Abstract
Introduction
Although IgA nephropathy (IgAN) is the most common recurrent glomerulonephritis encountered in the kidney allograft, the clinical and immunogenetic characteristics remain poorly understood. We sought to study determinants and prognosis of recurrent IgAN with special focus on human leukocyte antigens (HLAs).
Materials and Methods
Between 2005 and 2019, we identified 282 transplanted patients with failure secondary to IgAN from 2 North American and 1 European Medical Centers, including 80 with recurrent IgAN and 202 without recurrence. The prevalence of HLAs was compared to external healthy controls of European ancestry (n = 15,740). Graft survival was assessed by the Kaplan-Meier method and log rank test. Cox proportional hazards were used for multivariable analyses.
Results
Compared to external controls of European ancestry, kidney transplant recipients of European ancestry with kidney failure secondary to IgAN had higher frequency of HLA-DQ5 (42% vs. 30%, OR = 1.68, p = 0.002) and lower frequency of HLA-DR15 (15% vs. 28%, OR = 0.46, p < 0.001) and HLA-DQ6 (32% vs. 45%, OR = 0.59, p = 0.003); however, the frequency of these HLAs were similar in recurrent versus nonrecurring IgAN. Younger recipient age at transplantation was an independent predictor of recurrence. HLA matching was an independent predictor for recurrent IgAN only in recipients of living-related but not deceased or living-unrelated transplants. Recurrent IgAN was an independent predictor of allograft failure, along with acute rejection. In patients with recurrent IgAN, serum creatinine at biopsy, degree of proteinuria, and concurrent acute rejection were associated with inferior allograft survival.
Conclusion
Recurrent IgAN negatively affects allograft survival. Younger recipient age at transplantation is an independent predictor of recurrent IgAN, while the presence of HLAs associated with IgAN in the native kidney and HLA matching in recipients of deceased or living-unrelated transplants are not.
Keywords: IgA nephropathy, Kidney transplantation, Recurrent disease, Human leukocyte antigen matching, Pathology
Introduction
Observational studies have suggested an inherited component of IgA nephropathy (IgAN) by reporting large pedigrees with multiple affected individuals [1]. Moreover, several studies have shown associations of IgAN with certain human leukocyte antigen (HLA) alleles, including positive correlations with HLA-B35 and DQ5 and negative correlations with HLA-DR3, DR15, DQ6, and DQ2 [2, 3, 4, 5].
Our understanding of the determinants of recurrence and prognosis of IgAN in the kidney allograft is limited [6, 7]. Some [8, 9, 10], but not all [11], reports have shown that recurrent IgAN is associated with a guarded outcome. A few studies have suggested that younger recipient age [8, 10, 11, 12, 13, 14, 15], steroid-free regimen [16, 17, 18], allograft from living-related donor [8, 14, 19], and donor-recipient HLA matching [10, 20, 21] may predict recurrent disease. The latter findings propose that mismatching donor-recipient pairs may reduce recurrent IgAN and potentially improve allograft survival.
In this study, we report on a large cohort (n = 282) of kidney transplant patients with kidney failure secondary to IgAN from 2 medical centers in the USA (Columbia University Irving Medical Center [CUIMC] and Oregon Health & Science University [OHSU]) and 1 European medical center (Centro Hospitalar e Universitário de Coimbra [HUC]), including 80 patients with recurrent IgAN and 202 patients without evidence of recurrence. We analyzed predictors of recurrent IgAN and allograft failure in kidney transplant recipients with native kidney failure secondary to IgAN with particular focus on the impact of HLA matching and HLAs (B35, DQ5, DR3, DR15, DQ6, and DQ2) in the donor or recipient.
Materials and Methods
Study Population and Data Collection
A retrospective review to identify kidney transplant recipients with recurrent and nonrecurring IgAN was carried-out at 3 medical centers (CUIMC, OHSU, and HUC) with approval of each center's Institutional Review Board. The pathology dataset was used to identify patients with native kidney failure secondary to IgAN who underwent kidney transplantation and had allograft biopsies between 2005 and 2019, including recurrent and nonrecurring IgAN.
Recurrent IgAN was defined as kidney allograft biopsy with positive IgA-dominant immunofluorescence staining. Positive IgA staining was defined as strong IgA staining (>1 on a scale from 0 to 3 or ≥2 on a scale from 0 to 4). To avoid misclassifying patients as recurrent IgAN due to nondefinitive IgA staining, patients with biopsies showing weaker IgA staining were excluded from the study. According to these criteria, 80 patients were classified with recurrent IgAN (44 from CUIMC, 29 from OHSU, and 7 from HUC).
Nonrecurring IgAN was defined as all patients with IgAN in the native kidney who did not develop recurrent IgAN either on for-cause or protocol allograft biopsy. We identified 202 recipients with nonrecurring IgAN (CUIMC [n = 128], OHSU [n = 45], and HUC [n = 29]). Demographic and clinicopathologic data were extracted. Data collected at OHSU and HUC were de-identified and shared with CUIMC for analyses.
Renal allograft biopsies were performed for clinical indications at all centers or per-protocol in all OHSU patients (at 3 and 12 months after transplantation) and HUC patients (at 1 month after transplantation); CUIMC performed protocol biopsies in patients with pretransplant circulating donor-specific antibodies or positive flow cross-match at 1 week, 2 weeks, 1 month, 3 months, 6 months, 1 year, 2 years, 3 years, and 5 years after transplantation.
The primary outcome was recurrence of IgAN in the allograft. A secondary outcome of allograft failure was defined as the initiation of renal replacement therapy or re-transplantation. Steroid-free regimens were defined as steroid wean immediately post-transplantation. Patients with disease recurrence or rejection requiring steroid treatment were considered steroid-free, unless they were on steroids prior to the event. In 6 patients where only the spot urine protein was available at the time of biopsy, quantification of proteinuria of <20 mg/dL (trace), 30 mg/dL (1+), 100 mg/dL (2+), 300 mg/dL (3+), or >1,000 mg/dL (4+) was estimated for statistical purposes as 0.3 g/g, 0.5 g/g, 1 g/g, 2 g/g, and 3 g/g, respectively [22]. The enrolled individuals were censored at loss of follow-up or allograft failure.
HLA Typing
Serologic and/or molecular typing for HLA-A, -B, -DR, and -DQ was performed for both donors and recipients. The number of HLA matches between donor and recipient HLAs (A, B, and DR: scale 0–6) was recorded. Attention was directed to the presence of HLAs highlighted in the literature as having potential association with IgAN, namely, B35, DQ5, DR3, DR15, DQ6, and DQ2 in donors and recipients.
Pathological Evaluation
Allograft biopsies were stained with hematoxylin and eosin, periodic acid-Schiff, Masson trichrome, and Jones methenamine silver. Immunofluorescence staining for IgG, IgM, IgA, C3, C1q, albumin, fibrin, kappa, lambda, and C4d was performed. Allograft biopsies were evaluated for MEST-C histologic scores using modified Oxford criteria [23]. Acute rejection was defined by the presence of either acute T-cell-mediated rejection (grade IA or higher) or antibody-mediated rejection, according to Banff criteria [24, 25, 26].
