Abstract
Objective:
Altered hormonal regulation, including cortisol, is a proposed mechanism linking adiposity to obesity-related disorders. We examined the association of anthropometric, adipokine, and body fat distribution measures of adiposity with morning serum cortisol in an African American (AA) cohort.
Methods:
We investigated the cross-sectional associations of adiposity measures (BMI, waist circumference, leptin, adiponectin, leptin:adiponectin ratio, subcutaneous and visceral adipose tissue) and liver attenuation with cortisol in the Jackson Heart Study. Linear regression models were used to analyze the association between exposures and cortisol. Models were adjusted for multiple covariates.
Results:
Among 4,211 participants, a 1-SD higher BMI and waist circumference were associated with a 3.92% and 3.05% lower cortisol, respectively. A 1-SD higher leptin and leptin:adiponectin ratio were associated with a 6.48% and 4.97% lower morning serum cortisol, respectively. A 1-SD higher subcutaneous adipose tissue was associated with a 4.97% lower cortisol (all P < 0.001). There were no associations of liver attenuation or visceral adipose tissue with cortisol.
Conclusions:
Several measures of adiposity are associated with lower morning serum cortisol among AAs, with leptin having the greatest magnitude. Future studies examining the role of morning serum cortisol in the pathway from adiposity to cardiometabolic disease in AAs are warranted.
Introduction
Obesity impacts nearly 1 in 2 African American (AA) adults in the United States (1). Visceral adipose tissue (VAT), which surrounds the internal organs and which is hormonally active, is associated with an increased risk of coronary artery disease (2). Generally considered more benign, subcutaneous adipose tissue (SAT) is located directly beneath the skin in the abdomen, limbs, and gluteal region. AA adults have less VAT and more SAT compared with non-Hispanic White individuals and other racial/ethnic groups (3,4). However, despite having a lower burden of VAT, AAs have a significantly higher prevalence of cardiovascular disease than non-Hispanic White adults (5). This discrepancy suggests that adiposity may drive cardiometabolic disease through factors independent of visceral adiposity in AAs. Thus, there is a need to explore novel risk factors that are influenced by adiposity and are known to increase cardiovascular disease risk. One such risk factor is cortisol, whose excess and dysregulation with respect to normal circadian patterns are associated with cardiometabolic disease and mortality (6,7).
Perturbations of the hypothalamic-pituitary-adrenal (HPA) axis and cortisol have been cross-sectionally associated with obesity (BMI > 30 kg/m2) and obesity-related conditions including insulin resistance, type 2 diabetes, metabolic syndrome, and cardiovascular disease (8,9). A meta-analysis of smaller studies reported mixed associations between adiposity and morning serum/plasma cortisol; however, larger studies have found significant negative associations between anthropometric (body mass index [BMI], waist: hip ratio, and waist circumference [WC]) measures of adiposity and morning serum/plasma cortisol in Caucasian individuals (10–12). Higher BMI and greater WC are consistently associated with lower salivary wake-up cortisol and cortisol awakening responses (13,14). Additionally, a U-shaped relationship between BMI and morning serum cortisol has been identified (15). With the exception of one study (13), these associations have been investigated in predominantly non-Hispanic White and European individuals. Among community-dwelling individuals, longitudinal studies have indicated that changes in BMI drive perturbation of the HPA axis and cortisol production (16,17). These studies suggest that temporality favors changes in adiposity preceding alterations in systemic cortisol production.
Proteins produced and released by adipocytes are known as adipokines. Leptin is considered the primary peripheral protein involved in appetite regulation and it exerts its effects largely through the hypothalamus (18). Circulating levels of leptin have a strong positive correlation with overall fat mass, as leptin is secreted primarily by SAT (19). Previous studies investigating the relationship between leptin and cortisol have revealed mixed findings (10,20). Adiponectin, the most abundant adipokine in circulation, increases insulin sensitivity, reduces inflammatory cytokines, and is cardioprotective (21). It is exclusively produced by adipocytes and it decreases as adipocytes expand; thus it is inversely correlated with BMI, body fat percentage, WC, VAT, and SAT (22,23). Among Caucasians, adiponectin is associated with higher morning serum cortisol (24). Previous studies have been limited to anthropometric assessments of adiposity and thus they lacked the ability to examine more specific measures of adiposity, including adipokines and body fat distribution in association with cortisol in large populations. These studies have also suffered from a lack of racial/ethnic diversity or power to perform race stratified/specific analyses.
The Jackson Heart Study, a large prospective cohort study of AA adults, collected data on anthropometric as well as biomarker and depot-specific measures of adiposity. Given that significant racial/ ethnic differences exist in adipose tissue distribution (3,4), we examined the association between measures of adiposity and morning serum cortisol in AAs in the Jackson Heart Study. We hypothesized that anthropometric (WC and BMI), adipokine (leptin and leptin:adiponectin ratio), and body fat distribution (VAT, SAT, and liver attenuation) measures of adiposity would be negatively associated, while adiponectin would be positively associated with morning serum cortisol.
Methods
Study population
The Jackson Heart Study is a prospective cohort study of 5,306 AA adults, aged 21 to 94, from the tricounty area of metropolitan Jackson, Mississippi. The initial examination of participants was performed between 2000 and 2004, with 2 follow-ups then taking place between 2005 and 2008 and between 2009 and 2013. The design of the Jackson Heart Study has been described elsewhere (25). The study was approved by the institutional review boards of the University of Mississippi Medical Center, Jackson State University, and Tougaloo College. All participants provided informed consent. Participants were excluded if they had missing data on exposures (morning serum cortisol [n = 113]), outcomes (adiponectin [n = 91], leptin [n = 21], WC [n = 6], or BMI [n = 1 exam 1]) or important covariates at the initial exam (education [n = 23], systolic blood pressure [n = 15], smoking [n = 38], diabetes status [n = 4], or beta-blockers [n = 381]) or if cortisol measuring time was either missing or after 12 pm ([n = 402]) (Figure 1). In total, 1,095 participants were excluded, and the final analytical sample consisted of 4,211 participants. There were no statistically significant differences in either exposures or outcomes between included and excluded individuals. There were some statistically significant differences in confounders including age, sex, education, physical activity, systolic and diastolic blood pressures, and hormoe replacement therapy (HRT) medication use. However, the majority of these differences were small in magnitude and unlikely to be biologically significant. Further information regarding included versus excluded participants is reported in Supporting Information Table S1.
Figure 1.
