Abstract
Allosteric transcription factor (aTF) biosensors are valuable tools for engineering microbes toward a multitude of applications in metabolic engineering, biotechnology, and synthetic biology. One of the challenges toward constructing functional and diverse biosensors in engineered microbes is the limited toolbox of identified and characterized aTFs. To overcome this, extensive bioprospecting of aTFs from sequencing databases, as well as aTF ligand-specificity engineering are essential in order to realize their full potential as biosensors for novel applications. In this work, using the TetR-family repressor CmeR from Campylobacter jejuni, we construct aTF genetic circuits that function as salicylate biosensors in the model organisms Escherichia coli and Saccharomyces cerevisiae. In addition to salicylate, we demonstrate the responsiveness of CmeR-regulated promoters to multiple aromatic and indole inducers. This relaxed ligand specificity of CmeR makes it a useful tool for detecting molecules in many metabolic engineering applications, as well as a good target for directed evolution to engineer proteins that are able to detect new and diverse chemistries.
Keywords: CmeR, aTF, biosensor, aromatics, genetic circuits, salicylic acid, indoles
Bacteria have evolved single and multicomponent transcriptional regulatory networks in order to respond to numerous chemical cues.1 One example of these are allosteric transcription factors (aTFs), which are single-component regulators that play diverse roles in controlling various metabolic processes.2 For biosensing, aTFs have been used to alter transcription in response to small molecules in many applications, such as screening large strain or enzyme libraries,3,4 disease diagnostics,5,6 screening for environmental contaminants,7,8 among many others. One major constraint toward the use of aTFs as biosensors is their limited diversity with respect to the repertoire of molecules that they can sense,9,10 which makes identifying or engineering aTFs with novel ligand specificities important in order to provide the tools for next-generation biological engineering. This could be achieved by continually bioprospecting to identify and characterize previously unknown aTFs,11,12 or by investigating the ligand promiscuity of known aTFs.13−16 Other solutions to this limitation include directed evolution of aTFs toward novel ligands,17−20 construction and screening of novel fusion proteins as engineered aTFs,21 or by de novo protein design.22
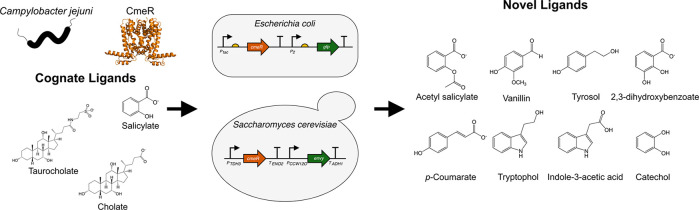
Synthetic biology is in constant need for characterized and robust aTF biosensors to implement in new applications. Toward this goal, we investigated the TetR-family repressor CmeR from the gastroenteric pathogen Campylobacter jejuni,23,24 which regulates the multidrug resistance efflux pump CmeABC in response to intestinal bile salts.23 In addition to cholate and taurocholate, CmeR was also shown to bind salicylate and glycerol.25,26 This diversity in ligands, which includes large aliphatic bile salts as well as small aliphatic and aromatic molecules, has inspired us to explore the binding specificity of CmeR, and further examine its potential application in synthetic biology as a part in genetic circuit biosensors. In this work, we constructed CmeR genetic circuits in the model organisms Escherichia coli and Saccharomyces cerevisiae using the identified aTF/operator pair and the inducer salicylate,25 which is a precursor to industrially relevant molecules such as muconate27 and acetyl salicylate.28,29 Using our circuits, we probed the ligand specificity of CmeR, and identified novel aromatic and indole molecules that induce expression from CmeR-regulated promoters in both prokaryotic and eukaryotic model systems. While many of these molecules have already been identified as inducers for other regulated promoters,30 tyrosol, tryptophol, and tyramine are, to our knowledge, novel inducers of aTF-regulated promoters. This work shows that CmeR is a broadly useful aTF candidate for directed evolution toward novel ligand specificities that belong to aromatics and indoles, and possibly other diverse functional groups.
Materials and Methods
Strains, Growth Conditions, and Transformations
E. coli DH5α (Thermo Fisher Scientific) was used for all genetic circuit construction and characterization purposes. Unless otherwise stated, E. coli cells were cultured at 37 °C for ∼16 h either in liquid or solid Luria–Bertani (LB) medium with or without kanamycin (50 μg mL–1) for plasmid selection. Chemically competent E. coli DH5α31 were transformed by heat shock for 50 s, and recovered in LB medium for 1 h without antibiotics, followed by plating on LB plates containing 50 μg mL–1 kanamycin
S. cerevisiae manipulations were conducted in the prototroph CEN.PK113–7D. Strains were grown at 30 °C for ∼16 or 48 h in liquid or solid yeast extract-peptone-dextrose (YPD) medium, respectively. Hygromycin (200 μg mL–1) and G418 (200 μg mL–1) were added for plasmid selection as needed. Yeast cells were transformed by heat shock following the standard Gietz PEG/LiOAc protocol scaled down to a 25 μL volume.32 Cells were incubated for 15 min at 30 °C, heat-shocked for 30 min at 42 °C, followed by a recovery step in YPD for 16 h, after which cells were plated on YPD plates containing the appropriate antibiotics for selection.