External Controls
To explore the association of HLAs with IgAN, we used HLA typing of 15,740 US residents of European descent with data available from the National Marrow Donor Program [27], who were designated as “external controls.” In these controls, the prevalence of a specific HLA serotype with an allelic frequency p was determined by summing up homozygote (p2) and heterozygote [2p(1 − p)] frequencies as calculated under the assumption of the Hardy-Weinberg equilibrium [p2 + 2p(1 − p) + (1 − p)2].
Statistical Analysis
Statistical analyses were performed using Prism 5 2007 (Graphpad Inc., San Diego, CA, USA), SPSS Statistics, version 26.0 (IBM, Armonk, NY, USA) and R v3.6.3. Continuous data were presented as median and interquartile range (IQR: 25th and 75th percentile). Continuous variables were compared using the Mann-Whitney test, while categorical variables were compared using Fisher's exact or χ2 tests as appropriate. The Kaplan-Meier methodology and log rank test were utilized to assess allograft survival. Cox proportional hazards models were constructed to account for confounders. All factors that demonstrated a suggestive association with the outcome (p value <0.1) at the univariable analysis were included in the multivariable cox proportional hazards models.
Recruitment site and year of transplantation were additionally included in multivariable analyses to account for differences in the medical approaches over time and among centers. Of note, the variable “year of transplant” was only included to adjust for confounders since it may not be a clinically meaningful predictor per se given the unique design of this study (pathology database was utilized to retrospectively select patients and controls with allograft biopsies within a specific period of time [2005–2019]. Given the relatively late occurrence of recurrent IgAN, patients with biopsies showing recurrent IgAN are expected to have older transplant date in non-time-to-event analyses. In contrast, when time-to-event analyses are performed, it is not completely surprising to observe opposite results [association with more recent transplant date], given the effects of a few recent and early events, whereas events from older transplant year would only include late events [events occurring before 2005 would not be captured]).
A forward stepwise selection method was applied on factors with p value <0.1 at the univariable analysis to avoid overfitting in the multivariable analysis of allograft failure in the small subgroup of patients with recurrent IgAN (n = 80). p values <0.05 were considered statistically significant. Since 6 HLAs have been studied, a Bonferroni-corrected significance cut-off of 0.008 was used for assessment of these antigens. Individuals with missing information on a tested predictor were excluded from the corresponding univariable time-to-event analysis; individuals with missing data in one or more predictors at the multivariable analyses were also excluded from the latter analyses.
Results
Demographic, Clinical, and Pathological Features of Patients with Kidney Failure Secondary to IgAN
Our cohort included 282 patients with kidney failure secondary to IgAN who underwent kidney transplantation. The majority of the recipients were first-time kidney transplant recipients (89%) who were maintained on both tacrolimus and mycophenolate mofetil (82%). The median age at transplantation was 42 years. The cohort included 31% women, 61% recipients of European ancestry, 47% recipients of allografts from deceased donors, and a median of 2 donor HLAs matched with the recipients. Fifty perecentage of patients received induction therapy with thymoglobulin, and 41% received steroid-free regimen (Table 1).
Table 1.
Demographics and clinical characteristics for patients with kidney failure secondary to IgAN
| Total (n = 282) | Recurrent IgAN (n = 80) | Nonrecurring IgAN (n = 202) | p values (recurrent vs. not) | |
|---|---|---|---|---|
| Age at transplant | 42 (33, 50) | 36 (28, 43) | 45 (36, 54) | <0.001 |
| <35 yr, n / N (%) | 82/282 (29) | 36/80 (45) | 46/202 (23) | <0.001 |
| 35–45 yr, n / N (%) | 87/282 (31) | 27/80 (34) | 60/202 (30) | 0.57 |
| >45 yr, n / N (%) | 113/282 (40) | 17/80 (21) | 96/202 (47) | <0.001 |
| Female sex, n / N (%) | 87/282 (31) | 28/80 (35) | 59/202 (29) | 0.39 |
| Recipient ancestry,1 n / N (%) | ||||
| European | 169/279 (61) | 54/79 (68) | 115/200 (58) | 0.10 |
| Hispanic/Latinx | 43/279 (15) | 10/79 (13) | 33/200 (16) | 0.85 |
| East Asian | 38/279 (14) | 7/79 (9) | 31/200 (15) | 0.18 |
| Black | 14/279 (5) | 4/79 (5) | 10/200 (5) | 1.0 |
| Other | 15/279 (5) | 4/79 (5) | 11/200 (6) | 1.0 |
| Allograft source, n / N (%) | ||||
| Living-related | 97/282 (34) | 34/80 (43) | 63/202 (31) | 0.10 |
| Living-unrelated | 54/282 (19) | 14/80 (17) | 40/202 (20) | 0.74 |
| Deceased donor | 131/282 (47) | 32/80 (40) | 99/202 (49) | 0.19 |
| Year of transplantation | 2011 (2006, 2015) | 2008 (2003, 2011) | 2012 (2008, 2015) | <0.001 |
| Donor ancestry,2 n / N (%) | ||||
| European | 172/237 (73) | 45/62 (73) | 127/175 (73) | 1.0 |
| Hispanic/Latinx | 32/237 (13) | 8/62 (13) | 24/175 (14) | 1.0 |
| East Asian | 7/237 (3) | 3/62 (5) | 4/175 (2) | 0.38 |
| Black | 17/237 (7) | 4/62 (6) | 13/175 (7) | 1.0 |
| Other | 9/237 (4) | 2/62 (3) | 7/175 (4) | 1.0 |
| Induction therapy,3 n / N (%) | ||||
| Thymoglobulin | 135/267 (50) | 27/69 (39) | 108/198 (55) | 0.04 |
| IL2R inhibitors | 76/267 (29) | 25/69 (36) | 51/198 (26) | 0.12 |
| Alemtuzumab | 37/267 (14) | 9/69 (13) | 28/198 (14) | 1.0 |
| No induction, n / N (%) | 14/267 (5) | 5/69 (7) | 9/198 (4) | 0.36 |
| Other induction, n / N (%) | 5/267 (2) | 3/69 (5) | 2/198 (1) | 0.11 |
| Steroid-free regimen, n / N (%) | 116/282 (41) | 28/80 (35) | 88/202 (44) | 0.23 |
| # HLA matches (0–6) | 2 (1, 3) | 3 (1, 4) | 2 (1, 3) | <0.001 |
| Recipient HLAs,4 n / N (%) | ||||
| B35 | 72/282 (26) | 24/80 (30) | 47/202 (23) | 0.29 |
| DQ5 | 99/250 (40) | 26/71 (37) | 73/179 (41) | 0.57 |
| DR3 | 37/282 (13) | 12/80 (15) | 25/202 (12) | 0.56 |
| DR15 | 51/282 (18) | 14/80 (18) | 37/202 (18) | 1.00 |
| DQ6 | 76/250 (30) | 20/71 (28) | 56/179 (31) | 0.65 |
| DQ2 | 77/250 (31) | 23/71 (32) | 54/179 (30) | 0.76 |
| Donor HLAs,5 n / N (%) | ||||
| B35 | 59/282 (21) | 17/80 (21) | 42/202 (21) | 1.0 |
| DQ5 | 86/248 (35) | 25/66 (38) | 61/182 (34) | 0.55 |
| DR3 | 51/282 (18) | 16/80 (20) | 35/202 (17) | 0.61 |
| DR15 | 60/282 (21) | 15/80 (19) | 45/202 (22) | 0.63 |
| DQ6 | 96/248 (39) | 25/66 (38) | 71/182 (39) | 1.0 |
| DQ2 | 90/248 (36) | 20/66 (30) | 70/182 (38) | 0.30 |
| Pretransplant DSA, n / N (%) | 15/276 (5) | 4/76 (5) | 11/200 (5.5) | 0.9 |
HLA match is calculated based on A, B, and DR antigens.
