Introduction
Adenoid cystic carcinoma (ACC) is a rare malignancy, making up 10% of salivary gland tumors overall. ACC is a rare cause of head and neck cancer, accounting for approximately 1% of malignancies in the region [1]. Nonetheless, it is the most common malignant tumor of the minor salivary glands [2,3], although the submandibular and parotid glands are the most common individual sites [4]. In addition to major and minor salivary glands, ACC may arise at a variety of sites throughout the head and neck including the lacrimal gland, sinonasal cavity, external auditory canal, larynx, and trachea [5–9]. Its clinical behavior often follows a slow growth, aggressive local invasion with early perineural invasion, infrequent regional lymph node metastasis, and common hematogenous spread to the lung, liver, and bone [10–12].
Despite encouraging early survival, patients with ACC experience poor long-term outcomes. Reported 5-year survival rates range from 68 to 83% falling to 21–25% at 20 years of follow up [1,13]. Reported 5, 10, and 20-year recurrence rates are 48%, 61%, and 74%, respectively [13]. Well-established predictors of survival include T-classification, age, and skull base involvement [14–16]. Perineural invasion (PNI), a hallmark of ACC, has been regarded as a negative prognostic indicator; however, a recent international review found no relationship between PNI and survival [17–19]. A recent meta-analysis found lymphovascular invasion (LVI) to be a significant predictor of poor prognosis [20]. Because ACC is a rare disease with variable biologic phenotypes requiring long-term follow up, the literature is limited by small single-institution analyses. Because of this, it inherently lacks large cohorts that would facilitate analysis of prognostic factors and potential interventions. Furthermore, advanced ACC currently lacks effective long-term treatments.
The influence of type-2 diabetes mellitus on ACC has seldom been assessed, though it is typically thought to be a negative prognostic factor for survival due to diabetic patients’ poorer overall health [21]. Untreated type-2 diabetes is associated not only with increased cardiovascular disease and mortality, but also with an increased cancer risk, thought to be due to chronically elevated glucose and insulin levels [22]. Metformin is often prescribed as a safe and affordable first-line therapy for type-2 diabetes.
The combination of the metabolic effects of metformin, directing cellular pathways toward catabolism, with its favorable side-effect profile has led to interest in its potential protective roles in cancer. It neither causes hyperinsulinemia nor carries a risk of hypoglycemia, making it a viable adjuvant therapy for cancer patients [23,24]. Recent systematic reviews have found that metformin may provide a survival benefit in pancreatic, colorectal, and prostate cancer as an adjuvant therapy [25,26] and it has been shown to lower the risk of developing head and neck squamous cell carcinoma in diabetics [27]. There are currently fewer than ten active clinical trials investigating metformin’s potential as an adjuvant therapy in head and neck cancer [28], all focused on squamous cell carcinoma. However, no studies have assessed metformin’s effects in salivary gland malignancies such as ACC.
This study aimed to determine prognostic factors associated with overall survival (OS) and disease-free survival (DFS), and further delineate the time course of recurrence and long-term progression of ACC in the head and neck, with special attention to the associations of outcome with metformin and diabetes. Hereafter, this paper presents the first report of a protective effect of metformin in salivary gland malignancy.
Materials and Methods:
The Ohio State Cancer Institutional Review Board (IRB) approved this study (protocol number 2018C0033). ICD9/ICD10 codes were used to identify patients with the diagnosis of ACC treated at the OSUCCC—James from 1990 to 2017. Date of initial diagnosis spanned from 1978 to 2017. Two hundred thirty-six patients with a diagnosis of ACC were initially identified. Patients with ACC originating outside of the head and neck were excluded, leaving 206 adults with ages ranging from 20 to 92 years. Data related to patient characteristics, tumor characteristics, clinical features, and treatment modalities was collected via a retrospective review of medical records. Major salivary glands were defined as parotid, submandibular, and sublingual, while remaining cases in the head and neck were classified as minor gland disease. Pathologic grading scale was determined by degree of solid patterns as described by Szanto et al [29]. Patients with diabetes were defined as having an established diagnosis of type-2 diabetes at the time of their ACC diagnosis. Metformin users were those confirmed to be taking metformin at the time of their ACC diagnosis throughout follow up. Follow up was through April 2019.
Descriptive statistics were used to characterize the study population. For survival analysis, nine patients with metastasis at presentation, two with inoperable disease, and two with no post-operative follow-up data were excluded, leaving 193. OS was defined from time of diagnosis to time of death. Patients were censored for OS at last follow up if still alive. DFS was defined from time of surgery to time of recurrence. Both biopsy-proven local relapse and radiographic evidence of distant metastasis were considered recurrence for DFS calculations. Patients were censored for DFS at last follow up if no recurrence. Survival curves were plotted using the Kaplan-Meier method. Cox proportional hazards models were first used to assess univariate associations between potential predictors and DFS or OS. Variables with a p-value < 0.10 (excluding baseline diabetes status and metformin use) were then entered into multivariable models for OS and DFS. Variables were removed sequentially from the multivariable models via backward selection. The associations of diabetes or metformin with OS or DFS were modeled by Cox proportional hazards regression, controlling for the significant predictors maintained after backward selection. Adjusted hazard ratios (HR) and profile likelihood-based p-values and 95% confidence intervals (CI) were reported. A p-value of < 0.05 in the multivariable model was considered statistically significant. All analyses were conducted using the SAS system, version 9.4 (SAS Institute Inc., Cary, NC).
