Abstract
IMPORTANCE
Imaging biomarkers in Parkinson disease (PD) are increasingly important for monitoring progression in clinical trials and also have the potential to improve clinical care and management. This Review addresses a critical need to make clear the temporal relevance for diagnostic and progression imaging biomarkers to be used by clinicians and researchers over the clinical course of PD. Magnetic resonance imaging (diffusion imaging, neuromelanin-sensitive imaging, iron-sensitive imaging, T1-weighted imaging), positron emission tomography/single-photon emission computed tomography dopaminergic, serotonergic, and cholinergic imaging as well as metabolic and cerebral blood flow network neuroimaging biomarkers in the preclinical, prodromal, early, and moderate to late stages are characterized.
OBSERVATIONS
If a clinical trial is being carried out in the preclinical and prodromal stages, potentially useful disease-state biomarkers include dopaminergic imaging of the striatum; metabolic imaging; free-water, neuromelanin-sensitive, and iron-sensitive imaging in the substantia nigra; and T1-weighted structural magnetic resonance imaging. Disease-state biomarkers that can distinguish atypical parkinsonisms are metabolic imaging, free-water imaging, and T1-weighted imaging; dopaminergic imaging and other molecular imaging track progression in prodromal patients, whereas other established progression biomarkers need to be evaluated in prodromal cohorts. Progression in early-stage PD can be monitored using dopaminergic imaging in the striatum, metabolic imaging, and free-water and neuromelanin-sensitive imaging in the posterior substantia nigra. Progression in patients with moderate to late-stage PD can be monitored using free-water imaging in the anterior substantia nigra, R2* of substantia nigra, and metabolic imaging. Cortical thickness and gyrification might also be useful markers or predictors of progression. Dopaminergic imaging and free-water imaging detect progression over 1 year, whereas other modalities detect progression over 18 months or longer. The reliability of progression biomarkers varies with disease stage, whereas disease-state biomarkers are relatively consistent in individuals with preclinical, prodromal, early, and moderate to late-stage PD.
CONCLUSIONS AND RELEVANCE
Imaging biomarkers for various stages of PD are readily available to be used as outcome measures in clinical trials and are potentially useful in multimodal combination with routine clinical assessment. This Review provides a critically important template for considering disease stage when implementing diagnostic and progression biomarkers in both clinical trials and clinical care settings.
Multiple neuroimaging biomarkers in Parkinson disease (PD) have emerged. Yet specific biomarkers may be better suited to assess the neurodegenerative process at different stages of PD progression. The purpose of this Review is to elucidate the temporal relevance for magnetic resonance imaging (MRI)–based and positron emission tomography (PET)/single-photon emission computed tomography (SPECT) neuroimaging biomarkers.
Biomarkers that separate patients with PD from healthy controls and atypical parkinsonisms (ie, disease state) in preclinical, prodromal, early, and moderate to late stages will first be described. Biomarkers of disease progression at each stage will also be summarized. The following biomarkers will be characterized: (1) PET and SPECT of nigrostriatal dopaminergic, serotonergic, and cholinergic systems; (2) metabolic imaging (18F–labeled fluorodeoxyglucose PET) or cerebral blood flow network imaging; and (3) structural MRI markers, including single-tensor diffusion imaging, free-water imaging, neuromelanin-sensitive imaging, iron-sensitive techniques, and T1-weighted methods (cortical thickness, volumetric-based morphometry [VBM], and deformation-based morphometry) (Box).
Box. Neuroimaging Methods for Each Biomarker.
T1-weighted structural MRI
Methods that index cortical and subcortical volumetric changes and brain atrophy:
Volumetric-based morphometry
Deformation-based morphometry
Cortical thickness
Dopaminergic PET/SPECT
Tracers that mark various aspects of presynaptic nigrostriatal dopaminergic pathology in the midbrain and striatum:
CFT PET and 123I-CIT SPECT reflect striatal membrane dopamine transporters and dopaminergic denervation
FD reflects dopa decarboxylase activity and dopamine storage
DTBZ reflects packaging of dopamine and other monoamines into synaptic vesicles by the VMAT2 and is a marker of dopaminergic (monoamergic) nerve terminal density
Nondopaminergic PET
Tracers that capture serotonergic and cholinergic denervation and neuroinflammation:
[11C]-DASB binds to serotonin transporters and reflects serotonergic denervation (other serotonergic tracers target 5-HT receptors)
[11C]-PMP and [11C]-MP4A both bind to acetylcholinesterase and reflect cholinergic activity [18F]FEOBV binds to the vesicular acetylcholine transporter and may be a more reliable marker for cholinergic nerve terminal density
[11C]-(R)-PK11195 (first generation) and several other second-generation ligands bind to the translocator protein 18kDa and are biomarkers for neuroinflammation
Metabolic and network imaging
Various methods that reveal functional brain changes and pathological covariance patterns:
Metabolic imaging (fluorodeoxyglucose PET)
Cerebral blood flow network imaging (PET, SPECT, and perfusion MRI)
Iron-sensitive MRI
Captures iron deposition as well as loss of dopaminergic cells in the nigrosome-1 in the substantia nigra:
R2* relaxation imaging reflects iron content in the substantia nigra
SWI highlights the nigrosome-1 formation in the dorsolateral substantia nigra pars compacta
QSM reflects iron content in the substantia nigra
Free-water imaging
Quantitative 2-compartment diffusion imaging modality that reflects neurodegeneration and/or neuroinflammation in cortical and subcortical regions:
Free water reflects extracellular fluid
Free water–corrected fractional anisotropy indexes integrity of white and gray matter brain tissue
Neuromelanin-sensitive MRI
Specialized T1-weighted imaging method that is sensitive to neuromelanin-iron complexes:
Loss of neuromelanin-containing dopaminergic neurons in the substantia nigra
Catecholaminergic neurons in the locus coeruleus/subcoeruleus complex
Abbreviations: 5-HT, 5-hydroxytryptamine; CIT, 2-β-carbomethoxy-3-β-(4-iodophenyl)tropane; CFT, 2-β-[11C]-carbomethoxy-3-β-(4-fluorophenyl) tropane; DASB, 3-amino-4-(2-dimethylaminomethyl-phenylsulfanyl)-benzonitrile; DTBZ, [11C]-dihydrotetrabenazine; FD, 6-[18F]fluoro-L-DOPA; FEOBV, fluoroethoxybenzovesamicol; MP4A, methylpiperidin-4-yl acetate; MRI, magnetic resonance imaging; PET, positron emission tomography; PK11195, 1-[2-chlorophenyl]-N-[1-methyl-propyl]-3-iso-quinoline carboxamide; PMP, methylpiperidin-4-yl propionate; QSM, quantitative susceptibility mapping; SPECT, single-photon emission computed tomography; SWI, susceptibility-weighted imaging; VMAT2, vesicular monoamine transporter type 2.
Dopaminergic PET/SPECT
Preclinical and Prodromal PD
PET/SPECT imaging of striatal membrane dopamine transporters (DAT) reveals dopaminergic deficit in approximately 50% of patients with idiopathic rapid eye movement sleep behavior disorder (iRBD) (Figure 1A).1–5 Nonmanifesting leucine-rich kinase 2 (LRRK2) variant carriers also have reduced DAT binding compared with noncarriers6,7 as well as reduced fluorodopa uptake (Figure 1B).8 Using extrapolative methods, it is estimated DAT, fluorodopa, and imaging of vesicular monoamine transporter type 2 (VMAT2) begin to decline years before motor symptoms arise.9,10 However, this estimation should be interpreted cautiously since it varies and is related to other factors, such as age at onset and which tracer is used.10 Moreover, PET markers of presynaptic dopaminergic denervation show potential as preclinical and prodromal disease-state markers with some limitations (Figure 2A; Table).
Figure 1.
