so a novel purified method was developed. The supernatant was added to washed blue Sepharose 6 fast flow beads (Sigma-Aldrich) and incubated at room temperature for 90 min. The beads were then packed into a high-performance liquid chromatography (HPLC) column and washed with buffer A (50 mM glycine, pH 9.9, and 0.2 M NaCl) followed by buffer B (50 mM glycine, pH 9.9, and 1 M NaCl). The D179Y KPC-2 protein elutes with the column wash in buffer A. HPLC fractions 1 to 28 were concentrated to 8 mL, followed by ammonium sulfate precipitation by addition of 8 mL of 3 M ammonium sulfate being slowly added at 4°C. This step brought the concentration of ammonium sulfate to 1.5 M; the suspension was stirred for 40 min and then spun down at 16,000 rpm. The pellet was resuspended in 3 mL of 10 mM Tris, pH 8, 60 mM NaCl, and 10 mM sorbitol. The resuspended protein was concentrated to 1.5 mL and injected onto a Superdex 200 column (3 rounds of 500 μL each; the running buffer is 10 mM Tris, pH 8, 60 mM NaCl, and 10 mM sorbitol). Fractions that contain the protein were collected and concentrated and then reinjected back over Superdex 200 with the same running buffer. The elution fractions of the D179Y KPC-2 protein were concentrated to 8 mg/mL prior to crystallization.
Bocillin SDS-PAGE analysis.
Purified protein samples of WT and D179 variants of KPC-2 were reacted with 80 μM Bocillin for 15 min followed by boiling to stop the reaction. The protein samples were loaded and separated using an Any kD Mini-Protean SDS-PAGE gel (Bio-Rad) using a nonreducing loading dye. The fluorescence detection of the Bocillin-protein complexes was carried out using a ChemiDoc MP imaging system (Bio-Rad) at 488 nm, as we previously described for PBP3 (64).
DSF/thermal shift analysis.
The reaction volume was 30 μL containing 8 μM KPC-2 or D179N KPC-2 protein in 50 mM Tris, pH 7, 100 mM NaCl2 buffer and 1 μL SYPRO orange (Thermo Fisher) to a final concentration of 10×, with or without vaborbactam (0.5 mM final concentration). The thermal gradient was conducted between 25 and 90°C with 0.2°C/min intervals. The DSF thermal shift assay and fluorescence readout were performed in duplicate on a CFX96 Touch real-time PCR detection system (Bio-Rad), similar to what was done previously (64, 65).
Crystallization and vaborbactam soaking.
Crystals of D179N KPC-2 were grown using the sitting drop method. The protein was concentrated to 16 mg/mL in 10 mM Tris, pH 8, 60 mM NaCl2, and 10 mM sorbitol; 1 μL of protein was mixed with 0.5 μL of reservoir containing a standard KPC-2 crystallization condition (66) (20% polyethylene glycol 6000 [PEG 6000], 100 mM citrate, pH 4.0, 100 mM potassium thiocyanate [KSCN], and 10 mM CdCl2). To obtain the complex with vaborbactam, crystals of D179N KPC-2 were used for soaking experiments. Apo D179N crystals were grown in 0.1 M sodium citrate-HCl, pH 4, and 0.8 M ammonium sulfate and soaked in mother liquor containing 0.1 M sodium citrate, pH 5.0, 1.0 M ammonium sulfate, and 2.1 mM vaborbactam for 17 h at room temperature. D179Y KPC-2 crystals were grown using the sitting drop method at room temperature in 1 M ammonium citrate dibasic and 0.1 M sodium acetate trihydrate, pH 4.6, with microcrystal seeds from WT KPC-2. The D179 KPC-2 variant crystals were cryo-protected in perfluoropolyether and frozen in liquid nitrogen prior to synchrotron data collection.
Data collection and refinement.