Results:
Cohort demographics and disease course
One-hundred-ninety-three patients were included in statistical analysis (Table 1). The cohort was composed of 88 men (46%) and 105 women (54%), with race distributed between 24 black (12%), 164 white (85%) and 5 other races (3%). The median age at diagnosis was 55 years (range 20–92). The most common presenting symptom was a painless mass (n = 129, 67%). Pain (36%) was another common presenting symptom, and otalgia was observed in 14% of patients. Additional presenting symptoms covered a wide range, including nasal obstruction, chronic sinusitis, facial numbness, tooth pain, headache, facial twitching, odynophagia, and eustachian tube dysfunction.
Table 1.
Patient demographics and disease characteristics.
| Patient Characteristics | No. of Patients (%) |
|---|---|
| No. of Patients | 193 |
| Male | 88 (46) |
| Female | 105 (54) |
| Median Age (range) | 55 (20 – 92) |
| ≤50 | 77 (40) |
| >50 | 116 (60) |
| Race | |
| Black | 24 (12) |
| White | 164 (85) |
| Other | 5 (3) |
| Alcohol | Missing 14 |
| Never | 99 (55) |
| Past | 34 (19) |
| Current | 46 (26) |
| Smoking | Missing 13 |
| Never | 71 (39) |
| Past | 80 (44) |
| Current | 29 (16) |
| Baseline Diabetes | 20 (10) |
| Metformin | 16 (8) |
| History of MI | 4 (2) |
| Primary Site | |
| Parotid | 53 (27) |
| Submandibular | 26 (13) |
| Sinonasal | 50 (26) |
| Oral Cavity/pharynx | 43 (22) |
| Larynx/Trachea | 10 (5) |
| Other | 11 (6) |
| Major Salivary Gland | 80 (42) |
| Minor Salivary Gland | 111 (58) |
| T-Classification | Missing 8 |
| 1 | 55 (30) |
| 2 | 39 (21) |
| 3 | 27 (15) |
| 4 | 64 (34) |
| N-Classification | Missing 3 |
| 0 | 174 (91) |
| 1 | 9 (5) |
| 2 | 7 (4) |
| Resection Margin | Missing 6 |
| Negative | 82 (44) |
| Positive | 105 (56) |
| Pathologic Grade | Missing 82 |
| 1 | 52 (47) |
| 2 | 29 (26) |
| 3 | 30 (27) |
| PNI (Missing 7) | 130 (70) |
| LVI (Missing 10) | 43 (24) |
| Adjuvant Therapy | Missing 1 |
| None | 63 (33) |
| Radiation | 117 (61) |
| Chemoradiation | 12 (6) |
| Recurrence | 90 (47) |
| Locoregional | 38 (20) |
| Distant | 28 (15) |
| Combined Locoregional and Distant | 24 (13) |
The most common primary tumor site was parotid gland (n = 53, 27%), followed by sinonasal tumors (n = 50, 26%), and oral cavity/oropharynx (n = 43, 22%). The remaining 47 tumors were in the submandibular gland, larynx, trachea, ear, and lacrimal gland. Seventy-six patients (40%) underwent neck dissection, with 16 (21%) nodal positive. As noted above, 9 patients presenting with distant metastasis, all in the lung, were excluded from the study cohort.
Twenty patients had diabetes at the time of their ACC diagnosis, and 16 of these were being treated with metformin. All patients taking metformin continued taking it throughout the study period. Non-metformin diabetics were insulin dependent at baseline and did not take metformin at any point during the study. Two metformin users were on insulin during the study with one beginning insulin 12 months after diagnosis. However, both patients remained on metformin the entire study period. A subgroup demographic analysis of the diabetic population versus remaining patients showed diabetics were significantly more likely to be obese (p = 0.001) and have hyperlipidemia (p = 0.031), however no other variables exhibited a statistically significant difference, demonstrating they otherwise closely represent the overall study population.
Recurrence of disease was observed in 90 patients (47%). Of these 90 patients with recurrence, 28 had distant metastatic disease, 38 had locoregional recurrence, and 24 had combined locoregional and distant recurrence. The most common site of distant metastasis was the lung (n = 48, 75%), followed by bone (n = 16, 25%) and liver (n = 8, 12.5%). The median time to initial recurrence was 36 months (IQR 16–70 months). Locoregional recurrence was observed as at up to 210 months after initial diagnosis, while distant metastasis was discovered at 240 months for one patient. Among patients who developed metastasis, those with bone and liver metastasis had substantially shorter median OS (45.5 months (95% CI: 24.3, 64.8)) than patients with only lung metastasis (93.3 months (95% CI: 55.9, 163.9)). The 17 patients with metastasis to bone and/or liver had a median DFS of 18 months (95% CI: 11.3, 36). Conversely, patients with only lung metastasis had a median DFS of 41 months (95% CI: 23, 60).
Median time to death was 77 months (inter-quartile range (IQR) 26–138 months), and median follow up for surviving patients was 79 months (IQR 44–128 months).
Prognostic Factors, including diabetes and metformin
Five and 10-year DFS were 63% and 46%, respectively, while 5- and 10-year OS were 87% and 61%. The median OS was 155 months (95% CI: 125, 180) and the median DFS was 96 months (95%CI: 70, 144). Univariate analysis (Table 2) for OS found that PNI, lymph node disease, positive resection margins, T4, advanced overall stage, pathologic grade 3, sinonasal disease, and history of myocardial infarction (MI) were associated with decreased survival. Those with diabetes showed no significant difference in OS despite their expected poorer health in comparison to the remainder of the cohort (Figure 1). Positive resection margins, LVI, PNI, T4, overall stage, sinonasal site, and pathologic grade were associated with shorter DFS on univariate analysis, while diabetes (HR (95% CI) = 0.4 (0.16, 0.98)) p = 0.0295 and metformin use HR (95% CI) = 0.29 (0.09, 0.91) p = 0.0180 were both significantly associated with decreased risk of recurrent disease (Figure 2).