DSA, donor-specific autoantibodies; IgAN, IgA nephropathy; HLA, human leukocyte antigen.
Information on recipient ancestry was not available for 3 patients (1 recurrent and 2 nonrecurring).
Information on donor ancestry was not available for 45 patients (18 recurrent and 27 nonrecurring).
Information on induction therapy was not available for 15 patients (11 recurrent and 4 nonrecurring).
Recipient HLA-DQ typing was not available for 32 patients (9 recurrent and 23 nonrecurring).
Donor HLA-DQ typing was not available for 34 patients (14 recurrent and 20 nonrecurring).
There were 80 patients with recurrent IgAN and 202 patients with IgAN without recurrence. Recurrent IgAN occurred at a median of 43 months (IQR: 13, 111 months) post-transplantation, and these patients were followed for a median of 92 months (IQR: 60, 147 months) after transplantation, while patients without IgAN recurrence were followed for a median of 74 months (IQR: 36, 123 months) post-transplantation. Compared to nonrecurring IgAN, patients with recurrent IgAN were younger (36 vs. 45 years at transplantation, p < 0.001), had greater donor-recipient HLA matching scores (median 3 vs. 2, p < 0.001), were transplanted earlier (median year of transplantation 2008 vs. 2012, p < 0.001), and were less likely to receive induction with thymoglobulin (39% vs. 55%, p = 0.04) (Table 1).
Recipients with Kidney Failure Secondary to IgAN Are Associated with High Frequency of HLA-DQ5 but Low Frequency of HLA-DR15 and HLA-DQ6
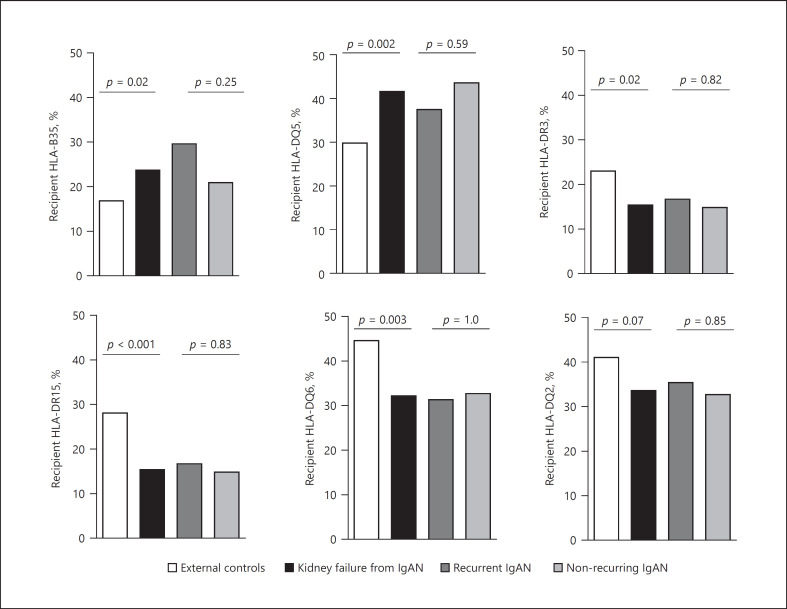
We compared the frequencies of HLA-B35, HLA-DQ5, HLA-DR3, HLA-DR15, HLA-DQ6, and HLA-DQ2 antigens in our recipients of European ancestry with kidney failure secondary to IgAN (n = 169/279) (including patients with recurrent [n = 54] and nonrecurring IgAN [n = 115]), to a group of external healthy controls of European descent from the US National Marrow Donor Program (n = 15,740) [27]. Kidney transplant recipients with renal failure secondary to IgAN had higher frequency of HLA-DQ5 (OR = 1.68 [1.21–2.33], p = 0.002) and lower frequency of both HLA-DR15 (OR = 0.46 [0.31–0.71], p < 0.001) and HLA-DQ6 (OR = 0.59 [0.42–0.84], p = 0.003) (shown in Fig. 1). The latter 2 (DR15 and DQ6) are in linkage disequilibrium to form a common HLA haplotype. Notably, recipients with recurrent and nonrecurring IgAN had similar frequency of these HLAs (shown in Fig. 1).
Fig. 1.
Prevalence of specific HLAs in US controls of European ancestry and transplant recipients of European ancestry who had kidney failure secondary to IgAN in the native kidney, including these with recurrent and nonrecurring disease. Patients of European ancestry with kidney failure secondary to IgAN (n = 169) [combining 54 recurrent IgAN and 115 nonrecurring IgAN] were compared to controls from European ancestry (n = 15,740) from US donors of stem cell transplantation. DQ typing was only available for 149 patients with native kidney failure secondary to IgAN, including 48 recurrent IgAN and 101 nonrecurring disease. Another comparison was performed between recurrent and nonrecurring IgAN. Since 6 HLAs were compared, the Bonferroni-corrected cut-off of 0.008 was considered significant. Of note, DR15 and DQ6 are in linkage disequilibrium to form a common haplotype. IgAN, IgA nephropathy; HLA, human leukocyte antigen.
Younger Recipient Age at Transplantation Is Associated with Recurrent IgAN
Transplant patients with kidney failure secondary to IgAN (n = 282) were assessed for variables predictive of recurrent disease (Table 2). Univariable time-to-event analyses showed that younger recipient age at transplantation and donor-recipient HLA matching were risk factors for recurrence. On multivariable analysis, only younger recipient age (adjusted HR [aHR] = 0.96 per year, p = 0.001) and more recent year of transplantation (aHR = 1.08, p = 0.006) were significantly associated with IgAN recurrence (Table 2).
Table 2.