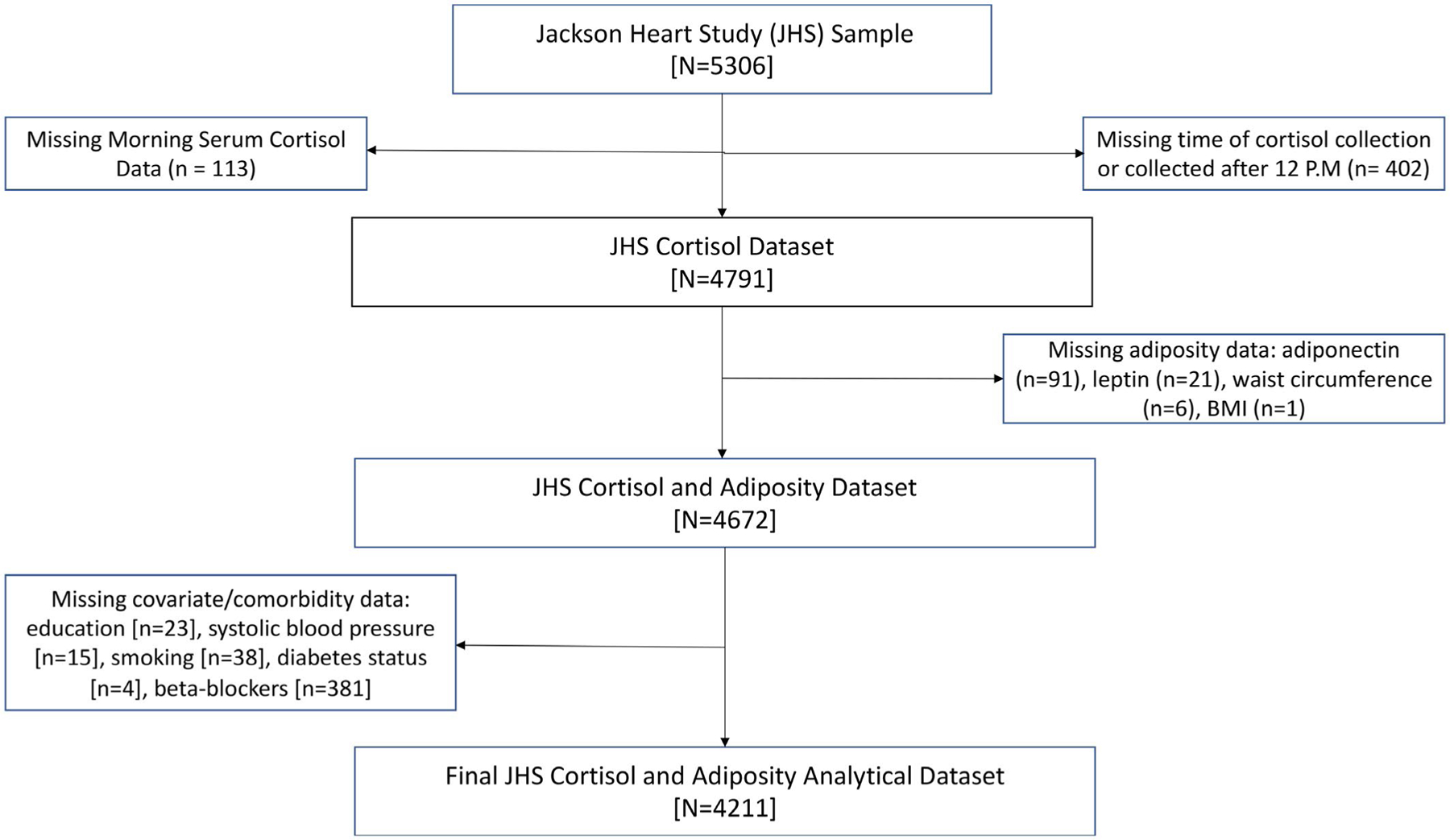
The Jackson Heart Study cohort included 5,306 participants. Individuals were excluded from the analysis because of missing data on morning serum cortisol (n = 113) or on the basis of missing time of cortisol collection/collection occurring after 12 PM (n = 402). Furthermore, participants were excluded if they had missing outcome or covariate data (adiponectin [n = 91], leptin [n = 21], waist circumference [n = 6], BMI [n = 1], education [n = 23], systolic blood pressure [n = 15], smoking [n = 38], diabetes status [n = 4] or beta-blockers [n = 381]).
Assessment of adiposity measures
WC was measured at the level of the umbilicus in centimeters, and BMI was calculated as weight in kilograms divided by height in meters squared. VAT and SAT volume as well as liver attenuation was measured via multidetector computed tomography (CT). The protocol for CT assessment of adiposity in the Jackson Heart Study has been previously described (26). Briefly, a 16-channel multidetector CT system equipped with cardiac gating (Lightspeed 16 Pro, GE Healthcare) was utilized to scan the heart and lower abdomen. Given that CT measures were collected at exam 2, we tested for change in adiposity between exam 1 and 2 (median 4 years). To determine the variance of adiposity levels in the time between exam 1 and 2, the intraclass correlation coefficients (ICC) were analyzed for BMI and WC. The ICC for BMI and WC was 0.90 and 0.83, respectively. The intraclass correlations were greater than 80%, suggesting adiposity was consistent between visits. Thus, CT measures were included in cross-sectional analyses.
Assessment of adipokines
Leptin and adiponectin were assessed using venous blood, which was collected after a minimum of 8 hours of fasting and 20 minutes in the supine position. Blood samples were processed using a standardized protocol and were stored at −80°C before serum adiponectin and leptin were measured (25). Total adiponectin concentration was measured using an enzyme-linked immunosorbent assay (ELISA) system (R&D Systems). The interassay coefficient of variation was 8.8%. Leptin was measured with a human lptin radioimmunoassay (RIA) kit (LINCO Research). The coefficient of variation was 10%. The leptin:adiponectin ratio was calculated via the division of leptin by adiponectin.
Assessment of morning serum cortisol
Normal diurnal cortisol regulation follows a circadian pattern in which levels are typically high upon waking, rise during the first 30 to 40 minutes post awakening, and then decline across the day, reaching a nadir in the late evening around 11 pm to midnight (27). In the Jackson Heart Study, fasting serum cortisol was collected in the morning between 8 am and 12 pm. Serum cortisol levels were measured by chemiluminescent immunoassay performed on an immunoassay system (ADVIA Centaur, Siemens). Intra-assay coefficients of variation were 9.1% and 7.7% for high and low cortisol concentrations, respectively.
Assessment of covariates
Information regarding covariates was obtained at the baseline exam. Data were collected during visits to the clinic or at home using questionnaires designed to assess occupation (management/professional versus not), educational attainment (less than high school versus high school diploma/GED equivalent), smoking status (current smoking versus not), medical conditions, and medication use (beta-blocker/HRT). Systolic blood pressure was taken while seated and was measured twice in 5-minute intervals. The average of the 2 measurements was used for the analysis. Physical activity was categorized according to the AHA 2020 Cardiovascular Health Impact Goals as poor, intermediate, or ideal, as described previously (28).
Statistical analyses
Baseline characteristics of participants were presented and compared across tertiles of BMI using analysis of variance (ANOVA) for parametric continuous variables, the Kruskal-Wallis test for nonparametric continuous variables, and the chi-square test for categorical variables. Because of skewed distributions, cortisol, adiponectin, leptin, leptin:adiponectin ratio, SAT, VAT, and liver attenuation measures were log-transformed prior to the analysis. We used multivariable linear regression models to assess the association between anthropometric (BMI and WC), CT (VAT, SAT, and liver attenuation) and biomarker (adiponectin, leptin, and leptin:adiponectin ratio) measures of adiposity after testing for a nonlinear association between measures of adiposity and log-morning serum cortisol (Supporting Information Figure S1). Relative magnitudes of associations between exposures and morning serum cortisol were assessed by standardizing each exposure variable. Various demographic, socioeconomic, and biological factors that have previously been shown to be associated with the exposure, outcome, or both were controlled for in the model. Both age and male sex are consistently associated with higher total daily cortisol output (29). Higher levels of education are associated with greater wake-up cortisol levels, and higher total daily output across the day among AAs (30). Higher socioeconomic status, a proxy for both greater occupational and educational attainment, is associated with a lower wake-up cortisol (31). Individuals who actively smoke have higher total cortisol output throughout the day (32). Systolic blood pressure is well known to increase with adiposity and it may be a proxy for increased sympathetic nervous system activity (33). Beta-blocker medications have significant interactions with the HPA axis. Estrogen monotherapy increases circulating plasma cortisol while an estrogen and progestin combination shows a trend toward increasing cortisol (34). Furthermore, HRT influences body composition in postmenopausal women (35). Finally, because of the circadian nature of cortisol output, we controlled for time of cortisol collection. Effect modification was tested by age and sex by inserting multiplicative interaction terms in the models and using the likelihood ratio test. Statistical significance was defined as 2-sided alpha < 0.05 in the main analysis and < 0.10 for interactions. Analyses were performed using SAS 9.4 (SAS Institute Inc.).
