Abstract
This study describes a pharmacokinetic evaluation of amphotericin B (AMB) lipid complex injection (ABLC or Abelcet) in 17 patients with systemic fungal infection administered 5 mg/kg of body weight/day by infusion for 10 to 17 days. The results showed that AMB exhibited multiexponential disposition with high clearance, large volume of distribution at steady state, and long apparent elimination half-life but no evidence of accumulation in the blood after multiple daily doses. The results confirm previous observations and further reinforce the suggestion that ABLC may exist as a depot in the tissues from which free AMB is slowly released to limit exposure.
Amphotericin B (AMB) remains the most effective systemic therapy for serious fungal infection (4, 12). The dose-limiting toxicity of the conventional formulation prepared as a mixed micellar dispersion with deoxycholate (Fungizone; Bristol-Myers Squibb) (13) is being addressed by new formulations that utilize either liposomes or lipid complexes as drug carriers and that limit the availability of “free” AMB (2, 5–7, 9). One of the most widely used of these new formulations is amphotericin B lipid complex injection (ABLC) (8). It has been shown to be clinically effective but to have reduced toxicity (11, 15) and is approved for use in the treatment of invasive fungal infections in patients who are refractory to or intolerant of conventional AMB therapy. This study was undertaken as a prospective, multiple-dose pharmacokinetic study to evaluate the pharmacokinetics of AMB after intravenous administration of ABLC at the recommended dose and dosing schedule in patients for whom it is indicated. This is in contrast to previous smaller and less than ideal studies that have been used to describe its disposition (1, 10, 16).
(This work was presented in part at the 13th Congress of the International Society for Human and Animal Mycology, Salsomaggiore, Parma, Italy, June 1997.)
This was a phase II, open-label, noncomparative, multiple-dose pharmacokinetic study in patients with proven or suspected systemic fungal infection. It was conducted, after obtaining informed consent from each patient, in accordance with the European Community Good Clinical Practices Guidelines. Patients were excluded if they had a positive serum pregnancy test or were lactating females, had a life expectancy of less than 10 days, had received AMB within the previous 3 months, or had renal impairment (serum creatinine > 2.5 mg/dl). The study was conducted at two sites: Hematology Department, Medical University of Gdansk, and the Provincial Hospital of Infectious Diseases, both in Gdansk, Poland.
ABLC (Abelcet; The Liposome Company, Inc., Princeton, N.J.) was administered once daily as an intravenous infusion at a dose of 5 mg/kg of body weight/day over 2 h. Patients received the drug for 10 to 17 days. Blood samples (3 ml each) for the determination of total AMB (both free and lipid complexed) were collected through a multilumen central line but using a port other than that used for study drug administration. They were collected in heparinized tubes after the first and last doses, at 5 min before the infusion, at 60 min after the start of the infusion, and at 5, 15, and 30 min and 1.5, 3, 6, 9, 12, and 22 h after the end of the infusion. In addition, samples were collected on days 2, 4, 5, 6, 8, and 10 after the last dose. Also, during the treatment period, blood samples were collected on days 2, 4, and 7 at 5 min before (trough) and 5 min after the infusion (peak). The whole-blood samples were kept frozen at −70°C and shipped on dry ice to the analytical laboratory where AMB was determined in whole blood by a validated high-pressure liquid chromatography method as previously described (1, 3).
Whole-blood concentration-time data were analyzed to estimate the pharmacokinetic parameters by standard noncompartmental methods. Data are expressed as the means ± standard deviations (SD). One subject on day 1 and one on day 2 who were otherwise comparable to the other members of the group at subsequent times had maximum concentrations of AMB in blood (Cmax) that were outside a three-SD range of the respective means for the group. They were thus excluded from the calculation of the mean Cmax reported for the group. This similarly affected the area under the concentration-time curve from 0 to 24 h (AUC0–24) after the first dose and was similarly treated. Statistical comparison of data obtained on different days of dosing was accomplished by analysis of variance with the level of significance (P) set at < 0.05.