Univariable and multivariable analyses of the associations with recurrent disease in patients with kidney failure secondary to IgAN
| Variable | Univariable (n = 282) |
Multivariable (n = 267), N events = 69 |
|||
|---|---|---|---|---|---|
| N events | HR (95% CI) | p value | aHR (95% CI) | p value | |
| Recipient age at transplant, per each year | 80 | 0.96 (0.94–0.98) | <0.001 | 0.96 (0.94–0.98) | 0.001 |
| Recipient, female gender | 80 | 1.16 (0.73–1.85) | 0.52 | ||
| Recipient, European ancestry | 79 | 1.39 (0.86–2.25) | 0.18 | ||
| Donor, European ancestry | 62 | 1.09 (0.62–1.93) | 0.77 | ||
| Allograft source | 80 | ||||
| Deceased donor | Ref | − | |||
| Living-related | 1.29 (0.80–2.11) | 0.3 | |||
| Living-unrelated | 1.16 (0.62–2.19) | 0.64 | |||
| Medical sites | 80 | ||||
| CUIMC | Ref | − | Ref | − | |
| OHSU | 1.36 (0.83–2.22) | 0.23 | 1.06 (0.56–2.02) | 0.86 | |
| HUC | 0.70 (0.31–1.55) | 0.37 | 0.59 (0.25–1.37) | 0.22 | |
| Year of transplantation | 80 | 1.05 (1.00–1.08) | 0.06 | 1.08 (1.02–1.14) | 0.006 |
| # HLA matches (per antigen: 0–6) | 80 | 1.15 (1.02–1.30) | 0.02 | 1.12 (0.98–1.29) | 0.11 |
| Induction with thymoglobulin1 | 69 | 0.65 (0.40–1.06) | 0.08 | 0.65 (0.36–1.15) | 0.14 |
| Steroid-free regimens | 80 | 0.77 (0.48–1.24) | 0.28 | ||
| Acute rejection* | 80 | 0.90 (0.55–1.46) | 0.66 | ||
| Recipient HLA-B35 | 80 | 1.40 (0.87–2.27) | 0.17 | ||
| Recipient HLA-DQ5 | 71 | 1.00 (0.61–1.64) | 0.99 | ||
| Recipient HLA-DR3 (DR17 or 18) | 80 | 1.20 (0.65–2.23) | 0.56 | ||
| Recipient HLA-DR15 | 80 | 0.99 (0.56–1.78) | 0.98 | ||
| Recipient HLA-DQ6 | 71 | 1.04 (0.62–1.75) | 0.89 | ||
| Recipient HLA-DQ2 | 71 | 1.09 (0.66–1.80) | 0.74 | ||
| Donor HLA-B35 | 80 | 1.09 (0.63–1.89) | 0.77 | ||
| Donor HLA-DQ5 | 66 | 1.24 (0.75–2.04) | 0.41 | ||
| Donor HLA-DR3 (DR17 or 18) | 80 | 1.38 (0.80–2.40) | 0.25 | ||
| Donor HLA-DR15 | 80 | 0.77 (0.44–1.36) | 0.37 | ||
| Donor HLA-DQ6 | 66 | 0.88 (0.54–1.46) | 0.63 | ||
| Donor HLA-DQ2 | 66 | 0.90 (0.53–1.53) | 0.7 | ||
| Pretransplant DSA | 76 | 1.25 (0.46–3.43) | 0.7 | ||
CUIMC, Columbia University Irving Medical center; DSA, donor-specific antibodies; OHSU, Oregon Health & Science University; HUC, Hospitais da Universidade de Coimbra; DSA, donor-specific autoantibodies; IgAN, IgA nephropathy; HLA, human leukocyte antigen.
Acute rejection is defined as any episode of acute rejection that occurred before recurrence of disease in recurrent IgAN or any time before the end of follow-up in nonrecurring controls.
Even when induction therapy with thymoglobulin was not included in the main multivariable analysis (information on induction therapy was lacking 15 patients, including 11 with recurrent IgAN), the new multivariable analysis (n = 282) revealed similarly that only younger age at transplant (0.96 [0.94–0.98], p < 0.001) and year of transplantation (1.10 [1.04–1.15], p < 0.001) remained independent predictors for IgAN but not HLA matches (1.13 [0.99–1.29], p = 0.06) or medical sites (OHSU: 1.38 [0.83–2.29], p = 0.21, HUC: 0.65 [0.29–1.45], p = 0.29).
HLA Matching Can Predict IgAN Recurrence Only in Recipients of Living-Related Kidney Allograft
The degree of donor-recipient HLA matching was significantly higher in recurrent IgAN than in nonrecurring IgAN in recipients of kidneys from living-related donors (recurrent IgAN: 4 [3, 6] vs. nonrecurring IgAN: 3 [2, 4], p = 0.002), whereas this difference was not significant in recipients of kidney allograft from living-unrelated or deceased donors (recurrent IgAN: 2 [1, 4], nonrecurring IgAN: 1 [1, 2], p = 0.1).
In recipients of living-related renal transplant, the number of HLA matches (aHR = 1.40, p = 0.01), younger age at transplant (aHR = 0.95, p = 0.006), and a more recent year of transplant (aHR = 1.16, p = 0.004) were independent risk factors for IgAN recurrence (online suppl. Table 1; for all online suppl. material, see www.karger.com/doi/10.1159/000519834). In recipients of living-unrelated or deceased donor transplants, younger age at transplant (aHR = 0.97, p = 0.03) was significant predictor for recurrent IgAN, while induction therapy with thymoglobulin (aHR = 0.46, p = 0.05) exerted a protective effects against IgAN recurrence (online suppl. Table 2). In this subgroup of recipients, the number of HLA matches was not associated with the recurrent IgAN (p = 0.9).
Recurrent IgAN Is Independently Associated with Inferior Allograft Survival
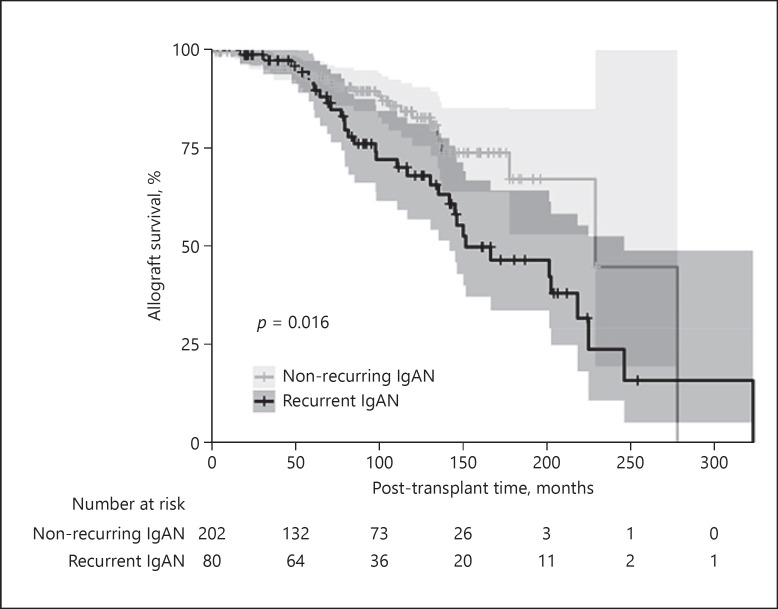
Recurrent IgAN was significantly associated with inferior post-transplant allograft survival (HR = 1.94 [1.13–3.31, p = 0.016]) (Fig. 2), where the survival curves began showing clear separation after 70 months of transplantation. Subsequently, variables associated with allograft survival were analyzed in all transplant patients with kidney failure secondary to IgAN. By univariable Cox regression analysis, living donor allografts, HLA matching, and steroid-free regimens were associated with superior allograft survival, while recurrent IgAN, acute rejection episodes, and more recent transplantation were associated with inferior allograft survival (Table 3).
Fig. 2.
Allograft survival in recurrent and nonrecurring IgAN. Kaplan-Meier curves for cumulative kidney allograft survival from time of transplant in patients with recurrent versus nonrecurring IgAN. Shaded areas around the curves represent the 95% confidence interval. IgAN, IgA nephropathy.