Results
Among 4,211 participants, the majority were female (64%), had a level of education equivalent to or greater than a high school diploma (81%), were nonsmokers (87%), and did not have diabetes (79%). The average participant was 54.9 years old (SD 12.7) and had BMI of 31.7 kg/m2 (SD 7.2) and WC of 100.6 cm (SD 16.2). Participants in higher tertiles of BMI were significantly less likely to smoke, and they had lower levels of morning serum cortisol, greater prevalence of diabetes, and higher systolic blood pressure. Individuals in higher categories of BMI had lower adiponectin and liver attenuation as well as higher leptin, SAT, and VAT (Table 1).
TABLE 1.
Demographic summary by BMI categories
| < 25 kg/m2 (n = 596) | 25–30 kg/m2 (n = 1,373) | 30+ kg/m2 (n = 2,242) | Total (n = 4,211) | P value | |
|---|---|---|---|---|---|
|
| |||||
| Age (y) | |||||
| Mean (SD) | 54.5 (14.5) | 56.3 (12.6) | 54.2 (12.2) | 54.9 (12.7) | < 0.001 |
| Sex | |||||
| Female | 320 (54%) | 762 (55%) | 1,626 (73%) | 2,708 (64%) | < 0.001 |
| Male | 276 (46%) | 611 (45%) | 616 (27%) | 1,503 (36%) | |
| Education | |||||
| Less than high school | 124 (21%) | 265 (19%) | 432 (19%) | 821 (19%) | 0.68 |
| Greater than or equal to high school | 472 (79%) | 1,108 (81%) | 1,810 (81%) | 3,390 (81%) | |
| Occupation | |||||
| Management/professional | 207 (35%) | 524 (38%) | 774 (35%) | 1,505 (36%) | 0.07 |
| Other | 389 (65%) | 849 (62%) | 1,468 (65%) | 2,706 (64%) | |
| Physical activity LS7 category | |||||
| Poor health | 300 (50%) | 611 (45%) | 1,135 (51%) | 2,046 (49%) | < 0.001 |
| Intermediate health | 183 (31%) | 427 (31%) | 712 (32%) | 1,322 (31%) | |
| Ideal health | 113 (19%) | 335 (24%) | 395 (18%) | 843 (20%) | |
| Current smoking | |||||
| No | 458 (77%) | 1,198 (87%) | 2,011 (90%) | 3,667 (87%) | < 0.001 |
| Yes | 138 (23%) | 175 (13%) | 231 (10%) | 544 (13%) | |
| Systolic blood pressure (mm Hg) | |||||
| Mean (SD) | 125.7 (17.3) | 126.5 (16.5) | 127.9 (16.1) | 127.2 (16.4) | < 0.001 |
| Min, max | 88.1, 188 | 78, 199 | 88.1, 228.4 | 78, 228.4 | |
| Diastolic blood pressure (mm Hg) | |||||
| Mean (SD) | 75.5 (9) | 75.6 (8.5) | 76.1 (8.6) | 75.8 (8.6) | 0.18 |
| Min, max | (50.1, 110.7) | (48.5, 110.7) | (36, 112.4) | (36, 112.4) | |
| BMI (kg/m2) | |||||
| Mean (SD) | 22.7 (1.9) | 27.6 (1.4) | 36.7 (6.1) | 31.7 (7.2) | < 0.001 |
| Min, max | (14.6, 24.9) | (25, 29.9) | (30, 75.1) | (14.6, 75.1) | |
| Waist circumference (cm) | |||||
| Mean (SD) | 81.8 (8.1) | 92.8 (8.1) | 110.4 (14.4) | 100.6 (16.2) | < 0.001 |
| Min, max | (60, 114) | (32, 134) | (66, 244) | (32, 244) | |
| Adiponectina (ng/dL) | |||||
| Mean (SD) | 8.6 (0.7) | 8.4 (0.7) | 8.3 (0.7) | 8.3 (0.7) | < 0.001 |
| Min, max | (6.1, 10.9) | (5.9, 11) | (5.9, 10.6) | (5.9, 11) | |
| Leptina (ng/dL) | |||||
| Mean (SD) | 2 (0.9) | 2.6 (0.8) | 3.5 (0.7) | 3 (1) | < 0.001 |
| Min, max | (−0.1, 3.9) | (0.2, 4.5) | (0.5, 5.9) | (−0.1, 5.9) | |
| SAT (cm3) a | |||||
| Missing | 286 | 568 | 1,017 | 1,871 | < 0.001 |
| Mean (SD) | 7 (0.6) | 7.4 (0.3) | 7.9 (0.3) | 7.6 (0.5) | |
| Min, max | (3.4, 8.1) | (4.8, 8.3) | (6.2, 8.6) | (3.4, 8.6) | |
| VAT (cm3) a | |||||
| Missing | 286 | 568 | 1,016 | 1,870 | < 0.001 |
| Mean (SD) | 6.1 (0.6) | 6.5 (0.5) | 6.8 (0.4) | 6.6 (0.5) | |
| Min, max | (4.2, 7.3) | (4.4, 7.6) | (4.5, 7.9) | (4.2, 7.9) | |
| Liver attenuation (HU) a | |||||
| Missing | 278 | 557 | 989 | 1,824 | < 0.001 |
| Mean (SD) | 4.1 (0.1) | 4.1 (0.2) | 4 (0.2) | 4.1 (0.2) | |
| Min, max | (3.1, 4.5) | (1.8, 4.4) | (2.3, 4.6) | (1.8, 4.6) | |
| Morning serum cortisola (μg/dL) | |||||
| HRT medication | |||||
| No | 516 (87%) | 1,183 (86%) | 1,876 (84%) | 3,575 (85%) | 0.06 |
| Yes | 36 (6%) | 141 (10%) | 251 (11%) | 428 (10%) | |
| Beta-blocker medication | |||||
| No | 560 (94%) | 1,232 (90%) | 1,991 (89%) | 3,783 (90%) | 0.001 |
| Yes | 36 (6%) | 141 (10%) | 251 (11%) | 428 (10%) | |
Ideal, intermediate, and poor health were defined by AHA 2020 Cardiovascular Health Impact Goals. P values calculated using χ2 (categorical variables), ANOVA (parametric continuous variables), and Kruskal-Wallis test (nonparametric continuous variables).
Indicates logged value.
HRT, hormone replacement therapy; HU, Hounsfield units; LS7, Life’s Simple Seven; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Examining the continuous relationships between adiposity measures and cortisol (Table 2), a 1% higher adiponectin was found to be associated with a 0.034% higher morning serum cortisol. A 1% higher leptin and leptin:adiponectin ratio were associated with a 0.07% and 0.045% lower cortisol, respectively, in the fully adjusted models (both P < 0.001). A 1-kg/m2 higher BMI and 1-cm higher WC were associated with 0.6% and 0.2% lower cortisol, respectively (both P < 0.001). A 1% higher SAT was associated with a 0.1% lower morning serum cortisol in the fully adjusted model (P < 0.001). No associations were observed between visceral adipose tissue and liver attenuation with baseline cortisol.
