Abstract
Objective:
To identify predictors of overall survival (OS) and to stratify patients according to significant prognostic variables.
Methods:
A retrospective study of 274 consecutive patients with primary Oral Cavity Squamous Cell Carcinoma. Kaplan-Meier, Cox proportional hazard models, and recursive partitioning analysis (RPA) were used for analysis of OS. These results were further validated using National Cancer Database cohort of 21 895 patients.
Results:
Median OS was 3.65 years. T-classification and N-classification, alcoholic beverages/week, age, and adjuvant treatment were significant predictors of OS. RPA identified high-risk subpopulations: N0–1 patients with CCI ≥ 4.5 and N2–3 patients ordered by those not receiving adjuvant treatment, those with T3–4 disease despite adjuvant therapy, and those having T1–2 disease with adjuvant therapy.
Conclusions:
This study utilized significant prognostic indicators and RPA to highlight the importance of age, N-classification, T-classification, comorbidity, and adjuvant therapy in conjunction with American Joint Committee on Cancer staging to improve preoperative counseling.
Keywords: comorbidity, head and neck cancer, National Cancer Database, NCDB, oral cavity cancer, overall survival, squamous cell carcinoma, staging
1 |. INTRODUCTION
In 2014, approximately 28 000 new cases of oral cavity cancer—the majority being oral cavity squamous cell carcinoma (OCSCC)—were expected to occur in the United States and to result in 5800 deaths.1,2 Worldwide, the annual estimated incidence of oral cavity cancer is 198 975, with a 5-year prevalence of 467 157 cases.3 The average 5-year survival is approximately 50% for patients with advanced-stage disease because of locoregional recurrences and distant metastases.4 Clinicians typically counsel their patients using standard overall American Joint Committee on Cancer (AJCC) statistics. However, major factors are often omitted from survival discussions, and these are individual T-stage, N-classification, age, and comorbidities. In the present study, we factored in these criteria, along with standard prognostic data points, to develop an adjusted multivariable model and a regression classification tree to clarify patient outcomes and risk factors for poor outcomes. Our objective was to offer better prognostic and overall survival (OS) discussions via patient stratifications. Although, the most recent edition of AJCC (eighth edition) released in January 2018 includes an additional variable, depth of invasion (DOI)—which primarily applies to early stage OCSCC—we did not include DOI in this study, as data on this variable were not available.5,6
2 |. PATIENTS AND METHODS
We conducted a retrospective study of 274 patients with primary OCSCC who were treated surgically with or without adjuvant therapy at The James Cancer Hospital and Solove Research Institute Wexner Medical Center at Ohio State University (OSU) from 2000 through 2007. Staging was based on the seventh edition of AJCC.7 IRB approval was obtained to conduct this retrospective analysis, with a waiver of HIPAA research authorization. Overall survival (OS) was defined as the time from the date of surgery to the date of death. Patients who were alive at the date of last observation were censored. The national cancer registry was used to obtain survival data from patients lost to follow-up. Survival curves were plotted using the Kaplan-Meier method. Cox proportional hazards models were used to assess univariate associations between potential predictors of death. Unadjusted hazard ratios (HRs) and 95% confidence intervals (CIs) are reported. Variables that were found to be significant at α = .10 in the univariate models were included in a multivariable model to estimate adjusted HRs.
To validate the results obtained from The OSU dataset, we accessed the National Cancer Database (NCDB).8 A retrospective cohort study was conducted using the NCDB for adults with previously untreated OCSCC without distant metastatic disease, who were diagnosed between 2004 through 2015. Patients diagnosed in 2015 did not have OS data and were excluded from the analysis.
To profile the risk of death, a recursive partitioning analysis (RPA) was used. R package “rpart” was used to fit a regression tree with the OS data.9,10 After evaluating prognostic factors for OS, the predictors used in the multivariable Cox proportional hazard model were included in the building of the tree.11 The regression tree was pruned using cross-validation to select the tree with the lowest cross-validation error. To evaluate predictive accuracy of the tree, we performed 1000 splits of the data into training (2/3) and test (1/3) sets. The process of building a regression tree (including pruning) was replicated in the training data and the predictive accuracy on the basis of the C-index, which was calculated using tree-based model predictions in the test data by splitting patients into high-risk and low-risk groups using the median score from the RT model.11 The C-index estimates the probability that, for a randomly selected pair of individuals, the individual with the higher risk score (shorter predicted survival time) has the shorter actual event time. This process evaluates the predictive ability of the model-building procedure performed on the entire data set and avoids the over-fitting issue that results from using re-substitution (ie, using the data to both fit the model and evaluate model performance). To interpret the model, we used R package “randomForestSRC” to fit survival random forests to the Head and Neck OSU data and to obtain variable importance measures based on minimal depth, which is the average distance from the root node to first split for a variable across all trees in the forest.12 All analyses were conducted in SAS, version 9.3 (SAS Institute, Cary, North Carolina) or the R language environment for statistical computing (R version:3.1.0).
See Supporting Information for further details on NCDB selection criteria and RPA.
3 |. RESULTS
Mean age of the cohort was 60.3 years (eTable 1). All four AJCC Cancer Stages were well represented, with stage IV being the most prevalent (34.1%). Majority of tumors (70.4%) were classified T1/T2. N0 was most prevalent at 60.8% and distant metastases were rare (2.6%). Extracapsular spread (ECS) was present in 15.8% of patients. Follow-up time ranged from 1 day to 11.1 years with a median of 3.6 years.
