ABSTRACT
The primary objective of the study was to evaluate the safety and tolerability of single oral doses of sutezolid tablets administered under fasting conditions in healthy adult subjects. The secondary objective was to determine the pharmacokinetics (PK) of sutezolid and two metabolites, PNU-101603 and PNU-101244. Overall, sutezolid was well tolerated when administered as a 300-mg, 600-mg, 1,200-mg, or 1,800-mg dose in healthy adult subjects under fasting conditions. Maximum concentration (Cmax) of sutezolid, PNU-101603, and PNU-101244 increased in a less-than-proportional manner with an increase in sutezolid dose between 300 mg and 1,800 mg. Total exposure (AUClast [area under the concentration-time curve from time zero to the time of the last quantifiable concentration] and AUCinf [area under the plasma concentration time curve from time zero extrapolated to infinity]) of sutezolid, PNU-101603, and PNU-101244 increased proportionally with an increase in sutezolid dose.
KEYWORDS: sutezolid, tuberculosis, pharmacokinetics, phase 1, safety, antimicrobial safety
INTRODUCTION
Tuberculosis (TB) is one of the top 10 causes of death worldwide and the leading cause of death from a single infectious agent in 2019 (1). The World Health Organization (WHO) estimated that 10 million people became ill with TB in 2019, and 1.4 million died (1). An estimated 465,000 incident cases were attributed to rifampicin-resistant TB or multidrug-resistant TB (MDR-TB), which is resistance to both rifampicin and isoniazid (2).
Two main challenges in the treatment of TB disease are the duration and complexity of drug regimens, both of which affect the following: adherence; toxicity, especially of second-line drugs used to treat drug-resistant TB (DR-TB); and the limited availability of pediatric drug formulations for second-line treatment. Current treatment regimens for TB disease require combinations of multiple drugs, ranging from a duration of 6 to 20 months for MDR-TB (3).
While host genetic factors might contribute to the development of MDR-TB, incomplete and inadequate treatment is the most important factor leading to its development (4). Poor adherence to treatment contributes to prolonged infectiousness, drug resistance, relapse, and death (5). Safe, affordable, and easily administered drugs that are effective against DR-TB are urgently needed.
In April 2000, the FDA approved linezolid (Zyvox) to treat serious Gram-positive bacterial infections for up 28 days (6). In recent years, linezolid has been increasingly used to treat difficult cases of MDR-TB and extensively drug-resistant TB (XDR-TB) with apparent clinical benefit (7). However, reports of uncontrolled studies indicate that linezolid’s dose must be reduced often, and/or the duration of its use must be limited due to potentially serious neurologic, ophthalmologic, and hematologic toxicities (8, 9). These toxicities, which typically occur only after months of treatment, are thought to be due to inhibition of mitochondrial protein synthesis (10). Hence, oxazolidinones that may be administered over long periods with reduced toxicity are needed for the treatment of DR-TB.
Sutezolid (PNU-100480), a sulfur-containing oxazolidinone analog of linezolid, has been evaluated as a promising TB drug candidate in preclinical efficacy studies in vitro and in vivo. Like linezolid, sutezolid inhibits the growth of TB by blocking microbial RNA translation and, thereby, protein synthesis, and it appears to be effective against MDR-TB and XDR-TB (11). Because bacterial protein synthesis is not targeted by any of the drugs in the first-line standard of care for treatment of tuberculosis (isoniazid, rifampicin, pyrazinamide, ethambutol), oxazolidinones have no known preexisting resistance or cross-resistance with other TB drug classes (12).
Sutezolid is metabolized to an active sulfoxide metabolite (PNU-101603) and another active but much less prevalent sulfone metabolite (PNU-101244) by flavin-containing monooxygenases (13). In mice dosed with 100 mg/kg once a day (QD), the sulfoxide metabolite achieved five and 10 times the exposure of the parent on the 1st and 24th days of dosing, respectively (14). In cynomolgus macaques, sulfoxide exposure was approximately four times that of parent (15). A one-compartment model for parent with an additional compartment for the sulfoxide characterized the cynomolgus data, although different parameter estimates were reported for 20 mg/kg and 40 mg/kg QD versus 40 mg/kg twice-a-day (BID) dosing (15).
Plasma protein binding of sutezolid, the sulfoxide, and the sulfone is 48%, 4%, and 6%, respectively, at 1 μg/mL (16).
Prior clinical studies have been conducted with sutezolid manufactured by Pfizer. Between 2009 and 2011, Pfizer tested sutezolid in healthy human subjects in a single-ascending-dose (SAD) study (16) and a multiple-ascending-dose (MAD) study (17) and in sputum smear-positive tuberculosis patients in an early bactericidal activity (EBA) study (12). The SAD study made use of a Pfizer suspension formulation, whereas the MAD study was conducted with the company’s suspension and tablet formulation. The EBA study was conducted with the Pfizer tablet formulation. No significant adverse events (AEs) were observed in the SAD study (up to 1,500 mg), MAD study (up to 1,200 mg once daily for 14 days and 600 mg twice daily for 28 days), and EBA study (600 mg twice daily and 1,200 mg once daily for 14 days). There was no effect on the QT interval. However, in the EBA study, seven sutezolid-treated patients (14%) had transient, asymptomatic alanine transaminase (ALT) elevations to 173 ± 34 U/L on day 15, none met Hy’s criteria for serious liver injury, and values for all patients had returned to normal on day 42.