TABLE 2.
Association of continuous anthropometric, adipokines, and body fat distribution measures with cortisol
| Measure of adiposity | Cortisola (n = 4,211), Beta (95% CI), P value |
|---|---|
|
| |
| Adiponectin (ng/dL) a | 0.034 (0.015, 0.053), 0.001 |
| Leptin (ng/dL) a | −0.07 (−0.088, −0.052), < 0.001 |
| Leptin:adiponectin ratio a | −0.045 (−0.058, −0.033), < 0.001 |
| Waist circumference (cm) | −0.002 (−0.003, −0.001), < 0.001 |
| BMI (kg/m2) | −0.006 (−0.007, −0.004), < 0.001 |
| SAT (cm3)a (n = 2,340) | −0.1 (−0.14, −0.06), < 0.001 |
| VAT (cm3)a (n = 2,341) | −0.014 (−0.048, 0.02), 0.43 |
| Liver attenuation (HU)a (n = 2,387) | 0.029 (−0.054, 0.112), 0.50 |
Indicates logged value.
Adjusted for age, sex, education, occupation, systolic blood pressure, smoking, physical activity, hormone replacement therapy medications, beta-blocker medica- tions and cortisol collect time.
Logged-dependent variable, level-independent: % ΔY = ( eβ − 1) ∗ 100.
Logged-dependent variable, logged-independent: % ΔY = ( 1. 01β − 1) ∗ 100 %. Interpretations: A 1% higher adiponectin is associated with a 0.034% higher morning serum cortisol. A 1% higher leptin is associated with a 0.07% lower morning serum cortisol. A 1% higher leptin:adiponectin ratio is associated with a 0.045% lower corti- sol. A 1-kg/m2 higher BMI is associated with a 0.6% lower cortisol. A 1-cm higher WC is associated with a 0.2% lower cortisol. A 1% higher SAT is associated with a 0.1% lower morning serum cortisol.
SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; HU, Hounsfield units.
A 1-SD unit increase in leptin was associated with a 6.48% (β = −0.067, 95% CI: −0.084, −0.05, P < 0.001) lower morning serum cortisol and a 1-SD higher adiponectin was associated with a 2.33% (β = 0.023, 95% CI: 0.01, 0.036, P < 0.001) higher morning serum cortisol. A 1-SD unit increase in both leptin:adiponectin ratio and SAT was associated with a 4.97% (leptin:adiponectin ratio [β = −0.051, 95% CI −0.065, −0.038, P < 0.001] and SAT [β = −0.051, 95% CI, −0.07, −0.032, P < 0.001]) lower morning serum cortisol. A 1-SD higher WC and BMI were associated with a 3.05% (β = −0.031, 95% CI: −0.043, −0.018) and 3.92% (β = −0.04, 95% CI: −0.052, −0.027) lower morning serum cortisol, respectively (both P < 0.001, Table 3, Figure 2).
TABLE 3.
Association of standardized continuous anthropometric, adipokine, and body fat distribution measures with cortisol
| Measure of adiposity | Cortisol (n = 4,211), Beta (95% CI), P value |
|---|---|
|
| |
| z-Log-adiponectin (ng/dL) | 0.023 (0.01, 0.036), 0.001 |
| z-Log-leptin (ng/dL) | −0.067 (−0.084, −0.05), < 0.001 |
| z-Log-leptin:adiponectin ratio | −0.051 (−0.065, −0.038), < 0.001 |
| z-Waist circumference (cm) | −0.031 (−0.043, −0.018), < 0.001 |
| z-BMI (kg/m2) | −0.04 (−0.052, −0.027), < 0.001 |
| z-Log-SAT (cm3) (n = 2,340) | −0.051 (−0.07, −0.032), < 0.001 |
| z-Log-VAT (cm3) (n = 2,341) | −0.007 (−0.024, 0.01), 0.43 |
| z-Liver attenuation (HU) (n = 2,387) | 0.006 (−0.011, 0.022), 0.50 |
Adjusted for age, sex, education, occupation, systolic blood pressure, smoking, physical activity, hormone replacement therapy medications, beta-blocker medica- tions, and cortisol collect time.
Logged-dependent variable, level-independent: % ΔY = ( eβ − 1) ∗ 100. Interpretations: A 1-SD higher adiponectin is associated with a 2.33% higher morn- ing serum cortisol. A 1-SD higher leptin is associated with a 6.48% lower morning serum cortisol. A 1-SD higher leptin:adiponectin ratio is associated with a 4.97% lower morning serum cortisol. A 1-SD higher WC is associated with a 3.05% lower morning serum cortisol. A 1-SD higher BMI is associated with a 3.92% lower morning serum cortisol. A 1-SD higher SAT is associated with a 4.97% lower morning serum cortisol. SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; HU, Hounsfield units.
Figure 2.
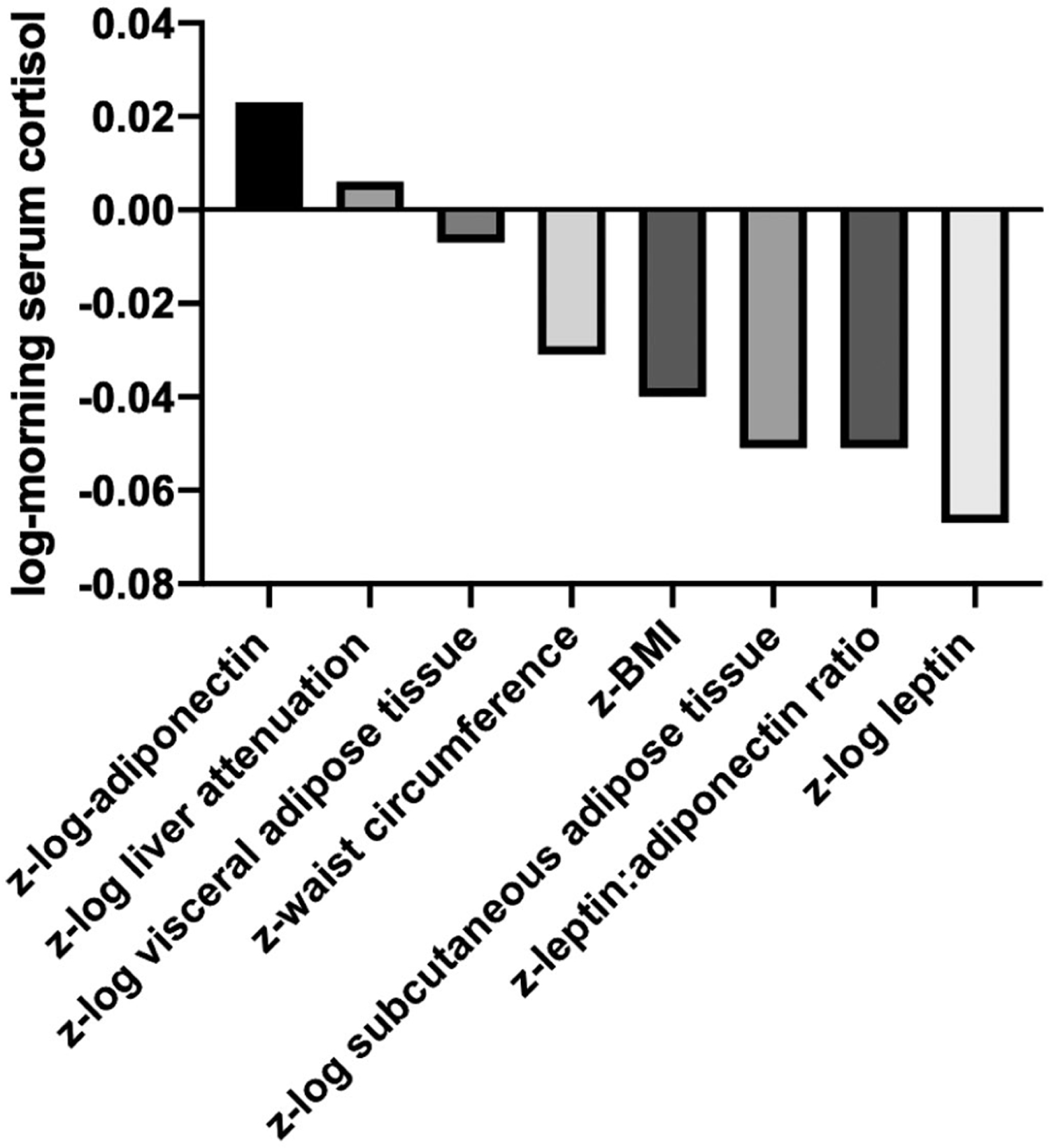
Relative magnitude of associations of standardized adiposity measures with morning serum cortisol. Leptin had the greatest relative magnitude of association with morning serum cortisol. The data corresponding with the figure is presented in Table 3. All associations P < 0.001 except for z-log liver attenuation and z-log visceral adipose tissue which were nonsignificant.
