ABSTRACT
The C4-dicarboxylates (C4-DC) l-aspartate and l-malate have been identified as playing an important role in the colonization of mammalian intestine by enteric bacteria, such as Escherichia coli and Salmonella enterica serovar Typhimurium, and succinate as a signaling molecule for host-enteric bacterium interaction. Thus, endogenous and exogenous fumarate respiration and related functions are required for efficient initial growth of the bacteria. l-Aspartate represents a major substrate for fumarate respiration in the intestine and a high-quality substrate for nitrogen assimilation. During nitrogen assimilation, DcuA catalyzes an l-aspartate/fumarate antiport and serves as a nitrogen shuttle for the net uptake of ammonium only, whereas DcuB acts as a redox shuttle that catalyzes the l-malate/succinate antiport during fumarate respiration. The C4-DC two-component system DcuS-DcuR is active in the intestine and responds to intestinal C4-DC levels. Moreover, in macrophages and in mice, succinate is a signal that promotes virulence and survival of S. Typhimurium and pathogenic E. coli. On the other hand, intestinal succinate is an important signaling molecule for the host and activates response and protective programs. Therefore, C4-DCs play a major role in supporting colonization of enteric bacteria and as signaling molecules for the adaptation of host physiology.
KEYWORDS: C4-dicarboxylates, l-aspartate, succinate, fumarate respiration, nitrogen assimilation, Escherichia coli, Salmonella Typhimurium, intestine colonization, initial growth, Salmonella
INTRODUCTION
Escherichia coli and Salmonella enterica serovar Typhimurium belong to the Enterobacteriaceae, which are characterized by their metabolic versatility. Hexoses represent the preferred carbon source of enterobacteria and are degraded by glycolysis (1) followed by complete oxidation in the citric acid cycle under aerobic conditions, or by mixed acid fermentation under anaerobic conditions (2–4). C4-dicarboxylic acids (C4-DCs) such as succinate, l-malate, l-tartrate, and l-aspartate represent alternative substrates for growth (5–9). C4-DCs are degraded by the citric acid cycle in combination with the pyruvate bypass under aerobic conditions and by fumarate respiration under anaerobic conditions. C4-DC degradation is subject to glucose repression, and central genes for the degradation of C4-DC substrates are induced by the C4-DC two-component system DcuS-DcuR (10). Major targets of regulation by DcuS-DcuR are the genes encoding the aerobic C4-DC transporter DctA, and of the anaerobic fumarate respiration, namely, the C4-DC/succinate antiporter DcuB, fumarate reductase FrdABCD, and fumarase FumB (10, 11). Fumarate respiration represents an important mode of energy conservation during anaerobic growth of enteric and proteobacteria (12–14).
The physiology, biochemistry, and regulation of hexose and C4-DC metabolism have been studied in detail for enteric bacteria, particularly under defined (laboratory) conditions. The significance of hexoses and hexose derivatives was confirmed for growth in the intestine of mice or the mucus covering the cecal epithelium by commensal and pathogenic E. coli (15, 16). On the other hand, the role of C4-DCs for the enteric bacteria in the intestine has remained uncertain. Under anaerobic conditions in the intestine, fumarate respiration has been shown to be important for fitness and initial growth of commensal E. coli and the enteropathogenic S. Typhimurium (17–20). The levels of fumarate in the cecum and intestinal lumen were negligible, whereas those for l-aspartate and l-malate are notable and reach levels that are sufficient to induce DcuS-DcuR dependent genes of fumarate respiration (19, 20). Therefore, C4-DCs represented by l-aspartate and l-malate have an important role for microbiota by driving fumarate respiration (19–22). Under the oxidative conditions in an inflamed intestine, S. Typhimurium also oxidizes succinate (and other C4-DCs) by an oxidative central metabolism (23). C4-DCs also serve as a carbon source during aerobic and anaerobic growth of E. coli and S. Typhimurium (8, 9, 24). l-Aspartate is used as an important nitrogen source (20, 21). The key enzyme of the assimilatory pathway, aspartase AspA, is subject to regulation by the general nitrogen regulatory system (22).
Recent findings suggest that, in addition to its role as substrates, succinate coordinates reactions related to virulence and survival of S. Typhimurium in macrophages and in mice (25). Moreover, succinate is an important signaling molecule for the host. Accumulation of microbiota-derived succinate affects transcriptional and posttranslational modifications in the host and activates inflammatory programs, epigenetic regulation, and ROS production (26–28).
Overall, C4-DCs have a major and specific role in the interaction of the mammalian host with enteric bacteria. l-Aspartate and l-malate are important for gut colonization of E. coli and S. Typhimurium due to their role in fumarate respiration, nitrogen assimilation, and regulation of virulence. On the other hand, succinate produced by the microbiota represents a substrate and signaling molecule for host luminal cells and macrophages. This review will discuss the role of C4-DCs in the growth and colonization of bacteria in the intestine with an overview of recent aspects of succinate as a signaling molecule in host-microbiota interaction and physiology.
l-ASPARTATE AND l-MALATE AS SUBSTRATES FOR FUMARATE RESPIRATION IN THE INTESTINE
E. coli and other enteric bacteria perform fumarate respiration in the absence of electron acceptors like O2 or nitrate (2, 12, 14, 29). Fumarate reductase FrdABCD is membrane-integral and perceives the electrons for fumarate reduction from menaquinol (30). During fermentation of hexoses and other carbohydrates, menaquinone (MK) is reduced at the expense of NADH, formate, or H2 originating from fermentation (1, 3, 31). NADH and H2 are oxidized by NADH dehydrogenase I (NuoA-N) and respiratory hydrogenase (mostly hydrogenase 2 or Hyb in E. coli and S. Typhimurium) to reduce MK. NuoA-N and Hyb conserve the redox energy in H+ potential (32–35).
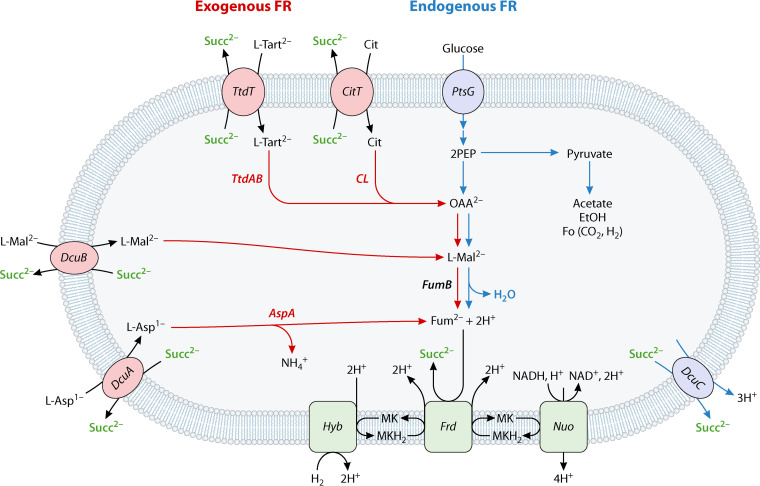
E. coli ferments hexoses to ethanol, acetate, and formate (or H2 + CO2) as the main products; additionally, 0.11 to 0.29 mol of succinate are formed per mol of glucose (36, 37). Succinate is the product of endogenous fumarate respiration; the fumarate for this reaction is derived from the phospho-enol-pyruvate of glycolysis by carboxylation and the reductive branch of the (anaerobic) citric acid cycle (Fig. 1) (38). Succinate is excreted by transporter DcuC (39, 40). E. coli also utilizes exogenous C4-DCs such as fumarate in the medium as the electron acceptor for fumarate respiration (8, 12, 31). The electrons for fumarate respiration are derived mostly from NADH or H2 that originate from glucose fermentation, or glycerol-3P (from glycerol) and H2 as exogenous electron donors (3, 8, 31) (Fig. 1). During fumarate respiration, uptake of external C4-DCs is catalyzed by the antiporters DcuA or DcuB in antiport against succinate (Fig. 1). In addition to fumarate, l-malate, l-aspartate, l-tartrate, and citrate can be utilized by E. coli as precursors of fumarate for fumarate respiration. l-Malate, l-tartrate, and citrate are found in significant amounts in fruit or plant materials, while l-aspartate is derived from proteins. Fumarate/succinate and l-malate/succinate antiport is performed preferentially by DcuB, and l-aspartate/succinate antiport by DcuA (8, 21, 39, 40). At high concentrations of fumarate, l-malate, and l-aspartate (>0.1 mM), the transporters are mutually active. In addition, DcuC, which mainly functions in succinate excretion during fermentation, can substitute for DcuA and DcuB (41, 42). The feeding reactions for fumarate formation from the latter C4-DCs are shown in Fig. 1. After uptake, l-malate and l-aspartate are converted to fumarate by fumarase FumB and aspartase AspA. It appears that FumB and AspA are organized in metabolons with the transporters for efficient channeling and supply of fumarate for respiration (Fig. 2) (43). l-Tartrate and citrate are transported by TtdT and CitT, respectively, in antiport against succinate (6, 44–47). l-Tartrate is dehydrated to oxaloacetate by l-tartrate dehydratase TtdAB, while citrate is cleaved by citrate lyase CL, also producing oxaloacetate (OAA). The OAA is converted to fumarate in E. coli by the anaerobic reductive part of the tricarboxylic acid (TCA) cycle and used for fumarate respiration (Fig. 1) (6, 8, 45), while in S. Typhimurium, the OAA is decarboxylated by the Na+-translocating OAA decarboxylase into pyruvate (48, 49).