Studies of novel regimens in mice have shown that sutezolid contributes at least as much as linezolid to bactericidal activity (18). Therefore, it has the potential to be a replacement for linezolid.
TB Alliance has developed a new synthetic drug-substance process and tablet formulation of sutezolid in 100-mg and 600-mg strengths. These tablets have been release-tested via International Council for Harmonization (ICH) guidelines. A randomized, double-blind, placebo-controlled, SAD study has been conducted to evaluate the safety, tolerability, and pharmacokinetics (PK) of single oral doses of these new tablets in healthy adult subjects under fasting conditions. The objective here is to describe the results of that study and thereby characterize the properties of the new sutezolid tablets.
RESULTS
Safety.
Overall, sutezolid was well tolerated when administered as a single 300-mg, 600-mg, 1,200-mg, or 1,800-mg dose in healthy adult subjects under fasting conditions. There were no serious AEs or AEs that led to subject discontinuation; all subjects completed the study. Twenty-one of 32 subjects (65.6%) reported a total of 30 treatment-emergent AEs (TEAEs), all mild. Eight (25.0%) subjects reported nine TEAEs that the investigator considered possibly or probably treatment related. There were no significant electrocardiogram (ECG) changes, and no AEs related to clinically significant ECGs or physical examinations. The number of subjects with TEAEs and the number of subjects with TEAEs assessed by the investigator as related to study treatment are presented by treatment group in Table 1. The treatment-related TEAEs are further identified in Table 2.
TABLE 1.
Subjects with TEAEs and TEAEs related to study treatment following single dose of placebo or sutezolida
| MedDRA primary system organ, class/preferred term | Placebo (n = 8) | Sutezolid, 300 mg (n = 6) | Sutezolid, 600 mg (n = 6) | Sutezolid, 1,200 mg (n = 6) | Sutezolid, 1,800 mg (n = 6) | Overall (n = 32) |
|---|---|---|---|---|---|---|
| Subjects with any TEAEs, n (%) | 5 (62.5) | 4 (66.7) | 5 (83.3) | 3 (50.0) | 4 (66.7) | 21 (65.6) |
| Subjects with any treatment-relatedb TEAEs, n (%) | 2 (25.0) | 1 (16.7) | 0 | 2 (33.3) | 3 (50.0) | 8 (25.0) |
Each cohort consisted of six subjects who took sutezolid and two subjects who took placebo. There were no serious AEs and no AEs that led to subject discontinuation. All events were of mild intensity. n, number of subjects; AE, adverse event; TEAE, treatment-emergent AE.
Possibly or probably related.
TABLE 2.
Treatment-emergent adverse events assessed as related to study treatment by the blinded site investigator following a single dose of placebo or sutezolida
| Preferred term | Placebo (n = 8) | Sutezolid, 300 mg (n = 6) | Sutezolid, 600 mg (n = 6) | Sutezolid, 1,200 mg (n = 6) | Sutezolid, 1,800 mg (n = 6) |
|---|---|---|---|---|---|
| Abdominal distension | 0 | 0 | 0 | 0 | 1 |
| Abdominal pain | 0 | 0 | 0 | 1 | 0 |
| Myalgia | 0 | 0 | 0 | 0 | 1 |
| Postural orthostatic tachycardia syndrome | 2 | 1 | 0 | 1 | 1 |
| Transaminases increased | 0 | 0 | 0 | 1 | 0 |
Each cohort consisted of six subjects who took active drug (sutezolid) and two subjects who took placebo. n = number of subjects administered placebo or sutezolid in each dosing group.
One subject who received 1,200 mg sutezolid experienced elevated transaminases. Aspartate transaminase (AST) values on days −1, 2, and 3 were 28, 25, and 35 U/L, respectively, with an upper limit of normal (ULN) of 32 U/L. ALT values were 47, 42, and 51 U/L, respectively, with a ULN of 44 U/L. When the subject returned for an unscheduled follow-up visit on day 6, values were within the normal range, 27 and 43 U/L for AST and ALT, respectively.
PK results.
The study enrolled 32 healthy adult subjects, 24 who received study drug and 8 who received placebo. Data from all 24 subjects who received active treatment were included in the PK analyses.
