ABSTRACT
Botulism is a rare, life-threatening paralytic disease caused by botulinum neurotoxin (BoNT). Available treatments including an equine antitoxin and human immune globulin are given postexposure and challenging to produce and administer. NTM-1633 is an equimolar mixture of 3 human IgG monoclonal antibodies, E1, E2, and E3, targeting BoNT serotype E (BoNT/E). This first-in-human study assessed the safety, tolerability, pharmacokinetics (PK), and immunogenicity of NTM-1633. This double-blind, single-center, placebo-controlled dose escalation study randomized 3 cohorts of healthy volunteers to receive a single intravenous dose of NTM-1633 (0.033, 0.165, or 0.330 mg/kg) or saline placebo. Safety monitoring included physical examinations, clinical laboratory studies, and vital signs. Blood sampling was performed at prespecified time points for PK and immunogenicity analyses. Twenty-four subjects received study product (18 NTM-1633; 6 placebo), and no deaths were reported. An unrelated serious adverse event was reported in a placebo subject. Adverse events in the NTM-1633 groups were generally mild and similar in frequency and severity to the placebo group, and no safety signal was identified. NTM-1633 has a favorable PK profile with a half-life >10 days for the 0.330 mg/kg dose and an approximately linear relationship with respect to maximum concentration and area under the concentration-time curve (AUC0→t). NTM-1633 also demonstrated low immunogenicity. NTM-1633 is well tolerated at the administered doses. The favorable safety, PK, and immunogenicity profile supports further development as a treatment for BoNT/E intoxication and postexposure prophylaxis.
KEYWORDS: Clostridium botulinum, clinical trials, monoclonal antibodies
INTRODUCTION
Botulinum toxin (BoNT) is the most potent toxin known to humans and is produced by obligate anaerobic Clostridium species (1). To date, 7 distinct serotypes of botulinum toxin have been identified (A–H) (2, 3), of which serotypes A and B account for the majority of cases and serotype E accounts for a small percentage of cases reported in the U.S. (4). There are several mechanisms of botulinum intoxication, specifically, ingestion of food contaminated with preformed toxin, wound infection following traumatic injury or intravenous drug use (5), toxicoinfection in infants and adults with in vivo toxin production, and iatrogenic following therapeutic or cosmetic chemodenervation (6). An additional theoretical mechanism considers dispersal and subsequent inhalation of weaponized toxin (7, 8). While toxicoinfection in infants is the most common, there is a rising incidence of wound botulism among intravenous drug users (4).
Current therapies for BoNT intoxication are given postexposure to symptomatic individuals. These include an equine antitoxin (investigational heptavalent botulinum antitoxin [HBAT]) (9) and a human immune globulin reserved for infant use (Botulism Immune Globulin Intravenous [BIG-IV]) (10). Production of both the equine antitoxin and the human immune globulin is labor-intensive and costly, leading to restricted availability and unstainable production. The equine antitoxin, in particular, is highly immunogenic with a short half-life.
These inherent challenges of the current antitoxin and immune globulin supply and manufacture indicate an urgent unmet need for novel therapeutics in this area both for treatment of diagnosed patients and for potential postexposure prophylaxis in high-risk situations. Over the past 15 years, new methodologies, such as small peptides and peptide mimetics, receptor mimics, and humanized monoclonal antibodies have emerged as attractive strategies (11). Novel mechanisms including inhibitors of neurotoxin uptake and processing as well as endopeptidases and neuronal modulation also remain attractive (12). While high-throughput screening and structure-based drug design have led to identification of potential therapeutics, the favorable in vitro profiles do not translate to in vivo efficacy (13). Small molecules, such as 3,4-diaminopyridine, are also emerging as potential symptomatic options for BoNT intoxication (14).
Of the above strategies, humanized monoclonal antibodies are among the more promising. Comixtures of monoclonal antibodies are currently in development by Ology Biosciences with the support of the National Institute of Allergy and Infectious Diseases (NIAID) to target multiple BoNT Serotypes. The specific products targeting BoNT serotypes A (BoNT/A NTM-1631 [formely XOMA 3AB]), serotype B (BoNT/B, NTM-1632 [formerly XOMA 3B]), C and D (BoNT/C and BoNT/D, NTM-1634 [formerly XOMA 4CD]), and E (BoNT/E, NTM-1633 [formerly XOMA 3E]) are being developed for the prevention and treatment of botulism (15–17). NTM-1633 is an equimolar mixture of 3 IgG monoclonal antibodies; each antibody is engineered to have distinct light and heavy chain variable regions that bind unique nonoverlapping epitopes on BoNT/E and common human gamma-1 and kappa constant regions. Since NTM-1633 is structurally similar to NTM-1631, NTM-1632, and NTM-1634, the predicted mechanism is likely similar: high affinity binding of the mixture to toxin, blockade of cellular binding epitopes on the toxin, and increased hepatic clearance of the toxin-Ab immune complexes (15, 18, 19). Based on promising preclinical testing (20, 21) and positive results from the Phase I trials of NTM-1631 (15), NTM-1632 (17), and NTM-1634 (16), the human investigational product NTM-1633 is in clinical development for the treatment and prophylaxis of botulism caused by BoNT/E. The purpose of this study was to assess the safety, tolerability, pharmacokinetics (PK), and immunogenicity of intravenously administered single escalating doses of NTM-1633.