FIG 1.
Exogenous and endogenous fumarate respiration (FR) by E. coli. For endogenous FR (fumarate produced during hexose fermentation) up to 15% of the PEP formed during hexose fermentation (37) is carboxylated to yield OAA, which is then converted by the reductive branch of the anaerobic citric acid cycle to succinate. For exogenous FR, l-aspartate, l-malate, fumarate, l-tartrate, or citrate are taken up by antiporters from the medium, and succinate is excreted in an electroneutral antiport. Enzymes and feeding reactions for fumarate formation are shown in blue and red, respectively, joint reactions in green. Details are described in the text and in reviews (3, 8, 31, 35). At concentrations > 0.1 mM fumarate, l-aspartate or l-malate, the transporters DcuA, DcuB, and DcuC are able to replace each other. DcuA, DcuB, and DcuC are present in S. Typhimurium as well (19), whereas citrate and tartrate are used only by E. coli (6, 46), but not by S. Typhimurium for FR (47, 48, 100). AspA, aspartase; CitT, citrate/succinate antiporter; CL, citrate lyase; DcuA, C4-DC antiporter DcuA; DcuB, C4-DC antiporter DcuB; DcuC, C4-DC transporter DcuC; FR, fumarate respiration; Frd, fumarate reductase FrdABCD; FumB, fumarase B; Hyb, hydrogenase; Nuo, NuoA-N; PtsG, glucose transporter of the phosphotransferase system; TtdAB, tartrate dehydratase; TtdT, l-tartrate/succinate antiporter; MK, menaquinone; MKH2, menaquinol.
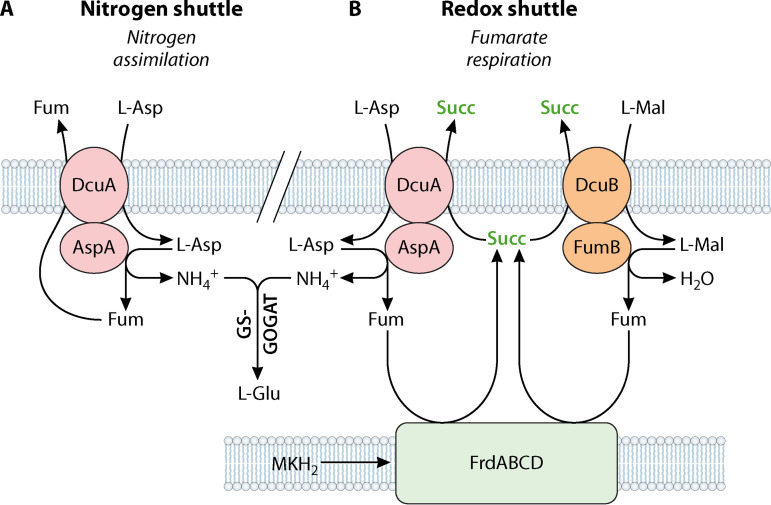
FIG 2.
Scheme for the DcuA/AspA, DcuB/AspA, and DcuB/FumB metabolons of E. coli. Complex formation between AspA and FumB with the Dcu transporters is based on interaction studies (43), suggesting metabolon formation and metabolic channeling. The l-aspartate/fumarate antiport used during nitrogen assimilation by DcuA results in net uptake of ammonium (“nitrogen or ammonium shuttle”), the fumarate/succinate or l-malate/succinate antiport in the net uptake of 2 [H] (“H or redox shuttle”) for the sake of fumarate respiration. Figure modified from Schubert and Unden (43). AspA, aspartase; DcuA, C4-DC transporter; DcuB, C4-DC transporter; FrdABCD, fumarate reductase; GS-GOGAT, glutamine synthetase (GS)-glutamine 2-oxoglutarate aminotransferase (GOGAT) pathway; Fum, fumarate; FumB, fumarase B; L-Asp, l-aspartate; L-Mal, l-malate; MKH2, menaquinol; Succ, succinate.
E. coli or S. Typhimurium deficient in frdA, dcuSR, dcuB, and aspA are severely impaired in their capacity to colonize mouse intestine. The most pronounced effects are observed in frdA deficiency (18–20), followed by the loss of dcuS dcuR, while the effects of dcuB and aspA deletion are more moderate. Also, the mRNA levels of not only frdA but also of aspA, dcuB, and dcuC are greatly increased in the cecum and colon of mice in a DcuR-dependent manner (20). The expression of dcuA, on the other hand, was constant, as previously shown in vitro (50). In the presence of E. coli, S. Typhimurium has reduced initial growth in the gut lumen, which is not the case for dcuAB- and frdA-deficient E. coli strains, suggesting that fumarate respiration promotes colonization of E. coli and establishes colonization resistance against S. Typhimurium (19). Therefore, fumarate respiratory genes, including the regulatory genes dcuS and dcuR, are important for gut colonization. Remarkably, frdA and dcuS dcuR, central for both exogenous and endogenous fumarate respiration, were most important for gut colonization; dcuB and dcuA, required for only externally supplied C4-DCs, were less significant for colonization efficiency but support initial growth (19, 20). The hydrogenase Hyb, the major uptake hydrogenase for MK reduction, is also essential for intestinal colonization of S. Typhimurium and E. coli (51, 52). The H2 could originate from formate that is converted by E. coli formate-hydrogen lyase to H2 and CO2, or from the fermentative metabolism of gut microbiota. In an inflamed gut, S. Typhimurium utilizes formate directly as an electron donor for aerobic and nitrate respiration. The reaction involves formate dehydrogenases of aerobic (fdo genes) and (fdn genes) nitrate respiration, as concluded from the decreased fitness of the corresponding mutants (53). Taken together, the data demonstrate the significance of fumarate respiration for colonization, and in particular of the genes that are also required for endogenous fumarate respiration. The frdA is expressed almost constitutively under anaerobic conditions in the interest of endogenous fumarate respiration and is stimulated by external C4-DCs by a factor of 1.5 to 2 only (10, 20), in contrast to the stimulation of dcuB by a factor of 5.6 to 11.6 (10, 20).
