Abstract
Hierarchical synchronization of sleep oscillations establishes communication pathways to support memory reactivation, transfer and consolidation. From an information-theoretical perspective, oscillations constitute highly structured network states that provide limited information coding capacity. Recent findings indicate that sleep oscillations occur in transient bursts that are interleaved with aperiodic network states, which had previously been considered random noise. Here we argue that aperiodic activity exhibits unique and variable spatiotemporal patterns, providing an ideal information rich neurophysiological substrate to imprint new mnemonic patterns onto existing circuits. We discuss novel avenues in conceptualizing and quantifying aperiodic network states during sleep to further understand their relevance and interplay with sleep oscillations in support of memory consolidation.
Keywords: Sleep-dependent memory consolidation, Slow oscillation spindle coupling, Hippocampal ripples, Non-oscillatory broadband activity, Excitation-inhibition-balance, Hippocampal-neocortical network interactions
Multi-scale rhythms support memory reactivation, transformation and consolidation during sleep
How does the human brain turn novel experiences into long-lasting stable memories? Over the last four decades, a large body of work has established that sleep plays a key role in memory formation [1,2]. The influential active systems memory consolidation hypothesis suggests a two-stage process where novel information is initially encoded in the hippocampus and the neocortex, which subsequently becomes neocortex-dependent as new information is consolidated [1,3–5]. The sleeping brain exhibits rich temporal dynamics, which are thought to subserve this transformation of mnemonic representations [6–9]. During sleep, prominent neuronal oscillations emerge, which serve as an intrinsic clocking mechanism for self-organized timed reorganization. Specifically, non-rapid eye movement (NREM) sleep is characterized by slow oscillations (< 1.25 Hz; sometimes also including delta activity < 4 Hz), sleep spindles (~12–16 Hz), and ripple oscillations (~100–200 Hz). Importantly, these cardinal sleep oscillations are thought to emerge from different neural circuits: slow oscillations are most prominent in prefrontal cortex, spindles are thought to emerge in thalamo-cortical loops, and ripples are most prevalent within the hippocampus [1,7,8,10–12]. The selective synchronization of these oscillations, which span several spatial and temporal scales, has been suggested to trigger a processing cascade [8,10,13]. Specifically, the occurrence of hippocampal ripples has been associated with the selective reactivation and replay (see Glossary) of spiking sequences, which reflect novel mnemonic content [7,14,15]. The repeated activation is thought to trigger plasticity, thus, imprinting a permanent mnemonic trace (also termed engram).
In this perspective, we will first review recent evidence supporting and extending the active systems consolidation theory. We discuss evidence that temporally precise cross-frequency coupling indexes the integrity of memory pathways but might not convey only memory-specific information. Next, we will review evidence that sleep oscillations constitute discrete oscillatory bursts that are interleaved with episodes that are not characterized by prominent spectral signatures and are often considered to reflect quiescence or noise [16]. To date the physiologic origins of these non-oscillatory episodes are not fully understood [17]. We utilize the terms desynchronized, aperiodic and non-oscillatory network state interchangeably to describe episodes where prominent oscillations are absent. This encompasses time points before and after an oscillatory (e.g. spindle) burst, where the network switches from a synchronized to a desynchronized (or vice versa) state, as well as prolonged episodes without a defining periodicity (such as during REM sleep) where no clear oscillatory peak is discernible and are therefore referred to as aperiodic [18,19].
The prevalence of oscillatory and aperiodic states in the sleeping brain
While oscillations constitute the most salient feature of electrophysiological sleep recordings, one cannot assume that this also implies their degree of functional significance. During a typical night of 8 hours of sleep, we spend approximately ~25% (2 hours) in REM sleep, which in humans is not characterized by continuous oscillations, unlike the very prominent ongoing theta (4–10 Hz) activity observed in rodent REM sleep [20]. Furthermore, we spend ~50% (4 hours) in NREM2 and another 25% (2 hours) in deep slow wave (NREM3) sleep [21]. NREM2 is characterized by 1 to 2 seconds long spindle bursts, which periodically reoccur approximately every 3 to 6 seconds [9,13,22]. Hence, one can assume a ratio of ~3:1 of time spent in an aperiodic over a synchronized state in NREM2 sleep. Likewise, sleep slow oscillations constitute the hallmark of NREM3, but are per definition only present ~50% of the time on average. Taken together, using a conservative estimate, one has to conclude that approximately ~50% of total sleep are spent in an asynchronous network state. Note, several definitions for detection of oscillatory events have been utilized, which might yield varying duration estimates. It is now considered best practice to employ algorithms that establish the presence of oscillations based on relative and not on absolute amplitude criteria, thus, resulting in more comparable estimates across different cohorts [21,23].
Collectively, these considerations raise a question: do these non-oscillatory time periods have a functional relevance or can we discard them as sleep intermissions reflecting random noise? Given that information encoding typically takes place during wakefulness, which is characterized by a predominately aperiodic states, it is conceivable that desynchronization during sleep might play a similar role benefiting further processing of newly encoded events [19,24].
Here, we discuss these aperiodic network states and argue that episodes without prominent oscillations reflect a less ordered neural state. From an information-theoretical perspective, random neural activity provides more unique patterns and increased complexity, thus, constituting the optimal milieu to imprint new information onto existing circuits. In contrast, during oscillatory bursts, the underlying population is firing in synchrony, thus, limiting their information processing capacity. This consideration implies that optimal information processing necessarily needs to occur after discrete oscillatory bursts. We will discuss recent empirical evidence in support of this consideration and will conclude by discussing how aperiodic activity can be conceptualized and how it might relate to the underlying circuit architecture, specifically with respect to the balance of excitation and inhibition (E/I-balance). Given the high degree of inter-species similarity of sleep oscillations, we speculate whether aperiodic activity might reflect a suitable level of abstraction to understand increased coding capacity in the human brain. Jointly, this will provide a perspective on how oscillations in concert with broadband aperiodic activity provide the necessary substrate for plasticity and imprinting of novel information onto existing circuits.
Recent trends in understanding systems memory consolidation
The conceptualization of the two-stage process of memory formation typically takes a hippocampus-centric perspective [6,7,25]. Specifically, the hippocampal ripple is proposed to serve as a conductor orchestrating the network organization supporting sleep-dependent memory formation [7,26]. For instance, electrical or optogenetic manipulation of hippocampal ripple expression has profound impact on cortical slow oscillation-spindle timing and subsequent memory formation [10,27]. Similarly to work in rodents, replay of novel mnemonic information constitutes a ripple-triggered process in humans [15]. In line with these considerations, evidence suggested that longer ripples exert a stronger influence on neocortical circuits and subsequent behavior [28,29]. In the same vein, low frequency activity also exhibits multiple distinct facets. It has been reported that slow oscillations (< 1.25 Hz) and delta activity (~1–4 Hz) constitute two distinguishable phenomena, which also subserve distinct functions [30]. Optogenetic manipulation of spiking that was coupled to slow oscillations strengthened the hierarchical oscillatory coupling and improved mnemonic performance. In contrast, optogenetic manipulation of delta activity weakened memory consolidation, supporting dissociable roles of slow oscillations and delta activity for memory formation with opposite effects on behavior [30].