RESULTS
Demographic characteristics.
A total of 24 subjects were randomized. All subjects received full study treatment and completed follow-up per protocol.
The demographic characteristics of the individual cohorts and total study population are summarized in Table 1.
TABLE 1.
Demographics of the study population
| Cohort |
||||||
|---|---|---|---|---|---|---|
| Characteristica | 0.033 mg/kg n = 6 |
0.165 mg/kg n = 6 |
0.330 mg/kg n = 6 |
Combined n = 18 |
Placebo n = 6 |
All subjects n = 24 |
| Age | ||||||
| Mean (SD) | 32.8 (7.8) | 29.7 (5.4) | 32.2 (7.9) | 31.6 (6.8) | 31.5 (7.5) | 31.5 (6.8) |
| Median | 35.0 | 29.0 | 32.0 | 30.5 | 30.5 | 30.5 |
| Min, Maxb | 22, 41 | 24, 39 | 21, 42 | 21, 42 | 23, 45 | 21, 45 |
| BMIc (kg/m2) | ||||||
| Mean (SD) | 25.9 (5.6) | 27.0 (3.5) | 29.0 (4.8) | 27.3 (4.6) | 22.8 (3.2) | 26.2 (4.7) |
| Median | 25.2 | 26.2 | 30.0 | 26.8 | 22.3 | 25.7 |
| Min, Max | 19.3, 32.5 | 22.7, 32.8 | 21.8, 33.6 | 19.3, 33.6 | 18.6, 28.0 | 18.6, 33.6 |
| Sex | ||||||
| Male | 2 (33) | 2 (33) | 1 (17) | 5 (27.7) | 1 (17) | 6 (25) |
| Female | 4 (67) | 4 (67) | 5 (83) | 13 (72.2) | 5 (83) | 18 (75) |
| Race | ||||||
| White | 5 (83) | 4 (67) | 5 (83) | 14 (77.8) | 5 (83) | 19 (79) |
| Black or African American | 1 (17) | 2 (33) | 1 (17) | 4 (22.2) | 1 (17) | 5 (21) |
| Ethnicity | ||||||
| Hispanic or Latino | 2 (33) | 0 (0) | 0 (0) | 2 (11.1) | 1 (17) | 3 (13) |
| Non-Hispanic or Latino | 4 (67) | 6 (100) | 6 (100) | 16 (88.9) | 5 (83) | 21 (88) |
Data are expressed as no. (%) unless stated otherwise.
Min, minimum; Max, maximum.
BMI, body mass index.
Safety profile.
No deaths were reported. A total of 36 adverse events (AEs; 33 mild and 3 moderate severity) were reported for 16 (67%) subjects: 4 (67%) in the 0.033 mg/kg NTM-1633 group, 4 (67%) in the 0.165 mg/kg NTM-1633 group, 2 (33%) in the 0.330 mg/kg NTM-1633 group, and 6 (100%) subjects in the placebo group. Of the reported events, 9 (25%) were deemed treatment emergent and related; they were all of mild severity and consisted of 3 headaches, 3 episodes of sinus tachycardia, 1 episode of chest pain, 1 episode of systolic hypotension, and 1 episode of intermittent diastolic hypertension. A total of 3 (8%) unrelated, moderate severity AEs were reported: 1 by a placebo subject, 1 by a 0.033 mg/kg NTM-1633 subject, and 1 by a 0.330 mg/kg NTM-1633 subject. A single serious adverse event (SAE) of severe tachycardia was reported in 1 subject who received placebo; this event occurred after the confinement period and was considered not-related to study product with a suspected etiology of illicit drug (cocaine) use.
AEs reported by 2 or more subjects included sinus bradycardia (21%), upper respiratory tract infection (12.5%), and headache (12.5%). A total of 5 subjects experienced 7 bradycardia events; only 1 event was deemed related to study product. All subjects who reported upper respiratory tract infection had received NTM-1633 (2 in 0.033 mg/kg group and 1 in 0.165 mg/kg group). No other MedDRA preferred term was reported by more than one subject in each treatment group. A breakdown of all AEs occurring in at least 10% of subjects is shown in Table 2.