Table 2.
Univariate analysis for DFS and OS variables, hazard ratio and 95% confidence interval.
| DFS |
OS |
|||||
|---|---|---|---|---|---|---|
| Variable | # Events | HR (95% CI) | p value | # Events | HR (95% CI) | p value |
| Age Group | 0.098 | 0.07 | ||||
| ≤50 (N = 77) | 43 | ref | 32 | ref | ||
| >50 (N = 116) | 47 | 0.7 (0.46, 1.07) | 58 | 1.51 (0.97, 2.34) | ||
| Sex | 0.08 | 0.68 | ||||
| Female (N = 105) | 56 | ref | 54 | ref | ||
| Male (N = 88) | 34 | 0.68 (0.44, 1.04) | 36 | 0.91 (0.59, 1.41) | ||
| Race | 0.25 | 0.40 | ||||
| Black (N = 24) | 14 | ref | 12 | ref | ||
| White (N = 164) | 76 | 0.71 (0.4, 1.26) | 78 | 0.77 (0.42, 1.42) | ||
| Smoking | 0.61 | 0.45 | ||||
| Never (n = 71) | 32 | ref | 29 | ref | ||
| Past (N = 80) | 42 | 1.08 (0.68, 1.71) | 43 | 1.35 (0.84, 2.18) | ||
| Current (N = 29) | 7 | 0.72 (0.31, 1.63) | 7 | 1.34 (0.57, 3.13) | ||
| LVI | 0.02 | 0.15 | ||||
| No (N = 140) | 61 | ref | 64 | ref | ||
| Yes (N = 43) | 25 | 1.73 (1.08, 2.76) | 20 | 1.45 (0.87, 2.41) | ||
| PNI | <0.001 | 0.006 | ||||
| No (N = 56) | 22 | ref | 28 | ref | ||
| Yes (N = 130) | 66 | 2.45 (1.47, 4.08) | 59 | 2.0 (1.23, 3.25) | ||
| Node Status | 0.36 | 0.009 | ||||
| N0 (N = 169) | 80 | ref | 78 | ref | ||
| N1+ (N = 16) | 8 | 1.41 (0.68, 2.93) | 10 | 2.44 (1.25, 4.77) | ||
| Resection Margin | <0.001 | <0.001 | ||||
| Negative (N = 82) | 32 | ref | 34 | ref | ||
| Positive (N = 105) | 56 | 2.39 (1.5, 3.82) | 53 | 2.18 (1.38, 3.43) | ||
| Gland Type | 0.89 | 0.08 | ||||
| Major (N = 80) | 39 | ref | 36 | ref | ||
| Minor (N = 111) | 50 | 1.03 (0.67, 1.57) | 54 | 1.48 (0.96, 2.28) | ||
| T Stage | <0.001 | <0.001 | ||||
| 1–3 (N = 121) | 52 | ref | 48 | ref | ||
| 4 (N = 64) | 35 | 2.29 (1.46, 3.6) | 37 | 3.56 (2.24, 5.66) | ||
| N Stage | 0.49 | 0.009 | ||||
| 0 (N = 174) | 80 | ref | 79 | ref | ||
| 1 (N = 9) | 5 | 1.28 (0.52, 3.16) | 6 | 1.94 (0.84, 4.49) | ||
| 2 (N = 7) | 3 | 1.92 (0.6, 6.11) | 4 | 4.2 (1.51, 11.7) | ||
| Overall Stage | 0.006 | <0.001 | ||||
| 1 (N = 54) | 19 | ref | 20 | ref | ||
| 2 (N = 41) | 21 | 1.35 (0.71, 2.58) | 17 | 1.12 (0.58, 2.16) | ||
| 3 (N = 26) | 12 | 1.43 (0.69, 2.98) | 11 | 1.7 (0.8, 3.63) | ||
| 4 (N = 63) | 35 | 2.64 (1.49, 4.67) | 37 | 3.98 (2,0.22, 7.12) | ||
| Pathologic Grade | 0.01 | 0.02 | ||||
| 1 (N = 52) | 17 | ref | 17 | ref | ||
| 2 (N = 29) | 12 | 1.61 (0.76, 3.41) | 8 | 1.24 (0.53, 2.92) | ||
| 3 (N = 30) | 19 | 2.78 (1.42, 5.43) | 19 | 2.48 (1.27, 4.85) | ||
| Sinonasal | 0.01 | 0.005 | ||||
| No (N = 143) | 60 | ref | 60 | ref | ||
| Yes (N = 50) | 30 | 1.79 (1.14, 2.8) | 30 | 1.93 (1.22, 3.04) | ||
| Adjuvant Therapy | 0.74 | 0.74 | ||||
| None (N = 63) | 27 | ref | 33 | ref | ||
| Radiation (N = 117) | 58 | 1.2 (0.76, 1.91) | 53 | 1.11 (0.71, 1.73) | ||
| Chemoradiation (N = 12) | 5 | 1.14 (0.43, 2.96) | 4 | 0.76 (0.27, 2.17) | ||
| Diabetes | 0.04 | 0.75 | ||||
| No (N = 173) | 85 | ref | 82 | ref | ||
| Yes (N = 20) | 5 | 0.4 (0.16, 0.98) | 8 | 0.89 (0.43, 1.85) | ||
| Metformin | 0.03 | 0.89 | ||||
| No (N = 177) | 87 | ref | 83 | ref | ||
| Yes (N = 16) | 3 | 0.29 (0.09, 0.91) | 7 | 0.95 (0.44, 2.05) | ||
| CAD | 0.35 | 0.47 | ||||
| No (N = 164) | 76 | ref | 75 | ref | ||
| Yes (N = 29) | 14 | 1.32 (0.74, 2.35) | 15 | 1.24 (0.69, 2.21) | ||
| Hx MI | 0.007 | 0.002 | ||||
| No (N = 189) | 86 | ref | 86 | ref | ||
| Yes (N = 4) | 4 | 4.07 (1.47, 11.3) | 4 | 5.09 (1.82, 14.19) | ||
| HLD | 0.13 | 0.86 | ||||
| No (N = 141) | 74 | ref | 71 | ref | ||
| Yes (N = 52) | 16 | 0.65 (0.38, 1.13) | 19 | 1.05 (0.62, 1.77) | ||
| HTN | 0.18 | 0.94 | ||||
| No (N = 114) | 58 | ref | 54 | ref | ||
| Yes (N = 79) | 32 | 0.74 (0.48, 1.15) | 36 | 1.02 (0.66, 1.57) | ||
| Obesity | 0.71 | 0.51 | ||||
| No (N = 173) | 81 | ref | 85 | ref | ||
| Yes (N = 20) | 9 | 1.14 (0.57, 2.28) | 5 | 0.74 (0.3, 1.82) | ||
| COPD | 0.94 | |||||
| No (N = 168) | 75 | ref | 0.4 | 77 | ref | |
| Yes (N = 25) | 15 | 1.28 (0.72, 2.27) | 13 | 1.02 (0.56, 1.86) | ||
Figure 1.