Taken together, these findings further refined our perspective on systems memory consolidation by highlighting previously underappreciated nuances. Critically, at first sight several recent findings seem to contradict the classic theory. For instance, ripples were thought to constitute a uniquely hippocampal feature, but have now also been observed in adjacent regions in the medial temporal lobe [7,13,31] as well as in neocortical association areas [32,33]. To date, the role of neocortical ripples is insufficiently understood, but the preliminary evidence suggests that a tight temporal coordination with their hippocampal counterparts is necessary for memory formation during wakefulness and sleep [32,33]. Critically, these findings also indicate that the neocortex might be more involved in encoding novel information than previously thought. These considerations are nicely paralleled by a set of recent imaging findings that collectively suggested that the neocortex is in fact a rapid learner and does not necessarily require hippocampal input [34]. In a related set of studies, several groups have demonstrated a reversed directionality for the hippocampal-neocortical dialogue and reported that in fact neocortical activations trigger and shape the hippocampal ripple expression and associated replay. For instance, activity in the prefrontal cortex [13,29,35], anterior cingulate [36] or temporal cortex [37] has been shown to modulate hippocampal reactivations, which in turn again modulate neocortical areas, thus, constituting bidirectional interactions.
Currently, less is known about the role of REM sleep for memory consolidation. While there is growing evidence that REM sleep is also involved in memory formation [20,38], the precise mechanisms are unknown. A particular focus has been on the hippocampal theta rhythm (~3–8 Hz), which is less pronounced in humans than in rodents [39] and to date it is unclear if both constitute functional homologues. In a related line of inquiry, it had been argued that REM might play a key function in maintaining network hemostasis by adjusting the balance of excitation and inhibition [40,41], which will be discussed in detail below.
Out of sync, out of memory: Integrity of memory pathways is indexed by precision of the temporal coordination
Sleep oscillations do not occur in isolation, but often emerge sequentially on a rapid timescale [1,8]. Critically, cardinal sleep signatures do not just coincide in time, but are synchronized to each other through phase-amplitude cross-frequency coupling (PAC), where the oscillatory phase of the slower frequency modulates the amplitude of the faster component [1,8,13,42]. For instance, it has repeatedly been shown that the slow oscillation phase predicts the spindle amplitude (Figure 1A; [8,43–45]). Likewise, the slow oscillation phase also modulates ripple amplitude, which in turn is also comodulated by the spindle phase [8,13]. Most commonly, the coupling strength (magnitude of how strongly these events are coupled to each other as determined by correlation) was quantified and linked to metrics of behavioral performance [42,46]. Recently, it became apparent that the precise timing is critical [43,44]: It does not only matter that a spindle always peaks at a consistent slow oscillation phase, but the spindle needs to occur at a specific phase angle within the slow oscillation cycle (Figure 1B). The subsequent processing cascade in the hippocampus is only triggered if the spindle hits this narrow ‘sweet-spot’ [13]. In recent years, it has been demonstrated that multiple age- as well as disease-related deficiencies impair this coupling, which in turns predicts mnemonic deficits and cognitive decline (Figure 1C–E).
Figure 1. Slow oscillation-spindle coupling across the lifespan tracks integrity of memory pathways.
(A) Spindle activity (grey) is nested in the slow oscillation (SO, black), preferably in the up-state (close to the SO peak or as shown in the inset close to 0°) (B) Coupling between SOs and spindles can be quantified by their coupling strength (length of the vector in red) as well as their coupling direction (where the vector is pointing). Several studies suggested that there is a narrow (< 90°, in green) optimal window to successfully initiate the hippocampal-neocortical dialogue in support of memory formation. Critically, SOs and spindle first grow into synchrony during maturation, wile a temporal dispersion can be observed later in life as a function of age- or disease-related cognitive decline. Examples include age-related prefrontal atrophy [43,44], mild cognitive impairment (MCI, [53]), hippocampal damage after autoimmune encephalitis [51] as well as precursors of neurodegenerative disease [52,55]. (C-E) SO-spindle coupling has been found to track both task-specific behavioral performance (panel C), as well as task-independent metrics, such as neuropsychological test scores (panel D) or the burden of precursors of imminent cognitive decline (panel E). Jointly, these findings suggest that SO-spindle coupling indexes the integrity of memory pathways, which constitute the foundation for information transfer in the PFC-MTL network. The graphs in C-E are adapted from data reported in [43,45,52] with permission.
The brain generates slow oscillations and spindles from early childhood, but they only become strongly coupled during maturation and development of the memory system (Figure 1C; [45,47,48]). In contrast, during healthy aging, these oscillations undergo a temporal dispersion and become increasingly uncoupled and less precise in their timing [21,43,44,49,50], thus leading to increased forgetting. Critically, pathological insults at multiple network nodes can impair the coupling. For instance, age-related atrophy in the medial prefrontal cortex (PFC) diminishes slow oscillation-spindle interactions and diminishes cognitive efficacy (Figure 1D; [43]). Similar effects can be observed after autoimmune-mediated encephalitis affecting hippocampal functioning [51] or deposition of precursors of neurodegenerative disease [52], with increasing errors in coupling precision as degeneration commences [53–55].
Importantly, slow oscillation-spindle coupling has repeatedly been linked to mnemonic performance. Depending on the task, both coupling strength as well as coupling direction are predictive of either declarative or procedural memory tasks, respectively [43–45,56,57]. Critically, most studies ruled out confounding factors such as oscillatory amplitude, number of events or broadband changes. Despite these careful controls, slow oscillation-spindle coupling correlates with performance, mostly irrespective of task. Furthermore, several related findings reported that coupling metrics also correlated with overall neuropsychological test scores (Figure 1D; [43]) or metrics of structural integrity (Figure 1E; [52]). Given that correlations can be seen for both task-specific (performance in a given task or reactivation of specific mnemonic traces [45,58,59]) as well as task-unspecific variables (trait-like characteristics such as overall neuropsychological test scores of metrics of structural integrity (i.e. grey matter density), [43,52]), one might conclude that coupling metrics do not necessarily index specific mnemonic content, but more likely non-specifically index the overall functionality of memory pathways. This consideration is in line with the observation that transient electrical volleys in the form of either direct brain stimulation [60] or endogenous epileptic activity [61] may engage these circuits as indicated by spindles that exhibit physiologic waveform shapes. It is currently unknown if these events constitute pathologic pseudo-spindles that lack function or if this phenomenon is the result of network resonance in response to the electrical volley [61–63].
This consideration is paralleled by a set of recent findings that revealed a variable memory response following hippocampal ripples [64]. Here, variability reflects the fact that a single ripple oscillation can trigger multiple responses depending on the state of the receiving region, reflecting either the reactivation of specific mnemonic trace or a variable, unspecific and potentially ‘content-free’ response. This lack of specificity could either imply that an oscillatory burst conveys unspecific information and mainly indexes the network engagement.