TABLE 2.
Summary of adverse events by subject with an overall rate of ≥10%
| Cohort |
||||||
|---|---|---|---|---|---|---|
| Adverse event | 0.033 mg/kg n = 6 |
0.165 mg/kg n = 6 |
0.330 mg/kg n = 6 |
Combined n = 18 |
Placebo n = 6 |
All subjects n = 24 |
| Sinus bradycardia | 1 (16.7) | 0 (0) | 1 (16.7) | 2 (11.1) | 3 (50.0) | 5 (20.8) |
| Upper respiratory tract infection | 2 (33.3) | 1 (16.7) | 0 (0) | 3 (16.7) | 0 (0) | 3 (12.5) |
| Headache | 2 (33.3) | 0 (0) | 0 (0) | 2 (11.1) | 1 (5.6) | 3 (12.5) |
| Aldolase increased | 5 (83.3) | 1 (16.7) | 4 (66.7) | 10 (55.5) | 4 (66.7) | 14 (58.3) |
| Creatine kinase elevated | 2 (33.3) | 0 (0) | 0 (0) | 2 (11.1) | 2 (33.3) | 4 (16.7) |
| Hemoglobin decreased | 3 (50.0) | 2 (33.3) | 1 (16.7) | 6 (33.3) | 1 (16.7) | 7 (29.2) |
| Neutropenia | 0 (0) | 2 (33.3) | 1 (16.7) | 3 (16.7) | 1 (16.7) | 4 (16.7) |
There were no clinically significant ECG changes observed and no related physical exam findings.
Laboratory analyses.
A total of 57 related biochemistry results were reported in 19 (79%) of subjects, including all 6 placebo subjects. A total of 3 severe biochemistry results (aldolase and creatine kinase in 1 subject on Day 8 and low fasting serum glucose in a different subject on Day 4) occurred in 2 placebo recipients. A total of 7 (16.7%) subjects had moderate abnormalities: elevated aldolase was the most common moderate abnormality noted among 4 subjects, 2 who received NTM-1633 (Days 2 and 4; Day 91) and 2 who received placebo (Day 2; Day 91).
A total of 27 related hematology results were reported in 11 (45.8%) subjects, 10 of whom received NTM-1633. Of these, 26 were mild and primarily involved reductions in hemoglobin and hematocrit (7 subjects) as well as transient neutropenia (4 subjects) (Table 2). Only 1 moderate abnormality, transient neutropenia, was observed in a single NTM-1633 0.330 mg/kg recipient on Day 29. All hematologic abnormalities had resolved or stabilized by Day 91 and required no further monitoring.
No related moderate or severe coagulation or urine abnormalities were observed.
Pharmacokinetic analysis.
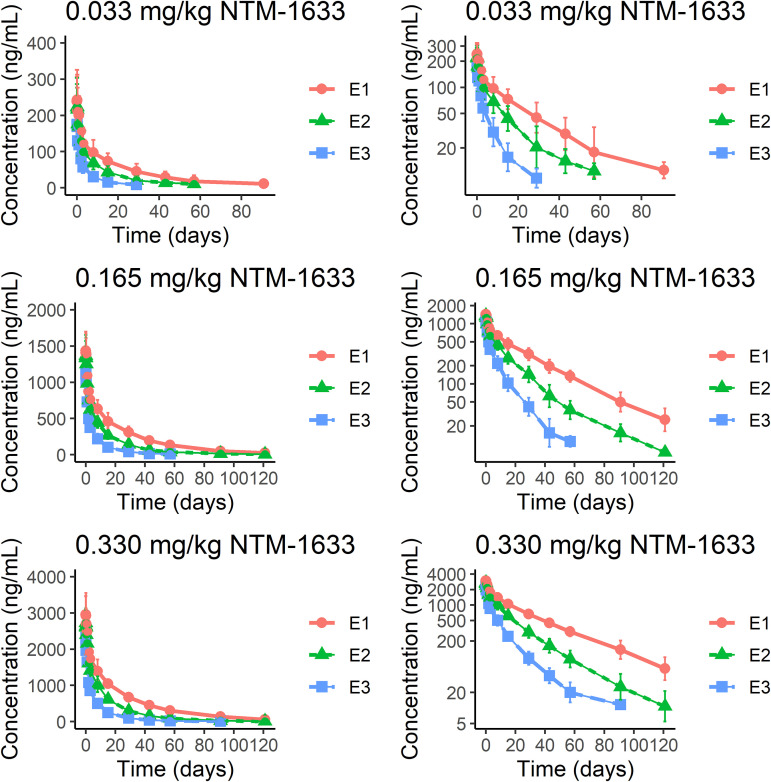
