Abstract
Aims/Introduction
In patients with pulmonary embolism (PE), the impact of diabetes mellitus on patient profile and outcome is not well investigated.
Material and Methods
The German nationwide inpatient sample of the years 2005–2018 was analyzed. Hospitalized PE patients were stratified for diabetes, and the impact of diabetes on in‐hospital events was investigated.
Results
Overall, 1,174,196 PE patients (53.8% aged ≥70 years, 53.5% women) and, among these, 219,550 (18.7%) diabetes patients were included. In‐hospital mortality rate amounted to 15.8%, and was higher in diabetes patients than in non‐diabetes patients (19.8% vs 14.8%, P < 0.001). PE patients with diabetes had a higher prevalence of cardiovascular risk factors, comorbidities, right ventricular dysfunction (31.8% vs 27.7%, P < 0.001), prolonged in‐hospital stay (11.0 vs 9.0 days, P < 0.001) and higher rates of adverse in‐hospital events. Remarkably, diabetes was independently associated with increased in‐hospital mortality (odds ratio [OR] 1.21, 95% confidence interval [CI] 1.20–1.23, P < 0.001) when adjusted for age, sex and comorbidities. Within the observation period of 2005–2018, a relevant decrease of in‐hospital mortality in PE patients with diabetes was observed (25.5% to 16.8%). Systemic thrombolysis was more often administered to diabetes patients (OR 1.18, 95% CI 1.01–3.49, P < 0.001), and diabetes was associated with intracerebral (OR 1.19, 95% CI 1.12–1.26, P < 0.001), as well as gastrointestinal bleeding (OR 1.11, 95% CI 1.07–1.15, P < 0.001). Type 1 diabetes mellitus was shown to be a strong risk factor in PE patients for shock, right ventricular dysfunction, cardiopulmonary resuscitation and in‐hospital death (OR 1.75, 95% CI 1.61–1.90, P < 0.001).
Conclusions
Despite the progress in diabetes treatments, diabetes is still associated with an unfavorable clinical patient profile and higher risk for adverse events, including substantially increased in‐hospital mortality in acute PE.
Keywords: Diabetes mellitus, Pulmonary embolism, Venous thromboembolism
In pulmonary embolism, the impact of diabetes mellitus on outcomes is not well investigated. The present study showed that diabetes mellitus is associated with an unfavorable clinical patient profile of pulmonary embolism patients. Diabetes mellitus affects complications and survival in pulmonary embolism patients negatively.

INTRODUCTION
Pulmonary embolism (PE) represents a leading cause of cardiovascular death worldwide 1 , 2 , with an improvement of mortality over the past years 2 , 3 , but increasing annual incidence rates 3 , 4 . The mortality risk of PE patients is associated with the patient’s clinical condition in the setting of acute PE, including hemodynamic status and acute cardiac adaptations (for example, right ventricular dysfunction [RVD]), pre‐existing comorbidities, method of treatment and adverse events 5 , 6 . Diabetes is known as a relevant cardiovascular risk factor 7 , 8 that has a tremendous impact on various organ systems, including the heart 9 and the coagulation system, inducing a shift from normal hemostasis to a hypercoagulable state with prothrombotic characteristics 10 , 11 , 12 . Various mechanisms have been proposed to explain the prothrombotic state in impaired glucose metabolism, including transcription of coagulation factors caused by hyperglycemia‐induced oxidative stress, loss of the endothelial glycocalyx layer that harbors coagulation factors, direct activation of coagulation factors and decreased fibrinolysis 13 , 14 , 15 , 16 , 17 . In consequence, individuals with diabetes experience an elevated risk for arterial, as well as venous, thrombosis 18 , including the risk for PE 19 . Until today, data on the impact of diabetes on clinical outcome including mortality of PE patients is scarce, and the findings of available studies are controversial 20 , 21 , 22 .
In a previously published analysis of our research group on patients with PE in the German nationwide inpatient sample, more diabetes patients were observed to be present in the non‐survivor group compared with PE patients who were discharged alive. Consequently, in this previously published study, diabetes was identified as an independent predictor of in‐hospital mortality in patients with PE 3 . Due to this relevant finding and the well‐known vast impact of diabetes on the cardiovascular system, the present in‐depth study was carried out with the aim to investigate the impact of diabetes on patient profile and clinical outcome of individuals hospitalized with PE, as well as thereon the impact of special diabetes subtypes, such as type 1 diabetes, on the outcomes in this patient group.
MATERIAL AND METHODS
We analyzed all in‐patient cases with PE in Germany between the years 2005 and 2018 (source: Research Data Center of the Federal Statistical Office and the Statistical Offices of the federal states, Diagnosis Related Groups Statistics 2005–2018, and our own calculations) including PE as the main reason for admission, as well as PE not being the main reason for admission or PE developed during hospitalization. As described previously 3 , 23 , in Germany, diagnoses are coded according to the coding guidelines, International Classification of Diseases, 10th Revision with German Modification (ICD‐10‐GM), and diagnostical, surgical and interventional procedures with OPS codes (surgery, diagnostic and procedures codes [Operationen‐ und Prozedurenschlüssel]). The data from all inpatient cases in Germany, which are processed according to the diagnosis related groups system, are collected by the Federal Statistical Office of Germany (Statistisches Bundesamt, Wiesbaden, Germany).
In the present study, we included all hospitalizations of patients with a PE event, who were identified by the ICD‐code I26 between the years 2005 and 2018 in Germany. Included hospitalizations of PE patients were further stratified for the presence of diabetes (ICD‐codes E10‐E14). PE patients coded with diabetes had diabetes as a chronic disease at admission or a new onset of diabetes was diagnosed during hospitalization (main or secondary diagnosis). We compared PE patients with and without diabetes regarding patient characteristics, venous thromboembolism (VTE) risk factors, risk stratification markers for PE, comorbidities, use of revascularization treatments and adverse in‐hospital events. In addition, temporal trends were investigated.
Study end‐points and in‐hospital adverse events
The primary study outcome was defined as death of all‐causes (in‐hospital death). Furthermore, the prevalence of adverse events during in‐hospital stay, such as pneumonia (ICD‐codes J12‐J18), high‐risk PE (defined as PE patients in shock and/or PE patients, who had to undergo cardiopulmonary resuscitation), acute kidney injury (AKI, ICD‐code N17), stroke (ischemic and hemorrhagic stroke, ICD‐codes I61‐64), intracerebral bleeding events (ICD‐code I61), gastrointestinal bleeding (ICD‐codes K920‐K922) and transfusion of blood components (OPS code 8‐800), were assessed. These adverse events were defined according to the relevant current guidelines 24 , 25 , 26 , 27 , 28 , 29 , and occurred during or shortly before the hospitalization and were treated during hospitalization.
Ethical aspects
In accordance with German law, approval by an ethics committee and informed consent were not required, as the present study did not involve direct access of the investigators to data of individual patients. The present analysis was carried out on our behalf by the Research Data Center of the Federal Statistical Office and the Statistical Offices of the federal states. Aggregated statistic results were provided on the basis of SPSS® codes (IBM SPSS® Statistics for Windows, version 20.0; IBM Corp. Armonk, NY, USA), which were supplied to the Research Data Center 3 .
Statistical analysis
Regarding baseline comparisons, comparison of treatments, and adverse outcomes of PE patients with and without diabetes, descriptive statistics are shown as the median and interquartile range (IQR) or absolute numbers and corresponding percentages. Continuous variables were assessed with the Mann–Whitney U‐test, and categorical variables using the Fisher’s exact or the χ2‐test, as appropriate.
The hospitalization rate of PE patients with and without diabetes as well as mortality rate (case‐fatality rate), revascularization treatments carried out (systemic thrombolysis [OPS code 8‐020.8] and/or surgical embolectomy [OPS code 5‐380.42]) and the rate of adverse in‐hospital events, were calculated on an annual basis. Linear regressions were used to assess trends over time, and the results are given as beta (β) with the corresponding 95% confidence intervals (CI).
The investigation of the impact of diabetes, including type 1 diabetes mellitus and type 2 diabetes, on in‐hospital events and in‐hospital death among PE patients was carried out using univariate and multivariate logistic regression models given as odds ratio (OR) and 95% CI. Logistic regression analysis is widely used in epidemiological studies concerned with quantifying an association between a study factor (i.e., an exposure variable) and a health outcome (i.e., disease status, binary data) 30 .