Out of the 25 patients enrolled in the study, 8 were unevaluable for pharmacokinetics. The remaining 17 patients were all Caucasians and consisted of 15 males and 2 females ranging in age from 19 to 60 years with a mean ± SD of 38.0 ±12.7 years and weighing between 45 and 87 kg with a mean of 65.5 ± 12.6 kg. The underlying conditions in these patients were hematologic disease (n = 7) and AIDS (n = 10), and fungal infection was due to Candida and/or Aspergillus spp. The drug was well tolerated. There were no significant differences in the data between the different patient groups; hence, the results from all patients were combined.
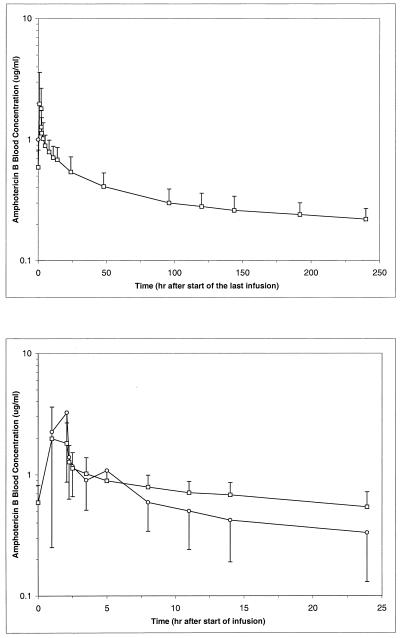
The blood concentration-time profile showed a multiexponential disposition of AMB after administration of ABLC, with a rapid initial decline in concentration from the peak attained immediately after the infusion and a slower terminal elimination phase lasting over several days (Fig. 1, top panel). The disposition of AMB after administration of ABLC was characterized by a high clearance from the blood, a large volume of distribution (V) and a long elimination half-life (Table 1). The weight-normalized clearance ranged from 0.19 to 0.44 liter/h · kg with a mean of 0.27 ± 0.07 liter/h · kg. The weight-normalized volume (Varea) ranged from 41 to 633 liters/kg with a mean of 147 ± 144 liters/kg.
FIG. 1.
Blood concentration-time profiles of AMB after an ABLC dose of 5 mg/kg/day by a 2-h infusion for 10 to 17 days in 17 patients with confirmed or suspected fungal infection following the last dose administration (top panel) and over 24 h following the first (○) and last (□) doses (bottom panel). Values are means ± SD.
TABLE 1.
Pharmacokinetic parameters of AMB after administration of ABLC at 5 mg/kg/day to 17 patients with proven or suspected fungal infections
| Parameterb | Valuea after:
|
|
|---|---|---|
| First dose | Last dose | |
| Cmax (μg/ml) | 3.67 ± 2.37 | 2.39 ± 1.58 |
| Tmax (h) | 2.24 ± 1.12 | 1.58 ± 0.56 |
| AUC0–24 (μg · h/ml) | 16.45 ± 7.22 | 19.17 ± 4.43 |
| AUC0–∞ (μg · h/ml) | 241.29 ± 122.93 | |
| CL (liters/h) | 17.8 ± 5.2 | |
| Varea (liters) | 9,301 ± 8,384 | |
| Vss (liters) | 865 ± 347 | |
| T1/2 (h) | 393 ± 486 | |
Values are means ± SD.
Tmax, time to maximum concentration of AMB; AUC0–∞, AUC from 0 h to ∞; CL, clearance; Vss, volume of distribution at steady state; T1/2, elimination half-life.