A summary of the PK data is presented in Table 3, and plots of concentration versus time are shown in Fig. 1. The median Cmax achieved following a single IV dose of 0.033 mg/kg, 0.165 mg/kg, and 0.330 mg/kg NTM-1633 was similar for E1 and E2; median Cmax for E3 at all doses was slightly lower. Median tmax was achieved in 1.03–2.01 h for E1, E2, and E3 at all doses. The median elimination half-life (t½) was 6.95–21.2 days in the 0.033 mg/kg dose and increased to 11.2–25.4 days in the 0.165 mg/kg and 0.330 mg/kg doses. The median AUC(0, t) (ng×h/mL) was highest for E1 and lowest for E3 at all doses. Dose proportionality curves for Cmax and AUC(0, ∞) revealed an approximately linear relationship with respect to both Cmax and total AUC exposure, suggesting that all 3 components of NTM-1633 exhibit linear kinetics. Median clearance was similar for E1 and E2 at all doses, ranging from 0.248 mL/h/kg to 0.656 mL/h/kg and increased for E3 at all doses, ranging from 1.07 mL/h/kg to 1.16 mL/h/kg. Apparent volume of distribution at steady-state (Vss) was consistent for E1 and E2, ranging from 205 to 271; Vss was increased for E3, ranging from 320 to 388 mL/kg.
TABLE 3.
Summary pharmacokinetic parameters for NTM-1633. Median values with (min, max) are reported
| MAb | Cohorta | Cmax (ng/mL) | Tmax (h) | T ½ (days) | AUC 0-t (ng × h/mL) | CLb (mL/h/kg) | VSS (mL/kg) |
|---|---|---|---|---|---|---|---|
| E1 | A B C |
268 (170, 332) 1490 (1190, 1790) 2960 (2560, 4110) |
2.01 (1.03, 3.95) 1.03 (1, 4.03) 1.55 (1.05, 4) |
21.2 (15.0, 30.1) 24.1 (22.2, 27.2) 25.4 (20.9, 34.5) |
89100 (43900, 135000) 638000 (447000, 691000) 1290000 (1180000, 1650000) |
0.329 (0.225, 0.690) 0.251 (0.225, 0.356) 0.248 (0.182, 0.271) |
238 (188, 342) 204 (173, 269) 205 (147, 251) |
| E2 | A B C |
248 (157, 306) 1420 (1090, 1680) 2740 (2420, 3530) |
2.01 (1.03, 3.95) 2 (1.03, 4) 1.55 (1, 7.9) |
13.5 (7.49, 20) 14.7 (11.6, 18.1) 17.3 (12.6, 22.4) |
45900 (22300, 67300) 302000 (223000, 381000) 658000 (589000, 895000) |
0.656 (0.451, 1.32) 0.54 (0.424, 0.71) 0.499 (0.359, 0.554) |
271 (202, 359) 237 (210, 271) 241 (180, 267) |
| E3 | A B C |
200 (118, 256) 1230 (947, 1450) 2370 (2090, 3010) |
2.01 (1.03, 3.95) 1.03 (1, 2.2) 1.54 (1, 2.03) |
6.95 (4.75, 9.15) 11.2 (7.9, 12.1) 11.2 (9.15, 16.7) |
17600 (9160, 28000) 139000 (104000, 163000) 300000 (261000, 361000) |
1.7 (1.46, 1.93) 1.16 (0.983, 1.55) 1.07 (0.899, 1.25) |
388 (242, 533) 327 (256, 369) 320 (237, 400) |
Dosing by cohort: A (0.033 mg/kg), B (0.165 mg/kg), C (0.330 mg/kg).
CL, clearance.
FIG 1.
The time course of serum monoclonal antibody (MAb) concentrations after single intravenous (IV) dose administration of NTM1633 is shown on the left with semi-log concentration plots shown on the right. NTM-1633 component MAbs are denoted as E1 (red), E2 (green), and E3 (blue). Mean concentration.
Immunogenicity analysis.