Chemicals for Genetic Circuit Induction
Chemicals for CmeR circuit characterization were purchased from Sigma-Aldrich and dissolved in water or 25% ethanol as specified in parentheses to obtain 25 mM stock solutions: resorcinol (water), catechol (water), adipic acid (water), epinephrine (water), dopamine (water), protocatechuate (water), salicylate (water), 2,3-dihydroxybenzoate (water), acetyl salicylate (ethanol), tyrosol (water), tryptophol (water), tyramine (ethanol), tryptamine (ethanol), caffeic acid (ethanol), phenyl acetate (ethanol), 4-hydroxyphenylacetate (ethanol), phloretic acid (ethanol), p-coumarate (ethanol), 1-phenyl ethanol (water), and homovanillic acid (water). IPTG 1 M stock solutions were prepared by dissolving in water.
Synthetic Genes, Oligonucleotides, and Plasmids
cmeR (cj0368c) from C. jejuni was codon-optimized for yeast expression using the IDT tool and purchased from Thermo Scientific. Oligonucleotides were purchased from Thermo Scientific and suspended in nuclease-free water to a concentration of 100 μM. Sequences of cmeR and the oligonucleotides used are listed in Supplementary Table S1.
E. coli Biosensor Genetic Circuit Construction, Characterization, and Data Analyses
Genetic circuits were constructed using the template plasmid pQacR-Q2, a gift from Christopher Voigt (Addgene plasmid #74690; http://n2t.net/addgene:74690; RRID:Addgene_74690).33qacR was replaced with the codon-optimized cmeR gene using primer sets 1–4 by polymerase incomplete primer extension (PIPE) cloning.34 Promoter PQacR was modified by replacing qaco with cmeO (TGTAATAAATATTACA) using primers 5–6, and further optimizing its −10 sequence to the consensus TATAAT using primers 7–8, creating promoter “P2” with the final sequence listed in Supplementary Table S1. As well, eyfp was mutated to egfp using primer sets 9–10 to introduce a Y203T mutation.
For biosensor response characterization, E. coli cells carrying genetic circuit plasmids were grown overnight in LB broth at 37 °C. The following day, different volumes of IPTG and small molecules were dispensed using a Labcyte Echo liquid handler or a Thermo Scientific electronic Finipipette, where applicable, into 96-well clear plates (Falcon). E. coli cells were diluted 1:100 in M9 minimal media35 with kanamycin, incubated at 37 °C for 4 h, and then diluted 1:200 in the presence of IPTG and putative ligands as needed. For all biosensor response characterization experiments here, M9 minimal media was supplemented with casamino acids (0.2%) thiamine hydrochloride (0.034%), and ascorbic acid (100 μM) as an antioxidant. Microtiter plates were then incubated in an Infors HT shaker at 30 °C for 20–24 h. The following day, eGFP fluorescence (excitation: 475–10 nm, emission: 510–10 nm) and OD600 of cultures were measured using a CLARIOstar microplate reader (BMG Labtech, Germany).
Unless otherwise stated, dose–response data points are averages of triplicates, shown either as three data points, or a single data point with an error bar representing standard deviation. Where applicable, curves were fitted using GraphPad Prism by modeling them to a four-parameter nonlinear regression equation.
Saccharomyces cerevisiae Strain Construction
S. cerevisiae strains were constructed using CRISPR-Cas9-mediated genomic integration and in vivo DNA homologous recombination.36,37 Yeast cells were transformed with a DNA pool that comprised: (1) PCR-amplified promoters, cmeR, Envy GFP and terminators with ∼40 bp homology to the preceding and/or following part, mixed in equimolar ratios (total DNA ∼1000 ng). (2) Two ∼500 bp homology arms to the genomic locus (200 ng each). (3) Linearized pCas-G418 and/or pCas-Hyg vectors encoding Cas938 (150 ng each). (4) gRNAs obtained by oligo-extension targeting the characterized chromosomal locus FgF1639 (in which all integrations in this work were made) with 40 bp homology to the Cas9 plasmids to allow plasmid recircularization by in vivo homologous recombination (2 μL of unpurified PCR reaction per transformation). For constructing the control strain, the full PCCW12 was amplified using primers MN527 and MN516. For the sensor strain with the operator insertion, PCCW12 was divided into two parts with the operator acting as the homology arm in between them. Primer sets MN527 and MN664, and sets MN663 and MN516 were used to amplify both promoter parts. Transformations were recovered for 16 h in YPD, after which they were plated on YPD plates with the appropriate selection (Hygromycin and/or G418 at 200 μg mL–1 each). Successful genomic integrations were confirmed by colony PCR and Sanger sequencing. Finally, the Cas9 plasmid was cured by streaking colonies two successive times on YPD plates with no selection. Sequences of promoters, gene expression cassettes, gRNA(s) and terminators are listed in Supplementary Table S1.