Table 3.
Univariable and multivariable analyses of the associations with allograft failure in transplant patients with kidney failure secondary to IgAN
| Variable | Univariable (n = 282) |
Multivariable (n = 282), N events = 60 |
|||
|---|---|---|---|---|---|
| N events | HR (95% CI) | p value | HR (95% CI) | p value | |
| Recipient age at transplant (per each year) | 60 | 0.98 (0.96–1.00) | 0.09 | 0.97 (0.94–0.99) | 0.02 |
| Recipient, female gender | 60 | 0.88 (0.50–1.54) | 0.66 | ||
| Recipient, European ancestry | 60 | 0.91 (0.54–1.56) | 0.74 | ||
| Donor recipient, European ancestry | 41 | 2.07 (0.87–4.94) | 0.1 | ||
| Allograft from living donor | 60 | 0.56 (0.34–0.95) | 0.03 | 0.36 (0.19–0.67) | 0.001 |
| Medical sites | 60 | ||||
| CUIMC | Ref | − | Ref | − | |
| OHSU | 1.70 (0.96–3.01) | 0.07 | 0.89 (0.41–1.92) | 0.77 | |
| HUC | 0.95 (0.39–2.29) | 0.9 | 0.39 (0.14–1.11) | 0.08 | |
| Year of transplantation | 60 | 1.07 (1.01–1.15) | 0.03 | 1.16 (1.08–1.25) | <0.001 |
| Recurrence of IgAN | 60 | 1.89 (1.12–3.19) | 0.02 | 2.45 (1.38–4.36) | 0.002 |
| # HLA matches (per antigen: 0–6) | 60 | 0.86 (0.74–0.99) | 0.04 | 0.87 (0.73–1.04) | 0.13 |
| Induction with thymoglobulin | 56 | 0.91 (0.52–1.59) | 0.73 | ||
| Steroid-free regimens | 60 | 0.54 (0.29–0.98) | 0.04 | 0.59 (0.26–1.30) | 0.19 |
| Acute rejection | 60 | 2.46 (1.46–4.16) | 0.001 | 2.68 (1.51–4.76) | 0.001 |
| Recipient HLA-B35 | 60 | 1.29 (0.74–2.24) | 0.37 | ||
| Recipient HLA-DQ5 | 57 | 1.07 (0.62–1.84) | 0.82 | ||
| Recipient HLA-DR3 (DR17 or 18) | 60 | 1.05 (0.49–2.22) | 0.91 | ||
| Recipient HLA-DR15 | 60 | 1.90 (1.08–3.35) | 0.02 | 1.90 (1.04–3.47) | 0.04 |
| Recipient HLA-DQ6 | 57 | 1.44 (0.83–2.51) | 0.2 | ||
| Recipient HLA-DQ2 | 57 | 0.63 (0.33–1.20) | 0.16 | ||
| Donor HLA-B35 | 60 | 0.97 (0.51–1.83) | 0.92 | ||
| DonorHLA-DQ5 | 57 | 1.07 (0.59–1.91) | 0.83 | ||
| Donor HLA-DR3 (DR17 or 18) | 60 | 1.06 (0.53–2.10) | 0.88 | ||
| Donor HLA-DR15 | 60 | 1.23 (0.71–2.30) | 0.42 | ||
| Donor HLA-DQ6 | 57 | 1.47 (0.84–2.56) | 0.18 | ||
| DonorHLA-DQ2 | 57 | 1.01 (0.55–1.86) | 0.97 | ||
| Pretransplant DSA | 55 | 1.02 (0.25–4.22) | 0.8 | ||
Rejection is defined as any episode of acute rejection encountered during the follow-up period.
CUIMC, Columbia University Irving Medical center; DSA, donor-specific antibodies; OHSU, Oregon Health & Science University; HUC, Hospitais da Universidade de Coimbra; DSA, donor-specific autoantibodies; IgAN, IgA nephropathy; HLA, human leukocyte antigen.
By multivariable Cox proportional hazards regression analysis, recurrent IgAN (aHR = 2.45, p = 0.002), acute rejection episodes (aHR = 2.68, p = 0.001), and younger recipient age at transplant (aHR = 0.97 per year, p = 0.02) were associated with shorter allograft survival, while receiving an allograft from a living donor (aHR = 0.36, p = 0.001) was associated with superior allograft survival (Table 3). There was a trend toward worse allograft survival in recipients with HLA-DR15 that did not reach statistical significance by the Bonferroni-corrected cut-off (Table 3). Given the design of the study, it was not surprising that more recent transplants were associated with worse survival in this cohort. Again, while the latter variable is probably not a meaningful predictor, it was used mainly to account to for confounders.
To confirm the importance of recurrence IgAN in graft failure, we used IgAN recurrence as a time-varying covariate in the multivariable Cox proportional hazards model for time from transplant to allograft failure. The latter confirmed the independent association between IgAN recurrence and graft failure (data not shown).
Characteristics, Treatment, and Outcome of Recurrent IgAN
Demographic, clinical, and pathological characteristics of patients with recurrent IgAN are presented in Table 1 and online supplementary Table 3. At the time of diagnosis, recipients had median post-transplant interval of 43 months, median serum creatinine of 1.9 mg/dL, and median proteinuria of 0.5 g/g. Seven IgAN recurrence events were detected on protocol biopsies, and the remaining 73 were detected on biopsies performed for clinical reasons. Histologically, concurrent acute rejection was present in 16 of 80 (20%) of index biopsies, including 11 T-cell-mediated rejection, 2 antibody-mediated rejection, and 3 mixed rejection. Diffuse mesangial proliferation, endocapillary proliferation, and crescents were seen in 46%, 33%, and 16% of index biopsies, respectively, while segmental sclerosis and >25% tubulointerstitial scarring were present in 51% and 48% of index biopsies, respectively. The median combined MEST-C score was 2 (IQR: 1, 3) (online suppl. Table 3).
Data on treatment of recurrent IgAN were available for 71 (88%) index biopsies. Treatment was variable and was initiated for recurrent IgAN and, to a lesser extent, for other concurrent conditions, such as acute rejection episodes (online suppl. Table 4). Thirty two (45%) of these patients were managed without adjustment of immunosuppression. Corticosteroids were used in 32 (45%) patients, while thymoglobulin and rituximab were administered to 3 (4%) and 2 (3%) patients, respectively. The mycophenolate mofetil dose was increased in 1 patient, and conversion from tacrolimus to belatacept was initiated in one subject given the presence of significant arteriolar hyalinosis.
We investigated variables associated with post-biopsy allograft failure in the 80 patients with recurrent IgAN (Table 4). On univariable analysis, longer post-transplant interval, serum creatinine values, degree of proteinuria, concurrent acute rejection, and histologic scores for each of mesangial proliferation, segmental sclerosis, tubulointerstitial scarring, as well as combined MEST-C scores in index allograft biopsies were all associated with inferior allograft survival (Table 4). Using Cox multivariable analysis that included the 6 variables with p < 0.1 in univariate analyses (combined MEST-C scores rather than individual scores was used as a histologic variable), serum creatinine at biopsy, degree of proteinuria, and concurrent acute rejection were predictors of inferior allograft survival, while HLA matching represented a significant predictor of superior allograft survival (Table 4). The same 4 variables remained independent predictors of allograft survival when forward stepwise analysis was performed (data presented in the legend of Table 4), supporting their role in determining the allograft outcome in patients with recurrent IgAN.