One subject in the NTM-1633 0.165 mg/kg group had preexisting ADA to E2; no subjects had preexisting ADA to E1 or E3. One subject in the placebo group developed a treatment-emergent response to E1. There were 7 treatment-emergent responses among subjects receiving NTM-1633: one subject in each dose group developed ADA to E1, and a total of 4 subjects (one 0.033 mg/kg, two 0.165 mg/kg, and one 0.330 mg/kg) developed ADA to E2. The E1 treatment-emergent responses were low-titer (18.6–77.6) and measurable on Day 15 for lowest and middle dose and on Days 15, 29, 57, and 121 for the highest dose. The E2 treatment emergent responses were low (10–58.9) in the lowest and highest dosing groups. It was higher for the middle dosing group (19.1–891.3) and detectable at Days 1, 15, 29, 57, 91, and 121. No subjects developed treatment-emergent ADA to E3 at any dose. Overall, in the highest dosing group only 2 subjects developed a treatment-emergent response, and those titers were low (10–77.6). Two subjects in the 0.165 mg/kg group developed a 9-fold increase in ADA, but this was not observed in the 0.330 mg/kg dosing group. There were no treatment-boosted responses.
DISCUSSION
This study was the first-in-human assessment of NTM-1633, a BoNT/E product, in healthy adult subjects. Single escalating doses of NTM-1633 administered intravenously to participants were well-tolerated over the investigational dose range. AEs in the NTM-1633 cohorts were generally mild and similar in frequency to the subjects receiving placebo. A single SAE was identified in a placebo subject (tachycardia in the setting of illicit drug use) and was deemed not related. Multiple subjects were identified as having bradycardia and transient elevations in aldolase; these more likely reflect the athletic nature of the healthy volunteer population, consistent with our prior experience (17), rather than an effect attributable to NTM-1633. No specific safety signal was identified in this study, and no serious infusion or hypersensitivity reactions occurred. Antibody levels were detectable up to 91 days in the 0.165 mg/kg group and 121 days in the 0.330 mg/kg dose group, and the estimated half-lives of the individual components for the highest dosing group were 11.2–25.4 days. This longevity offers an advantage over the current HBAT antitoxin, which has a documented half-life of approximately 7 h for components against serotype E (22). NTM-1633 has a benign immunogenicity profile. A total of 7 participants developed transient, low titer antibodies to the constituent components of NTM-1633 (3 to E1 and 4 to E2) following dosing, and there were no treatment-boosted ADA responses. The highest dose tested, 0.330 mg/kg, is well tolerated and achieved the highest blood concentrations with the longest half-life, and minimal immunogenicity. Based on the completed mouse protection assay and extrapolation to humans, a 0.330 mg/kg dose would be expected to neutralize circulating BoNT/E at the levels tested in preclinical models (23).
BoNT remains a significant threat, particularly as a potential weaponized agent, and has been prioritized as a Category A biothreat by NIAID (24). The destructive potential of weaponized BoNTs increases the urgency and necessity for highly efficacious, scalable, and rapidly deployable therapeutics (25). Investment in new therapeutic strategies and new structure-based designs for treatment and prevention of botulinum intoxication have advanced the therapeutic landscape over the last 10 years. In particular, human and humanized MAbs capable of neutralizing BoNTs show promise. They offer a number of key advantages over existing therapies, including in vitro production, low risk of hypersensitivity reactions and immunogenicity, and consistent potency and stability. Due to high-affinity binding with BoNT serotypes, BoNT monoclonal antibodies are less likely to cause off-target effects and can be administered postexposure without causing adverse effects (23). Completed first-in-human studies of the BoNT serotype A MAb, NTM-1631; BoNT serotype B, NTM-1632; BoNT serotype C/D MAb, NTM-1634; and BoNT serotype E, NTM-1633, revealed that the products were well tolerated with a favorable PK profile (15–17).
The current study has several limitations. NTM-1633 admixtures are active only against serotype E; the ideal therapeutic contains admixtures of MAbs that would offer broader coverage against multiple BoNT serotypes. Polyvalent combinations of NTM-1631, NTM-1632, and NTM-1633 in temperature-stable formulations will greatly increase the capability to rapidly deploy an antidote to empirically treat BoNT intoxication when the serotype is unknown.
In conclusion, the initial safety, PK, and immunogenicity profile of NTM-1633 supports further clinical development as a treatment for BoNT/E intoxication and its inclusion in a potential strategy to develop a new generation of therapeutics for post-exposure prophylaxis following suspected BoNT exposure.
MATERIALS AND METHODS
This is a first-in-human, double-blind, single-center, placebo-controlled dose-escalation study to evaluate the safety and PK of intravenous NTM-1633. Dose selection was based on conversion from the NOAEL dose of GLP studies in Sprague Dawley rats into a human equivalent dose (HED). A safety factor of 10 was used to establish a maximal recommended starting dose (MRSD) of 5 mg/kg and further reduced by 150-fold to establish the starting dose of 0.033 mg/kg. A total of three cohorts were planned at doses of 0.033 mg/kg (Cohort A), 0.165 mg/kg (Cohort B), and 0.330 mg/kg (Cohort C). Further dose escalation was deemed unnecessary based on preclinical murine studies demonstrating toxin neutralization in this dose range, which approximates expected human BoNT/E exposure levels.