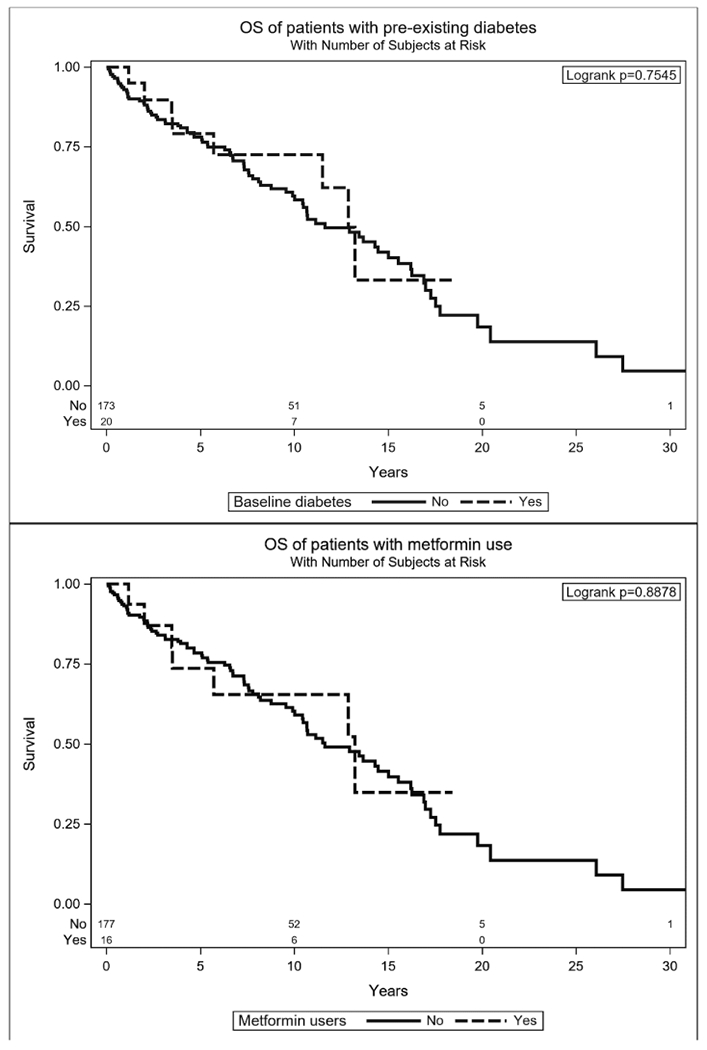
Kaplan-Meier plots for OS, separated by pre-existing diabetes and metformin users versus remaining cohort.
Figure 2.
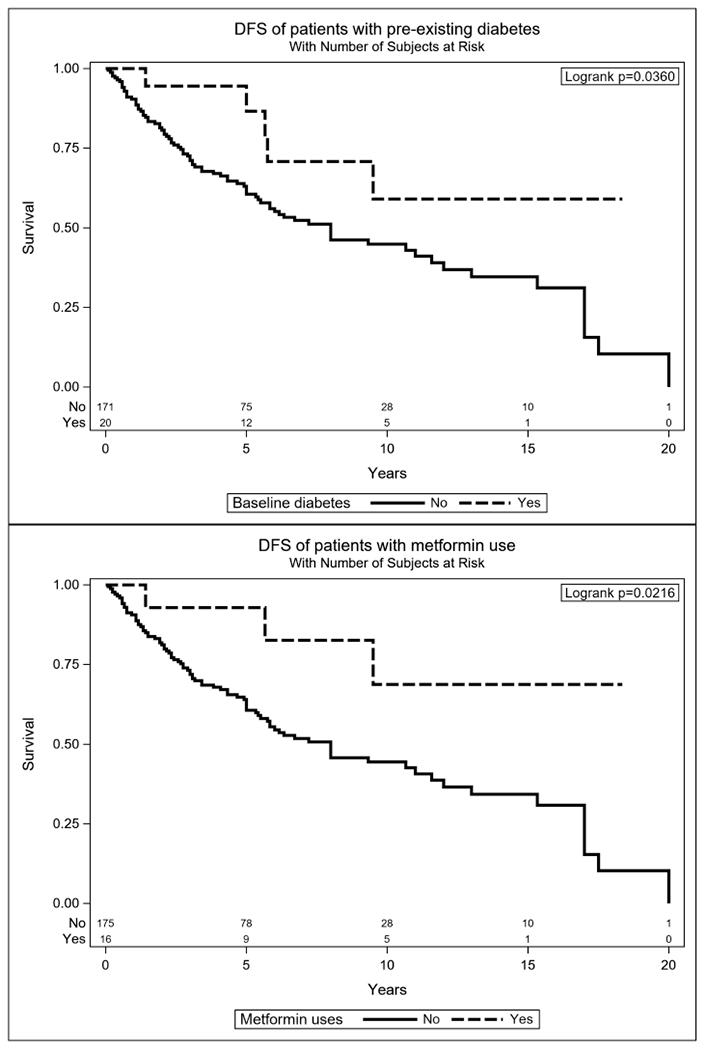
Kaplan-Meier plots for DFS, separated by pre-existing diabetes and metformin users versus remaining cohort.