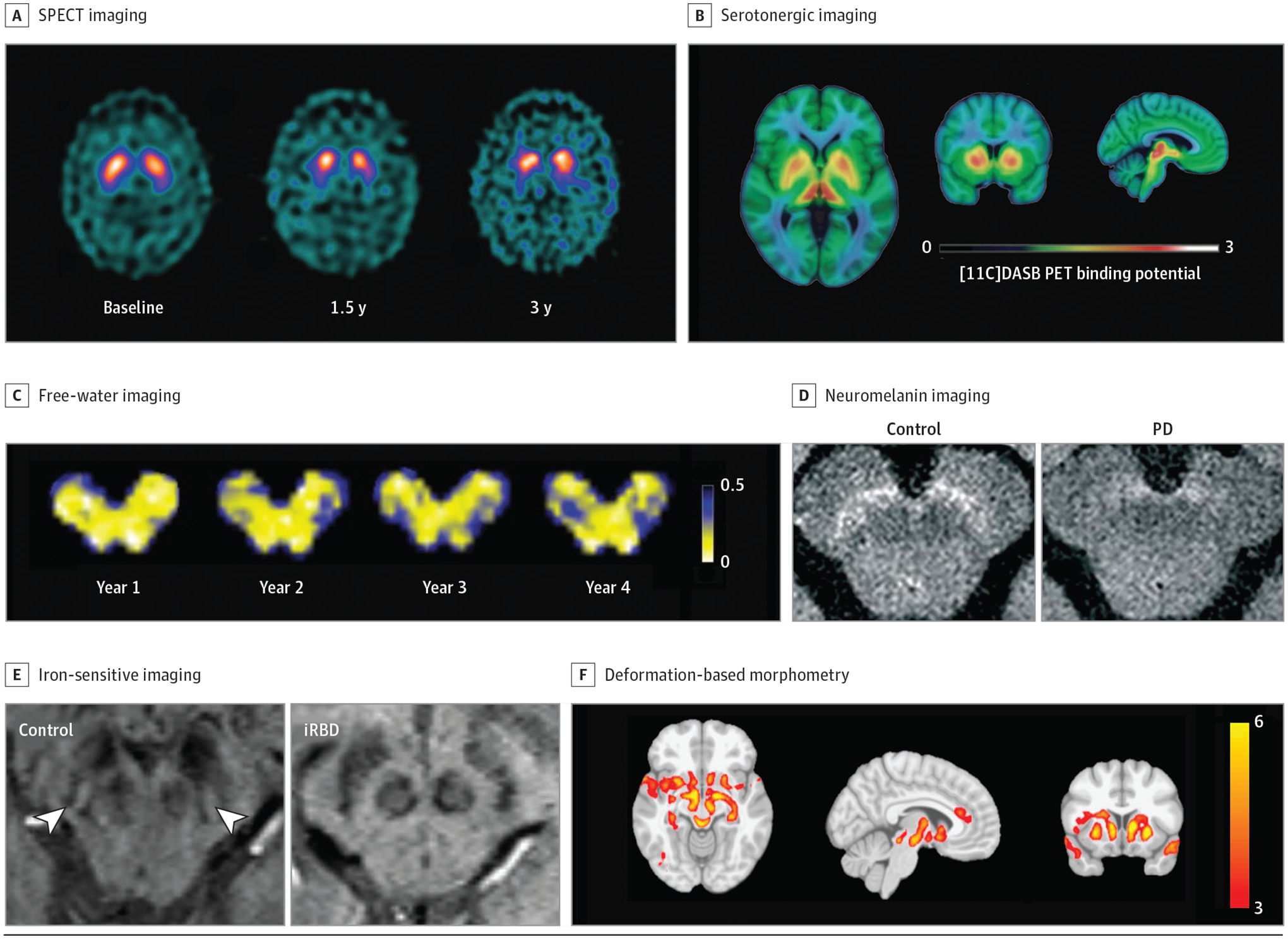
Neuroimaging Biomarkers
Neuroimaging techniques are reviewed. A, Single-photon emission computed tomography (SPECT) imaging in controls and those with early-stage, moderate, and late-stage Parkinson disease (PD; reprinted with permission from Schapira and Olanow1). B, Positron emission tomography (PET) fluorodopa imaging in controls and those with early-stage and late-stage PD (reprinted with permission from Schapira and Olanow1). C, Free-water imaging longitudinally over 4 years in an individual with de novo PD (provided with courtesy of Roxanna Burciu, PhD [Department of Kinesiology and Applied Physiology, University of Delaware, Newark], and used with permission). D, Neuromelanin-sensitive imaging in a healthy control and patient with PD (disease duration of 4 years; reprinted with permission from Biondetti et al2). E, Absence of the nigrosome formation hypointensity using iron-sensitive imaging in a patient with idiopathic rapid eye movement sleep behavior disorder (reprinted with permission from De Marzi et al3). F, Distribution of atrophy in a patient with PD measured by deformation-based morphometry (reprinted with permission from Zeighami et al4).
Figure 2.
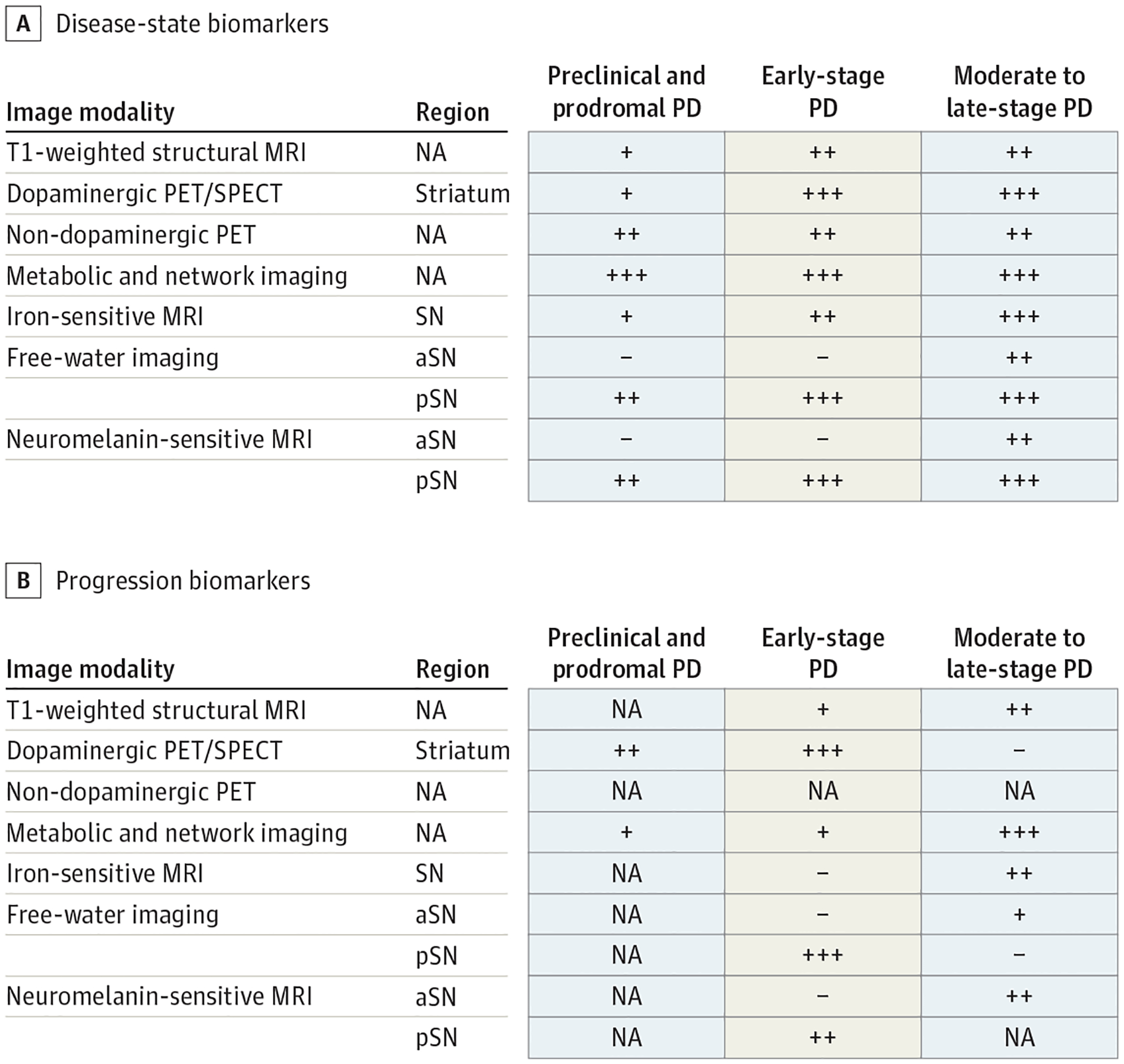
Disease State and Progression Biomarkers
A, Evidence for disease-state biomarkers in patients with preclinical and prodromal, early-stage, and moderate to late-stage Parkinson disease. B, Evidence for progression biomarkers in patients with preclinical and prodromal, early-stage, and moderate to late-stage Parkinson disease. + Indicates the effect was shown by only 1 article or that there are inconsistent results across studies; ++, effect shown in 2 to 3 articles using a single-site cohort; +++, effect shown consistently in 3 or more articles or in a multisite cohort; −, no effect of biomarker was shown. Gray-colored squares are used where there are no studies available. aSN indicates anterior substantia nigra; MRI, magnetic resonance imaging; NA, not applicable; PET, positron emission tomography; pSN, posterior substantia nigra; SN, substantia nigra; SPECT, single-photon emission computed tomography.
Table.