Information processing in the sleeping brain: Stable representations in a dynamic system
Sleep oscillations are not a continuous phenomenon, but appear in bursts. On every occurrence, the precise manifestation is variable and depends on the present network state. For instance, it has recently been shown that hippocampal ripple expression is dynamically shaped by the precision of cortical slow oscillation-spindle coupling. Specifically, if the spindle fails to peak within a narrow phase range, hippocampal ripple expression was diminished [13].
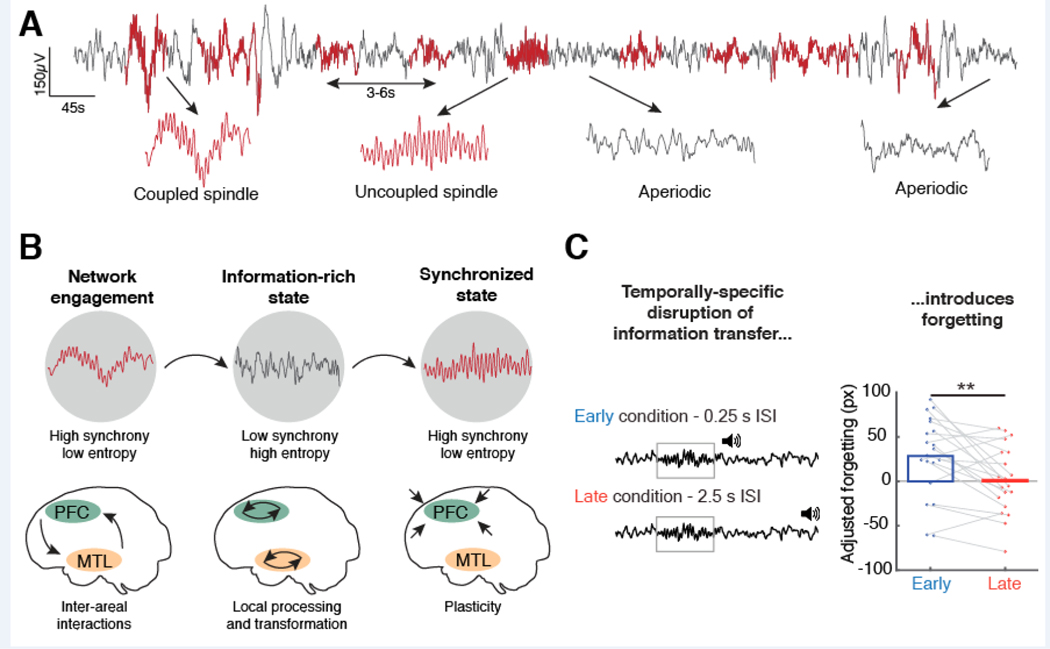
Furthermore, several lines of inquiry converge on the notion that spindle bursts do not occur at random time points, but follow a rhythm on a slower timescale [9,13,22]. Rhythmic spindle bursts relative to hippocampal ripples were first described in rodents [9], albeit on a slower timescale (approx. every ~12s). Recently, similar observations were made in humans (~3–6s; Figure 2A). This set of findings implies that this slower rhythm delineates episodes of high synchrony (oscillations) from aperiodic states (absence of oscillations), since e.g. ripple expression is intricately linked to spindles [7,8,10]. Notably, spindles might also occur in isolation and it is currently unknown if spindle trains differ in their function from isolated spindles [65].
Figure 2. Spindle timing and information transfer.
(A) 45 seconds of NREM sleep (electrode Cz, black) with superimposed spindle oscillations (in red). Arrows highlight distinct signatures of NREM sleep, including (left to right) spindles nested in SOs, uncoupled spindles as well as two examples of aperiodic states. Note, spindles exhibit a second-order temporal structure and re-occur rhythmically every ~3–6 seconds. (B) Schematic of how the different NREM states might contribute to memory consolidation. Note, it is currently unclear if coupled and uncoupled spindles are functionally distinct and it is unknown in which sequence oscillatory and aperiodic states alternate. Left: Coupled SO-spindles trigger bidirectional hippocampal-neocortical interactions (as indicated by the arrows). Center: During aperiodic states, networks might switch to local processing to enable transformation of mnemonic content (arrows indicate local processing loops). Right: Highly synchronized population activity is necessary to mediate synaptic plasticity to enable long-term neocortical storage. (C) Left: Auditory-triggered disruption of the information transfer. A sound was presented directly after an oscillatory (grey box) event (0.25s, early) or with a longer temporal delay (2.5s, late). Right: Only early sound presentation selectively disrupted information transfer as indicated by an increased forgetting rate. Panel C is reproduced with permission from [22].
It remains puzzling how a dynamic system, which exhibits constantly waxing and waning activity patterns, can implement the formation of stable and long-lasting mnemonic representations. Recently, several approaches were introduced to trace mnemonic representations during sleep. For instance, methodologies such as decoding/classification or similarity analyses have all been applied to sleep recordings (Box 1; [66,67]). Critically, most methods implicitly assume that the underlying pattern remains mostly stable with a fixed succession of e.g. firing cells [68]. While the majority of evidence for replay was obtained from single cell firing, similar approaches have been utilized at the level of field potentials [58,59]. To date, it remains unclear what level of abstraction is necessary to successfully track a memory trace throughout consolidation [69]. Importantly, recent advances show that population activity provides a comparable information content as the underlying single unit firing patterns [70]. Specifically, intracranial recordings in humans provide high explanatory power, since it had been shown that decoding in sensory and frontal areas based on high frequency activity (HFA; ~70–150 Hz; [71,72]) contains more decodable information than single unit firing or scalp EEG activity [73]. Furthermore, that this HFA signal does not solely reflect population-spiking activity, but captures additional afferent inputs, thus, further increasing its information content [74].
Box 1. Tools to estimate information content in the sleeping brain
Sleep is thought to subserve the processing of mnemonic information. However, it is non-trivial to study endogenous information processing during sleep in the absence of external input. Several studies circumvented this issue by presenting auditory or olfactory cues during sleep [91–93], which enabled an event-related analysis. However, this technique does not allow studying truly intrinsic processing of memory traces during sleep. Recently, several approaches moved away from an activation-based framework and adopted an information- or representation-based framework [94,95], which can also be applied to continuous recordings where no external events are present. Using either multivariate classification (sometimes also termed decoding or discrimination; [67]) or representational similarity correlation [96]) analyses, several groups started to assess whether neural patterns that were present during encoding are spontaneously recapitulated during sleep. These techniques assume a relatively stable representation during sleep, despite dramatically different electrophysiological features (cf. Figure 3B). An algorithm is trained on a specific pattern that was present during learning or awake retrieval. Next, sleep recordings are searched for evidence that a similar pattern reoccurs, which based on likelihood estimations (e.g. correlation with behavior or permutation statistics) is then considered as evidence for reactivation. The mutual information framework constitutes a related approach to detect similarities between encoding, retrieval and sleep patterns [13]. Recently, reactivation and decoding approaches have also been combined to study sleep-dependent learning [97]. The authors demonstrated that periods of memory reactivation span across several seconds, encompassing both oscillatory as well as aperiodic states. Furthermore, the results indicated that the duration of memory reactivation exceeded the typical duration of sleep spindles, further supporting the notion that processing entails aperiodic states. One shortcoming that applies to all techniques is that it is currently unclear what the most informative features are. In other words, there is no consensus on which level of abstraction is necessary to extract memory-specific information from neural recordings [98]. As an example, is it sufficient to quantify a spatial pattern or do we need to incorporate precise temporal information?