Five and 10-year DFS for those with diabetes at presentation was 87% and 59%, compared to 61% and 45% for the remaining cases. Five- and 10-year DFS for metformin users was 93% and 69%, respectively, compared to 61% and 44% for non-users. Five- and 10-year OS rates for those with diabetes were 79% and 73%, respectively, compared to 78% and 60% for the remaining cohort. Metformin users were comparable with 5- and 10-year OS rates of 74% and 65%, respectively.
To evaluate the associations of diabetes and metformin use with DFS and OS in more detail, we used Cox multiple regression analyses to control for other significant predictors identified through backward elimination. Due to significant overlap in the diabetic and metformin populations, diabetes and metformin were each examined separately with respect to DFS and OS. The results of these analyses showed a significant improvement in DFS for both baseline diabetes (HR (95% CI) = 0.44 (0.16, 0.99); p = 0.046) (Table 3) and metformin (HR (95% CI) = 0.35 (0.09, 0.93); p = 0.032) (Table 4). In the subgroup of 16 metformin users, only three experienced recurrence (18.8%), compared to 49.2% of the remaining cohort. Of 16 metformin users, seven were deceased and only three of these died of ACC. This is in contrast with 177 patients not taking metformin, of which 62 out of the 83 deceased died due to ACC.
Table 3.
Cox multiple regression analysis of the association between diabetes at presentation with DFS and OS.
| DFS |
OS |
||||
|---|---|---|---|---|---|
| Variable | HR (95% CI) | p value | Variable | HR (95% CI) | p value |
| Diabetes | 0.44 (0.16, 0.99) | 0.046 | Diabetes | 1.02 (0.44, 2.08) | 0.96 |
| PNI+ | 2.37 (1.45, 4.04) | 0.001 | Age > 50 | 1.8 (1.13, 2.9) | 0.01 |
| Sinonasal | 1.61 (1.01, 2.52) | 0.045 | Positive Nodes | 3.12 (1.4, 6.41) | 0.01 |
| T4 | 2.96 (1.77, 4.9) | <0.001 | |||
| Sinonasal | 1.85 (1.08, 3.14) | 0.02 | |||
| Hx MI | 3.58 (1.04, 9.4) | 0.04 | |||
Associations of diabetes with DFS and OS were controlled for the indicated variables, which had been maintained in backward elimination starting with the variables identified by univariate analysis and in Table 2.
N = 180, 13 removed due to missing variables.
Table 4.
Cox multiple regression analysis of the association between Metformin with DFS and OS.
| DFS |
OS |
||||
|---|---|---|---|---|---|
| Variable | HR (95% CI) | p value | Variable | HR (95% CI) | p value |
| Metformin | 0.35 (0.09, 0.93) | 0.03 | Metformin | 1.25 (0.52, 2.59) | 0.59 |
| PNI+ | 2.31 (1.41, 3.95) | 0.001 | Age > 50 | 1.79 (1.13, 2.88) | 0.01 |
| Sinonasal | 1.62 (1.02, 2.53) | 0.04 | Positive Nodes | 3.13 (1.41, 6.4) | 0.01 |
| T4 | 2.95 (1.76, 4.9) | <0.001 | |||
| Sinonasal | 1.88 (1.1, 3.2) | 0.02 | |||
| Hx MI | 3.64 (1.06, 9.6) | 0.04 | |||
Associations of diabetes with DFS and OS were controlled for the indicated variables, which had been maintained in backward elimination starting with the variables identified by univariate analysis and in Table 2.
N = 180, 13 removed due to missing variables.
Discussion:
Diabetes and metformin in ACC
This study, one of the largest and most comprehensive ACC cohorts with long-term follow up, is the first to demonstrate an association of metformin treatment and diabetes with improved DFS in ACC patients. These results held even after controlling for the major other variables associated with DFS, PNI and a sinonasal site. Despite the expected poorer overall health of patients having diabetes and using metformin, and a previous report suggesting that diabetes was associated with shorter OS in patients with ACC [21], they were not associated with shorter OS in the present study.
Our results suggest that metformin use rather than diabetes per se was the main factor accounting for the improved DFS. Within the diabetic subgroup, patients taking metformin had a lower rate of recurrence (18.8%) compared to diabetics not taking metformin (50%). The metformin group displayed significantly better disease control compared to all metformin non-users with a 32% 5-year DFS improvement, and 24% improvement at 10-years. The fact that improved DFS is more strongly associated with metformin use than diabetes supports the hypothesis that metformin is responsible for this protective effect.
To our knowledge, there is only one other report that even discusses a possible relationship between diabetes and outcome in ACC. In a study of 88 patients of whom only 5 (5.7%) had diabetes, Choi et al. reported that diabetes mellitus was associated with shorter DFS and OS [21]. That study, however included no information regarding metformin use. Thus, those results might be consistent with the present results if metformin is providing the protection from disease recurrence and diabetics in the Choi et al. study were less likely to have been taking metformin.