X-ray diffraction data for vaborbactam in complex with D179N KPC-2, apo D179N KPC-2, and 179Y KPC-2 structures were collected at the NSLS II AMX beamline and processed using XDS (67) (Table S1 in the supplemental material contains data collection statistics). The crystal structures were solved by molecular replacement using the program PHASER (68) with chain A of the KPC-2 structure (PDB accession number 2OV5) (19) as the search model. The crystallographic refinement for D179N KPC-2 and D179N KPC-2 in complex with vaborbactam was carried out using Refmac (69) and model building with Coot (70). The D179Y KPC-2 structure was refined using Phenix (71), and the two molecules in the asymmetric unit were refined using noncrystallographic restraints due to the lower resolution of the data set. Refinement parameter files for vaborbactam were generated using AceDRG (72). Molecular figures were generated using PyMOL 2.4.1. (www.pymol.org).
Data availability.
The coordinates and structure factors of the D179N KPC-2, D179Y KPC-2, and D179N KPC-2 vaborbactam complex have been deposited with the Protein Data Bank (PDB accession numbers 7TB7, 7TBX, and 7TC1, respectively).
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) to C.J.C. under award number R21AI142049. This study was also supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, award number 1I01BX001974, to R.A.B. from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development and the Geriatric Research Education and Clinical Center VISN 10.
We thank support personnel at NSLS synchrotron beamlines AMX and FMX for help with data collection.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Department of Veterans Affairs.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Bush K, Bradford PA. 2019. Interplay between β-lactamases and new β-lactamase inhibitors. Nat Rev Microbiol 17:295–306. 10.1038/s41579-019-0159-8. [DOI] [PubMed] [Google Scholar]
- 2.Drawz SM, Papp-Wallace KM, Bonomo RA. 2014. New β-lactamase inhibitors: a therapeutic renaissance in an MDR world. Antimicrob Agents Chemother 58:1835–1846. 10.1128/AAC.00826-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papp-Wallace KM, Mack AR, Taracila MA, Bonomo RA. 2020. Resistance to novel β-lactam-β-lactamase inhibitor combinations: the “price of progress.” Infect Dis Clin North Am 34:773–819. 10.1016/j.idc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shields RK, Nguyen MH, Press EG, Chen L, Kreiswirth BN, Clancy CJ. 2017. In vitro selection of meropenem resistance among ceftazidime-avibactam-resistant, meropenem-susceptible Klebsiella pneumoniae isolates with variant kpc-3 carbapenemases. Antimicrob Agents Chemother 61:e00079-17. 10.1128/AAC.00079-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giddins MJ, Macesic N, Annavajhala MK, Stump S, Khan S, McConville TH, Mehta M, Gomez-Simmonds A, Uhlemann AC. 2018. Successive emergence of ceftazidime-avibactam resistance through distinct genomic adaptations in blaKPC-2-harboring Klebsiella pneumoniae sequence type 307 isolates. Antimicrob Agents Chemother 62:e02101-17. 10.1128/AAC.02101-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaibani P, Campoli C, Lewis RE, Volpe SL, Scaltriti E, Giannella M, Pongolini S, Berlingeri A, Cristini F, Bartoletti M, Tedeschi S, Ambretti S. 2018. In vivo evolution of resistant subpopulations of KPC-producing Klebsiella pneumoniae during ceftazidime/avibactam treatment. J Antimicrob Chemother 73:1525–1529. 10.1093/jac/dky082. [DOI] [PubMed] [Google Scholar]