Neuroimaging Biomarkers of Disease State and Parkinson Disease (PD) Progression
| Imaging modality | Disease-state biomarker | Progression biomarker | Clinical application and potential limitations |
|---|---|---|---|
| T1-weighted structural MRI | Potential disease-state biomarker in preclinical, prodromal, early-stage, and moderate to late-stage PD | Potential progression biomarker in early-stage and moderate to late-stage PD (detected >18 mo) | PD-specific progression effects require long follow-up periods |
| Dopaminergic PET/SPECT | Potential disease-state marker in preclinical and prodromal PD, and established disease-state marker in early-stage and moderate to late-stage PD | Potential progression biomarker in preclinical and prodromal PD, and established marker in early-stage PD (<2 y postdiagnosis; detected over 1 y) | SPECT imaging has limited spatial resolution, and there are significant treatment effects of various drugs for dopaminergic PET/SPECT. There is a poor relationship between change in DA imaging and change in clinical outcome. Does not differentiate other parkinsonian syndromes. |
| Nondopaminergic PET | Potential disease-state biomarker in preclinical, prodromal, early-stage, and moderate to late-stage PD | NA | Still limited in clinical application for tracking progression and differentiating atypical parkinsonisms |
| Metabolic and network imaging | Established disease-state biomarker in preclinical, prodromal, early-stage, and moderate to late-stage PD | Potential progression biomarker in preclinical, prodromal, and early-stage PD, and established progression marker in moderate to late-stage PD (detected over 2 y) | Significant acute treatment effects with levodopa and dopamine agonists as well as other therapies, such as deep brain stimulation |
| Iron-sensitive MRI | Potential disease-state biomarker in preclinical, prodromal, and early-stage PD, and established marker in moderate to late-stage PD | Potential progression biomarker in moderate to late-stage PD (detected >18 mo) | Still unclear if useful in differentiating atypical parkinsonisms |
| Free-water imaging | Potential disease-state marker in preclinical and prodromal PD, and established marker in early-stage and moderate to late-stage PD | Established progression biomarker in early-stage PD (detected over 1 y), and potential progression in moderate to late-stage PD | Newer automated methods make these advanced analyses feasible for clinical application |
| Neuromelanin-sensitive MRI | Potential disease-state marker in preclinical and prodromal PD, and established marker in early-stage and moderate to late-stage PD | Potential progression biomarker in early-stage and moderate PD (detected over 2 y) | Only moderately distinguishes atypical parkinsonisms |
Abbreviations: DA, dopaminergic; MRI, magnetic resonance imaging; NA, not applicable; PET, positron emission tomography; SPECT, single photon emission computed tomography.
Longitudinal striatal DAT imaging measures a decline in dopaminergic function over 3 years in iRBD compared with healthy controls (Figure1A).11 Similarly, in individuals with both hyposmia and DAT deficiency, there was progressively reduced DAT binding over 4 years.12 However, several studies have demonstrated there is a poor relationship between change in DA imaging and change in clinical function over time, complicating interpretation of striatal DA imaging.13,14 Furthermore, possible acute and chronic confounding effects of drugs on DA imaging are still not resolved and have potentially significant limitations as a progression biomarker in clinical trials14 (Table). Although there is generally held to be a relationship between dopaminergic markers and nigral dopamine cell counts,15 this may be true for only mild cell loss, and the correlation may be stronger between imaging markers and striatal dopamine content.16 More recently, it has been suggested that striatal DAT binding does not correlate with either tyrosine hydroxylase nigral cell counts17 or striatal tyrosine hydroxylase immunolabeling.18
Early-Stage PD
In addition to DAT imaging, VMAT2 imaging and fluorodopa uptake can be used to detect presynaptic striatal dopaminergic denervation in individuals with early PD (Hoehn and Yahr Scale stage 1).19 These are robust biomarkers of disease state in early-stage PD. VMAT2 imaging is thought to be less subject to compensatory and other regulatory changes in expression than DAT or 6-[18F]fluoro-L-DOPA,20 but may nonetheless be sensitive to large changes in dopamine content,21 and DAT binding tends to be more sensitive to dopamine denervation. However, all dopaminergic tracers are limited to detecting nigrostriatal pathology, and there is also reduced dopaminergic activity in other parkinsonian syndromes, such as progressive supranuclear palsy (PSP) and multiple system atrophy (MSA). Furthermore, variance in dopaminergic binding in atypical parkinsonism seems unreliable for differential diagnosis, and clinical application of dopaminergic imaging is limited to identification of dopaminergic deficit (Figure 3).
Figure 3.
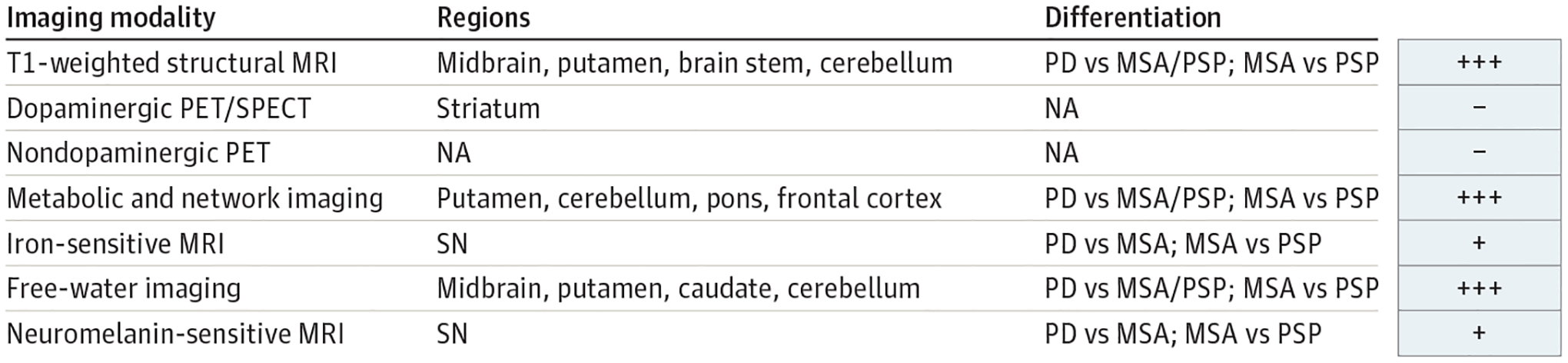
Biomarkers in Differentiating Parkinson Disease (PD) From Atypical Parkinsonisms
+ Indicates the effect was shown by only 1 article or that there are inconsistent results across studies; +++, effect shown consistently in 3 or more articles or in a multisite cohort; −, no effect of biomarker was shown. MRI indicates magnetic resonance imaging; MSA, multiple system atrophy; NA, not applicable; PET, positron emission tomography; PSP, progressive supranuclear palsy; SN, substantia nigra; SPECT, single-photon emission computed tomography.
Fluorodopa uptake declines faster during the first 2 years in de novo PD compared with the subsequent 3 years.22 In the Parkinson Disease Progression Marker Initiative (PPMI) cohort,23 there were similar findings of larger changes in striatal DAT binding over 1 year, with little change between 2 and 4 years.13 Furthermore, striatal dopaminergic markers follow an exponential decline and largely plateau within 5 years of diagnosis.9,19 Moreover, existing data suggest PET/SPECT imaging of dopaminergic function is not sensitive to progression beyond 2 years postdiagnosis. Some research suggests midbrain dopaminergic markers are better suited beyond this point and may also better correlate with clinical function.16
Moderate to Late-Stage PD
DA PET/SPECT measures are good disease-state biomarkers in individuals with moderate to late-stage PD. However, their ability to track progression in moderate to late-stage PD is unlikely (Figure2).19
Nondopaminergic PET Imaging
Preclinical and Prodromal PD
Several nondopaminergic PET modalities, including serotonergic and cholinergic systems and markers of neuroinflammation, are sensitive to disease state and are associated with nonmotor PD pathophysiology. Serotonin transporters are upregulated in nonmanifesting LRRK2 variant carriers compared with downregulated in manifesting LRRK2 variant carriers and those with idiopathic PD, a possibly compensatory or protective mechanism.24 In comparison, nonmanifesting A53T α-synuclein (SNCA) carriers had reduced binding of serotonin transporters in brain stem and subcortical regions compared with controls (Figure 1B).25 Cholinesterase activity was increased in nonmanifesting LRRK2 variant carriers in the cortex.26 In contrast, peripheral cholinesterase activity in the gastrointestinal tract is reduced in individuals with iRBD, along with evidence of cardiac sympathetic denervation and reduced central nervous system noradrenergic activity, even prior to the development of striatal dopamine deficiency.27 Both serotonergic and cholinergic PET variably change in different models of prodromal and preclinical PD, but their potential as early disease-state biomarkers is still unclear. Furthermore, translocator protein (TSPO) PET has shown microglial activation in nonmanifesting LRRK2 variant carriers8 as well as in those with iRBD,28 suggesting a possible role of neuroinflammation in prodromal PD. TSPO ligands have several challenging limitations, including low signal to noise, and in the case of second-generation ligands, binding is largely affected by a disease-independent polymorphism.14,29
Metabolic imaging using fluorodeoxyglucose PET or cerebral blood flow network imaging using PET, SPECT, and perfusion MRI has revealed a PD-related pattern (PDRP) also present in prodromal PD. In particular, the PDRP detects disease state in those with Irbd and is correlated with conversion to PD or dementia with Lewy bodies.30 In addition, PDRP is present in LRRK2 and GBA carriers.31 The ability of the PDRP in tracking progression in preclinical and prodromal stages has not been determined.