S. cerevisiae Biosensor Characterization
Cells were grown overnight at 30 °C and 300 rpm in 96 deep-well plates (Greiner) in an Infors HT shaker. The following day, different volumes of small molecules were dispensed using a Labcyte Echo liquid handler or a Thermo Finipipette, where applicable, into 96-well clear plates (Falcon). Yeast cells were then diluted 1/50 in synthetic complete media that was adjusted to pH 4, supplemented with freshly prepared 5 mM ascorbic acid (Sigma-Aldrich), and dispensed into the plates. Plates were incubated at 30 °C and 300 rpm in the same shaker for 8 h, after which Envy GFP fluorescence was measured by flow cytometry.
S. cerevisiae Biosensor Experiment and Flow Cytometry
Flow cytometry fluorescence characterization was performed using an Accuri C6 Cytometer (BD Biosciences). Cells were diluted 1:5 in deionized water and measured at an average rate of ∼2000 events/second for a total of 10 000 events, and the mean fluorescence of the total ungated population was plotted for each molecule and/or concentration tested. Unless otherwise stated, dose–response data points are averages of triplicates, shown either as 3 data points, or a single data point with an error bar representing standard deviation. When applicable, curves were fitted using GraphPad Prism by modeling them to a four-parameter nonlinear regression equation.
Results and Discussion
1. Constructing a CmeR Salicylate Biosensor in E. coli
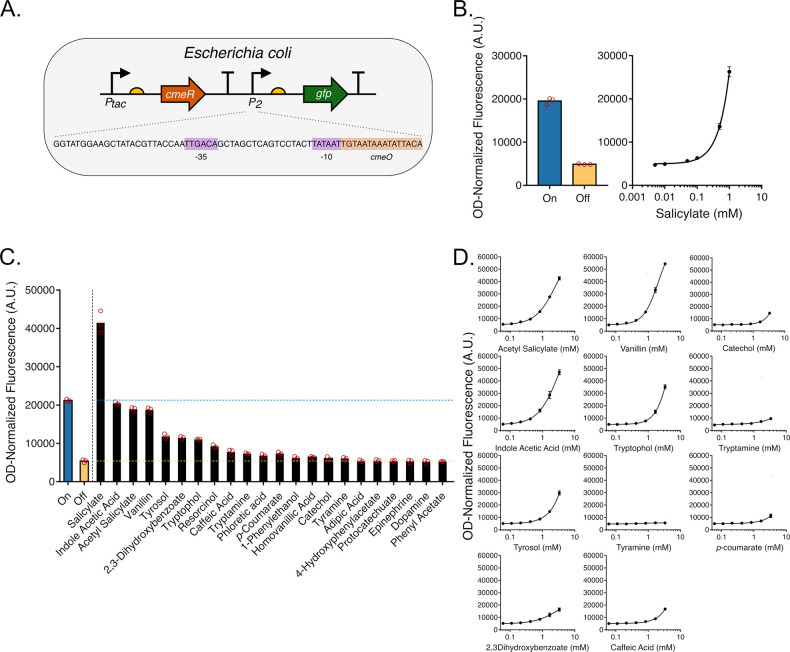
CmeR belongs to the TetR family of repressors, one of the major families of transcription factors in bacteria,40 which regulates expression by binding to the promoter region and physically blocking RNA polymerase binding or progression.10 In order to identify inducers for CmeR-regulated promoters in E. coli, we constructed a genetic circuit with CmeR expressed from an IPTG-inducible tac promoter and eGFP expressed from a second promoter that was modified for salicylate-responsiveness. In addition to having a consensus −35 (TTGACA) sequence, this promoter was engineered to have a consensus −10 (TATAAT) sequence followed by cmeO (Figure 1A). Adding IPTG to cultures carrying this plasmid repressed fluorescence by inducing CmeR expression (in turn repressing eGFP expression), and adding increasing concentrations of one of the known inducers, salicylate, resulted in a maximal induction of eGFP expression by >5 fold due to the reduction in CmeR affinity to its operator site25 (Figure 1B). Titrating IPTG and salicylate concentrations allowed us to further tune the level of expression of eGFP from this engineered promoter (Supplementary Figure S1).
Figure 1.
Construction and inducer profile of an E. coli salicylate biosensor. (A) One-plasmid genetic circuit structure. The IPTG-inducible tac promoter drives CmeR expression, followed by a second engineered salicylate-inducible promoter to drive GFP expression, which contains one copy of the cmeO operator sequence inserted immediately downstream of the −10 sequence. (B) Left panel: Fluorescence measurements of cells carrying the CmeR genetic circuit in the on and off states without and with 1 mM IPTG added, respectively. Right panel: Fluorescence measurements of cells in the presence of 1 mM IPTG and increasing concentrations of salicylate. (C) Fluorescence measurements of E. coli cultures carrying the CmeR genetic circuit exposed to 1 mM of one of 22 molecules in the presence of 1 mM of IPTG. (D) Dose response curves for 11 candidate inducer molecules in the presence of 1 mM IPTG. All data represents plate reader measurements of GFP fluorescence of E. coli cultures performed in triplicate.