Tobacco and alcohol were common risk factors in this patient population; 78.3% of patients were current or past smokers and 75.8% were current or past drinkers. Twelve comorbidities were indexed, with the most common being pulmonary diseases (COPD—47.4%) and hypertension (44.9%). Charlson Comorbidity Index (CCI) was calculated for all patients with a mean value of 3.2 and age-adjusted mean value of 4.9.13 Over 47% of the population received postoperative adjuvant radiation or chemoradiation based on postoperative recommended National Comprehensive Cancer Network (NCCN) guidelines.14
One-year OS was 75% and trending downward to 47% after 5 years. Stage I cancers had 1-year and 5-year OS of 93% and 70%, respectively whereas stage IV had poor 1 and 5-year OS of 53% and 28%, respectively (Figure 1).
FIGURE 1.
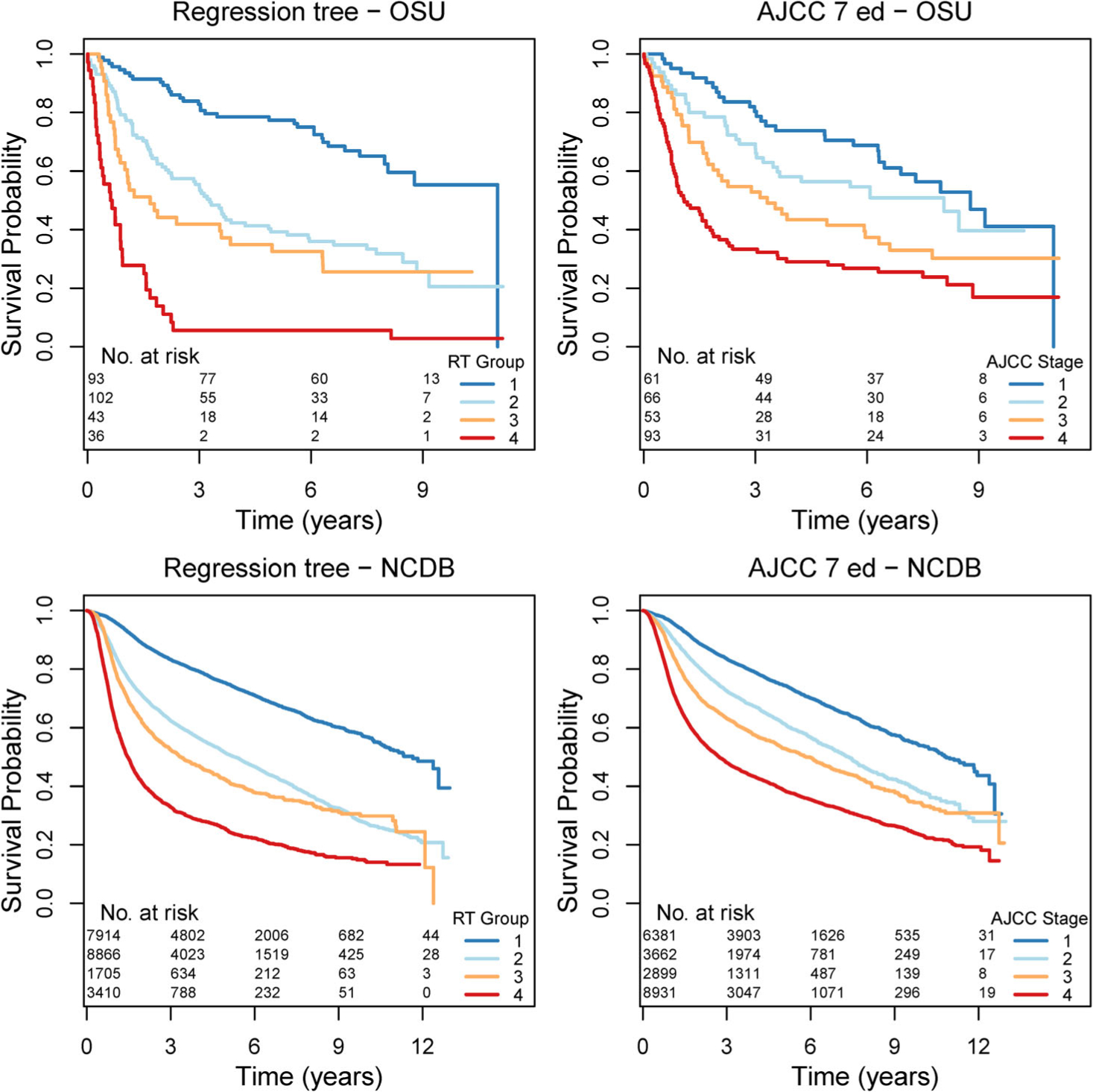
Comparison of Kaplan-Meier survival curves of the RT group and AJCC stage, 7th ed. for both OSU and NCDB cohorts suggests similar survival for RT risk group 1 and AJCC stage I. RT risk group 2 had similar survival to AJCC stage III. RT risk group 4 had much lower survival compared to AJCC stage IV
3.1 |. Univariate analysis
Univariate Cox regression results are shown in Table 1. Patients with T3 or T4 disease had a higher hazard of death compared to those with T1, with no significant difference between T1 and T2 disease. N-classification yielded similar results; there was no significant difference between N1 and N0 disease, but N2/3 had a higher hazard of death compared to N0 (HR: 3.065, 2.170–4.329, P < .001). Stage III and IV patients had a high hazard of death compared to stage I patients. There was no significant difference between stage I and II disease. Patients with nodal ECS spread had a hazard of death 3.02 times (2.088–4.376, P < .001) greater than patients without ECS.
TABLE 1.