- 7.Castanheira M, Arends SJR, Davis AP, Woosley LN, Bhalodi AA, MacVane SH. 2018. Analyses of a ceftazidime-avibactam-resistant Citrobacter freundii isolate carrying bla KPC-2 reveals a heterogenous population and reversible genotype. mSphere 3:e00408-18. 10.1128/mSphere.00408-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wozniak A, Paillavil B, Legarraga P, Zumaran C, Prado S, Garcia P. 2019. Evaluation of a rapid immunochromatographic test for detection of KPC in clinical isolates of Enterobacteriaceae and Pseudomonas species. Diagn Microbiol Infect Dis 95:131–133. 10.1016/j.diagmicrobio.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, Pandey R, Doi Y, Kreiswirth BN, Nguyen MH, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae Infections. Antimicrob Agents Chemother 61:e02097-16. 10.1128/AAC.02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taracila MA, Bethel CR, Hujer AM, Papp-Wallace KM, Barnes MD, Rutter JD, VanPelt J, Shurina BA, van den Akker F, Clancy CJ, Nguyen MH, Cheng S, Shields RK, Page RC, Bonomo RA. 2022. Different conformations revealed by NMR underlie resistance to ceftazidime/avibactam and susceptibility to meropenem and imipenem among D179Y variants of KPC β-lactamase. Antimicrob Agents Chemother. 10.1128/aac.02124-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hobson CA, Cointe A, Jacquier H, Choudhury A, Magnan M, Courroux C, Tenaillon O, Bonacorsi S, Birgy A. 2021. Cross-resistance to cefiderocol and ceftazidime-avibactam in KPC β-lactamase mutants and the inoculum effect. Clin Microbiol Infect 27:1172.e7–1172.e10. 10.1016/j.cmi.2021.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Bianco G, Boattini M, Comini S, Iannaccone M, Bondi A, Cavallo R, Costa C. 2022. In vitro activity of cefiderocol against ceftazidime-avibactam susceptible and resistant KPC-producing Enterobacterales: cross-resistance and synergistic effects. Eur J Clin Microbiol Infect Dis 41:63–70. 10.1007/s10096-021-04341-z. [DOI] [PubMed] [Google Scholar]
- 13.Tiseo G, Falcone M, Leonildi A, Giordano C, Barnini S, Arcari G, Carattoli A, Menichetti F. 2021. Meropenem-vaborbactam as salvage therapy for ceftazidime-avibactam-, cefiderocol-resistant ST-512 Klebsiella pneumoniae-producing KPC-31, a D179Y variant of KPC-3. Open Forum Infect Dis 8:ofab141. 10.1093/ofid/ofab141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsivkovski R, Lomovskaya O. 2020. Potency of vaborbactam is less affected than that of avibactam in strains producing KPC-2 mutations that confer resistance to ceftazidime-avibactam. Antimicrob Agents Chemother 64:e01936-19. 10.1128/AAC.01936-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes MD, Winkler ML, Taracila MA, Page MG, Desarbre E, Kreiswirth BN, Shields RK, Nguyen MH, Clancy C, Spellberg B, Papp-Wallace KM, Bonomo RA. 2017. Klebsiella pneumoniae carbapenemase-2 (KPC-2), substitutions at Ambler position Asp179, and resistance to ceftazidime-avibactam: unique antibiotic-resistant phenotypes emerge from β-lactamase protein engineering. mBio 8:e00528-17. 10.1128/mBio.00528-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro AB, Moussa SH, Carter NM, Gao N, Miller AA. 2021. Ceftazidime-avibactam resistance mutations V240G, D179Y, and D179Y/T243M in KPC-3 β-lactamase do not alter cefpodoxime-ETX1317 susceptibility. ACS Infect Dis 7:79–87. 10.1021/acsinfecdis.0c00575. [DOI] [PubMed] [Google Scholar]
- 17.Sun L, Chen W, Li H, Li L, Zou X, Zhao J, Lu B, Li B, Wang C, Li H, Liu Y, Cao B. 2020. Phenotypic and genotypic analysis of KPC-51 and KPC-52, two novel KPC-2 variants conferring resistance to ceftazidime/avibactam in the KPC-producing Klebsiella pneumoniae ST11 clone background. J Antimicrob Chemother 75:3072–3074. [DOI] [PubMed] [Google Scholar]
- 18.Venditti C, Butera O, Meledandri M, Balice MP, Cocciolillo GC, Fontana C, D'Arezzo S, Giuli Antonini DC, Capone M, Messina A, Nisii F, Di Caro C. 2021. Molecular analysis of clinical isolates of ceftazidime-avibactam-resistant Klebsiella pneumoniae. Clin Microbiol Infect 27:1040.e1–1040.e6. 10.1016/j.cmi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Ke W, Bethel CR, Thomson JM, Bonomo RA, van den Akker F. 2007. Crystal structure of KPC-2: insights into carbapenemase activity in class A β-lactamases. Biochemistry 46:5732–5740. 