We next sought to probe the ligand specificity of CmeR. In order to do this, we exposed a strain (E. coli DH5α) carrying our genetic circuit to 1 mM concentrations of various aromatic, indole, and aliphatic molecules in the presence of IPTG. This concentration range was chosen due to solubility limit and molecule toxicity constraints, and to be able to properly compare the circuit response to different molecules. We observed an increase in fluorescence with some of these molecules that ranged from ∼1.1 fold (catechol) to ∼3.8 fold (the plant auxin indole-3-acetic acid or IAA) (Figure 1C). Next, we characterized the dose–response of a subset of the tested molecules and show that in addition to the identified cognate inducers, expression from the CmeR-regulated promoter was induced by acetylsalicylate, vanillin, IAA, tryptophol, tyrosol, 2,3-dihydroxybenzoate (DHBA), catechol, caffeic acid, and p-coumarate. Expression from the CmeR-regulated promoter appeared to be minimally induced by tryptamine (the amine derivative of tryptophol), while remaining uninduced by tyramine (the amine derivative of tyrosol) (Figure 1D).
2. Constructing a CmeR Salicylate Biosensor in S. cerevisiae
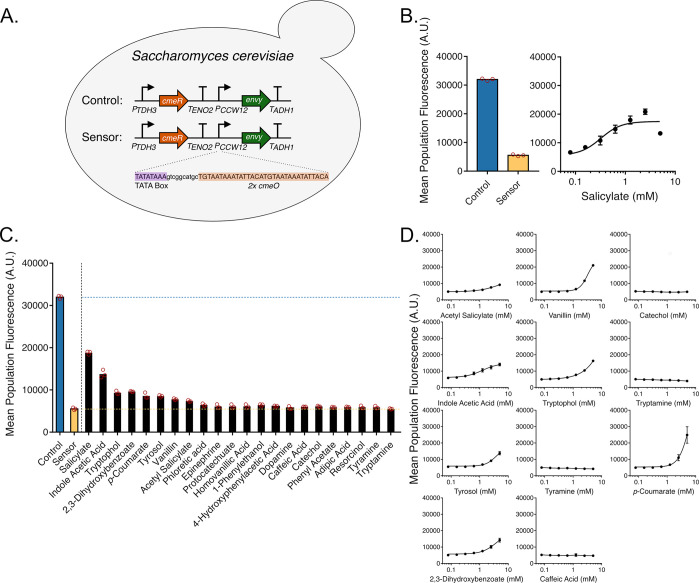
To expand its use as a biosensor, we transplanted CmeR into a eukaryotic chassis and tested for repression and subsequent induction activities using GFP as a reporter. Designing genetic circuits with transcription repressors in eukaryotes requires placing the operator sequence between the TATA box and the transcription start site in order to allow for the repressor to sterically interfere with the binding or progression of RNA polymerase.41 To this end, we designed and constructed a chromosomally integrated CmeR genetic circuit in S. cerevisiae. In this circuit, CmeR (without a nuclear localization signal) was expressed from the strong constitutive promoter PTDH3. A second strong constitutive promoter, PCCW12, was simultaneously used to express the Envy GFP variant,42 yielding the control strain (Figure 2A). PCCW12 was engineered to be salicylate-responsive by introducing two copies of the cmeO operator sequence 11 bp downstream of the TATA box consensus sequence TATA(A/T)A(A/T)(A/G)43 found at −133–125 nt relative to the transcription start site, thereby generating PCCW12O and the sensor strain (Figure 2A). Due to the toxicity of salicylate to S. cerevisiae,44,45 all experiments were conducted at a lower yeast culture dilution factor and were measured after 8 h. GFP fluorescence outputs of the control and sensor strains measured by flow cytometry showed a marked drop in fluorescence upon inserting cmeO, which in turn increased by adding increasing concentrations of salicylate to the culture media, reaching up to ∼4.3-fold induction in 2.5 mM salicylate (Figure 2B). This data shows that, in a manner similar to our E. coli genetic circuit, CmeR functions as a transcription repressor in S. cerevisiae, and is inducible by salicylate.
Figure 2.
Construction and inducer profile of a S. cerevisiae salicylate biosensor. (A) Chromosomally integrated genetic circuit design in S. cerevisiae. CmeR is expressed from the strong constitutive promoter PTDH3. PCCW12 is used for expressing Envy GFP in the control strain, which was subsequently engineered in the sensor strain for salicylate responsiveness by inserting two copies of cmeO downstream of the TATA box. (B) Left panel: Fluorescence measurements of cells carrying the control and sensor circuit. Right panel: Fluorescence measurements of the sensor strain in the presence of increasing concentrations of salicylate. (C) Fluorescence measurements of the sensor strain exposed to 1 mM of one of 22 molecules. (D) Dose response curves for 11 candidate inducer molecules. All data represents flow cytometry measurements of GFP fluorescence of S. cerevisiae cultures performed in triplicate.