The multivariate regression models were adjusted for.
Adjustment I: age, sex, cancer, heart failure, coronary artery disease, peripheral artery disease, chronic obstructive pulmonary disease, essential arterial hypertension, acute and chronic kidney disease, atrial fibrillation/flutter, and hyperlipidemia.
Adjustment II: age, sex, cancer, heart failure, coronary artery disease, peripheral artery disease, chronic obstructive pulmonary disease, essential arterial hypertension, acute and chronic kidney disease, atrial fibrillation/flutter, hyperlipidemia, and anemia.
This epidemiological approach regarding the adjustment was chosen to prove the widespread independence of these outstanding predictors of case fatality rate during hospitalization. Statistical significance was assumed in case of P‐value <0.05 (two‐sided). Statistical analyses were carried out with the software SPSS® (IBM SPSS® Statistics for Windows, version 20.0; : IBM Corp. Armonk, NY, USA).
RESULTS
The present study comprised 1,174,196 hospitalizations (53.8% aged ≥70 years, 53.5% women) of patients with PE in Germany during the years 2005–2018. Overall, 3.9% presented with shock, 28.5% had a RVD and 15.8% died in‐hospital. In general, 219,550 (18.7%) patients were coded with diabetes. The in‐hospital fatality rate was higher among patients with diabetes (43,411 [19.8%]) compared with those without diabetes (141,606 [14.8%]; Figure S1).
Clinical profile of PE patients with and without diabetes
PE patients with diabetes were older (75.0 vs 71.0 years, P < 0.001), more often women (54.6 vs 53.2%, P < 0.001) and required a prolonged in‐hospital stay (11.0 vs 9.0 days, P < 0.001) compared with those without diabetes. Diabetes patients showed an aggravated cardiovascular risk profile with an approximately doubled prevalence of obesity and hyperlipidemia, as well as a 1.5‐fold higher occurrence of arterial hypertension. Also, comorbidities were considerably more prevalent in diabetes patients with approximately doubled prevalence of coronary artery disease, tripled occurrence of peripheral artery disease and 1.5–2‐fold more heart failure, chronic obstructive pulmonary disease and atrial fibrillation/flutter. Anemia was 1.4‐fold more prevalent in PE patients with diabetes compared with those without (Table 1). Regarding risk factors for VTE, more often surgery had been carried out in diabetes patients (55.4 vs 49.8%, P < 0.001), whereas more patients without diabetes were affected by cancer (19.8 vs 20.2%, P < 0.001) and thrombophilia (0.7 vs 1.2%, P < 0.001). The Charlson Comorbidity Index was substantially higher in PE patients with diabetes than those without diabetes (6.0 [5.0–8.0] vs 4.0 [2.0–6.0], P < 0.001; Table 1).
Table 1.
Patients' characteristics, medical history, presentation and outcomes of the included 1,174,196 pulmonary embolism patients stratified according the presence of diabetes
| Parameters | PE patients with diabetes (n = 219,550; 18.7%) | PE patients without diabetes (n = 954,646; 81.3%) | P‐value |
|---|---|---|---|
| Age (years) | 75.0 (67.0–81.0) | 71.0 (58.0–79.0) | <0.001 |
| Age ≥70 years | 144,159 (65.7%) | 487,382 (51.1%) | <0.001 |
| Female sex* | 119,895 (54.6%) | 507,725 (53.2%) | <0.001 |
| In‐hospital stay (days) | 11.0 (7.0–19.0) | 9.0 (5.0–15.0) | <0.001 |
| Diabetes subtypes | |||
| Type 1 diabetes | 3,540 (1.6%) | ||
| Type 2 diabetes | 208,996 (94.9%) | ||
| Unknown/uncoded diabetes subtype | 7,014 (3.2%) | ||
| Traditional cardiovascular risk factors | |||
| Obesity | 36,407 (16.6%) | 76,087 (8.0%) | <0.001 |
| Essential arterial hypertension | 127,266 (58.0%) | 382,061 (40.0%) | <0.001 |
| Hyperlipidemia | 43,415 (19.8%) | 99,272 (10.4%) | <0.001 |
| Classical risk factors for venous thromboembolism | |||
| Cancer | 43,475 (19.8%) | 193,023 (20.2%) | <0.001 |
| Surgery | 121,631 (55.4%) | 475,276 (49.8%) | <0.001 |
| Thrombophilia | 1,443 (0.7%) | 11,842 (1.2%) | <0.001 |
| Comorbidities | |||
| Coronary artery disease | 51,004 (23.2%) | 110,384 (11.6%) | <0.001 |
| Heart failure | 71,280 (32.4%) | 183,728 (19.2%) | <0.001 |
| Peripheral artery disease | 13,299 (6.1%) | 20,346 (2.1%) | <0.001 |
| Atrial fibrillation/flutter | 47,941 (21.8%) | 130,898 (13.7%) | <0.001 |
| Chronic obstructive pulmonary disease | 29,779 (13.6%) | 90,808 (9.5%) | <0.001 |
| Acute and chronic kidney disease | 78,013 (35.5%) | 168,251 (17.6%) | <0.001 |
| Renal insufficiency (comprised diagnosis of chronic renal insufficiency stages 3–5 with glomerular filtration rate <60 mL/min/1.73 m2) | 44,322 (20.2%) | 81,112 (8.5%) | <0.001 |
| Anemia | 42,437 (19.3%) | 136,157 (14.2%) | <0.001 |
| Charlson comorbidity index | 6.0 (5.0–8.0) | 4.0 (2.0–6.0) | <0.001 |
| Risk stratification markers and presence of DVT | |||
| High‐risk PE (PE with hemodynamical instability) † | 25,514 (11.6%) | 79,845 (8.3%) | <0.001 |
| Right ventricular dysfunction | 69,912 (31.8%) | 264,910 (27.7%) | <0.001 |
| Shock | 11,344 (5.2%) | 34,294 (3.6%) | <0.001 |
| Syncope | 5,950 (2.7%) | 22,220 (2.3%) | <0.001 |
| Tachycardia | 7,236 (3.3%) | 26,291 (2.8%) | <0.001 |
| Deep venous thrombosis or thrombophlebitis | 73,615 (33.5%) | 367,838 (38.5%) | <0.001 |
| Reperfusion treatments | |||
| Systemic thrombolysis | 10,439 (4.8%) | 38,719 (4.1%) | <0.001 |
| Surgical embolectomy | 335 (0.2%) | 1,446 (0.2%) | 0.904 |
| Adverse events during hospitalization | |||
| In‐hospital death | 43,411 (19.8%) | 141,606 (14.8%) | <0.001 |
| Cardiopulmonary resuscitation | 18,736 (8.5%) | 59,278 (6.2%) | <0.001 |
| Pneumonia | 50,339 (22.9%) | 224,776 (23.5%) | <0.001 |
| Acute kidney injury | 21,045 (9.6%) | 48,605 (5.1%) | <0.001 |
| Stroke (ischemic or hemorrhagic) | 8,336 (3.8%) | 24,854 (2.6%) | <0.001 |
| Intracerebral bleeding | 1,579 (0.7%) | 5,512 (0.6%) | <0.001 |
| Gastrointestinal bleeding | 4,121 (1.9%) | 13,060 (1.4%) | <0.001 |
| Transfusion of blood constituents | 32,370 (14.7%) | 103,555 (10.8%) | <0.001 |
Information available for 1,174,135 patients.
High‐risk pulmonary embolism (PE) is defined as PE patients in shock and/or PE patients, who had to undergo cardiopulmonary resuscitation. DVT, deep venous thrombosis or thrombophlebitis of the leg veins;
Statistical significance was assumed in case of P‐value <0.05 (two‐sided) (in bold).
