ABSTRACT
Antimicrobial resistance is one of the greatest global health challenges today. For over 3 decades, antibacterial discovery research and development have been focused on cell-based and target-based high-throughput assays. Target-based screens use diagnostic enzymatic reactions to look for molecules that can bind directly to and inhibit the target. Target-based screens are applied only to proteins that can be successfully expressed and purified and the activity of which can be effectively measured using a biochemical assay. Often the molecules found in these in vitro screens are not active in cells due to poor permeability or efflux. On the other hand, cell-based screens use whole cells and look for growth inhibition. These screens give higher numbers of hits than target-based assays and can simultaneously test many targets of one process or pathway in their physiological context. Both strategies have advantages and disadvantages when used separately. In the past 15 years, our increasing knowledge of bacterial physiology has led to the development of innovative and sophisticated technologies to perform high-throughput screening combining these two strategies and thus minimizing their disadvantages. In this review, we discuss recent examples of high-throughput approaches that used both target-based and whole-cell screening to find new antibacterials, the new insights they have provided, and how this knowledge can be applied to other in vivo-validated targets to develop new antimicrobials.
KEYWORDS: high-throughput screening, antibacterial development, target based, whole cell based, rational screens
INTRODUCTION
Antibacterial resistance is one of the major public health challenges of our time. The incidence of antibiotic-resistant infections is increasing, and these infections are often difficult to treat. The Centers for Disease Control and Prevention (CDC) estimates that each year in the United States at least 2.8 million people acquire an antibiotic-resistant infection, and more than 35,000 people lose their lives (1). According to the World Health Organization (WHO), about 700,000 people die each year due to drug-resistant infections. These drug-resistant diseases are projected to increase to 10 million deaths each year by 2050, and antimicrobial resistance could force up to 24 million people into extreme poverty by 2030 (2). In addition, the sustainability of our modern lifestyle is threatened because all of the advances of modern medicine, from cancer treatment to surgery, are at risk if we are unable to treat bacterial infections (3).
As of December 2020, there were only 43 antibiotics in the global clinical pipeline, with 292 diverse antibacterial agents in preclinical development. While there is optimism regarding these drugs, antibiotic innovation and discovery have experienced a gap in approved antibiotics since the middle 1980s, and no new classes of antibiotics have successfully made it into the clinic for almost 4 decades (3–5). Thus, antibiotic discovery has not kept pace with the rise in antibiotic resistance, and we are in great need of novel antibacterials with novel mechanisms of action (MOAs). The reader is referred to several excellent reviews about the formidable challenges that drug discovery represents (6–11).
In order to face this resistance crisis, efforts to improve drug discovery are necessary (2, 3). The scientific challenges to identify and develop new antimicrobials remain significant. One approach taken by academic and industrial teams in antibiotic discovery has been to develop innovative screening methods to identify new drugs in unique ways by taking advantage of the knowledge generated by many decades of basic research focused on bacterial physiology. Additional approaches to combat this resistance crisis have been previously reviewed elsewhere (6–8, 11–13).
HIGH-THROUGHPUT SCREENING: THE BASICS
One of the early stages in antibacterial research and discovery is screening and lead identification. This stage allows the identification of a molecule with an acceptable profile of antimicrobial and pharmaceutical properties to warrant testing in humans (11). High-throughput screening (HTS) has been the most common approach to identify antibacterials in the past 3 decades. Unlike early screening that utilized natural products as sources (14), HTS often uses thousands of synthetic chemicals to identify active molecules (hits) and to discover potential leads. It involves miniaturized and automated assays that can evaluate thousands of molecules in weeks rather than years. The use of 384-well and 1,536-well plate formats is standard for HTS and ultra-HTS (uHTS), respectively (15).
HTS assays can be generally divided into (i) biochemical screens (also referred to as target-based [TB] assays), (ii) cell-based assays (also referred to as classic or empirical screens and, with the use of microorganisms, also named whole-cell screens [WCSs] or whole-organism screens), and (iii) combined TB and cell-based assays (also referred to as phenotypic screens) (9, 11, 14, 15).
Biochemical screens use enzymes and in vitro activity assays to identify molecules that can bind directly and inhibit the target. Biochemical screens can be effectively applied only to well-behaved proteins and in most instances require prior in vivo validation of the target (in animal infection models) (11, 16). Often the molecules found in these screens are not active in cells due to efflux or poor membrane permeability. This is particularly the case for Gram-negative bacteria, in which the outer membrane is a difficult barrier for drugs to cross (9, 11). Biochemical screens tend to have more nuisance compounds, also known as pan-assay interference molecules (PAINS) (including molecules that interfere with assay signaling or form aggregates, as well as reactive compounds), and thus require extensive secondary assays to sort them out (Table 1) (17).
TABLE 1.
Advantages and disadvantages of HTS
| Screen type | Definition | Advantages | Disadvantages |
|---|---|---|---|
| Biochemical screens | In vitro assay to look for enzyme inhibition | More sensitivity; assay format can be simple | Only well-behaved targets (excluding membrane proteins); biological activity is not guaranteed; more nuisance compounds with nonspecific activity (PAINS) |
| WCSs (classic screens) | Bacterial assay to look for inhibitors of growth | Biological activity is guaranteed in the model organism; assay format can be simple | Target identification can take time; difficult to prioritize due to high hit rates (20–30%) |
| TB-WCSs | Bacterial assay to look for inhibition of desirable target(s) in a pathway | Biological activity is guaranteed in the model organism; assay format can be simple; on-target activity is expected; low hit rate, making it easier to focus on a few hits | Bacterial physiology must be well understood; requires a model organism with sufficient genetic tools |
WCSs use bacterial cells to identify molecules that inhibit growth and were predominantly used in early screening and antibiotic discovery. WCSs give higher hit rates (20 to 30%) than TB assays (18). Even so, WCSs largely guarantee biological activity and can simultaneously test many targets in their physiological context. However, they require secondary assays to eliminate nonspecific cytotoxic compounds (such as detergents and uncouplers) and to identify the target in order to proceed to optimization. In most cases, without an MOA, hits may not be developed into leads. WCSs are preferred for finding a lead compound since it is easier to identify the target for a compound than it is to improve the permeability of the compound, because the physicochemical and structural rules that govern bacterial cell wall permeability are highly complex (9, 11, 19) (Table 1).
TB-WCSs combine the previous two categories to generate more rational strategies to find new antibacterials and to minimize their disadvantages (Table 1). TB-WCSs integrate genetic and biochemical information to use specific phenotypes in order to identify inhibitors of a desirable target (14). TB-WCSs can be directed to identify inhibitors of either a single target or multiple targets that belong to a pathway (pathway-directed screens) (16, 20).
TB-WCSs have been used in antibacterial development for almost 6 decades with various degrees of throughput, and they emerged as a method for dereplication or elimination of already known antibiotics (14, 21). Initial work to develop TB-WCSs used spheroplast visualization, hypersusceptible mutants, β-lactamase induction, and sensitization of bacteria, among other approaches (14, 21). Pioneer strategies to sensitize bacteria involved gene silencing. This approach is based on the assumption that reduced levels of a target should affect cell growth and make the strain more susceptible to inhibitors specific for that particular target, either directly or indirectly through effects on the interaction network of the target. These proof-of-concept technologies included (i) deleting the chromosomal copy of the target while having a second copy of the target under a regulatable promoter (22) or (ii) expressing an antisense RNA to silence the target (23, 24). A condition with less inducer in the former case or with the induction of antisense RNA expression in the latter case should make the bacteria more susceptible to inhibitors (22–24). Access to a copy of the gene under an inducible promoter also allows testing of upregulation, which should render the bacteria less sensitive to the molecule if a hit is target specific (see Perspectives) (22). Screens that used downregulation of the target by antisense technology led to the identification of natural product inhibitors of the fatty acid synthesis enzyme FabF (25, 26), the small ribosomal protein RpsD (27), and type II topoisomerase (28) in Staphylococcus aureus and the chromosome partitioning protein ParA in Mycobacterium tuberculosis (29). For more about the history and earlier rational screening, the reader is referred to excellent reviews (14, 18, 21).
