Abstract
Aims/Introduction
Sodium–glucose cotransporter 2 inhibitors (SGLT2i) were reported to increase hemoglobin levels in short‐term clinical trials. Whether it is also true in real clinical practice is unknown.
Materials and Methods
This is a retrospective cohort study. Inclusion criterion was diabetes patients who visited our outpatient clinic from January 2019 to August 2020. Exposure of interest was the use of SGLT2i. Outcomes were hemoglobin levels. For the cross‐sectional analyses, non‐linear regression models were fitted with restricted cubic splines to investigate the association between hemoglobin levels and estimated glomerular filtration rate (eGFR) for users and non‐users of SGLT2i. For the case–control study, cases (anemia defined as hemoglobin <120 g/L for men, <110 g/L for women or the use of erythropoiesis stimulating agents) and controls were matched by age, sex and eGFR.
Results
Among 2,063 diabetes patients, 723 were taking SGLT2i. In the cross‐sectional analyses, hemoglobin levels were higher among SGLT2i users compared with non‐users at eGFR >15 mL/min/1.73 m2. For the case–control study, 197 cases and controls were matched. Conditional logistic regression showed that the use of SGLT2i was associated with significantly lower prevalence of anemia (odd ratio 0.35, 95% confidence interval 0.21–0.58). Adjusted mean differences in hemoglobin levels between users and propensity score‐matched non‐users of SGLT2i were 7.0 g/L (95% confidence interval 3.0–10.0 g/L) at 6 months. Among SGLT2i users, the odds of an increase in 6‐month hemoglobin were similar across eGFR categories, except for eGFR <15 mL/min/1.73 m2.
Conclusions
The use of SGLT2i was associated with higher hemoglobin levels and lower prevalence of anemia in real clinical practice.
Keywords: Anemia, Diabetes mellitus, Sodium–glucose cotransporter 2 inhibitors
The use of sodium–glucose cotransporter 2 inhibitors was associated with higher hemoglobin levels and a lower prevalence of anemia in real‐world clinical practice, including those with advanced chronic kidney disease, active malignancy or acute illness. The results suggest that sodium–glucose cotransporter 2 inhibitors might be used for the treatment of anemia in chronic kidney disease, including patients with malignancy, and might reduce the use of high‐dose erythropoiesis‐stimulating agents or hypoxia‐inducible factor‐prolyl hydroxylase inhibitors.
![]()
INTRODUCTION
Sodium–glucose cotransporter 2 inhibitors (SGLT2i) inhibit the absorption of glucose from the proximal tubule of the kidney and, hence, cause glycosuria. They were initially developed for the treatment of diabetes. In addition to improving glycemic control, beneficial effects of these agents on cardiovascular and renal outcomes have been reported in large randomized clinical trials 1 , 2 , 3 , 4 , 5 , 6 .
SGLT2i are also reported to increase hemoglobin levels in multiple clinical trials 7 , 8 , 9 , 10 , 11 . The beneficial effects of SGLT2i on cardiovascular events seem to be at least partially mediated by their improvement of anemia 12 . However, participants in the clinical trials were relatively stable patients with good adherence. Additionally, these clinical trials excluded patients with active malignancies or advanced chronic kidney disease 7 , 8 , 9 , 10 , 11 . It is unknown whether SGLT2i improve anemia in real clinical practice in diabetes patients with various comorbidities and reduced kidney function.
In the present study, we investigated the association between the use of SGLT2i and hemoglobin levels or anemia in a cross‐sectional study and a case–control study among all diabetes patients in an outpatient setting.
MATERIALS AND METHODS
Participants
Inclusion criteria
Those with diabetes who visited the outpatient clinic of the Department of Endocrinology and Diabetes or Department of Nephrology at Nagoya City University Hospital from 1 January 2019 to 31 August 2020 were included in the study. Diabetes was defined as either taking glucose‐lowering agents or a hemoglobin A1c 6.5%.
Exclusion criteria
Those without data for estimated glomerular filtration rate (eGFR) or hemoglobin levels during the study period were excluded.
Study design
The present study was a retrospective observational study. The study protocol and waiver of consent were approved by Nagoya City University Graduate School of Medical Sciences and Nagoya City University Hospital Institutional Review Board, and the study was carried out in accordance with the Helsinki Declaration. Age, sex, smoking status, laboratory data and prescriptions were automatically extracted from electronic medical records. Other demographic details were obtained by chart review.