In the categorical analysis (Table 4), adiponectin in the 4th quartile was associated with a 5.7% higher cortisol than the 1st quartile with no evidence for a graded association. Leptin in the 2nd, 3rd, and 4th quartiles was associated with 7.6%, 11.6%, and 15.5% lower cortisol, respectively, compared with the 1st quartile. Increasing quartiles of leptin:adiponectin ratio and WC were also associated with lower morning serum cortisol. Compared with normal BMI, overweight and obesity BMI was associated with 7.7% and 13.2% lower cortisol, respectively. SAT in the 3rd and 4th quartiles was associated with a 10.1% and 10.0% lower cortisol, respectively (all P < 0.05). No significant associations were identified in the fully adjusted models assessing the association of VAT and liver attenuation with cortisol.
TABLE 4.
Categorical associations of anthropometric, adipokine, and body fat distribution measures with cortisol
| Measure of adiposity | Categories | Cortisola (n = 4,211), Beta (95% CI), P value |
|---|---|---|
|
| ||
| Adiponectin (ng/dL) | Q1 | ref |
| Q2 | −0.003 (−0.039, 0.032), 0.85 | |
| Q3 | 0.029 (−0.007, 0.065), 0.11 | |
| Q4 | 0.055 (0.019, 0.092), 0.003 | |
| Leptin (ng/dL) | Q1 | ref |
| Q2 | −0.079 (−0.118, −0.04), < 0.001 | |
| Q3 | −0.123(−0.168, −0.078), < 0.001 | |
| Q4 | −0.168 (−0.214, −0.121), < 0.001 | |
| Leptin:adiponectin ratio | Q1 | ref |
| Q2 | −0.08 (−0.117, −0.046), < 0.001 | |
| Q3 | −0.11 (−0.147, −0.073), < 0.001 | |
| Q4 | −0.126 (−0.164, −0.088), < 0.001 | |
| Waist circumference (cm) | Q1 | ref |
| Q2 | −0.049 (−0.085, −0.014), 0.011 | |
| Q3 | −0.069 (−0.104, −0.034), < 0.001 | |
| BMI (kg/m2) | Q4 | −0.09 (−0.126, −0.055), < 0.001 |
| 1: < 25 | ref | |
| 2: 25–30 | −0.08 (−0.124, −0.045), < 0.001 | |
| SAT (cm3) (n = 2340) | Q1 | ref |
| 3: 30+ | −0.037 (−0.09, 0.012), 0.13 | |
| Q2 | −0.141 (−0.179, −0.104), < 0.001 | |
| Q3 | −0.107 (−0.157, −0.056), < 0.001 | |
| VAT (cm3) (n = 2341) | Q4 | −0.105 (−0.157, −0.052), 0.001 |
| Q1 | ref | |
| Q2 | −0.037 (−0.084, 0.010), 0.13 | |
| Q3 | −0.004 (−0.05, 0.043), 0.86 | |
| Q4 | −0.015 (−0.063, 0.034), 0.55 | |
| Liver attenuation (HU) (n = 2387) | Q1 Q2 | ref 0.04 (−0.007, 0.086), 0.09 |
| Q3 | 0.01 (−0.037, 0.056), 0.68 | |
| Q4 | 0.023 (−0.023, 0.07), 0.32 | |
Adjusted for age, sex, education, occupation, systolic blood pressure, smoking, physical activity, hormone replacement therapy medications, beta-blocker medications and cortisol collection time.
Interpretations: % ΔY = ( eβ − 1) ∗ 100.
Examples: Adiponectin levels in the 4th quartile were associated with a 5.7% higher cortisol than the 1st quartile. Leptin levels in the 2nd, 3rd and 4th quartiles were associated with 7.6%, 11.6%, and 15.5% lower cortisol, respectively, compared with the 1st quartile. Compared with those with normal BMI, overweight participants (BMI = 25–29.99 kg/ m2) and participants with obesity (BMI ≥ 30 kg/m2) had 7.7% and 13.2% lower cortisol levels, respectively. Subcutaneous adipose tissue in the 3rd and 4th quartiles had 10.1% and 10.0% lower cortisol, respectively (P < 0.05).
SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; HU, Hounsfield units
No consistent effect modification was identified by age or sex. Additionally, there was no evidence of a U-shaped association between measures of adiposity and morning serum cortisol (Supporting Information Figure S1).
Discussion
In a large sample of AAs, we examined the association of adiposity measures, including anthropometric, adipokine, and body fat distribution measures, with morning serum cortisol. In this study, leptin, leptin:adiponectin ratio, BMI, WC, and SAT were negatively associated with morning serum cortisol, while adiponectin was positively associated. Leptin had the greatest magnitude of relative association with morning serum cortisol in standardized analyses.
Anthropometric measures
Previous studies have investigated the cross-sectional relationship between anthropometric adiposity measures and cortisol; however, to our knowledge this is the first to examine these associations in a large cohort of AAs and, more generally, with objective measures of adiposity including adipokines and CT measures of body fat distribution. Concordant with our study, BMI, WC, and hip circumference had significant negative correlations with morning serum cortisol among middle-aged Scandinavian men (10) and middle-aged White men (36). While there was no evidence of effect modification by sex in the Jackson Heart Study, differences in the association between adiposity and cortisol have previously been identified between men and women in other populations. In a larger Scandinavian cohort, fasting cortisol was negatively correlated with measures of central adiposity in women, but not in men (12). Similarly, body weight and mean 24-hour plasma cortisol were inversely correlated among women; however, the 2 were invariant in men (37). The lack of sex-specific differences may be related to the high degree of generalized and abdominal obesity in the Jackson Heart Study compared with other previous studies and the detailed phenotypic biological and imaging characterizations used in this study.
Some prior studies are discordant with those aforementioned, having identified nonlinear relationships between obesity and morning serum cortisol among White individuals. A U-shaped association between BMI and morning serum cortisol was identified, with cortisol reaching a nadir at a BMI of 32 among White women (15). However, another study identified a linear association of BMI with cortisol (14). In the current study, there was no evidence of a U-shaped association between either BMI or WC and morning serum cortisol. This may be due to the distribution of BMI in the Jackson Heart Study, with only 14% of the participants having BMI < 25 and a mean BMI of 22.7 among those participants. One potential hypothesis that may explain the previously identified U-shaped association is that the state of being “underweight” may drive HPA axis activation and cortisol output. In the current sample, very few participants had BMI < 18, which may have hindered our ability to detect a significant signal.