Early-Stage PD
Serotonergic imaging demonstrates reduced binding in individuals with early PD (patients with disease duration less than 5 years) and does not correlate to disease severity or duration.32 Furthermore, serotonergic denervation in early PD is correlated with increased dopamine turnover and reduced levodopa repsonse.33 Cholinergic denervation also occurs in early PD (disease duration less than 3 years) but is more pronounced in PD with dementia.34 The utility of these markers in tracking progression (Figure 2B) or differential diagnosis of atypical parkinsonism is not yet clear.
A recent large multisite study in de novo PD demonstrates the PDRP detects disease state prior to dopaminergic treatment35 aswell as atypical parkinsonism.36 Furthermore, the PDRP progresses in early PD (disease duration less than 2 years) over 24 months, suggesting it is also a good progression marker in early PD.37 A critical limitation is the acute effect of dopaminergic treatment that significantly diminishes the PDRP.38
Moderate to Late-Stage PD
In moderate to late-stage PD (disease duration of 5 to 10 and 10 or more years, respectively), serotonergic transporter binding is reduced compared with controls.32 The degree of serotonergic pathology is associated with cognitive decline in those with mild to moderate PD.39 Cholinergic activity is increased in the cortex and thalamus of manifesting LRRK2 variant carriers but affected to variable degrees in idiopathic PD (disease duration of 7 years).26 This could be because cholinergic pathology in PD is strongly related to clinical symptoms, such as cognitive impairment or postural and gait disorders.40,41 Noradrenergic activity is reduced in PD and is associated with the presence of RBD and cognitive impairment.42 Furthermore, PD variants and clinical subtypes should be considered in further development and utilization of serotonergic and cholinergic tracers.
In addition to the PDRP, the PD-related cognitive pattern progresses over time, with the PDRP typically progressing several years prior to the PD-related cognitive pattern.37,43 These separate metabolic networks are of interest particularly in those with moderate to late-stage PD to track progression.
Diffusion Imaging
Preclinical and Prodromal PD
Diffusion imaging in the substantia nigra (SN) measures early neurodegeneration in prodromal PD (Box). Using single-tensor diffusion imaging, there are changes in nigral, midbrain, and pontine fractional anisotropy in individuals with iRBD.44,45 Recently, an advanced 2-compartment diffusion model, free-water imaging,46 was implemented in iRBD demonstrating that free water is increased in posterior SN.47 This is particularly interesting because this subregion also has increased free water in individuals with early PD,48 highlighting posterior nigral free water in individuals with iRBD is indicative of early neurodegenerative processes and is a potentially good target for disease-modifying clinical trials (Figure 2A).
Early-Stage PD
Using single-tensor diffusion imaging, fractional anisotropy is reduced in the entire SN49 or a posterior/caudal subregion50,51 in de novo and early-stage PD, although studies have shown conflicting results. A meta-analysis of 10 studies found no significant association of single-tensor fractional anisotropy with the SN.52 In comparison, free water is increased in the posterior SN in de novo and early PD, a consistent finding replicated in multiple studies and at multiple sites.48,53 In addition, free-water imaging in basal ganglia, midbrain, and cerebellum can differentiate PSP and MSA from PD.54 Free-water imaging is a promising disease-state biomarker with potentially useful clinical applications.
In de novo PD from the multisite PPMI cohort, free water in the posterior SN increased from baseline to 1 year and over 2 and 4 years, and free water change over 1 year predicted 4-year Hoehn and Yahr Scale change (Figure 1C).53 Moreover, free water in the posterior SN is a robust progression marker in individuals with early-stage PD. Free-water imaging reflects neurodegeneration and/or neuroinflammation55 and other advanced diffusion imaging models may similarly be able to index neuroinflammation.56
Moderate to Late-Stage PD
Diffusion imaging in individuals with moderate to late-stage PD generally shows anterior and rostral SN are damaged later in the disease. Using single-tensor diffusion imaging, those with late-stage PD (disease duration of 10 years) had reduced fractional anisotropy in rostral/anterior SN in addition to the caudal SN.51 There were similar findings in those with moderate to late-stage PD (disease duration of 7 years), where free water was increased in both the PD posterior and anterior SN.57 In comparison, those with moderate PD (disease duration of 3 years) had no difference in fractional anisotropy in the rostral SN compared with controls. In addition, diffusion imaging of nucleus basalis of Meynert was shown to precede and predict cognitive impairment,58 suggesting other extranigral regions may index disease state in clinical subtypes.
Free-water imaging in patients with moderate to late-stage PD (disease duration of 7 years) shows there is a longitudinal increase in free water in the anterior but not posterior SN over 3 years.57 This study suggests effects of progression should be monitored in the anterior rather than posterior SN in individuals with late-stage PD (Figure 2B).
Neuromelanin-Sensitive MRI
Preclinical and Prodromal PD
Multiple studies using neuromelanin-sensitive MRI in individuals with iRBD show reduced neuromelanin signal in the SN, particularly in the ventrolateral segment congruent with the posterior subregion with increased free water.2,44 In addition, imaging of the locus coeruleus/subcoeruleus complex is a promising early nondopaminergic marker. Reduced signal identifies iRBD with 82.5% sensitivity and 81% specificity.59 Moreover, neuromelanin-sensitive MRI in the ventral or posterior SN and locus coeruleus have promise as prodromal markers of disease state (Figure 2A).
Early-Stage PD
Neuromelanin imaging in individuals with early-stage PD (disease duration of 1.5 years) reveals reduced signal in the posterior (ie, ventrolateral tier) SN.2 Moreover, neuromelanin-sensitive MRI in the posterior SN also seems to be a robust early-stage marker of PD. Preliminary evidence suggests neuromelanin signal in the SN and locus coeruleus detects some differences in MSA and PSP, although sensitivity and specificity were not optimal compared with PD (Figure 3).60
Neuromelanin-sensitive MRI has shown to track progression in those with mild to moderate PD (disease duration of 1 to 5 years) with varying follow-up times (1 to 5 years),61 as well as in a recent longitudinal study where volumes were derived from neuromelanin signal in both early and advanced PD,2 demonstrating neuromelanin-sensitive MRI is a promising progression biomarker in individuals with early and advanced PD.
Moderate to Late-Stage PD
There is reduced neuromelanin signal in individuals with moderate to late-stage PD (disease duration of 4 to 6 years) compared with controls62 (Figure 1D). Further, individuals with late-stage PD (disease duration of 10 years) had reduced signal in the anteromedial and ventrolateral segments of the SN.2 This pattern of neurodegeneration from ventrolateral to anteromedial SN, detected by free-water imaging and neuromelanin-sensitive imaging, is consistent with the well-established neuropathological pattern of cell loss in the SN. In summary, structural imaging markers of disease state in individuals with moderate to late-stage PD show neurodegeneration of both anterior and posterior SN. Neuromelanin-sensitive MRI also tracks progression in advanced PD.63
Iron-Sensitive MRI
Preclinical and Prodromal PD
Iron-sensitive techniques, including R2* relaxation imaging, susceptibility-weighted imaging (SWI), and quantitative susceptibility mapping (QSM), show promise in iRBD. QSM reveals increased iron content bilaterally in SN in individuals with iRBD.64 In comparison, one study showed nigral R2* mapping did not differentiate individuals with iRBD from controls; however, this may be due to the 2-mm3 resolution and imaging sequence used.44 R2* imaging demonstrates increased iron content in the SN of nonmanifesting LRRK2 and Parkin carriers.65 SWI also highlights the nigrosome formation in dorsolateral SN pars compacta. There is signal loss of this dorsolateral nigral hyperintensity (DNH) in PD, detected in approximately 60% of patients with iRBD (Figure 1E).3 While these imaging methods are promising, future studies are needed to substantiate their usefulness as prodromal disease-state biomarkers.