Table 4.
Univariable and multivariable analyses of the associations with post-biopsy allograft failure in patients with recurrent IgAN
| Variable | Univariable (n = 80) |
Multivariable (n = 67), N events = 33* |
|||
|---|---|---|---|---|---|
| N events | HR (95% CI) | p value | HR (95% CI) | p value | |
| Recipient age at biopsy (per each year) | 33 | 1.01 (0.98–1.04) | 0.55 | ||
| Recipient, female gender | 33 | 0.71 (0.33–1.54) | 0.39 | ||
| Recipient, European ancestry1 | 33 | 0.79 (0.37–1.70) | 0.55 | ||
| Donor, European ancestry2 | 23 | 0.99 (0.36–2.75) | 0.99 | ||
| Allograft from living donor | 33 | 0.62 (0.31–1.25) | 0.18 | ||
| Induction with thymoglobulin3 | 30 | 1.16 (0.55–2.45) | 0.7 | ||
| Steroid-free regimens | 33 | 0.68 (0.31–1.47) | 0.33 | ||
| Post-transplant interval (per month) | 33 | 1.01 (1.01–1.01) | <0.001 | 1.01 (0.99–1.01) | 0.14 |
| Serum creatinine at biopsy (mg/dL)4 | 33 | 1.94 (1.40–2.69) | <0.001 | 2.81 (1.69–4.68) | <0.001 |
| Proteinuria (g/g vs. mg/dL)5 | 33 | 1.15 (1.07–1.24) | <0.001 | 1.20 (1.07–1.34) | 0.002 |
| Concurrent acute rejection | 33 | 3.21 (1.54–6.70) | 0.002 | 3.51 (1.11–11.0) | 0.03 |
| # Of HLA matches (per antigen: 0–6) | 33 | 0.82 (0.67–1.01) | 0.06 | 0.68 (0.50–0.91) | 0.009 |
| Protocol biopsy | 33 | 0.4 (0.05–2.49) | 0.3 | ||
| Mesangial score (M: 0–1)6 | 33 | 2.14 (1.05–4.34) | 0.04 | ||
| Endocapillary proliferation score (E: 0–1)6 | 33 | 1.50 (0.74–3.05) | 0.26 | ||
| Cellular or fibrocellular crescent score (C0–2)6 | 33 | 1.71 (0.73–3.98) | 0.22 | ||
| Segmental sclerosis score (S: 0–1)5 | 33 | 2.79 (1.32–5.93) | 0.007 | ||
| Tubular atrophy/interstitial fibrosis score (T: 0–2)6 | 33 | 2.56 (1.64–4.00) | <0.001 | ||
| Combined MEST-C score (0–7)5 | 33 | 1.51 (1.23–1.85) | <0.001 | 1.20 (0.86–1.66) | 0.28 |
DSA, donor-specific autoantibodies; IgAN, IgA nephropathy; HLA, human leukocyte antigen.
To avoid potential overfitting in multivariable analysis, we also used a forward and backward stepwise multivariable analysis that included variables with p < 0.1. Only the combined MEST-C score, rather than individual components were entered in multivariable analysis. Forward stepwise analyses showed that each of serum creatinine (3.09 [1.88–5.10], p < 0.001), proteinuria (1.26 [1.14–1.38], p < 0.001), concurrent acute rejection (6.67 [2.54–17.6], p < 0.001), and # HLA matches (0.73 [0.55–0.97], p = 0.03) were associated with inferior allograft survival.
Information on recipient ancestry was not available for 1 patient.
Information on donor ancestry was not available for 18 patients.
Information on induction therapy was not available for 11 patients.
Information on serum creatinine was not available for 4 patients.
Information on proteinuria was not available for 11 patients.
Histologic Oxford scores could not be assessed in 1 patient.
Discussion
In contrast to IgAN in the native kidney, there are limited data on the prognosis and risk factors for recurrent IgAN in the kidney allograft. The available reports are largely restricted to small single center studies [7, 8, 10, 11, 12, 13, 28] or rely on data from registries [20, 29, 30, 31], where the information is incomplete and subject to bias. While prior studies have identified potential risk factors for recurrent IgAN, the results varied among studies. Furthermore, despite the described association of some HLAs with IgAN in the native kidney [2, 3, 4, 5], an evaluation of donor and recipient HLAs in recurrent and nonrecurring IgAN in the renal allograft has not been performed to date. To our knowledge, the current report represents one of the largest nonregistry studies to assess predictors for recurrent IgAN and is the first to analyze the association of HLAs in donors and recipients with recurrence and prognosis of IgAN.
Some [8, 9, 10], but not all [11], studies have shown detrimental impact of IgAN on allograft survival. In the current study, recurrent IgAN emerged as an independent predictor of inferior allograft survival, together with acute rejection. When predictors of recurrent IgAN were examined in multivariable analysis, younger recipient age at transplantation, which is a nonmodifiable extrarenal variable related to the recipient, was significantly associated with recurrent IgAN. Prior studies have shown an association between younger recipient age and recurrent IgAN [10, 11, 12, 13, 14, 15]. Notably, younger recipient age is not universally associated with recurrent glomerulonephritis. In contrast, older recipient age is an independent predictor for recurrent membranous nephropathy post-transplantation [32].
Data on genetic predisposing factors for recurrent IgAN are very limited. Despite the suggested association of HLA-B35, HLA-DQ5, HLA-DR3, HLA-DR15, HLA-DQ6, and HLA-DQ2 with IgAN in the native kidney [2, 3, 4, 5], these antigens have not been studied systematically in the context of recurrent IgAN. Compared to the general population of US residents of European descent [27], transplant recipients of European ancestry who had kidney failure secondary to IgAN showed higher frequencies of HLA-DQ5 and lower frequencies of HLA-DR15 and HLA-DQ6 (Fig. 1), supporting a predisposing role of DQ5 for IgAN in the native kidney and a protective role of DR15-DQ6 haplotype. In contrast, the lack of difference in frequencies of such HLAs in the recipients or the donors between recurrent and nonrecurring IgAN argue against an important role of these specific antigens in recurrence of IgAN and do not support donor selection based on presence/absence of any of these HLAs.
Previous studies have suggested that HLA mismatch may reduce the risk of recurrent IgAN [10, 20, 21]. Our findings do not support an approach that encourages HLA mismatching in patients with IgAN undergoing renal transplantation for 2 reasons. First, while donor-recipient pairs with recurrent IgAN were more HLA matched than their counterparts without recurring disease, HLA matching was not independently associated with protection from recurrence in time-to-event analyses, except in the subgroup of patients who received kidney allograft from living-related donors. Second, HLA mismatch may increase susceptibility to alloimmunity, which can negatively affect allograft survival. Indeed, more rigorous HLA matching remained an independent predictor for superior post-biopsy allograft survival in patients with recurring IgAN (Table 4). However, the association between HLA matching in recipients of a kidney from living-related donors and IgAN recurrence suggest that risk of recurrent IgAN may be mostly carried in HLA genetic regions in individuals from the same family with close genetic makeup. This finding may help in guiding future studies to explore genomic donor-recipient matching to reduce recurrent IgAN.