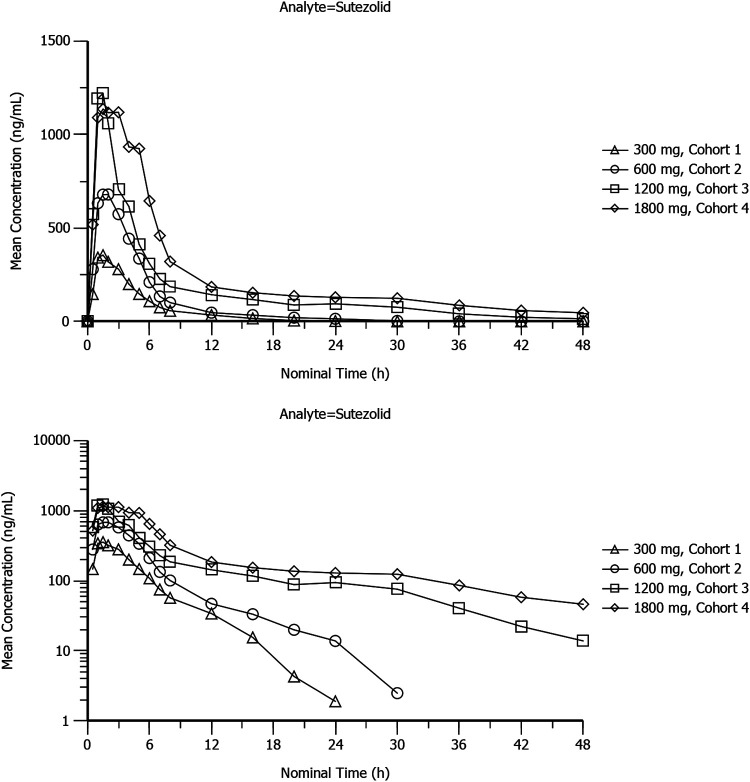
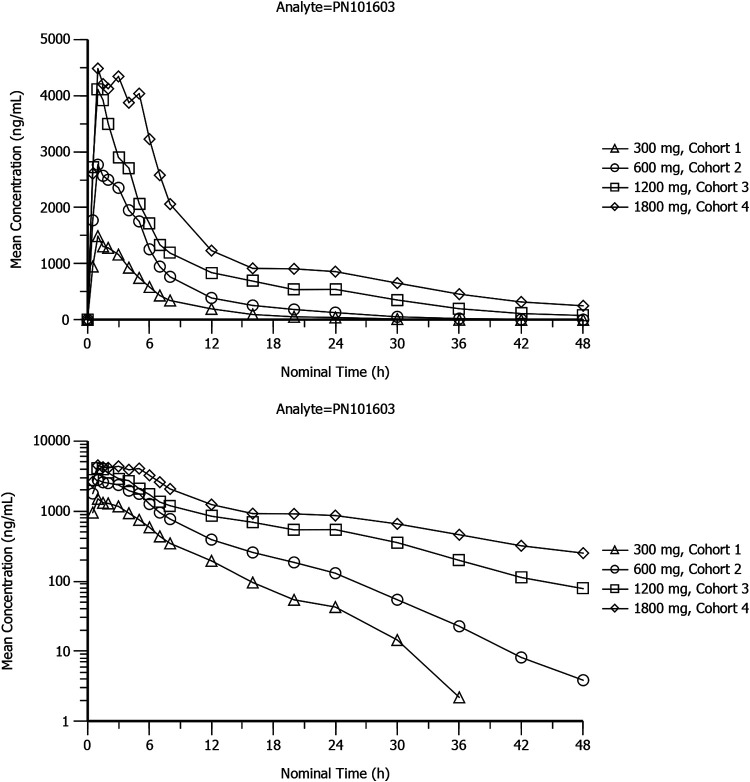
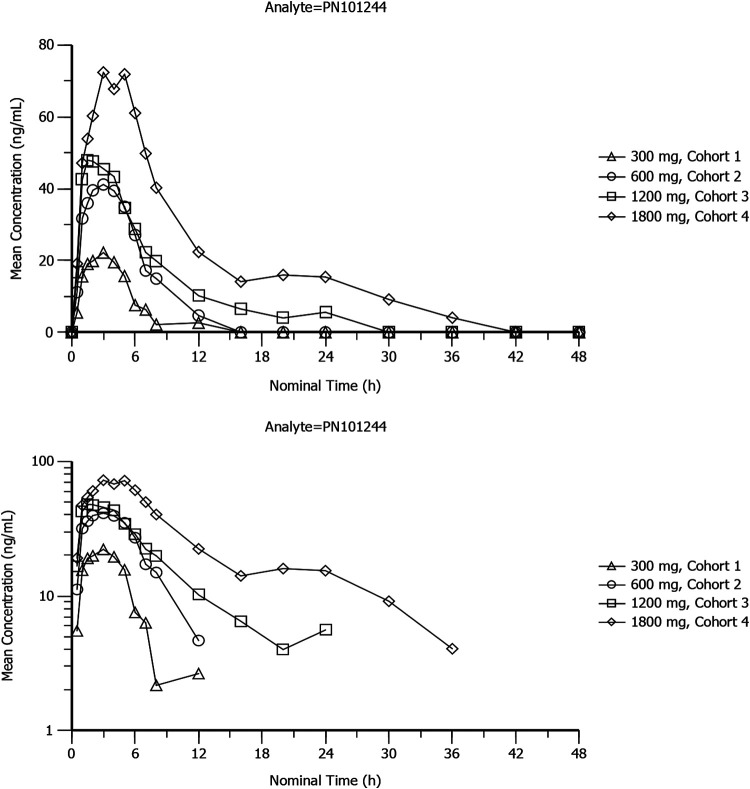
Mean plasma concentration-time profiles of sutezolid, PNU-101603, and PNU-10244 after single doses of 300 mg, 600 mg, 1,200 mg, and 1,800 mg sutezolid tablets are plotted on linear and semilogarithmic scales in Fig. 1, 2, and 3. Individual curves for sutezolid and its metabolites are provided in Fig. S1 and S2 in the supplemental material. Mean concentration curves of sutezolid and PNU-101603 exhibited multiphasic distribution/elimination, more prominently at the higher two doses, with a rapid decline after the peak followed by a somewhat prolonged plateau and then a return to a steeper slope. Such a pattern is also hinted at for PNU-10244, where many concentrations were below the limit of quantification (BLQ).
FIG 1.