Early-Stage PD
R2* and QSM in the SN are significantly different from healthy controls in individuals with both early (disease duration less than 1 year) and moderate to late-stage PD.66 Looking at the absence of the DNH, one study using SWI in individuals with early-stage PD (disease duration of 9 months) found signal loss of DNH was an excellent diagnostic marker with an accuracy of 94%.67 Another SWI study in a larger cohort of patients with primarily de novo PD replicated this finding and found 88% of patients had signal loss of DNH.68 Moreover, in early-stage and de novo PD, R2* imaging, SWI, and QSM seem to be robust disease-state biomarkers. For differential diagnosis, SWI reveals a putaminal hypointensity that can be quantified to distinguish MSA.69 However, some studies have shown overlap between MSA and PSP in the putamen, limiting the utility for clinical applications.70 Several studies also report SWI differences in PSP in several brain regions; however, more research is needed.70
Overall, there is insufficient evidence that iron-sensitive techniques track progression in individuals with early-stage PD (Figure 2B). Striatal and nigral R2* relaxation rate increased rapidly in early-stage PD after 2-year follow-up.71 Another study also found increased R2* in the globus pallidus and caudate in early-stage PD over 2 years.72 In comparison, another cohort of de novo PD showed no longitudinal changes in R2* after 3-year follow-up.73 Similarly, in individuals with both early PD (disease duration of 0.5 months) and moderate PD (disease duration of 3 years), there was no difference in rate of R2* or QSM progression compared with controls.66 Authors have suggested these inconsistencies could be due to gliosis and neurodegeneration potentially having a lengthening effect of relaxation times.74
Moderate to Late-Stage PD
R2* and QSM in the SN pars compacta have shown promise as disease-sensitive biomarkers in those with moderate to late-stage PD.66 A meta-analysis of 10 studies using iron-sensitive MRI sequences (SWI, multiecho data image combination MRI, fluid-attenuated in version recovery MRI, T2-weighted MRI) in mostly patients with moderate PD found absence of the DNH resulted in sensitivity and specificity of 97% and 94%, respectively, for differentiating those with PD from controls.75 A novel implementation of an advanced SWI sequence also had high sensitivity and specificity for separating those with PD from controls in those with moderate to late-stage PD.76 Moreover, iron-sensitive techniques of the SN are robust disease-state biomarkers for advanced PD.
One study observed patients with moderate PD (disease duration of 5 years) over 3 years using R2* mapping and found increased relaxation time in the SN.77 In a large cohort of individuals with early PD (disease duration of 0.5 years), moderate PD (disease duration of 3 years), and late-stage PD (disease duration of 10 years), distinct patterns of progression were observed in the SN pars compacta and SN pars reticulata. In the compacta, only those with late-stage PD demonstrated a faster increase in R2* than healthy controls.66 In contrast, in the reticulata, there was a trend for a longitudinal decline of R2* in those with late-stage PD only.66 In this study, QSM did not detect progression in any disease stage,66 suggesting R2* may be a more sensitive progression marker in patients with late-stage PD.
T1-Weighted Structural MRI
Preclinical and Prodromal PD
T1-based structural MRI methods, such as cortical thickness andVBM, provide sensitive disease-state measures in individuals with prodromal PD. VBM reveals reduced hippocampal volume in those with iRBD.45 In addition, patients with iRBD have reduced cortical thickness in frontal, temporal, cingulate, and occipital cortices, with pronounced atrophy in patients with concomitant mild cognitive impairment.78 Further studies and validation of these findings are necessary, particularly in patients with iRBD without cognitive impairment.
Early-Stage PD
T1-based structural methods are sensitive to disease state in patients with de novo or early-stage PD without dementia. Using deformation-based morphometry and independent component analysis in PPMI, a pattern of atrophy was identified in de novo PD,4 which predicted motor progression over 4.5 years (Figure 1F).79 Cortical thickness and VBM applied in a different cohort of patients with newly diagnosed PD found atrophy in subcortical and cortical regions that were more pronounced in individuals with cognitive impairment.80 Furthermore, a recent multisite cohort used machine learning in combination with volumetry and achieved high accuracy differentiating PSP and MSA, a potentially beneficial clinical application of T1-based methods (Figure 3).81
Structural imaging could track disease progression in individuals with early PD without dementia, although it needs to be explored further. In tensor-based morphometry, measures in those with de novo PD decline faster in prefrontal and cingulate cortices as well as the caudate and thalamus compared with controls over 3 years.82 Using cortical thickness, there is greater atrophy in those with mild PD (disease duration of 2 years) without cognitive impairment in frontal regions compared with healthy controls after 18 months.80 In comparison, another study with 18-month follow-up in individuals with mild PD (disease duration of 2 years) without cognitive impairment showed no differences from controls in whole brain atrophy or ventricular enlargement.83 Moreover, the progression effects of atrophy-related structural MRI in PD without cognitive impairment needs to be confirmed (Figure 2B).
Moderate to Late-Stage PD
T1-weighted MRI modalities have varying and conflicting findings in individuals with moderate to late-stage PD without dementia (Figure 2A). No difference in VBM between healthy controls and those with advanced PD without dementia (disease duration of 14 years) has been reported.84 Another study found reduced gray matter in the frontal lobe in patients with moderate PD (disease duration of 3.5 years).85 Currently, the utility of T1-based structural MRI biomarkers in individuals with PD without dementia remains unclear. More consistent methods across studies might help ameliorate this. In contrast, structural changes are more pronounced in patients with mild cognitive impairment and dementia.86 In those with mild cognitive impairment and dementia, there is consistent gray matter atrophy in frontal, temporal, and parietal cortices as well as the hippocampus.86
Longitudinal cortical thickness in those with moderate to late-stage PD (disease duration of 5 years) revealed atrophy of occipital and fusiform regions in PD without cognitive impairment. Patients with cognitive impairment had even greater and more widespread atrophy in supplementary motor area, temporal, parietal, and occipital cortices.87 A more recent study using cortical surface measures showed more accelerated loss of gyrification in bilateral frontal and parietal regions in the late stages (disease duration greater than 5 years) compared with those with early and moderate PD (disease duration less than 5 years).88 Moreover, current evidence suggests T1-weighted MRI methods are more sensitive to neurodegenerative disease processes in more advanced stages. Validation of T1-weighted MRI methods as progression biomarkers are still needed.
Conclusions and Implications for Clinical Care and Management
In summary, it is critical to consider disease stage when choosing an imaging biomarker as an outcome measure in clinical trials (Table). If a clinical trial is carried out in preclinical and prodromal stages, DA imaging and other molecular imaging track progression. Further research is needed to evaluate other imaging progression biomarkers in preclinical and prodromal cohorts. Progression in individuals with early-stage PD can be monitored using striatal DA imaging (less than 2 years postdiagnosis), metabolic imaging, and free-water or neuromelanin-sensitive imaging in posterior SN. Progression in those with moderate to late-stage PD can be monitored using free-water imaging in the anterior SN, R2* of SN, and metabolic imaging. DA imaging and free-water imaging detects progression over 1 year, whereas other modalities detect progression over 18 months or longer (Table). Cortical thickness and gyrification might also track progression in more advanced stages. Disease-state biomarkers that can distinguish atypical parkinsonisms are metabolic imaging, free-water imaging, and T1-weighted imaging. For clinical application of these emerging biomarkers, larger multisite studies with harmonized methods and ligands will help establish normative ranges and standardization that will facilitate federal agency approvals. Further, the US Food and Drug Administration biomarker qualification program require statistical rigor of the biomarker that would include quality control and standard operating procedures, performance reports (eg, mean, range, confidence intervals, sensitivity, specificity, and accuracy), proposed cutoff points, and interoperator and intraoperator variability. In addition, other important criteria would include how the biomarker relates to the clinical outcome or predicts a relevant clinical outcome. Federal agencies will be more likely to approve a medication as disease modifying that slows both clinical progression and a biomarker of progression.
A final consideration is the disease mechanisms one wants to consider in a clinical trial or clinical care. For example, free-water imaging reflects neurodegeneration and/or neuroinflammation55,89 and is linked to synuclein pathology in PD mouse models,90 providing a marker of health for dopaminergic and other neuronal subtypes. Diffusion imaging may be a better marker compared with DA imaging for non–dopamine-related therapies for disease progression. Neuromelanin imaging also reflects the integrity of the dopaminergic neurons within SN, another marker for DA neurodegeneration.91 Nonmotor features central to the clinical picture in PD will be better addressed with nondopaminergic imaging modalities. The potential future role of other PET tracers, such as tau for PSP and amyloid for PD dementia, is being considered. Meanwhile, a multimodal combination of structural (MRI), functional (DA and metabolic imaging), and potentially other modalities (inflammation, other neurotransmitters) would provide stronger interpretation of disease-modifying therapies. Future clinical trials and clinical management should consider these features in trial design and patient care.