Studies examining the association between adiposity and free salivary cortisol show similar results to those assessing plasma and serum cortisol. In the Whitehall II cohort, a linear trend between increasing categories of BMI (underweight, normal, overweight, and obese) and lower wake-up cortisol as well as blunted cortisol awakening responses were reported. These associations were mirrored with WC as the exposure (14). While the majority of investigations examining these associations utilized Caucasian participants, an analysis in the Multi-Ethnic Study of Atherosclerosis reported similar findings but was limited in power for racial/ethnic stratifications (13).
The hypothesized temporality of the associations between BMI and WC with altered HPA axis function is that adiposity precedes perturbations of the glucocorticoid-regulating system. This hypothesis is based on work from the Multi-Ethnic Study of Atherosclerosis, where investigators found that a 1% higher annual change in BMI was associated with a 2.9% lower wake-up and 3.1% lower 16-hour area under the curve salivary cortisol over a 7-year period (16), Similar associations were reported with WC as the exposure. These findings are corroborated by the Massachusetts Male Aging Study (17). Although the current study is cross-sectional, it is consistent with these previous findings as we found negative cross-sectional associations between continuous measures of both BMI and WC with morning serum cortisol.
Body fat distribution
The specific site of adipose tissue deposition has also been examined as a potential modulating factor of morning cortisol levels. In a comparison of women with abdominal versus peripheral obesity, those with abdominal body fat distribution had significantly lower wake-up cortisol and cortisol awakening responses than those with peripheral body fat distribution (38). This study, however, used less sophisticated measures of adipose tissue distribution than those used in the Jackson Heart Study. In the current study, SAT was associated with significantly lower morning serum cortisol while VAT and liver attenuation were unrelated to morning cortisol. Previously, a U-shaped relationship between VAT and morning serum cortisol was identified in women (15). Testing for a nonlinear association between both visceral and liver attenuation in the Jackson Heart Study revealed no significant findings (Supporting Information Figures S1C–S1D). VAT and liver attenuation may be associated to a greater degree with local cortisol production, regulated by 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), which may not be reflected in changes to circulating levels.
Although VAT is generally considered a greater component for cardiovascular disease risk because of its proinflammatory effects, SAT is also cross-sectionally associated with cardiometabolic risk factors (26). Given that AAs have the highest rates of obesity in the US, the absolute magnitude of SAT may play a larger role in cardiovascular disease burden than the relative ratio of VAT to SAT. SAT upregulates TNF-α indirectly through leptin production, which contributes to systemic inflammation (39). In addition to the relationship with systemic inflammation, the current analysis suggests that SAT and leptin are associated with HPA axis dysfunction, which may ultimately manifest itself in cardiometabolic disease (Figure 3). Lower levels of cortisol in the morning are generally considered indicative of HPA axisdysfunction. Further research is needed to explore cortisol as a potential mediator of the relationship between SAT-driven adiposity with cardiometabolic disease among AAs.
Figure 3.
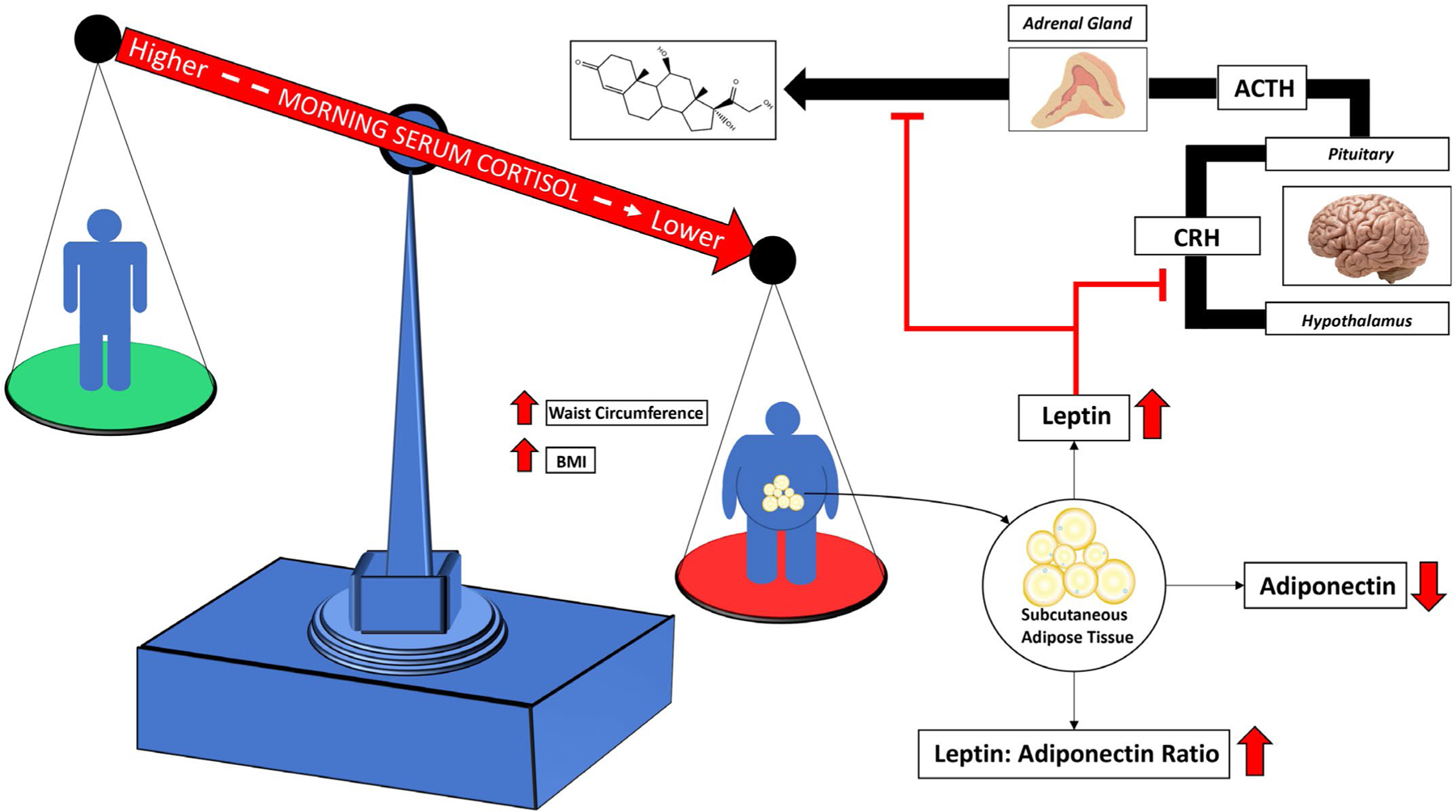
BMI and waist circumference were negatively associated with morning serum cortisol. The hypothesized mechanism between increasing adiposity and lower cortisol among African Americans involves the preferential storage of adipose tissue in subcutaneous depots. Subcutaneous adipose tissue produces leptin, which is able to suppress cortisol production at the hypothalamus and the adrenal gland.