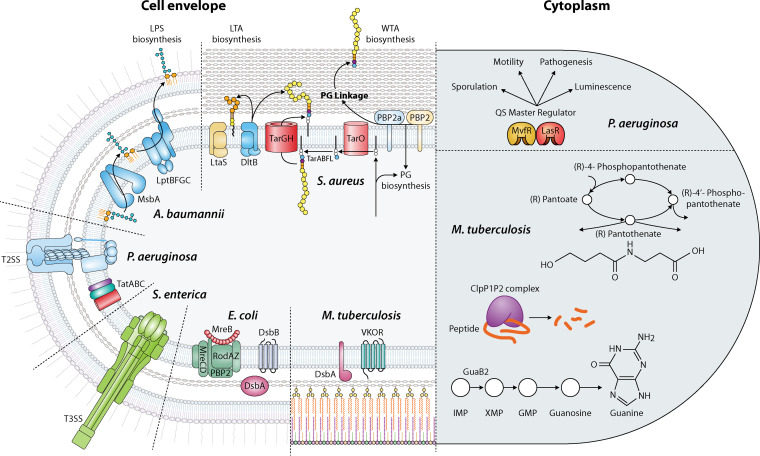
This review, rather than being comprehensive, discusses recent examples of TB-WCSs (≥384-well format) using chemical libraries in an array of bacterial physiological conditions, in order to illustrate the advantages of this category and the new insights the assays have provided. We also present perspectives on how such knowledge could be applied to other in vivo-validated targets to develop new antimicrobials and enable advances in their development. The technologies discussed below use parallel screening to identify molecules that differentially inhibit cells grown under two conditions by either genetically or chemically sensitizing cells to one target, by exploiting conditional lethality, or by using transcriptional and translational reporters. We have divided the discussion based on the target and its location, i.e., either cytoplasmic (Fig. 1, right) or in the cell envelope (Fig. 1, left).
FIG 1.
Targets used to develop TB and WCS. Cytoplasmic targets are represented on the right and cell envelope targets on the left for each organism. See text for further details of each pathway.
CYTOPLASMIC TARGETS
Targeting quorum sensing in Pseudomonas aeruginosa.
Quorum sensing (QS) is a cell-to-cell, population density-dependent communication system that is mediated via the production of and regulation by low-molecular-weight signaling molecules. QS is important for the development and maintenance of acute and chronic human infections, as well as the commonly observed antibiotic tolerance of many pathogenic bacteria. Two approaches have been developed to find inhibitors of QS master regulators in P. aeruginosa, using transcriptional fusions to either a fluorescent protein or a toxin to have fluorescence or growth as a readout, respectively.
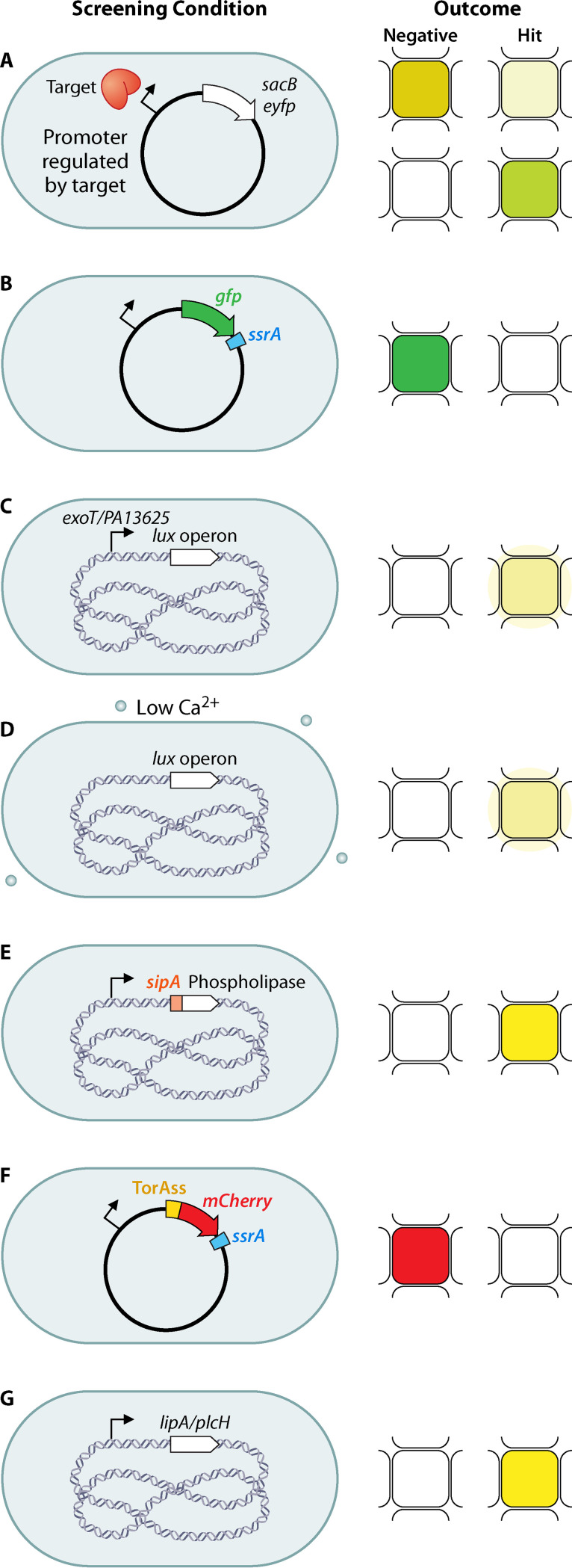
P. aeruginosa has three distinct QS systems mediated by cell-to-cell signals, including the acyl-homoserine lactones (AHLs), produced by the Las and Rhl systems, and the 4-hydroxy-2-alkylquinolines (HAQs), produced by the MvfR (PqsR) system (Fig. 1, right). Müh et al. (30) selected P. aeruginosa LasR as a target because LasR activates rhlR expression and inhibition of LasR should result in inhibition of the expression of both LasR- and RhlR-regulated genes. The HTS assay utilizes P. aeruginosa and nanowell technology (3,456 wells per plate). The P. aeruginosa reporter strain lacks the AHL synthases (LasI and RhlI) and thus does not produce AHLs. The strain also carries a reporter plasmid, which has the yellow fluorescent protein (yfp) under the control of a LasR-dependent rsaL promoter (highly inducible in the presence of N-3-oxo-dodecanoyl-homoserine lactone). The P. aeruginosa reporter strain is grown at a concentration of AHL that results in approximately half-maximal induction of yfp (Fig. 2a). Any compound that interferes with the AHL response should reduce the fluorescence and, since the yfp induction is half-maximal, only strong inhibitors are likely to be detected by lowering the endogenous signal about 30-fold. This technology identified 20 inhibitors of LasR (30).
FIG 2.
Conditions and possible outcomes of TB and single WCSs. (a) Transcriptional fusion of a toxin (top) or a fluorescent protein (bottom) to a promoter that is upregulated by a QS regulator. (b) Translational fusion of a fluorescent protein with the caseinolytic-protease-specific peptide SsrA. (c) Transcriptional fusion of the lux operon to promoters that are upregulated by inhibition of T3SS or Sec/T2SS. (d) Low calcium conditions induce T3SS and inhibit growth, which is detected as luminescence by having a chromosomal copy of the lux operon. (e) Translational fusion of the T3SS-secreted effector SipA to a phospholipase reporter. (f) Translational fusion of mCherry to a TorA signal peptide and SsrA peptide to secrete mCherry by the Tat pathway. (g) Monitoring native secretion of phospholipase with a chromogenic substrate. The cell shape is depicted as shown for simplicity, but shapes vary for the bacterial taxons discussed. Chromosomal DNA is depicted as blue DNA material in the center of the bacterial cell. Plasmid DNA is depicted as a circle. Pale mustard indicates no growth, dark mustard indicates growth, green and red indicate fluorescence, pale yellow indicates luminescence, dark yellow indicates positive phospholipase activity, and white indicates no fluorescence, no luminescence, or inactive phospholipase.
To target P. aeruginosa MvfR, a transcriptional regulator that directs the synthesis of 60 related low-molecular-weight HAQ molecules (Fig. 1, right), Starkey et al. (31) generated a P. aeruginosa reporter strain to detect inhibition of pqsA expression. The strain carries the pqsA promoter fused to the sacB gene. A noninhibitory compound results in bacterial death when sucrose is present in the culture medium, due to SacB-mediated conversion of the sugar to toxic levans in the periplasm, whereas compounds that inhibit pqsA promoter expression, and thus HAQ synthesis, permit bacterial growth (Fig. 2a). The inhibitors were verified for pqsA promoter repression using a pqsA-green fluorescent protein (GFP) reporter construct. This work allowed the identification of 17 compounds belonging to seven chemical families (31).