The significance of C4-DCs and of fumarate respiration for establishing growth in the intestine appears to be mostly related to its capacity to facilitate redox balancing in hexose fermentation. Thus, endogenous fumarate respiration provides an alternative means to consume reducing equivalents during fermentation, such as NADH or H2. Therefore, endogenous (and exogenous) fumarate respiration allows metabolic flexibility and the production of alternative substrates.
The lumen of the murine small intestine contains significant levels of l-aspartate (>1 mmol/kg wet mass), depending on diet and mouse breeding (19, 20), and the contents in the cecum are still notable (≥0.1 to 1 mmol/kg wet mass). In addition, l-aspartate-related compounds, such as l-asparagine or fructose-asparagine (Fruc-Asn) (54), can provide l-aspartate in the intestine. The contents of l-malate were lower but also significant, whereas those for fumarate were negligible (19, 20).
l-Aspartate and l-malate levels in the intestine exceed the Km values for uptake by DcuA and DcuB (43 and 110 μM, respectively) (21, 40), suggesting efficient uptake of C4-DCs by E. coli. The C4-DC-dependent stimulation of DcuS-DcuR occurs via the periplasmic sensor domain of DcuS (55, 56). The apparent Km for the activation of DcuS by C4-DCs is in the range of 0.5 to 3 mM (57). Schubert and coworkers confirmed that C4-DC concentrations of the small intestine or the cecum induce the expression of a dcuB-lacZ reporter fusion, which is in agreement with the high levels of mRNA of fumarate respiratory genes in mouse intestine (20). Most stimulation will be caused by l-aspartate, which stimulates DcuS with an apparent Km of 2 mM (57) and l-malate. In summary, endogenous and exogenous fumarate respiration contribute to initial growth of E. coli and S. Typhimurium in mouse intestine. l-Aspartate and, to a lesser extent, l-malate are the main substrates for fumarate respiration by external C4-DCs. Remarkably, l-aspartate is also a major regulator of chemotaxis in E. coli utilizing the chemoreceptor Tar for perception (58).
The mid small intestine and colon of mice are microaerobic with approximately 7.8 mbar O2 (59), which allows an almost half-maximal expression of FNR-regulated fumarate respiration (60, 61). Under the same O2 tension, the microaerobic oxidase encoded by cydAB is strongly expressed (61, 62), and the microaerobic conditions in the intestine are compatible with the concurrent expression of the genes for fumarate and microaerobic respiration (17–20). Fumarate and microaerobic respiration therefore coexist in the homeostatic mouse intestine. Under inflammatory conditions, reactive nitrogen species (RNS) and oxygen species (ROS) are formed (63, 64). RNS generate nitrate (NO3−), an important electron acceptor for facultative anaerobic bacteria (65). Nitrate represses fumarate respiration and frdABCD (66), indicating a decreased role for C4-DC utilization under inflammation (53). In support of this speculation, DcuS-DcuR is dispensable for E. coli fitness under inflammatory conditions (20).
l-ASPARTATE AS A HIGH-QUALITY NITROGEN SOURCE
l-Aspartate is a high-quality nitrogen source and is capable of saturating the nitrogen demand of E. coli under aerobic and anaerobic conditions (20–22). Nitrogen assimilation from l-aspartate requires the transporter DcuA and aspartate ammonia lyase AspA (Fig. 2). l-Asparagine (l-Asn) is deamidated to l-aspartate in the periplasmic space of E. coli by asparaginase AnsB (67, 68), and then utilized in the same way as l-aspartate. Fruc-Asn, a primary nutrient of S. Typhimurium in an inflamed intestine (54), is also an excellent source of nitrogen and carbon. Fruc-Asn is deamidated in the periplasmic space by FraE, transported into the bacterial cell by FraA, phosphorylated by FraD, and hydrolyzed to glucose-6-P (G6P) and l-aspartate by FraB (69).
AspA catalyzes the deamination of l-aspartate, producing fumarate and ammonium (21, 22). Assimilation of the ammonium occurs by glutamine synthetase GS (or GlnA) and glutamine 2-oxoglutarate aminotransferase GOGAT (or GltBD) yielding L-Glu (70, 71). The DcuA-AspA-GS-GOGAT pathway saturates the nitrogen requirement of E. coli efficiently (21, 22).
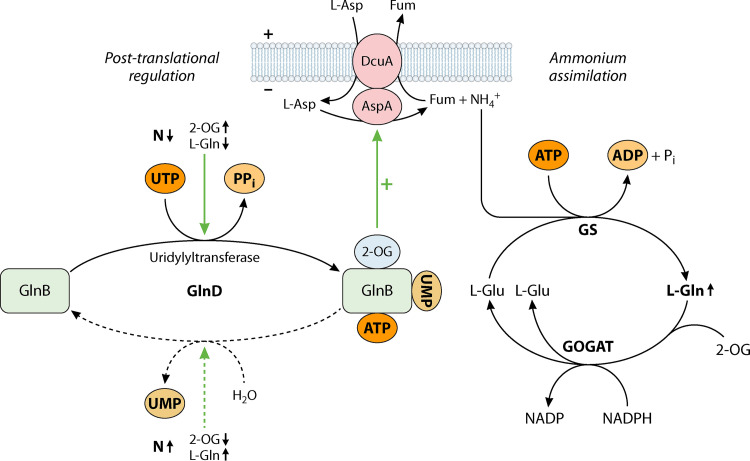
The expression of aspA and dcuA is essentially constitutive in E. coli (22, 50). AspA is integrated, however, into the nitrogen regulatory system of the central nitrogen regulator GlnB (alternative name PII) (72). GlnB regulates AspA activity in response to nitrogen availability in the cell (22). Under nitrogen-limited conditions, the deaminase activity of AspA and ammonium release is stimulated 2-fold by GlnB when the regulator is activated by uridylylation and binding of ATP and 2-oxoglutarate (Fig. 3). The stimulation is lost in the deuridylylated state of GlnB that prevails under nitrogen-saturated conditions. Overall, GlnB regulates the utilization of l-aspartate to ensure nitrogen supply under nitrogen-limited conditions. The presence of high levels of l-aspartate in mouse (20) or bovine (73) intestines, together with specific regulation, highlights the physiological relevance of l-aspartate as a source of nitrogen in E. coli.
FIG 3.
Ammonium assimilation from l-aspartate using DcuA-AspA for uptake and intracellular ammonium release, ammonium assimilation by GS-GOGAT, and the GlnB regulatory system. The scheme shows the uptake of l-aspartate by DcuA and ammonium release by the DcuA-AspA metabolon, the ammonium assimilation via the common GS-GOGAT pathway yielding L-Glu, and the regulation of AspA by the GlnB regulatory system and regulatory factors. N↓, nitrogen-limited conditions; N↑, nitrogen-saturated conditions; 2-OG, 2-oxoglutarate; UTP, uridine-triphosphate; PPi, diphosphate; Pi, phosphate; UMP, uridine-monophosphate; GlnD, uridylyltransferase/uridylyl-removing enzyme; Fum, fumarate; PII, nitrogen regulator GlnB; DcuA, aerobic l-aspartate transporter; AspA, aspartate ammonium-lyase; GS, glutamine synthetase GlnA; GOGAT, glutamine 2-oxoglutarate aminotransferase GltBD.
The fumarate released by the AspA reaction is excreted in aerobic growth nearly stoichiometrically by DcuA (Fig. 2) when other carbon sources are available (21). Under anaerobic conditions, the fumarate is used as a substrate for fumarate respiration and excreted only after reduction to succinate (8, 20, 39). DcuA therefore catalyzes an l-aspartate/fumarate or l-aspartate/succinate substrate/product antiport under aerobic and anaerobic conditions, respectively (Fig. 2). The l-aspartate/fumarate antiport results in the net uptake of ammonium and serves as an ammonium shuttle for the purpose of nitrogen assimilation. The l-aspartate/succinate antiport during fumarate respiration, on the other hand, represents a redox shuttle (in addition to its function as the ammonium shuttle), similar to DcuB catalyzing the fumarate/succinate antiport (Fig. 2B).
