Abstract
Background:
Treatment of wounds in diabetes often gets less than perfect healing. One of the reasons for the difficulty in treating wounds in diabetes is the growth of aerobic and anaerobic bacteria. This study aims to determine the pulse voltage and treatment time that can optimally inactivate bacteria, and their effect on wound healing in mice suffering from diabetes.
Methods:
The study used electrical stimulation with a direct voltage of 10 volts given a pulse voltage of 50–80 volts, a width of 50 μs, and the number of pulses of 65 per second. The research samples were Staphylococcus aureus (S. aureus) and Pseudomonas aeruginosa (P. aeruginosa) bacteria that grew on beef and mice (Mus musculus) with diabetes. The treatment for S. aureus and P. aeruginosa bacteria was carried out using a pulse voltage of 50–80 volts for 5–15 min/day and repeated for 3 days. Meanwhile, treatment of mice wounds was carried out with a pulse voltage of 80 volts for 15 min/day and repeated for 7 days.
Results:
The results showed that treatment with a pulse voltage of 50–80 volts and a treatment time of 5–15 min significantly reduced the number of S. aureus and P. aeruginosa bacteria in beef (p≤0.05). Treatment with a pulse voltage of 80 volts for 15 min made beef free from bacteria. Meanwhile, treatment with a pulse voltage of 80 volts for 15 min per day for seven days resulted in the wound state of three mice in the maturation phase and two mice in the proliferation phase on day 8 with an average wound area of 0.108 cm 2.
Conclusion:
The treatment with a pulse voltage of 80 volts for 15 min made the beef sterile, the mice wounds healed quickly, and the mice not stressed. The higher the blood glucose level, the slower the wound healing process.
Keywords: Bacteria, Electrical stimulation, Pulse voltage, Wound
Introduction
Diabetes is a severe threat to human health globally. In 2019 the number of people with diabetes globally was around 463 millions, and it is estimated that by 2030 it will increase significantly into 578.4 millions 1. Approximately 15–25% of diabetic patients have a lifetime risk of developing diabetic foot ulcers, 40–80% of whom will become infected so severely that the infection extends to the bone, causing osteomyelitis 2. One of the causes of difficulty in treating wounds in diabetics is the growth of aerobic and anaerobic bacteria such as Propionibacterium granulasum, Pseudomonas aeruginosa (P. aeruginosa), and Staphylococcus aureus (S. aureus), among many others 3. Therefore, efforts need to be made to minimize the occurrence of infection in the wound so that the healing process can occur faster.
Numerous researchers have made observations about the ability of electrical stimulation to influence bacterial activity. It has been reported that electrical stimulation can affect flagellar proteins and cause disruption of flagella, thereby inhibiting bacterial motility 4. The electric current in microampere can also damage the bacterial cell membrane and allow Propidium iodide to enter the bacteria 5. Therefore, electrical stimulation can accelerate wound healing in people with diabetes with lower side effects. Besides, it has been reported that electrical stimulation can reduce bacterial infection, increase local perfusion, and promote wound healing 6. Electrical stimulation is easy and safe to use, reduces infection, and improves local perfusion 7.
Previous studies have reported that electrical stimulation has a direct antibacterial effect on Gram-positive and Gram-negative bacteria, but this effect is much lower than that of wound antiseptics 8. The alternating low-frequency and low-voltage electric currents are likely to induce a significant change in the metabolic activity of the bacteria but not in their viability 9. This antibacterial activity is only effective on the surface of the electrode because bacteria adhering to the catheter area can still live even when 100 μA direct current is applied 10.
Previous studies have shown that direct current and alternating current with low voltage has little effect on bacterial growth and wound healing. The low effect of low-voltage electrical stimulation on bacterial growth occurs because the skin and surrounding tissue are resistive and capacitive 11. As a result, direct current with low voltage applied makes the impedance large, so it is difficult for the electric current to pass through the skin tissue. Therefore, for the inactivation of bacteria and fast wound healing with minimal side effects, this study used electrical stimulation with a direct voltage of 10 volts given a medium voltage pulse. The applied pulse voltage has a narrow pulse width with a long duration to minimize side effects, especially joule heating. Electrical stimulation with medium voltage pulses has never been encountered in previous studies. Electrical stimulation with medium voltage pulses will accelerate the decrease in bacteria, so the wound heals quickly. Nevertheless, the drawback of this healing method is the difficulty of placing electrodes on large and deep wounds.