Clinical manifestation, treatment and in‐hospital adverse events of PE patients with and without diabetes
Compared with PE patients without diabetes, PE patients with diabetes showed a higher prevalence of typical risk stratification markers, such as RVD (31.8 vs 27.7%, P < 0.001), tachycardia (3.3 vs 2.8%, P < 0.01) and syncope (2.7 vs 2.3%, P < 0.001). PE patients with diabetes showed a substantially higher rate of patients with high‐risk PE (PE with hemodynamical instability, defined as PE patients in shock and/or PE patients, who had to undergo cardiopulmonary resuscitation) in comparison with PE patients without diabetes (11.6 vs 8.3%, P < 0.001); consequently, systemic thrombolysis was more often carried out in diabetes patients (4.8 vs 4.1%, P < 0.001), whereas the frequency of surgical embolectomy was similar between both patient groups (both 0.2%). Individuals with diabetes suffered far more often from adverse in‐hospital events, including the need for cardiopulmonary resuscitation (CPR; 8.5 vs 6.2%, P < 0.001), the rate of AKI (9.6 vs 5.1%, P < 0.001) and ischemic or hemorrhagic stroke (3.8 vs 2.6%, P < 0.001), whereas pneumonia was more prevalent in patients without diabetes (22.9 vs 23.5%, P < 0.001). All investigated bleeding complications comprising intracerebral (0.7 vs 0.6%, P < 0.001) and gastrointestinal bleeding (1.9 vs 1.4%, P < 0.001), as well as the need for transfusion of blood constituents, more frequently occurred in PE patients with diabetes. Remarkably, in‐hospital mortality was 1.34‐fold higher in patients with diabetes compared with patients without diabetes (19.8 vs 14.8%, P < 0.001; Table 1). Diabetes was associated with in‐hospital mortality (adjustment I: OR 1.21, 95% CI 1.20–1.23, P < 0.001) independently of age, sex and comorbidities (Table 2). Although diabetes affected the frequency of RVD (adjustment I: OR 1.09, 95% CI 1.08–1.10, P < 0.001), high‐risk PE (adjustment I: OR 1.22, 95% CI 1.21–1.24, P < 0.001) and shock (adjustment I: OR 1.12, 95% CI 1.10–1.15, P < 0.001), tachycardia as well as syncope were not independently more often present in patients with diabetes (Table 2). All investigated bleeding events, including intracerebral (adjustment I: OR 1.19, 95% CI 1.12–1.26, P < 0.001) and gastrointestinal (adjustment I: OR 1.11, 95% CI 1.07–1.15, P < 0.001), as well as necessity for blood transfusions (adjustment I: OR 1.15, 95% CI 1.14–1.17, P < 0.001) were associated with diabetes in PE patients, maybe in part driven by the higher use of systemic thrombolysis (4.8 vs 4.1%, P < 0.001; adjustment I: OR 1.18, 95% CI 1.15–1.21, P < 0.001; Table 2). Diabetes was further associated with an increased risk for CPR (adjustment I: OR 1.26, 95% CI 1.24–1.29, P < 0.001) and stroke events (adjustment I: OR 1.28, 95% CI 1.24–1.31, P < 0.001) (Table 2). These results of the multivariate regression models for the association of diabetes with the different end‐points remained stable after additional adjustment for anemia (adjustment II; Table 2).
Table 2.
Impact of diabetes on the different adverse in‐hospital events in pulmonary embolism patients (univariable and multivariable logistic regression models)
| Univariable regression model | Multivariable regression model (adjustment I)* | Multivariable regression model (adjustment II)† | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| In‐hospital death | 1.42 (1.40–1.43) | <0.001 | 1.21 (1.20–1.23) | <0.001 | 1.20 (1.19–1.22) | <0.001 |
| Cardiopulmonary resuscitation | 1.41 (1.39–1.43) | <0.001 | 1.26 (1.24–1.29) | <0.001 | 1.24 (1.22–1.26) | <0.001 |
| Syncope | 1.17 (1.14–1.20) | <0.001 | 1.02 (0.99–1.05) | 0.206 | 1.02 (0.99–1.05) | 0.251 |
| Tachycardia | 1.20 (1.17–1.24) | <0.001 | 1.01 (0.99–1.04) | 0.379 | 1.00 (0.98–1.03) | 0.824 |
| Right ventricular dysfunction | 1.22 (1.20–1.23) | <0.001 | 1.09 (1.08–1.10) | <0.001 | 1.09 (1.07–1.10) | <0.001 |
| Pneumonia | 0.97 (0.96–0.98) | <0.001 | 0.93 (0.91–0.94) | <0.001 | 0.92 (0.91–0.93) | <0.001 |
| Deep venous thrombosis or thrombophlebitis | 0.81 (0.80–0.81) | <0.001 | 0.92 (0.91–0.93) | <0.001 | 0.93 (0.92–0.94) | <0.001 |
| Acute kidney injury | 1.98 (1.94–2.01) | <0.001 | – | – | – | – |
| High‐risk PE (PE with hemodynamical instability) ‡ | 1.44 (1.42–1.46) | <0.001 | 1.22 (1.21–1.24) | <0.001 | 1.20 (1.18–1.22) | <0.001 |
| Shock | 1.46 (1.43–1.49) | <0.001 | 1.12 (1.10–1.15) | <0.001 | 1.10 (1.07–1.12) | <0.001 |
| Stroke (ischemic or hemorrhagic) | 1.48 (1.44–1.51) | <0.001 | 1.28 (1.24–1.31) | <0.001 | 1.26 (1.23–1.29) | <0.001 |
| Intracerebral bleeding | 1.25 (1.18–1.32) | <0.001 | 1.19 (1.12–1.26) | <0.001 | 1.16 (1.10–1.23) | <0.001 |
| Gastrointestinal bleeding | 1.38 (1.33–1.43) | <0.001 | 1.11 (1.07–1.15) | <0.001 | 1.05 (1.02–1.09) | 0.006 |
| Transfusion of blood constituents | 1.42 (1.40–1.44) | <0.001 | 1.15 (1.14–1.17) | <0.001 | 1.06 (1.03–1.08) | <0.001 |
| Systemic thrombolysis | 1.18 (1.16–1.21) | <0.001 | 1.18 (1.15–1.21) | <0.001 | 1.17 (1.14–1.20) | <0.001 |
| Surgical embolectomy | 1.01 (0.89–1.14) | 0.904 | 0.99 (0.87–1.12) | 0.841 | 0.90 (0.80–1.02) | 0.112 |
Adjustment I: Adjusted for age, sex, cancer, heart failure, coronary artery disease, peripheral artery disease, chronic obstructive pulmonary disease, essential arterial hypertension, acute and chronic kidney disease, atrial fibrillation/flutter, and hyperlipidemia.
Adjustment II: adjusted for age, sex, cancer, heart failure, coronary artery disease, peripheral artery disease, chronic obstructive pulmonary disease, essential arterial hypertension, acute and chronic kidney disease, atrial fibrillation/flutter, and hyperlipidemia, anemia.
High‐risk pulmonary embolism (PE) is defined as PE patients in shock and/or PE patients who had to undergo cardiopulmonary resuscitation.
Statistical significance was assumed in case of P‐value <0.05 (two‐sided) (in bold).
In PE patients with diabetes, heart failure (univariate: OR 1.63, 95% CI 1.59–1.66, P < 0.001; adjustment I: OR 1.32, 95% CI 1.29–1.35, P < 0.001; adjustment II: OR 1.30, 95% CI 1.27–1.33, P < 0.001), anemia (univariate: OR 1.87, 95% CI 1.83–1.92, P < 0.001; adjustment I: OR 1.61, 95% CI 1.57–1.65, P < 0.001) and the Charlson Comorbidity Index (univariate: OR 1.18, 95% CI 1.18–1.19] P < 0.001) were associated with increased in‐hospital mortality.
Temporal trends of hospitalization, accompanying diseases, revascularization treatment, bleeding and case ‐fatality rate in PE patients with diabetes
Hospitalized PE patients with diabetes increased over time (Figure 1a) from 12,368 (17.6% of all PE patients annually) in the year 2005, to 17,447 (18.4% of all PE patients of this year) in 2018 (β 457, 95% CI 378–535, P < 0.001). Additionally, total numbers of PE patients with diabetes increased with age, showing a peak in the 8th decade of life (Figure 1c; β 0.24, 95% CI 0.23–0.25, P < 0.001).
Figure 1.