Conflict of Interest Disclosures:
Dr Lehéricy has received grants from Biogen outside the submitted work. Dr Strafella has received personal fees from Hoffman–La Roche and GE Healthcare Canada outside the submitted work. Dr Stoessl has received personal fees from Voyager/Neurocrine and SioGene outside the submitted work. Dr Vaillancourt has received grants from the National Institutes of Health and National Science Foundation outside the submitted work and is a cofounder of Neuroimaging Solutions LLC. No other disclosures were reported.
Funding/Support:
This work was supported by grants R01 NS058487, R01 NS052318, T32 NS0821696 from the National Institutes of Health.
Role of the Funder/Sponsor:
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
REFERENCES
- 1.Schapira AH, Olanow CW. Neuroprotection in Parkinson disease: mysteries, myths, and misconceptions. JAMA. 2004;291(3):358–364. doi: 10.1001/jama.291.3.358 [DOI] [PubMed] [Google Scholar]
- 2.Biondetti E, Gaurav R, Yahia-Cherif L, et al. Spatiotemporal changes in substantia nigra neuromelanin content in Parkinson’s disease. Brain. 2020;143(9):2757–2770. doi: 10.1093/brain/awaa216 [DOI] [PubMed] [Google Scholar]
- 3.De Marzi R, Seppi K, Högl B, et al. Loss of dorsolateral nigral hyperintensity on 3.0 tesla susceptibility-weighted imaging in idiopathic rapid eye movement sleep behavior disorder. Ann Neurol. 2016;79(6):1026–1030. doi: 10.1002/ana.24646 [DOI] [PubMed] [Google Scholar]
- 4.Zeighami Y, Ulla M, Iturria-Medina Y, et al. Network structure of brain atrophy in de novo Parkinson’s disease. Elife. 2015;4:4. doi: 10.7554/eLife.08440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iranzo A, Stefani A, Niñerola-Baizan A, et al. ; Sleep Innsbruck Barcelona (SINBAR) Group. Left-hemispheric predominance of nigrostriatal deficit in isolated REM sleep behavior disorder. Neurology. 2020;94(15):e1605–e1613. doi: 10.1212/WNL.0000000000009246 [DOI] [PubMed] [Google Scholar]
- 6.Pont-Sunyer C, Tolosa E, Caspell-Garcia C, et al. ; LRRK2 Cohort Consortium. The prodromal phase of leucine-rich repeat kinase 2-associated Parkinson disease: clinical and imaging studies. Mov Disord. 2017;32(5):726–738. doi: 10.1002/mds.26964 [DOI] [PubMed] [Google Scholar]
- 7.Adams JR, van Netten H, Schulzer M, et al. PET in LRRK2 mutations: comparison to sporadic Parkinson’s disease and evidence for presymptomatic compensation. Brain. 2005;128(pt 12):2777–2785. doi: 10.1093/brain/awh607 [DOI] [PubMed] [Google Scholar]
- 8.Gersel Stokholm M, Garrido A, Tolosa E, et al. Imaging dopamine function and microglia in asymptomatic LRRK2 mutation carriers. J Neurol. 2020;267(8):2296–2300. doi: 10.1007/s00415-020-09830-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nandhagopal R, Kuramoto L, Schulzer M, et al. Longitudinal evolution of compensatory changes in striatal dopamine processing in Parkinson’s disease. Brain. 2011;134(pt 11):3290–3298. doi: 10.1093/brain/awr233 [DOI] [PubMed] [Google Scholar]
- 10.de la Fuente-Fernández R, Schulzer M, Kuramoto L, et al. Age-specific progression of nigrostriatal dysfunction in Parkinson’s disease. Ann Neurol. 2011;69(5):803–810. doi: 10.1002/ana.22284 [DOI] [PubMed] [Google Scholar]
- 11.Iranzo A, Valldeoriola F, Lomeña F, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2011;10(9):797–805. doi: 10.1016/S1474-4422(11)70152-1 [DOI] [PubMed] [Google Scholar]
- 12.Jennings D, Siderowf A, Stern M, et al. ; PARS Investigators. Conversion to Parkinson disease in the PARS hyposmic and dopamine transporter-deficit prodromal cohort. JAMA Neurol. 2017;74(8):933–940. doi: 10.1001/jamaneurol.2017.0985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simuni T, Siderowf A, Lasch S, et al. ; Parkinson’s Progression Marker Initiative. Longitudinal change of clinical and biological measures in early Parkinson’s disease: Parkinson’s Progression Markers Initiative Cohort. Mov Disord. 2018;33(5): 771–782. doi: 10.1002/mds.27361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merchant KM, Cedarbaum JM, Brundin P, et al. ; The Michael J. Fox Foundation Alpha Synuclein Clinical Path Working Group. A proposed roadmap for Parkinson’s disease proof of concept clinical trials investigating compounds targeting alpha-synuclein. J Parkinsons Dis. 2019;9(1):31–61. doi: 10.3233/JPD-181471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraemmer J, Kovacs GG, Perju-Dumbrava L, Pirker S, Traub-Weidinger T, Pirker W. Correlation of striatal dopamine transporter imaging with post mortem substantia nigra cell counts. Mov Disord. 2014;29(14):1767–1773. doi: 10.1002/mds.25975 [DOI] [PubMed] [Google Scholar]
- 16.Perlmutter JS, Norris SA. Neuroimaging biomarkers for Parkinson disease: facts and fantasy. Ann Neurol. 2014;76(6):769–783. doi: 10.1002/ana.24291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saari L, Kivinen K, Gardberg M, Joutsa J, Noponen T, Kaasinen V. Dopamine transporter imaging does not predict the number of nigral neurons in Parkinson disease. Neurology. 2017;88 (15):1461–1467. doi: 10.1212/WNL.0000000000003810 [DOI] [PubMed] [Google Scholar]
- 18.Honkanen EA, Saari L, Orte K, et al. No link between striatal dopaminergic axons and dopamine transporter imaging in Parkinson’s disease. Mov Disord. 2019;34(10):1562–1566. doi: 10.1002/mds.27777 [DOI] [PubMed] [Google Scholar]
- 19.Lee CS, Samii A, Sossi V, et al. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson’s disease. Ann Neurol. 2000; 47(4):493–503. doi: [DOI] [PubMed] [Google Scholar]
- 20.Vander Borght T, Kilbourn M, Desmond T, Kuhl D, Frey K. The vesicular monoamine transporter is not regulated by dopaminergic drug treatments. Eur J Pharmacol. 1995;294(2–3):577–583. doi: 10.1016/0014-2999(95)00594-3 [DOI] [PubMed] [Google Scholar]
- 21.De La Fuente-Fernández R, Furtado S, Guttman M, et al. VMAT2 binding is elevated in dopa-responsive dystonia: visualizing empty vesicles by PET. Synapse. 2003;49(1):20–28. doi: 10.1002/syn.10199 [DOI] [PubMed] [Google Scholar]
- 22.Brück A, Aalto S, Rauhala E, Bergman J, Marttila R, Rinne JO. A follow-up study on 6-[18F]fluoro-L-dopa uptake in early Parkinson’s disease shows nonlinear progression in the putamen. Mov Disord. 2009;24(7):1009–1015. doi: 10.1002/mds.22484 [DOI] [PubMed] [Google Scholar]
- 23.Parkinson’s Progression Markers Initiative. Home page. Accessed March 3, 2021. https://www.ppmi-info.org/
- 24.Wile DJ, Agarwal PA, Schulzer M, et al. Serotonin and dopamine transporter PET changes in the premotor phase of LRRK2 parkinsonism: cross-sectional studies. Lancet Neurol. 2017;16(5): 351–359. doi: 10.1016/S1474-4422(17)30056-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson H, Dervenoulas G, Pagano G, et al. Serotonergic pathology and disease burden in the premotor and motor phase of A53T α-synuclein parkinsonism: a cross-sectional study. Lancet Neurol. 2019;18(8):748–759. doi: 10.1016/S1474-4422(19)30140-1 [DOI] [PubMed] [Google Scholar]
- 26.Liu SY, Wile DJ, Fu JF, et al. The effect of LRRK2 mutations on the cholinergic system in manifest and premanifest stages of Parkinson’s disease: a cross-sectional PET study. Lancet Neurol. 2018;17 (4):309–316. doi: 10.1016/S1474-4422(18)30032-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knudsen K, Fedorova TD, Hansen AK, et al. In-vivo staging of pathology in REM sleep behaviour disorder: a multimodality imaging case-control study. Lancet Neurol. 2018;17(7):618–628. doi: 10.1016/S1474-4422(18)30162-5 [DOI] [PubMed] [Google Scholar]
- 28.Stokholm MG, Iranzo A, Østergaard K, et al. Assessment of neuroinflammation in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study. Lancet Neurol. 2017; 16(10):789–796. doi: 10.1016/S1474-4422(17)30173-4 [DOI] [PubMed] [Google Scholar]