OS univariate analyses
| Predictor | Hazard ratio | 95% confidence interval | P-value | N | |
|---|---|---|---|---|---|
| T | |||||
| 1 | Reference | ||||
| 2 | 1.25 | 0.848 | 1.842 | .26 | |
| 3 | 2.663 | 1.626 | 4.361 | <.001 | |
| 4 | 2.568 | 1.661 | 3.97 | <.001 | 274 |
| N | |||||
| 0 | Reference | ||||
| 1 | 1.311 | 0.861 | 1.995 | .21 | |
| 2/3 | 3.065 | 2.170 | 4.329 | <.001 | 273 |
| AJCC stage | |||||
| I | Reference | ||||
| II | 1.262 | 0.766 | 2.08 | .36 | |
| III | 1.881 | 1.152 | 3.069 | .011 | |
| IV | 3.014 | 1.954 | 4.651 | <.001 | 273 |
| ECS | 3.022 | 2.088 | 4.376 | <.001 | 273 |
| CCI—age adjusted | 1.241 | 1.145 | 1.346 | <.001 | 274 |
| CCI | 1.281 | 1.158 | 1.416 | <.001 | 274 |
| Age | 1.017 | 1.005 | 1.03 | .006 | 274 |
| CAD | 1.649 | 1.152 | 2.359 | .006 | 274 |
| Adjuvant therapy | |||||
| None | Reference | ||||
| Post rad | 1.529 | 1.104 | 2.118 | .010 | |
| Post rad + post chemo | 1.615 | 0.977 | 2.67 | .062 | 269 |
| Pulmonary diagnosis | 1.431 | 1.056 | 1.938 | .021 | 272 |
| CVS | 1.833 | 1.059 | 3.174 | .030 | 273 |
| EtOH per week | 1.008 | 1 | 1.016 | .040 | 250 |
| Marital status | |||||
| Married | Reference | ||||
| Divorced/single/widowed | 1.352 | 1 | 1.828 | .05 | 273 |
| Complications | |||||
| No | Reference | ||||
| Yes | 1.353 | 0.957 | 1.915 | .09 | 274 |
| Smoke pack-years | 1.004 | 0.998 | 1.01 | .19 | 265 |
| Smoking status | |||||
| Never | Reference | ||||
| Current | 1.122 | 0.757 | 1.665 | .57 | |
| Past | 0.998 | 0.653 | 1.525 | .99 | 271 |
| Smoking status (pack-years) | |||||
| 10 pack years or less | Reference | ||||
| More than 10 pack years | 1.032 | 0.74 | 1.44 | .85 | 265 |
| EtOH consumption | |||||
| Never | Reference | ||||
| Current | 0.783 | 0.543 | 1.129 | .19 | |
| Past | 1.03 | 0.669 | 1.586 | .89 | 268 |
| EtOHism | 1.16 | 0.823 | 1.635 | .40 | 265 |
| HTN | 1.052 | 0.779 | 1.421 | .74 | 274 |
| Diabetes | 1.17 | 0.809 | 1.69 | .40 | 274 |
| Hypothyroidism | 0.905 | 0.548 | 1.494 | .70 | 273 |
| PVD | 1.044 | 0.648 | 1.683 | .86 | 273 |
| Hyperlipidemia | 0.95 | 0.637 | 1.417 | .80 | 273 |
| Obesity | 0.934 | 0.653 | 1.335 | .71 | 271 |
| Race | |||||
| White | Reference | ||||
| Other | 1.21 | 0.682 | 2.147 | .51 | 233 |
Abbreviations: CAD, coronary artery disease; CCI, Charlson Comorbidity Index; CVS, cerebrovascular stroke; ECS, extracapsular spread; ETOHism, defined by >14 drinks per week; HR, hazard ratio; HTN, hypertension; OS, overall survival; Post rad, adjuvant radiation therapy; PVD, peripheral vascular disease.
Alcohol use, as a continuous variable, weekly self-reported alcohol intake prior to diagnosis resulted in an increased hazard of death for each additional drink/week (HR: 1.008, 1–1.016, P = .04), however there was no difference in survival when evaluating lifetime drinking status and clinical alcoholism (>14 drinks/week). Smoking status as both a discrete and continuous variable did not achieve a significant difference in survival.
Age and comorbidity were evaluated both individually and via combined indices. Each 1-year advancement in age was associated with a higher hazard of death (HR: 1.017, 1.005–1.03, P = .006). Coronary artery disease (CAD), cerebrovascular disease (CVS), and pulmonary disease diagnoses were associated with a higher hazard of death as well. Each additional point in CCI for composite comorbidity was associated with a higher hazard of death (HR: 1.281, 1.158–1.416, P < .001). Age was then integrated into an age-adjusted CCI and was associated with higher hazard of death.
Patients who received adjuvant treatment were compared to those who did not. Patients receiving adjuvant therapy corresponded to a greater hazard of death than those who did not receive treatment, reflecting the more advance disease.
3.2 |. Multivariate analysis
Multivariate Cox regression analysis (see eTable 2) was built upon variables from the univariate analyses that were significantly associated with OS (P ≤ .1). T-classification was a predictor of survival when comparing T1/T2 against T3/T4. Similarly, N-classification remained a significant predictor of survival when N2/3 was compared to N0 disease. Alcohol consumption per week as a continuous variable maintained significance with each additional drink/week being associated with a higher hazard of death. Age was also significantly associated with survival while CAD was associated with a higher hazard of death.
3.3 |. Recursive partitioning analysis
RPA was done to stratify patients using predictors of survival from the univariate model (Figure 2). Each branchpoint represents divisions of the population by the most important variables. For instance, N-stage was the most important variable (best able to separate patients with good vs poor prognosis), therefore it is the first branch point of the entire cohort.
FIGURE 2.
