Abstract
Fluoroquinolone-resistant avian Escherichia coli isolates from northern Georgia were investigated for gyrA and parC mutations. All isolates contained a mutation in GyrA replacing Ser83 with Leu; seven isolates also contained mutations replacing Asp87 with either Gly or Tyr. Random amplified polymorphic DNA analysis revealed that quinolone-resistant E. coli isolates were genetically diverse.
Colibacillosis continues to significantly contribute to increased mortality and economic losses in the poultry industry (1, 4, 6, 11). Sarafloxacin and enrofloxacin were approved in 1995 and 1996 in the United States for veterinary use to help control morbidity and mortality associated with Escherichia coli-related colibacillosis infections (14).
Quinolone resistance mechanisms employed by gram-negative bacteria include chromosomal mutations that reduce membrane permeability and decrease drug accumulation or alter DNA topoisomerases (9, 12, 17, 22, 23, 25). Clinical resistance to fluoroquinolones in E. coli, however, is mostly associated with mutations that result in amino acid changes in the A subunit (gyrA) and the B subunit (gyrB) of the DNA gyrase and in the parC-encoded subunit of topoisomerase IV (5, 8, 17, 18, 20, 23, 25).
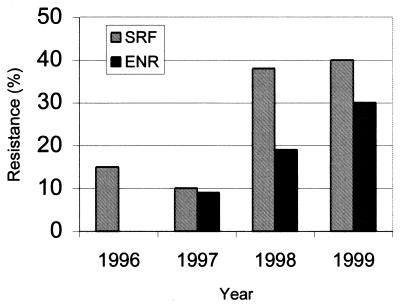
This study was undertaken to investigate the genetic mechanisms involved in the emergence of bacterial fluoroquinolone resistance among pathogenic avian E. coli isolates. Furthermore, isolates were genetically characterized via random amplified polymorphic DNA (RAPD) analysis to determine if fluoroquinolone resistance was associated with specific E. coli clones. Fluoroquinolone resistance was surveyed among avian E. coli organisms isolated at the Poultry Diagnostic and Research Center (PDRC), University of Georgia, during a 37-month period from May 1996 to June 1999 (Fig. 1). Five hundred thirty-five E. coli isolates from clinical cases of avian colibacillosis were identified at the PDRC during this time. The percentage of sarafloxacin-resistant avian E. coli isolates steadily increased from 15% in 1996 to 40% in 1999 (Fig. 1). Dual resistance to sarafloxacin and enrofloxacin increased from 9% in 1997 to 30% in 1999.
FIG. 1.
Emergence of fluoroquinolone resistance among clinical E. coli isolates in northern Georgia. Five-hundred thirty-five pathogenic avian E. coli isolates implicated in colibacillosis were submitted to the PDRC diagnostic laboratory during a 37-month period from May 1996 to June 1999. Forty-one isolates were submitted from May to December 1996, 189 isolates in 1997, 228 isolates in 1998, and 77 isolates from January to June 1999. Sarafloxacin and enrofloxacin susceptibilities were determined according to NCCLS standards (16). SRF, sarafloxacin; ENR, enrofloxacin.
Antimicrobial susceptibilities of 29 nalidixic acid-resistant avian E. coli isolates were determined with agar dilution and broth microdilution methods and interpreted according to the National Committee for Clinical Laboratory Standards (NCCLS) guidelines (15, 16). The majority of E. coli isolates were also resistant to several other antimicrobials tested, particularly sulfamethoxazole (n = 27), tetracycline (n = 25), streptomycin (n = 24), gentamicin (n = 18), and ampicillin (n = 16). Resistance to the cephalosporins cephalothin (n = 6) and ceftiofur (n = 4) was observed as well. Additionally, nine E. coli isolates were resistant to chloramphenicol. Sixty-six percent (19 of 29) of quinolone-resistant E. coli isolates exhibited multiple resistance to five or more antimicrobials. MICs of nalidixic acid, enrofloxacin, sarafloxacin, and ciprofloxacin were then determined using an agar plate dilution method (Table 1). The MIC of the antibiotic was defined as the concentration (in micrograms per milliliter of agar) at which no more than two colonies were detected. Ninety-three percent (27 of 29) of the isolates required >256 μg of nalidixic acid/ml for inhibition, whereas two isolates required 64 μg/ml (resistant MIC breakpoint, ≥32 μg/ml). Three isolates required 32 μg of enrofloxacin/ml (resistant MIC breakpoint, ≥2 μg/ml) and 32 μg of sarafloxacin/ml (resistant MIC breakpoint, ≥0.25 μg/ml) for inhibition, and 13 isolates required 8 μg of sarafloxacin/ml or more for inhibition. Six isolates displayed intermediate susceptibility to ciprofloxacin (MIC = 2 μg/ml), and one isolate was cross-resistant to ciprofloxacin (MIC = 16 μg/ml; resistant MIC breakpoint, ≥4 μg/ml) as well as to enrofloxacin (≥32 μg/ml) and sarafloxacin (≥32 μg/ml).