10.1021/bi700300u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pemberton OA, Noor RE, Kumar MVV, Sanishvili R, Kemp MT, Kearns FL, Woodcock HL, Gelis I, Chen Y. 2020. Mechanism of proton transfer in class A β-lactamase catalysis and inhibition by avibactam. Proc Natl Acad Sci USA 117:5818–5825. 10.1073/pnas.1922203117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egorov A, Rubtsova M, Grigorenko V, Uporov I, Veselovsky A. 2019. The role of the Ω-loop in regulation of the catalytic activity of TEM-type β-lactamases. Biomolecules 9:854. 10.3390/biom9120854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furey IM, Mehta SC, Sankaran B, Hu L, Prasad BVV, Palzkill T. 2021. Local interactions with the Glu166 base and the conformation of an active site loop play key roles in carbapenem hydrolysis by the KPC-2 β-lactamase. J Biol Chem 296:100799. 10.1016/j.jbc.2021.100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrosino JF, Palzkill T. 1996. Systematic mutagenesis of the active site Ω loop of TEM-1 beta-lactamase. J Bacteriol 178:1821–1828. 10.1128/jb.178.7.1821-1828.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levitt PS, Papp-Wallace KM, Taracila MA, Hujer AM, Winkler ML, Smith KM, Xu Y, Harris ME, Bonomo RA. 2012. Exploring the role of a conserved class A residue in the Ω-loop of KPC-2 β-lactamase: a mechanism for ceftazidime hydrolysis. J Biol Chem 287:31783–31793. 10.1074/jbc.M112.348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livermore DM, Warner M, Jamrozy D, Mushtaq S, Nichols WW, Mustafa N, Woodford N. 2015. In vitro selection of ceftazidime-avibactam resistance in Enterobacteriaceae with KPC-3 carbapenemase. Antimicrob Agents Chemother 59:5324–5330. 10.1128/AAC.00678-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sampson JM, Ke W, Bethel CR, Pagadala SR, Nottingham MD, Bonomo RA, Buynak JD, van den Akker F. 2011. Ligand-dependent disorder of W-loop observed in extended-spectrum SHV-type β-lactamase. Antimicrob Agents Chemother 55:2303–2309. 10.1128/AAC.01360-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bös F, Pleiss J. 2008. Conserved water molecules stabilize the Ω-loop in class A β-lactamases. Antimicrob Agents Chemother 52:1072–1079. 10.1128/AAC.01035-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haidar G, Clancy CJ, Shields RK, Hao B, Cheng S, Nguyen MH. 2017. Mutations in blaKPC-3 that confer ceftazidime-avibactam resistance encode novel KPC-3 variants that function as extended-spectrum β-lactamases. Antimicrob Agents Chemother 61:e02534-16. 10.1128/AAC.02534-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Compain F, Arthur M. 2017. Impaired inhibition by avibactam and resistance to the ceftazidime-avibactam combination due to the D(179)Y substitution in the KPC-2 β-lactamase. Antimicrob Agents Chemother 61:e00451-17. 10.1128/AAC.00451-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winkler ML, Papp-Wallace KM, Bonomo RA. 2015. Activity of ceftazidime/avibactam against isogenic strains of Escherichia coli containing KPC and SHV β-lactamases with single amino acid substitutions in the Ω-loop. J Antimicrob Chemother 70:2279–2286. 10.1093/jac/dkv094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gottig S, Frank D, Mungo E, Nolte A, Hogardt M, Besier S, Wichelhaus TA. 2019. Emergence of ceftazidime/avibactam resistance in KPC-3-producing Klebsiella pneumoniae in vivo. J Antimicrob Chemother 74:3211–3216. 10.1093/jac/dkz330. [DOI] [PubMed] [Google Scholar]