This present study aims to inactivate S. aureus and P. aeruginosa bacteria that grow on beef and treat wounds in mice suffering from diabetes. The application of electrical stimulation on S. aureus and P. aeruginosa bacteria aims to determine its optimal dose in the bacteria inactivation, while wound care on mice aims to examine the healing effect of such electrical stimulation.
Materials and Methods
Sample preparation
This study used samples of S. aureus and P. aeruginosa bacteria that grew on beef with a size of 1×1×1 cm 3. The bacterial growth process was carried out by taking pure isolates of S. aureus and P. aeruginosa bacteria, and then each isolate was grown in a test tube containing 50 ml of sterile liquid Nutrient Broth (NB) media. The bacteria in the test tube were then incubated for 24 hr at 37°C. Following this, the sterile cut of beef was inserted into the NB medium, which had been overgrown with bacteria and incubated for 5 hr 12.
This study used a sample of male mice aged between 60 to 65 days. The weight of the mice was between 20–24 g. Initially, alloxan tetrahydrate was injected into mice, resulting in diabetes 13,14. Afterwards, ten mice were randomly divided into two groups, five as the control group and five as the treatment group. Seven days after the injection, the mice's blood glucose levels were measured to ensure that the mice had diabetes. The measurement of blood glucose levels was carried out after the mice had been fasting for 18 hr. Seven days after the measurement of the blood glucose levels, mice were injured on the back as deep as 1.3 mm and 2.0 cm long. One day after the injury, the treatment group was given electrical stimulation for 15 min a day, while the control group was not given stimulation. The use of experimental animal samples in this study has received approval from the Ethics Commission of the Faculty of Science and Technology, State Islamic University of Malang, Number 02/KEP.FST/PP.02/09/2019.
Electrical stimulation
The electrical stimulation device used is Electronic Pulse Massager Physiotherapy, model JR-309A manufactured by: Shenzhen Shunkanglai Technology Co., Ltd, with electrode shape adjustment. The electrode is made of aluminum. The pulse voltage for stimulation uses a combination of positive and negative polarization in the RUB mode. The application of electrical stimulation to samples of S. aureus and P. aeruginosa bacteria growing on beef was carried out with a direct voltage of 10 volts given a pulse voltage of 50, 60, 70, and 80 volts, a width of 50 μs and the number of pulses per second of 65 pulses. Electrical stimulation was given for 5, 10, and 15 min/day for three days.
Similarly, electrical stimulation was performed on the back of the injured mice. The position of the two electrodes was right on the outside of the wound. Electrical stimulation was given a day after the mice's back was injured. Stimulation was given for 15 min/day for seven days in the treatment group, while the control group was not given stimulation. The stimulation was carried out with a direct voltage of 10 volts given a pulse voltage of 80 volts.
Bacteria counting
Shortly after the electrical stimulation, the beef slices were removed with sterile tweezers, and then rinsed three times with clean, purified water so that the planktonic cells were released. The beef slices were then put in 10 ml of 0.9% NaCl in a test tube. Following this, 0.5 grams of glass beads were added and then vibrated for 2 min to release the bacterial cells 15. Further dilution was carried out by taking 1.0 ml of culture and putting it in a bottle containing 9.0 ml of sterile distilled water. The dilution was repeated 2–3 times according to the calculation requirements. After dilution, 1.0 ml of culture was taken and scratched on a petri dish that had been given liquid Plate Count Agar (PCA) media, and then incubated for 24 hr at a temperature of 37°C. Afterwards, the count of bacteria was carried out using a Colony Counter 16.