Materials.
NTM-1633 is a sterile, clear, colorless, preservative-free, pH 6.0 buffered solution containing a 5 mg/mL equimolar mixture of E1, E2, and E3. The drug was supplied by Ology Bioservices, Inc. through a NIAID-Clinical Agents Repository Contract (CAR) by Fisher BioServices (Germantown, MD). Normal saline was used as placebo. Drug stability testing was developed and performed by Ology Bioservices, Inc. (Alameda, CA).
Participants.
A total of 24 healthy volunteers and a maximum of 4 alternates per cohort were enrolled. Eligible participants included healthy, nonpregnant, nonlactating female and male subjects aged 18–45 with body mass index (BMI) between 18.5 and 30 kg/m2 and a negative illicit drug and alcohol screen who agreed to use contraception per protocol. Exclusion criteria included history of recent febrile illness, chronic medical conditions, severe allergic reaction to any type of medication or any study product components, blood donation within 2 months of enrollment, receipt of a blood product within 6 months of enrollment or an antibody within 5 half-lives, treatment with another investigational drug within 1 month of dosing, and use of H1 antihistamines or beta blockers within 5 days of dosing. Additional exclusion criteria included laboratory abnormalities (urinalysis, hematology, and biochemistry analysis), positive serology for HIV, Hepatitis B or C, and a clinically significant abnormality on electrocardiogram including baseline QT/QTc prolongation.
Study design.
Research participants were randomized to sequential dose-escalation cohorts consisting of 8 subjects. Within each cohort, participants were randomized in a 3:1 ratio to receive a single intravenous infusion of the investigational medicinal product (IMP) or placebo administered over 1 h. Each participant underwent screening followed within 4 weeks by a 3-day inpatient stay at the Duke Early Phase Clinical Research Unit (DEPRU) of Duke University Medical Center. The study was approved by the Duke Health Institutional Review Board, and written informed consent was obtained from all participants prior to screening. Biosafety committee approval was not required because study participants received only a monoclonal antibody targeting botulinum toxin E and no botulinum toxin was used. This trial is registered with ClinicalTrials.gov, number NCT03603665.
Study procedures.
Following screening, eligible participants were admitted to the DEPRU 1 day prior to dosing for a complete physical examination, electrocardiogram, and laboratory testing including routine biochemistry, hematology, urinalysis, and urine pregnancy test (for females of childbearing potential). Two sentinel subjects in each dosing cohort were randomized 1:1 to receive IMP or placebo and monitored for at least 24 h prior to dosing the remaining subjects. All study participants who received IMP were monitored in the DEPRU for 24 h postdose. Subjects in Cohort A were followed for 91 Days and those in Cohorts B and C for 121 days.
Safety analyses.
(i) Adverse events. Adverse events (AEs) were assessed by targeted interviews for symptoms, physical examination, and laboratory studies from the day of infusion through Day 57 for all cohorts and classified in accordance with the Medical Dictionary for Regulatory Activities (MedDRA) Version 22.0. Relationship to IMP, alternate plausible explanation or etiology, severity, and outcome were documented for each AE.
(ii) Laboratory analyses. Safety laboratory studies (hematology, biochemistry, and urinalysis) were conducted on Days −1, 2, 4, 8, 15, 29, and 91. Values outside the reference range were recorded as AEs.
Pharmacokinetics and immunogenicity analyses.
(i) Pharmacokinetics. Blood PK samples were drawn prior to infusion, at end of infusion, and at 1, 3, 7, 23, 47, and 71 h following end of infusion. Additional PK samples were drawn at Days 8, 15, 29, 43, 57, and 91 (all cohorts) and 121 (Cohorts B and C only). Serum samples were analyzed by 3 validated bridging electrochemiluminescence (ECL) assays for the concentrations of each MAb, E1, E2, and E3 (15, 26). Testing was developed and performed by Ology Bioservices, Inc. (Alameda, CA). The lower limit of quantification for E1, E2, and E3 was 6.67 ng/mL as expressed in 100% human serum. Concentrations below the lower limit of quantification were set to missing if they occurred after the first positive concentration or imputed to 0 if no positive concentration was ever detected. Missing samples were excluded from the PK analysis.