Mean plasma concentration-time profiles of sutezolid after single doses of 300-mg (cohort 1), 600-mg (cohort 2), 1,200-mg (cohort 3), and 1,800-mg (cohort 4) sutezolid tablets on linear and semilogarithmic scales (top and bottom panels, respectively).
FIG 2.
Mean plasma concentration-time profiles of PNU-101603 after single doses of 300-mg (cohort 1), 600-mg (cohort 2), 1,200-mg (cohort 3), and 1,800-mg (cohort 4) sutezolid tablets on linear and semilogarithmic scales (top and bottom panels, respectively).
FIG 3.
Mean plasma concentration-time profiles of PNU-101244 after single doses of 300-mg (cohort 1), 600-mg (cohort 2), 1,200-mg (cohort 3), and 1,800-mg (cohort 4) sutezolid tablets on linear and semilogarithmic scales (top and bottom panels, respectively).
The PK parameters of sutezolid and PNU-101603 determined in this study are summarized below in Tables 3 and 4. See Table S1 in the supplemental material for a summary of plasma PK parameters of PNU-101224.
TABLE 3.
Plasma pharmacokinetic parameters of sutezolid
| Parameter | Cohort 1 (300 mg) |
Cohort 2 (600 mg) |
Cohort 3 (1,200 mg) |
Cohort 4 (1,800 mg) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | CV% | n | Mean | SD | CV% | n | Mean | SD | CV% | n | Mean | SD | CV% | |
| Tmax (h) | 6 | 2.18 | 0.94 | 43.15 | 6 | 2.25 | 1.54 | 68.49 | 6 | 1.75 | 1.13 | 64.52 | 6 | 2.50 | 1.52 | 60.66 |
| Cmax (ng/mL) | 6 | 408 | 109 | 26.81 | 6 | 782 | 211 | 27.06 | 6 | 1,440 | 571 | 39.62 | 6 | 1,550 | 633 | 40.85 |
| AUClast (h · ng/mL) | 6 | 1,810 | 490 | 27.11 | 6 | 3,690 | 694 | 18.80 | 6 | 7,720 | 3,060 | 39.63 | 6 | 11,400 | 5,350 | 47.08 |
| AUCinf (h · ng/mL) | 6 | 1,880 | 482 | 25.56 | 6 | 3,810 | 686 | 18.01 | 6 | 8,020 | 3,160 | 39.45 | 5 | 14,100 | 5,880 | 41.66 |
| AUCextrap (%) | 6 | 4.43 | 1.90 | 42.90 | 6 | 3.14 | 1.12 | 35.52 | 6 | 3.80 | 1.64 | 43.17 | 5 | 7.23 | 6.31 | 87.33 |
| λz (h−1) | 6 | 0.175 | 0.0308 | 17.67 | 6 | 0.134 | 0.0585 | 43.59 | 6 | 0.0851 | 0.0194 | 22.83 | 5 | 0.0774 | 0.0442 | 57.13 |
| t1/2 (h) | 6 | 4.08 | 0.773 | 18.93 | 6 | 5.74 | 1.61 | 28.00 | 6 | 8.48 | 1.79 | 21.07 | 5 | 11.7 | 6.80 | 58.05 |
| Tlast (h) | 6 | 17.3 | 4.13 | 23.83 | 6 | 23.7 | 4.46 | 18.83 | 6 | 40.0 | 9.03 | 22.58 | 6 | 42.0 | 9.30 | 22.13 |
| Clast (ng/mL) | 6 | 13.2 | 1.31 | 9.92 | 6 | 14.0 | 2.45 | 17.53 | 6 | 23.8 | 11.6 | 48.84 | 6 | 51.2 | 42.0 | 81.99 |
| CL/F (L/h) | 6 | 167 | 38.2 | 22.83 | 6 | 161 | 27.1 | 16.78 | 6 | 167 | 54.8 | 32.89 | 5 | 145 | 55.4 | 38.25 |
| Vz/F (L) | 6 | 990 | 335 | 33.82 | 6 | 1,360 | 436 | 32.09 | 6 | 2,060 | 898 | 43.60 | 5 | 2,040 | 382 | 18.71 |
TABLE 4.
Plasma pharmacokinetic parameters of PNU-101603
| Parameter | Cohort 1 (300 mg) |
Cohort 2 (600 mg) |
Cohort 3 (1,200 mg) |
Cohort 4 (1,800 mg) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | CV% | n | Mean | SD | CV% | n | Mean | SD | CV% | n | Mean | SD | CV% | |
| Tmax (h) | 6 | 1.68 | 1.05 | 62.60 | 6 | 2.33 | 1.72 | 73.82 | 6 | 1.50 | 1.26 | 84.33 | 6 | 2.50 | 1.79 | 71.55 |
| Cmax (ng/mL) | 6 | 1,620 | 513 | 31.64 | 6 | 3,070 | 782 | 25.50 | 6 | 4,770 | 858 | 17.99 | 6 | 5,850 | 1,730 | 29.51 |
| AUClast (h · ng/mL) | 6 | 9,210 | 2,550 | 27.73 | 6 | 20,100 | 4,730 | 23.55 | 6 | 36,900 | 2,070 | 5.60 | 6 | 57,800 | 12,800 | 22.16 |
| AUCinf (h · ng/mL) | 6 | 9,320 | 2,580 | 27.67 | 6 | 20,200 | 4,730 | 23.42 | 6 | 38,200 | 3,340 | 8.75 | 6 | 64,100 | 19,300 | 30.17 |
| AUCextrap (%) | 6 | 1.21 | 0.327 | 27.03 | 6 | 0.595 | 0.187 | 31.38 | 6 | 3.04 | 3.56 | 117.05 | 6 | 7.90 | 7.99 | 101.18 |
| λz (h−1) | 6 | 0.159 | 0.0360 | 22.65 | 6 | 0.149 | 0.0484 | 32.44 | 6 | 0.117 | 0.0579 | 49.31 | 6 | 0.0889 | 0.0623 | 70.05 |
| t1/2 (h) | 6 | 4.54 | 0.919 | 20.26 | 6 | 5.07 | 1.62 | 31.90 | 6 | 7.61 | 4.42 | 58.04 | 6 | 11.9 | 8.09 | 68.00 |
| Tlast (h) | 6 | 29.0 | 4.50 | 15.52 | 6 | 39.0 | 9.10 | 23.33 | 6 | 47.0 | 2.45 | 5.21 | 6 | 47.0 | 2.45 | 5.21 |
| Clast (ng/mL) | 6 | 16.9 | 3.58 | 21.19 | 6 | 16.7 | 5.67 | 33.97 | 6 | 80.3 | 75.3 | 93.80 | 6 | 253 | 219 | 86.76 |
Sutezolid.