We then aimed to determine whether the CmeR ligands identified using the E. coli circuit would induce GFP expression in S. cerevisiae as well using our modified CCW12 promoter. Introducing 1 mM solutions of putative inducers to our S. cerevisiae sensor strain followed by flow cytometric analyses showed that many of the same ligands that induced GFP expression in E. coli successfully induce expression from PCCW12O by ∼1.1–2.5 fold (Figure 2C). Further characterization of the circuit dose–response with the same 11 molecules used in the previous section highlights some of the differences between the CmeR induction profile in E. coli and S. cerevisiae, the latter which exhibited maximal fluorescence inductions ranging from ∼1.8 fold (acetyl salicylate) to ∼5 fold (p-coumarate). Overall, acetylsalicylate, IAA, vanillin, DHBA, tyrosol, tryptophol, and p-coumarate, maintained their capacity to induce expression from PCCW12O in S. cerevisiae. Catechol, caffeic acid, and tryptamine fail to induce GFP in the tested conditions (Figure 2D). Doubling the maximum concentration of these molecules to 10 mM resulted in a modest increase in fluorescence of ∼1.3 fold in 10 mM catechol, but no changes in fluorescence with 10 mM caffeic acid and 10 mM tryptamine were detected (Supplementary Figure S2), suggesting that catechol is indeed a very weak inducer of CmeR in S. cerevisiae as it also is in E. coli. Furthermore, like in E. coli, tyramine failed to induce CmeR in S. cerevisiae (Figure 2D). Notably, the response to many of these molecules in our S. cerevisiae CmeR circuit, including to the cognate inducer salicylate, appears to be dampened in general relative to the E. coli circuit, which has been shown previously with other biosensors in E. coli and S. cerevisiae.18 In the case of CmeR, this could be due the many differences between both circuits, such as the E. coli one being expressed from a multicopy vector as opposed to a single copy genomic integration in the case of S. cerevisiae, or the number of cmeO operator copies, which is 1 in E. coli, and 2 copies in S. cerevisiae. Furthermore, p-coumarate appear to be an exception to this rule, as it exhibits a stronger response in S. cerevisiae than in E. coli. This variability could be a result of the effects the differences in membrane transport mechanisms and cellular metabolism across both organisms, in addition to other unknown mechanisms. Such processes need to be taken into account when designing and engineering aTF biosensors, especially when transplanting them between different organisms. Possible ways of optimizing the response of the S. cerevisiae CmeR biosensor could be by varying the operator copy number and distance from the TATA box,41 as well as the number of genomically integrated copies of the genetic circuit.
Conclusion
One of the aims of synthetic biology is to reprogram new cellular functions, for which aTFs serve as excellent tools. As a result, there is a constant need of new aTFs that are functional and have predictable responses in microbial systems. In this work, we describe the construction and characterization of a CmeR-based salicylate whole cell biosensor in the prokaryotic and eukaryotic model systems E. coli and S. cerevisiae, respectively. In addition to salicylate and the bile salts cholate and taurocholate, we identified new ligands for CmeR in E. coli (acetylsalicylate, vanillin, catechol, IAA, tryptophol, tyrosol, caffeic acid, catechol, p-coumarate, DHBA, and to a lesser extent, tryptamine) and in S. cerevisiae (acetylsalicylate, vanillin, IAA, tryptophol, tyrosol, p-coumarate, DHBA, and to a lesser extent, catechol). In particular, tyrosol, tryptophol, and tyramine, to our knowledge, have never been described before as inducers of aTF-regulated promoters. The ability to detect these molecules would be useful for metabolic engineering applications, since a lot of these molecules are either intermediates or final products of pathways toward many commodity chemicals and value-added products.46−48 The caveat of this multiligand specificity however is that direct use of CmeR in metabolic engineering applications would be limited due to the significant crosstalk which might occur between different molecules in the same pathway. Furthermore, this work shows that the ligand promiscuity exhibited by CmeR makes it an excellent candidate for directed evolution and protein engineering efforts to detect novel molecules, as well as eliminate crosstalk between existing inducers. Although the inducer-binding site of TetR-family repressors is spatially conserved in a triangle formed by helices 5–7, exceptions to this rule exist.2,49 Therefore, solving the crystal structure of CmeR with salicylate in addition to these novel ligands would greatly facilitate these protein engineering efforts. Finally, this work also offers insights into additional roles of CmeR in C. jejuni pathogenesis. Salicylate has been shown to increase the minimum inhibitory concentrations of various antibiotics for C. jejuni.25,50,51 Given the ligand promiscuity demonstrated here, it would be tempting to investigate whether other aromatic and indole molecules play a role in the induction of antibiotic resistance in C. jejuni.
Acknowledgments
This study was financially supported by NSERC Discovery Grants RGPIN-2016-05464 to D.H.K., and RGPIN-2017-06703 to V.J.J.M.; M.A.N. is supported by a Concordia International Tuition Award of Excellence, and an FRQNT B2X Doctoral Research Award. L.R.T. was supported by an PROTEO undergraduate research award. V.J.J.M. is supported by a Concordia University Research Chair.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acssynbio.2c00063.
Additional response measurements of E. coli CmeR sensors; List of DNA and oligonucleotide sequences employed in this study (PDF)
Author Present Address
∥ Life Sciences Institute, Department of Biomedical Engineering, University of British Columbia, Vancouver, British Columbia V6T 1Z3, Canada
Author Contributions
M.A.N., D.H.K., and V.J.J.M. designed the research. M.A.N. performed the experiments. L.R.T. assisted in preliminary experiments. D.H.K. and V.J.J.M. supervised the research. M.A.N. wrote the manuscript with editing help from L.R.T., D.H.K., and V.J.J.M.