Measurement and analysis
Wound conditions were observed and photographed on days 2, 5, and 8 after the mice were injured. Observations were carried out to determine the healing phase, which in its implementation was assisted by experts. Observations also aimed to measure the length and width of the wound. The photo of the wound was taken using a camera with a resolution of 20 mega-pixels. In addition, statistical analysis to determine differences in the number of bacteria in beef samples was carried out using One Way Anova and Post Hoc analysis using Tukey's test. Verification of the results of the calculation of the wound area of mice was carried out using an Image Processing Program.
Results
S. aureus bacteria
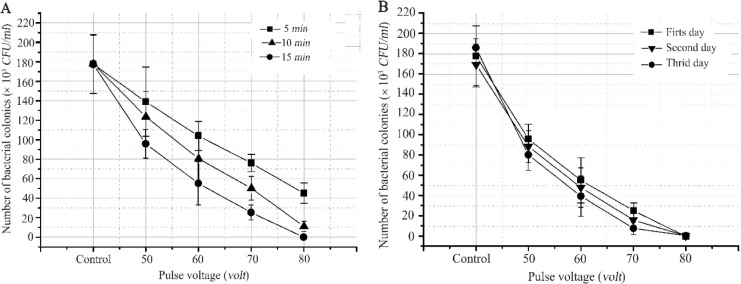
S. aureus is a Gram-positive bacterium, with a diameter of 0.5–1.5 μm 17. S. aureus is pathogenic and is known to be resistant to antibiotics 18. As a sample, bacteria were grown on beef so that the conductivity of the growth medium was low. Based on the results of the study, the provision of electrical stimulation changed the number of bacterial colonies. Figure 1 shows a reduction in the number of S. aureus bacteria colonies present in beef. Moreover, different pulse voltages caused a significant difference (p≤0.05) in reducing the number of bacteria. Initially, the number of bacteria in the untreated beef was (177.6±30.1)×.103 CFU/ml, but after being treated with a pulse voltage of 80 volts for 5, 10, and 15 min it became (45±10.44) ×103 CFU/ml, (11±3.00)×103 CFU/ml and 0.00 CFU/ml, respectively. The difference in treatment time also caused a significant difference in the number of bacteria, as shown in Figure 1A. The longer the treatment was given, the fewer the number of bacteria was present. It has also been previously reported that inactivation increases with increasing treatment time and electric current intensity 19. Gusmão et al in their study reported that the inactivation of Escherichia coli (E. coli) and S. aureus increased along with time and current intensity 20. Meanwhile, giving treatment with electrical stimulation periodically for 15 min a day did not show any significant difference in the number of bacteria on the first, second, and third day, as shown in Figure 1B. This condition occurred because the remaining bacteria divided, so their number increased. Meanwhile, different conditions occurred in treatment using a pulse voltage of 80 volts where the number of bacteria on the first day of treatment was equal to zero, so there was no division.
Figure 1.
The effect of electrical stimulation pulse voltage on the number of S. aureus bacteria growing on beef, where the number of bacteria decreased significantly with increasing pulse voltage with p≤0.05. A) Graph of the number of bacterial colonies due to electrical stimulation for 5, 10, and 15 min, where the number of bacterial colonies became zero in the treatment with a pulse voltage of 80 volts for 15 min. B) Graph of the number of bacterial colonies on the first, second, and third day, where the treatment with a pulse voltage of 80 volts for 15 min made the number of bacterial colonies zero from the first day to the third day.