Other results on ACC
The majority of our other findings are fairly consistent with the existing literature (Table 5). This study adds to the patients included in Oplatek et at. [30], more than doubling the sample size and increasing both follow-up times and the number of variables analyzed. Advanced stage has been a consistent predictor of decreased OS across multiple studies [13–15,30,31]. In this study we found age > 50, T- and N-classification, sinonasal disease, and history of MI to be associated with shorter OS. Consistent with prior reports, nodal positivity was found in<10% of patients with ACC (16 of 193) but was strongly associated with shorter OS when present.
Table 5.
Comparison of findings in recent single-institution analyses.
| Author | Year | N | Survival | OS Factors | DFS Factors |
|---|---|---|---|---|---|
| Present Study | 2019 | 193 | OS 5 = 78% DFS 5 = 63% |
PNI+, T4, prior MI, N+, age > 50 | PNI, prior MI, diabetes, metformin protective |
| Fordice et al. [35] | 1999 | 160 | DSS 5, 10, 15 years = 89%, 67.4%, and 39.6% | 4+ symptoms at presentation, N-status, solid histology, PNI of major nerves | PNI of major nerves, positive margins, solid histology |
| Van Weert et al. [13] | 2013 | 105 | OS 5, 10, 20 years = 68%, 52%, 28% DFS 5, 10, 20 years = 56%, 39%, 26% |
T-stage, N-status, positive margins, histologic subtype, increasing age | T-stage, N-status, positive margins, histologic subtype |
| Oplatek et at. [30] | 2010 | 99 | OS 5, 10-years = 78%, 55% | Increasing age, minor salivary gland, N+, AJCC stage | N+, LVI, advanced T-stage |
| Sung et al. [31] | 2003 | 94 | DSS 5, 10, 15 years = 80%, 58%, 48% | Distant metastasis (bone worse than lung), solid histology, T-stage | — |
| Choi et al. [21] | 2013 | 88 | OS 5-year = 89.7% DFS 5-year = 52.9% |
Age > 60, Diabetes | Diabetes, histologic grade 3, N+ |
Multiple regression analysis was used to determine all predictors with the exception of Fordice et al. (Kaplan-Meier and log-rank test).
Patients analyzed by Oplatek et al. are included in the present study.
An international multicenter study by Amit et al. reported significantly decreased OS of patients with ACC of the paranasal sinuses [32]. We similarly found sinonasal location to be a significant independent predictor of death, as sinonasal tumors had a lower median overall survival (97 months (95% CI: 79, 155)) than all other sites (173 months (95% CI: 128, 204)). ACC of the major salivary glands may be detected earlier in disease progression due to anatomic location, potentially accounting for this finding. In our cohort major gland disease was less likely to be locally advanced at presentation (23% T4) than was minor gland disease (40% T4), supporting this hypothesis.
We found distant metastasis, a common late complication of ACC and an important predictor of survival [31], in 58 patients (28%). Within this group of patients, we separated lung from bone or liver metastasis, as the latter sites have been identified as a potential subset of ACC patients with more aggressive disease strongly associated with activating NOTCH1 mutations [33]. Our findings also suggest that patients with bone and/or liver metastasis represent a distinct group with a more aggressive clinical course. The 17 patients with metastasis to bone and/or liver had substantially decreased survival intervals in both DFS and OS compared to those with only lung metastasis.
Strengths and weaknesses of the present study
There are several limitations to acknowledge for this study in addition to its retrospective nature. Some patients treated nearly three decades ago may have received slightly different care than under modern standards. Patients diagnosed through 2017 were included, meaning a subset of the cohort has relatively short follow up considering the indolent nature of ACC, and recurrence rates could be expected to increase over time. Nevertheless, this study represents the largest single-institution ACC cohort to date.
Although the number of metformin users was small, the association of metformin with longer DFS even after controlling for other clinical variables is a striking finding for a rare disease that has lacked significant progress in treatment. Additionally, the lack of association with OS supports the improvement related to metformin use in a population that typically has worse OS in long term studies. Furthermore, patients who were diagnosed with diabetes or started metformin at times after presentation with ACC were not considered diabetics or metformin users for survival analysis, potentially missing some additional association of those clinical variables with DFS and OS. This exclusion, however, was important to avoid survivorship bias that might occur if patients who simply lived long enough to develop diabetes later were included as diabetics. Our results thus were able to highlight important prognostic factors and further define the long-term course of this disease.
Implications for research
Metformin is the most widely used oral antihyperglycemic medication for type-2 diabetes. Its mechanism as an anticancer agent has been studied extensively in recent years and dozens of clinical trials are currently evaluating its therapeutic role in cancer. Current reviews in the literature cites the regulation of AMP-activated protein kinase (AMPK) as a main mechanistic target of metformin among other pathways [22,24,25,34]. This and other proposed mechanisms are largely tied to the overall benefit of tight glycemic control, avoidance of hyperglycemia, and directing cellular pathways towards catabolism [22]. In pancreatic cancer, metformin causes upregulation of hsa-miR-150, leading to a decrease in MYB and inducing cell cycle arrest and apoptosis [36]. Rearrangements of the MYB gene are common in ACC and are thought to be a driver of pathogenesis [37]. This mechanistic link offers a potential explanation for the improved outcomes observed in the metformin subgroup. Metformin is a compelling adjuvant therapy in patients without diabetes because it is well tolerated without many side effects and does not induce hypoglycemia [24].