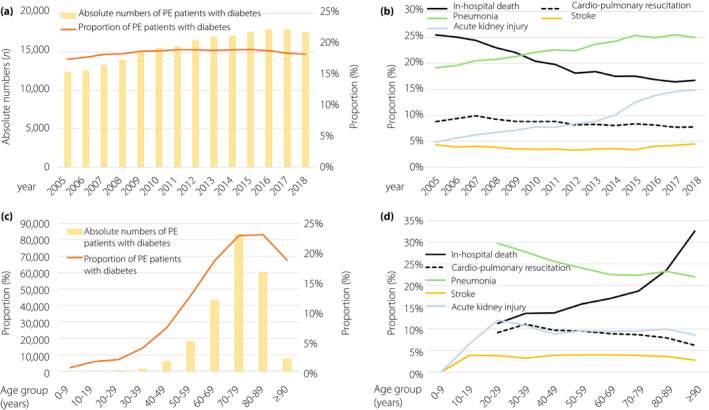
Temporal trends regarding absolute numbers and relative rate of pulmonary embolism (PE) with diabetes and adverse outcomes. (a) Temporal trends regarding absolute numbers of PE with diabetes (yellow bars) and proportion of PE patients with diabetes related to all PE patients (orange line) stratified for treatment year. (b)Temporal trends regarding rates of in‐hospital mortality (solid black line), cardiopulmonary resuscitation (dashed black line), pneumonia (green line), stroke (yellow line) and acute kidney injury (blue line) stratified for treatment year. (c) Temporal trends regarding absolute numbers of PE patients with diabetes (yellow bars), proportion of PE patients with diabetes related to all PE patients (orange line) and proportion of PE patients with type 1 diabetes related to all PE patients (grey line) stratified for age decade. (d) Temporal trends regarding rates of in‐hospital mortality (solid black line), cardiopulmonary resuscitation (dashed black line), pneumonia (green line), stroke (yellow line) and acute kidney injury (blue line) stratified for age decade.
Fortunately, in‐hospital mortality decreased from 25.5% in the year 2005 to 16.8% in the year 2018 among PE patients with diabetes (β −0.73, 95% CI −0.77 to −0.69] P < 0.001; Figure 1b, Table S1). With higher age, a rise of in‐hospital mortality was observed, whereas the rates of pneumonia, CPR, stroke and AKI declined (Figure 1d). The portion of women increased during the observational period in parallel with cardiac risk factors, comorbidities (Figures S1 and S3) and the prevalence of deep venous thrombosis or thrombophlebitis of the leg veins (2005: 32.1 vs 2018: 33.1%, P < 0.001), whereas the rate of RVD decreased (40.9 vs 25.8%, P < 0.001) in the same period. The proportion of PE patients with diabetes in the high‐risk status increased slightly from 2005 to 2018 (2005: 11.0% to 2018: 12.1%; β 0.08, 95% CI 0.03 to 0.13, P = 0.003), but decreased with increasing age (3rd decade: 16.5% to >9th decade: 8.3%; β −0.13, 95% CI −0.14 to −0.11, P < 0.001; Figure S3, Table S1). With rising age, the rate of deep venous thrombosis or thrombophlebitis of the leg veins and shock decreased from the third to the greater than ninth decade, whereas regarding the prevalence of RVD, as well as syncope, a small increase could be detected (Figure S4, Table S1). Within the investigated time period, the rates of pneumonia (β 0.45, 95% CI 0.41–0.49, P < 0.001), stroke and AKI increased, whereas the need for CPR (β −0.27, 95% CI −0.33 to −0.21, P < 0.001) decreased (Figures 1b, 2a; Table S1).
Figure 2.
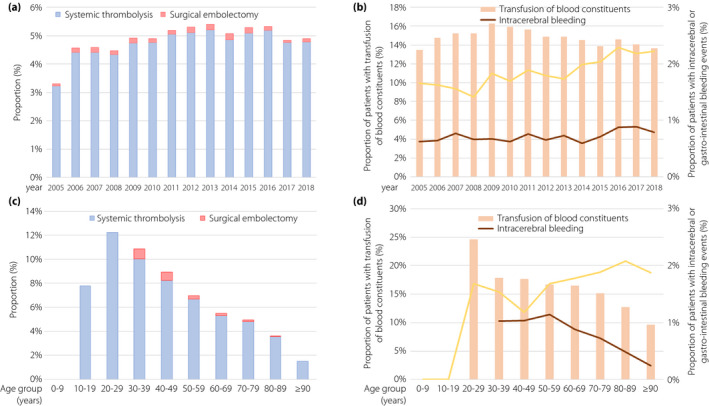
Temporal trends regarding revascularization treatments and bleeding. (a) Temporal trends regarding systemic thrombolysis (blue bars) and surgical embolectomy (red bars) in pulmonary embolism (PE) patients with diabetes stratified for treatment year. (b) Temporal trends regarding transfusion of blood constituents (red bars), intracerebral bleeding (red line) and gastrointestinal bleeding (yellow line) in PE patients with diabetes stratified for treatment year. (c) Temporal trends regarding systemic thrombolysis (blue bars) and surgical embolectomy (red bars) in diabetes patients with diabetes stratified for age decade. (d) Temporal trends regarding transfusion of blood constituents (red bars), intracerebral bleeding (red line) and gastrointestinal bleeding (yellow line) in PE patients with diabetes stratified for age decade.
The use of revascularization of PE patients with diabetes increased from 2005 to 2018 (Figure 2a), whereby only an increase of systemic thrombolysis (β 0.26, 95% CI 0.19–0.34, P < 0.001) was present with unchanged rates of surgical embolectomy (Table S1). In line with these findings, an increase of intracerebral (β 0.30, 95% CI 0.10–0.49, P = 0.003) and gastrointestinal (β 0.48, 95% CI 0.36–0.60, P < 0.001) bleeding was present (Figure 2b, Table S1). Revascularization was predominantly carried out in younger patients (peak decade 20–29 years with a revascularization rate of 12.3%), and declined with increasing age (Figure 2c). Concomitant to revascularization, the need for transfusion of blood constituents was highest in patients aged 20–29 years, but intracerebral bleeding occurred most frequently in patients aged between 30 and 59 years. In contrast, the portion of gastrointestinal bleeding events was 1.5% in patients aged 20–29 years, with rising prevalence in older age groups (Figure 2d).
Impact of diabetes subtypes on adverse in‐hospital events in patients with PE
Among the PE patients with diabetes, 3,540 (1.6%) were coded with type 1 diabetes , 208,996 (94.9%) with type 2 diabetes, and in 7,014 (3.2%) patients, the subtype was unknown or not coded (Table 1). The proportion of PE patients with type 1 diabetes subtype decreased over time (β −0.99, 95% CI −1.12 to −0.86, P < 0.001) and with increasing age (β −1.10, 95% CI −1.14 to −1.06, P < 0.001; Figure 3; Table S1).
Figure 3.
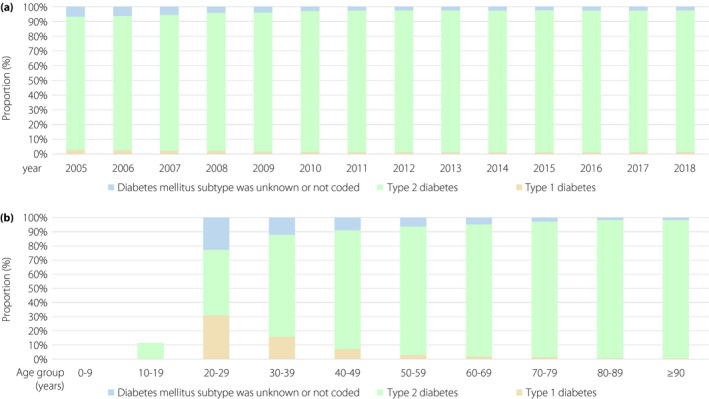
Temporal trends regarding diabetes subtypes. (a) Temporal trends regarding diabetes subtypes stratified for treatment year. (b) Temporal trends regarding diabetes subtypes stratified for age decade.