(ii) Immunogenicity. Serum samples were drawn at Days −1, 29, 57, 91 (all cohorts), and 121 (Cohorts B and C) for measurement of anti-drug antibody (ADA) using 3 validated ECL assays, one each for anti-E1, anti-E2, and anti-E3. Testing was developed and performed by Ology Bioservices, Inc. (Alameda, CA). A treatment-emergent response was defined as negative ADA status at baseline, and positive ADA status at any point after baseline. A treatment-boosted response was defined as positive ADA status at baseline with increase in titer in any subsequent posttreatment serum samples, that was at least 9-fold over baseline levels.
Statistical analysis.
Categorical variables were summarized as numbers and percentages and continuous variables as standard deviation, means, and medians. PK parameters included area under the concentration-time curve (AUC), maximum observed concentration (Cmax), time to maximum concentration (Tmax), elimination rate constant (ke), terminal half-life (t1/2), clearance, and volume of distribution (VD) for each monoclonal component for each dosing cohort. Descriptive statistics were calculated using SAS (v9.4; SAS Institute, Cary, NC), and PK parameters were estimated by noncompartmental methods using WinNonLin (v8.0 or higher, Certara, Princeton, NJ).
ACKNOWLEDGMENTS
This project has been funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), U.S. Department of Health and Human Services, through contract no. HHSN272201500002C (Emmes Corporation), and a Vaccine and Treatment Evaluation Units (VTEU) award under contract no. HHSN272201300017I (Duke University). This work was also supported by the Clinical and Translational Science Awards (CTSA) Program from the National Center for Advancing Translational Sciences, NIH UL1TR002553 #A03-0077 (Duke University—Early Phase Research Unit). Ology Biosciences, Inc. provided postclinical trial DP stability testing and NOAEL for reports on toxicity. The authors and participating faculty and staff were compensated for their work on this project through the U.S. government contracts to their institutions listed above. J.T.G. is supported by the National Institute of Neurological Disorders and Stroke, NIH, under award number K23NS085049.
G.K.S. has been an investigator for clinical trials sponsored by Novavax, GlaxoSmithKline, and Regeneron and is currently an investigator for a clinical trial sponsored by Pfizer. She serves as chair of Independent Data Monitoring Committees for GlaxoSmithKline and Pfizer. E.B.W. is a principal investigator for a study funded by Pfizer and is a coinvestigator for a study funded by Moderna.
REFERENCES
- 1.Lamanna C. 1959. The most poisonous poison. Science 130:763–772. 10.1126/science.130.3378.763. [DOI] [PubMed] [Google Scholar]
- 2.Rossetto O, Montecucco C. 2019. Tables of toxicity of botulinum and tetanus neurotoxins. Toxins 11:686. 10.3390/toxins11120686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan Y, Barash JR, Conrad F, Lou J, Tam C, Cheng LW, Arnon SS, Marks JD. 2019. The novel clostridial neurotoxin produced by strain IBCA10-7060 is immunologically equivalent to BoNT/HA. Toxins 12:9. 10.3390/toxins12010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2019. Botulism annual summary, 2017. Department of Health and Human Services, CDC, Atlanta, Georgia. [Google Scholar]
- 5.Peak CM, Rosen H, Kamali A, Poe A, Shahkarami M, Kimura AC, Jain S, McDonald E. 2019. Wound botulism outbreak among persons who use black tar heroin—San Diego County, California, 2017–2018. MMWR Morb Mortal Wkly Rep 67:1415–1418. 10.15585/mmwr.mm675152a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yiannakopoulou E. 2015. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology 95:65–69. 10.1159/000370245. [DOI] [PubMed] [Google Scholar]
- 7.Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, Lillibridge S, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Swerdlow DL, Tonat K, Working Group on Civilian Biodefense. 2001. Botulinum toxin as a biological weapon: medical and public health management. JAMA 285:1059–1070. 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- 8.Adalja AA, Toner E, Inglesby TV. 2015. Management of potential bioterrorism-related conditions. N Engl J Med 372:2272–2273. 10.1056/NEJMc1504248. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2010. Investigational heptavalent botulinum antitoxin (HBAT) to replace licensed botulinum antitoxin AB and investigational botulinum antitoxin E. MMWR Morb Mortal Wkly Rep 59:299. [PubMed] [Google Scholar]