There are two possibilities to consider as potential explanations for the decrease in recurrence observed with metformin. The first is that metformin users develop less aggressive primary tumors as a result of their medication regimen. In an attempt to determine if this was the case, a subgroup analysis of diabetics versus the remainder of our cohort was performed. This showed the diabetic patients to have higher incidence of obesity and hyperlipidemia, but otherwise closely represent the demographics of the overall population without significant differences in TNM classification or pathologic grade. This does not address potential differences in tumor biology and genetics. The second possibility is that, by some currently unidentified mechanism, metformin causes ACC to follow a less aggressive disease course and to recur or metastasize less frequently. If this is the case, then metformin would serve as an excellent adjuvant therapy for patients suffering from ACC who are marred by frequent recurrence despite optimal therapy. These findings warrant further mechanistic studies in the laboratory and humans to discern if this is a viable treatment option for ACC patients.
Conclusions
In conclusion, we present clinicopathologic predictors of survival and recurrence for ACC of the head and neck based on a large cohort with long-term follow up from a tertiary care comprehensive cancer center. We found metformin usage to be protective against recurrence and significantly improved DFS. Our results necessitate further research to uncover the mechanism of metformin’s protective effect and its influence on tumor cell physiology. Additionally, we believe these findings warrant further studies and clinical trial investigation into the potential therapeutic use of metformin in patients with ACC.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
We acknowledge the generous champions and philanthropic contributions that support this effort including Santino Carnevale and supporters to Santino’s Crusade for a Cancer-Free World, Sally Millet and The Douglas Tyler Millett Endowed Fund for Research in Head/Neck Cancer as well as the generous donors to the Head and Neck Strategic Initiative Fund and The Joan Bisesi Fund for Head and Neck Oncology Research.
Footnotes
Appendix A.: Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.oraloncology.2020.104726.
References:
- [1].Dodd RL, Slevin NJ. Salivary gland adenoid cystic carcinoma: a review of chemotherapy and molecular therapies. Oral Oncol. 2006;42(8):759–69. [DOI] [PubMed] [Google Scholar]
- [2].Kim KH, Sung MW, Chung PS, Rhee CS, Park CI, Kim WH. Adenoid cystic carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1994;120(7):721–6. [DOI] [PubMed] [Google Scholar]
- [3].Vander Poorten VL, Balm AJ, Hilgers FJ, et al. Prognostic factors for long term results of the treatment of patients with malignant submandibular gland tumors. Cancer 1999;85(10):2255–64. [DOI] [PubMed] [Google Scholar]
- [4].Bonaparte JP, Hart R, Trites J, Taylor MS. Incidence of adenoid cystic carcinoma in nova scotia: 30-year population-based epidemiologic study. J Otolaryngol Head Neck Surg. 2008;37(5):642–8. [PubMed] [Google Scholar]
- [5].Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck–An update. Oral Oncol. 2015;51(7):652–61. [DOI] [PubMed] [Google Scholar]
- [6].Azar T, Abdul-Karim FW, Tucker HM. Adenoid cystic carcinoma of the trachea. Laryngoscope. 1998;108(9):1297–300. [DOI] [PubMed] [Google Scholar]
- [7].Friedrich RE, Bleckmann V. Adenoid cystic carcinoma of salivary and lacrimal gland origin: localization, classification, clinical pathological correlation, treatment results and long-term follow-up control in 84 patients. Anticancer Res. 2003;23(2A):931–40. [PubMed] [Google Scholar]
- [8].Gu FM, Chi FL, Dai CF, Chen B, Li HW. Surgical outcomes of 43 cases with adenoid cystic carcinoma of the external auditory canal. Am J Otolaryngol. 2013;34(5):394–8. [DOI] [PubMed] [Google Scholar]
- [9].Argyris PP, Pambuccian SE, Cayci Z, Singh C, Tosios KI, Koutlas IG. Lacrimal gland adenoid cystic carcinoma with high-grade transformation to myoepithelial carcinoma: report of a case and review of literature. Head Neck Pathol. 2013;7(1):85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kokemueller H, Eckardt A, Brachvogel P, Hausamen JE. Adenoid cystic carcinoma of the head and neck-a 20 years experience. Int J Oral Maxillofac Surg. 2004;33(1):25–31. [DOI] [PubMed] [Google Scholar]
- [11].Spiro RH, Huvos AG, Strong EW. Adenoid cystic carcinoma of salivary origin. A clinicopathologic study of 242 cases. Am J Surg. 1974;128(4):512–20. [DOI] [PubMed] [Google Scholar]
- [12].Bradley PJ. Adenoid cystic carcinoma of the head and neck: a review. Curr Opin Otolaryngol Head Neck Surg. 2004;12(2):127–32. [DOI] [PubMed] [Google Scholar]
- [13].van Weert S, Bloemena E, van der Waal I, et al. Adenoid cystic carcinoma of the head and neck: a single-center analysis of 105 consecutive cases over a 30-year period. Oral Oncol. 2013;49(8):824–9. [DOI] [PubMed] [Google Scholar]