Adipokines
To our knowledge, this is the first study to examine the association between leptin and cortisol in a large AA cohort. Leptin and cortisol share an inverse circadian rhythm, which is suggestive of potential counter-regulation (20). In support of this hypothesis, leptin is able to directly suppress the activity of the HPA axis at the hypothalamus by blunting corticotropin releasing hormone (CRH) in response to hypoglycemic stress (40). Additionally, incubation of adrenocortical cells with leptin inhibits both basal and adrenocorticotropin hormone (ACTH) stimulated cortisol release, further linking leptin to attenuated systemic cortisol production (41). Two previous investigations have examined the relationship between leptin as the exposure and cortisol as the outcome. First, among 11 healthy, young men the influence of sleep duration on 24-hour measures of leptin and cortisol showed an inverse relationship throughout daytime hours (20). In the other, leptin did not significantly correlate with cortisol among middle-aged Scandinavian men (10). In the current study, the negative association between leptin and morning serum cortisol may explain the relationship observed with SAT as the exposure. SAT was negatively associated with morning serum cortisol in the continuous model. Given the ability of leptin to suppress the activation of the HPA axis at the hypothalamus and cortisol production at the adrenal gland, the association may be explained through this shared molecular physiology.
Adiponectin, the most abundant adipokine in circulation, increases insulin sensitivity, is an anti-inflammatory protein, and is protective against cardiovascular disease (21). It is exclusively produced by adipocytes and decreases as adipocytes expand; thus it is inversely correlated with BMI, body fat percentage, WC, VAT, and SAT (22,23). Within a small study of Caucasian men and women, those with adiponectin levels in the 4th quartile had significantly higher morning serum cortisol levels than those in lower quartiles of adiponectin, which is consistent with our study (24).
To our knowledge, this is also the first investigation to examine the association between the leptin:adiponectin ratio and cortisol. The leptin:adiponectin ratio may be a potential surrogate measure estimating adipose tissue dysfunction (42). Increasing fat cell size is associated with lower adiponectin expression (43). Furthermore, leptin is also primarily expressed by SAT (19). This suggests that the leptin:adiponectin ratio may have more specificity for SAT dysfunction. Interestingly, the standardized leptin:adiponectin ratio and SAT had the same relative magnitude of effect on morning serum cortisol. The degree of HPA axis suppression in the morning may thus be a function of the magnitude of subcutaneous adipocyte dysfunction.
Adiposity, cortisol, and cardiovascular disease
Contextualizing these findings shows that multiple measures of adiposity are associated with a lower morning serum cortisol. Many studies have shown an inverse relationship between morning cortisol and subclinical or clinical cardiovascular disease. Smaller studies have shown that lower morning plasma cortisol trended toward being associated with required intervention following angiography (P = 0.07) and predicted those with more symptoms of angina (P = 0.01) (44). The relationship between morning serum cortisol and the dynamic nature of the diurnal cortisol curve has, to our knowledge, not been elucidated. However, lower morning serum cortisol may be indicative of a flatter, less dynamic diurnal cortisol curve. A recent meta-analysis and systematic review found that obesity has a significant relationship with flatter diurnal cortisol curves. Flatter diurnal cortisol curves have been associated cross-sectionally with coronary artery calcium and longitudinally with cardiovascular disease mortality (45). Thus, investigations of cortisol in the pathophysiology linking obesity with cardiovascular disease are paramount.
Our study has several strengths. First, our study uses data from the Jackson Heart Study, which has a large sample of AAs, a population that had limited data on the relationship between HPA axis dysfunction and measures of adiposity. Next, we were able to assess tissue-specific depots of adiposity via CT scans of VAT, SAT, and liver attenuation. Furthermore, we were able to assess adipokines’ levels of importance among study participants, thus giving greater insight into the role of adiposity on potential biochemical links to the HPA axis. However, this investigation should be interpreted in light of some limitations. First, given the cross-sectional nature of the study, the temporality between the associations cannot be determined. Second, we were limited by a single measure of morning serum cortisol and were thus unable to assess the diurnal cortisol profile and its relation to the exposure. Finally, the Jackson Heart Study is a single-site study, which precludes the results from being generalizable to AAs throughout the United States. Even though the strength of this study is to allow a greater understanding of adiposity and cortisol in AAs, our work lays the foundation for future studies to explore the potential differences between AAs and other racial groups in adiposity, hormonal and adipokine dysregulation, and cardiovascular disease.
Conclusion
In conclusion, this is the first study to examine the association between anthropometric, adipokine, and body fat distribution measures with morning serum cortisol in a large group of AAs. Leptin, leptin:adiponectin ratio, BMI, WC, and SAT were negatively associated with morning serum cortisol, while adiponectin had a positive association with cortisol. The strongest relative association was found between leptin and cortisol. AAs have the highest rates of obesity in the US based on BMI, and they preferentially store adipose tissue in SAT. Leptin is primarily produced from SAT and is able to suppress HPA axis activity, leading to potential long-term dysfunction of the system. Further studies are needed to identify the biological mediators of the association between adiposity and cortisol as well as examine the pathophysiology of cortisol in the relationship between adiposity and cardiovascular disease in AAs.
Supplementary Material
Study Importance.
What is already known?
The majority of studies in non-Hispanic White individuals have found negative associations between anthropometric measures of adiposity (BMI and waist circumference) and cortisol.
There are limited studies using other measures of adiposity, such as subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT), liver attenuation, and adipokines.
African Americans have a different body fat distribution compared with non-Hispanic White individuals and the relationship of adiposity with cortisol is not well understood in African Americans.
What does this study add?
BMI, waist circumference, SAT, leptin, and leptin:adiponectin ratio were all associated with lower morning serum cortisol in African Americans.
Among adiposity measures, leptin had the greatest magnitude of association with cortisol in African Americans.
There was no evidence of effect modification by age or sex.
How might these results change the direction of research or the focus of clinical practice?
The interactions and underlying mechanisms between adiposity and the cortisol axis warrant further investigation to develop novel approaches to cardiometabolic disease prevention and treatment in African Americans.
Funding:
The Jackson Heart Study is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I), and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD). The authors also wish to thank the staff and participants of the Jackson Heart Study (USA). BK was supported by a research grant from the Endocrine Society Summer Research Fellowship Program. JJJ was supported by K23DK117041 from the National Institute of Diabetes and Digestive and Kidney Diseases (USA). The funding sources had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Disclosure: The authors declared no conflict of interest.
Supporting information: Additional Supporting Information may be found in the online version of this article.