The authors declare no competing financial interest.
Supplementary Material
References
- Seshasayee A. S.; Bertone P.; Fraser G. M.; Luscombe N. M. Transcriptional regulatory networks in bacteria: from input signals to output responses. Curr. Opin. Microbiol. 2006, 9, 511. 10.1016/j.mib.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Fernandez-López R.; Ruiz R.; de la Cruz F.; Moncalián G. Transcription factor-based biosensors enlightened by the analyte. Front. Microbiol. 2015, 6, 648. 10.3389/fmicb.2015.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchler M. M.; Garcia J. M.; Montero N. E.; Williams G. J. Transcription factor-based biosensors: a molecular-guided approach for natural product engineering. Curr. Opin. Biotechnol. 2021, 69, 172. 10.1016/j.copbio.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J. C. H.; Pawar S. V.; Hallam S. J.; Yadav V. G. An Improved Whole-Cell Biosensor for the Discovery of Lignin-Transforming Enzymes in Functional Metagenomic Screens. ACS Synth. Biol. 2018, 7, 392–398. 10.1021/acssynbio.7b00412. [DOI] [PubMed] [Google Scholar]
- Lin C.; Jair Y. C.; Chou Y. C.; Chen P. S.; Yeh Y. C. Transcription factor-based biosensor for detection of phenylalanine and tyrosine in urine for diagnosis of phenylketonuria. Anal. Chim. Acta 2018, 1041, 108–113. 10.1016/j.aca.2018.08.053. [DOI] [PubMed] [Google Scholar]
- Wen K. Y.; Cameron L.; Chappell J.; Jensen K.; Bell D. J.; Kelwick R.; Kopniczky M.; Davies J. C.; Filloux A.; Freemont P. S. A Cell-Free Biosensor for Detecting Quorum Sensing Molecules in P. aeruginosa -Infected Respiratory Samples. ACS Synth. Biol. 2017, 6, 2293–2301. 10.1021/acssynbio.7b00219. [DOI] [PubMed] [Google Scholar]
- Jung J. K.; Alam K. K.; Verosloff M. S.; Capdevila D. A.; Desmau M.; Clauer P. R.; Lee J. W.; Nguyen P. Q.; Pastén P. A.; Matiasek S. J.; Gaillard J. F.; Giedroc D. P.; Collins J. J.; Lucks J. B. Cell-free biosensors for rapid detection of water contaminants. Nat. Biotechnol. 2020, 38, 1451–1459. 10.1038/s41587-020-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereza-Malcolm L. T.; Mann G.; Franks A. E. Environmental Sensing of Heavy Metals Through Whole Cell Microbial Biosensors: A Synthetic Biology Approach. ACS Synth. Biol. 2015, 4, 535–546. 10.1021/sb500286r. [DOI] [PubMed] [Google Scholar]
- Libis V.; Delépine B.; Faulon J. L. Sensing new chemicals with bacterial transcription factors. Curr. Opin. Microbiol. 2016, 33, 105–112. 10.1016/j.mib.2016.07.006. [DOI] [PubMed] [Google Scholar]
- De Paepe B.; Peters G.; Coussement P.; Maertens J.; De Mey M. Tailor-made transcriptional biosensors for optimizing microbial cell factories. J. Ind. Microbiol. Biotechnol. 2017, 44, 623–645. 10.1007/s10295-016-1862-3. [DOI] [PubMed] [Google Scholar]
- Grazon C.; Baer R. C.; Kuzmanović U.; Nguyen T.; Chen M.; Zamani M.; Chern M.; Aquino P.; Zhang X.; Lecommandoux S.; Fan A.; Cabodi M.; Klapperich C.; Grinstaff M. W.; Dennis A. M.; Galagan J. E. A progesterone biosensor derived from microbial screening. Nat. Commun. 2020, 11, 1–10. 10.1038/s41467-020-14942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.; Zhao H.; Ang E. L. A New Biosensor for Stilbenes and a Cannabinoid Enabled by Genome Mining of a Transcriptional Regulator. ACS Synth. Biol. 2020, 9, 698–705. 10.1021/acssynbio.9b00443. [DOI] [PubMed] [Google Scholar]
- Ruegg T. L.; Pereira J. H.; Chen J. C.; DeGiovanni A.; Novichkov P.; Mutalik V. K.; Tomaleri G. P.; Singer S. W.; Hillson N. J.; Simmons B. A.; Adams P. D.; Thelen M. P. Jungle Express is a versatile repressor system for tight transcriptional control. Nat. Commun. 2018, 9, 1–13. 10.1038/s41467-018-05857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K.; Tokunaga Y.; Imai M.; Takahashi H.; Shimada I. Dynamic multidrug recognition by multidrug transcriptional repressor LmrR. Sci. Rep. 2015, 4, 1–12. 10.1038/srep06922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Barajas J. F.; Burdu M.; Ruegg T. L.; Dias B.; Keasling J. D. Development of a Transcription Factor-Based Lactam Biosensor. ACS Synth. Biol. 2017, 6, 439–445. 10.1021/acssynbio.6b00136. [DOI] [PubMed] [Google Scholar]
- Dietrich J. A.; Shis D. L.; Alikhani A.; Keasling J. D. Transcription Factor-Based Screens and Synthetic Selections for Microbial Small-Molecule Biosynthesis. ACS Synth. Biol. 2013, 2, 47–58. 10.1021/sb300091d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellefson J. W.; Ledbetter M. P.; Ellington A. D. Directed evolution of a synthetic phylogeny of programmable Trp repressors. Nat. Chem. Biol. 2018, 14, 361–367. 10.1038/s41589-018-0006-7. [DOI] [PubMed] [Google Scholar]