TABLE 1.
GyrA and ParC mutations and fluoroquinolone susceptibility profiles of avian E. coli isolates
| No. of isolates | Mutation in:
|
MIC range (μg/ml)a
|
||||
|---|---|---|---|---|---|---|
| GyrA proteinb | ParC genec | Nal | Cip | Enr | Sar | |
| 18 | Ser83 → Leu | AGC → AGT | 64–>256 | 0.5 to 16 | 1–32 | 1–32 |
| 4 | Ser83 → Leu | None (wild type) | >256 | 1 | 2 | 4 |
| 3 | Ser83 → Leu; Asp87 → Gly | AGC → AGT | >256 | 1 | 2 | 4–8 |
| 2 | Ser83 → Leu; Asp87 → Gly | None (wild type) | >256 | 1 | 2 | 2–4 |
| 2 | Ser83 → Leu; Asp87 → Tyr | AGC → AGT | >256 | 1 | 2 | 4–8 |
Single-stranded conformational polymorphism PCR was employed to investigate the presence of mutations in the quinolone resistance-determining regions (QRDR) of the gyrA gene of DNA gyrase and the parC gene of topoisomerase IV as previously described (8). DNA sequencing of the gyrA and parC regions using previously described primers (8) confirmed the initial single-stranded conformation polymorphism analysis and interpretations (Table 1). All 29 nalidixic acid-resistant E. coli isolates contained the amino acid substitution Ser83 → Leu in the GyrA QRDR (Table 1). However, many of these E. coli isolates displayed variable fluoroquinolone susceptibility patterns. Seven isolates had the additional amino acid substitution Asp87 → Tyr (n = 2) or Asp87 → Gly (n = 5) within the GyrA QRDR. Twenty-three of 29 quinolone-resistant E. coli isolates assayed contained a silent mutation at Ser85 (AGC→AGT) in parC. However, no mutations conferring amino acid substitutions were detected in the QRDR of parC among the quinolone-resistant avian E. coli isolates (Table 1).
Fluoroquinolone-resistant avian E. coli isolates have also been previously identified in Saudi Arabia and Spain (3, 4). However, neither study identified the specific mutations associated with the fluoroquinolone resistance phenotypes. Additionally, Everett et al. observed that the majority of veterinary E. coli isolates (six of eight) resistant to fluoroquinolones isolated in the United Kingdom had mutations only in the QRDR of the gyrA gene (8). The present study suggests that avian E. coli isolates recovered from diseased poultry in the United States display similar resistance phenotypes as well as sharing common resistance mechanisms with those isolates previously described by other European investigators. However, we cannot exclude additional mechanisms that have yet to be identified which may contribute to fluoroquinolone resistance, especially in isolates with high-level resistance.
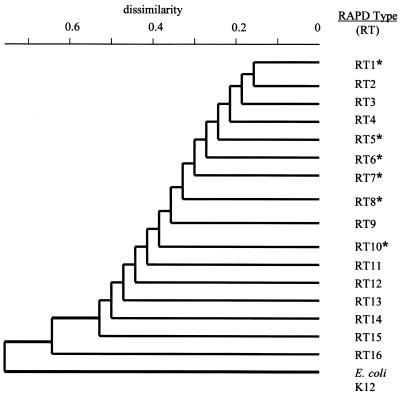
Avian E. coli isolates were further typed by RAPD using previously published primers (13). Grouping of E. coli isolates into each cluster or branch in the dendrogram correlated with similarities in their RAPD DNA pattern (Fig. 2). A total of 16 different clusters or RAPD types (RT) were identified from 184 avian E. coli isolates that have been previously described (13). The 29 fluoroquinolone-resistant avian E. coli isolates were typed by RAPD and could be assigned to six different RT groups: RT1, RT5, RT6, RT7, RT8, and RT10 (Fig. 2). The random association of fluoroquinolone-resistant avian E. coli isolates among multiple RTs indicates a genetically diverse population and suggests that fluoroquinolone resistance has appeared among independent chromosomal backgrounds and is not due to the emergence of particular resistant clonal genotypes.