References
- 1.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief 2017;1–8. [PubMed] [Google Scholar]
- 2.Després J-P. Body fat distribution and risk of cardiovascular disease: an update. Circulation 2012;126:1301–1313. [DOI] [PubMed] [Google Scholar]
- 3.Katzmarzyk PT, Bray GA, Greenway FL, et al. Racial differences in abdominal depot– specific adiposity in white and African American adults. Am J Clin Nutr 2010;91:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll JF, Chiapa AL, Rodriquez M, et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity 2008;16:600–607. [DOI] [PubMed] [Google Scholar]
- 5.Carnethon MR, Pu J, Howard G, et al. Cardiovascular Health in African Americans: a scientific statement from the American Heart Association. Circulation 2017;136:e393–e423. [DOI] [PubMed] [Google Scholar]
- 6.Hackett RA, Kivimäki M, Kumari M, Steptoe A. Diurnal cortisol patterns, future diabetes, and impaired glucose metabolism in the whitehall II cohort study. J Clin Endocrinol Metab 2016;101:619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogelzangs N, Beekman ATF, Milaneschi Y, Bandinelli S, Ferrucci L, Penninx BWJH. Urinary cortisol and six-year risk of all-cause and cardiovascular mortality. J Clin Endocrinol Metab 2010;95:4959–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab 2009;94:2692–2701. [DOI] [PubMed] [Google Scholar]
- 9.Incollingo Rodriguez AC, Epel ES, White ML, Standen EC, Seckl JR, Tomiyama AJ. Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: a systematic review. Psychoneuroendocrinology 2015;62:301–318. [DOI] [PubMed] [Google Scholar]
- 10.Haffner SM, Miettinen H, Karhapää P, Mykkänen L, Laakso M. Leptin concentrations, sex hormones, and cortisol in nondiabetic men. J Clin Endocrinol Metab 1997;82:1807–1809. [DOI] [PubMed] [Google Scholar]
- 11.Tenk J, Mátrai P, Hegyi P, et al. In obesity, HPA axis activity does not increase with BMI, but declines with aging: a meta-analysis of clinical studies. PLoS One 2016;11:e0166842. doi: 10.1371/journal.pone.0166842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker BR, Soderberg S, Lindahl B, Olsson T. Independent effects of obesity and cortisol in predicting cardiovascular risk factors in men and women. J Intern Med 2000;247:198–204. [DOI] [PubMed] [Google Scholar]
- 13.Champaneri S, Xu X, Carnethon MR, et al. Diurnal salivary cortisol is associated with body mass index and waist circumference: the multiethnic study of atherosclerosis. Obesity 2013;21:E56–E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumari M, Chandola T, Brunner E, Kivimaki M. A nonlinear relationship of generalized and central obesity with diurnal cortisol secretion in the whitehall II study. J Clin Endocrinol Metab 2010;95:4415–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schorr M, Lawson EA, Dichtel LE, Klibanski A, Miller KK. Cortisol measures across the weight spectrum. J Clin Endocrinol Metab 2015;100:3313–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joseph JJ, Wang X, Roux AVD, et al. Antecedent longitudinal changes in body mass index are associated with diurnal cortisol curve features: the multi-ethnic study of atherosclerosis. Metabolism 2017;68:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Travison TG, O’Donnell AB, Araujo AB, Matsumoto AM, McKinlay JB. Cortisol levels and measures of body composition in middle-aged and older men. Clin Endocrinol (Oxf) 2007;67:71–77. [DOI] [PubMed] [Google Scholar]
- 18.Havel PJ. Role of adipose tissue in body-weight regulation: mechanisms regulating leptin production and energy balance. Proc Nutr Soc 2000;59:359–371. [DOI] [PubMed] [Google Scholar]
- 19.Harmelen VV, Reynisdottir S, Eriksson P, et al. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes 1998;47:913–917. [DOI] [PubMed] [Google Scholar]
- 20.Spiegel K, Leproult R, L’Hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 2004;89:5762–5771. [DOI] [PubMed] [Google Scholar]
- 21.Robinson K, Prins J, Venkatesh B. Clinical review: adiponectin biology and its role in inflammation and critical illness. Crit Care 2011;15:221. doi: 10.1186/cc10021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guenther M, James R, Marks J, Zhao S, Szabo A, Kidambi S. Adiposity distribution influences circulating adiponectin levels. Transl Res 2014;164:270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drolet R, Bélanger C, Fortier M, et al. Fat depot-specific impact of visceral obesity on adipocyte adiponectin release in women. Obesity 2009;17:424–430. [DOI] [PubMed] [Google Scholar]
- 24.Fernández-Real JM, López-Bermejo A, Casamitjana R, Ricart W. Novel interactions of adiponectin with the endocrine system and inflammatory parameters. J Clin Endocrinol Metab 2003;88:2714–2718. [DOI] [PubMed] [Google Scholar]
- 25.Taylor HA, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis 2005;15:S6–4-17. [PubMed] [Google Scholar]
- 26.Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: The Jackson heart study. J Clin Endocrinol Metab 2010;95:5419–5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joseph JJ, Golden SH. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann N Y Acad Sci 2017;1391:20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joseph JJ, Echouffo-Tcheugui JB, Talegawkar SA, et al. Modifiable lifestyle risk factors and incident diabetes in African Americans. Am J Prev Med 2017;53:e165–e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlamangla AS, Friedman EM, Seeman TE, Stawksi RS, Almeida DM. Daytime trajectories of cortisol: demographic and socioeconomic differences. findings from the national study of daily experiences. Psychoneuroendocrinology 2013;38: 2585–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dowd JB, Ranjit N, Do DP, Young EA, House JS, Kaplan GA. Education and levels of salivary cortisol over the day in U.S. adults. Ann Behav Med 2011;41:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hajat A, Diez-Roux A, Franklin TG, et al. Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology 2010;35:932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. J Clin Endocrinol Metab 2007;92:819–824. [DOI] [PubMed] [Google Scholar]
- 33.Dua S, Bhuker M, Sharma P, Dhall M, Kapoor S. Body mass index relates to blood pressure among adults. N Am J Med Sci 2014;6:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards KM, Mills PJ. Effects of estrogen versus estrogen and progesterone on cortisol and interleukin-6. Maturitas 2008;61:330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yüksel H, Odabasi AR, Demircan S, Köseoglu K, Kizilkaya K, Onur E. Effects of postmenopausal hormone replacement therapy on body fat composition. Gynecol Endocrinol 2007;23:99–104. [DOI] [PubMed] [Google Scholar]
- 36.Ljung T, Andersson B, Bengtsson B-Å, Björntorp P, Mårin P. Inhibition of cortisol secretion by dexamethasone in relation to body fat distribution: a dose-response study. Obes Res 1996;4:277–282. [DOI] [PubMed] [Google Scholar]
- 37.Strain GW, Zumoff B, Kream J, Strain JJ, Levin J, Fukushima D. Sex difference in the influence of obesity on the 24 hr mean plasma concentration of cortisol. Metabolism 1982;31:209–212. [DOI] [PubMed] [Google Scholar]
- 38.Duclos M, Pereira PM, Barat P, Gatta B, Roger P. Increased cortisol bioavailability, abdominal obesity, and the metabolic syndrome in obese women. Obes Res 2005;13:1157–1166. [DOI] [PubMed] [Google Scholar]
- 39.Shen J, Sakaida I, Uchida K, Terai S, Okita K. Leptin enhances TNF-alpha production via p38 and JNK MAPK in LPS-stimulated Kupffer cells. Life Sci 2005;77:1502–1515. [DOI] [PubMed] [Google Scholar]
- 40.Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology 1997;138:3859–3863. [DOI] [PubMed] [Google Scholar]
- 41.Bornstein SR, Uhlmann K, Haidan A, Ehrhart-Bornstein M, Scherbaum WA. Evidence for a novel peripheral action of leptin as a metabolic signal to the adrenal gland: leptin inhibits cortisol release directly. Diabetes 1997;46:1235–1238. [DOI] [PubMed] [Google Scholar]
- 42.Frühbeck G, Catalán V, Rodríguez A, Gómez-Ambrosi J. Adiponectin-leptin ratio: a promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 2018;7:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer LK, Ciaraldi TP, Henry RR, Wittgrove AC, Phillips SA. Adipose tissue depot and cell size dependency of adiponectin synthesis and secretion in human obesity. Adipocyte 2013;2:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reynolds RM, Ilyas B, Price JF, et al. Circulating plasma cortisol concentrations are not associated with coronary artery disease or peripheral vascular disease. QJM 2009;102:469–475. [DOI] [PubMed] [Google Scholar]
- 45.Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, Gilbert KE. Diurnal cortisol slopes and mental and physical health outcomes: a systematic review and meta-analysis. Psychoneuroendocrinology 2017;83:25–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