Exposure of interest
The exposure of interest was the use of SGLT2i. The SGLT2i available during the study period at Nagoya City University Hospital were ipragliflozin, empagliflozin, canagliflozin, dapagliflozin, tofogliflozin and luseogliflozin. Of note, during the study period, SGLT2i were not approved for patients without diabetes in Japan.
Outcomes
Outcomes were the patient’s hemoglobin level or anemic status, defined as hemoglobin <120 g/L for men and <110 g/L for women or the use of erythropoiesis‐stimulating agents (ESAs). The ESAs available at Nagoya City University Hospital during the study period were darbepoetin and epoetin β pegol. Of note, during the study period, hypoxia‐inducible factor‐prolyl hydroxylase (HIF‐PH) inhibitors were not approved for patients with non‐dialysis‐dependent chronic kidney disease (CKD) in Japan. When a patient visited our clinics multiple times during the study period, the data on the last visit were used for the cross‐sectional analyses, and the data on the last visit were used to define cases versus controls for the case–control study.
Statistical analysis
The data are shown as the number (%) or median (interquartile range). Categorical variables were compared using the χ2‐test, and continuous variables were compared using the Mann‐Whitney U‐test. Glycated albumin levels were converted to hemoglobin A1c using the following formula 13 : hemoglobin A1c (%) = 0.216 × glycated albumin (%) + 2.978. The eGFR was calculated by the formula developed for the Japanese population 14 . For the cross‐sectional analyses, non‐linear or logistic regression models were fitted with restricted cubic splines to investigate the association between hemoglobin levels or the probability of anemia and eGFR among users and non‐users of SGLT2i. The data were adjusted for age, sex, history of smoking, types of diabetes (type 1 or type 2), hospitalization during a study period, diagnosis of malignancy, and the use of angiotensin converting enzyme inhibitors and or angiotensin receptor blockers, dosage of iron supplementation, average monthly dose of darbepoetin or epoetin β pegol, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), platelet counts and white blood cell counts. The data for ferritin and transferrin saturation were available for only a small portion of participants, so MCV, MCHC, MCH and RDW were used instead as assessments of iron status. Additional adjustment for duration of diabetes, the presence of retinopathy, dipstick proteinuria levels as a categorical variable and C‐reactive protein levels (log‐transformed) were also carried out. C‐reactive protein levels and urine dipstick proteinuria levels were included in a separate model as the number of participants with both data were small. For the case–control study, cases (with anemia as defined above) and controls were matched 1:1 by age within 5 years, sex and eGFR within 1 mL/min/1.73 m2. Associations between the use of SGLT2i until the last visit and cases (anemia) were analyzed using conditional logistic regression. The data were further adjusted for the duration of diabetes, body mass index, history of smoking (missing data were treated as a separate category), comorbidities (including active or previous malignancy), medications and hemoglobin A1c level nearest the SGLT2i start time. Active malignancy was defined as treatment for malignancy (including surgery, radiation and chemotherapy) within 3 months or the presence of malignancy (before treatment, under observation or under best supportive care). Previous malignancy was defined as the presence of malignancy at any time before the last visit (those with active malignancy were also included). Missing values for body mass index and CRP were imputed by multiple imputation by a chained equation using predictive mean matching. Five imputed datasets were created. The variables included in the model were age, sex, history of smoking, cardiovascular morbidities, active and previous malignancy, the use of chemotherapy, antihypertensives, antidiabetic medications, statins, aspirin, white blood cell counts, hemoglobin levels, and platelet counts.
The following analyses were also carried out. Changes in hemoglobin levels after initiation of SGLT2i were compared with temporary changes in hemoglobin levels among non‐users who were followed up more than a year. Propensity scores for SGLT2i use were estimated by a logistic regression model. Variables included in the model were age, sex, an interaction term for age and sex, baseline eGFR, baseline hemoglobin levels, MCV, MCHC, MCH, platelet counts, RDW, history of smoking, the use of antidiabetic medications and the average dose of ESA. SGLT2i users and non‐users were matched on the logit of propensity scores (±0.25 SD). Baseline eGFR and hemoglobin levels were defined as those before and closest to the initiation of the SGLT2i for SGLT2i users and the first measurements of these values during the study period for SGLT2i non‐users. Adjusted mean values and adjusted mean differences in hemoglobin levels at 3, 6 and 12 months were estimated by analysis of covariance with baseline hemoglobin levels as a covariate. In addition, among SGLT2i users, variables associated with an increase in hemoglobin levels, defined as an increase in hemoglobin more than the mean change in hemoglobin at 6 months after initiation of SGLT2i, were examined by logistic regression analysis. Association between increase in hemoglobin at 6 months and baseline hemoglobin levels were examined by restricted cubic spline analyses. Statistical analyses were carried out using Stata version 15 (StataCorp, College Station, TX, USA).