FIG. 2.
Fluoroquinolone resistance occurs in genetically diverse avian E. coli isolates. Similarities among E. coli RAPD patterns were identified by cluster analysis using the neighbor-joining method to draw a phylogenetic tree (13). E. coli isolates with similar RAPD patterns were placed into the same cluster or branch and designated as an RT. Phylogenetic analysis identified 16 RTs based on differences in RAPD patterns among 184 avian E. coli isolates that have been previously described (13). An asterisk indicates an RAPD type identified among fluoroquinolone-resistant avian E. coli isolates.
This study reports the first occurrence of fluoroquinolone resistance in veterinary E. coli isolates recovered from clinical cases of avian colibacillosis in the United States. A marked increase in sarafloxacin and enrofloxacin resistance was observed among pathogenic avian E. coli in northern Georgia from 1996 to 1999. This trend coincides with the approval of these fluoroquinolones in 1995 and 1996 for treatment of E. coli-related poultry infections. Similar results have been seen among other pathogenic E. coli strains and most likely reflect the selection of antibiotic-resistant populations due to therapeutic use of antimicrobials (3, 4, 6, 7, 20, 24). There is mounting evidence that antimicrobial use in veterinary medicine may select for antimicrobial-resistant zoonotic bacterial pathogens (e.g., Salmonella and Campylobacter) (2, 7, 10, 19, 21). This has led to increased pressure to limit fluoroquinolones in animals to preserve the value of these drugs in the treatment of human infections (2, 10, 19, 21). However, the proposed linkage between fluoroquinolone use in agriculture and the occurrence of resistant human enteric bacterial pathogens is still being debated (19). Regardless, the detection of fluoroquinolone bacterial resistance in a veterinary situation stresses the need for the judicious use of these antimicrobials.
Acknowledgments
We thank Marie Maier and Julie Sherwood for their technical assistance.
This work was supported by grants from the U.S. Poultry and Egg Association (to J.M., D.W., and L.J.V.P.) and USDA-NRICGP grant 9902829 (to D.W., J.M., and S.Z.).
REFERENCES
- 1.Amara A, Ziani Z, Bouzoubaa K. Antibiotic resistance of Escherichia coli strains isolated in Morocco from chickens with colibacillosis. Vet Microbiol. 1995;43:325–330. doi: 10.1016/0378-1135(94)00101-2. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. Use of quinolones in food animals and potential impact on human health. Report and proceedings of a WHO meeting, Geneva, Switzerland. Geneva Switzerland: World Health Organization; 1998. pp. 1–17. [Google Scholar]
- 3.Bazile-Pham-Khac S, Truong Q C, Lafont J-P, Gutmann L, Zhou X Y, Osman M, Moreau N J. Resistance to fluoroquinolones in Escherichia coli isolated from poultry. Antimicrob Agents Chemother. 1996;40:1504–1507. doi: 10.1128/aac.40.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco J E, Blanco M, Mora A, Blanco J. Prevalence of bacterial resistance to quinolones and other antimicrobials among avian Escherichia coli strains isolated from septicemic and healthy chickens in Spain. J Clin Microbiol. 1997;35:2184–2185. doi: 10.1128/jcm.35.8.2184-2185.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61:377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emery D A, Nagaraja K V, Shaw D P, Newman J A, White D G. Virulence factors of Escherichia coli associated with colisepticemia in chickens and turkeys. Avian Dis. 1992;36:504–511. [PubMed] [Google Scholar]
- 7.Endtz H P, Ruijs G J, Van Klingeren B. Quinolone resistance in Campylobacter isolated from man and poultry following the introduction of fluoroquinolones in veterinary medicine. J Antimicrob Chemother. 1991;27:199–209. doi: 10.1093/jac/27.2.199. [DOI] [PubMed] [Google Scholar]
- 8.Everett M J, Jin Y F, Ricci V, Piddock L J V. Contributions of individual mechanisms to fluoroquinolone resistance in 36 Escherichia coli strains isolated from humans and animals. Antimicrob Agents Chemother. 1996;40:2380–2386. doi: 10.1128/aac.40.10.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh A S, Ahamed J, Chauhan K K, Kundu M. Involvement of an efflux system in high-level fluoroquinolone resistance of Shigella dysenteriae. Biochem Biophys Res Commun. 1998;242:54–56. doi: 10.1006/bbrc.1997.7902. [DOI] [PubMed] [Google Scholar]