Although some prior studies suggested a benefit of steroid maintenance in preventing recurrent IgAN [16, 17, 18], others have not [8, 12]. Our study did not show any benefit of steroids maintenance on recurrent disease or allograft survival. Varying immunosuppressive regimens and definitions of steroid-free regimens may account for some of the different results. Prospective trials will be needed to address this issue with certainty.
A few studies have shown that allografts from living-related donors [8, 14, 19] are associated with an increased risk for recurrent IgAN, while others [8, 10, 12, 13], including our study, do not support an independent association. The lack of correlation between acute rejection and recurrent IgAN is an interesting finding that argues against such association, especially in a cohort where most allograft biopsies were performed for allograft dysfunction.
Regarding the prognosis of patients with recurrent IgAN, serum creatinine, proteinuria, and concurrent acute rejection were all significant predictors for allograft failure. Whereas, a previous report, focusing on allograft survival in patients with post-transplant IgAN, revealed a significant negative impact of MEST-C scores on allograft survival [33]; our study, albeit smaller, failed to demonstrate such an association on multivariable analyses. However, the aforementioned study combined both de novo and recurrent IgAN and did include proteinuria or serum creatinine at biopsy together with histologic score in the same multivariable analysis.
Unique strengths of the study includes its relatively large sample size with prolonged follow-up and exclusion of de uncertain cases with low IgA staining intensity to include pure cases of recurrent IgAN. However, the findings in this study need to be interpreted in light of several limitations, including the retrospective nature of the data, center-based bias, and incomplete data. Other limitations include case ascertainment due to inability to precisely exclude potential cases with subclinical recurrence of IgAN that did not reach threshold needed to trigger follow-up allograft biopsies and shorter follow-up for non-recurring IgAN compared with recurrent IgAN. To overcome some of these biases, recurrence of IgAN was treated as a time-dependent variable and year of transplantation and medical sites were included in multivariable analyses. Additionally, the median follow-up time in patients with no evidence of recurrent IgAN is longer than the median time of recurrence (median 74 vs. 43 months after transplantation, respectively). Finally, while using the pathology dataset may be regarded as a limitation, it should be stressed that the search included post-reperfusion biopsies (performed routinely for CUIMC patients) and protocol biopsies (performed routinely for OHSU and HUC patients). This would have assured inclusion of all nonrecurring patients with native kidney failure secondary to IgAN who underwent kidney transplant at CUIMC, OHSU, and HUC.
In conclusion, recurrent IgAN is an important predictor of long-term allograft survival. Younger recipient age at transplantation is independently associated with recurrent IgAN. While HLA matching may play a role in recurrent IgAN, it may only be important in the setting of living-related transplantation. Whereas, our results support the predisposing role of HLA-DQ5 and the protective role of HLA-DR15 and HLA-DQ6 in IgAN in the native kidney, the presence of any of these antigens in the recipients or in the donors did not appear to affect recurrent disease post-transplantation.
Statement of Ethics
This study is in compliance with the guidelines for human subjects and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The study was approved by each site's Institutional Review Board (Columbia University [Protocol Number AAAO2107], OHSU [STUDY00017467], and HUC [CHUC 167-20]) and granted waiver of informed consent.
Conflict of Interest Statement
The authors declare no conflicts of interest.
Funding Sources
I.B. is supported from 2020 Mendez National Institute of Transplantation Foundation Research Grant. K.K. is supported by R01-DK105124 from the NIH/NIDDK.
Author Contributions
Catherine R. Kavanagh MD, Namrata G. Jain MD, Ibrahim Batal MD, Nicole K. Andeen MD, and Krzysztof Kiryluk MD participated in research design. Catherine R. Kavanagh MD, Namrata G. Jain MD, Ibrahim Batal MD, Nicole K. Andeen MD, Krzysztof Kiryluk MD, Satoru Kudose MD, Dominick Santoriello MD, Pietro A. Canetta MD, David Cohen MD, Jai Radhakrishnan MD, Gerald B. Appel MD, Michael B. Stokes MD, Glen S. Markowitz MD, Vivette D. D'Agati MD, Rita Leal MD, Francesca Zanoni MD, Elena-Rodica Vasilescu MD, Geo Serban PhD, Megan Stack FNP, Carley Shaut PhD, António Martinho MS, Rui Alves PhD, and Jeanne Kamal MD participated in interpretation of results, writing of the paper, and final review. Catherine R. Kavanagh MD, Namrata G. Jain MD, Ibrahim Batal MD, Nicole K. Andeen MD, Rita Leal MD, Francesca Zanoni MD, Elena-Rodica Vasilescu MD, Geo Serban PhD, Megan Stack FNP, Carley Shaut PhD, António Martinho MS, Rui Alves PhD, and Jeanne Kamal MD participated in the performance of the research. Krzysztof Kiryluk MD, Ibrahim Batal MD, and Nicole K. Andeen MD contributed to new analytic tools. Francesca Zanoni MD, Catherine R. Kavanagh MD, Ibrahim Batal MD, and Nicole K. Andeen MD participated in data analysis.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary files. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Acknowledgments
The work was presented in part as oral presentation at the ASN 2019 Kidney Week (Washington, DC). I.B. is supported from 2020 Mendez National Institute of Transplantation Foundation Research Grant. K.K. is supported by R01-DK105124 from the NIH/NIDDK.
Catherine R. Kavanagh and Francesca Zanoni contributed equally to this manuscript.
Funding Statement
I.B. is supported from 2020 Mendez National Institute of Transplantation Foundation Research Grant. K.K. is supported by R01-DK105124 from the NIH/NIDDK.