- Snoek T.; Chaberski E. K.; Ambri F.; Kol S.; Bjørn S. P.; Pang B.; Barajas J. F.; Welner D. H.; Jensen M. K.; Keasling J. D. Evolution-guided engineering of small-molecule biosensors. Nucleic Acids Res. 2020, 48, e3. 10.1093/nar/gkz954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L F. M.; Currin A.; Dixon N. Directed evolution of the PcaV allosteric transcription factor to generate a biosensor for aromatic aldehydes. J. Biol. Eng. 2019, 13, 91. 10.1186/s13036-019-0214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. D.; Garruss A. S.; Moretti R.; Chan S.; Arbing M. A.; Cascio D.; Rogers J. K.; Isaacs F. J.; Kosuri S.; Baker D.; Fields S.; Church G. M.; Raman S. Engineering an allosteric transcription factor to respond to new ligands. Nat. Methods 2016, 13, 177–183. 10.1038/nmeth.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez J. F.; Lecube-Azpeitia B.; Brown S. L.; Johnston C. D.; Church G. M. Biosensor libraries harness large classes of binding domains for construction of allosteric transcriptional regulators. Nat. Commun. 2018, 9, 3101. 10.1038/s41467-018-05525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester B. W.; Tinberg C. E.; Rich M. S.; Baker D.; Fields S. Engineered Biosensors from Dimeric Ligand-Binding Domains. ACS Synth. Biol. 2018, 7, 2457–2467. 10.1021/acssynbio.8b00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.; Cagliero C.; Guo B.; Barton Y. W.; Maurel M. C.; Payot S.; Zhang Q. Bile salts modulate expression of the CmeABC multidrug efflux pump in Campylobacter jejuni. J. Bacteriol. 2005, 187, 7417–7424. 10.1128/JB.187.21.7417-7424.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs-Reitsma W.; Lyhs U.; Wagenaar J.. Campylobacter in the Food Supply. In Campylobacter, 3rd ed.; Nachamkin I., Szymanski C. M., Blaser M. J., Ed.; American Society for Microbiology Press: Washington, D.C., 2008; pp 625–644. [Google Scholar]
- Shen Z.; Pu X.-Y.; Zhang Q. Salicylate Functions as an Efflux Pump Inducer and Promotes the Emergence of Fluoroquinolone-Resistant Campylobacter jejuni Mutants. Appl. Environ. Microbiol. 2011, 77, 7128–7133. 10.1128/AEM.00763-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H. T.; Shen Z.; Surana P.; Routh M. D.; Su C. C.; Zhang Q.; Yu E. W. Crystal structures of CmeR-bile acid complexes from Campylobacter jejuni. Protein Sci. 2011, 20, 712–723. 10.1002/pro.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.; Sun X.; Yuan Q.; Yan Y. Extending shikimate pathway for the production of muconic acid and its precursor salicylic acid in Escherichia coli. Metab. Eng. 2014, 23, 62–69. 10.1016/j.ymben.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Huccetogullari D.; Luo Z. W.; Lee S. Y. Metabolic engineering of microorganisms for production of aromatic compounds. Microb. Cell Fact. 2019, 10.1186/s12934-019-1090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Wang H.; Ding D.; Liu Y.; Fang H.; Chang Z.; Chen T.; Zhang D. Metabolic engineering of Escherichia coli for production of chemicals derived from the shikimate pathway. J. Ind. Microbiol. Biotechnol. 2020, 47, 525. 10.1007/s10295-020-02288-2. [DOI] [PubMed] [Google Scholar]
- Matilla M. A.; Elix Velando F.; Martín-Mora D.; Monteagudo-Cascales E.; Krell T. A catalogue of signal molecules that interact with sensor kinases, chemoreceptors and transcriptional regulators. FEMS Microbiol. Rev. 2022, 10.1093/femsre/fuab043. [DOI] [PubMed] [Google Scholar]
- Nakata Y.; Tang X.; Yokoyama K. K. Preparation of competent cells for high-efficiency plasmid transformation of Escherichia coli. Methods Mol. Biol. 1996, 69, 129–137. 10.1385/0-89603-383-X:129. [DOI] [PubMed] [Google Scholar]
- Gietz R. D.; Schiestl R. H. Quick and easy yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2007, 2, 35–37. 10.1038/nprot.2007.14. [DOI] [PubMed] [Google Scholar]
- Nielsen A. A. K.; Der B. S.; Shin J.; Vaidyanathan P.; Paralanov V.; Strychalski E. A.; Ross D.; Densmore D.; Voigt C. A. Genetic circuit design automation. Science 2016, 352, aac7341. 10.1126/science.aac7341. [DOI] [PubMed] [Google Scholar]