- 10.Glynn M K, Bopp C, Dewitt W, Dabney P, Mokhtar M, Angulo F J. Emergence of multidrug-resistant Salmonella enterica serotype typhimurium DT104 infections in the United States. N Engl J Med. 1999;338:1333–1338. doi: 10.1056/NEJM199805073381901. [DOI] [PubMed] [Google Scholar]
- 11.Gross W G. Diseases due to Escherichia coli in poultry. In: Gyles C L, editor. Escherichia coli in domestic animals and humans. Wallingford, United Kingdom: CAB International; 1994. pp. 237–259. [Google Scholar]
- 12.Heisig P. Genetic evidence for a role of parC mutations in development of high-level fluoroquinolone resistance in Escherichia coli. Antimicrob Agents Chemother. 1996;40:879–885. doi: 10.1128/aac.40.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurer J J, Lee M D, Lobsinger C, Brown T, Maier M, Thayer S G. Molecular typing of avian Escherichia coli isolates by random amplification of polymorphic DNA. Avian Dis. 1998;42:431–451. [PubMed] [Google Scholar]
- 14.Medders W M, Wooley R E, Gibbs P S, Shotts E B, Brown J. Mutation rate of avian intestinal coliform bacteria when pressured with fluoroquinolones. Avian Dis. 1998;42:146–153. [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed., Approved standard. NCCLS document M7-A5. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. Approved standard. NCCLS document M31-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1999. [Google Scholar]
- 17.Ouabdesselam S, Hooper D C, Tankovic J, Soussy C J. Detection of gyrA and gyrB mutations in quinolone-resistant clinical isolates of Escherichia coli by single-strand conformational polymorphism analysis and determination of levels of resistance conferred by two different single gyrA mutations. Antimicrob Agents Chemother. 1995;39:1667–1670. doi: 10.1128/aac.39.8.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piddock L J V. Mechanisms of resistance to fluoroquinolones: state of the art 1992–1994. Drugs. 1995;49(Suppl. 2):29–35. doi: 10.2165/00003495-199500492-00006. [DOI] [PubMed] [Google Scholar]
- 19.Piddock L J V. Does the use of antimicrobial agents in veterinary medicine and animal husbandry select antibiotic-resistant bacteria that infect man and compromise antimicrobial chemotherapy? J Antimicrob Chemother. 1996;38:1–3. doi: 10.1093/jac/38.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Piddock L J V, Ricci V, McLaren I, Griggs D J. Role of mutation in the gyrA and parC genes of nalidixic-acid-resistant salmonella serotypes isolated from animals in the United Kingdom. J Antimicrob Chemother. 1998;41:635–641. doi: 10.1093/jac/41.6.635. [DOI] [PubMed] [Google Scholar]
- 21.Smith K E, Besser J M, Hedberg C W, Leano F T, Bender J B, Wicklund J H, Johnson B P, Moore K A, Osterholm M T. Quinolone-resistant Campylobacter jejuni infections in Minnesota, 1992–1998. N Engl J Med. 1999;340:1525–1532. doi: 10.1056/NEJM199905203402001. [DOI] [PubMed] [Google Scholar]
- 22.Vila J, Ruiz J, Marco F, Barcelo A, Goñi P, Giralt E, Jimenez De Anta T. Association between double mutation in gyrA gene of ciprofloxacin-resistant clinical isolates of Escherichia coli and MICs. Antimicrob Agents Chemother. 1994;38:2477–2479. doi: 10.1128/aac.38.10.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolfson J S, Hooper D C. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev. 1989;2:378–424. doi: 10.1128/cmr.2.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wooley R E, Spears K R, Brown J, Nolan L K, Dekich M A. Characteristics of conjugative R-plasmids from pathogenic avian Escherichia coli. Avian Dis. 1992;36:348–352. [PubMed] [Google Scholar]
- 25.Yoshida H, Bogaki M, Nakamura M, Yamanaka L M, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrB gene of Escherichia coli. Antimicrob Agents Chemother. 1991;35:1647–1650. doi: 10.1128/aac.35.8.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]