Identifying inhibitors of pantothenate biosynthesis in Mycobacterium tuberculosis.
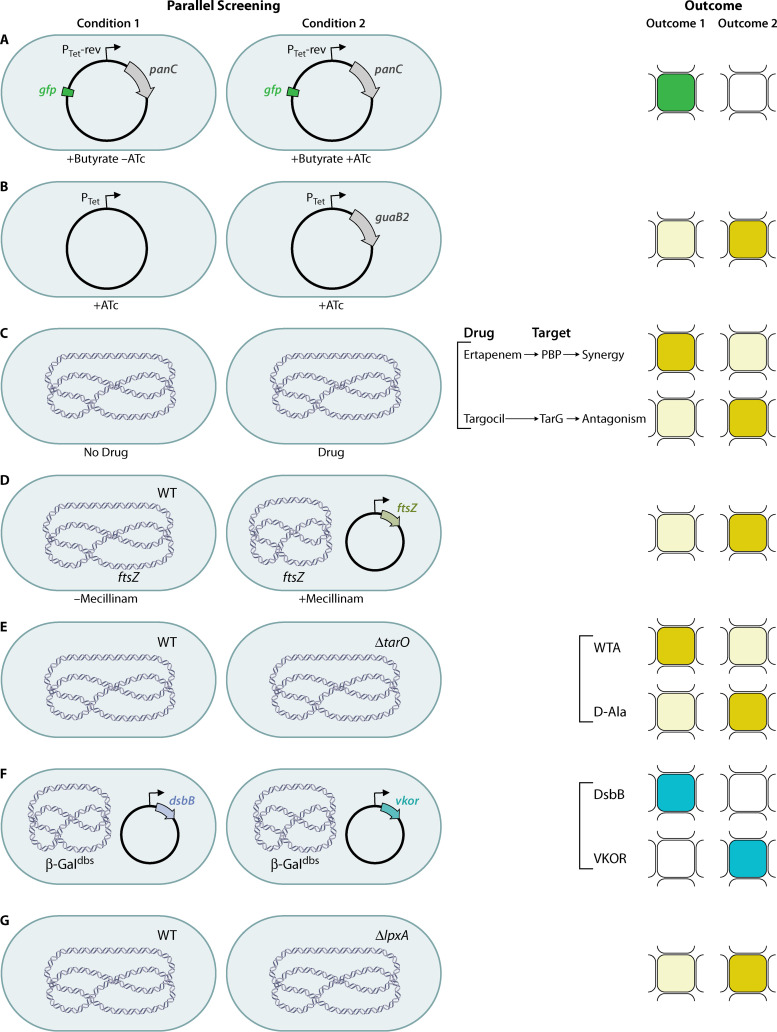
Pantothenate synthetase (panC) participates in the biosynthesis of pantothenate (vitamin B5), an essential precursor of coenzyme A (CoA) and acyl carrier protein (Fig. 1, right). PanC is essential for M. tuberculosis growth. Abrahams et al. (32) constructed a conditionally lethal mutant by replacing the native promoter of the panC gene with the tet promoter, while a reverse TetR regulator (harboring E15A, L17G, and L25V mutations that affect TetR allostery and turn anhydrotetracycline [ATc] into a corepressor) was expressed from a plasmid that also contained the other genes in the pan operon to prevent downstream effects. To make the assay quantitative, gfp was fused to a constitutive promoter to measure growth as fluorescence emission (Fig. 3a). Compounds at seven different concentrations were screened in parallel against a PanC conditional mutant grown on butyrate, either without (nonsensitized strain) or with (sensitized strain) ATc, to identify molecules to which the strain was specifically hypersensitive. Butyrate was selected as a fatty acid carbon source to increase the sensitivity of the screen, because it requires CoA-dependent β-oxidation to acetyl-CoA for growth. Replacing native promoters is unlikely to be equally applicable to all essential targets, since the promoter strength and its leakiness would have a major impact on developing an effective screen (32).
FIG 3.
Conditions and outcomes of TB and parallel WCSs. (a) Downregulation of PanC using a regulatable promoter and growth using a fatty acid as a carbon source to increase sensitivity; a fluorescent protein allows measurement of growth to find inhibitors of pantothenate biosynthesis. (b) Upregulation of GuaB2 to make bacteria more resistant to inhibitors of purine biosynthesis. (c) Sensitization of bacteria by adding a drug with a known target to find synergistic inhibitors (top) or antagonists (bottom). (d) Synthetic lethality assay to reverse the lethality of Rod inhibition by overexpression of FtsZ. WT, wild-type. (e) Synthetic lethality assay with a ΔtarO mutant to find inhibitors in late steps of WTA synthesis (top) or d-alanylation of LTA and WTA (bottom). (f) Comparison of two nonhomologous disulfide-bond-forming proteins that catalyze the same step of the pathway to find inhibitors of one (top) or the other (bottom) using β-Galdbs. (g) Synthetic lethality assay with a ΔlpxA mutant that is more resistant to inhibitors of late steps in LPS biogenesis. The cell shape is depicted as shown for simplicity, but shapes vary for the bacterial taxons discussed. Chromosomal DNA is depicted as blue DNA material in the center of the bacterial cell. Plasmid DNA is depicted as a circle. Pale mustard indicates no growth, dark mustard indicates growth, green indicates fluorescence, blue indicates active β-galactosidase, and white indicates no fluorescence or inactive β-galactosidase.
Technology to identify inhibitors of caseinolytic protease in M. tuberculosis.
Caseinolytic proteases are serine proteases that form a degradative complex involved in the removal of partially synthesized and misfolded proteins. The protease complex is composed of catalytic protease subunits (ClpP) and regulatory subunits (ATPases). The catalytic ClpP subunits form a degradative chamber in which proteolysis occurs, while the regulatory subunits recognize substrates and provide the energy for protein degradation. In M. tuberculosis, the catalytic chamber consists of two subunits, ClpP1 and ClpP2, which are both essential for the growth of M. tuberculosis in culture and in an animal model. Aborted translation products are tagged with a caseinolytic-protease-specific (SsrA) degradation peptide, which the caseinolytic protease recognizes to degrade (Fig. 1, right). Moreira et al. (19) used this peptide to find inhibitors of intracellular ClpP1P2 activity via accumulation of SsrA-tagged GFP. The rationale is that ClpP1P2 would degrade SsrA-GFP to background fluorescence levels, while an inhibitor of ClpP1P2 activity would block the degradation of tagged GFP, resulting in an increase in fluorescence (Fig. 2b). The screen uses Mycobacterium smegmatis with an episomal SsrA-tagged GFP gene placed under the control of a tetracycline-inducible promoter and a control strain carrying an untagged episomal GFP gene under the control of the same promoter. This screen identified one inhibitor of the protease (19).
Identifying inhibitors of purine biosynthesis in Mycobacterium bovis and M. tuberculosis.
IMP is a metabolic precursor in the biosynthesis of the purine nucleotides adenine and guanine. GMP requires the synthesis of XMP from IMP by IMP dehydrogenase (Fig. 1, right). In M. tuberculosis, there are three homologs of the IMP dehydrogenase, GuaB1, GuaB2, and GuaB3, among which GuaB2 is the only essential enzyme. This screen used upregulation rather than downregulation, which should render the bacteria less sensitive to the molecule. Wild-type M. bovis BCG and a strain overexpressing GuaB2 were used together with an ATP-dependent thermostable luciferase reporter assay to measure cell viability in a 1,536-well format (Fig. 3b). Known inhibitors of M. tuberculosis growth were tested against the two strains, and hits were identified based on a shift in the apparent inhibition, calculated as inhibition of the wild-type strain minus inhibition of M. bovis overexpressing guaB2. This study identified seven new chemical series that inhibit GuaB2 (33).
CELL ENVELOPE TARGETS
Targeting secretion systems in P. aeruginosa, Yersinia pestis, Salmonella enterica, and Acinetobacter baumannii.
Bacterial secretion is an essential and conserved mechanism of protein export. Proteins are translocated across the inner membrane by either the Sec or Tat (twin arginine translocation) pathways. In the periplasm, certain proteins are transported into the extracellular milieu by an outer membrane secretin (type 2 secretion system [T2SS]). This system is responsible for the secretion of numerous degradative enzymes and toxins that contribute to survival in the environment and in the mammalian hosts. In contrast, a type 3 secretion system (T3SS) is a multiprotein secretory mechanism that also facilitates the secretion and translocation of effector proteins (proteins that help in host invasion and immune evasion) from the bacterial cytoplasm directly into the mammalian cytosol (Fig. 1, left). Both T2SS and T3SS are conserved among multiple Gram-negative pathogens. Screens to find inhibitors of secretion used transcriptional fusions to monitor induction of the secretion system or translational fusions to monitor the secretion itself of the effector proteins.