The proportion of PE patients aged ≥70 years was substantially higher in patients with type 2 diabetes than PE patients without diabetes and compared with those with type 1 diabetes (Table S2). Traditional cardiovascular risk factors, as well as atherosclerotic diseases, such as coronary artery disease and peripheral arterial disease, but also heart failure, were all more prevalent in PE patients with both diabetes subtypes. PE patients with type 1 diabetes (15.7 vs 8.3%, P < 0.001), as well as PE patients with type 2 diabetes (11.4 vs 8.3%, P < 0.001) presented more often with a high‐risk PE compared with PE patients without diabetes (Table S2). Consequently, patients with type 1 diabetes, as well as patients with type 2 diabetes were more frequently treated with systemic thrombolysis. The Charlson Comorbidity Index was higher in both subgroups of diabetes patients. PE patients with a type 1 diabetes died more often in hospital (22.1 vs 14.8%, P < 0.001), and showed a higher number of strokes, bleeding and acute kidney injury. Similarly, PE patients with type 2 diabetes showed a higher mortality rate compared with PE patients without diabetes (19.6 vs 14.8%, P < 0.001). Remarkably, the in‐hospital mortality of PE patients with type 1 diabetes was significantly higher than in the type 2 diabetes subgroup (P < 0.001; Table S2).
The vast clinical impact of type 1 diabetes mellitus in PE patients was emphasized by multivariate regression analyses: patients with type 1 diabetes mellitus were independently associated with a significantly increased risk for in‐hospital death (adjustment I: OR 1.91, 95% CI 1.75–2.09, P < 0.001), high‐risk PE (adjustment I: OR 1.84, 95% CI 1.67–2.03, P < 0.001) and RVD (adjustment I: OR 1.13, 95% CI 1.04–1.22, P = 0.003) as well as intracerebral bleeding (adjustment I: OR 1.69, 95% CI 1.21–2.37, P = 0.002) in comparison with PE patients without diabetes (Table S3).
The impact of type 2 diabetes on the in‐hospital outcomes was less strong: PE patients with type 2 diabetes were independently associated with increased mortality risk (adjustment I: OR 1.19, 95% CI 1.18–1.21, P < 0.001), high‐risk PE (adjustment I: OR 1.19, 95% CI 1.17–1.21, P < 0.001) and all investigated bleeding complications compared with PE patients without diabetes (Table S4).
DISCUSSION
In the present study assessing >1.1 million hospitalizations of patients with PE, vast differences regarding cardiovascular profile and adverse in‐hospital mortality between PE patients with and without diabetes were identified.
The results can be summarized as follows. First, diabetes patients were 4 years older (in median), and showed an unbeneficial clinical profile with a higher prevalence of cardiovascular risk factors and atherosclerotic comorbidities compared with non‐diabetes patients. Second, regarding typical VTE risk factors, diabetes patients were more often operated on, whereas cancer and thrombophilia were more prevalent in non‐diabetes patients. Third, patients with diabetes were more often afflicted with risk stratification markers, such as RVD, tachycardia and syncope, and were more often hemodynamically unstable. Consequently, treatment with systemic thrombolysis was more often administered in patients with diabetes, and higher rates of intracerebral and gastrointestinal bleeding complications were detected. Fourth, in‐hospital mortality was substantially elevated by approximately 30% compared with non‐diabetes patients, but declined from 25.5 to 16.8% within the investigated time period. Fifth, remarkably, in the present study, type 1 diabetes was identified as a strong risk factor independently associated with a higher occurrence of shock, stroke, RVD, CPR and in‐hospital death in hospitalized PE patients, despite the younger age of this patient group in comparison with the other PE patients with diabetes.
The annual incidence of VTE in patients with diabetes was estimated as 0.12% 19 , 31 . Nevertheless, data on the impact of diabetes on the occurrence of PE remain controversial in the literature. In several investigations, diabetes was associated with an increased risk for PE 19 , 22 , 32 , 33 , 34 , 35 , 36 , whereas in other studies, no correlation was present 37 , 38 . The Atherosclerosis Risk in Communities (ARIC) study on four communities of the USA showed a more than doubled incidence of VTE (including deep vein thrombosis and PE) in people with diabetes 32 , and an investigation of US Veteran Health Administration Hospitals reported that diabetes was identified as an independent risk factor for the development of PE 35 , which was in line with study results from Sweden 33 , Taiwan 19 , 36 , Italy 22 and previously published studies from the USA 32 . A meta‐analysis of this topic confirmed an elevated VTE risk in participants with diabetes 34 . In contrast, a large USA epidemiological study with a 25‐year observational period found no correlation between diabetes and the occurrence of VTE 37 , and another large population‐based case–control study from the Netherlands including approximately 5,000 VTE patients was also unable to confirm an association between diabetes and the occurrence of VTE 38 . In addition, a study of the US National Hospital Discharge Survey including >92 million patients investigating a follow‐up observational period of 26 years showed an increased age‐dependent VTE risk only in patients aged <59 years (with a peak in the age decade of 20–29 years) 39 . In line with these findings, in a large nationwide population‐based cohort study in Taiwan, people with diabetes were associated with an elevated prevalence for PE, with the highest risk in patients aged ≤49 years 19 . These data stand in contrast to the results of the present study, where the peak co‐prevalence of PE and diabetes was found in the eighth decade of life. Interestingly, the aforementioned large USA study further detected a similar risk for VTE in patients with type 1 diabetes and type 2 diabetes 39 . These findings are contradictory to a recent British study of >1.5 million participants with special focus on this issue in which an elevated risk for VTE was especially found in patients with type 1 diabetes, but not in patients with type 2 diabetes 40 . In addition, the impact of type 1 diabetes on the occurrence of VTE was recently confirmed by a National Health Insurance Research Database study from Taiwan 36 . Importantly, antidiabetes treatments are also associated with VTE occurrence 31 . In particular, dipeptidyl peptidase‐4 inhibitors were found to be associated with an elevated occurrence of VTE (OR 2.0, 95% CI 1.7–2.3) compared with other non‐insulin glucose‐lowering drugs 31 .
Besides the association of diabetes with the development of VTE, diabetes is a well‐recognized important driver for cardiovascular morbidity and mortality 7 , 41 , 42 , 43 , 44 , 45 . Nevertheless, data on the impact of diabetes on mortality and other adverse outcomes of PE patients are sparse and contradictory 20 , 21 , 22 . Although, in accordance with the present results, the majority of studies showed an increased risk of adverse outcomes and especially mortality among PE patients with diabetes, other studies were not able to confirm this finding. Diabetes is associated with higher prevalences of other cardiovascular risk factors, such as arterial hypertension and dyslipidemia, often leading to the metabolic syndrome. Furthermore, diabetes promotes various morbidities, such as coronary artery disease, atrial fibrillation, kidney disease and peripheral artery disease 46 . Also, in the present study, patients with diabetes showed a crucially unbeneficial clinical profile, but additionally, diabetes affected mortality substantially (25.5 vs 16.8%) in PE patients, and was identified as an independent risk factor for increased in‐hospital mortality with an OR of 1.21 (95% CI 1.20–1.23).
In accordance with the present results, a recently published analysis of the Spanish National Health System Hospital Discharge Database in the timescale 2016–2018 comprising 47,190 patients aged ≥40 years who were hospitalized due to PE showed that diabetes was also associated with increased in‐hospital mortality (OR 1.15, 95% CI 1.05–1.26) 21 . These results from Spain (2016–2018) confirmed a former analysis comprising data of >120,000 PE patients in which diabetes was also related to higher in‐hospital mortality (men OR 1.22, 95% CI 1.12–1.32; women OR 1.24, 95% CI 1.15–1.33) 47 . Interestingly, a study of 577 PE patients aiming to investigate sex differences in outcome also showed an elevated mortality of PE patients with diabetes, but after sex‐specific assessment, the association between diabetes and increased mortality among PE patients was attenuated in women who were associated with a doubled risk of death compared with patients without diabetes 48 . In contrast to the aforementioned studies, in a matched retrospective cohort study (RIETI registry) comprising 2,010 diabetes patients with PE who were compared with two age‐ and sex‐matched controls, diabetes was not independently associated with mortality 20 .