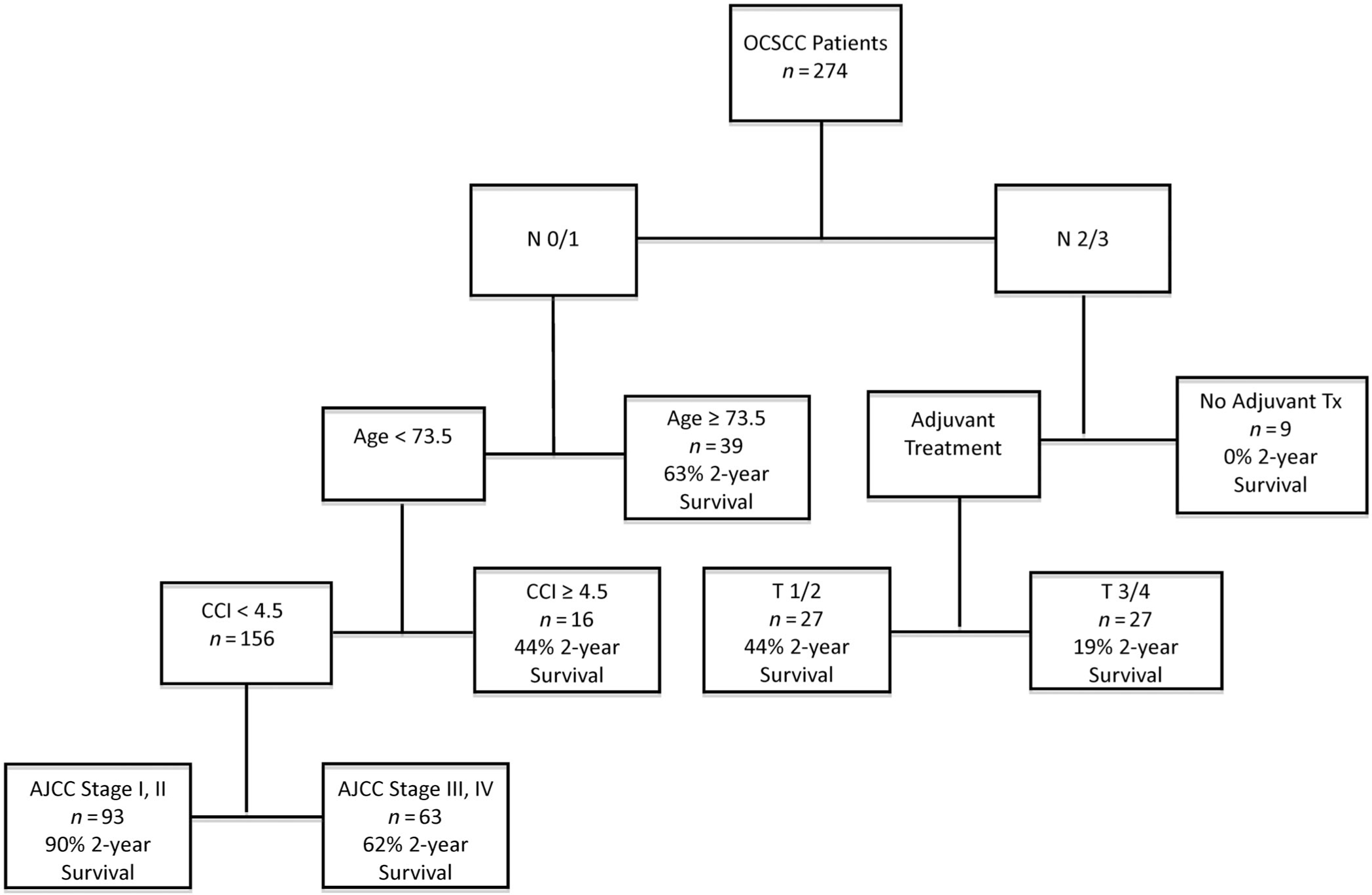
The optimal RT for the OSU cohort after pruning had six splits—nodal staging being the first branch. In N0/N1 group, age <73.5 years was important, followed by CCI with a terminal branch based on AJCC stage. In the N2/N3 group, receipt of adjuvant therapy was the next most important branch followed by the T-classification
The optimal RT after pruning had six splits (Figure 2). Patients 74 years and older with N0–1 disease had 63% 2-year OS. Moreover, patients 73 years and younger were stratified again via CCI and AJCC stage which revealed that among these patients, those with a CCI ≥4.5 had 44% 2-year OS. Those patients with CCI < 4.5 were subsequently stratified by stage I, II (90% 2-year OS) vs stage III, IV (62% 2-year OS). N2–3 patients were stratified based on receipt of adjuvant treatment. Those who did not receive adjuvant treatment had no survivors at 2 years. Of these patients who did receive some form of adjuvant treatment, patients with T1/T2 disease had reduced morality (44% 2-year OS) compared to those with T3/T4 disease (19% 2-year OS). The nodes of the RT were further grouped by similarity in 2-year OS (Figure 3) into four groups (RT risk groups 1–4). KM curves for these groups were then compared to the survival by AJCC stage (Figure 1). Contrasting the two stratifications, the survival curves for RT risk group 1 and stage I were very similar as were RT risk group 2 and stage III. The biggest difference between the two risk stratifications is that RT risk group 4 has much lower survival compared to the corresponding stage IV.
FIGURE 3.