As dose increased from 300 mg to 1,800 mg, the mean sutezolid Cmax (maximum concentration of drug in plasma) values increased from 408 ng/mL to 1,550 ng/mL, and mean area under the concentration-time curve from time zero extrapolated to infinity (AUCinf) increased from 1,880 h · ng/mL to 14,100 h · ng/mL. Mean values of Tmax (time to maximum concentration of drug in plasma) ranged between 1.75 h and 2.50 h postdose.
Mean sutezolid clearance (CL/F) values were similar across cohorts, ranging from 145 L/h to 167 L/h, with no dose dependence. But mean half-life (t1/2) increased with dose from 4.08 h to 11.7 h, and mean volume of distribution (Vz/F) increased from 990 L at 300 mg to around 2,000 L at both 1,200 and 1,800 mg. Differences observed in t1/2 and Vz/F values reflect the greater elaboration of the central plateau portion of the elimination phase at higher doses.
Variability (percent coefficient of variation [CV%]) in maximum and total sutezolid exposure was lower after 300-mg and 600-mg doses (18% to 27%) compared to after 1,200-mg and 1,800-mg doses (39% to 47%). Similarly, variability of t1/2 increased with dose. These patterns reflect the increase of variability in the individual concentration profiles evident in Fig. S1 and S2 in the supplemental material.
Results of the power model for assessing dose proportionality for sutezolid are summarized in Table 5. The slope value for Cmax was 0.74, and the 90% confidence interval did not include 1 (0.56, 0.93). The mean Cmax values seem consistent with dose proportionality through 1,200 mg, approximately doubling as the doses doubled, but with a lower mean Cmax at 1,800 mg than continued dose proportionality would predict. Slopes for AUClast (area under the concentration-time curve from time zero to the time of the last quantifiable concentration) and AUCinf were 0.99 and 1.08, respectively, and 90% confidence intervals included 1 for both parameters: AUClast (0.81, 1.17) and AUCinf (0.92, 1.24).
TABLE 5.
Assessment of dose proportionality of sutezolid following single-dose administration of sutezolidc
| Dependent variable | Model variable | Estimate (β1) | Lower CIa | Upper CIa | Rho1b |
|---|---|---|---|---|---|
| ln(Cmax) | ln(dose) | 0.7449 | 0.5613 | 0.9285 | 1.9266 |
| ln(AUClast) | ln(dose) | 0.9878 | 0.8098 | 1.1658 | 4.5390 |
| ln(AUCinf) | ln(dose) | 1.0791 | 0.9242 | 1.2340 | 3.4200 |
90% confidence intervals (lower and upper).
High/low dose ratio in which dose proportionality can be demonstrated definitely, relative to the lowest dose in the analysis data set. Rho1 was calculated as Rho1 = (ϴH) ^ (1/max(1 − lower, upper − 1)), in which ϴH = 1.333.
Power model: ln(PK) = ln(β0) + β1 × ln(dose) + ε, where PK is the pharmacokinetic parameter tested, ln(β0) is the y-intercept, β1 is the slope, and ε is an error term.
PNU-101603.
Mean PNU-101603 maximum and total exposure increased with increases in sutezolid dose, Cmax ranging from 1,620 ng/mL to 5,850 ng/mL and AUCinf ranging from 9,320 h · ng/mL to 64,100 h · ng/mL. AUCinf values for PNU-101603 were around five times the values for sutezolid at each dose. Mean values of Tmax ranged between 1.50 h and 2.50 h postdose. CV% was consistently below around 30%. As was observed for sutezolid, and for the same reason, mean PNU-101603 t1/2 increased with dose, from 4.54 h to 11.9 h (1,800 mg). As with sutezolid, Cmax increased in a less-than-proportional manner, while AUClast and AUCinf increased proportionally with dose (see Table S2 in the supplemental material).
PNU-101244.
AUCinf values for PNU-101244 were around 2% of the values for sutezolid at each dose. As with the other two analytes, Cmax increased in a less-than-proportional manner, while AUClast and AUCinf increased proportionally with dose (see Table S3 in the supplemental material).