P. aeruginosa bacteria
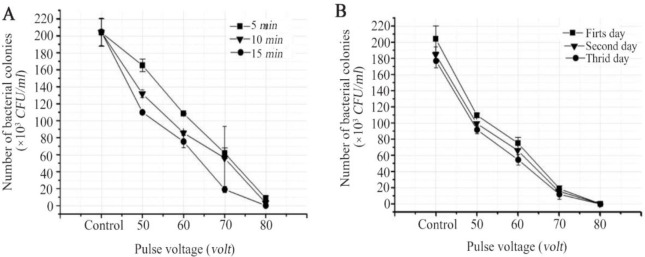
P. aeruginosa is anaerobic, rod-shaped Gram-negative bacterium 21. P. aeruginosa is resistant to a wide variety of antimicrobial agents and the expression of various molecular epidemiologies to various well-established antibiotic classes 22. Electrical stimulation with a pulse voltage of 50–80 volts has been shown to reduce the number of bacterial colonies in beef. Figure 2A. shows that treatment with a pulse voltage of 50–80 volts made the number of bacteria significantly reduce (p≤0.05). Specifically, the number of bacterial colonies in untreated beef was (204.33±16.25)×103 CFU/ml, while the number of bacterial colonies treated with a pulse voltage of 80 volts for 5, 10, and 15 min became (9±2.6)×103 CFU/ml, (3±1.7)×103 CFU/ml, and zero, respectively. Besides, the length of treatment also affected the number of bacteria. The longer the treatment was given, the fewer the number of bacteria was present in beef. Thus, regular treatment every day was proven to be able to inhibit and reduce the number of bacteria in beef. Specifically, treatment with a pulse voltage of 80 volts for 15 min a day was proven to be able to make beef free from bacteria, as shown in Figure 2B.
Figure 2.
The effect of electrical stimulation pulse voltage on the number of P. aeruginosa bacteria growing on beef, where the number of bacteria decreased significantly with increasing pulse voltage p≤ 0,05. A) Graph of the number of bacterial colonies due to electrical stimulation for 5, 10, and 15 min, where the number of bacterial colonies became zero in the treatment with a pulse voltage of 80 volts for 15 min. B) Graph of the number of bacterial colonies on the first, second, and third day, where the treatment with a pulse voltage of 80 volts for 15 min made the number of bacterial colonies zero from the first day to the third day.
Treatment of wounds
The samples were male mice which were aged between 60 to 65 days, had an average body weight of 22.2 g, and had fasting blood glucose levels before and after treatment of 159–264 mg/dL and 141–211 mg/dL, respectively. Mice were injured on the back with a length of 2.0 cm and a depth of 1.3 mm. Afterwards, wound treatment was performed using an electric stimulator with a pulse voltage of 80 volts for 15 min a day. Treatment with electrical stimulation was carried out for seven days, starting from the second to the eighth day after injury to the mice's back. Stimulation was done by attaching the electrodes to the outer area of the wound, bordering the wound.
Table 1 shows the condition of the mice's wounds on days 2, 5, and 8 post-injury. On the second day after injury, both the control group and the treatment group experienced inflammation of the wound with a wound area of more than 0.6 cm 2. On the fifth day, the wound conditions of the control and treatment groups were still in the proliferation phase, with a smaller wound area in the treatment group. After eight days, the wound conditions of the control group were all at the proliferation phase, while the treatment group was three animals at the maturation phase and two animals at the proliferation phase. Meanwhile, the average wound area for the control group was 0.58 cm2, while the treatment group was 0.108 cm2. After eight days, the wound area of the treatment group sample number 4 was 0.36 cm2 due to the high blood glucose level of mice, which was 264 mg/dL. Identical conditions also occurred in the control group sample number 5, which had a wound area of 1.5 cm2 because the blood glucose level was 236 mg/dL. High blood glucose levels cause loss of glycocalyx in the endothelium and accelerate the recruitment of leukocytes, creating a pro-inflammatory environment 23. In addition, blood glucose levels of more than 200 mg/dL are sufficient to cause leukocyte dysfunction 24.
Table 1.