Box 2. How to quantify non-oscillatory brain activity
Time-series analysis in neuroscience often utilizes spectral analyses, such as Fourier, Hilbert or Wavelet transforms, to study the activity in distinct frequency bands [86]. For instance in sleep EEG, the Fourier transform is commonly used to obtain a power spectrum where individual peaks can be detected (e.g. in the spindle-band, [99]). Subsequently activity in this frequency band can be extracted using band-pass filtering combined with a Hilbert transform to extract amplitude- and phase-specific information. However, it is less common to study time points in sleep electrophysiology where no prominent spectral signatures are present [100]. This raises the question how to quantify network states where strong oscillations are absent. One approach that has been motivated by physics is the analyses of 1/f characteristics, which have often been interpreted as a power law [17]. Several tools have been introduced in recent years to disentangle oscillatory activity (which exhibits a defining temporal scale with a fixed period) from non-oscillatory or aperiodic activity [18,19,87,101,102], which lacks defining temporal characteristics (thus, sometimes also termed scale-free). These approaches include polynomial fitting of the power spectrum, Gaussian-modeling [19] or irregular resampling [101] of the time series. What all approaches have in common is that they enable extracting parameters that describe the statistical regularities of the background activity and are well suited to quantify states that lack prominent oscillations. Critically, these tools still require a spectral transformation at some point in the process. Currently, there are only a few approaches that solely operate in the time- and not frequency-domain [103,104]. Future developments will need to take techniques into account that can quantify temporal regularities, for instance based on waveform shape [105], which are not obvious to the naked eye, but might contain complementary information to more established algorithms.
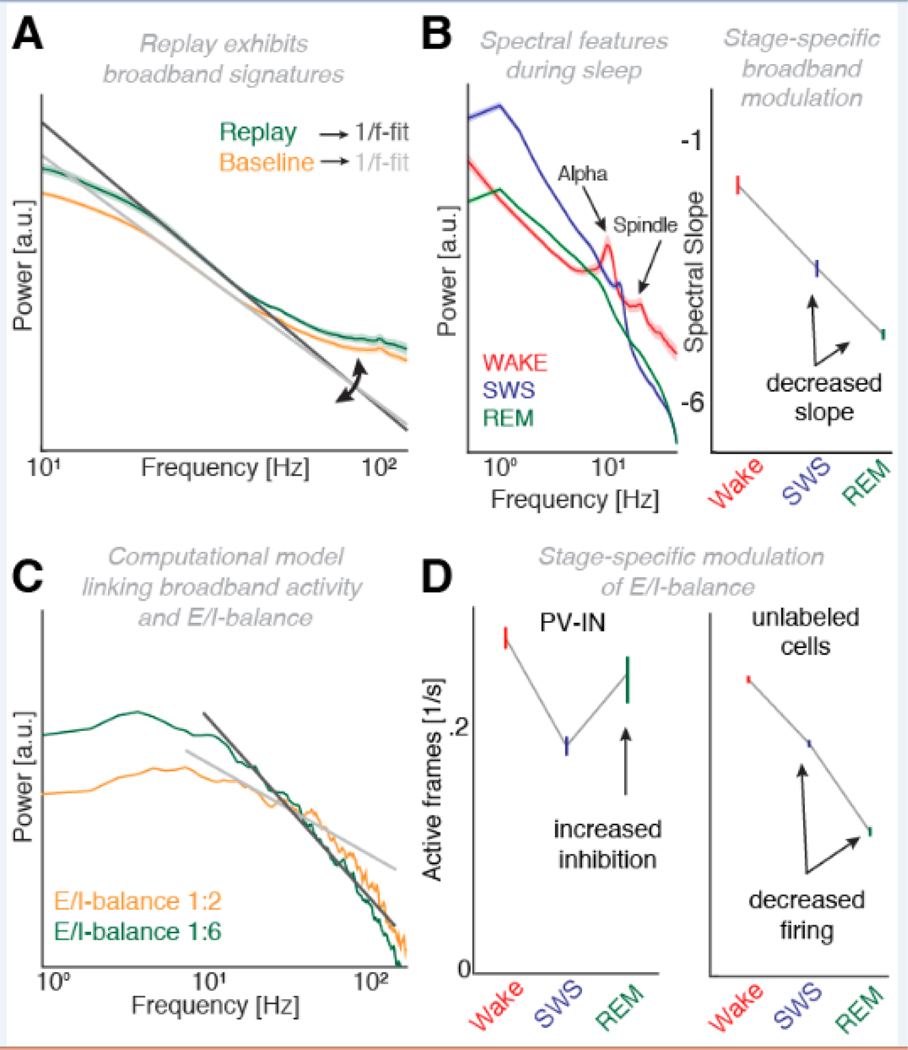
Collectively, oscillations reflect highly temporally- and often also spatially structured sequences that can be captured by a few parameters. In the Shannon information theory framework [75,76], oscillations reflect states of high order and consequently, low Shannon entropy [77,78]. Critically, low entropy states reflect limited information capacities and are suboptimal to imprint novel information [79]. In contrast, aperiodic states reflect higher levels of entropy providing additional capacity to encode new information [24,77]. In line with this consideration, several recent studies showed that inter-areal information flow is actually enhanced after an oscillation and not during the oscillation itself [13,22,59]. These findings contribute to the notion that oscillations serve as a messenger signal, but information transformation might occur during a desynchronized state with synchrony and shared information cycling in anti-phase (Figure 2A/B). After a hippocampal ripple, the directed influence from hippocampus to neocortex does not peak immediately, but ramps up only after 1 to 2 seconds preceded by information flow from prefrontal cortex to hippocampus [13]. Critically, it has been observed that if a sound is presented directly following an oscillatory event (< 1 second), the processing cascade is disrupted, resulting in less information flow and leading to increased forgetting (Figure 2C). One testable hypothesis that emerges from these considerations is that oscillation-locked decoding should peak after and not during the spindle or ripple event [58,59]. Moreover, recent evidence revealed that replay signatures at the level of brain-wide networks exhibit broadband and not narrow-band signatures [80], further substantiating the notion that information processing is maximized during asynchronous network states (Figure 3A).
Figure 3. Non-oscillatory electrophysiological features track excitability during sleep.