One of the pioneer studies that developed a medium-throughput screen to identify inhibitors of T3SS in Yersinia pseudotuberculosis used a transcriptional luciferase reporter to monitor expression of the effector protein (yopE) as an indicator of T3SS function (34, 35). An analogous reporter was used in HTS to find inhibitors of T3SS in P. aeruginosa, with a transcriptional fusion of the lux operon to the promoter of the exoT effector (Fig. 2c). To eliminate molecules that inhibit luminescence by other mechanisms, the hits were tested against a strain that had the lux operon under a lac-regulated promoter. This screen identified five inhibitors of T3SS-mediated secretion and one inhibitor of T3SS-mediated translocation of effectors into mammalian cells (36). An alternative method, compared to the transcriptional reporter, used the observation that in Y. pestis low-calcium-concentration-dependent induction of T3SS is accompanied by the inhibition of growth; therefore, inhibition of T3SS would reverse this phenotype and allow growth. To prevent the loss of T3SS and a false-positive result in the screening, the T3SS-encoding megaplasmid was stably inserted into the chromosome of an avirulent Y. pestis strain that also expressed the lux operon, to measure growth by luminescence (Fig. 2d). Therefore, the screening worked by requiring at least a 2-fold increase in luminescence and led to the identification of four inhibitors of Y. pestis T3SS (37). Finally, Felise et al. (38) used a reporter of T3SS by fusing the effector SipA of S. enterica serovar Typhimurium with the signal sequence-less T3SS-secreted phospholipase of Y. enterocolitica, YplA (Fig. 2e). The screen uses S. enterica expressing the phospholipase reporter and a phospholipase substrate that produces a fluorescence signal upon cleavage. Hence, molecules that target secretion of the phospholipase were found by decreases in fluorescence. The screen yielded a potent inhibitor of the secretin-containing transmembrane complex (38).
The Tat pathway transports fully folded proteins and is required for optimal growth and virulence in many bacteria. Bageshwar et al. (39) developed an assay to find inhibitors of the Tat system in Escherichia coli using a fusion of the signal sequence of TorA to mCherry and SsrA in the carboxy-terminal region. This reporter guides mCherry-SsrA to the Tat system for export to the periplasm, and the C-terminal SsrA peptide promotes Clp-dependent degradation of the protein fusion if it remains in the cytoplasm. Due to degradation of the cytoplasmic fusion, the total mCherry fluorescence comes from the periplasmic mCherry-SsrA (Fig. 2f). Therefore, when the Tat machinery is inhibited, a decrease in fluorescence is observed. Although this screen identified seven inhibitors of Tat transport by collapsing the electrical field gradient of the proton motive force, unfortunately none of the inhibitors directly targeted the TatABC proteins (39).
To identify inhibitors of the Sec/T2SS in P. aeruginosa, Moir et al. (40) used a transcriptional fusion as a reporter of SecA inhibition. This type of reporter requires previous identification of promoters that are responsive to depletion of the target. The screen uses P. aeruginosa cells deleted of the secA gene and harboring a plasmid with a copy of the P. aeruginosa secA gene or the homologous Burkholderia pseudomallei secA gene. In addition, the strain contains a chromosomal transcriptional fusion of the lux operon to a regulatory element upstream of PA1365 (a gene of unknown function), which is upregulated when secA is depleted (Fig. 2c). Hence, inhibition of secretion results in upregulation of the lux operon and should result in growth inhibition, depending on which step was blocked. This screen allowed the identification of nine inhibitors of T2SS that were confirmed through secondary assays testing secretion of β-lactamase into the periplasm and elastase and phospholipase C into the extracellular medium (40). Three additional approaches used a native Tat-secreted substrate to identify inhibitors of the T2SS, phospholipase C (plcH), or lipase A (lipA) in P. aeruginosa (41, 42) or A. baumannii (43) (Fig. 2g). The lipase activity produced by cells is quantified by using the chromogenic substrate p-nitrophenyl-phosphorylcholine or p-nitrophenyl-myristate. Hence, inhibition of the T2SS displays low phospholipase activity, compared to conditions lacking a drug. Counterscreens using in vitro lipase assays are necessary for this type of screen, to eliminate inhibitors of the lipase itself. Using these approaches, two inhibitors of Tat (41) and one of T2SS (42) were identified.
Identifying inhibitors of peptidoglycan biogenesis in S. aureus and E. coli.
Methicillin-resistant S. aureus (MRSA) displays resistance to β-lactams by the cooperative function of penicillin-binding proteins (PBPs), i.e., PBP2A and PBP2. These enzymes provide transpeptidation and transglycosylation activities, respectively, required to assemble peptidoglycan (PG) precursors and to cross-link these polymers within the cell wall (Fig. 1, left). Transposon screens have identified genes involved in PG precursor biosynthesis and turnover to be required for maximal resistance by PBP2A. If these genes are targeted, then inhibitors can restore β-lactam susceptibility. Thus, to identify molecules that restore the activity of the carbapenem class of β-lactams against MRSA, Huber et al. (44) performed a whole-cell synergism-based HTS assay using the MRSA strain grown in the presence of a sub-MIC concentration of ertapenem. The rationale is that β-lactam potentiators should phenocopy their genetic inactivation and inhibit growth (Fig. 3c, top). This screen identified two indole-class potentiators of β-lactams (44). The team then used an antisense-induced strain sensitivity profiling assay that uses a xylose-inducible promoter to reduce expression of the cognate target, thus sensitizing the strain to the two potentiators (45). The profile with 245 essential genes led to the identification of one target, SAV1754, an uncharacterized gene product that is potentially involved in cell wall synthesis (44). This innovative approach demonstrated the ability to develop synergistic drugs and to identify the target which enabled the identification of a gene with a previously uncharacterized function.
The PG biogenesis complex in Gram-negative bacteria, also called the Rod system, includes five integral membrane proteins organized by filaments of the actin-like MreB protein. The complex is essential in E. coli and other rod-shaped bacteria and is required to elongate the cell wall and maintain a capsule-like rod shape (Fig. 1, left). Buss et al. (46) designed a pathway-directed screen to identify inhibitors of the Rod system (i.e., MreB, PBP2, MreC, MreD, RodA, and RodZ) that promote growth under special conditions but are lethal against wild-type bacteria. This is achieved by screening for molecules that suppress the toxic effect of amdinocillin (mecillinam) in a strain that overexpresses FtsZ. The rationale is that amdinocillin induces toxicity by blocking PBP2 and hence PG cross-linking, while increased expression of FtsZ in cells that have inactivated components of the Rod system compensates for the essentiality of these genes and results in viable but small spherical cells. Thus, blocking the Rod system in FtsZ-overexpressing cells would make cells viable when treated with amdinocillin (Fig. 3d). The screen included the identification of molecules that inhibited the growth of an E. coli strain with a sensitized outer membrane (i.e., lower expression levels of genes involved in the lipopolysaccharide [LPS] transport system, lptFG) to increase the number of lethal molecules identified. The hits were then tested for suppression of amdinocillin toxicity in the FtsZ-overexpressing strain, indicative of inhibition of the Rod system. Finally, the hits were tested for their ability to alter cell shape, another confirmation of Rod system inhibition. One class of molecules with similar structure were found to target MreB (46). This technology demonstrated a powerful strategy to reverse the traditional outcome of inhibitors of essential targets by utilizing conditions under which compounds that would typically block growth instead promote it.
Targeting wall teichoic acid biosynthesis in S. aureus.