Weight and blood glucose levels of mice and the healing phase and wound area (A) of mice, both control and treatment on days 2, 5, and 8 after injury
| No. | Weight (gr) | Blood glucose level (mg/dL) | The condition of the mice's wounds on days 2, 5, and 8 after injury | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Before | After | Day 2 | Day 5 | Day 8 | ||
|
| ||||||
| Treatment | ||||||
| Control group | ||||||
| 1 | 22 | 159 | 172 | Inflammation | Proliferation | Proliferation |
| A = 0.62 cm2 | A = 0.49 cm2 | A = 0.32 cm2 | ||||
| 2 | 20 | 177 | 198 | Inflammation | Proliferation | Proliferation |
| A = 0.62 cm2 | A = 0.60 cm2 | A = 0.60 cm2 | ||||
| 3 | 22 | 173 | 184 | Inflammation | Proliferation | Proliferation |
| A = 0.64 cm2. | A = 0.40 cm2 | A = 0.32 cm2 | ||||
| 4 | 21 | 155 | 141 | Inflammation | Proliferation | Proliferative |
| A = 0.63 cm2 | A = 0.58 cm2 | A = 0.14 cm2 | ||||
| 5 | 24 | 236 | 204 | Inflammation wound area | Proliferation wound area | Proliferation wound area |
| A = 1.60 cm2 | A = 1.6 cm2 | A = 1.5 cm2 | ||||
| Treatment group | ||||||
| 1 | 23 | 197 | 172 | Inflammation | Proliferation | Proliferation |
| A = 0.70 cm2 | A = 0.38 cm2 | A = 0.10 cm2 | ||||
| 2 | 24 | 194 | 150 | Inflammation | Proliferation | Maturation |
| A = 0.70 cm2 | A = 0.13 cm2 | A = 0.01 cm2 | ||||
| 3 | 22 | 154 | 166 | Inflammation | Proliferation | Maturation |
| A = 0.68 cm2 | A = 0.20 cm2 | A = 0.03 cm2 | ||||
| 4 | 23 | 264 | 211 | Inflammation | Proliferation | Proliferation |
| A = 0.88 cm2 | A = 0.57 cm2 | A = 0.36 cm2 | ||||
| 5 | 21 | 187 | 177 | Inflammation | Proliferation | Maturation |
| A = 0.70 cm2 | A = 0.36 cm2 | A = 0.04 cm2 | ||||
Discussion
Electrical stimulation using medium voltage pulses (50–80 volts) for 5–15 min significantly decreased the number of S. aureus and P. aeruginosa bacteria growing on beef. Even stimulation with a pulse voltage of 80 volts for 15 min made beef free from bacteria. Furthermore, electrical stimulation was carried out using a voltage of 10 volts with a pulse voltage of 80 volts for 15 min every day on the wounds of mice suffering from diabetes. The results showed that on the eighth day, the wound conditions of three mice were in the maturation phase, and two mice were in the proliferative phase. Different conditions occurred in mice that were not given electrical stimulation where the wounds of 5 mice were still in the proliferation phase. Therefore, electrical stimulation with a pulse voltage of 80 volts for 15 min a day could accelerate wound healing in mice suffering from diabetes.
Similar results have been reported in previous studies. One study suggested that electrical stimulation with a voltage of 220 volts significantly reduced the number of bacteria in chickens and ducks 25. Another study reported that treatment for 4 hr on bacteria with an electric current of 4 μA, 40 μA, and 400 μA and a voltage of 3 volts could reduce the growth rate of bacteria up to 5 times 26, electrical stimulation for 24 hr with an electric current of 20 mA could inhibit the growth of E. coli bacteria in soybean broth 27, and treatment using an electric current of 75 mA/cm2 and a voltage of 5.6 volts for 60 min could reduce the survival rate of E. coli in water by 85% 20. In addition, using electrical stimulation with medium voltage pulses of 50–80 volts has been shown to reduce the number of bacteria faster than using low voltages. This condition occurs because the cell is surrounded by an insulating cell membrane, while the cytosol and extracellular fluid are electrolytes 28; the electrolyte behaves like a resistor, while the membrane forms a capacitive element 28. The low-voltage direct current passing through the beef makes the impedance of the beef huge, making it difficult to pass through it. Therefore, direct current electrical stimulation with low voltage takes a long time to reduce bacteria significantly, while at the medium voltage, it is faster.