(A) Replay of information (green; baseline in orange) is not frequency-specific but exhibits broadband spectral signatures. Superimposed (dark and light grey) spectral slope estimates that show a shift along the y-axis and imply a rotation of the power spectrum. (B) Left: Electrophysiological power spectra during sleep. During wakefulness alpha oscillations at ~11 Hz are prominent (red), while slow wave sleep (SWS, blue) is characterized by increased delta (< 4 Hz) and spindle power (~16 Hz). Note that REM (green) does not exhibit any prominent oscillations as indicated by the absence of a characteristic bump exceeding the 1/f drop-off. Right: Quantification of the slope of the 1/f drop-off reliably distinguishes sleep stages. (C) Computational model linking the shape of the power spectrum to the balance between excitation and inhibition (E/I-balance). Increasing the level of inhibition from 1:2 (orange) to 1:6 (green) resulted in a steepening of the power spectrum slope. (D) Two-photon calcium imaging demonstrated a relative increase in inhibition. Left: Inhibitory parvalbumin+ interneuron (PV-IN) activity was increased during REM sleep. Right: Overall firing rates were reduced during SWS and REM sleep. This effect was highly significant for unlabeled cells, including pyramidal cells as well as somatostatin interneurons, thus, nicely mimicking the distribution as observed for human slope estimates at the population level (cf. right side in panel B). In summary, this set of findings indicates that a relative increase in inhibition is mediated by a decrease in overall firing as well as an increase in inhibitory drive. Panel A was modified with permission under the Creative Commons CC-BY license from [80], panel B was published under the CC-BY license and adapted from [18], panel C was adapted with permission from [87] and panel D was adapted with permission from [88].
Collectively, this set of findings suggests that the neocortical interplay of slow oscillations and spindles triggers hippocampal reactivations. Thereafter, the cortex goes from a synchronized into a desynchronized state to maximize processing capacities when reactivating mnemonic information. Similar considerations might apply to episodic memory formation during wakefulness [77,81], but is currently unknown if the outlined principles are generalizable between wakefulness and sleep.
The physiologic basis of non-oscillatory brain activity during sleep
Neuronal oscillations are thought to emerge from the synchronized firing of multiple neurons within a population [82,83]. Conversely, it could be assumed that desynchronized electrophysiological field patterns should also be the result of asynchronous neuronal firing [84]. However, to date it is not established how the collective firing of different neuron types gives rise to the local field potential [82]. Several lines or research suggest that there might be a region- and state-specific transfer function between spiking activity and field potentials [85].
Traditionally, spectral analyses have been used to determine neuronal oscillations and similar methods have been adopted to quantify the absence of oscillations (Box 2; [86]). It is also evident that spectral transformations of sleep data exhibit rich state-dependent signatures (Figure 3B; [18]). While oscillations can be identified as bumps exceeding the 1/f drop-off of the power spectrum, several recent attempts have parameterized the 1/f decay and linked it to physiology [19]. When displayed in log-log space, the power spectrum can be estimated by a linear fit where both the offset on the y-axis as well as the slope have been linked to population firing [84,87]. One model proposes that the steepness of the slope of the power spectrum is linked to the balance of excitation and inhibition in the underlying population (Figure 3C). While the model only accounted for ~24% of the variance in empirical data, it has provided an additional perspective into both monkey and human recordings. Here, inhibition was boosted through administration of the GABAergic anesthetic propofol. In both species, the slope got steeper when inhibition increased [18,87]. When being applied to sleep data, it was found that the slope distinguished different sleep stages and specifically delineated REM from both wakefulness as well as NREM sleep, solely based on the electrophysiological recordings (Figure 3B; [18]). Critically, the slope during REM was found to be more negative than during NREM, indicating an increased level of inhibition. This consideration was paralleled by a recent two-photon calcium imaging study, which revealed a comparable pattern at the level of population firing [88]: First, overall firing activity was decreased during REM sleep, but firing of inhibitory cells was selectively increased, thus, constituting a relative shift of the E/I-balance towards inhibition (Figure 3D). Moreover, a recent study employing magnetic resonance spectroscopy provided complementary results by demonstrating a learning-specific shift towards inhibition during REM sleep [89]. A functional and behavioral dissociation in NREM sleep was observed, where excitability and overall performance were increased, irrespective of pre-sleep learning. Previously, it had been observed that REM sleep subserves downscaling of excitability in cortical networks, which was accompanied by changes in both firing as well as spectral power [40,41]. Critically, this effect spanned almost all frequency bands, which implied a change in the aperiodic background activity.
Collectively, while we are at the beginning of understanding how unit firing, population activity and network oscillations are related, several recent empirical findings have provided a perspective on how to conceptualize both oscillatory as well non-oscillatory brain states in relationship to network homeostasis between excitation and inhibition.
Concluding Remarks
The mechanisms that support the self-organization of the sleeping brain to optimally support information reactivation, processing, transfer and consolidation remain elusive. Converging evidence suggests that sleep oscillations may provide key messengers to coordinate memory consolidation in space and time. These oscillations likely constitute an endogenous timing mechanism providing distinct windows-of-opportunity for information processing as exemplified by the spindle pulsing every 3–6s, which might segregate reactivation, transfer and consolidation. Importantly, selective synchronization within narrow time windows might serve as a tuning mechanism – similar to a radio dial – to selectively engage communication channels [13,90] as indicated by slow oscillation-spindle coupling that precisely indexes hippocampal ripple expression and subsequent reactivation [8,10,13,30,58].
Critically, we argue that aperiodic states, which are interleaved with oscillatory states, provide an information-rich state to transform, consolidate and eventually imprint new information onto neuronal circuits. However, our understanding of aperiodic episodes is limited and many questions remain (see Outstanding Questions). We reviewed evidence that suggests that the absence of temporal structure might actually be beneficial for information coding. This concept also requires novel tools to capture the absence of oscillations.
Outstanding Questions Box.
How does the sleeping brain achieve specific reactivations? Does slow oscillation-spindle-ripple coupling precision serve as a temporal code to selectively retrieve information?
What is the functional role of short- or long-duration ripples? At which point in the processing hierarchy is the duration of the oscillatory burst read out and used for subsequent computations? Likewise, do the same principles extend to slow oscillations and spindles?
What are the precise functional differences between slow oscillation-coupled and uncoupled spindles? Do spindles that occur in trains differ in their function from spindles that occur in isolation? What is their relationship with aperiodic activity and what are the organizing principles of their interplay?
Why are sleep oscillations highly similar in different species, despite dramatically different cognitive capacities? Which neural markers reflect the increased information coding capacity in the human brain?
How can time-varying, ever-changing neural activity support the emergence of stable and long-lasting mnemonic representations?
Are aperiodic network states a brain-wide phenomenon or do they occur locally as well? What is the interplay of aperiodic activity in different network nodes, such as the hippocampus and prefrontal cortex?
Are aperiodic states as observed during wakefulness, NREM and REM sleep related? Do different aperiodic episodes exhibit similar functional properties or do they reflect a multiplexed signal? Does the duration of aperiodicity play a functional role for memory consolidation?
How does aperiodicity relate to plasticity, which typically requires highly synchronized inputs? Alternatively, is plasticity only mediated by oscillations?
What is the spatial structure of aperiodic network states? Do co-occuring desynchronized states delineate distinct large-scale networks? How are oscillatory and aperiodic networks related?
Does spindle timing and inter-spindle intervals correlate with mnemonic capacities?
Do ripples in humans and rodents support the same functions, despite pronounced differences in their peak frequency?