COORDINATION OF l-ASPARTATE AND C4-DC METABOLISM BY C4-DCS, CATABOLITE CONTROL, RESPIRATION, AND AMINO ACID AVAILABILITY
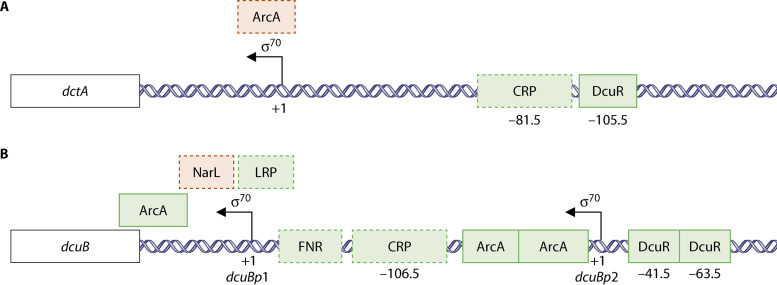
Transcriptional regulation by C4-DCs is the result of direct regulation by the DcuS-DcuR two-component system (8, 10, 11, 74). DcuS-DcuR-regulated genes encode proteins catalyzing uptake and initial catabolic steps of C4-DC catabolism (DctA, DcuB, FumB, and FrdABCD proteins). Most DcuS-DcuR-regulated genes are subject to multiple regulation, including FNR for aerobic regulation, NarX-NarL for nitrate regulation, cAMP-CRP for catabolite control, and Lrp responding to amino acids. Regulation of frdA, dcuB, and dctA by electron acceptors O2, nitrate, and C4-DCs by FNR, NarX-NarL, and DcuS-DcuR has been discussed earlier (10, 11, 50, 66, 75, 76), whereas regulation by cAMP-CRP and Lrp has been analyzed more recently (11, 20, 77). Many proteins that display altered levels in response to fumarate are not members of the DcuS-DcuR regulon but are subject to catabolite regulation by cAMP-CRP (77, 78). This includes proteins of the citric acid cycle and associated pathways, proteins involved in motility and chemotaxis under anaerobic conditions, and oxidative stress (77, 78). Cellular cAMP levels are known to increase during growth on low-quality carbon and energy sources, such as C4-DCs and acetate (79–81), which has been related to the fumarate effect on the expression of genes that are not under direct DcuS-DcuR control (77). Genes regulated by DcuS-DcuR are apparently often under the control of cAMP-CRP or FNR also, as shown for the dctA and dcuB promoters in Fig. 4. Transcriptional activation by cAMP-CRP and FNR is related to DNA-bending of the promoter regions (82, 83). It has been suggested that regulation by DcuR requires DNA-bending by cAMP-CRP to induce expression in response to C4-DCs via DcuS-DcuR (81).
FIG 4.
Promoter regions of dctA (A) and dcuB (B) and binding sites for transcriptional regulators DcuR, cAMP-CRP, FNR, ArcA, NarL and Lrp. The binding sites have been determined experimentally (solid line) (101–103) or by the presence of consensus sites (broken lines). Transcriptional regulators exerting positive (green) or negative (red) regulation on the promoter are annotated. Numbering gives the position relative to the transcriptional start sites of the promoters. The location of the binding sites for NarL and Lrp at dcuB have not been identified.
Growth on C4-dicarboxylates also requires gluconeogenesis for the synthesis of the glycolytic substrates and cell components derived from these. The gluconeogenic switch is known for the transition from glucose to acetate-grown E. coli (84–86). A similar switch and lower growth rates are observed for growth on C4-DCs, with an increase in all TCA cycle enzymes, the pyruvate bypass malic enzyme MaeB, and PEP-carboxykinase PckA (77). In the same way, the enzymes for the degradation of amino acids and fatty acids are increased to feed the TCA cycle (77).
The dcuB and frdA genes are targets for regulation by the transcriptional regulator Lrp (20), and the promoter regions of dcuB (Fig. 4) and frdA contain putative Lrp binding sites. Lrp is a global transcriptional regulator that responds to l-leucine and controls the expression of about 10% of E. coli genes; it is presumed to function by interaction with other regulators (87, 88). Mammalian intestine is an amino acid-rich environment (20, 73) containing almost all proteinogenic amino acids. The regulation of dcuB and frdA by Lrp is suggested to coordinate the utilization of l-aspartate as a source for fumarate respiration, for ammonium, and for degradation and feeding into the citric acid cycle.
SUCCINATE AND DcuB AS TRIGGERS FOR INTRACELLULAR INFECTION AND HOST-BACTERIUM SIGNALING
Intestinal colonization by enteric bacteria is established in two main steps—initial growth and growth in the inflamed intestine. Commensal E. coli grows, in contrast to S. Typhimurium or pathogenic E. coli strains, without initiation of inflammation. Inflammation drastically alters the intestinal environment and causes the release of host-derived electron acceptors, such as oxygen, nitrate, and tetrathionate (17, 64, 89). Electron acceptors promote the blooming of enterobacteria by conveying a growth advantage over the resident microbiota. Under anaerobic conditions in the normal intestine, the TCA cycle is repressed, but the reductive branch leading from oxaloacetate to succinate is active (19, 38), which is important for fumarate respiration and initial growth of E. coli and S. Typhimurium. The oxidative conditions in an inflamed gut, however, allow expression of the complete TCA cycle, which enables the utilization of the microbiota-derived fermentation product succinate as a carbon source (23). Bacteroides strains are the major succinate producers in the microbiota.
Apart from its benefit for efficient colonization of S. Typhimurium in an inflamed intestine, succinate is an activation signal for virulence of S. Typhimurium (25). Uptake of host succinate induces Salmonella pathogenicity island 2 (SPI-2) and antimicrobial resistance, which is vital for intracellular survival in macrophages. The response depends on the presence of DcuB, suggesting that DcuB is involved in the uptake or sensing of succinate (25). SPI-2 is required to translocate effector proteins from vacuolar-resident bacteria into host cells (90). In contrast, SPI-1 is essential for triggering gastrointestinal diseases, whereas it is dispensable for systemic infections (90). Therefore, in addition to the role of C4-DCs as substrates for initial growth of E. coli and S. Typhimurium in the intestine, succinate appears to function as a trigger inducing survival of the bacteria in the host cell.
Besides its role in bacteria in the activation of virulence factors and as a nutrient for colonization (23, 24), succinate is an important signaling molecule for the host. Accumulation of succinate in the host cytosol affects posttranslational modification by succinylation and activates inflammatory programs, epigenetic regulation, and ROS production (26–28). In addition, microbiota-derived succinate is used for gluconeogenesis (91–93) and thermogenesis (94, 95) by luminal host cells. Moreover, higher levels of circulating succinate have been associated with obesity and gut dysbiosis disorders. The gut microbiota is the predominant producer of luminal succinate (96–99). Understanding the role and control of bacterial succinate production could be a starting point for the development of probiotic interventions to modulate gut-derived succinate and to target obesity-related diseases (95).
CONCLUSION
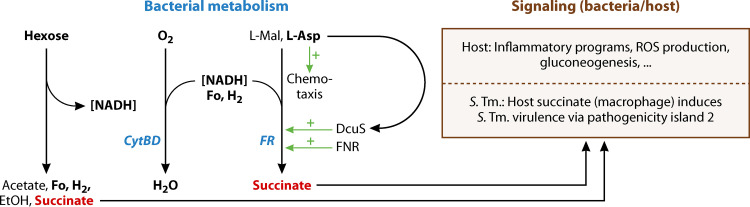
Fumarate respiration, whether using endogenously produced fumarate from hexose fermentation or consuming exogenously-supplied C4-DCs, was found to promote initial growth and colonization of the mammalian gut by intestinal bacteria. The significance of C4-DCs contrasts their rather low levels found in the intestine compared to sugars and sugar derivatives. It is suggested that a major role of endogenous and exogenous fumarate respiration is to provide a means for redox balancing under anaerobic conditions (Fig. 5). For the same reason, microaerobic respiration might be important for efficient colonization of the intestine by E. coli.