RESULTS
Patient characteristics
Among 5,111 patients who visited the Department of Nephrology or the Department of Endocrinology and Diabetes at Nagoya City University Hospital during the study period, 2,063 patients had diabetes after exclusion of 158 with missing data for eGFR or hemoglobin (Figure S1). Among those with diabetes, 723 were taking an SGLT2i, and 581 had anemia. The most commonly used SGLT2i was canagliflozin followed by dapagliflozin and tofogliflozin (Figure S2). The demographics of patients with diabetes are shown in Table 1. SGLT2i users were significantly younger. The proportion of men was significantly higher, and the proportion of patients taking darbepoetin was significantly lower among SGLT2i users. The estimated GFR, proportion of patients with a history of smoking and proportion of patients taking iron supplementation were not significantly different between SGLT2i users and non‐users. MCV, MCH and RDW were significantly lower among SGLT2i users.
Table 1.
Demographics of patients
|
No SGLT2 inhibitors (n = 1,340) |
SGLT2 inhibitors (n = 723) |
P | |
|---|---|---|---|
| Age (years) | 72.0 (61.5–79.0) | 64.0 (52.0–74.0) | <0.001 |
| Male sex | 769 (57.4) | 460 (63.6) | 0.006 |
| Duration of diabetes (years) |
10 (2–19) n = 1,164 |
10 (4–17) n = 644 |
0.24 |
| Type 2 diabetes | 1,228 (91.6) | 701 (97.0) | <0.001 |
| Retinopathy |
286 (36.9) n = 776 |
202 (39.0) n = 518 |
0.44 |
| Malignancy | 612 (54.7) | 245 (33.9) | <0.001 |
| Hospitalization during a study period | 811 (60.5) | 413 (57.1) | 0.13 |
| Smoking |
145 (33.8) n = 429 |
89 (39.6) n = 225 |
0.32 |
| eGFR (mL/min/1.73 m2) | 63.1 (44.6–80.3) | 63.7 (41.5–81.7) | 0.98 |
| eGFR >60 | 735 (54.8) | 401 (55.5) | |
| eGFR >30 and ≤60 | 434 (32.4) | 220 (30.4) | |
| eGFR >15 and ≤30 | 72 (5.4) | 66 (9.1) | |
| eGFR ≤15 | 99 (7.4) | 36 (5.0) | |
| Dipstick proteinuria | |||
| Negative | 367 (51.8) | 281 (57.8) | 0.01 |
| (+/−) | 138 (19.5) | 63 (13.0) | |
| (+) | 100 (14.1) | 52 (10.7) | |
| (2+) | 67 (9.4) | 60 (12.3) | |
| (3+) | 37 (5.2) | 30 (6.2) | |
| n = 709 | n = 486 | ||
| Use of iron supplementation | 82 (6.1) | 53 (7.3) | 0.33 |
| Use of epoetin β pegol | 47 (3.5) | 38 (5.3) | 0.07 |
| Use of darbepoetin | 35 (2.6) | 4 (0.6) | 0.003 |
| Use of ACE‐I/ARB | 426 (31.8) | 358 (49.5) | <0.001 |
| MCV (fL) | 93.3 (89.8–96.8) | 92.3 (89.2–95.7) | <0.001 |
| MCHC (%) | 33.3 (32.8–33.7) | 33.2 (32.8–33.7) | 0.82 |
| MCH (pg) | 31.0 (29.8–32.2) | 30.8 (29.6–32.0) | 0.001 |
| RDW (%) | 46.4 (43.8–50.3) | 45.3 (42.9–48.1) | <0.001 |
| Platelet (×109/L) | 224 (178–270) | 223 (183–274) | 0.60 |
| White blood cell (×109/L) | 6.2 (5.0–7.9) | 6.5 (5.0–7.9) | 0.02 |
| C‐reactive protein (mg/L) |
2.6 (0.7–13.7) n = 686 |
1.6 (0.6–7.0) n = 279 |
0.003 |
| Ferritin (μg/L) |
135 (79–236) n = 104 |
120 (62–202) n = 81 |
0.23 |
| Transferrin saturation (%) |
28 (21–36) n = 81 |
28.5 (21.5–35.5) n = 84 |
0.60 |
| HbA1c (mmol/mol) (%) |
53 (47–62) [7.1 (6.5–7.9)] ( n = 1,239) |
56 (48–65) [7.3 (6.6–8.1)] (n = 705) |
0.01 |
Data are shown as n (%) or median (interquartile range). P‐values were by the χ2‐test or Mann‐Whitney U‐test. When some of the data are missing, the numbers of available data are shown in parentheses.