What is the functional significance of 1/f-scaled background activity? How is population firing linked to spectral parameters? Do distinct spectral parameters (slope, offset, autocorrelation function, band limited features) index homeostasis?
What is the appropriate level of abstraction to study memory reactivation and its relationship to aperiodic activity: Neural firing, field potentials, local circuit activity or large-scale population activity as measured at the network level?
Do the principles of the interplay between synchronization and desynchronization during sleep generalize to wakefulness?
Finally, it is important to highlight that this theory is fully compatible with previous considerations, since it implies that oscillations in concert with broadband activity jointly support information coding. Collectively, we propose an oscillation-mediated dynamic process with rapid cycling between synchronized and desynchronized states that each subserve distinct elements of sleep-dependent memory formation, namely triggered reactivation and subsequent processing for consolidation.
Highlights.
The quality of cortical SO-spindle coupling shapes hippocampal ripple expression and indexes the integrity of memory pathways
Synchronized and desynchronized network states alternate on a slow time scale to temporally segregate reactivation and subsequent information processing
Temporally-specific disruption of bidirectional network interactions impairs information transfer and leads to increased forgetting
Desynchronized network states are characterized by broadband electrophysiological signatures potentially reflecting rapid shifts in population excitation-inhibition balance
Novel methods are required to conceptualize and analyze aperiodic network states
Acknowledgements
This work was supported by the German Research Foundation (DFG HE 8329/2–1, R.F.H. and DFG LE 3863/2–1, J.D.L.), the Hertie Foundation (Hertie Network for Excellence in Clinical Neuroscience, R.F.H.), NINDS Javits Award R37NS21135 (R.T.K.) and U19 Brain Initiative 1U19NS107609–01 (R.T.K.). The authors would like to thank Jan Weber, Frank van Schalkwijk and Michael Hahn for valuable feedback on the manuscript as well as Joe Winer, Cameron Higgins and James Antony for supplying data for illustrations.
Glossary
- Aperiodic activity
Desynchronized network states are characterized by the absence of strong oscillations; thus, lacking one prominent periodic signal and reflecting increased variability. Aperiodic activity follows a power law (1/frequency) and indicates that no predominant temporal activity, such as an oscillation, is present. See also under Desynchronization
- Cross-frequency coupling (CFC)
Interaction between oscillations at different frequencies. The most common type is phase-amplitude-coupling (PAC) where the phase of the slower rhythm modulates the amplitude of the faster oscillation
- Complexity
Here we define complexity in the temporal domain as a state of high Shannon entropy, i.e. with high variability and low redundancy. In the spatial domain at the network level, it refers to low synchrony and therefore primarily local processing
- Decoding
Classification of a neural activity patterns to infer e.g. different cortical states or mnemonic representations. Decoding requires a training dataset where the decoder has access to the ground truth, while novel patterns can be classified according to the learned signal characteristics
- Desynchronization
Refers to states after an oscillatory burst and used interchangeably with aperiodic and non-oscillatory activity. Occasionally these phenomena are also termed scale-free activity, 1/f or fractal behavior
- Engram
The physical trace of a memory after encoding and consolidation. Engrams can be found at all levels of abstraction from single cell to population activity to network connectivity
- E/I-balance (Excitation-inhibition-balance)
Ratio between excitatory (E) and inhibitory (I) cell activity in a neural population. In the cortex, pyramidal neurons typically reflect excitatory and interneurons inhibitory drive. A balance between both is observed under physiological conditions, while a shift towards either one extreme impairs function
- Oscillatory burst
Oscillations in the brain are not continuous, ongoing phenomena but periodically occurring as discrete events. On the cellular level, bursts are associated with coherent population firing
- Replay
Periodic reoccurrence of neuronal firing patterns, which first occurred during initial encoding and are now repeated, preferentially during NREM sleep, and are thought to strengthen mnemonic representations. Replay can occur time-compressed and/or forward and backwards
- Shannon entropy and information theory
Quantification of signal variability, effectively assessing the uncertainty about a signal or the probability to observe a distinct state. Information theory builds on the entropy framework and quantifies information as the degree to which uncertainty about a signal can be reduced given a known input signal
- Power law
One parameter scales as a function of the power of a second parameter. In the brain typically amplitude scales as a function of 1/frequencyX, where x denotes an exponent ranging between −2 and −4
- Two-photon calcium imaging
Modified fluorescence microscopy. Different cell types can be labeled in vivo. Laser light is then used to excite molecules of a fluorescent dye and local calcium concentrations can be inferred from fluorescence intensity changes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rasch B. and Born J. (2013) About sleep’s role in memory. Physiol. Rev. 93, 681–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stickgold R. and Walker MP (2013) Sleep-dependent memory triage: evolving generalization through selective processing. Nat. Neurosci. 16, 139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buzsáki G. (1989) Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience 31, 551–570 [DOI] [PubMed] [Google Scholar]
- 4.Frankland PW and Bontempi B. (2005) The organization of recent and remote memories. Nat. Rev. Neurosci. 6, 119–130 [DOI] [PubMed] [Google Scholar]
- 5.Eichenbaum H. (2017) Prefrontal-hippocampal interactions in episodic memory. Nat Rev Neurosci 18, 547–558 [DOI] [PubMed] [Google Scholar]
- 6.Buzsáki G. (1996) The hippocampo-neocortical dialogue. Cereb. Cortex 6, 81–92 [DOI] [PubMed] [Google Scholar]
- 7.Buzsáki G. (2015) Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staresina BP et al. (2015) Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat. Neurosci. 18, 1679–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sirota A. et al. (2003) Communication between neocortex and hippocampus during sleep in rodents. Proc. Natl. Acad. Sci. U.S.A. 100, 2065–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latchoumane C-FV et al. (2017) Thalamic Spindles Promote Memory Formation during Sleep through Triple Phase-Locking of Cortical, Thalamic, and Hippocampal Rhythms. Neuron DOI: 10.1016/j.neuron.2017.06.025 [DOI] [PubMed] [Google Scholar]
- 11.Murphy M. et al. (2009) Source modeling sleep slow waves. Proc. Natl. Acad. Sci. U.S.A. 106, 1608–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Gennaro L. and Ferrara M. (2003) Sleep spindles: an overview. Sleep Med Rev 7, 423–440 [DOI] [PubMed] [Google Scholar]
- 13.Helfrich RF et al. (2019) Bidirectional prefrontal-hippocampal dynamics organize information transfer during sleep in humans. Nat Commun 10, 3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster DJ (2017) Replay Comes of Age. Annu. Rev. Neurosci. 40, 581–602 [DOI] [PubMed] [Google Scholar]