Comparison of data after the first and last doses showed that, generally, the blood concentration-time profiles were very similar (Fig. 1, bottom panel). The first and last dosing interval AUC values (AUCfirst and AUClast, respectively) are similar in magnitude with an AUCfirst/AUClast ratio ranging from 0.49 to 1.22 (mean of 0.83 ± 0.21). The Cmax was higher in most patients after the first infusion than after subsequent infusions. The trough and peak concentrations obtained in the intervening days between the first and last doses were comparable throughout the dosing period, showing no evidence of accumulation in the blood and suggesting the attainment and maintenance of a steady state during the dosing period.
Previous studies (1, 10, 16) have shown the distinct dispositional characteristics of AMB after ABLC compared to the conventional formulation. However, they suffer from some limitations including small sample sizes and inadequate study conditions. The present study represents a prospective, multiple-dose study devoid of the limitations of these earlier studies with a larger sample size and better study design under the conditions to be expected in clinical practice. The results are generally consistent with these earlier studies. The disposition of AMB after ABLC is multiexponential and characterized by a high clearance from blood and a large V, consistent with its rapid uptake from blood and storage in tissues for subsequent slow release. The slow terminal elimination and long half-life are consistent with this slow release from the tissue depot.
Contrary to expectation based on its long half-life (about 390 h or 16 days) and a daily dosing regimen, there was little or no accumulation of AMB in blood after administration for 10 days or more. This was shown by the observations that the Cmax after the last dose was lower than that after the first dose and that the ratio of the AUC0–24 for the first dose to that for the last dose was close to unity (0.83 ± 0.21). This unusual behavior is also reflected in the observation that trough and peak concentrations remained almost unchanged from about the 3rd day of dosing, suggesting that steady state had been reached within 3 days in spite of a half-life of about 16 days. The reasons for this unusual pharmacokinetic behavior are still unclear but may be related to extensive and almost unsaturable tissue uptake within the dose range and dosing period used in this study.
Collectively, these observations are consistent with the view that ABLC is rapidly removed from the circulation and distributed into tissues, thus achieving high tissue concentrations (as observed in preclinical studies where elevated AMB levels were observed in liver, spleen, and lung) and providing a slow-release drug depot. Active drug release may occur at the site of infection as the result of phospholipases secreted by the invading fungus itself or surrounding activated inflammatory cells (14). These data may provide a rationale for a regimen that would include a loading dose or doses and then a reduction in the dose or frequency of dosing, as the large tissue depot would provide a sustained antifungal effect. Preliminary support for such a suggestion comes from evidence of sustained response after discontinuation of ABLC in an experimental model of hepatosplenic candidiasis in rabbits (J. Lee, M. Allende, A. Francesconi, T. Sein, H. Dollenberg, K. Garrett, C. Lyman, P. Francis, P. Pizzo, and T. Walsh, Abstr. 7th Int. Symp. Infect. Immunocompromised Host, abstr. 52, 1992) and in a small clinical study in children (16). Such a regimen will also enhance the safety profile of ABLC due to a reduction in exposure to AMB, as previous data have suggested that its toxicity is related to exposure in blood.
Acknowledgments
The support by The Liposome Company, Inc., of the studies described in this paper and by PHS grant GM47124 from the NIH for research in the author's (R.A.B.) laboratory are gratefully acknowledged.