FIG 5.
C4-dicarboxylates as substrates or products of metabolism, and as signaling molecules for host/microbiota interaction in the intestine. Hexose fermentation, fumarate respiration (FR) and microaerobic respiration run in parallel under the microaerobic conditions of the intestine. The intestinal C4-DCs (black) serve as stimuli of the DcuS regulated metabolism of enteric bacteria, and of chemotaxis by Tar. Succinate produced by the enteric bacteria (red) or other microbiota is used for signaling or for communication with host cell, and succinate of host cells (macrophages) stimulates virulence and pathogenicity of S. Typhimurium. See the text for details. CytBD, microaerobic Cyt bd oxygen reductase; Fo, formate; ROS, reactive oxygen species; other abbreviations as in Fig. 1 to 4.
C4-DCs serve as important stimuli for regulating metabolism and physiology of enteric bacteria, using the two-component system DcuS-DcuR and the chemotaxis receptor Tar for perception (Fig. 5). Succinate produced by the microbiota also represents an important signaling molecule for host-microbiota interaction and a nutrient for host cells that may be involved in intestinal dysbiosis disorders (Fig. 5). On the other hand, succinate of host cells, such as macrophages, stimulates virulence and pathogenicity of S. Typhimurium. Identifying these roles and functions will open up new avenues for understanding and controlling host-microbiota interaction.
ACKNOWLEDGMENTS
We are grateful to Deutsche Forschungsgemeinschaft for funding (DFG UN 49/19-1 and 49/21-1).
Contributor Information
Gottfried Unden, Email: unden@uni-mainz.de.
Laurie E. Comstock, Duchossois Family Institute
REFERENCES
- 1.Mayer C, Boos W. 29 March 2005, posting date. Hexose/pentose and hexitol/pentitol metabolism. EcoSal Plus 2013 10.1128/ecosalplus.3.4.1. [DOI] [Google Scholar]
- 2.Unden G, Bongaerts J. 1997. Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta Bioenerg 1320:217–234. 10.1016/S0005-2728(97)00034-0. [DOI] [PubMed] [Google Scholar]
- 3.Sawers RG, Clark DP. 27 July 2004, posting date. Fermentative pyruvate and acetyl-coenzyme A metabolism. EcoSal Plus 2013 10.1128/ecosalplus.3.5.3. [DOI] [PubMed] [Google Scholar]
- 4.Cronan JE, Laporte D. 11 September 2005, posting date. Tricarboxylic acid cycle and glyoxylate bypass. EcoSal Plus 2013 10.1128/ecosalplus.3.5.2. [DOI] [PubMed] [Google Scholar]
- 5.Vaughn RH, Marsh GL, Stadtman TC, Cantino BC. 1946. Decomposition of tartrates by the coliform bacteria. J Bacteriol 52:311–325. 10.1128/jb.52.3.311-325.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reaney SK, Begg C, Bungard SJ, Guest JR. 1993. Identification of the l-tartrate dehydratase genes (ttdA and ttdB) of Escherichia coli and evolutionary relationship with the class I fumarase genes. J Gen Microbiol 139:1523–1530. 10.1099/00221287-139-7-1523. [DOI] [PubMed] [Google Scholar]
- 7.Kay WW, Kornberg HL. 1971. The uptake of C4‐dicarboxylic acids by Escherichia coli. Eur J Biochem 18:274–281. 10.1111/j.1432-1033.1971.tb01240.x. [DOI] [PubMed] [Google Scholar]
- 8.Unden G, Strecker A, Kleefeld A, Kim OB. 2016. C4-dicarboxylate utilization in aerobic and anaerobic growth. EcoSal Plus 7. 10.1128/ecosalplus.ESP-0021-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macy J, Kulla H, Gottschalk G. 1976. H2-dependent anaerobic growth of Escherichia coli on l-malate: succinate formation. J Bacteriol 125:423–428. 10.1128/jb.125.2.423-428.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zientz E, Bongaerts J, Unden G. 1998. Fumarate regulation of gene expression in Escherichia coli by the DcuSR (dcuSR Genes) two-component regulatory system. J Bacteriol 180:5421–5425. 10.1128/JB.180.20.5421-5425.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golby P, Davies S, Kelly DJ, Guest JR, Andrews SC. 1999. Identification and characterization of a two-component sensor-kinase and response-regulator system (DcuS-DcuR) controlling gene expression in response to C4-dicarboxylates in Escherichia coli. J Bacteriol 181:1238–1248. 10.1128/JB.181.4.1238-1248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guest JR. 1979. Anaerobic growth of Escherichia coli K12 with fumarate as terminal electron acceptor: genetic studies with menaquinone and fluoroacetate-resistant mutants. J Gen Microbiol 115:259–271. 10.1099/00221287-115-2-259. [DOI] [PubMed] [Google Scholar]
- 13.Kröger A, Biel S, Simon J, Gross R, Unden G, Lancaster CD. 2002. Fumarate respiration of Wolinella succinogenes: enzymology, energetics and coupling mechanism. Biochim Biophys Acta Bioenerg 1553:23–38. 10.1016/S0005-2728(01)00234-1. [DOI] [PubMed] [Google Scholar]
- 14.Cole ST, Condon C, Lemire BD, Weiner JH. 1985. Molecular biology, biochemistry and bionergetics of fumarate reductase, a complex membrane-bound iron-sulfur flavoenzyme of Escherichia coli. Biochim Biophys Acta Rev Bioenerg 811:381–403. 10.1016/0304-4173(85)90008-4. [DOI] [PubMed] [Google Scholar]
- 15.Chang D-E, Smalley DJ, Tucker DL, Leatham MP, Norris WE, Stevenson SJ, Anderson AB, Grissom JE, Laux DC, Cohen PS, Conway T. 2004. Carbon nutrition of Escherichia coli in the mouse intestine. Proc Natl Acad Sci USA 101:7427–7432. 10.1073/pnas.0307888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabich AJ, Jones SA, Chowdhury FZ, Cernosek A, Anderson A, Smalley D, McHargue JW, Hightower GA, Smith JT, Autieri SM, Leatham MP, Lins JJ, Allen RL, Laux DC, Cohen PS, Conway T. 2008. Comparison of carbon nutrition for pathogenic and commensal Escherichia coli strains in the mouse intestine. Infect Immun 76:1143–1152. 10.1128/IAI.01386-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones SA, Chowdhury FZ, Fabich AJ, Anderson A, Schreiner DM, House AL, Autieri SM, Leatham MP, Lins JJ, Jorgensen M, Cohen PS, Conway T. 2007. Respiration of Escherichia coli in the mouse intestine. Infect Immun 75:4891–4899. 10.1128/IAI.00484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones SA, Gibson T, Maltby RC, Chowdhury FZ, Stewart V, Cohen PS, Conway T. 2011. Anaerobic respiration of Escherichia coli in the mouse intestine. Infect Immun 79:4218–4226. 10.1128/IAI.05395-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen BD, Cuenca M, Hartl J, Gül E, Bauer R, Meile S, Rüthi J, Margot C, Heeb L, Besser F, Escriva PP, Fetz C, Furter M, Laganenka L, Keller P, Fuchs L, Christen M, Porwollik S, McClelland M, Vorholt JA, Sauer U, Sunagawa S, Christen B, Hardt W-D. 2020. Import of aspartate and malate by DcuABC drives H2/fumarate respiration to promote initial Salmonella gut-lumen colonization in mice. Cell Host Microbe 27:922–936. 10.1016/j.chom.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schubert C, Winter MG, Ebert-Jung A, Nagel-Wolfrum K, Kierszniowska S, Schramm T, Link H, Winter SE, Unden G. 2021. C4-dicarboxylates and l-aspartate utilization by Escherichia coli K-12 in the mouse intestine: l-aspartate as a major substrate for fumarate respiration and as a nitrogen source. Environ Microbiol 23:2564–2577. 10.1111/1462-2920.15478. [DOI] [PubMed] [Google Scholar]