The decrease in the number of bacteria due to electrical stimulation may occur due to mechanical, thermal or chemical effects, or a combination of these three factors. Stratford revealed that electrical stimulation could cause hyperpolarization in E. coli bacterial cells and open K+ channels in the cell membrane 29. Meanwhile, Krishnamurthi demonstrated that micro-ampere currents cause membrane damage significant enough to allow molecules to enter the bacterial cell 5. The cell membrane is a viscoelastic fluid, so the membrane can rupture due to electrical pressure 30. The thermal effect occurs because an electric current flows through the resistor, causing joules of heating. However, the stimulation uses a pulse voltage with a narrow pulse and long duration; the heating effect is very low. Meanwhile, the chemical effect occurs because the mechanism of electric current activity can disrupt the integrity of the bacterial membrane or molecular electrolysis on the cell surface.
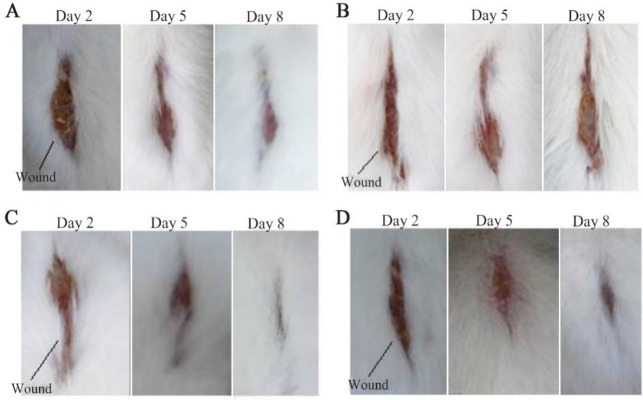
Regarding electrical stimulation for wound healing, Thakral revealed that electrical stimulation is known to accelerate wound healing and improve skin perfusion 6. Electrical stimulation can also inhibit the growth of possible pathogens, accelerate the recovery of damaged nerve tissue, increase the concentration of Adenosine Triphosphate (ATP) in the skin 31, increase DNA synthesis 32, attract epithelial cells and fibroblasts to the wound site, reduce edema, and increase blood flow 33. This study also showed that electrical stimulation with a pulse voltage of 80 volts and a treatment time of 15 min per day did not stress mice, making it possible to apply it as a wound therapy tool in humans. Figure 3 shows the wound conditions of control and treatment mice on days 2, 5, and 8 after injury. Figures 3A and 3B are the wound conditions of control mice, where on the eighth day, both were still in the proliferative phase. Figures 3C and 3D are the wound conditions of mice that were treated using electrical stimulation with a pulse voltage of 80 volts for 15 min a day. The eighth day showed that the wounds in the treated mice were narrower than those not treated.
Figure 3.
Wound condition of mice on days 2, 5, and 8 after being injured. Taking pictures using a camera with a resolution of 20 Megapixel. A) Wound condition of control mice that had blood glucose levels measured before treatment was 159 mg/dL and after treatment 172 mg/dL, where the wound area on day 8 was 0.32 cm2. B) Wound condition of control mice that had blood glucose levels measured before treatment was 177 mg/dL and after treatment 198 mg/dL, where the wound area on day 8 was 0.60 cm2. C) The condition of the mice's wounds that were given electrical stimulation and had blood glucose levels measured before treatment was 187 mg/dL and after treatment 177 mg/dL, where the wound area on day 8 was 0.04 cm2. D) The wound condition of mice that were given electrical stimulation and had glucose levels measured before treatment was 197 mg/dL and after treatment 172 mg/dL, where the wound area on day 8 was 0.10 cm2.
Wound healing with electrical stimulation was carried out directly at the wound position without affecting other organs so that the side effects caused were lower. Electrical stimulation can inhibit the growth of bacteria, thereby reducing the risk of infection in the wound even though the injury is in an open condition without a wound dressing. Therefore, the effect of peeling the wound due to the opening of the dressing can be avoided. Economically, wound therapy with electrical stimulation is cheaper because the cost of procuring equipment is cheap, easy to obtain, and can be used repeatedly.
Conclusion
The results showed that electrical stimulation with a pulse voltage of 50–80 volts significantly reduced the number of Gram-positive and Gram-negative bacteria growing on beef. The pulse voltage and treatment time also influenced the magnitude of the decrease in bacteria. Specifically, stimulation with a pulse voltage of 80 volts for 15 min could keep beef free from bacteria. Likewise, treating wounds in mice with diabetes using electrical stimulation could accelerate the healing process. The results also showed that higher blood glucose levels in mice required a longer healing process. Electrical stimulation of long and wide wounds requires electrode adjustment so that the placement of the electrodes does not interfere with the wound. It is necessary to understand the micro conditions of the wound healing process with electrical stimulation. Therefore, histological observation is required.