Cell envelope polysaccharides are potential targets for new antibacterials; however, the discovery of inhibitors that block their synthesis had been hindered due to the difficulty of reconstituting the enzymatic machinery and obtaining the lipid substrates. The wall teichoic acid (WTA) biosynthetic pathway is not required for cell viability but is required to colonize host tissue and establish infections in animal models. WTAs from S. aureus are disaccharide-linked anionic polymers of poly(ribitol-phosphate) that are covalently attached to PG (Fig. 1, left). Swoboda and colleagues (20, 47) developed a pathway-directed HTS approach to identify small molecules that inhibit the WTA pathway, based on the synthetic essentiality of these genes. The first and second steps in the WTA synthesis pathway (tarO and tarA) are not essential and can be deleted. However, the downstream genes (tarBDFGHIJL) can be deleted only in a strain that cannot initiate polymer synthesis, i.e., in a ΔtarO strain. This synthetic essentiality is due to the fact that blocking a late step in WTA biosynthesis depletes lipid II, the PG precursor, which is synthesized on the same undecaprenyl phosphate carrier lipid as the WTA precursor. Therefore, by comparing the growth of a wild-type S. aureus strain and its isogenic ΔtarO strain in the presence of a chemical library, compounds that inhibit a conditionally essential enzyme in the WTA pathway can be identified (Fig. 3e, top). Those molecules are expected to inhibit growth of the wild-type strain but not of the ΔtarO strain, which cannot initiate polymer synthesis. This study led to the identification of an inhibitor (targocil) of TarG, the membrane component of the ABC transporter that exports WTAs to the cell surface (20, 47). WTA is a cell wall polymer of roughly equal abundance, compared to PG, and the activity of β-lactams against MRSA is restored by blocking WTA biogenesis. Thus, another group used an approach to find potentiators of β-lactams by using S. aureus wild-type and ΔtarO strains. Wang et al. screened a library of bioactive synthetic compounds against wild-type and ΔtarO strains in a 1,536-well plate format and found another class of TarG inhibitors that strongly synergize with the β-lactam imipenem (48).
Farha et al. used HTS to screen for molecules that antagonize the activity of a lethal concentration of the TarG inhibitor targocil, in order to identify inhibitors of different targets than the early steps of WTA synthesis, TarO or TarA, for which inhibitors had already been discovered (49). Hence, the selection of hits was based on molecules that allowed growth of S. aureus in the presence of targocil but prevented growth of the wild-type strain (Fig. 3c, bottom). The HTS identified 12 inhibitors and one of them, clomiphene, was found to inhibit UppS (through antisense RNA technology), blocking the synthesis of undecaprenyl phosphate, which acts as the lipid carrier in the synthesis of PG and WTA polymers, possibly because inhibition of UppS decreases the undecaprenyl phosphate pool available for WTA synthesis such that fewer WTA polymers are synthesized, thereby rendering targocil less effective (50).
Finally, by inverting the process of hit selection in the differential growth screening of wild-type and ΔtarO strains, Matano et al. (16) realized they could identify inhibitors of other pathways that are synthetically lethal to WTA depletion. The group focused on the same differential growth screen but this time selected compounds that killed the ΔtarO strain rather than the wild-type strain in order to identify inhibitors of d-alanylation of WTA, the lipoteichoic acid (LTA) pathway, or the GraRS/VraFG stress response pathway, to mention some (Fig. 3e, bottom). To distinguish inhibitors of the d-alanylation pathway, hits were tested against three additional mutants involved in the LTA synthesis pathway (ypfP, ltaS, and ORF_01050 mutants), based on the fact that inhibitors of d-alanylation would not affect growth of the wild-type or ΔltaS strains but would inhibit growth of the other three strains. Three molecules showed the expected pattern, with growth of the wild-type and ΔltaS strains but not the ΔtarO, ΔypfP, or Δ1050 strains. These hits were identified to target DltB, a polytopic membrane protein that is a core component of the d-alanylation machinery (16).
Identifying inhibitors of disulfide bond formation in E. coli, P. aeruginosa, and M. tuberculosis.
Structural disulfide bonds are found in proteins of the cell envelope, and they play a role in folding and stability of proteins involved in important cellular processes such as cell division, motility, virulence, transport of virulence proteins into host cells, responses to environmental threats, and assembly of the outer membrane of Gram-negative bacteria. Inhibition of this process would result in the simultaneous inactivation of several types of essential proteins and virulence factors. The formation of disulfide bonds in bacteria requires the DsbA/DsbB or DsbA/VKOR proteins, which catalyze the formation of disulfide bonds between pairs of cysteines in substrate proteins (Fig. 1, left). Our group exploited the functional conservation of the nonhomologous enzymes DsbB and VKOR and their ability to function in E. coli despite their lack of homology. We also used an agar-based assay for detecting defects in disulfide bond formation to identify compounds that target DsbB and VKOR enzymes by using a translational fusion of E. coli β-galactosidase to the membrane protein MalF, which localizes β-galactosidase in the periplasm (β-Galdbs). β-Galdbs is active only when the disulfide bond formation pathway is inactivated. The screen used E. coli mutants lacking dsbB and complemented with either P. aeruginosa or E. coli DsbB or M. tuberculosis VKOR while also expressing the β-Galdbs reporter. By parallel screening of DsbB- and VKOR-complemented E. coli strains, we identified inhibitors for one or the other membrane protein (Fig. 3f). The parallel screening provides reciprocal controls that eliminate inhibitors that are influencing β-Galdbs by acting directly on DsbA or affecting membrane protein assembly, since those molecules appear as hits in both screens. While it is possible that a particular compound could inhibit both DsbB and VKOR, the protein sequence and structural dissimilarity between the two make it unlikely. Thus, specific inhibitors of DsbB and VKOR that register as hits against one strain or the other but not both can be prioritized (51, 52). The HTS identified one potent inhibitor of DsbB enzymes of several Gram-negative pathogens (51, 53) and few other inhibitors of P. aeruginosa DsbB and M. tuberculosis VKOR enzymes (52).
Technology to identify inhibitors of LPS biogenesis in A. baumannii.
Gram-negative bacteria have an outer membrane, which is an asymmetric bilayer containing phospholipids in the inner leaflet and LPS in the outer leaflet. The biosynthesis of LPS is completed at the outer leaflet of the inner membrane following translocation. Then, LPS is transported from the inner membrane and delivered to the cell surface by the Lpt complex, which accomplishes LPS periplasmic transit and translocation through the outer membrane (Fig. 1, left). Additionally, LPS biogenesis is highly conserved across Gram-negative bacteria. Zhang et al. (54) developed a pathway-directed screen to find inhibitors of LPS biogenesis using the synthetic lethality phenotypes observed in Acinetobacter species. The intermediate genes in LPS biogenesis (lpxH, lpxB, lpxK, waaA, msbA, lptB, lptF, lptG, lptC, and lptA) are essential unless the genes involved in the first steps in LPS biogenesis (lpxA or lpxC) are removed, likely due to toxic accumulation of LPS intermediates. Thus, a strain lacking lpxA would tolerate higher concentrations of inhibitors of the conditionally essential steps of LPS biogenesis than would the wild-type strain (Fig. 3g). On the other hand, inhibitors in unrelated pathways would function at lower concentrations in the lpxA mutant than in the wild-type strain because of more accessibility to other targets due to LPS deficiency. The screen identified molecules that inhibited growth of the wild-type strain, and then those molecules were counterscreened against the lpxA mutant strain to identify the compounds for which inhibition was nullified in the mutant. This allowed the identification of a class of inhibitors of MsbA, which encodes the ABC transporter that flips LPS across the inner membrane during biogenesis (54). Similar to the Rod system screen described above, this screen highlights the use of conferring resistance rather than sensitivity when inhibiting the target, which helps to better define hits, decrease the number of false-positive results, and decrease the use of secondary assays.
PERSPECTIVES
Modern society has benefitted from antibiotics since the 1930s. With the increase in antibiotic resistance in recent decades, the benefits of these valuable molecules are threatened for future generations. More and more “superbugs” have been encountered, and these organisms are resistant to last-line antibiotics. The antibiotics most commonly used were discovered mainly by WCSs over 60 years ago, and very few new classes of antibiotics have been discovered in the past 4 decades. Hence, the discovery of novel molecules is important in order to fight the antimicrobial resistance problem. In the past decades, there has been a focus on generating innovative methods against unconventional targets to develop new therapies. This strategy has great potential because no preexisting TB resistance mechanisms exist; however, it also represents a great risk of failure due to the unknowns during drug development, including drug optimization, gene essentiality, and toxicology (10). While traditional antibiotics target essential pathways, nonessential pathways that contribute to virulence are also being used as targets (55, 56).
In the past 15 years, creative TB-WCSs (≥384-well plates) have been developed to test chemical libraries. The targets range from essential genes to genes that contribute to virulence (Fig. 1). In the case of virulence-related targets, the use of phenotypes other than growth inhibition was established in order to evaluate candidate hits. The TB-WCSs presented here focused on developing powerful assays to find inhibitors against one or multiple possible targets in one pathway (16, 20).