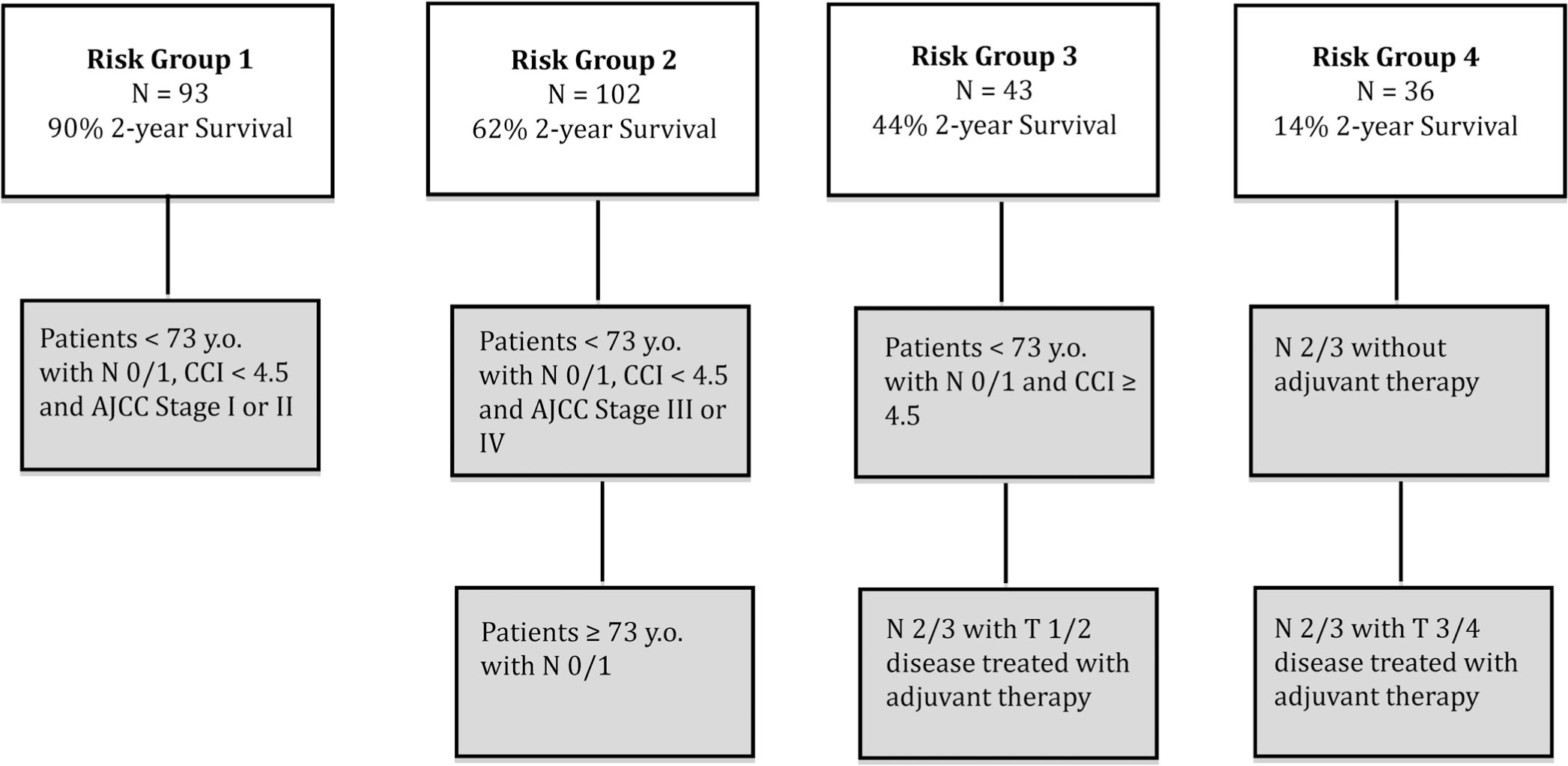
Based on the RT, the OSU cohort was divided into four risk groups. The risk groups had a clear separation of 2 year OS with risk group 1 having a 2 year OS of 90%, risk group 2 with 2 year OS of 62%, risk group 3 with 2 year OS of 44% and finally risk group 4 with 2 year OS of 14%
A boxplot of the test data accuracy (C-indexes) of the pruned regression tree models fitted to the 1000 training data sets is given in the Supporting Information eFigure 1 (median value of 0.612). Predictive accuracy was calculated for models based on dichotomizing patients into high/low risk groups with median C-index value of 0.60. KM curves of these high/low-risk splits of the test data are provided in eFigure 2 and show consistent separation between the two groups.
Variable importance measures based on minimal depth were calculated from the R package “randomForestSRC” and displayed in eFigure 3. The minimal depth is the average distance from the root node to first split for a variable across all trees in the forest. A smaller value is better and indicates the split occurred earlier on in the tree. Based on the plot the top variables are N-classification, CCI, and then age.
3.4 |. Validation of regression tree using NCDB data
The OSU RT model was validated using external data obtained from the NCDB. 21 895 patients were identified with OCSCC which passed our selection criteria. They were assigned to corresponding nodes in Figure 2 and further assigned to risk groups as outlined in Figure 3. Since NCDB only records the CCI as 0, 1, 2, and 3 or more, all subjects in the N0/1, age < 73.5 branch of the tree were subsequently passed through the CCI < 4.5 node. However, a sensitivity analysis which assigned NCDB subjects with a CCI of 3 or more to have a CCI of 5 or greater did not significantly alter the risk group stratification and corresponding survival curves. Figure 1 displays the risk group stratification in the NCDB data based on both the RT model (RT groups 1–4) and AJCC stage. These findings are similar to those based on The OSU training data. In particular, RT group 4 has much lower survival compared to stage IV, while the survival for the lowest risk groups in each case are largely similar. The overall separation between the survival curves in the RT risk groups is larger than that for AJCC stage, as is the predictive accuracy (C-index of 0.669, 95% CI 0.663–0.674 for RT risk groups compared to 0.649, 95% CI 0.643–0.655 for AJCC stage). Cross-tabulation between the RT risk groups and AJCC stage is given in eTable 3. RT group 1 consists of patients in stages I and II, RT group 2 has patients from across all four AJCC stages, while RT groups 3 and 4 are subgroups of stage IV patients.