- [14].Jang S, Patel PN, Kimple RJ, McCulloch TM. Clinical Outcomes and Prognostic Factors of Adenoid Cystic Carcinoma of the Head and Neck. Anticancer Res. 2017;37(6):3045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Prokopakis EP, Snyderman CH, Hanna EY, Carrau RL, Johnson JT, D’Amico F. Risk factors for local recurrence of adenoid cystic carcinoma: the role of postoperative radiation therapy. Am J Otolaryngol. 1999;20(5):281–6. [DOI] [PubMed] [Google Scholar]
- [16].Issing PR, Hemmanouil I, Stover T, et al. Adenoid cystic carcinoma of the skull base. Skull Base Surg. 1999;9(4):271–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Amit M, Binenbaum Y, Trejo-Leider L, et al. International collaborative validation of intraneural invasion as a prognostic marker in adenoid cystic carcinoma of the head and neck. Head Neck. 2015;37(7):1038–45. [DOI] [PubMed] [Google Scholar]
- [18].Sequeiros Santiago G, Rodrigo Tapia JP, Llorente Pendas JL, Suarez Nieto C. Prognostic factors in adenoid cystic carcinoma of salivary glands. Acta Otorrinolaringol Esp. 2005;56(8):361–7. [DOI] [PubMed] [Google Scholar]
- [19].Huang M, Ma D, Sun K, Yu G, Guo C, Gao F. Factors influencing survival rate in adenoid cystic carcinoma of the salivary glands. Int J Oral Maxillofac Surg. 1997;26(6):435–9. [DOI] [PubMed] [Google Scholar]
- [20].Martins-Andrade B, Dos Santos Costa SF, Sant’ana MSP, et al. Prognostic importance of the lymphovascular invasion in head and neck adenoid cystic carcinoma: A systematic review and meta-analysis. Oral Oncol. 2019;93:52–8. [DOI] [PubMed] [Google Scholar]
- [21].Choi Y, Kim SB, Yoon DH, Kim JY, Lee SW, Cho KJ. Clinical characteristics and prognostic factors of adenoid cystic carcinoma of the head and neck. Laryngoscope. 2013;123(6):1430–8. [DOI] [PubMed] [Google Scholar]
- [22].Vancura A, Bu P, Bhagwat M, Zeng J, Vancurova I. Metformin as an Anticancer Agent. Trends Pharmacol Sci. 2018;39(10):867–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee AJ. Metformin in noninsulin-dependent diabetes mellitus. Pharmacotherapy. 1996;16(3):327–51. [PubMed] [Google Scholar]
- [24].Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. CellMetab. 2014;20(6):953–66. [DOI] [PubMed] [Google Scholar]
- [25].Coyle C, Cafferty FH, Vale C, Langley RE. Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Ann Oncol. 2016;27(12):2184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhou PT, Li B, Liu FR, et al. Metformin is associated with survival benefit in pancreatic cancer patients with diabetes: a systematic review and meta-analysis. Oncotarget. 2017;8(15):25242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yen YC, Lin C, Lin SW, Lin YS, Weng SF. Effect of metformin on the incidence of head and neck cancer in diabetics. Head Neck. 2015;37(9):1268–73. [DOI] [PubMed] [Google Scholar]
- [28].ClinicalTrials.gov. Search results. https://clinicaltrials.gov/ct2/results?cond=Head+and+Neck+Cancer&term=metformin+&cntry=&state=&city=&dist=. Published 2019, December 2. Accessed.
- [29].Szanto PA, Luna MA, Tortoledo ME, White RA. Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer 1984;54(6):1062–9. [DOI] [PubMed] [Google Scholar]
- [30].Oplatek A, Ozer E, Agrawal A, Bapna S, Schuller DE. Patterns of recurrence and survival of head and neck adenoid cystic carcinoma after definitive resection. Laryngoscope. 2010;120(1):65–70. [DOI] [PubMed] [Google Scholar]
- [31].Sung MW, Kim KH, Kim JW, et al. Clinicopathologic predictors and impact of distant metastasis from adenoid cystic carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2003;129(11):1193–7. [DOI] [PubMed] [Google Scholar]
- [32].Amit M, Binenbaum Y, Sharma K, et al. Analysis of failure in patients with adenoid cystic carcinoma of the head and neck. An international collaborative study. Head Neck. 2014;36(7):998–1004. [DOI] [PubMed] [Google Scholar]
- [33].Ferrarotto R, Mitani Y, Diao L, et al. Activating NOTCH1 Mutations Define a Distinct Subgroup of Patients With Adenoid Cystic Carcinoma Who Have Poor Prognosis, Propensity to Bone and Liver Metastasis, and Potential Responsiveness to Notch 1 Inhibitors. J Clin Oncol. 2017;35(3):352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Adak T, Samadi A, Unal AZ, Sabuncuoglu S. A reappraisal on metformin. Regul Toxicol Pharmacol. 2018;92:324–32. [DOI] [PubMed] [Google Scholar]
- [35].Fordice J, Kershaw C, El-Naggar A, Goepfert H. Adenoid cystic carcinoma of the head and neck: predictors of morbidity and mortality. Arch Otolaryngol Head Neck Surg. 1999;125(2):149–52. [DOI] [PubMed] [Google Scholar]
- [36].Kato K, Iwama H, Yamashita T, Kobayashi K, Fujihara S, Fujimori T, Kamada H, Kobara H, Masaki T. The anti-diabetic drug metformin inhibits pancreatic cancer cell proliferation in vitro and in vivo: Study of the microRNAs associated with the antitumor effect of metformin. Oncol Rep. 2016; 35(3):1582–92. [DOI] [PubMed] [Google Scholar]
- [37].Ho AS, Ochoa A, Jayakumaran G, Zehir A, Valero Mayor C, Tepe J, Makarov V, Dalin MG, He J, Bailey M, Montesion M, Ross JS, Miller VA, Chan L, Ganly I, Dogan S, Katabi N, Tsipouras P, Ha P, Agrawal N, Solit DB, Futreal PA, El Naggar AK, Reis-Filho JS, Weigelt B, o AL, Schultz N, Chan TA, Morris LG. Genetic hallmarks of recurrent/metastatic adenoid cystic carcinoma. J Clin Invest. 2019. Oct 1;129(10):4276–4289. doi: 10.1172/JCI128227. [DOI] [PMC free article] [PubMed] [Google Scholar]