One of the early approaches used downregulation and upregulation of the target to increase or decrease drug sensitivity, respectively. This approach has been used to find inhibitors of PanC (Fig. 3a) and GuaB2 (Fig. 3b) by using regulatable promoters. This requires careful evaluation of promoter strength to determine whether levels of expression are suitable for the assay. While this approach has had some success, it is important to keep in mind that sensitivity to antibiotics does not always monotonically increase with target expression levels, and often this is indicative of a more complex MOA (57).
An additional approach used transcriptional fusions to find inhibitors of QS regulators (LasR and MvfR) (Fig. 2a) and secretion systems (T2SS and T3SS) (Fig. 2a). Transcriptional fusion methods require prior determination of genes that respond to inhibition of the target, as well as secondary assays to eliminate inhibitors of the reporter or its expression by unrelated mechanisms. Translational fusions were also used to find inhibitors of the proteasome (Fig. 2b) and secretion systems (Fig. 2e and f). In targeting secretion systems (T3SS), it is also possible to use the native substrate to monitor secretion if there is an enzymatic assay that can be adapted to HTS (Fig. 2g). Secondary assays are also needed to determine inhibitors of the enzymatic activity of the reporter itself.
Our approach used a comparison of nonhomologous enzymes that perform the same reaction and are functional when expressed in E. coli. Having nonhomologous proteins compared directly helps to eliminate molecules that may not be specific to the target (Fig. 3f), and this could be adapted to other proteins that perform the same function in different bacteria but are not homologs. Our assay also used a translational fusion as a reporter for inhibition of disulfide-bond-forming enzymes, which had been used in bacterial genetic studies for over 3 decades. The many studies with β-Galdbs reaffirmed that only null mutations (or inhibition) in Dsb proteins restore high levels of β-Galdbs activity. Furthermore, an advantage of having expressed exogenous targets in nonpathogenic bacteria is avoiding the need to work with biosafety level 3 (BSL3) organisms during screening (e.g., expressing M. tuberculosis vkor in E. coli [51, 52] or B. pseudomallei secA into P. aeruginosa [40]). Thus, the use of secondary assays to confirm the whole-cell activity of hits in the pathogen is required to validate hits found in these screens.
Lastly, synthetic lethality has been exploited to find inhibitors of WTA biosynthesis, d-alanylation of WTA (Fig. 3c and e), and PG and LPS biogenesis (Fig. 3d and g). This approach uses a sophisticated understanding of biological pathways in which a phenotype is caused when two gene products contribute to the essentiality or dispensability of a particular pathway. In the first scenario, a mutant would be more sensitive than the wild-type strain if the inhibitor is targeting other steps related to the pathway; in the second scenario, a mutant would be more resistant than the wild-type strain if the inhibitor is blocking steps in the pathway that contribute to toxicity. The use of interactions between steps of the pathway makes the assay more powerful and decreases the number of false-positive results (46, 54). A similar approach could be adapted for other cellular processes if the same essentiality/dispensability pattern is observed.
One clear advantage of TB-WCSs is that the hit rate for most screening campaigns remains low (0.007 to 0.6%) (Table 2), as opposed to the huge numbers of active molecules found in whole-cell assays, which are difficult to prioritize. The low hit rate suggests selectivity, and it is influenced by the sensitivity of the assay and the druggability of the target(s). For instance, finding inhibitors of the TatABC system has proved to be difficult by implementing the screen in which mCherry is fused to a TorA signal sequence, perhaps reflecting lower druggability of the Tat proteins (39). An opposite example was observed in the screens that found inhibitors of only TarG and DltB in the WTA pathway. Thus, some targets in a pathway tend to be more druggable than others, possibly due to their accessibility and contribution to the pathway (16).
TABLE 2.
Summary of screening sizes and hit rates of TB-WCSs
| Process | Targeta | Organism | No. of compounds screened | Concn | Hit rate (%) | Agarb | Reference(s) |
|---|---|---|---|---|---|---|---|
| QS | LasR | P. aeruginosa | 200,000 | 33 μM | 0.01 | N | 30 |
| MvfR | P. aeruginosa | 284,256 | 50 μg/mL | 0.19 | N | 31 | |
| Pantothenate biosynthesis | PanC | M. tuberculosis | 13,549 | 0.27 | N | 32 | |
| Proteome housekeeping | ClpP1P2 | M. tuberculosis | 503,879 | 10 μM | 0.04 | N | 19 |
| Purine biosynthesis | GuaB2 | M. tuberculosis | 10,000 | 1 and 10 μM | 2.56c | N | 33 |
| Secretion | Tat | E. coli | 389,481 | 5–20 μM | 0.016–0.6 | N | 39 |
| T3SS | Y. pestis | 70,966 | ∼17 μg/mL | 0.6 | N | 37 | |
| P. aeruginosa | 80,000 | 50 μM | 0.4 | N | 36 | ||
| S. enterica | 92,000 | ∼160 μg/mL | 0.097 | N | 38 | ||
| T2SS | P. aeruginosa | 75,000 | 50 μM | 0.15 | N | 40 | |
| P. aeruginosa | 83,986 | 0.17 | N | 41 | |||
| A. baumannii | 6,400 | 10 μM | 3c | N | 43 | ||
| P. aeruginosa | 40,000 | 10–20 μM | 0.15 | N | 42 | ||
| PG biogenesis | SAV1754 | S. aureus | 8 μM | N | 44 | ||
| MreB | E. coli | 690,000 | 25 μM | 0.006–0.01 | N | 46 | |
| WTA synthesis | TarG | S. aureus | 55,000 | 38 μM | 0.08 | N | 20 |
| TarG | S. aureus | 20,000 | 3 and 16 μM | 0.02 | N | 48 | |
| UppS | S. aureus | 1,600 | 20 μM | 4.25c | N | 50 | |
| DltB | S. aureus | 258,000 | 15 μM | 0.08 | N | 16 | |
| Oxidative protein folding | DsbB | E. coli, P. aeruginosa | 342,109 | 16–33 μM | 0.015 | Y | 51, 52 |
| VKOR | M. tuberculosis | 342,109 | 16–33 μM | 0.06–0.27 | Y | 51, 52 | |
| LPS biogenesis | MsbA | A. baumannii | 150,000 | 5 μM | 0.007 | N | 54 |
For pathway-directed screens, a target (underlined) of the most promising inhibitor in the screen was selected. However, these screens could detect inhibitors in other steps of the pathway or its regulation.
Agar-based versus liquid-based screens. Y, yes; N, no.
Higher hit rates are likely due to the use of preselected libraries known to target M. tuberculosis (first case) or the size of the libraries tested (second and third cases).
In summary, the use of TB-WCSs has opened a way to find new druggable targets and novel active molecules to develop next-generation therapeutics. TB-WCSs require a better understanding of the cellular process to target but decrease the number of molecules to prioritize. While these technologies have not yet yielded a registered drug, they have been providing the basis for better design and deployment of new screening approaches. TB-WCSs are still highly desirable to contribute to consistent antibacterial innovation, which should be sustained in the long term since microorganisms outpace drug development by evolving rapidly and developing resistance. Due to the formidably fast adaptability of microorganisms, it is our responsibility to create a solid foundation for future generations to continually respond to this challenge.
ACKNOWLEDGMENTS
We are thankful to Karen Bush and Clay Fuqua for helpful comments on the manuscript. We apologize to those whose work we omitted in writing this minireview. Due to space constraints, we selected TB-WCSs (in HTS ≥384-well format) with the use of chemical libraries and left out creative screens that used medium-throughput assays (96-well format).
We acknowledge research efforts in our laboratory supported by the Department of Biology, Indiana University Bloomington.
We declare no conflicts of interest.
Contributor Information
Cristina Landeta, Email: clandeta@iu.edu.
George O'Toole, Geisel School of Medicine at Dartmouth.