DISCUSSION
Overall, sutezolid was found to be safe and well tolerated when administered as single doses of the TB Alliance tablet at 300 mg, 600 mg, 1,200 mg, or 1,800 mg in healthy adult subjects under fasting conditions. In particular, the safety and PK results encompassed the 1,800-mg dose, a dose level not previously tested in a clinical study with sutezolid.
Maximum sutezolid, PNU-101603, and PNU-101244 exposure (Cmax) increased in a less-than-proportional manner with an increase in sutezolid dose between 300 mg and 1,800 mg. Total sutezolid, PNU-101603, and PNU-101244 exposure (AUClast and AUCinf) increased proportionally with an increase in sutezolid dose.
The dose-proportional increases in AUC, the less-than-proportional increases in Cmax, and the complex and variable concentration-profile shapes suggest the possibility that, at least under fasting conditions, as sutezolid dose increases, absorption saturates in the upper gastrointestinal (GI) tract but is eventually completed more slowly through the lower GI tract.
In the SAD study (16) performed by Pfizer with a suspension formulation under fasting conditions, Cmax of sutezolid increased approximately proportionally between 300 mg and 1,000 mg but then declined at 1,500 mg, and similarly for the sulfoxide. Results were not reported for AUCs. Pfizer then conducted a MAD study of the suspension formulation under both fed and fasting conditions in four cohorts: 100 mg BID (fasted), 300 mg BID (fasted), 600 mg BID (fed), and 1,200 mg QD (fed). As shown in Table 6 below, AUCinf from the present study was comparable with AUCtau of the Pfizer study in the 300-mg, 600-mg, and 1,200-mg cohorts. And Cmax and AUC in Pfizer’s MAD study exhibited approximately dose-proportional behavior, unlike in the company’s SAD study. Thus, it may be the case that the fed conditions at the higher doses of Pfizer’s MAD study facilitated more complete, early absorption. However, t1/2 in the present study (4 to 12 h) was longer than that observed in the Pfizer study (3 h). This may be because Pfizer computed t1/2 using only concentrations through 12 or 24 h postdose for the multiple-dose BID or QD treatment arms, respectively. A more relevant metric may be the effective t1/2 determined from the accumulation ratio Rac. As shown in Table 6, values of effective t1/2 from the Pfizer MAD study range from 3 to 8 h, more in the range of values of t1/2 from the present study.
TABLE 6.
Selected sutezolid PK parameters of the TB Alliance SAD study compared with PK parameters of Pfizer MAD study
| Pfizer MADg | 100 mg BID (n = 8) (suspension fasted) | 300 mg BID (n = 8) (suspension fasted) | 600 mg BID (n = 7) (suspension fed) | 1,200 mg QD (n = 8) (suspension fed) | |
|---|---|---|---|---|---|
| AUCtau (ng · h/mL)a | 845.8 (43) | 2,133 (23) | 4,294 (23) | 10,100 (30) | |
| Cmax (ng/mL)a | 252 (43) | 459 (45) | 943 (20) | 2,020 (50) | |
| Tmax (h)b | 1.0 (0.5–2.0) | 2.0 (0.5–2.0) | 3.0 (2.0–3.0) | 3.0 (2.0–3.0) | |
| t1/2 (h)c,d | 2.72 (5) | 2.55 (27) | 2.92 (41) | 3.38 (10) | |
| Raca,e | 1.565 (12) | 1.080 (19) | 1.194 (14) | 1.051 (14) | |
| Effective t1/2f | 8.16 | 3.20 | 4.58 | 5.50 |
Data represent the geometric mean (percent coefficient of variation).
Data represent the median (range).
Data represent the arithmetic mean (percent coefficient of variation).
t1/2 = half-life.
Rac = accumulation ratio, AUC0–tau, Day 14/AUC0–tau, Day 1, where tau = 12 h for BID and tau = 24 h for QD.
Effective t1/2 computed from the geometric mean Rac as −log(2) × tau/log((Rac − 1)/Rac).
All results for the Pfizer MAD study are from reference 14 except for effective t1/2.
TBA, TB Alliance.
Results from this first study of the TB Alliance tablet formulation of sutezolid support further investigation of multiple doses and of safety and efficacy in patients.
MATERIALS AND METHODS
Study design.
The protocol and other study documents, including the informed consent document, were reviewed and approved by the institutional review board (IRB), IntegReview IRB, located in Austin, TX. The study was conducted by Worldwide Clinical Trials Early Phase Services, LLC, at a single clinical site in San Antonio, TX.
Four cohorts of healthy subjects (n = 8 each) were randomly assigned at a 3:1 ratio to receive sutezolid tablet (n = 6) or placebo (n =2) under fasting conditions. The number of subjects selected for the study was based on the number considered adequate to provide sufficient safety data; this study was not formally powered. Cohort 1 was separated into two groups: a sentinel group of 3 subjects (2 sutezolid and 1 placebo) were dosed at least 24 h before the remaining 5 subjects (4 sutezolid and 1 placebo). All subjects in cohort 1 received a single dose of 300 mg sutezolid or placebo. Subjects in the other three cohorts received placebo or single ascending doses of sutezolid (600 mg, 1,200 mg, or 1,800 mg). Each subject participated in only 1 dose level. The study enrolled subjects who were healthy males or females of nonchildbearing potential, between the ages of 19 and 50 years (inclusive), with a body mass index of ≥18.5 and ≤32.0 kg/m2, and a body weight of at least 50.0 kg.