ACE‐I, angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; RDW, red cell distribution width; SGLT2, sodium–glucose cotransporter 2.
Cross‐sectional analysis
Hemoglobin levels and the probability of anemia estimated by restricted cubic spline analysis for SGLT2i users and non‐users are shown in Figure 1. The hemoglobin levels of SGLT2i users were higher at an eGFR above approximately 15 mL/min/1.73 m2, and the probability of anemia was lower at an eGFR above approximately 30 mL/min/1.73 m2. Subgroup analyses showed that the use of SGLT2i was associated with higher hemoglobin levels irrespective of the history of malignancy (P for interaction 0.67) or recent hospitalization (P for interaction 0.48; Figures S3 and S4). Further adjustment for duration of diabetes, the presence of retinopathy, proteinuria or C‐reactive protein levels did not change the results significantly (Figure S5).
Figure 1.
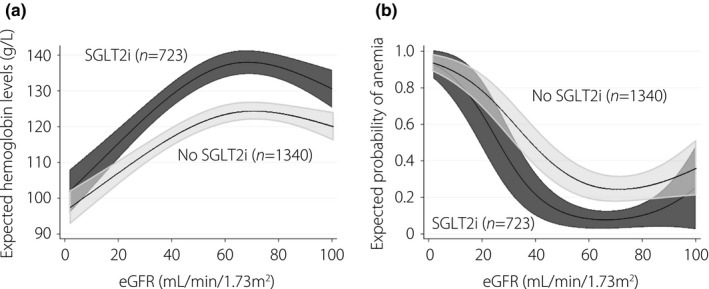
Relationship between (a) estimated glomerular filtration rate (eGFR) and hemoglobin levels and (b) probability of anemia modeled by restricted cubic splines (cross‐sectional analyses). The data were adjusted for age, sex, history of smoking, types of diabetes (type 1 or type 2), history of hospitalization during a study period, diagnosis of malignancy, use of angiotensin converting enzyme inhibitors and/or angiotensin receptor blockers, use of iron supplementation, average monthly dose of erythropoiesis stimulating agents, mean corpuscular volume, mean corpuscular hemoglobin concentration, mean corpuscular hemoglobin, red cell distribution width, white blood cell counts, and platelet counts. Anemia was defined as hemoglobin <120 g/L for men and hemoglobin <110 g/L for women or the use of erythropoiesis‐stimulating agents. SGLT2i, sodium‐glucose cotransporter 2 inhibitors.
Case–control study
A total of 197 cases and 197 controls were matched by age, sex and eGFR. The demographics of cases and controls are shown in Table S1. Age, sex and eGFR were well matched. Body mass index was significantly lower among cases. The proportion of patients with previous or active malignancy or who were on chemotherapy or diuretics was significantly higher among cases. The proportion of patients taking SGLT2i, biguanides, glucagon‐like peptide‐1 agonists or statins was significantly lower among cases. The duration of diabetes, the number of cardiovascular comorbidities and the use of other antihypertensives or glucose‐lowering agents were not significantly different. The use of an SGLT2i was associated with a significantly lower incidence of anemia (odds ratio 0.35, 95% confidence interval [CI] 0.21–0.58; Table 2) in univariate analysis. There might be potential indication biases. For example, those with malignancy and poor appetite were unlikely to be prescribed an SGLT2i, and they were more likely to have anemia. In contrast, obese patients were more likely to be prescribed an SGLT2i for weight loss, and they were more likely to have obstructive sleep apnea and high hemoglobin levels.
Table 2.