- Klock H. E.; Lesley S. A. The polymerase incomplete primer extension (PIPE) method applied to high-throughput cloning and site-directed mutagenesis. Methods Mol. Biol. 2009, 498, 91–103. 10.1007/978-1-59745-196-3_6. [DOI] [PubMed] [Google Scholar]
- M9 minimal medium (standard). Cold Spring Harb. Protoc. 2010. 10.1101/pdb.rec12295. [DOI] [Google Scholar]
- Horwitz A. A.; Walter J. M.; Schubert M. G.; Kung S. H.; Hawkins K.; Platt D. M.; Hernday A. D.; Mahatdejkul-Meadows T.; Szeto W.; Chandran S. S.; Newman J. D. Efficient Multiplexed Integration of Synergistic Alleles and Metabolic Pathways in Yeasts via CRISPR-Cas. Cell Syst. 2015, 1, 88–96. 10.1016/j.cels.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Shao Z.; Zhao H.; Zhao H. DNA assembler, an in vivo genetic method for rapid construction of biochemical pathways. Nucleic Acids Res. 2009, 37, e16–e16. 10.1093/nar/gkn991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. D. M.; Whiteway M.; Martin V. J. J.. The MyLo CRISPR-Cas9 Toolkit: A Markerless Yeast Localization and Overexpression CRISPR-Cas9 Toolkit. bioRxiv, December 16, 2021. 10.1101/2021.12.15.472800. [DOI] [PMC free article] [PubMed]
- Flagfeldt D. B.; Siewers V.; Huang L.; Nielsen J. Characterization of chromosomal integration sites for heterologous gene expression in Saccharomyces cerevisiae. Yeast 2009, 26, 545–551. 10.1002/yea.1705. [DOI] [PubMed] [Google Scholar]
- Cuthbertson L.; Nodwell J. R. The TetR Family of Regulators. Microbiol. Mol. Biol. Rev. 2013, 77, 440–475. 10.1128/MMBR.00018-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K. F.; Balázsi G.; Collins J. J. Combinatorial promoter design for engineering noisy gene expression. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 12726–12731. 10.1073/pnas.0608451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slubowski C. J.; Funk A. D.; Roesner J. M.; Paulissen S. M.; Huang L. S. Plasmids for C-terminal tagging in Saccharomyces cerevisiae that contain improved GFP proteins, Envy and Ivy. Yeast 2015, 32, 379–387. 10.1002/yea.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basehoar A. D.; Zanton S. J.; Pugh B. F. Identification and distinct regulation of yeast TATA box-containing genes. Cell 2004, 116, 699–709. 10.1016/S0092-8674(04)00205-3. [DOI] [PubMed] [Google Scholar]
- Baroni M. D.; Colombo S.; Martegani E. Antagonism between salicylate and the cAMP signal controls yeast cell survival and growth recovery from quiescence. Microb. Cell 2018, 5, 344–356. 10.15698/mic2018.07.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff T. G.; Perry A. C. The Effects of Salicylic Acid on Metabolism and Potassium Ion Content in Yeast. Proc. Soc. Exp. Biol. Med. 1976, 151, 72–77. 10.3181/00379727-151-39146. [DOI] [PubMed] [Google Scholar]
- Pyne M. E.; Kevvai K.; Grewal P. S.; Narcross L.; Choi B.; Bourgeois L.; Dueber J. E.; Martin V. J. J. A yeast platform for high-level synthesis of tetrahydroisoquinoline alkaloids. Nat. Commun. 2020, 11, 1–10. 10.1038/s41467-020-17172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averesch N. J. H.; Krömer J. O. Metabolic engineering of the shikimate pathway for production of aromatics and derived compounds-Present and future strain construction strategies. Front. Bioeng. Biotechnol. 2018, 6, 32. 10.3389/fbioe.2018.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q.; Yu T.; Li X.; Chen Y.; Campbell K.; Nielsen J.; Chen Y. Rewiring carbon metabolism in yeast for high level production of aromatic chemicals. Nat. Commun. 2019, 10, 1–13. 10.1038/s41467-019-12961-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Nafría J.; Baumgart M.; Turkenburg J. P.; Wilkinson A. J.; Bott M.; Wilson K. S. Crystal and solution studies reveal that the transcriptional regulator AcnR of Corynebacterium glutamicum is regulated by citrate-Mg2+ binding to a non-canonical pocket. J. Biol. Chem. 2013, 288, 15800–12. 10.1074/jbc.M113.462440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Tawab A. A.; Ammar A. M.; Ahmed H. A.; Hefny A. A. Efflux pump inhibitors, alpha-tocopherol and aspirin: Role in Campylobacter jejuni and Campylobacter coli Fluoroquinolone Resistance. Microb. Drug Resist. 2019, 25, 203–211. 10.1089/mdr.2018.0086. [DOI] [PubMed] [Google Scholar]
- Hannula M.; Hänninen M. L. Effect of putative efflux pump inhibitors and inducers on the antimicrobial susceptibility of Campylobacter jejuni and Campylobacter coli. J. Med. Microbiol. 2008, 57, 851–855. 10.1099/jmm.0.47823-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.