4 |. DISCUSSION
Some of the most robust indicators of OS belong to the AJCC TNM classification criteria. Review of the literature reveals 5-year OS data ranging from 38% to 60% consistent with our series.15–22 However, as a predictor of survival, composite AJCC stage was significant in univariate but not multivariate analysis. The literature is conflicting regarding AJCC stage as a predictor of OS.18,23,24 Our data suggest that the major factors in predicting survival is not the composite AJCC stage, but rather advanced N-classification, CCI, age, need for adjuvant therapy, and advanced T-classification. This is reflected by the variable importance measures based on minimal depth, which is the average distance from the root node to first split for variable across all tree in the forest. See eFigure 3. A smaller value is better—which means the split occurred earlier on the in the tree. Based on the plot the top variables are CCI, N-stage, and then age.
Both univariate and multivariate analysis reported that T3/4 patients have over double the hazard of death than T1/2 patients. The same holds true for N2/3 patients who have at least double the hazard of death as N0/1 patients (eTables 2 and 3) which is consistent with other studies.23–29 The AJCC stage may be an imperfect prognostication tool because it can have varying T-classification and N-classifications within the same stage, that is, a patient with stage III disease could be T-classification 1, 2, or 3; or a stage IVa patient could be N-classification 0, 1, or 2. In this study, T3 patients have a hazard of death over twice that of T1 patients, and that N2 patients have a hazard of death three times that of N0 patients. Consequently, it may be of value to place higher emphasis on advanced N-classification and T-classification when reporting patient disease prognosis.
Smoking and alcohol are known risk factors for the development of OCSCC yet the relationship these factors have with OS and prognosis is less clear.26 Analysis of our population shows alcohol consumption around the time of diagnosis in drinks/week at the time of diagnosis rather than alcohol consumption history, may be the better of the two in terms of predictors of OS. Surprisingly, smoking was not found to be a significant predictor of survival. Despite this, other studies have shown a relationship between OS and smoking, especially in patients who continue to smoke after HNC diagnosis.29–32 It is conceivable that some patients with smoking history did in fact quit after the diagnosis which resulted in smoking status as not being a significant factor in the analysis. Smoking cessation status was not collected as part of the dataset. It is difficult to consider smoking and alcohol in a vacuum given the complex and often causative relationship they have on oral cancer as well as comorbidities. In addition, this was a retrospective dataset and is not a case-controlled study for specific variables. We have included a robust comorbidity variables, such as coronary artery disease, congestive heart failure, peripheral vascular disease, pulmonary disease all of which are associated with smoking. CCI did in fact impact survival in our analysis. Nevertheless, more important than any secondary prevention, there remains a need for primary prevention that highlights these entities in relation to oral cancer and other malignancies.29
Age proved to be a significant predictor of OS in all models. Recent literature search reveals evidence confirming these findings and refuting them.23,24,27,33 Comorbidity, with the exception of multivariate analysis (P = .24), showed promise as a predictor of survival as well. We chose to evaluate comorbidity utilizing the CCI, in both its native and age-adjusted forms, because of its previous validation in HNC, its ease of use and the frequency of use in the literature.34 Of note, the presence of solid tumor generates a score of 2 using the CCI, however because all patients start with this value and because of its validation in HNC, any bias that this would introduce is negligible. Analyzing these factors independently to predict survival may have value, but more insight is derived via patient stratification. Focusing on younger patients with N0/1 disease, CCI was a major driver of OS (Figure 2). In this specific subset, CCI appears to be the most important predictor. Although younger patients have less comorbidities, those who do have advanced comorbidity have worse prognosis.35 As reported by Singh et al in patients 45 years of age and under, those who had more advanced comorbidity were more likely to develop high-grade complications.35 This was also noted for median disease-free interval and disease specific survival where patients with advanced morbidity had worse outcomes. Comorbidity played a larger role in determining OS in younger patients, and data from this study confirms this finding.36 Furthermore, N0/1 patients of age 73 and below with CCI ≥4.5 had lower OS at 2-years compared to N0/1 patients of age 74 and older. Reid et al using SEER/HCFA (Surveillance, Epidemiology, and End Results Program/Health Care Finance Administration Medicare) data noted comorbidity as a predictor of survival in the elderly population with adjusted relative hazards ratio of 1.00 for CCI of 0, 1.33 (95% CI: 1.21–1.47) for CCI of 1 and 1.83 (95% CI: 1.64–2.05) for CCI of 2 or more.37 Similar findings were noted by Ribeiro et al where addition of clinical factors to the TNM classification, provided a better estimation of prognosis.38 Thus, findings from this study and others suggest that comorbidity score has an important prognostic role and should be included in staging systems.
Subset analysis of patients with N2/3 disease raises important questions. Postoperative adjuvant therapy plays a major role in survival of this subset, with 0% of patients with N2/3 disease surviving at 1 year in patients with N2/3 disease that did not receiver adjuvant therapy (Figure 2). Disease biology and comorbidity can disable these patients and impeded them from receiving adjuvant therapy, however those who are able to complete adjuvant therapy have increased survival. Accordingly, it is imperative during preoperative counseling to impress that completion of adjuvant therapy is necessary for optimal outcomes. Moreover, of the N2/3 patients who did receive adjuvant therapy. T-classification was the major driver of OS, with T3/4 patients having approximately 2.5 times the hazard of death compared to T1/2 patients.