- 29.Varnäs K, Cselényi Z, Jucaite A, et al. PET imaging of [11C]PBR28 in Parkinson’s disease patients does not indicate increased binding to TSPO despite reduced dopamine transporter binding. Eur J Nucl Med Mol Imaging. 2019;46(2): 367–375. doi: 10.1007/s00259-018-4161-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holtbernd F, Gagnon JF, Postuma RB, et al. Abnormal metabolic network activity in REM sleep behavior disorder. Neurology. 2014;82(7):620–627. doi: 10.1212/WNL.0000000000000130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schindlbeck KA, Vo A, Nguyen N, et al. LRRK2 and GBA variants exert distinct influences on Parkinson’s disease-specific metabolic networks. Cereb Cortex. 2020;30(5):2867–2878. doi: 10.1093/cercor/bhz280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Politis M, Wu K, Loane C, et al. Staging of serotonergic dysfunction in Parkinson’s disease: an in vivo 11C-DASB PET study. Neurobiol Dis. 2010;40 (1):216–221. doi: 10.1016/j.nbd.2010.05.028 [DOI] [PubMed] [Google Scholar]
- 33.Fu JF, Matarazzo M, McKenzie J, et al. Serotonergic system impacts levodopa response in early Parkinson’s and future risk of dyskinesia. Mov Disord. 2021;36(2):389–397. doi: 10.1002/mds.28340 [DOI] [PubMed] [Google Scholar]
- 34.Shimada H, Hirano S, Shinotoh H, et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology. 2009;73(4): 273–278. doi: 10.1212/WNL.0b013e3181ab2b58 [DOI] [PubMed] [Google Scholar]
- 35.Schindlbeck KA, Lucas-Jiménez O, Tang CC, et al. Metabolic network abnormalities in drug-naïve Parkinson’s disease. Mov Disord. 2020; 35(4):587–594. doi: 10.1002/mds.27960 [DOI] [PubMed] [Google Scholar]
- 36.Tang CC, Poston KL, Eckert T, et al. Differential diagnosis of parkinsonism: a metabolic imaging study using pattern analysis. Lancet Neurol. 2010;9 (2):149–158. doi: 10.1016/S1474-4422(10)70002-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang C, Tang C, Feigin A, et al. Changes in network activity with the progression of Parkinson’s disease. Brain. 2007;130(pt 7):1834–1846. doi: 10.1093/brain/awm086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asanuma K, Tang C, Ma Y, et al. Network modulation in the treatment of Parkinson’s disease. Brain. 2006;129(pt 10):2667–2678. doi: 10.1093/brain/awl162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kotagal V, Spino C, Bohnen NI, Koeppe R, Albin RL. Serotonin, β-amyloid, and cognition in Parkinson disease. Ann Neurol. 2018;83(5):994–1002. doi: 10.1002/ana.25236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Zee S, Müller MLTM, Kanel P, van Laar T, Bohnen NI. Cholinergic denervation patterns across cognitive domains in Parkinson’s disease. Mov Disord. 2021;36(3):642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bohnen NI, Kanel P, Zhou Z, et al. Cholinergic system changes of falls and freezing of gait in Parkinson’s disease. Ann Neurol. 2019;85(4):538–549. doi: 10.1002/ana.25430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sommerauer M, Fedorova TD, Hansen AK, et al. Evaluation of the noradrenergic system in Parkinson’s disease: an 11C-MeNER PET and neuromelanin MRI study. Brain. 2018;141(2):496–504. doi: 10.1093/brain/awx348 [DOI] [PubMed] [Google Scholar]
- 43.Mattis PJ, Niethammer M, Sako W, et al. Distinct brain networks underlie cognitive dysfunction in Parkinson and Alzheimer diseases. Neurology. 2016;87(18):1925–1933. doi: 10.1212/WNL.0000000000003285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pyatigorskaya N, Gaurav R, Arnaldi D, et al. Magnetic resonance imaging biomarkers to assess substantia nigra damage in idiopathic rapid eye movement sleep behavior disorder. Sleep. 2017;40 (11). doi: 10.1093/sleep/zsx149 [DOI] [PubMed] [Google Scholar]
- 45.Scherfler C, Frauscher B, Schocke M, et al. ; SINBAR (Sleep Innsbruck Barcelona) Group. White and gray matter abnormalities in idiopathic rapid eye movement sleep behavior disorder: a diffusion-tensor imaging and voxel-based morphometry study. Ann Neurol. 2011;69(2):400–407. doi: 10.1002/ana.22245 [DOI] [PubMed] [Google Scholar]
- 46.Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med. 2009;62(3):717–730. doi: 10.1002/mrm.22055 [DOI] [PubMed] [Google Scholar]
- 47.Zhou L, Li G, Zhang Y, et al. Increased free water in the substantia nigra in idiopathic REM sleep behaviour disorder. Brain. 2021;144(5):1488–1497. doi: 10.1093/brain/awab039 [DOI] [PubMed] [Google Scholar]
- 48.Ofori E, Pasternak O, Planetta PJ, et al. Increased free water in the substantia nigra of Parkinson’s disease: a single-site and multi-site study. Neurobiol Aging. 2015;36(2):1097–1104. doi: 10.1016/j.neurobiolaging.2014.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rolheiser TM, Fulton HG, Good KP, et al. Diffusion tensor imaging and olfactory identification testing in early-stage Parkinson’s disease. J Neurol. 2011;258(7):1254–1260. doi: 10.1007/s00415-011-5915-2 [DOI] [PubMed] [Google Scholar]
- 50.Vaillancourt DE, Spraker MB, Prodoehl J, et al. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology. 2009;72(16):1378–1384. doi: 10.1212/01.wnl.0000340982.01727.6e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du G, Lewis MM, Sen S, et al. Imaging nigral pathology and clinical progression in Parkinson’s disease. Mov Disord. 2012;27(13):1636–1643. doi: 10.1002/mds.25182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwarz ST, Abaei M, Gontu V, Morgan PS, Bajaj N, Auer DP. Diffusion tensor imaging of nigral degeneration in Parkinson’s disease: a region-of-interest and voxel-based study at 3 T and systematic review with meta-analysis. Neuroimage Clin. 2013;3:481–488. doi: 10.1016/j.nicl.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burciu RG, Ofori E, Archer DB, et al. Progression marker of Parkinson’s disease: a 4-year multi-site imaging study. Brain. 2017;140(8):2183–2192. doi: 10.1093/brain/awx146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Archer DB, Bricker JT, Chu WT, et al. Development and validation of the Automated Imaging Differentiation in Parkinsonism (AID-P): a multi-site machine learning study. Lancet Digit Health. 2019;1(5):e222–e231. doi: 10.1016/S2589-7500(19)30105-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Febo M, Perez PD, Ceballos-Diaz C, et al. Diffusion magnetic resonance imaging-derived free water detects neurodegenerative pattern induced by interferon-γ. Brain Struct Funct. 2020;225(1): 427–439. doi: 10.1007/s00429-019-02017-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61(4):1000–1016. doi: 10.1016/j.neuroimage.2012.03.072 [DOI] [PubMed] [Google Scholar]
- 57.Guttuso T Jr, Bergsland N, Hagemeier J, Lichter DG, Pasternak O, Zivadinov R. Substantia nigra free water increases longitudinally in Parkinson disease. AJNR Am J Neuroradiol. 2018;39(3):479–484. doi: 10.3174/ajnr.A5545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schulz J, Pagano G, Fernández Bonfante JA, Wilson H, Politis M. Nucleus basalis of Meynert degeneration precedes and predicts cognitive impairment in Parkinson’s disease. Brain. 2018;141 (5):1501–1516. doi: 10.1093/brain/awy072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ehrminger M, Latimier A, Pyatigorskaya N, et al. The coeruleus/subcoeruleus complex in idiopathic rapid eye movement sleep behaviour disorder. Brain. 2016;139(pt 4):1180–1188. doi: 10.1093/brain/aww006 [DOI] [PubMed] [Google Scholar]
- 60.Ohtsuka C, Sasaki M, Konno K, et al. Differentiation of early-stage parkinsonisms using neuromelanin-sensitive magnetic resonance imaging. Parkinsonism Relat Disord. 2014;20(7): 755–760. doi: 10.1016/j.parkreldis.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 61.Matsuura K, Maeda M, Tabei KI, et al. A longitudinal study of neuromelanin-sensitive magnetic resonance imaging in Parkinson’s disease. Neurosci Lett. 2016;633:112–117. doi: 10.1016/j.neulet.2016.09.011 [DOI] [PubMed] [Google Scholar]