- 15.Zhang H. et al. (2018) Electrophysiological mechanisms of human memory consolidation. Nat Commun 9, 4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voytek B. et al. (2015) Age-Related Changes in 1/f Neural Electrophysiological Noise. J. Neurosci. 35, 13257–13265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He BJ (2014) Scale-free brain activity: past, present, and future. Trends Cogn. Sci. (Regul. Ed.) 18, 480–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lendner JD et al. (2020) An electrophysiological marker of arousal level in humans. Elife 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donoghue T. et al. (2020) Parameterizing neural power spectra into periodic and aperiodic components. Nat Neurosci 23, 1655–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyce R. et al. (2017) REM sleep and memory. Curr. Opin. Neurobiol. 44, 167–177 [DOI] [PubMed] [Google Scholar]
- 21.Djonlagic I. et al. (2020) Macro and micro sleep architecture and cognitive performance in older adults. Nature Human Behaviour DOI: 10.1038/s41562-020-00964-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antony JW et al. (2018) Sleep Spindle Refractoriness Segregates Periods of Memory Reactivation. Curr. Biol. 28, 1736–1743.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muehlroth BE and Werkle-Bergner M. (2020) Understanding the interplay of sleep and aging: Methodological challenges. Psychophysiology 57, e13523 [DOI] [PubMed] [Google Scholar]
- 24.Hanslmayr S. et al. (2016) Oscillations and Episodic Memory: Addressing the Synchronization/Desynchronization Conundrum. Trends Neurosci. 39, 16–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joo HR and Frank LM (2018) The hippocampal sharp wave-ripple in memory retrieval for immediate use and consolidation. Nat. Rev. Neurosci. DOI: 10.1038/s41583-018-0077-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logothetis NK et al. (2012) Hippocampal-cortical interaction during periods of subcortical silence. Nature 491, 547–553 [DOI] [PubMed] [Google Scholar]
- 27.Maingret N. et al. (2016) Hippocampo-cortical coupling mediates memory consolidation during sleep. Nat. Neurosci. 19, 959–964 [DOI] [PubMed] [Google Scholar]
- 28.Fernández-Ruiz A. et al. (2019) Long-duration hippocampal sharp wave ripples improve memory. Science 364, 1082–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ngo H-V et al. (2020) Sleep spindles mediate hippocampal-neocortical coupling during long-duration ripples. Elife 9, e57011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J. et al. (2019) Competing Roles of Slow Oscillations and Delta Waves in Memory Consolidation versus Forgetting. Cell 179, 514–526.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox R. et al. (2020) Sharp Wave-Ripples in Human Amygdala and Their Coordination with Hippocampus during NREM Sleep. Cereb Cortex Commun 1, tgaa051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khodagholy D. et al. (2017) Learning-enhanced coupling between ripple oscillations in association cortices and hippocampus. Science 358, 369–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaz AP et al. (2019) Coupled ripple oscillations between the medial temporal lobe and neocortex retrieve human memory. Science 363, 975–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brodt S. et al. (2018) Fast track to the neocortex: A memory engram in the posterior parietal cortex. Science 362, 1045–1048 [DOI] [PubMed] [Google Scholar]
- 35.Tang W. et al. (2017) Hippocampal-Prefrontal Reactivation during Learning Is Stronger in Awake Compared with Sleep States. J. Neurosci. 37, 11789–11805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang DV and Ikemoto S. (2016) Coordinated Interaction between Hippocampal Sharp-Wave Ripples and Anterior Cingulate Unit Activity. J. Neurosci. 36, 10663–10672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothschild G. et al. (2017) A cortical-hippocampal-cortical loop of information processing during memory consolidation. Nat. Neurosci. 20, 251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boyce R. et al. (2016) Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 352, 812–816 [DOI] [PubMed] [Google Scholar]
- 39.Cantero JL et al. (2003) Sleep-dependent theta oscillations in the human hippocampus and neocortex. J. Neurosci. 23, 10897–10903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grosmark AD et al. (2012) REM sleep reorganizes hippocampal excitability. Neuron 75, 1001–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watson BO et al. (2016) Network Homeostasis and State Dynamics of Neocortical Sleep. Neuron 90, 839–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canolty RT and Knight RT (2010) The functional role of cross-frequency coupling. Trends Cogn. Sci. (Regul. Ed.) 14, 506–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helfrich RF et al. (2018) Old Brains Come Uncoupled in Sleep: Slow Wave-Spindle Synchrony, Brain Atrophy, and Forgetting. Neuron 97, 221–230.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muehlroth BE et al. (2019) Precise Slow Oscillation-Spindle Coupling Promotes Memory Consolidation in Younger and Older Adults. Sci Rep 9, 1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hahn MA et al. (2020) Slow oscillation-spindle coupling predicts enhanced memory formation from childhood to adolescence. eLife 9, e53730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aru J. et al. (2015) Untangling cross-frequency coupling in neuroscience. Curr. Opin. Neurobiol. 31, 51–61 [DOI] [PubMed] [Google Scholar]
- 47.Gorgoni M. et al. (2020) Sleep electroencephalography and brain maturation: developmental trajectories and the relation with cognitive functioning. Sleep Med 66, 33–50 [DOI] [PubMed] [Google Scholar]
- 48.Joechner A-K et al. (2020) Electrophysiological Indicators of Sleep-associated Memory Consolidation in 5- to 6-year-old Children. bioRxiv DOI: 10.1101/2020.09.04.283606 [DOI] [PubMed] [Google Scholar]
- 49.Muehlroth BE et al. (2020) Episodic memory consolidation during sleep in healthy aging. Sleep Med Rev 52, 101304 [DOI] [PubMed] [Google Scholar]
- 50.Purcell SM et al. (2017) Characterizing sleep spindles in 11,630 individuals from the National Sleep Research Resource. Nat Commun 8, 15930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spanò G. et al. (2020) Sleeping with Hippocampal Damage. Curr Biol 30, 523–529.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winer JR et al. (2019) Sleep as a potential biomarker of tau and β-amyloid burden in the human brain. J. Neurosci. DOI: 10.1523/JNEUROSCI.0503-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ladenbauer J. et al. (2017) Promoting sleep oscillations and their functional coupling by transcranial stimulation enhances memory consolidation in mild cognitive impairment. J. Neurosci. DOI: 10.1523/JNEUROSCI.0260-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benthem SD et al. (2020) Impaired Hippocampal-Cortical Interactions during Sleep in a Mouse Model of Alzheimer’s Disease. Curr Biol 30, 2588–2601.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sunwoo J-S et al. (2021) Nonrapid eye movement sleep electroencephalographic oscillations in idiopathic rapid eye movement sleep behavior disorder: a study of sleep spindles and slow oscillations. Sleep 44, zsaa160 [DOI] [PubMed] [Google Scholar]
- 56.Mikutta C. et al. (2019) Phase-amplitude coupling of sleep slow oscillatory and spindle activity correlates with overnight memory consolidation. J Sleep Res 28, e12835. [DOI] [PubMed] [Google Scholar]