- 10.Arnon SS, Schechter R, Maslanka SE, Jewell NP, Hatheway CL. 2006. Human botulism immune globulin for the treatment of infant botulism. N Engl J Med 354:462–471. 10.1056/NEJMoa051926. [DOI] [PubMed] [Google Scholar]
- 11.Cai S, Singh BR. 2007. Strategies to design inhibitors of Clostridium botulinum neurotoxins. Infect Disord Drug Targets 7:47–57. 10.2174/187152607780090667. [DOI] [PubMed] [Google Scholar]
- 12.Kiris E, Burnett JC, Kane CD, Bavari S. 2014. Recent advances in botulinum neurotoxin inhibitor development. Curr Top Med Chem 14:2044–2061. 10.2174/1568026614666141022093350. [DOI] [PubMed] [Google Scholar]
- 13.Lin L, Olson ME, Eubanks LM, Janda KD. 2019. Strategies to counteract botulinum neurotoxin A: nature's deadliest biomolecule. Acc Chem Res 52:2322–2331. 10.1021/acs.accounts.9b00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradford AB, Machamer JB, Russo TM, McNutt PM. 2018. 3,4-diaminopyridine reverses paralysis in botulinum neurotoxin-intoxicated diaphragms through two functionally distinct mechanisms. Toxicol Appl Pharmacol 341:77–86. 10.1016/j.taap.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Nayak SU, Griffiss JM, McKenzie R, Fuchs EJ, Jurao RA, An AT, Ahene A, Tomic M, Hendrix CW, Zenilman JM. 2014. Safety and pharmacokinetics of XOMA 3AB, a novel mixture of three monoclonal antibodies against botulinum toxin A. Antimicrob Agents Chemother 58:5047–5053. 10.1128/AAC.02830-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snow DM, Riling K, Kimbler A, Espinoza Y, Wong D, Pham K, Martinez Z, Kraus CN, Conrad F, Garcia-Rodriguez C, Cobb RR, Marks JD, Tomic MT. 2019. Safety and pharmacokinetics of a four monoclonal antibody combination against botulinum C and D neurotoxins. Antimicrob Agents Chemother 63:e01270-19. 10.1128/AAC.01270-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guptill JT, Raja SM, Juel VC, Walter EB, Cohen-Wolkowiez M, Hill H, Sendra E, Hauser B, Jackson P, Swamy GK. 2021. Safety, tolerability and pharmacokinetics of NTM-1632, a novel mixture of three monoclonal antibodies against botulinum toxin B. Antimicrob Agents Chemother 65:e02329-20. 10.1128/AAC.02329-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowakowski A, Wang C, Powers DB, Amersdorfer P, Smith TJ, Montgomery VA, Sheridan R, Blake R, Smith LA, Marks JD. 2002. Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody. Proc Natl Acad Sci USA 99:11346–11350. 10.1073/pnas.172229899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pless DD, Torres ER, Reinke EK, Bavari S. 2001. High-affinity, protective antibodies to the binding domain of botulinum neurotoxin type A. Infect Immun 69:570–574. 10.1128/IAI.69.1.570-574.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espinoza Y, Wong D, Ahene A, Der K, Martinez Z, Pham J, Cobb RR, Farr-Jones S, Marks JD, Tomic MT. 2019. Pharmacokinetics of human recombinant anti-botulinum toxin antibodies in rats. Toxins 11:345. 10.3390/toxins11060345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomic MT, Espinoza Y, Martinez Z, Pham K, Cobb RR, Snow DM, Earnhart CG, Pals T, Syar ES, Niemuth N, Kobs DJ, Farr-Jones S, Marks JD. 2019. Monoclonal antibody combinations prevent serotype A and serotype B inhalational botulism in a guinea pig model. Toxins (Basel) 11:208. 10.3390/toxins11040208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anonymous. 2017. BAT(R) [botulism antitoxin heptavalent (A, B, C, D, E, F, G) - (equine)] sterile solution for injection [package insert]. Cangene Corportation, Winnipeg, Manitoba (Canada). [Google Scholar]
- 23.Ology Bioservices Incorporated. 2018. Investigational brochure: anti-BoNT E antibody (NTM-1633) treatment and prevention of type E botulism poisoning, version 1.0. Ology Bioservices, Alameda, CA. [Google Scholar]
- 24.NIAID. 2002. NIAID biodefense research agenda for CDC category A agents. U.S. Department of Health and Human Services, Washington, DC. http://sp.xoma.com/sites/RefLib/Library/niaid-2002.pdf. [Google Scholar]
- 25.Barras V, Greub G. 2014. History of biological warfare and bioterrorism. Clin Microbiol Infect 20:497–502. 10.1111/1469-0691.12706. [DOI] [PubMed] [Google Scholar]
- 26.Meng Q, Li M, Silberg MA, Conrad F, Bettencourt J, To R, Huang C, Ma J, Meyer K, Shimizu R, Cao L, Tomic MT, Marks JD. 2012. Domain-based assays of individual antibody concentrations in an oligoclonal combination targeting a single protein. Anal Biochem 421:351–361. 10.1016/j.ab.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]