The results in this study were based on AJCC seventh edition. AJCC eighth edition was released in January of 2018 which includes DOI as an additional factor in the staging system and since then has undergone revisions.5 According to AJCC eighth edition staging for OCSCC: T1: ≤2 cm in the greatest dimension or ≤5 mm DOI, T2: ≤2 cm and DOI >5 mm but ≤10 mm, or tumor >2 cm but ≤4 cm and ≤10 mm DOI, T3: >4 cm or any tumor with >10 mm DOI. In the NCDB, “tumor depth” was available in our dataset but was only limited to 2010 and onwards. Additionally, a large percentage of NCDB cohort had missing tumor depth data. Moreover, the NCDB does not distinguish between tumor thickness vs DOI in recording this variable, nor how these variables are pathologically sectioned and measured. Given the degree of missing values and ambiguity of variable definition with potential heterogeneity of pathologic measurement/reporting practices across 1500+ hospitals, DOI was not included in the analysis. Moreover, The OSU cohort also did not have DOI information. Therefore, the analysis was done based on early T-classification (T1/T2) vs late T-classification (T3/T4). DOI has shown to been a prognosticator factor in early T-classification tumors (T1/T2),39–41 however the DOI becomes less important in late T-classification (T3/T4) tumors given the size of the tumors and other invasive parameters that are accounted for in the staging system.6 In the RPA, the most important branching pattern was the nodal extent, followed by age in the early N-classification patients and adjuvant therapy in the advanced N-classification patients. Thus, although it is conceivable that there would be additional risk groups that could be stratified based on DOI, this may not be the most important factor therefore attesting to the applicability of our findings and conclusion.
Certain limitations that should be considered when evaluating our findings include its retrospective nature. All patients in the population were treated surgically at a single institution with curative intention. Disease-specific survival data was not available given the majority of patients were lost to long-term follow-up. Additionally, there did exist patients in the cohort with short follow-up (1 day to 6 months). These patients were not excluded from the analysis in order to evaluate early mortality from comorbid conditions. The treatment bias of patients being treated at single institution is mitigated by the data from NCDB. The NCDB records only timing of primary site surgery with other treatment modalities, without explicit information of primary treatment modality with cervical lymphadenopathy, and whether surgery was for primary treatment vs persistent disease. NCDB also does not capture environmental exposures such as smoking or alcohol use and thus these were not included in the analysis of the NCDB dataset.
As demonstrated, the predictive factors of OS for OCSCC are not simply independent and vary in importance depending on the patient subset. Patient stratification is necessary to determine which factors are most predictive for a specific patient. In addition, the traditional AJCC staging system that has long been the gold standard prognostic indicator in OCSCC is imperfect. Improving survival outcomes and patient care in OCSCC may depend on better patient selection, preoperative optimization and risk stratification based upon subpopulation stratification. We provide a risk stratification system to predict survival based up our subpopulation analysis. Looking toward the future, subpopulation tumor biology analysis based upon these risk groups will be important to identify biomarkers and potential targets for additional postoperative therapy or alternative treatment strategies. Finally, as we deepen our understanding of the disease, unique treatment strategies should be considered based upon specific patient characteristics in order to maximize survival and improve outcomes.
Supplementary Material
ACKNOWLEDGMENT
The authors would like to thank Jeffrey Schord and Syed Ali for their help with the project.
Footnotes
CONFLICT OF INTEREST
No authors have conflicts of interest relevant to the subject of this article.
Meeting: Poster Presentation—Combined Otolaryngology Spring Meetings, Boston, MA.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman HT, Karnell LH, Funk GF, Robinson RA, Menck HR. The National Cancer Data Base report on cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124:951–962. [DOI] [PubMed] [Google Scholar]
- 3.Warnakulasuriya S Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–316. [DOI] [PubMed] [Google Scholar]
- 4.Leon X, Quer M, Orus C, del Prado VM, Lopez M. Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck. 2000;22:680–686. [DOI] [PubMed] [Google Scholar]
- 5.Amin MB, Edge SB, American Joint Committee on Cancer, Springer Science+Business Media. AJCC Cancer Staging Manual. Chicago: American Joint Committee on Cancer/Springer International Publishing AG Switzerland; 2017. [Google Scholar]
- 6.Kantola S, Parikka M, Jokinen K, et al. Prognostic factors in tongue cancer—relative importance of demographic, clinical and histopathological factors. Br J Cancer. 2000;83:614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edge SBD, Compton C, Fritz A, Greene F, Trotti A. AJCC Cancer Staging Manual. 7th ed. New York: Springer-Verlag; 2010. [Google Scholar]
- 8.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15: 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Team RC. R: A Language and Environment for Statistical Computing. www.r-project.org/. Accessed November 10, 2018.
- 10.Therneau T, Atkinson B, Ripley B Recursive Partitioning and Regression Trees. R package version 4.1–8; 2014. [Google Scholar]
- 11.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 12.Ishwaran H, Kogalur UB, Blackstone EH, Lauer MS. Random survival forests. Ann Appl Stat. 2008;2:841–860. [Google Scholar]
- 13.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245–1251. [DOI] [PubMed] [Google Scholar]
- 14.NCCN Clinical Practice Guidelines in Oncology in Head and Neck Cancers; 2017. http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf [type]. Accessed November 10, 2018.