- 21.Strecker A, Schubert C, Zedler S, Steinmetz P, Unden G. 2018. DcuA of aerobically grown Escherichia coli serves as a nitrogen shuttle (l‐aspartate/fumarate) for nitrogen uptake. Mol Microbiol 109:801–811. 10.1111/mmi.14074. [DOI] [PubMed] [Google Scholar]
- 22.Schubert C, Zedler S, Strecker A, Unden G. 2021. l-Aspartate as a high-quality nitrogen source in Escherichia coli: regulation of L-aspartase by the nitrogen regulatory system and interaction of L-aspartase with GlnB. Mol Microbiol 115:526–538. 10.1111/mmi.14620. [DOI] [PubMed] [Google Scholar]
- 23.Spiga L, Winter MG, Furtado de Carvalho T, Zhu W, Hughes ER, Gillis CC, Behrendt CL, Kim J, Chessa D, Andrews-Polymenis HL, Beiting DP, Santos RL, Hooper LV, Winter SE. 2017. An oxidative central metabolism enables Salmonella to utilize microbiota-derived succinate. Cell Host Microbe 22:291–301. 10.1016/j.chom.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curtis MM, Hu Z, Klimko C, Narayanan S, Deberardinis R, Sperandio V. 2014. The gut commensal Bacteroides thetaiotaomicron exacerbates enteric infection through modification of the metabolic landscape. Cell Host Microbe 16:759–769. 10.1016/j.chom.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg G, Yehezkel D, Hoffman D, Mattioli CC, Fremder M, Ben-Arosh H, Vainman L, Nissani N, Hen-Avivi S, Brenner S, Itkin M, Malitsky S, Ohana E, Ben-Moshe NB, Avraham R. 2021. Host succinate is an activation signal for Salmonella virulence during intracellular infection. Science 371:400–405. 10.1126/science.aba8026. [DOI] [PubMed] [Google Scholar]
- 26.Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, Eyassu F, Shirley R, Hu C-H, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa ASH, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, Murphy MP. 2014. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515:431–435. 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O’Neill LAJ. 2013. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496:238–242. 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy MP, O'Neill LAJ. 2018. Krebs cycle reimagined: the emerging roles of succinate and itaconate as signal transducers. Cell 174:780–784. 10.1016/j.cell.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 29.Gunsalus RP. 1992. Control of electron flow in Escherichia coli: coordinated transcription of respiratory pathway genes. J Bacteriol 174:7069–7074. 10.1128/jb.174.22.7069-7074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomasiak TM, Cecchini G, Iverson TM. 2007. Succinate as donor; fumarate as acceptor. EcoSal Plus 2. 10.1128/ecosal.3.2.6. [DOI] [PubMed] [Google Scholar]
- 31.Pinske C, Sawers RG. 4 October 2016, posting date. Anaerobic formate and hydrogen metabolism. EcoSal Plus 2016 10.1128/ecosalplus.ESP-0011-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stolpe S, Friedrich T. 2004. The Escherichia coli NADH: ubiquinone oxidoreductase (complex I) is a primary proton pump but may be capable of secondary sodium antiport. J Biol Chem 279:18377–18383. 10.1074/jbc.M311242200. [DOI] [PubMed] [Google Scholar]
- 33.Unden G, Steinmetz PA, Degreif-Dünnwald P. 18 July 2014, posting date. The aerobic and anaerobic respiratory chain of Escherichia coli and Salmonella enterica: enzymes and energetics. EcoSal Plus 2014 10.1128/ecosalplus.ESP-0005-2013. [DOI] [PubMed] [Google Scholar]
- 34.Lubek D, Simon AH, Pinske C. 2019. Amino acid variants of the HybB membrane subunit of Escherichia coli [NiFe]‐hydrogenase‐2 support a role in proton transfer. FEBS Lett 593:2194–2203. 10.1002/1873-3468.13514. [DOI] [PubMed] [Google Scholar]
- 35.Pinske C. 2019. Bioenergetic aspects of archaeal and bacterial hydrogen metabolism. Adv Microb Physiol 74:487–514. 10.1016/bs.ampbs.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Thimann KV. 1955. The life of bacteria. Macmillan, New York, NY. [Google Scholar]
- 37.Woods WA. 1961. Fermentation of carbohydrates and related compounds, p 59–149, In Gunsalus IC, Stanier RY (ed), The bacteria, vol 1. Academic Press, New York, NY. [Google Scholar]
- 38.Guest JR. 1992. Oxygen-regulated gene expression in Escherichia coli. J Gen Microbiol 138:2253–2263. 10.1099/00221287-138-11-2253. [DOI] [PubMed] [Google Scholar]
- 39.Six S, Andrews SC, Unden G, Guest JR. 1994. Escherichia coli possesses two homologous anaerobic C4-dicarboxylate membrane transporters (DcuA and DcuB) distinct from the aerobic dicarboxylate transport system (Dct). J Bacteriol 176:6470–6478. 10.1128/jb.176.21.6470-6478.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engel P, Krämer R, Unden G. 1994. Transport of C4‐dicarboxylates by anaerobically grown Escherichia coli. Eur J Biochem 222:605–614. 10.1111/j.1432-1033.1994.tb18903.x. [DOI] [PubMed] [Google Scholar]
- 41.Zientz E, Six S, Unden G. 1996. Identification of a third secondary carrier (DcuC) for anaerobic C4-dicarboxylate transport in Escherichia coli: roles of the three Dcu carriers in uptake and exchange. J Bacteriol 178:7241–7247. 10.1128/jb.178.24.7241-7247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zientz E, Janausch IG, Six S, Unden G. 1999. Functioning of DcuC as the C4-dicarboxylate carrier during glucose fermentation by Escherichia coli. J Bacteriol 181:3716–3720. 10.1128/JB.181.12.3716-3720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schubert C, Unden G. 2021. C4-dicarboxylate metabolons: interaction of C4 -dicarboxylate transporters of Escherichia coli with cytosolic enzymes and regulators. bioRxiv 10.1101/2021.03.01.433382. [DOI] [PubMed]
- 44.Kim OB, Lux S, Unden G. 2007. Anaerobic growth of Escherichia coli on D-tartrate depends on the fumarate carrier DcuB and fumarase, rather than the l-tartrate carrier TtdT and l-tartrate dehydratase. Arch Microbiol 188:583–589. 10.1007/s00203-007-0279-9. [DOI] [PubMed] [Google Scholar]
- 45.Kim OB, Unden G. 2007. The l-tartrate/succinate antiporter TtdT (YgjE) of l-tartrate fermentation in Escherichia coli. J Bacteriol 189:1597–1603. 10.1128/JB.01402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lütgens M, Gottschalk G. 1980. Why a co-substrate is required for anaerobic growth of Escherichia coli on citrate. J Gen Microbiol 119:63–70. 10.1099/00221287-119-1-63. [DOI] [PubMed] [Google Scholar]
- 47.Pos KM, Dimroth P, Bott M. 1998. The Escherichia coli citrate carrier CitT: a member of a novel eubacterial transporter family related to the 2-oxoglutarate/malate translocator from spinach chloroplasts. J Bacteriol 180:4160–4165. 10.1128/JB.180.16.4160-4165.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woehlke G, Dimroth P. 1994. Anaerobic growth of Salmonella Typhimurium on L(+)- and D(+)-tartrate involves an oxaloacetate decarboxylase Na+ pump. Arch Microbiol 162:233–237. 10.1007/s002030050130. [DOI] [PubMed] [Google Scholar]
- 49.Dimroth P. 6 July 2004, posting date. Molecular basis for bacterial growth on citrate or malonate. EcoSal Plus 2013 10.1128/ecosalplus.3.4.6. [DOI] [PubMed] [Google Scholar]