The association between diabetes and deep vein thrombosis is controversially discussed in the literature, varying from diabetes as a high‐risk factor to minor associated risk to no independent risk factor for deep vein thrombosis 49 . In line, a nationwide cohort study from Taiwan, for instance, found an elevated risk for patients with diabetes for developing deep vein thrombosis 19 , whereas in a German nationwide study congruent with the present results, diabetes was not associated with a higher risk for deep vein thrombosis 46 . A possible reason for this difference might lie in the differing cohort characteristics, as the Taiwan study focused on adults aged ≤49 years, whereas in the present study, the median patient age was >70 years. By all means, as proposed by Nielsen 50 , the controversy on this issue, including the present results, shows that PE and deep vein thrombosis should rather be regarded as separate clinical entities than as one consequence of the other, with differing risk factors – probably including diabetes – as suggested by the results of the present investigation and further data 46 .
Regarding the safety outcomes of the anticoagulant therapy, the present study showed higher bleeding rates in PE patients with diabetes in comparison with those without, that could only in part be explained by higher use of revascularization treatments. Diabetes was independently associated with an increased risk of intracerebral (OR 1.19, 95% CI 1.12–1.26), as well as gastrointestinal bleeding (OR 1.11, 95% CI 1.07–1.15). This finding is in line with a study from Piazza et al. 51 , who reported that patients with diabetes more likely suffer from a complicated clinical course with a higher frequency of recurrent deep vein thrombosis and major bleeding complications. Similarly, the study of Zhang et al. 52 confirmed that diabetes was an independent risk factor for major bleeding (OR 2.11, 95% CI 1.10–4.12) and clinically relevant non‐major bleeding (OR 2.11, 95% CI 1.10–4.12) in PE patients under anticoagulation therapy in a 3‐month follow‐up period.
Remarkably, although data from large studies about the impact of type 1 diabetes on adverse outcome and mortality in PE patients were missing up to now, the present study gives new insights for relevant clinical associations between both diseases. The results of the present study suggest that type 1 diabetes mellitus is a strong risk factor for adverse outcomes in PE patients. Type 1 diabetes was shown to be a substantial risk factor in the setting of PE, with higher risk for shock, stroke, RVD, CPR and in‐hospital death. In this context, studies reported that diabetes affected cardiac structure and function, and this might contribute to higher RVD rate in PE patients with diabetes 9 .
Pathophysiologically, diabetes causes endothelial dysfunction, inflammation and hypercoagulability, resulting in vascular complications and a shift to a prothrombotic state 13 . Hyperglycemia induces insulin‐independent passive glucose diffusion into endothelial cells, leading to consecutive activation of secondary metabolic pathways, which, besides other consequences, imitates the effects of hypoxia by converting nicotinamide adenine dinucleotide phosphate to oxidized nicotinamide adenine dinucleotide phosphate, causing endothelial cytotoxicity and decreased nitric oxide availability with the result of endothelial dysfunction 13 , 53 , 54 . These alterations promote both a pro‐coagulatory and pro‐inflammatory condition by endothelial cell release of thrombogenic factors, such as tromboxan A2, plasminogen activator inhibitor‐1 and von‐Willebrand factor, as well as expression of inflammatory adhesion molecules, such as P‐selectin, E‐selectin, vascular adhesion molecular‐1 and intercellular adhesion molecule‐1 13 , 55 . Oxidative stress and endothelial activation are further induced by advanced glycation end‐products, which irreversibly result from protein glycation 13 , 56 , 57 . In addition, the diabetic state promotes alterations on the fibrin network structure and causes affects fibrinolysis 15 , 58 . These hyperglycemia‐induced mechanisms orchestrate the induction of both a pro‐coagulatory and pro‐inflammatory condition, potentially leading to a pro‐thrombotic state in people with diabetes 11 , 13 , 58 .
Diabetes patients who suffer from VTE are at elevated risk for adverse events and a complicated clinical course 51 , which was also confirmed in the present study. Due to the important impact of diabetes on the risk for VTE and outcome of PE patients, including mortality and bleeding, risk stratification and identification of vulnerable patient groups are essential to optimize patient treatment in the setting of PE. For this, the development and continuous improvement of risk assessment strategies represent a vital tool aiming to identify the best individual treatment option in consideration of relevant comorbidities, such as diabetes, for each of these PE patient 6 , 59 , 60 . Beyond this, in view of the contradictory data on this issue, further investigations are required to elucidate the underlying mechanisms and impact of disturbed glucose metabolism on the generation and clinical outcome of PE. Improved understanding of the influence of diabetes on the coagulation system might help to prevent VTE events and enhance treatment modalities, as well as health consequences for affected patients. Although the adjustments of the multivariate regression models were very widely chosen, aiming to address most of the cofounders in light of the differences in PE patients’ characteristics between patients with and without diabetes, we could not be completely sure that we purposefully addressed all of these differences by these adjustments.
In summary, the present study involves by far the largest cohort, investigating the impact of diabetes on mortality within hospitalized PE patients. Diabetes was found to be independently associated with an increase of in‐hospital mortality in patients with PE. This stands in line with other findings on the influence of glucose metabolism on outcome of PE patients. In this context, a large study investigating the association between mortality and serum glucose level at admission in PE patients showed increased elevated admission glucose levels to be independently associated with increased mortality, even in patients without diabetes 61 . Furthermore, elevated admission glucose levels were shown to be associated with increased mortality in the treatment of PE patients within thrombolytic therapy 62 .
There were certain limitations of the present study, which need to be mentioned. First, the study was based on ICD and OPS discharge codes of hospitalized patients, which might lead to an under‐reporting/under‐coding. Nevertheless, the coding carried out by hospitals to receive their remuneration (in Germany, primarily carried out by physicians or administrative specialists) has to follow high‐quality standards, in order not to compromise and lower the remuneration payments. Second, detailed baseline data, such as concomitant medications, cardiac troponin plasma concentrations and echocardiographic parameters, and outcome data, such as major bleeding, were not available. Third, due to the data structure including only the in‐hospital stay, follow‐up evaluation after discharge was not possible.
Diabetes is common in patients with PE in Germany. PE patients with diabetes showed an unfavorable clinical profile, including higher prevalence of cardiovascular risk factors and atherosclerotic comorbidities, but also typical risk stratification markers, such as RVD, were found to be elevated in this patient group. In PE patients, diabetes was associated with hemodynamic instability and higher rates of adverse in‐hospital events, such as bleeding events and, in particular, increased in‐hospital mortality. Diabetes was identified as an independent and important risk factor for short‐term mortality, as well as bleeding events in PE patients. Additionally, type 1 diabetes was shown to be a strong independent risk factor in the setting of PE, with higher risk for shock, stroke, RVD, CPR and in‐hospital death. The present findings might help to identify patients with PE at higher risk for in‐hospital mortality and should draw more attention to the concomitant presence of diabetes.
DISCLOSURE
LH reports having received lecture honoraria from MSD. TG has received grant support (CARIMA study) and speaker’s honoraria from Novartis; and speaker’s honoraria from Boehringer Ingelheim, Daiichi‐Sankyo, MSD, Pfizer – Bristol‐Myers Squibb and Astra Zeneca. SVK reports having received consultancy and lecture honoraria from Bayer, Boehringer Ingelheim, Daiichi‐Sankyo, MSD and Pfizer – Bristol‐Myers Squibb; and institutional grants from Actelion, Bayer, Boehringer Ingelheim, Daiichi‐Sankyo and Pfizer – Bristol‐Myers Squibb. The other authors declare no conflict of interest.
Approval of the research protocol: N/A.
Informed consent: N/A.
Approval date of registry and the registration no. of the study/trial: N/A.
Animal Studies: N/A.
Supporting information
Figure S1 | Flowchart.
Figure S2 | Temporal trend on cardiovascular risk factors and comorbidities in pulmonary embolism patients with diabetes mellitus. (a) Temporal trends between the years 2005 and 2018. (b) Temporal trends stratified by age‐decades (cumulative 2005–2018).
Figure S3 | Temporal trends on anemia, high‐risk pulmonary embolism (PE) and heart failure. (a) Temporal trends on anemia (solid red line), high‐risk PE (solid black line) and heart failure (solid orange line) in PE patients with diabetes from 2005 to 2018 in Germany. (b) Temporal trends on anemia (solid red line), high‐risk PE (solid black line) and heart failure (solid orange line) in PE patients with diabetes mellitus stratified for age‐decades (cumulative 2005–2018).