Acknowledgement
This research was carried out under the financial assistance from the Directorate of Islamic Higher Education, Indonesian Ministry of Religious Affairs. Therefore, we would like to say our great gratitude to the Minister of Religious Affairs along with his staff. We also thank the research and community service institution of State Islamic University of Maulana Malik Ibrahim Malang for their assistance in the successful accomplishment of this research.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.IDF Diabetes Atlas International Diabetes Federation 2019;266(6881). [Google Scholar]
- 2.Geraghty T, LaPorta G. Current health and economic burden of chronic diabetic osteomyelitis. Expert Rev Pharmacoecon Outcomes Res 2019;19(3):279–86. [DOI] [PubMed] [Google Scholar]
- 3.Alsaimary IEA. Bacterial wound infections in diabetic patients and their therapeutic implications. Medical Practice and Review 2010;1(2):12–5. [Google Scholar]
- 4.Berthelot R, Neethirajan S. Harnessing electrical energy for anti-biofilm therapies: Effects of current on cell morphology and motility. J Exp Nanosci 2017;12(1):197–207. [Google Scholar]
- 5.Krishnamurthi VR, Rogers A, Peifer J, Niyonshuti I I, Chen J, Wang Y. Microampere electric current causes bacterial membrane damage and two-way leakage in a short period of time. Appl Environ Microbiol 2020;86 (16):e01015–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thakral G, LaFontaine J, Najafi B, Talal TK, Kim P, Lavery LA. Electrical stimulation to accelerate wound healing. Diabet Foot Ankle 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ud-Din S, Bayat A. Electrical stimulation and cutaneous wound healing: A review of clinical evidence. Healthcare (Basel) 2014;2(4):445–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitten FA, Werner HP, Kramer A. A standardized test to assess the impact of different organic challenges on the antimicrobial activity of antiseptics. J Hosp Infect 2003; 55(2):108–15. [DOI] [PubMed] [Google Scholar]
- 9.Mirzaii M, Alfi A, Kasaeian A, Norozi P, Nasiri M, Sarokhalil DD, et al. Antibacterial effect of alternating current against Staphylococcus aureus and Pseudomonas aeroginosa. Russian Open Medical Journal 2015;4(2):2–6. [Google Scholar]
- 10.Liu WK, Brown MR, Elliott TS. Mechanisms of the bactericidal activity of low amperage electric current (DC). J Antimicrob Chemother 1997;39(6):687–95. [DOI] [PubMed] [Google Scholar]
- 11.Bjorklund S, Ruzgas T, Nowacka A, Dahi I, Topgaard D, Sparr E, et al. Skin membrane electrical impedance properties under the influence of a varying water gradient. Biophys J 2013;104(12):2639–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cárdenas FC, Giannuzzi L, Zaritzky NE. Mathematical modelling of microbial growth in ground beef from Argentina. Effect of lactic acid addition, temperature and packaging film. Meat Sci 2008;79(3):509–20. [DOI] [PubMed] [Google Scholar]
- 13.Rahman SS, Yasmin N, Rahman ATMM, Zaman A, Rahman MH, Rouf SMA. Evaluation and optimization of effective-dose of alloxan for inducing type-2 diabetes mellitus in long evans rat. Indian J Pharmaceutical Education and Research 2017;51(4):661–6. [Google Scholar]
- 14.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008;51(2):216–26. [DOI] [PubMed] [Google Scholar]
- 15.Krysinski EP, Brown LJ, Marchisello TJ. Effect of cleaners and sanitizers on Listeria monocytogenes attached to product contact surfaces. J Food Prot 1992;55(4); 246–51. [DOI] [PubMed] [Google Scholar]