- 50.Golby P, Kelly DJ, Guest JR, Andrews SC. 1998. Transcriptional regulation and organization of the dcuA and dcuB genes, encoding homologous anaerobic C4-dicarboxylate transporters in Escherichia coli. J Bacteriol 180:6586–6596. 10.1128/JB.180.24.6586-6596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maier L, Vyas R, Cordova CD, Lindsay H, Schmidt TSB, Brugiroux S, Periaswamy B, Bauer R, Sturm A, Schreiber F, von Mering C, Robinson MD, Stecher B, Hardt W-D. 2013. Microbiota-derived hydrogen fuels Salmonella Typhimurium invasion of the gut ecosystem. Cell Host Microbe 14:641–651. 10.1016/j.chom.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Hughes ER, Winter MG, da Silva LA, Muramatsu MK, Jimenez AG, Gillis CC, Spiga L, Chanin RB, Santos RL, Zhu W, Winter SE. 2021. Reshaping of bacterial molecular hydrogen metabolism contributes to the outgrowth of commensal E. coli during gut inflammation. Elife 10:e58609. 10.7554/eLife.58609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hughes ER, Winter MG, Duerkop BA, Spiga L, de Carvalho TF, Zhu W, Gillis CC, Büttner L, Smoot MP, Behrendt CL, Cherry S, Santos RL, Hooper LV, Winter SE. 2017. Microbial respiration and formate oxidation as metabolic signatures of inflammation-associated dysbiosis. Cell Host Microbe 21:208–219. 10.1016/j.chom.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ali MM, Newsom DL, González JF, Sabag-Daigle A, Stahl C, Steidley B, Dubena J, Dyszel JL, Smith JN, Dieye Y, Arsenescu R, Boyaka PN, Krakowka S, Romeo T, Behrman EJ, White P, Ahmer BMM. 2014. Fructose-asparagine is a primary nutrient during growth of Salmonella in the inflamed intestine. PLoS Pathog 10:e1004209. 10.1371/journal.ppat.1004209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pappalardo L, Janausch IG, Vijayan V, Zientz E, Junker J, Peti W, Zweckstetter M, Unden G, Griesinger C. 2003. The NMR structure of the sensory domain of the membranous two-component fumarate sensor (histidine protein kinase) DcuS of Escherichia coli. J Biol Chem 278:39185–39188. 10.1074/jbc.C300344200. [DOI] [PubMed] [Google Scholar]
- 56.Monzel C, Unden G. 2015. Transmembrane signaling in the sensor kinase DcuS of Escherichia coli: a long-range piston-type displacement of transmembrane helix 2. Proc Natl Acad Sci USA 112:11042–11047. 10.1073/pnas.1507217112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kneuper H, Janausch IG, Vijayan V, Zweckstetter M, Bock V, Griesinger C, Unden G. 2005. The nature of the stimulus and of the fumarate binding site of the fumarate sensor DcuS of Escherichia coli. J Biol Chem 280:20596–20603. 10.1074/jbc.M502015200. [DOI] [PubMed] [Google Scholar]
- 58.Clarke S, Koshland DE. 1979. Membrane receptors for aspartate and serine in bacterial chemotaxis. J Biol Chem 254:9695–9702. 10.1016/S0021-9258(19)83572-X. [DOI] [PubMed] [Google Scholar]
- 59.He G, Shankar RA, Chzhan M, Samouilov A, Kuppusamy P, Zweier JL. 1999. Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc Natl Acad Sci USA 96:4586–4591. 10.1073/pnas.96.8.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Becker S, Holighaus G, Gabrielczyk T, Unden G. 1996. O2 as the regulatory signal for FNR-dependent gene regulation in Escherichia coli. J Bacteriol 178:4515–4521. 10.1128/jb.178.15.4515-4521.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tseng C-P, Albrecht J, Gunsalus RP. 1996. Effect of microaerophilic cell growth conditions on expression of the aerobic (cyoABCDE and cydAB) and anaerobic (narGHJI, frdABCD, and dmsABC) respiratory pathway genes in Escherichia coli. J Bacteriol 178:1094–1098. 10.1128/jb.178.4.1094-1098.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fu H-A, Iuchi S, Lin EC. 1991. The requirement of ArcA and Fnr for peak expression of the cyd operon in Escherichia coli under microaerobic conditions. Mol Gen Genet 226:209–213. 10.1007/BF00273605. [DOI] [PubMed] [Google Scholar]
- 63.Lundberg J, Lundberg JM, Alving K, Hellström PM. 1994. Greatly increased luminal nitric oxide in ulcerative colitis. Lancet 344:1673–1674. 10.1016/s0140-6736(94)90460-x. [DOI] [PubMed] [Google Scholar]
- 64.Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, Popova IE, Parikh SJ, Adams LG, Tsolis RM, Stewart VJ, Bäumler AJ. 2013. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 339:708–711. 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Szabó C, Ischiropoulos H, Radi R. 2007. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 6:662–680. 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 66.Jones HM, Gunsalus RP. 1985. Transcription of the Escherichia coli fumarate reductase genes (frdABCD) and their coordinate regulation by oxygen, nitrate, and fumarate. J Bacteriol 164:1100–1109. 10.1128/jb.164.3.1100-1109.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cedar H, Schwartz JH. 1967. Localization of the two L-asparaginases in anaerobically grown Escherichia coli. J Biol Chem 242:3753–3755. 10.1016/S0021-9258(18)95875-8. [DOI] [PubMed] [Google Scholar]
- 68.Willis RC, Woolfolk CA. 1974. Asparagine utilization in Escherichia coli. J Bacteriol 118:231–241. 10.1128/jb.118.1.231-241.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sabag-Daigle A, Sengupta A, Blunk HM, Biswas PK, Cron MC, Bogard AJ, Behrman EJ, Gopalan V, Ahmer BMM. 2017. Salmonella FraE, an asparaginase homolog, contributes to fructose-asparagine but not asparagine utilization. J Bacteriol 199:e00330-17. 10.1128/JB.00330-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Heeswijk WC, Westerhoff HV, Boogerd FC. 2013. Nitrogen assimilation in Escherichia coli: putting molecular data into a systems perspective. Microbiol Mol Biol Rev 77:628–695. 10.1128/MMBR.00025-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Michal G. 1999. Biochemical pathways. Spektrum Akad, Heidelberg, Germany. [Google Scholar]
- 72.Huergo LF, Chandra G, Merrick M. 2013. PII signal transduction proteins: nitrogen regulation and beyond. FEMS Microbiol Rev 37:251–283. 10.1111/j.1574-6976.2012.00351.x. [DOI] [PubMed] [Google Scholar]
- 73.Bertin Y, Segura A, Jubelin G, Dunière L, Durand A, Forano E. 2018. Aspartate metabolism is involved in the maintenance of enterohaemorrhagic Escherichia coli O157: H7 in bovine intestinal content. Environ Microbiol 20:4473–4485. 10.1111/1462-2920.14380. [DOI] [PubMed] [Google Scholar]
- 74.Janausch IG, Zientz E, Tran QH, Kröger A, Unden G. 2002. C4-dicarboxylate carriers and sensors in bacteria. Biochim Biophys Acta Bioenerg 1553:39–56. 10.1016/S0005-2728(01)00233-X. [DOI] [PubMed] [Google Scholar]
- 75.Jones HM, Gunsalus RP. 1987. Regulation of Escherichia coli fumarate reductase (frdABCD) operon expression by respiratory electron acceptors and the fnr gene product. J Bacteriol 169:3340–3349. 10.1128/jb.169.7.3340-3349.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davies SJ, Golby P, Omrani D, Broad SA, Harrington VL, Guest JR, Kelly DJ, Andrews SC. 1999. Inactivation and regulation of the aerobic C4-dicarboxylate transport (dctA) gene of Escherichia coli. J Bacteriol 181:5624–5635. 10.1128/JB.181.18.5624-5635.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Surmann K, Stopp M, Wörner S, Dhople VM, Völker U, Unden G, Hammer E. 2020. Fumarate dependent protein composition under aerobic and anaerobic growth conditions in Escherichia coli. J Proteomics 212:103583. 10.1016/j.jprot.2019.103583. [DOI] [PubMed] [Google Scholar]