- 32.Tooke CL, Hinchliffe P, Bonomo RA, Schofield CJ, Mulholland AJ, Spencer J. 2021. Natural variants modify Klebsiella pneumoniae carbapenemase (KPC) acyl-enzyme conformational dynamics to extend antibiotic resistance. J Biol Chem 296:100126. 10.1074/jbc.RA120.016461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrella S, Ziental-Gelus N, Mayer C, Renard M, Jarlier V, Sougakoff W. 2008. Genetic and structural insights into the dissemination potential of the extremely broad-spectrum class A β-lactamase KPC-2 identified in an Escherichia coli strain and an Enterobacter cloacae strain isolated from the same patient in France. Antimicrob Agents Chemother 52:3725–3736. 10.1128/AAC.00163-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pemberton OA, Zhang X, Chen Y. 2017. Molecular basis of substrate recognition and product release by the Klebsiella pneumoniae carbapenemase (KPC-2). J Med Chem 60:3525–3530. 10.1021/acs.jmedchem.7b00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ke W, Bethel CR, Papp-Wallace KM, Pagadala SR, Nottingham M, Fernandez D, Buynak JD, Bonomo RA, van den Akker F. 2012. Crystal structures of KPC-2 β-lactamase in complex with 3-nitrophenyl boronic acid and the penam sulfone PSR-3–226. Antimicrob Agents Chemother 56:2713–2718. 10.1128/AAC.06099-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao G, Meier TI, Kahl SD, Gee KR, Blaszczak LC. 1999. BOCILLIN FL, a sensitive and commercially available reagent for detection of penicillin-binding proteins. Antimicrob Agents Chemother 43:1124–1128. 10.1128/AAC.43.5.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldberg JA, Kumar V, Spencer EJ, Hoyer D, Marshall SH, Hujer AM, Hujer KM, Bethel CR, Papp-Wallace KM, Perez F, Jacobs MR, van Duin D, Kreiswirth BN, van den Akker F, Plummer MS, Bonomo RA. 2021. A γ-lactam siderophore antibiotic effective against multidrug-resistant Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter spp. Eur J Med Chem 220:113436. 10.1016/j.ejmech.2021.113436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papp-Wallace K, Bonomo RA. 2013. Reply to Frère: covalent trapping and bacterial resistance to ceftazidime. J Biol Chem 288:26968. 10.1074/jbc.l113.502237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernandez-Garcia M, Sanchez-Lopez J, Martinez-Garcia L, Becerra-Aparicio F, Morosini MI, Ruiz-Garbajosa P, Canton R. 2021. Emergence of the new KPC-49 variant conferring an ESBL phenotype with resistance to ceftazidime-avibactam in the ST131-H30R1 Escherichia coli high-risk clone. Pathogens 10:67. 10.3390/pathogens10010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Padayatti PS, Sheri A, Totir MA, Helfand MS, Carey MP, Anderson VE, Carey PR, Bethel CR, Bonomo RA, Buynak JD, van den Akker F. 2006. Rational design of a β-lactamase inhibitor achieved via stabilization of the trans-enamine intermediate: 1.28Å crystal structure of wt SHV-1 complex with a penam sulfone. J Am Chem Soc 128:13235–13242. 10.1021/ja063715w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang P, Shi Q, Hu H, Hong B, Wu X, Du X, Akova M, Yu Y. 2020. Emergence of ceftazidime/avibactam resistance in carbapenem-resistant Klebsiella pneumoniae in China. Clin Microbiol Infect 26:124.e1–124.e4. 10.1016/j.cmi.2019.08.020. [DOI] [PubMed] [Google Scholar]
- 42.Shields RK, Nguyen MH, Press EG, Chen L, Kreiswirth BN, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance and restoration of carbapenem susceptibility in Klebsiella pneumoniae carbapenemase-producing K pneumoniae: a case report and review of literature. Open Forum Infect Dis 4:ofx101. 10.1093/ofid/ofx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hemarajata P, Humphries RM. 2019. Ceftazidime/avibactam resistance associated with L169P mutation in the Ω loop of KPC-2. J Antimicrob Chemother 74:1241–1243. 10.1093/jac/dkz026. [DOI] [PubMed] [Google Scholar]