REFERENCES
- 1.Mohammed A, Ghebreyesus TA. 2019. No time to wait: securing the future from drug-resistant infections. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.World Health Organization. 2021. 2020 antibacterial agents in clinical and preclinical development: an overview and analysis. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.The Pew Charitable Trusts. 2016. A scientific roadmap for antibiotic discovery. The Pew Charitable Trusts, Philadelphia, PA. [Google Scholar]
- 4.Pucci MJ, Page MGP, Bush K. 2014. Cautious optimism for the antibacterial pipeline. Microbe 9:147–152. 10.1128/microbe.9.147.1. [DOI] [Google Scholar]
- 5.Deak D, Outterson K, Powers JH, Kesselheim AS. 2016. Progress in the fight against multidrug-resistant bacteria? A review of US Food and Drug Administration-approved antibiotics, 2010–2015. Ann Intern Med 165:363–372. 10.7326/M16-0291. [DOI] [PubMed] [Google Scholar]
- 6.Jackson N, Czaplewski L, Piddock LJV. 2018. Discovery and development of new antibacterial drugs: learning from experience? J Antimicrob Chemother 73:1452–1459. 10.1093/jac/dky019. [DOI] [PubMed] [Google Scholar]
- 7.Brown ED, Wright GD. 2016. Antibacterial drug discovery in the resistance era. Nature 529:336–343. 10.1038/nature17042. [DOI] [PubMed] [Google Scholar]
- 8.Walsh CT, Wencewicz TA. 2014. Prospects for new antibiotics: a molecule-centered perspective. J Antibiot (Tokyo) 67:7–22. 10.1038/ja.2013.49. [DOI] [PubMed] [Google Scholar]
- 9.Silver LL. 2011. Challenges of antibacterial discovery. Clin Microbiol Rev 24:71–109. 10.1128/CMR.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gwynn MN, Portnoy A, Rittenhouse SF, Payne DJ. 2010. Challenges of antibacterial discovery revisited. Ann N Y Acad Sci 1213:5–19. 10.1111/j.1749-6632.2010.05828.x. [DOI] [PubMed] [Google Scholar]
- 11.Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. 2007. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov 6:29–40. 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- 12.Brooks BD, Brooks AE. 2014. Therapeutic strategies to combat antibiotic resistance. Adv Drug Deliv Rev 78:14–27. 10.1016/j.addr.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Moellering RC. 2011. Discovering new antimicrobial agents. Int J Antimicrob Agents 37:2–9. 10.1016/j.ijantimicag.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Silver LL. 2012. Rational approaches to antibacterial discovery: pre-genomic directed and phenotypic screening, p 33–75. In Dougherty TJ, Pucci MJ (ed), Antibiotic discovery and development. Springer, New York, NY. [Google Scholar]
- 15.An WF, Tolliday N. 2010. Cell-based assays for high-throughput screening. Mol Biotechnol 45:180–186. 10.1007/s12033-010-9251-z. [DOI] [PubMed] [Google Scholar]
- 16.Matano LM, Morris HG, Wood BMK, Meredith TC, Walker S. 2016. Accelerating the discovery of antibacterial compounds using pathway-directed whole cell screening. Bioorg Med Chem 24:6307–6314. 10.1016/j.bmc.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baell JB, Holloway GA. 2010. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem 53:2719–2740. 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]
- 18.Singh SB, Young K, Miesel L. 2011. Screening strategies for discovery of antibacterial natural products. Expert Rev Anti Infect Ther 9:589–613. 10.1586/eri.11.81. [DOI] [PubMed] [Google Scholar]
- 19.Moreira W, Ngan GJY, Low JL, Poulsen A, Chia BCS, Ang MJY, Yap A, Fulwood J, Lakshmanan U, Lim J, Khoo AYT, Flotow H, Hill J, Raju RM, Rubin EJ, Dick T. 2015. Target mechanism-based whole-cell screening identifies bortezomib as an inhibitor of caseinolytic protease in mycobacteria. mBio 6:e00253-15. 10.1128/mBio.00253-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swoboda JG, Meredith TC, Campbell J, Brown S, Suzuki T, Bollenbach T, Malhowski AJ, Kishony R, Gilmore MS, Walker S. 2009. Discovery of a small molecule that blocks wall teichoic acid biosynthesis in Staphylococcus aureus. ACS Chem Biol 4:875–883. 10.1021/cb900151k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mills SD, Dougherty TJ. 2012. Cell-based screening in antibacterial discovery, p 901–929. In Dougherty TJ, Pucci MJ (ed), Antibiotic discovery and development. Springer, New York, NY. [Google Scholar]
- 22.DeVito JA, Mills JA, Liu VG, Agarwal A, Sizemore CF, Yao Z, Stoughton DM, Cappiello MG, Barbosa MDFS, Foster LA, Pompliano DL. 2002. An array of target-specific screening strains for antibacterial discovery. Nat Biotechnol 20:478–483. 10.1038/nbt0502-478. [DOI] [PubMed] [Google Scholar]
- 23.Forsyth RA, Haselbeck RJ, Ohlsen KL, Yamamoto RT, Xu H, Trawick JD, Wall D, Wang L, Brown-Driver V, Froelich JM, GC K, King P, McCarthy M, Malone C, Misiner B, Robbins D, Tan Z, Zhu Zy Z-Y, Carr G, Mosca DA, Zamudio C, Foulkes JG, Zyskind JW. 2002. A genome-wide strategy for the identification of essential genes in Staphylococcus aureus. Mol Microbiol 43:1387–1400. 10.1046/j.1365-2958.2002.02832.x. [DOI] [PubMed] [Google Scholar]
- 24.Yin D, Fox B, Lonetto ML, Etherton MR, Payne DJ, Holmes DJ, Rosenberg M, Ji Y. 2004. Identification of antimicrobial targets using a comprehensive genomic approach. Future Med Pharmacogenomics 5:101–113. 10.1517/phgs.5.1.101.25679. [DOI] [PubMed] [Google Scholar]
- 25.Young K, Jayasuriya H, Ondeyka JG, Herath K, Zhang C, Kodali S, Galgoci A, Painter R, Brown-Driver V, Yamamoto R, Silver LL, Zheng Y, Ventura JI, Sigmund J, Ha S, Basilio A, Vicente F, Tormo JR, Pelaez F, Youngman P, Cully D, Barrett JF, Schmatz D, Singh SB, Wang J. 2006. Discovery of FabH/FabF inhibitors from natural products. Antimicrob Agents Chemother 50:519–526. 10.1128/AAC.50.2.519-526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Soisson SM, Young K, Shoop W, Kodali S, Galgoci A, Painter R, Parthasarathy G, Tang YS, Cummings R, Ha S, Dorso K, Motyl M, Jayasuriya H, Ondeyka J, Herath K, Zhang C, Hernandez L, Allocco J, Basilio Á, Tormo JR, Genilloud O, Vicente F, Pelaez F, Colwell L, Lee SH, Michael B, Felcetto T, Gill C, Silver LL, Hermes JD, Bartizal K, Barrett J, Schmatz D, Becker JW, Cully D, Singh SB. 2006. Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature 441:358–361. 10.1038/nature04784. [DOI] [PubMed] [Google Scholar]
- 27.Singh SB, Zink DL, Huber J, Genilloud O, Salazar O, Diez MT, Basilio A, Vicente F, Byrne KM. 2006. Discovery of lucensimycins A and B from Streptomyces lucensis MA7349 using an antisense strategy. Org Lett 8:5449–5452. 10.1021/ol062041r. [DOI] [PubMed] [Google Scholar]
- 28.Phillips JW, Goetz MA, Smith SK, Zink DL, Polishook J, Onishi R, Salowe S, Wiltsie J, Allocco J, Sigmund J, Dorso K, Lee S, Skwish S, de la Cruz M, Martín J, Vicente F, Genilloud O, Lu J, Painter RE, Young K, Overbye K, Donald RGK, Singh SB. 2011. Discovery of kibdelomycin, a potent new class of bacterial type II topoisomerase inhibitor by chemical-genetic profiling in Staphylococcus aureus. Chem Biol 18:955–965. 10.1016/j.chembiol.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Nisa S, Blokpoel MCJ, Robertson BD, Tyndall JDA, Lun S, Bishai WR, O'Toole R. 2010. Targeting the chromosome partitioning protein ParA in tuberculosis drug discovery. J Antimicrob Chemother 65:2347–2358. 10.1093/jac/dkq311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müh U, Schuster M, Heim R, Singh A, Olson ER, Greenberg EP. 2006. Novel Pseudomonas aeruginosa quorum-sensing inhibitors identified in an ultra-high-throughput screen. Antimicrob Agents Chemother 50:3674–3679. 10.1128/AAC.00665-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starkey M, Lepine F, Maura D, Bandyopadhaya A, Lesic B, He J, Kitao T, Righi V, Milot S, Tzika A, Rahme L. 2014. Identification of anti-virulence compounds that disrupt quorum-sensing regulated acute and persistent pathogenicity. PLoS Pathog 10:e1004321. 