REFERENCES
- 1.Adedoyin A, Bernardo J F, Swenson C E, Bolcsak L E, Horwith G, DeWitt S, Kelly E, Klastersky J, Sculier J P, DeValeriola D, Anaissie E, Lopez-Berestein G, Llanos-Cuentas A, Boyle A, Branch R A. Pharmacokinetic profile of ABELCET (amphotericin B lipid complex injection): combined experience from phase I and phase II studies. Antimicrob Agents Chemother. 1997;41:2201–2208. doi: 10.1128/aac.41.10.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler-Moore J P, Proffitt R T. Development, characterization, efficacy and mode of action of AmBisome, a unilamellar liposomal formulation of amphotericin B. J Liposome Res. 1993;3:429–450. [Google Scholar]
- 3.Bhamra R, Sa'ad A, Bolcsak L E, Janoff A S, Swenson C E. Behavior of amphotericin B lipid complex in plasma in vitro and in the circulation of rats. Antimicrob Agents Chemother. 1997;41:886–892. doi: 10.1128/aac.41.5.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallis H A, Drew R H, Pickard W W. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990;12:27–38. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 5.Gates C, Pinney R J. Amphotericin B and its delivery by liposomal and lipid formulations. J Clin Pharm Ther. 1993;18:147–153. doi: 10.1111/j.1365-2710.1993.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 6.Guo L S S, Working P K. Complexes of amphotericin B and cholesteryl sulfate. J Liposome Res. 1993;3:473–490. [Google Scholar]
- 7.Janknegt R, de Marie S, Baker-Wonderberg I A J M, Crommelin D J A. Liposomal and lipid formulations of amphotericin B. Clinical pharmacokinetics. Clin Pharmacokinet. 1992;23:279–291. doi: 10.2165/00003088-199223040-00004. [DOI] [PubMed] [Google Scholar]
- 8.Janoff A S, Boni L T, Popescu M C, Minchey S R, Cullis P R, Madden T D, Taraschi T, Gruner S M, Shyamsunder E, Tate M W, Mendelsohn R, Bonner D. Unusual lipid structures selectively reduce the toxicity of amphotericin B. Proc Natl Acad Sci USA. 1988;85:6122–6126. doi: 10.1073/pnas.85.16.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janoff A S, Perkins W R, Saletan S L, Swenson C E. Amphotericin B lipid complex (ABLC): a molecular rationale for the attenuation of amphotericin B related toxicities. J Liposome Res. 1993;3:451–472. [Google Scholar]
- 10.Kan V L, Bennett J E, Amantea M A, Smolskis M C, McManus E, Grasela D M, Sherman J W. Comparative safety, tolerance and pharmacokinetics of amphotericin B lipid complex and amphotericin B deoxycholate in healthy male volunteers. J Infect Dis. 1991;164:418–421. doi: 10.1093/infdis/164.2.418. [DOI] [PubMed] [Google Scholar]
- 11.Luke R G, Boyle J A. Renal effects of amphotericin B lipid complex. Am J Kidney Dis. 1998;31:780–785. doi: 10.1016/s0272-6386(98)70046-0. [DOI] [PubMed] [Google Scholar]
- 12.Patel R. Antifungal agents. Part I. Amphotericin B preparations and flucytosine. Mayo Clin Proc. 1998;73:1205–1225. doi: 10.4065/73.12.1205. [DOI] [PubMed] [Google Scholar]
- 13.Sabra R, Branch R A. Amphotericin B nephrotoxicity. Drug Safety. 1990;5:94–108. doi: 10.2165/00002018-199005020-00003. [DOI] [PubMed] [Google Scholar]
- 14.Swenson C E, Perkins W R, Roberts P, Ahmad I, Stevens R, Stevens D A, Janoff A S. In vitro and in vivo antifungal activity of amphotericin B lipid complex: are phospholipases important? Antimicrob Agents Chemother. 1998;42:767–771. doi: 10.1128/aac.42.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh T J, Hiemenz J W, Seibel N L, Perfect J R, Horwith G, Lee L, Silber J L, DiNubile M J, Reboli A, Bow E, Lister J, Anaissie E J. Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin Infect Dis. 1998;26:1383–1396. doi: 10.1086/516353. [DOI] [PubMed] [Google Scholar]
- 16.Walsh T J, Whitcomb P, Piscitelli S, Figg W D, Hill S, Chanock S J, Jarosinski P, Gupta R, Pizzo P A. Safety, tolerance and pharmacokinetics of amphotericin B lipid complex in children with hepatosplenic candidiasis. Antimicrob Agents Chemother. 1997;41:1944–1948. doi: 10.1128/aac.41.9.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]