Dose escalation to the next cohort (dose level) did not take place until the sponsor, in conjunction with the principal investigator, determined that adequate safety, tolerability, and PK data from the previous cohorts demonstrated confidence to proceed.
Blood was collected for PK analysis prior to study drug administration and at serial time points through 48 h after study drug administration. Blood and urine were collected for clinical laboratory evaluations. Female subjects had blood collected for serum pregnancy testing. Postmenopausal females had blood collected to measure follicle-stimulating hormone (FSH) levels. Subjects were housed in the clinic from at least 24 h prior (day −1) until 48 h (day 3) after dosing. Subjects were contacted via a phone call for follow-up questioning about AEs on day 10 (+1 day).
Criteria for evaluation. (i) Safety.
The investigator evaluated safety using the following assessments: physical and neurological examinations, vital signs, ECGs, cardiac monitoring, clinical laboratory tests (including hematology, serum chemistry, coagulation, and urinalysis), and reported or observed AEs.
(ii) Pharmacokinetics.
Blood samples for determination of plasma concentrations of sutezolid, PNU-101603, and PNU-101244 were collected at predose (0 h) and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 7, 8, 12, 16, 20, 24, 30, 36, 42, and 48 h postdose in each treatment period.
Blood was collected into two prechilled 6-mL Vacutainer tubes containing K2-EDTA, gently inverted 8 to 10 times, and immediately placed on wet ice. Samples were maintained on wet ice throughout processing. Within 60 min of blood collection, samples were centrifuged at approximately 1,500 × g at approximately 4°C (±10°C) for 10 min. After centrifugation, plasma was harvested and transferred in approximately equal amounts into four appropriately labeled 5-mL amber cryovials and immediately placed on dry ice. The two primary aliquots contained at least 0.5 mL of plasma. Within 90 min of blood sample collection, aliquots were stored upright in a freezer set at approximately −20°C (±10°C) until transferred on dry ice to the Alliance Pharma, Inc., bioanalytical laboratory for analysis.
Plasma samples were analyzed using a validated LC-MS/MS (liquid chromatography-tandem mass spectrometry) method. Accuracy for all three analytes ranged from 96.4% to 105.5% over 10 to 10,000 ng/mL. Of reassayed samples, 95.7% to 100% met acceptance criteria.
Concentration-time data that were below the limit of quantification (BLQ) were treated as zero in the data summarization and descriptive statistics. During the pharmacokinetic analysis, concentrations that were BLQ up to the time of the first quantifiable concentration were treated as zero. Embedded (values between 2 quantifiable concentrations) and/or terminal BLQ concentrations were treated as “missing.” Actual sample times were used for all pharmacokinetic and statistical analysis. The PK analysis set (all subjects with sufficient data to derive PK parameters) was used in the pharmacokinetic and statistical analyses.
The following PK parameters were calculated for sutezolid, PNU-101603, and PNU-101244, using default settings in WinNonlin except where noted: peak concentration in plasma (Cmax), time to peak concentration (Tmax), elimination rate constant (λz), terminal half-life (t1/2), area under the concentration-time curve from time zero to the time of the last quantifiable concentration (AUClast, using the linear trapezoidal rule), area under the plasma concentration time curve from time zero extrapolated to infinity (AUCinf = AUClast + Clast/λz), percentage of AUCinf based on extrapolation (AUCextrap), apparent total body clearance (CL/F; calculated for sutezolid only), apparent volume of distribution (Vz/F; calculated for sutezolid only), the last quantifiable concentration (Clast), and the time of the last quantifiable concentration (Tlast).
(iii) Dose proportionality.
The PK parameters Cmax, AUClast, and AUCinf for sutezolid and metabolites were compared across doses to assess dose proportionality. Statistical analyses were done using a power model (19) of the following general form:
where PK is the PK parameter tested (e.g., Cmax or AUC), ln(β0) is the y-intercept, β1 is the slope (a value of β1 ≈ 1 indicates dose proportionality), and ε is an error term. The estimate of β1 with the 90% confidence interval and the dose range for proportionality were reported. Statistical analysis was performed in SAS (Version 9.4, SAS Institute Inc.) using the PROC MIXED procedure.
Data availability.
TB Alliance is committed to making sutezolid data and drug material available to the research community and has established an independent review committee to facilitate requests for access. Please contact SIRCquery@tballiance.org for additional information.
ACKNOWLEDGMENTS
This study was supported by TB Alliance (Global Alliance for TB Drug Development) with funding from Australia’s Department of Foreign Affairs and Trade, Bill & Melinda Gates Foundation, the Foreign, Commonwealth and Development Office (United Kingdom), Germany’s Federal Ministry of Education and Research through KfW, Irish Aid, Netherlands Ministry of Foreign Affairs, and the United States Agency for International Development.
We thank Tzvi Jacobs for providing medical writing and publishing support.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.World Health Organization. 2020. WHO global tuberculosis report 2020. Section 1. Introduction. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.World Health Organization. 2020. WHO global tuberculosis report 2020. Section 4.3.2. Estimates of the disease burden caused by drug-resistant TB. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.World Health Organization. 2020. WHO global tuberculosis report 2020. Section 9.3. New drugs and drug regimens to treat TB disease. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 4.Sharma SK, Mohan A. 2006. Multidrug-resistant tuberculosis: a menace that threatens to destabilize tuberculosis control. Chest 130:261–272. doi: 10.1016/S0012-3692(15)50981-1. [DOI] [PubMed] [Google Scholar]
- 5.Munro SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, Volmink J. 2007. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med 4:e238. doi: 10.1371/journal.pmed.0040238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfizer. 2022. Zyvox physician prescribing information. Pfizer, New York, NY. https://www.pfizer.com/products/product-detail/Zyvox.