References
- 1.Julian BA, Quiggins PA, Thompson JS, Woodford SY, Gleason K, Wyatt RJ. Familial IgA nephropathy. Evidence of an inherited mechanism of disease. N Engl J Med. 1985;312:202–208. doi: 10.1056/NEJM198501243120403. [DOI] [PubMed] [Google Scholar]
- 2.Doxiadis II, De Lange P, De Vries E, Persijn GG, Claas FH. Protective and susceptible HLA polymorphisms in IgA nephropathy patients with end-stage renal failure. Tissue Antigens. 2001;57:344–347. doi: 10.1034/j.1399-0039.2001.057004344.x. [DOI] [PubMed] [Google Scholar]
- 3.Feehally J, Farrall M, Boland A, Gale DP, Gut I, Heath S, et al. HLA has strongest association with IgA nephropathy in genome-wide analysis. J Am Soc Nephrol. 2010;21:1791–1797. doi: 10.1681/ASN.2010010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiryluk K, Li Y, Sanna-Cherchi S, Rohanizadegan M, Suzuki H, Eitner F, et al. Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet. 2012;8:e1002765. doi: 10.1371/journal.pgen.1002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiryluk K, Li Y, Scolari F, Sanna-Cherchi S, Choi M, Verbitsky M, et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46:1187–1196. doi: 10.1038/ng.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moroni G, Belingheri M, Frontini G, Tamborini F, Messa P. Immunoglobulin A nephropathy. Recurrence after renal transplantation. Front Immunol. 2019;10:1332. doi: 10.3389/fimmu.2019.01332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berthoux F, Suzuki H, Mohey H, Maillard N, Mariat C, Novak J, et al. Prognostic value of serum biomarkers of autoimmunity for recurrence of IgA nephropathy after kidney transplantation. J Am Soc Nephrol. 2017;28:1943–1950. doi: 10.1681/ASN.2016060670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nijim S, Vujjini V, Alasfar S, Luo X, Orandi B, Delp C, et al. Recurrent IgA nephropathy after kidney transplantation. Transplant Proc. 2016;48:2689–2694. doi: 10.1016/j.transproceed.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Moroni G, Longhi S, Quaglini S, Gallelli B, Banfi G, Montagnino G, et al. The long-term outcome of renal transplantation of IgA nephropathy and the impact of recurrence on graft survival. Nephrol Dial Transplant. 2013;28:1305–1314. doi: 10.1093/ndt/gfs472. [DOI] [PubMed] [Google Scholar]
- 10.Rodas LM, Ruiz-Ortiz E, Garcia-Herrera A, Pereira A, Blasco M, Ventura-Aguiar P, et al. IgA nephropathy recurrence after kidney transplantation: role of recipient age and human leukocyte antigen-B mismatch. Am J Nephrol. 2020;51:357–365. doi: 10.1159/000506853. [DOI] [PubMed] [Google Scholar]
- 11.Ponticelli C, Traversi L, Feliciani A, Cesana BM, Banfi G, Tarantino A. Kidney transplantation in patients with IgA mesangial glomerulonephritis. Kidney Int. 2001;60:1948–1954. doi: 10.1046/j.1523-1755.2001.00006.x. [DOI] [PubMed] [Google Scholar]
- 12.Ahn S, Min SI, Min SK, Ha IS, Kang HG, Kim YS, et al. Different recurrence rates between pediatric and adult renal transplant for immunoglobulin A nephropathy: predictors of posttransplant recurrence. Exp Clin Transplant. 2015;13:227–232. [PubMed] [Google Scholar]
- 13.Cazorla-Lopez JM, Wu J, Villanego-Fernandez F, Naranjo-Muñoz J, Vigara-Sánchez LA, García-García-Doncel A, et al. IgA nephropathy after renal transplant: recurrences and de novo cases. Transplant Proc. 2020;52:515–518. doi: 10.1016/j.transproceed.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Han SS, Huh W, Park SK, Ahn C, Han JS, Kim S, et al. Impact of recurrent disease and chronic allograft nephropathy on the long-term allograft outcome in patients with IgA nephropathy. Transpl Int. 2010;23:169–175. doi: 10.1111/j.1432-2277.2009.00966.x. [DOI] [PubMed] [Google Scholar]
- 15.Sato K, Ishida H, Uchida K, Nitta K, Tanabe K. Risk factors for recurrence of immunoglobulin a nephropathy after renal transplantation: single center study. Ther Apher Dial. 2013;17:213–220. doi: 10.1111/j.1744-9987.2012.01139.x. [DOI] [PubMed] [Google Scholar]
- 16.Von Visger JR, Gunay Y, Andreoni KA, Bhatt UY, Nori US, Pesavento TE, et al. The risk of recurrent IgA nephropathy in a steroid-free protocol and other modifying immunosuppression. Clin Transplant. 2014;28:845–854. doi: 10.1111/ctr.12389. [DOI] [PubMed] [Google Scholar]
- 17.Di Vico MC, Messina M, Fop F, Barreca A, Segoloni GP, Biancone L. Recurrent IgA nephropathy after renal transplantation and steroid withdrawal. Clin Transplant. 2018;32:e13207. doi: 10.1111/ctr.13207. [DOI] [PubMed] [Google Scholar]
- 18.Clayton P, McDonald S, Chadban S. Steroids and recurrent IgA nephropathy after kidney transplantation. Am J Transplant. 2011;11:1645–1649. doi: 10.1111/j.1600-6143.2011.03667.x. [DOI] [PubMed] [Google Scholar]
- 19.Andresdottir MB, Hoitsma AJ, Assmann KJ, Wetzels JF. Favorable outcome of renal transplantation in patients with IgA nephropathy. Clin Nephrol. 2001;56:279–288. [PubMed] [Google Scholar]
- 20.Andresdottir MB, Haasnoot GW, Doxiadis II, Persijn GG, Claas FH. Exclusive characteristics of graft survival and risk factors in recipients with immunoglobulin A nephropathy: a retrospective analysis of registry data. Transplantation. 2005;80:1012–1018. doi: 10.1097/01.tp.0000179150.84803.56. [DOI] [PubMed] [Google Scholar]
- 21.McDonald SP, Russ GR. Recurrence of IgA nephropathy among renal allograft recipients from living donors is greater among those with zero HLA mismatches. Transplantation. 2006;82:759–762. doi: 10.1097/01.tp.0000230131.66971.45. [DOI] [PubMed] [Google Scholar]
- 22.Proteinuria measurement and urine-based markers for preeclampsia diagnosis Technology Opportunity Assessment. 2014 [Google Scholar]
- 23.Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, et al. Oxford classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91:1014–1021. doi: 10.1016/j.kint.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, et al. Antibody-mediated rejection criteria − an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708–714. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 25.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 26.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 27.Maiers M, Gragert L, Klitz W. High-resolution HLA alleles and haplotypes in the United States population. Hum Immunol. 2007;68:779–788. doi: 10.1016/j.humimm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Avasare RS, Rosenstiel PE, Zaky ZS, Tsapepas DS, Appel GB, Markowitz GS, et al. Predicting post-transplant recurrence of IgA nephropathy: the importance of crescents. Am J Nephrol. 2017;45:99–106. doi: 10.1159/000453081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang SH, Kennard AL, Walters GD. Recurrent glomerulonephritis following renal transplantation and impact on graft survival. BMC Nephrol. 2018;19:344. doi: 10.1186/s12882-018-1135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen PJ, Chadban SJ, Craig JC, Lim WH, Allen RDM, Clayton PA, et al. Recurrent glomerulonephritis after kidney transplantation: risk factors and allograft outcomes. Kidney Int. 2017;92:461–469. doi: 10.1016/j.kint.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 31.Kennard AL, Jiang SH, Walters GD. Increased glomerulonephritis recurrence after living related donation. BMC Nephrol. 2017;18:25. doi: 10.1186/s12882-016-0435-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batal I, Vasilescu ER, Dadhania DM, Adel AA, Ali Husain S, Avasare R, et al. Association of HLA typing and alloimmunity with posttransplantation membranous nephropathy: a multicenter case series. Am J Kidney Dis. 2020;76((3)):374–383. doi: 10.1053/j.ajkd.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park S, Go H, Baek CH, Kim YH, Kim YC, Yang SH, et al. Clinical importance of the updated Oxford classification in allograft IgA nephropathy. Am J Transplant. 2019;19:2855–2864. doi: 10.1111/ajt.15400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary files. Further inquiries can be directed to the corresponding author.