- 62.Castellanos G, Fernández-Seara MA, Lorenzo-Betancor O, et al. Automated neuromelanin imaging as a diagnostic biomarker for Parkinson’s disease. Mov Disord. 2015;30(7):945–952. doi: 10.1002/mds.26201 [DOI] [PubMed] [Google Scholar]
- 63.Gaurav R, Yahia-Cherif L, Pyatigorskaya N, et al. Longitudinal changes in neuromelanin MRI signal in Parkinson’s disease: a progression marker. Mov Disord. 2021;36(7):1592–1602. doi: 10.1002/mds.28531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun J, Lai Z, Ma J, et al. Quantitative evaluation of iron content in idiopathic rapid eye movement sleep behavior disorder. Mov Disord. 2020;35(3): 478–485. doi: 10.1002/mds.27929 [DOI] [PubMed] [Google Scholar]
- 65.Pyatigorskaya N, Sharman M, Corvol JC, et al. High nigral iron deposition in LRRK2 and Parkin mutation carriers using R2* relaxometry. Mov Disord. 2015;30(8):1077–1084. doi: 10.1002/mds.26218 [DOI] [PubMed] [Google Scholar]
- 66.Du G, Lewis MM, Sica C, et al. Distinct progression pattern of susceptibility MRI in the substantia nigra of Parkinson’s patients. Mov Disord. 2018;33(9):1423–1431. doi: 10.1002/mds.27318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noh Y, Sung YH, Lee J, Kim EY. Nigrosome 1 detection at 3T MRI for the diagnosis of early-stage idiopathic Parkinson disease: assessment of diagnostic accuracy and agreement on imaging asymmetry and clinical laterality. AJNR Am J Neuroradiol. 2015;36(11):2010–2016. doi: 10.3174/ajnr.A4412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bae YJ, Kim JM, Kim E, et al. Loss of nigral hyperintensity on 3 Tesla MRI of parkinsonism: comparison with (123) I-FP-CIT SPECT. Mov Disord. 2016;31(5):684–692. doi: 10.1002/mds.26584 [DOI] [PubMed] [Google Scholar]
- 69.Meijer FJ, van Rumund A, Fasen BA, et al. Susceptibility-weighted imaging improves the diagnostic accuracy of 3T brain MRI in the work-up of parkinsonism. AJNR Am J Neuroradiol. 2015;36 (3):454–460. doi: 10.3174/ajnr.A4140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sjöström H, Granberg T, Westman E, Svenningsson P. Quantitative susceptibility mapping differentiates between parkinsonian disorders. Parkinsonism Relat Disord. 2017;44:51–57. doi: 10.1016/j.parkreldis.2017.08.029 [DOI] [PubMed] [Google Scholar]
- 71.Hopes L, Grolez G, Moreau C, et al. Magnetic resonance imaging features of the nigrostriatal system: biomarkers of Parkinson’s disease stages? PLoS One. 2016;11(4):e0147947. doi: 10.1371/journal.pone.0147947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rossi ME, Ruottinen H, Saunamäki T, Elovaara I, Dastidar P. Imaging brain iron and diffusion patterns: a follow-up study of Parkinson’s disease in the initial stages. Acad Radiol. 2014;21(1):64–71. doi: 10.1016/j.acra.2013.09.018 [DOI] [PubMed] [Google Scholar]
- 73.Wieler M, Gee M, Martin WR. Longitudinal midbrain changes in early Parkinson’s disease: iron content estimated from R2*/MRI. Parkinsonism Relat Disord. 2015;21(3):179–183. doi: 10.1016/j.parkreldis.2014.11.017 [DOI] [PubMed] [Google Scholar]
- 74.Lehericy S, Vaillancourt DE, Seppi K, et al. ; International Parkinson and Movement Disorder Society (IPMDS)-Neuroimaging Study Group. The role of high-field magnetic resonance imaging in parkinsonian disorders: pushing the boundaries forward. Mov Disord. 2017;32(4):510–525. doi: 10.1002/mds.26968 [DOI] [PubMed] [Google Scholar]
- 75.Mahlknecht P, Krismer F, Poewe W, Seppi K. Meta-analysis of dorsolateral nigral hyperintensity on magnetic resonance imaging as a marker for Parkinson’s disease. Mov Disord. 2017;32(4):619–623. doi: 10.1002/mds.26932 [DOI] [PubMed] [Google Scholar]
- 76.Cheng Z, He N, Huang P, et al. Imaging the nigrosome 1 in the substantia nigra using susceptibility weighted imaging and quantitative susceptibility mapping: an application to Parkinson’s disease. Neuroimage Clin. 2020;25: 102103. doi: 10.1016/j.nicl.2019.102103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ulla M, Bonny JM, Ouchchane L, Rieu I, Claise B, Durif F. Is R2* a new MRI biomarker for the progression of Parkinson’s disease? a longitudinal follow-up. PLoS One. 2013;8(3):e57904. doi: 10.1371/journal.pone.0057904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rahayel S, Postuma RB, Montplaisir J, et al. Cortical and subcortical gray matter bases of cognitive deficits in REM sleep behavior disorder. Neurology. 2018;90(20):e1759–e1770. doi: 10.1212/WNL.0000000000005523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zeighami Y, Fereshtehnejad SM, Dadar M, Collins DL, Postuma RB, Dagher A. Assessment of a prognostic MRI biomarker in early de novo Parkinson’s disease. Neuroimage Clin. 2019;24: 101986. doi: 10.1016/j.nicl.2019.101986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mak E, Su L, Williams GB, et al. Baseline and longitudinal grey matter changes in newly diagnosed Parkinson’s disease: ICICLE-PD study. Brain. 2015;138(pt 10):2974–2986. doi: 10.1093/brain/awv211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chougar L, Faouzi J, Pyatigorskaya N, et al. Automated categorization of parkinsonian syndromes using magnetic resonance imaging in a clinical setting. Mov Disord. 2021;36(2):460–470. [DOI] [PubMed] [Google Scholar]
- 82.Tessa C, Lucetti C, Giannelli M, et al. Progression of brain atrophy in the early stages of Parkinson’s disease: a longitudinal tensor-based morphometry study in de novo patients without cognitive impairment. Hum Brain Mapp. 2014;35 (8):3932–3944. doi: 10.1002/hbm.22449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mak E, Su L, Williams GB, et al. Longitudinal whole-brain atrophy and ventricular enlargement in nondemented Parkinson’s disease. Neurobiol Aging. 2017;55:78–90. doi: 10.1016/j.neurobiolaging.2017.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Agosta F, Canu E, Stojković T, et al. The topography of brain damage at different stages of Parkinson’s disease. Hum Brain Mapp. 2013;34(11): 2798–2807. doi: 10.1002/hbm.22101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain. 2004;127(pt 4):791–800. doi: 10.1093/brain/awh088 [DOI] [PubMed] [Google Scholar]
- 86.Delgado-Alvarado M, Gago B, Navalpotro-Gomez I, Jiménez-Urbieta H, Rodriguez-Oroz MC. Biomarkers for dementia and mild cognitive impairment in Parkinson’s disease. Mov Disord. 2016;31(6):861–881. doi: 10.1002/mds.26662 [DOI] [PubMed] [Google Scholar]
- 87.Hanganu A, Bedetti C, Degroot C, et al. Mild cognitive impairment is linked with faster rate of cortical thinning in patients with Parkinson’s disease longitudinally. Brain. 2014;137(pt 4):1120–1129. doi: 10.1093/brain/awu036 [DOI] [PubMed] [Google Scholar]
- 88.Sterling NW, Wang M, Zhang L, et al. Stage-dependent loss of cortical gyrification as Parkinson disease “unfolds.” Neurology. 2016;86 (12):1143–1151. doi: 10.1212/WNL.0000000000002492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shimony JS, Rutlin J, Karimi M, et al. Validation of diffusion tensor imaging measures of nigrostriatal neurons in macaques. PLoS One. 2018; 13(9):e0202201. doi: 10.1371/journal.pone.0202201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chu WT, DeSimone JC, Riffe CJ, et al. α-Synuclein induces progressive changes in brain microstructure and sensory-evoked brain function that precedes locomotor decline. J Neurosci. 2020; 40(34):6649–6659. doi: 10.1523/JNEUROSCI.0189-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cassidy CM, Zucca FA, Girgis RR, et al. Neuromelanin-sensitive MRI as a noninvasive proxy measure of dopamine function in the human brain. Proc Natl Acad Sci U S A. 2019;116(11):5108–5117. doi: 10.1073/pnas.1807983116 [DOI] [PMC free article] [PubMed] [Google Scholar]