- 15.Gil Z, Carlson DL, Boyle JO, et al. Lymph node density is a significant predictor of outcome in patients with oral cancer. Cancer. 2009;115:5700–5710. [DOI] [PubMed] [Google Scholar]
- 16.Rose BS, Jeong JH, Nath SK, Lu SM, Mell LK. Population-based study of competing mortality in head and neck cancer. J Clin Oncol. 2011;29:3503–3509. [DOI] [PubMed] [Google Scholar]
- 17.Patel SC, Carpenter WR, Tyree S, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol 2011;29:1488–1494. [DOI] [PubMed] [Google Scholar]
- 18.Amit M, Yen TC, Liao CT, et al. Improvement in survival of patients with oral cavity squamous cell carcinoma: an international collaborative study. Cancer. 2013;119:4242–4248. [DOI] [PubMed] [Google Scholar]
- 19.Chan AK, Huang SH, Le LW, et al. Postoperative intensity-modulated radiotherapy following surgery for oral cavity squamous cell carcinoma: patterns of failure. Oral Oncol. 2013;49: 255–260. [DOI] [PubMed] [Google Scholar]
- 20.Daly ME, Le QT, Kozak MM, et al. Intensity-modulated radiotherapy for oral cavity squamous cell carcinoma: patterns of failure and predictors of local control. Int J Radiat Oncol Biol Phys. 2011;80:1412–1422. [DOI] [PubMed] [Google Scholar]
- 21.Zelefsky MJ, Harrison LB, Fass DE, et al. Postoperative radiotherapy for oral cavity cancers: impact of anatomic subsite on treatment outcome. Head Neck. 1990;12:470–475. [DOI] [PubMed] [Google Scholar]
- 22.Peters LJ, O’Sullivan B, Giralt J, et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: results from TROG 02.02. J Clin Oncol 2010;28:2996–3001. [DOI] [PubMed] [Google Scholar]
- 23.Choi KK, Kim MJ, Yun PY, et al. Independent prognostic factors of 861 cases of oral squamous cell carcinoma in Korean adults. Oral Oncol. 2006;42:208–217. [DOI] [PubMed] [Google Scholar]
- 24.Kademani D, Bell RB, Bagheri S, et al. Prognostic factors in intraoral squamous cell carcinoma: the influence of histologic grade. J Oral Maxillofac Surg. 2005;63:1599–1605. [DOI] [PubMed] [Google Scholar]
- 25.Gorsky M, Epstein JB, Oakley C, Le ND, Hay J, Stevenson-Moore P. Carcinoma of the tongue: a case series analysis of clinical presentation, risk factors, staging, and outcome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:546–552. [DOI] [PubMed] [Google Scholar]
- 26.Montero PH, Yu C, Palmer FL, et al. Nomograms for preoperative prediction of prognosis in patients with oral cavity squamous cell carcinoma. Cancer. 2014;120:214–221. [DOI] [PubMed] [Google Scholar]
- 27.Sargeran K, Murtomaa H, Safavi SM, Vehkalahti MM, Teronen O. Survival after diagnosis of cancer of the oral cavity. Br J Oral Maxillofac Surg. 2008;46:187–191. [DOI] [PubMed] [Google Scholar]
- 28.Vallecillo Capilla M, Romero Olid MN, Olmedo Gaya MV, Reyes Botella C, Bustos RV. Factors related to survival from oral cancer in an Andalusian population sample (Spain). Med Oral Patol Oral Cir Bucal 2007;12:E518–E523. [PubMed] [Google Scholar]
- 29.Yeole BB, Ramanakumar AV, Sankaranarayanan R. Survival from oral cancer in Mumbai (Bombay), India. Cancer Causes Control 2003;14:945–952. [DOI] [PubMed] [Google Scholar]
- 30.Dias GS, Almeida AP. A histological and clinical study on oral cancer: descriptive analyses of 365 cases. Med Oral Patol Oral Cir Bucal 2007;12:E474–E478. [PubMed] [Google Scholar]
- 31.El-Husseiny G, Kandil A, Jamshed A, et al. Squamous cell carcinoma of the oral tongue: an analysis of prognostic factors. Br J Oral Maxillofac Surg. 2000;38:193–199. [DOI] [PubMed] [Google Scholar]
- 32.van Imhoff LC, Kranenburg GG, Macco S, et al. Prognostic value of continued smoking on survival and recurrence rates in patients with head and neck cancer: A systematic review. Head Neck. 2016;38(suppl 1):E2214–E2220. [DOI] [PubMed] [Google Scholar]
- 33.Lacy PD, Piccirillo JF, Merritt MG, Zequeira MR. Head and neck squamous cell carcinoma: better to be young. Otolaryngol Head Neck Surg. 2000;122:253–258. [DOI] [PubMed] [Google Scholar]
- 34.Singh B, Bhaya M, Stern J, et al. Validation of the Charlson comorbidity index in patients with head and neck cancer: a multi-institutional study. Laryngoscope. 1997;107:1469–1475. [DOI] [PubMed] [Google Scholar]
- 35.Singh B, Bhaya M, Zimbler M, et al. Impact of comorbidity on outcome of young patients with head and neck squamous cell carcinoma. Head Neck. 1998;20:1–7. [DOI] [PubMed] [Google Scholar]
- 36.Paleri V, Wight RG, Silver CE, et al. Comorbidity in head and neck cancer: a critical appraisal and recommendations for practice. Oral Oncol. 2010;46:712–719. [DOI] [PubMed] [Google Scholar]
- 37.Reid BC, Alberg AJ, Klassen AC, et al. Comorbidity and survival of elderly head and neck carcinoma patients. Cancer. 2001;92:2109–2116. [DOI] [PubMed] [Google Scholar]
- 38.de Cassia Braga Ribeiro K, Kowalski LP, Latorre Mdo R. Perioperative complications, comorbidities, and survival in oral or oropharyngeal cancer. Arch Otolaryngol Head Neck Surg. 2003; 129:219–228. [DOI] [PubMed] [Google Scholar]
- 39.Huang SH, Hwang D, Lockwood G, Goldstein DP, O’Sullivan B. Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity: a meta-analysis of reported studies. Cancer. 2009; 115:1489–1497. [DOI] [PubMed] [Google Scholar]
- 40.Ganly I, Patel S, Shah J. Early stage squamous cell cancer of the oral tongue—clinicopathologic features affecting outcome. Cancer. 2012;118:101–111. [DOI] [PubMed] [Google Scholar]
- 41.Almangush A, Bello IO, Keski-Santti H, et al. Depth of invasion, tumor budding, and worst pattern of invasion: prognostic indicators in early-stage oral tongue cancer. Head Neck. 2014; 36:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