- 16.Brugger SD, Baumberger C, Jost M, Jenni W, Brugger U, Mühlemann K. Automated counting of bacterial colony forming units on agar plates. PLoS One 2012; 7(3):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris LG, Foster SJ, Richards RG. An introduction to Staphylococcus aureus, and techniques for identifying and quantifying S. aureus adhesins in relation to adhesion to biomaterials: review. Eur Cells Mater 2002;4:39–60. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi SD, Malachowa N, Deleo FR. Pathogenesis of Staphylococcus aureus abscesses. Am J Pathol 2015; 185(6):1518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayoub GM, Zayyat R, Naji N. Electric current induced bacterial inactivation in seawater: effects of various operating conditions. Int J Environmental Science and Technology 2019;16(8):4749–60. [Google Scholar]
- 20.Gusmão ED, Moraes ICCP, Bidoia PB. Wind power characterization in the Lages City - SC, Brazil. Brazilian Arch Biol Technol 2010;53(5):155–60. [Google Scholar]
- 21.Rocha AJ, Barsottini MRDO, Rocha RR, Laurindo MV, De Moraes FLL, Da Rocha SL. Pseudomonas aeruginosa: Virulence factors and antibiotic resistance genes. Brazilian Arch Biol Technol 2019;62(January):1–15. [Google Scholar]
- 22.Mohanty S, Baliyarsingh B, Nayak SK. Antimicrobial Resistance in Pseudomonas aeruginosa: A concise review. IntechOpen 2020. [Google Scholar]
- 23.Shakya S, Wang Y, MacK JA, Maytin EV. Hyperglycemia-induced changes in hyaluronan contribute to impaired skin wound healing in diabetes: review and perspective. Int J Cell Biol 2015;2015:701738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bongiovanni CM. Nonsurgical management of chronic wounds in patients with diabetes. J Vascular Ultrasound 2006;30(4):215–8. [Google Scholar]
- 25.Al-Hmedawy N K, Al-Asadi MH, Al-Hilphy AR. Destruction of bacteria using electric stimulation of old duck and chicken carcasses. Basrah J Agric Sci 2019;31(2): 31–5. [Google Scholar]
- 26.Freebairn D, Linton D, Harkin-Jones E, Jones DS, Gilmore BF, Gorman SP. Electrical methods of controlling bacterial adhesion and biofilm on device surfaces. Expert Rev Med Devices 2013;10(1):85–103. [DOI] [PubMed] [Google Scholar]
- 27.Chakroborty SJ, Laymon M, Banerjee W, Chungi KCTY. Effect of electrical stimulation on carabeef quality. J Food Sci Technol 2007;44(5):487–90. [Google Scholar]
- 28.Pliquett U, Joshi RP, Sridhara V, Schoenbach KH. High electrical field effects on cell membranes. Bioelectro-chemistry 2007;70(2):275–82. [DOI] [PubMed] [Google Scholar]
- 29.Stratforda JP, Edwardsa CLA, Ghanshyama M J, Malysheva D, Delisea M A, Hayashic Y, et al. Electrically induced bacterial membrane-potential dynamics correspond to cellular proliferation capacity. Proc Natl Acad Sci USA 2019;116(19):9552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park JC, Lee MS, Lee DH, Park BJ, Han DW, Uzawa M, et al. Inactivation of bacteria in seawater by low-amperage electric current. Appl Environ Microbiol 2003;69 (4):2405–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Tong S, Chen N, Liu Y, Feng C, Hu Q. Effect of electro-stimulation on activity of heterotrophic denitrifying bacteria and denitrification performance. Bioresour Technol 2015;196:123–8. [DOI] [PubMed] [Google Scholar]
- 32.Hernández-Bule ML, Paíno CL, Trillo MÁ, Úbeda A. Electric stimulation at 448 kHz promotes proliferation of human mesenchymal stem cells. Cell Physiol Biochem 2014;34(5):1741–55. [DOI] [PubMed] [Google Scholar]
- 33.Jin HK, Hwang TY, Cho S H. Effect of electrical stimulation on blood flow velocity and vessel size. Open Med 2017;12(1):5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]