10.1371/journal.ppat.1004321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abrahams GL, Kumar A, Savvi S, Hung AW, Wen S, Abell C, Barry CE, Sherman DR, Boshoff HIM, Mizrahi V. 2012. Pathway-selective sensitization of Mycobacterium tuberculosis for target-based whole-cell screening. Chem Biol 19:844–854. 10.1016/j.chembiol.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox JAG, Mugumbate G, Del Peral LVG, Jankute M, Abrahams KA, Jervis P, Jackenkroll S, Perez A, Alemparte C, Esquivias J, Lelièvre J, Ramon F, Barros D, Ballell L, Besra GS. 2016. Novel inhibitors of Mycobacterium tuberculosis GuaB2 identified by a target based high-throughput phenotypic screen. Sci Rep 6:38986. 10.1038/srep38986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kauppi A, Nordfelth R, Uvell H, Wolf-Watz H, Elofsson M. 2003. Targeting bacterial virulence: inhibitors of type III secretion in Yersinia. Chem Biol 10:241–249. 10.1016/s1074-5521(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 35.Nordfelth R, Kauppi AM, Norberg HA, Wolf-Watz H, Elofsson M. 2005. Small-molecule inhibitors specifically targeting type III secretion. Infect Immun 73:3104–3114. 10.1128/IAI.73.5.3104-3114.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aiello D, Williams JD, Majgier-Baranowska H, Patel I, Peet NP, Huang J, Lory S, Bowlin TL, Moir DT. 2010. Discovery and characterization of inhibitors of Pseudomonas aeruginosa type III secretion. Antimicrob Agents Chemother 54:1988–1999. 10.1128/AAC.01598-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan NJ, Brady MJ, Leong JM, Goguen JD. 2009. Targeting type III secretion in Yersinia pestis. Antimicrob Agents Chemother 53:385–392. 10.1128/AAC.00670-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felise HB, Nguyen HV, Pfuetzner RA, Barry KC, Jackson SR, Blanc MP, Bronstein PA, Kline T, Miller SI. 2008. An inhibitor of Gram-negative bacterial virulence protein secretion. Cell Host Microbe 4:325–336. 10.1016/j.chom.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bageshwar UK, VerPlank L, Baker D, Dong W, Hamsanathan S, Whitaker N, Sacchettini JC, Musser SM. 2016. High throughput screen for Escherichia coli twin arginine translocation (Tat) inhibitors. PLoS One 11:e0149659. 10.1371/journal.pone.0149659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moir DT, Di M, Wong E, Moore RA, Schweizer HP, Woods DE, Bowlin TL. 2011. Development and application of a cellular, gain-of-signal, bioluminescent reporter screen for inhibitors of type II secretion in Pseudomonas aeruginosa and Burkholderia pseudomallei. J Biomol Screen 16:694–705. 10.1177/1087057111408605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vasil ML, Tomaras AP, Pritchard AE. 2012. Identification and evaluation of twin-arginine translocase inhibitors. Antimicrob Agents Chemother 56:6223–6234. 10.1128/AAC.01575-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Massai F, Saleeb M, Doruk T, Elofsson M, Forsberg Å. 2019. Development, optimization, and validation of a high throughput screening assay for identification of Tat and type II secretion inhibitors of Pseudomonas aeruginosa. Front Cell Infect Microbiol 9:250. 10.3389/fcimb.2019.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waack U, Johnson TL, Chedid K, Xi C, Simmons LA, Mobley HLT, Sandkvist M. 2017. Targeting the type II secretion system: development, optimization, and validation of a high-throughput screen for the identification of small molecule inhibitors. Front Cell Infect Microbiol 7:380. 10.3389/fcimb.2017.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huber J, Donald RGK, Lee SH, Jarantow LW, Salvatore MJ, Meng X, Painter R, Onishi RH, Occi J, Dorso K, Young K, Park YW, Skwish S, Szymonifka MJ, Waddell TS, Miesel L, Phillips JW, Roemer T. 2009. Chemical genetic identification of peptidoglycan inhibitors potentiating carbapenem activity against methicillin-resistant Staphylococcus aureus. Chem Biol 16:837–848. 10.1016/j.chembiol.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Donald RGK, Skwish S, Forsyth RA, Anderson JW, Zhong T, Burns C, Lee S, Meng X, LoCastro L, Jarantow LW, Martin J, Lee SH, Taylor I, Robbins D, Malone C, Wang L, Zamudio CS, Youngman PJ, Phillips JW. 2009. A Staphylococcus aureus fitness test platform for mechanism-based profiling of antibacterial compounds. Chem Biol 16:826–836. 10.1016/j.chembiol.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Buss JA, Baidin V, Welsh M, Flores-Kim J, Cho H, Wood BM, Uehara T, Walker S, Kahne D, Bernhardt TG. 2019. Pathway-directed screen for inhibitors of the bacterial cell elongation machinery. Antimicrob Agents Chemother 63:e01530-18. 10.1128/AAC.01530-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell J, Singh AK, Swoboda JG, Gilmore MS, Wilkinson BJ, Walker S. 2012. An antibiotic that inhibits a late step in wall teichoic acid biosynthesis induces the cell wall stress stimulon in Staphylococcus aureus. Antimicrob Agents Chemother 56:1810–1820. 10.1128/AAC.05938-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang H, Gill CJ, Lee SH, Mann P, Zuck P, Meredith TC, Murgolo N, She X, Kales S, Liang L, Liu J, Wu J, Santa Maria J, Su J, Pan J, Hailey J, McGuinness D, Tan CM, Flattery A, Walker S, Black T, Roemer T. 2013. Discovery of wall teichoic acid inhibitors as potential anti-MRSA β-lactam combination agents. Chem Biol 20:272–284. 10.1016/j.chembiol.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farha MA, Leung A, Sewell EW, D'Elia MA, Allison SE, Ejim L, Pereira PM, Pinho MG, Wright GD, Brown ED. 2013. Inhibition of WTA synthesis blocks the cooperative action of PBPs and sensitizes MRSA to β-lactams. ACS Chem Biol 8:226–233. 10.1021/cb300413m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farha MA, Czarny TL, Myers CL, Worrall LJ, French S, Conrady DG, Wang Y, Oldfield E, Strynadka NCJ, Brown ED. 2015. Antagonism screen for inhibitors of bacterial cell wall biogenesis uncovers an inhibitor of undecaprenyl diphosphate synthase. Proc Natl Acad Sci USA 112:11048–11053. 10.1073/pnas.1511751112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Landeta C, Blazyk JL, Hatahet F, Meehan BM, Eser M, Myrick A, Bronstain L, Minami S, Arnold H, Ke N, Rubin EJ, Furie BC, Furie B, Beckwith J, Dutton R, Boyd D. 2015. Compounds targeting disulfide bond forming enzyme DsbB of Gram-negative bacteria. Nat Chem Biol 11:292–298. 10.1038/nchembio.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Landeta C, McPartland L, Tran NQ, Meehan BM, Zhang Y, Tanweer Z, Wakabayashi S, Rock J, Kim T, Balasubramanian D, Audette R, Toosky M, Pinkham J, Rubin EJ, Lory S, Pier G, Boyd D, Beckwith J. 2019. Inhibition of Pseudomonas aeruginosa and Mycobacterium tuberculosis disulfide bond forming enzymes. Mol Microbiol 111:918–937. 10.1111/mmi.14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Landeta C, Meehan BM, McPartland L, Ingendahl L, Hatahet F, Tran NQ, Boyd D, Beckwith J. 2017. Inhibition of virulence-promoting disulfide bond formation enzyme DsbB is blocked by mutating residues in two distinct regions. J Biol Chem 292:6529–6541. 10.1074/jbc.M116.770891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang G, Baidin V, Pahil KS, Moison E, Tomasek D, Ramadoss NS, Chatterjee AK, McNamara CW, Young TS, Schultz PG, Meredith TC, Kahne D. 2018. Cell-based screen for discovering lipopolysaccharide biogenesis inhibitors. Proc Natl Acad Sci USA 115:6834–6839. 10.1073/pnas.1804670115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clatworthy AE, Pierson E, Hung DT. 2007. Targeting virulence: a new paradigm for antimicrobial therapy. Nat Chem Biol 3:541–548. 10.1038/nchembio.2007.24. [DOI] [PubMed] [Google Scholar]
- 56.Rasko DA, Sperandio V. 2010. Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov 9:117–128. 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]
- 57.Palmer AC, Kishony R. 2014. Opposing effects of target overexpression reveal drug mechanisms. Nat Commun 5:4296. 10.1038/ncomms5296. [DOI] [PMC free article] [PubMed] [Google Scholar]