- 44.Venditti C, Nisii C, Ballardini M, Meledandri M, Di Caro A. 2019. Identification of L169P mutation in the Ω loop of KPC-3 after a short course of ceftazidime/avibactam. J Antimicrob Chemother 74:2466–2467. 10.1093/jac/dkz201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krishnan NP, Nguyen NQ, Papp-Wallace KM, Bonomo RA, van den Akker F. 2015. Inhibition of Klebsiella β-lactamases (SHV-1 and KPC-2) by avibactam: a structural study. PLoS One 10:e0136813. 10.1371/journal.pone.0136813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ehmann DE, Jahic H, Ross PL, Gu RF, Hu J, Kern G, Walkup GK, Fisher SL. 2012. Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor. Proc Natl Acad Sci USA 109:11663–11668. 10.1073/pnas.1205073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tooke CL, Hinchliffe P, Krajnc A, Mulholland AJ, Brem J, Schofield CJ, Spencer J. 2020. Cyclic boronates as versatile scaffolds for KPC-2 β-lactamase inhibition. RSC Med Chem 11:491–496. 10.1039/c9md00557a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papp-Wallace KM, Taracila M, Wallace CJ, Hujer KM, Bethel CR, Hornick JM, Bonomo RA. 2010. Elucidating the role of Trp105 in the KPC-2 β-lactamase. Protein Sci 19:1714–1727. 10.1002/pro.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. 2008. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol Rev 32:234–258. 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 50.Sauvage E, Terrak M. 2016. Glycosyltransferases and transpeptidases/penicillin-binding proteins: valuable targets for new antibacterials. Antibiotics (Basel) 5:12. 10.3390/antibiotics5010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yousif SY, Broome-Smith JK, Spratt BG. 1985. Lysis of Escherichia coli by β-lactam antibiotics: deletion analysis of the role of penicillin-binding proteins 1A and 1B. J Gen Microbiol 131:2839–2845. 10.1099/00221287-131-10-2839. [DOI] [PubMed] [Google Scholar]
- 52.Moya B, Barcelo IM, Cabot G, Torrens G, Palwe S, Joshi P, Umarkar K, Takalkar S, Periasamy H, Bhagwat S, Patel M, Bou G, Oliver A. 2019. In vitro and in vivo activities of β-lactams in combination with the novel β-lactam enhancers zidebactam and WCK 5153 against multidrug-resistant metallo-β-lactamase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 63:e00128-19. 10.1128/AAC.00128-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sutaria DS, Moya B, Green KB, Kim TH, Tao X, Jiao Y, Louie A, Drusano GL, Bulitta JB. 2018. First penicillin-binding protein occupancy patterns of β-lactams and β-lactamase inhibitors in Klebsiella pneumoniae. Antimicrob Agents Chemother 62:e00282-18. 10.1128/AAC.00282-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Asli A, Brouillette E, Krause KM, Nichols WW, Malouin F. 2016. Distinctive binding of avibactam to penicillin-binding proteins of Gram-negative and Gram-positive bacteria. Antimicrob Agents Chemother 60:752–756. 10.1128/AAC.02102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farmer TH, Degnan BA, Payne DJ. 1999. Penetration of β-lactamase inhibitors into the periplasm of Gram-negative bacteria. FEMS Microbiol Lett 176:11–15. 10.1111/j.1574-6968.1999.tb13635.x. [DOI] [PubMed] [Google Scholar]
- 56.Kim TH, Tao X, Moya B, Jiao Y, Basso KB, Zhou J, Lang Y, Sutaria DS, Zavascki AP, Barth AL, Reeve SM, Schweizer HP, Deveson Lucas D, Boyce JD, Bonomo RA, Lee RE, Shin BS, Louie A, Drusano GL, Bulitta JB. 2020. Novel cassette assay to quantify the outer membrane permeability of five β-lactams simultaneously in carbapenem-resistant Klebsiella pneumoniae and Enterobacter cloacae. mBio 11:e03189-19. 10.1128/mBio.03189-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nikaido H, Liu W, Rosenberg EY. 1990. Outer membrane permeability and β-lactamase stability of dipolar ionic cephalosporins containing methoxyimino substituents. Antimicrob Agents Chemother 34:337–342. 10.1128/AAC.34.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen Z, Ding B, Ye M, Wang P, Bi Y, Wu S, Xu X, Guo Q, Wang M. 2017. High ceftazidime hydrolysis activity and porin OmpK35 deficiency contribute to the decreased susceptibility to ceftazidime/avibactam in KPC-producing Klebsiella pneumoniae. J Antimicrob Chemother 72:1930–1936. 10.1093/jac/dkx066. [DOI] [PubMed] [Google Scholar]