- 78.Deutscher J, Francke C, Postma PW. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev 70:939–1031. 10.1128/MMBR.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bennett BD, Kimball EH, Gao M, Osterhout R, van Dien SJ, Rabinowitz JD. 2009. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat Chem Biol 5:593–599. 10.1038/nchembio.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hogema BM, Arents JC, Bader R, Eijkemans K, Yoshida H, Takahashi H, Aiba H, Postma PW. 1998. Inducer exclusion in Escherichia coli by non-PTS substrates: the role of the PEP to pyruvate ratio in determining the phosphorylation state of enzyme IIAGlc. Mol Microbiol 30:487–498. 10.1046/j.1365-2958.1998.01053.x. [DOI] [PubMed] [Google Scholar]
- 81.Schubert C, Unden G. 2021. Regulation of dctA and DctA by cAMP-CRP and EIIAGlc at the transcriptional and post-translational levels in E. coli: consequences for aerobic uptake and metabolism of C4-dicarboxylates. bioRxiv 10.1101/2021.12.01.470772. [DOI]
- 82.Ebright RH, Ebright YW, Gunasekera A. 1989. Consensus DNA site for the Escherichia coli catabolite gene activator protein (CAP): CAP exhibits a 450-fold higher affinity for the consensus DNA site than for the E. coli lac DNA site. Nucleic Acids Res 17:10295–10305. 10.1093/nar/17.24.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parkinson G, Wilson C, Gunasekera A, Ebright YW, Ebright RH, Ebright RE, Berman HM. 1996. Structure of the CAP-DNA complex at 2.5 Å resolution: a complete picture of the protein-DNA interface. J Mol Biol 260:395–408. 10.1006/jmbi.1996.0409. [DOI] [PubMed] [Google Scholar]
- 84.Oh M-K, Rohlin L, Kao KC, Liao JC. 2002. Global expression profiling of acetate-grown Escherichia coli. J Biol Chem 277:13175–13183. 10.1074/jbc.M110809200. [DOI] [PubMed] [Google Scholar]
- 85.Kao KC, Yang Y-L, Boscolo R, Sabatti C, Roychowdhury V, Liao JC. 2004. Transcriptome-based determination of multiple transcription regulator activities in Escherichia coli by using network component analysis. Proc Natl Acad Sci USA 101:641–646. 10.1073/pnas.0305287101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kao KC, Tran LM, Liao JC. 2005. A global regulatory role of gluconeogenic genes in Escherichia coli revealed by transcriptome network analysis. J Biol Chem 280:36079–36087. 10.1074/jbc.M508202200. [DOI] [PubMed] [Google Scholar]
- 87.Hart BR, Blumenthal RM. 2011. Unexpected coregulator range for the global regulator Lrp of Escherichia coli and Proteus mirabilis. J Bacteriol 193:1054–1064. 10.1128/JB.01183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kroner GM, Wolfe MB, Freddolino PL. 2019. Escherichia coli Lrp regulates one-third of the genome via direct, cooperative, and indirect routes. J Bacteriol 201:e00411-18. 10.1128/JB.00411-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM, Roth JR, Bäumler AJ. 2010. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467:426–429. 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jennings E, Thurston TLM, Holden DW. 2017. Salmonella SPI-2 type III secretion system effectors: molecular mechanisms and physiological consequences. Cell Host Microbe 22:217–231. 10.1016/j.chom.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 91.de Vadder F, Kovatcheva-Datchary P, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. 2016. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab 24:151–157. 10.1016/j.cmet.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 92.Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, de Vadder F, Arora T, Hallen A, Martens E, Björck I, Bäckhed F. 2015. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab 22:971–982. 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 93.Wang K, Liao M, Zhou N, Bao L, Ma K, Zheng Z, Wang Y, Liu C, Wang W, Wang J, Liu S-J, Liu H. 2019. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep 26:222–235. 10.1016/j.celrep.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 94.Mills EL, Pierce KA, Jedrychowski MP, Garrity R, Winther S, Vidoni S, Yoneshiro T, Spinelli JB, Lu GZ, Kazak L, Banks AS, Haigis MC, Kajimura S, Murphy MP, Gygi SP, Clish CB, Chouchani ET. 2018. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 560:102–106. 10.1038/s41586-018-0353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fernández-Veledo S, Vendrell J. 2019. Gut microbiota-derived succinate: friend or foe in human metabolic diseases? Rev Endocr Metab Disord 20:439–447. 10.1007/s11154-019-09513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Faith JJ, Ahern PP, Ridaura VK, Cheng J, Gordon JI. 2014. Identifying gut microbe–host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci Transl Med 6:220ra11. 10.1126/scitranslmed.3008051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nagao-Kitamoto H, Shreiner AB, Gillilland IIM, Kitamoto S, Ishii C, Hirayama A, Kuffa P, El-Zaatari M, Grasberger H, Seekatz AM, Higgins PDR, Young VB, Fukuda S, Kao JY, Kamada N. 2016. Functional characterization of inflammatory bowel disease–associated gut dysbiosis in gnotobiotic mice. Cell Mol Gastroenterol Hepatol 2:468–481. 10.1016/j.jcmgh.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim YA, Keogh JB, Clifton PM. 2018. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr Res Rev 31:35–51. 10.1017/S095442241700018X. [DOI] [PubMed] [Google Scholar]
- 99.Serena C, Ceperuelo-Mallafré V, Keiran N, Queipo-Ortuño MI, Bernal R, Gomez-Huelgas R, Urpi-Sarda M, Sabater M, Pérez-Brocal V, Andrés-Lacueva C, Moya A, Tinahones FJ, Fernández-Real JM, Vendrell J, Fernández-Veledo S. 2018. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J 12:1642–1657. 10.1038/s41396-018-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bott M. 1997. Anaerobic citrate metabolism and its regulation in enterobacteria. Arch Microbiol 167:78–88. 10.1007/s002030050419. [DOI] [PubMed] [Google Scholar]
- 101.Abo-Amer AE, Munn J, Jackson K, Aktas M, Golby P, Kelly DJ, Andrews SC. 2004. DNA interaction and phosphotransfer of the C4-dicarboxylate-responsive DcuS-DcuR two-component regulatory system from Escherichia coli. J Bacteriol 186:1879–1889. 10.1128/JB.186.6.1879-1889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Federowicz S, Kim D, Ebrahim A, Lerman J, Nagarajan H, Cho BK, Zengler K, Palsson B. 2014. Determining the control circuitry of redox metabolism at the genome-scale. PLoS Genet 10:e1004264. 10.1371/journal.pgen.1004264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Scheu PD, Witan J, Rauschmeier M, Graf S, Liao YF, Ebert-Jung A, Basché T, Erker W, Unden G. 2012. CitA/CitB two-component system regulating citrate fermentation in Escherichia coli and its relation to the DcuS/DcuR system in vivo. J Bacteriol 194:636–645. 10.1128/JB.06345-11. [DOI] [PMC free article] [PubMed] [Google Scholar]