- 7.Singh B, Cocker D, Ryan H, Sloan DJ. 2019. Linezolid for drug-resistant pulmonary tuberculosis. Cochrane Database Syst Rev 3:Cd012836. doi: 10.1002/14651858.CD012836.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conradie F, Diacon AH, Ngubane N, Howell P, Everitt D, Crook AM, Mendel CM, Egizi E, Moreira J, Timm J, McHugh TD, Wills GH, Bateson A, Hunt R, Van Niekerk C, Li M, Olugbosi M, Spigelman M, Nix-TB Trial Team . 2020. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med 382:893–902. doi: 10.1056/NEJMoa1901814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beekmann SE, Gilbert DN, Polgreen PM, IDSA Emerging Infections Network . 2008. Toxicity of extended courses of linezolid: results of an Infectious Diseases Society of America Emerging Infections Network survey. Diagn Microbiol Infect Dis 62:407–410. doi: 10.1016/j.diagmicrobio.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 10.De Vriese AS, Coster RV, Smet J, Seneca S, Lovering A, Van Haute LL, Vanopdenbosch LJ, Martin JJ, Groote CC, Vandecasteele S, Boelaert JR. 2006. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin Infect Dis 42:1111–1117. doi: 10.1086/501356. [DOI] [PubMed] [Google Scholar]
- 11.Barbachyn MR, Hutchinson DK, Brickner SJ, Cynamon MH, Kilburn JO, Klemens SP, Glickman SE, Grega KC, Hendges SK, Toops DS, Ford CW, Zurenko GE. 1996. Identification of a novel oxazolidinone (U-100480) with potent antimycobacterial activity. J Med Chem 39:680–685. doi: 10.1021/jm950956y. [DOI] [PubMed] [Google Scholar]
- 12.Wallis RS, Dawson R, Friedrich SO, Venter A, Paige D, Zhu T, Silvia A, Gobey J, Ellery C, Zhang Y, Eisenach K, Miller P, Diacon AH. 2014. Mycobactericidal activity of sutezolid (PNU-100480) in sputum (EBA) and blood (WBA) of patients with pulmonary tuberculosis. PLoS One 9:e94462. doi: 10.1371/journal.pone.0094462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanoix J-P, Nuermberger E. 2013. Sutezolid. Drugs Future 38:387–394. doi: 10.1358/dof.2013.038.06.1965098. [DOI] [Google Scholar]
- 14.Williams KN, Stover CK, Zhu T, Tasneen R, Tyagi S, Grosset JH, Nuermberger E. 2009. Promising antituberculosis activity of the oxazolidinone PNU-100480 relative to that of linezolid in a murine model. Antimicrob Agents Chemother 53:1314–1319. doi: 10.1128/AAC.01182-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim S, Scanga CA, de Miranda Silva C, Zimmerman M, Causgrove C, Stein B, Dartois V, Peloquin CA, Graham E, Louie A, Flynn JL, Schmidt S, Drusano GL. 2020. Pharmacokinetics of tedizolid, sutezolid, and sutezolid-M1 in non-human primates. Eur J Pharm Sci 151:105421. doi: 10.1016/j.ejps.2020.105421. [DOI] [PubMed] [Google Scholar]
- 16.Wallis RS, Jakubiec WM, Kumar V, Silvia AM, Paige D, Dimitrova D, Li X, Ladutko L, Campbell S, Friedland G, Mitton-Fry M, Miller PF. 2010. Pharmacokinetics and whole-blood bactericidal activity against Mycobacterium tuberculosis of single doses of PNU-100480 in healthy volunteers. J Infect Dis 202:745–751. doi: 10.1086/655471. [DOI] [PubMed] [Google Scholar]
- 17.Wallis RS, Jakubiec W, Kumar V, Bedarida G, Silvia A, Paige D, Zhu T, Mitton-Fry M, Ladutko L, Campbell S, Miller PF. 2011. Biomarker-assisted dose selection for safety and efficacy in early development of PNU-100480 for tuberculosis. Antimicrob Agents Chemother 55:567–574. doi: 10.1128/AAC.01179-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tasneen R, Betoudji F, Tyagi S, Li S-Y, Williams K, Converse PJ, Dartois V, Yang T, Mendel CM, Mdluli KE, Nuermberger EL. 2016. Contribution of oxazolidinones to the efficacy of novel regimens containing bedaquiline and pretomanid in a mouse model of tuberculosis. Antimicrob Agents Chemother 60:270–277. doi: 10.1128/AAC.01691-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith BP, Vandenhende FR, DeSante KA, Farid NA, Welch PA, Callaghan JT, Forgue ST. 2000. Confidence interval criteria for assessment of dose proportionality. Pharm Res 17:1278–1283. doi: 10.1023/A:1026451721686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S3 and Fig. S1 and S2. Download aac.02108-21-s0001.pdf, PDF file, 0.2 MB (249.3KB, pdf)
Data Availability Statement
TB Alliance is committed to making sutezolid data and drug material available to the research community and has established an independent review committee to facilitate requests for access. Please contact SIRCquery@tballiance.org for additional information.