- 57.Hahn MA et al. (2021) Slow oscillation-spindle coupling strength predicts real-life gross-motor learning in adolescents and adults. bioRxiv DOI: 10.1101/2021.01.21.427606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schreiner T. et al. (2020) Endogenous memory reactivation during sleep in humans is clocked by slow oscillation-spindle complexes. bioRxiv DOI: 10.1101/2020.09.16.299545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cairney SA et al. (2018) Memory Consolidation Is Linked to Spindle-Mediated Information Processing during Sleep. Curr. Biol. 28, 948–954.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eckert MJ et al. (2021) Reliable induction of sleep spindles with intracranial electrical pulse stimulation. Learn Mem 28, 7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gelinas JN et al. (2016) Interictal epileptiform discharges induce hippocampal-cortical coupling in temporal lobe epilepsy. Nat. Med. 22, 641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dahal P. et al. (2019) Interictal epileptiform discharges shape large-scale intercortical communication. Brain 142, 3502–3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Helfrich RF et al. (2019) Neural entrainment and network resonance in support of top-down guided attention. Curr Opin Psychol 29, 82–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swanson RA et al. (2020) Variable specificity of memory trace reactivation during hippocampal sharp wave ripples. Current Opinion in Behavioral Sciences 32, 126–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boutin A. and Doyon J. (2020) A sleep spindle framework for motor memory consolidation. Philosophical Transactions of the Royal Society B 375, 20190232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tingley D. and Peyrache A. (2020) On the methods for reactivation and replay analysis. Philosophical Transactions of the Royal Society B 375, 20190231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quian Quiroga R. and Panzeri S. (2009) Extracting information from neuronal populations: information theory and decoding approaches. Nat. Rev. Neurosci. 10, 173–185 [DOI] [PubMed] [Google Scholar]
- 68.Ólafsdóttir HF et al. (2018) The Role of Hippocampal Replay in Memory and Planning. Curr Biol 28, R37–R50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dudai Y. (2004) The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol 55, 51–86 [DOI] [PubMed] [Google Scholar]
- 70.Sandhaeger F. et al. (2019) Monkey EEG links neuronal color and motion information across species and scales. Elife 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parvizi J. and Kastner S. (2018) Promises and limitations of human intracranial electroencephalography. Nat. Neurosci. 21, 474–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rich EL and Wallis JD (2017) Spatiotemporal dynamics of information encoding revealed in orbitofrontal high-gamma. Nat Commun 8, 1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kanth ST and Ray S. (2020) Electrocorticogram (ECoG) Is Highly Informative in Primate Visual Cortex. J Neurosci 40, 2430–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leszczyński M. et al. (2020) Dissociation of broadband high-frequency activity and neuronal firing in the neocortex. Sci Adv 6, eabb0977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gray RM (2011) Entropy and information theory, Springer Science & Business Media. [Google Scholar]
- 76.Timme NM and Lapish C. (2018) A Tutorial for Information Theory in Neuroscience. eNeuro 5, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanslmayr S. et al. (2012) Oscillatory power decreases and long-term memory: the information via desynchronization hypothesis. Front. Hum. Neurosci. 6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sejnowski TJ and Paulsen O. (2006) Network Oscillations: Emerging Computational Principles. J. Neurosci. 26, 1673–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Borst A. and Theunissen FE (1999) Information theory and neural coding. Nature Neuroscience 2, 947–957 [DOI] [PubMed] [Google Scholar]
- 80.Higgins C. et al. (2020) Replay bursts in humans coincide with activation of the default mode and parietal alpha networks. Neuron DOI: 10.1016/j.neuron.2020.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fellner M-C et al. (2019) Spectral fingerprints or spectral tilt? Evidence for distinct oscillatory signatures of memory formation. PLOS Biology 17, e3000403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pesaran B. et al. (2018) Investigating large-scale brain dynamics using field potential recordings: analysis and interpretation. Nat. Neurosci. 21, 903–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buzsáki G. et al. (2012) The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 13, 407–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manning JR et al. (2009) Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J. Neurosci. 29, 13613–13620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Watson BO et al. (2017) Temporal coupling of field potentials and action potentials in the neocortex. Eur. J. Neurosci. DOI: 10.1111/ejn.13807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bruns A. (2004) Fourier-, Hilbert- and wavelet-based signal analysis: are they really different approaches? J. Neurosci. Methods 137, 321–332 [DOI] [PubMed] [Google Scholar]
- 87.Gao R. et al. (2017) Inferring synaptic excitation/inhibition balance from field potentials. Neuroimage 158, 70–78 [DOI] [PubMed] [Google Scholar]
- 88.Niethard N. et al. (2016) Sleep-Stage-Specific Regulation of Cortical Excitation and Inhibition. Curr. Biol. 26, 2739–2749 [DOI] [PubMed] [Google Scholar]
- 89.Tamaki M. et al. (2020) Complementary contributions of non-REM and REM sleep to visual learning. Nature Neuroscience 23, 1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Niethard N. et al. (2018) Cortical circuit activity underlying sleep slow oscillations and spindles. PNAS DOI: 10.1073/pnas.1805517115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rasch B. et al. (2007) Odor cues during slow-wave sleep prompt declarative memory consolidation. Science 315, 1426–1429 [DOI] [PubMed] [Google Scholar]
- 92.Bar E. et al. (2020) Local Targeted Memory Reactivation in Human Sleep. Curr Biol 30, 1435–1446.e5 [DOI] [PubMed] [Google Scholar]
- 93.Lewis PA and Bendor D. (2019) How Targeted Memory Reactivation Promotes the Selective Strengthening of Memories in Sleep. Curr Biol 29, R906–R912 [DOI] [PubMed] [Google Scholar]
- 94.Hebart MN and Baker CI (2018) Deconstructing multivariate decoding for the study of brain function. Neuroimage 180, 4–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.King J-R and Dehaene S. (2014) Characterizing the dynamics of mental representations: the temporal generalization method. Trends Cogn. Sci. (Regul. Ed.) 18, 203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kriegeskorte N. et al. (2008) Representational similarity analysis - connecting the branches of systems neuroscience. Front Syst Neurosci 2, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang B. et al. (2019) Targeted Memory Reactivation during Sleep Elicits Neural Signals Related to Learning Content. J Neurosci 39, 6728–6736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Panzeri S. et al. (2015) Neural population coding: combining insights from microscopic and mass signals. Trends Cogn. Sci. (Regul. Ed.) 19, 162–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Prerau MJ et al. (2017) Sleep Neurophysiological Dynamics Through the Lens of Multitaper Spectral Analysis. Physiology (Bethesda) 32, 60–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Voytek B. and Knight RT (2015) Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biol. Psychiatry 77, 1089–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wen H. and Liu Z. (2016) Separating Fractal and Oscillatory Components in the Power Spectrum of Neurophysiological Signal.Brain Topogr 29, 13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kosciessa JQ et al. (2020) Single-trial characterization of neural rhythms: Potential and challenges. Neuroimage 206, 116331. [DOI] [PubMed] [Google Scholar]
- 103.Vidaurre D. et al. (2020) Dissociable components of oscillatory activity underly information encoding in human perception. bioRxiv DOI: 10.1101/2020.09.10.291294 [DOI] [Google Scholar]
- 104.Quinn AJ et al. (2021) EMD: Empirical Mode Decomposition and Hilbert-Huang Spectral Analyses in Python. Journal of Open Source Software 6, 2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cole SR and Voytek B. (2017) Brain Oscillations and the Importance of Waveform Shape. Trends Cogn. Sci. (Regul. Ed.) 21, 137–149 [DOI] [PubMed] [Google Scholar]