Figure S4 | Temporal trends on risk stratification markers of acute pulmonary embolism (PE) and deep venous thrombosis or thrombophlebitis of the leg veins. (a) Temporal trends on deep venous thrombosis or thrombophlebitis of the leg veins (solid dark blue line), right ventricular dysfunction (solid grey line), shock (solid black line), tachycardia (solid light blue line) and syncope (dashed black line) in PE patients with diabetes from 2005 to 2018 in Germany. (b) Temporal trends on deep venous thrombosis or thrombophlebitis of the leg veins (solid dark blue line), right ventricular dysfunction (solid grey line), shock (solid black line), tachycardia (solid light blue line) and syncope (dashed black line) in PE patients with diabetes stratified for age decades (cumulative 2005–2016).
Table S1 | Annual time trends in pulmonary embolism patients with diabetes 2005–2018 in Germany.
Table S2 | Patients’ characteristics, medical history, presentation and outcomes of the pulmonary embolism patients stratified according the presence of diabetes subtypes 1 and 2 in comparison with pulmonary embolism patients without diabetes mellitus.
Table S3 | Impact of type 1 diabetes on the different adverse in‐hospital events in pulmonary embolism patients (univariate and multivariate logistic regression model) in comparison with pulmonary embolism patients without diabetes.
Table S4 | Impact of type 2 diabetes type on the different adverse in‐hospital events in pulmonary embolism patients (univariate and multivariate logistic regression model) in comparison with pulmonary embolism patients without diabetes mellitus.
ACKNOWLEDGMENTS
We thank the Federal Statistical Office of Germany (Statistisches Bundesamt, DEStatis) for providing the data/results and the kind permission to publish these data/results (source: Research Data Center of the Federal Statistical Office and the Statistical Offices of the federal states, Diagnosis Related Groups Statistics 2005‐2018, own calculations).
J Diabetes Investig.2022; 13: 725–737
REFERENCES
- 1. Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res 2016; 118: 1340–1347. [DOI] [PubMed] [Google Scholar]
- 2. Jiménez D, de Miguel‐Díez J, Guijarro R, et al. Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE registry. J Am Coll Cardiol 2016; 67: 162–170. [DOI] [PubMed] [Google Scholar]
- 3. Keller K, Hobohm L, Ebner M, et al. Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur Heart J 2020; 41: 522–529. [DOI] [PubMed] [Google Scholar]
- 4. Konstantinides SV, Barco S, Rosenkranz S, et al. Late outcomes after acute pulmonary embolism: rationale and design of FOCUS, a prospective observational multicenter cohort study. J Thromb Thrombolysis 2016; 42: 600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35: 69a–69k. [DOI] [PubMed] [Google Scholar]
- 6. Hobohm L, Hellenkamp K, Hasenfuss G, et al. Comparison of risk assessment strategies for not‐high‐risk pulmonary embolism. Eur Respir J 2016; 47: 1170–1178. [DOI] [PubMed] [Google Scholar]
- 7. Schmitt VH, Hobohm L, Munzel T, et al. Impact of diabetes mellitus on mortality rates and outcomes in myocardial infarction. Diabetes Metab 2021; 47: 101211. [DOI] [PubMed] [Google Scholar]
- 8. Schmitt VH, Leuschner A, Jünger C, et al. Cardiovascular profiling in the diabetic continuum: results from the population‐based Gutenberg Health Study. Clin Res Cardiol 2021. Online ahead of print. 10.1007/s00392-021-01879-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmitt VH, Billaudelle A‐M, Schulz A, et al. Disturbed glucose metabolism and left ventricular geometry in the general population. J Clin Med 2021; 10: 3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tripodi A, Branchi A, Chantarangkul V, et al. Hypercoagulability in patients with type 2 diabetes mellitus detected by a thrombin generation assay. J Thromb Thrombolysis 2011; 31: 165–172. [DOI] [PubMed] [Google Scholar]
- 11. Beijers HJBH, Ferreira I, Spronk HMH, et al. Impaired glucose metabolism and type 2 diabetes are associated with hypercoagulability: potential role of central adiposity and low‐grade inflammation–the Hoorn Study. Thromb Res 2012; 129: 557–562. [DOI] [PubMed] [Google Scholar]
- 12. Carr ME. Diabetes mellitus: a hypercoagulable state. J Diabetes Complications 2001; 15: 44–54. [DOI] [PubMed] [Google Scholar]
- 13. Domingueti CP, Dusse LM, Carvalho M, et al. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications 2016; 30: 738–745. [DOI] [PubMed] [Google Scholar]
- 14. Shi Y, Vanhoutte PM. Macro‐ and microvascular endothelial dysfunction in diabetes. J Diabetes 2017; 9: 434–449. [DOI] [PubMed] [Google Scholar]
- 15. Alzahrani SH, Ajjan RA. Coagulation and fibrinolysis in diabetes. Diab Vasc Dis Res 2010; 7: 260–273. [DOI] [PubMed] [Google Scholar]
- 16. Goligorsky MS. Vascular endothelium in diabetes. Am J Physiol Renal Physiol 2017; 312: F266–F275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldberg RB. Cytokine and cytokine‐like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab 2009; 94: 3171–3182. [DOI] [PubMed] [Google Scholar]
- 18. Yang G, Meng F, Liu Y, et al. Diabetes mellitus and risk of deep vein thrombosis after total knee replacement: a meta‐analysis of cohort studies. Int J Clin Exp Med 2015; 8: 9086–9092. [PMC free article] [PubMed] [Google Scholar]
- 19. Chung WS, Lin CL, Kao CH. Diabetes increases the risk of deep‐vein thrombosis and pulmonary embolism. A population‐based cohort study. Thromb Haemost 2015; 114: 812–818. [DOI] [PubMed] [Google Scholar]
- 20. de Miguel‐Diez J, Lopez‐de‐Andres A, Jimenez‐Trujillo I, et al. Mortality after pulmonary embolism in patients with diabetes. Findings from the RIETE registry. Eur J Intern Med 2019; 59: 46–52. [DOI] [PubMed] [Google Scholar]
- 21. Jimenez‐Garcia R, Albaladejo‐Vicente R, Hernandez‐Barrera V, et al. Type 2 diabetes is a risk factor for suffering and for in‐hospital mortality with pulmonary embolism. A population‐based study in Spain (2016‐2018). Int J Environ Res Public Health 2020; 17: 2016–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fabbian F, Gallerani M, Pala M, et al. In‐hospital mortality for pulmonary embolism: relationship with chronic kidney disease and end‐stage renal disease. The hospital admission and discharge database of the Emilia Romagna region of Italy. Intern Emerg Med 2013; 8: 735–740. [DOI] [PubMed] [Google Scholar]
- 23. Keller K, Hobohm L, Munzel T, et al. Sex‐specific differences regarding seasonal variations of incidence and mortality in patients with myocardial infarction in Germany. Int J Cardiol 2019; 287: 132–138. [DOI] [PubMed] [Google Scholar]
- 24. Konstantinides SV, Meyer G. The 2019 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2019; 40: 3453–3455. [DOI] [PubMed] [Google Scholar]
- 25. Perkins GD, Gräsner J‐T, Semeraro F, et al. European resuscitation council guidelines 2021: executive summary. Resuscitation 2021; 161: 1–60. [DOI] [PubMed] [Google Scholar]
- 26. Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from The American Heart Association/American Stroke Association. Stroke 2021; 52: e364–e467. [DOI] [PubMed] [Google Scholar]
- 27. Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost 2005; 3: 692–694. [DOI] [PubMed] [Google Scholar]
- 28. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital‐acquired and ventilator‐associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63: e61–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community‐acquired pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200: e45–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Langlois PH, Canfield MA, Swartz MD. Poisson versus logistic regression in a descriptive epidemiologic analysis of data from a Birth Defects Registry. Birth Defects Res A Clin Mol Teratol 2013; 97: 702–707. [DOI] [PubMed] [Google Scholar]
- 31. Gouverneur A, Lair A, Arnaud M, et al. DPP‐4 inhibitors and venous thromboembolism: an analysis of the WHO spontaneous reporting database. Lancet Diabetes Endocrinol 2020; 8: 365–367. [DOI] [PubMed] [Google Scholar]