- 59.Han S, Zaniewski RP, Marr ES, Lacey BM, Tomaras AP, Evdokimov A, Miller JR, Shanmugasundaram V. 2010. Structural basis for effectiveness of siderophore-conjugated monocarbams against clinically relevant strains of Pseudomonas aeruginosa. Proc Natl Acad Sci USA 107:22002–22007. 10.1073/pnas.1013092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar V, Tang C, Bethel CR, Papp-Wallace KM, Wyatt J, Desarbre E, Bonomo RA, van den Akker F. 2020. Structural insights into ceftobiprole inhibition of Pseudomonas aeruginosa penicillin-binding protein 3. Antimicrob Agents Chemother 64:e00106-20. 10.1128/AAC.00106-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klumpp S, Krieglstein J. 2002. Phosphorylation and dephosphorylation of histidine residues in proteins. Eur J Biochem 269:1067–1071. 10.1046/j.1432-1033.2002.02755.x. [DOI] [PubMed] [Google Scholar]
- 62.Keyhani NO, Boudker O, Roseman S. 2000. Isolation and characterization of IIAChb, a soluble protein of the enzyme II complex required for the transport/phosphorylation of N,N'-diacetylchitobiose in Escherichia coli. J Biol Chem 275:33091–33101. 10.1074/jbc.M001044200. [DOI] [PubMed] [Google Scholar]
- 63.Tierney AR, Rather PN. 2019. Roles of two-component regulatory systems in antibiotic resistance. Future Microbiol 14:533–552. 10.2217/fmb-2019-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar V, Viviani SL, Ismail J, Agarwal S, Bonomo RA, van den Akker F. 2021. Structural analysis of the boronic acid β-lactamase inhibitor vaborbactam binding to Pseudomonas aeruginosa penicillin-binding protein 3. PLoS One 16:e0258359. 10.1371/journal.pone.0258359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rajavel M, Kumar V, Nguyen H, Wyatt J, Marshall SH, Papp-Wallace KM, Deshpande P, Bhavsar S, Yeole R, Bhagwat S, Patel M, Bonomo RA, van den Akker F. 2021. Structural characterization of diazabicyclooctane β-lactam “enhancers” in complex with penicillin-binding proteins PBP2 and PBP3 of Pseudomonas aeruginosa. mBio 12:e03058-20. 10.1128/mBio.03058-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Papp-Wallace KM, Nguyen NQ, Jacobs MR, Bethel CR, Barnes MD, Kumar V, Bajaksouzian S, Rudin SD, Rather PN, Bhavsar S, Ravikumar T, Deshpande PK, Patil V, Yeole R, Bhagwat SS, Patel MV, van den Akker F, Bonomo RA. 2018. Strategic approaches to overcome resistance against Gram-negative pathogens using β-lactamase inhibitors and β-lactam enhancers: activity of three novel diazabicyclooctanes WCK 5153, zidebactam (WCK 5107), and WCK 4234. J Med Chem 61:4067–4086. 10.1021/acs.jmedchem.8b00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kabsch W. 2010. Xds. Acta Crystallogr D Biol Crystallogr 66:125–132. 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. 2007. Phaser crystallographic software. J Appl Crystallogr 40:658–674. 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murshudov GN, Skubak P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, Winn MD, Long F, Vagin AA. 2011. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr 67:355–367. 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Emsley P, Cowtan K. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60:2126–2132. 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 71.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66:213–221. 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Long F, Nicholls RA, Emsley P, Graulis S, Merkys A, Vaitkus A, Murshudov GN. 2017. AceDRG: a stereochemical description generator for ligands. Acta Crystallogr D Struct Biol 73:112–122. 10.1107/S2059798317000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naas T, Oueslati S, Bonnin RA, Dabos ML, Zavala A, Dortet L, Retailleau P, Iorga BI. 2017. β-Lactamase database (BLDB) - structure and function. J Enzyme Inhib Med Chem 32:917–919. 10.1080/14756366.2017.1344235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 and S2 and Table S1. Download aac.02414-21-s0001.pdf, PDF file, 0.4 MB (449.3KB, pdf)
Data Availability Statement
The coordinates and structure factors of the D179N KPC-2, D179Y KPC-2, and D179N KPC-2 vaborbactam complex have been deposited with the Protein Data Bank (PDB accession numbers 7TB7, 7TBX, and 7TC1, respectively).