- 32. Tsai AW, Cushman M, Rosamond WD, et al. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med 2002; 162: 1182–1189. [DOI] [PubMed] [Google Scholar]
- 33. Petrauskiene V, Falk M, Waernbaum I, et al. The risk of venous thromboembolism is markedly elevated in patients with diabetes. Diabetologia 2005; 48: 1017–1021. [DOI] [PubMed] [Google Scholar]
- 34. Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism: a meta‐analysis. Circulation 2008; 117: 93–102. [DOI] [PubMed] [Google Scholar]
- 35. Movahed MR, Hashemzadeh M, Jamal MM. The prevalence of pulmonary embolism and pulmonary hypertension in patients with type II diabetes mellitus. Chest 2005; 128: 3568–3571. [DOI] [PubMed] [Google Scholar]
- 36. Peng Y‐H, Lin Y‐S, Chen C‐H, et al. Type 1 diabetes is associated with an increased risk of venous thromboembolism: a retrospective population‐based cohort study. PLoS One 2020; 15: e0226997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heit JA, Leibson CL, Ashrani AA, et al. Is diabetes mellitus an independent risk factor for venous thromboembolism?: a population‐based case‐control study. Arterioscler Thromb Vasc Biol 2009; 29: 1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li‐Gao R, Morelli VM, Lijfering WM, et al. Glucose levels and diabetes are not associated with the risk of venous thrombosis: results from the MEGA case‐control study. Br J Haematol 2019; 184: 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stein PD, Goldman J, Matta F, et al. Diabetes mellitus and risk of venous thromboembolism. Am J Med Sci 2009; 337: 259–264. [DOI] [PubMed] [Google Scholar]
- 40. Hinton W, Nemeth B, Lusignan S, et al. Effect of type 1 diabetes and type 2 diabetes on the risk of venous thromboembolism. Diabetes Med 2021; 38: e14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause‐specific death. N Engl J Med 2011; 364: 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet 2010; 375: 2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Faulds MH, Dahlman‐Wright K. Metabolic diseases and cancer risk. Curr Opin Oncol 2011; 24: 58–61. [DOI] [PubMed] [Google Scholar]
- 44. Goldberg RB. Cardiovascular disease in diabetic patients. Med Clin North Am 2000; 84: 81–93. viii. [DOI] [PubMed] [Google Scholar]
- 45. Rawshani A, Rawshani A, Franzén S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 2017; 376: 1407–1418. [DOI] [PubMed] [Google Scholar]
- 46. Schmitt VH, Hobohm L, Munzel T, et al. Impact of diabetes mellitus on mortality rates and outcomes in myocardial infarction. Diabetes Metab 2020; 47: 101211. [DOI] [PubMed] [Google Scholar]
- 47. de Miguel‐Díez J, Muñoz‐Rivas N, Jiménez‐García R, et al. Type 2 diabetes is associated with a higher incidence of hospitalization for pulmonary embolism in Spain: analysis of hospital discharge data during 2004–2013. Respirology 2016; 21: 1277–1284. [DOI] [PubMed] [Google Scholar]
- 48. Oliveira D, Brito T, Elias C, et al. The influence of gender in the prognostic impact of diabetes mellitus in acute pulmonary embolism. J Clin Med 2020; 9: 3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang Y, Shi Y, Ye R, et al. Diabetes mellitus‐associated hyperglycemia is a risk factor for recurring deep vein thrombosis and post‐thrombotic syndrome‐a cohort study. Int J Clin Exp Med 2016; 9: 17739–17749. [Google Scholar]
- 50. Nielsen JD. The incidence of pulmonary embolism during deep vein thrombosis. Phlebology 2013; 28: 29–33. [DOI] [PubMed] [Google Scholar]
- 51. Piazza G, Goldhaber SZ, Kroll A, et al. Venous thromboembolism in patients with diabetes mellitus. Am J Med 2012; 125: 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang Z, Zhai Z, Yang Y, et al. Diabetes mellitus is associated with increased bleeding in pulmonary embolism receiving conventional anticoagulant therapy: findings from a "real‐world" study. J Thromb Thrombolysis 2017; 43: 540–549. [DOI] [PubMed] [Google Scholar]
- 53. Giannini C, Mohn A, Chiarelli F, et al. Macrovascular angiopathy in children and adolescents with type 1 diabetes. Diabetes Metab Res Rev 2011; 27: 436–460. [DOI] [PubMed] [Google Scholar]
- 54. Kessler L, Wiesel ML, Attali P, et al. Von Willebrand factor in diabetic angiopathy. Diabetes Metab 1998; 24: 327–336. [PubMed] [Google Scholar]
- 55. Margetic S. Inflammation and haemostasis. Biochem Med 2012; 22: 49–62. [PMC free article] [PubMed] [Google Scholar]
- 56. Wautier JL, Guillausseau PJ. Diabetes, advanced glycation endproducts and vascular disease. Vasc Med 1998; 3: 131–137. [DOI] [PubMed] [Google Scholar]
- 57. Yamagishi S, Matsui T. Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxid Med Cell Longev 2010; 3: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sobel BE, Schneider DJ. Platelet function, coagulopathy, and impaired fibrinolysis in diabetes. Cardiol Clin 2004; 22: 511–526. [DOI] [PubMed] [Google Scholar]
- 59. Becattini C, Agnelli G, Lankeit M, et al. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J 2016; 48: 780–786. [DOI] [PubMed] [Google Scholar]
- 60. Hobohm L, Becattini C, Konstantinides SV, et al. Validation of a fast prognostic score for risk stratification of normotensive patients with acute pulmonary embolism. Clin Res Cardiol 2020; 109: 1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Scherz N, Labarere J, Aujesky D, et al. Elevated admission glucose and mortality in patients with acute pulmonary embolism. Diabetes Care 2012; 35: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bozbay M, Uyarel H, Avsar S, et al. Admission glucose level predicts in‐hospital mortality in patients with acute pulmonary embolism who were treated with thrombolytic therapy. Lung 2016; 194: 219–226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Flowchart.
Figure S2 | Temporal trend on cardiovascular risk factors and comorbidities in pulmonary embolism patients with diabetes mellitus. (a) Temporal trends between the years 2005 and 2018. (b) Temporal trends stratified by age‐decades (cumulative 2005–2018).
Figure S3 | Temporal trends on anemia, high‐risk pulmonary embolism (PE) and heart failure. (a) Temporal trends on anemia (solid red line), high‐risk PE (solid black line) and heart failure (solid orange line) in PE patients with diabetes from 2005 to 2018 in Germany. (b) Temporal trends on anemia (solid red line), high‐risk PE (solid black line) and heart failure (solid orange line) in PE patients with diabetes mellitus stratified for age‐decades (cumulative 2005–2018).
Figure S4 | Temporal trends on risk stratification markers of acute pulmonary embolism (PE) and deep venous thrombosis or thrombophlebitis of the leg veins. (a) Temporal trends on deep venous thrombosis or thrombophlebitis of the leg veins (solid dark blue line), right ventricular dysfunction (solid grey line), shock (solid black line), tachycardia (solid light blue line) and syncope (dashed black line) in PE patients with diabetes from 2005 to 2018 in Germany. (b) Temporal trends on deep venous thrombosis or thrombophlebitis of the leg veins (solid dark blue line), right ventricular dysfunction (solid grey line), shock (solid black line), tachycardia (solid light blue line) and syncope (dashed black line) in PE patients with diabetes stratified for age decades (cumulative 2005–2016).
Table S1 | Annual time trends in pulmonary embolism patients with diabetes 2005–2018 in Germany.
Table S2 | Patients’ characteristics, medical history, presentation and outcomes of the pulmonary embolism patients stratified according the presence of diabetes subtypes 1 and 2 in comparison with pulmonary embolism patients without diabetes mellitus.
Table S3 | Impact of type 1 diabetes on the different adverse in‐hospital events in pulmonary embolism patients (univariate and multivariate logistic regression model) in comparison with pulmonary embolism patients without diabetes.
Table S4 | Impact of type 2 diabetes type on the different adverse in‐hospital events in pulmonary embolism patients (univariate and multivariate logistic regression model) in comparison with pulmonary embolism patients without diabetes mellitus.