Association between the use of sodium–glucose cotransporter 2 inhibitors and anemia (case–control analyses)
| Odds ratio (95% confidence interval) | |
|---|---|
| Univariate | 0.35 (0.21–0.58) |
| Model 1 | 0.28 (0.10–0.81) |
| Model 2 | 0.22 (0.05–0.94) |
| Analysis limited to those with eGFR <60 mL/min/1.73 m2 | 0.28 (0.14–0.56) |
| Analysis limited to those with eGFR <45 mL/min/1.73 m2 | 0.30 (0.12–0.74) |
The data were analyzed using conditional logistic regression. Anemia was defined as hemoglobin <120 g/L for men and <110 g/L for women or the use of erythropoiesis stimulating agents. Model 1: the data were adjusted for duration of diabetes, body mass index, smoking, hypertension, congestive heart failure, coronary artery disease, cerebrovascular disease, peripheral arterial disease, history of malignancy, active malignancy, chemotherapy, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, β‐blockers, diuretics, calcium channel blockers, statin, aspirin, dipeptidyl peptidase‐4 inhibitor, biguanides, glucagon‐like peptide‐1 agonist, sulfonylurea, α‐glucosidase inhibitors, thiazolidinediones, glinides and insulin. Model 2: the data were adjusted for variables in model 1, mean corpuscular volume, mean corpuscular hemoglobin concentration, mean corpuscular hemoglobin, red cell distribution width, white blood cell counts and platelet counts.
eGFR, estimated glomerular filtration rate.
Adjusting for potential confounders, including malignancy and body mass index, did not substantially change the results (odds ratio 0.28, 95% CI 0.10–0.81; Table 2). Further adjustment for MCV, MCHC, MCH, RDW, white blood cell counts and platelet counts did not change the results substantially (Table 2). Limiting the analysis to those with eGFR <60 mL/min/1.73 m2 or eGFR <45 mL/min/1.73 m2 yielded similar results (Table 2). Further adjustment for CRP levels (log‐transformed) in the imputed datasets did not change the effect size of SGLT2i use significantly (data not shown).
Propensity score‐matched analyses
There were 688 participants (262 users and 426 non‐users of SGLT2i) who were followed up more than a year and who had data on hemoglobin levels before and after the initiation of the SGLT2i (for SGLT2i users) or underwent hemoglobin measurements more than once (for SGLT2i nonusers). Among them, 98 users and 98 nonusers of SGLT2i were matched by propensity score. The baseline characteristics for the propensity score‐matched cohort were well matched (Table S2). The changes in hemoglobin levels among the propensity score‐matched cohort are shown in Table 3. Hemoglobin levels gradually declined among non‐users of SGLT2i, whereas hemoglobin levels gradually increased among users of SGLT2i and plateaued at 6 months. The adjusted mean differences in hemoglobin levels were 5.0 (95% CI 1.0–8.0), 7.0 (95% CI 3.0–10.0) and 7.0 (95% CI 4.0–11.0) g/L at 3, 6 and 12 months, respectively (Table 3). Changes in hemoglobin levels for those with eGFR ≤60 mL/min/1.73 m2 and >60 mL/min/1.73 m2 are also shown in Table 3.
Table 3.
Comparison of changes in hemoglobin levels on users and non‐users of sodium–glucose cotransporter 2 inhibitors among propensity‐matched cohort (propensity score‐matched analyses)
| Mean hemoglobin levels (95% confidence interval) (g/L) † | Adjusted mean difference (95% confidence interval) | ||
|---|---|---|---|
| No SGLT2i | SGLT2i | ||
| Total | |||
| Baseline ‡ |
135 (131–140) (n = 98) |
135 (131–139) (n = 98) |
|
| 3 months § |
132 (129–134) (n = 81) |
136 (134–139) (n = 73) |
5.0 (1.0–8.0) |
| 6 months § |
131 (129–134) (n = 76) |
138 (135–140) (n = 80) |
7.0 (3.0–10.0) |
| 12 months § |
130 (128–132) (n = 84) |
137 (135–140) (n = 69) |
7.0 (4.0–11.0) |
| Those with estimated glomerular filtration rate ≤60 mL/min/1.73 m2 | |||
| Baseline ‡ |
131 (124–138) (n = 41) |
134 (127–141) (n = 39) |
|
| 3 months § |
128 (124–132) (n = 36) |
138 (132–140) (n = 32) |
7.6 (2.0–13.2) |
| 6 months § |
128 (124–132) (n = 34) |
138 (134–142) (n = 33) |
10.2 (4.1–16.3) |
| 12 months § |
127 (123–131) (n = 34) |
136 (132–141) (n = 31) |
8.9 (2.6–15.2) |
| Those with estimated glomerular filtration rate >60 mL/min/1.73 m2 | |||
| Baseline ‡ |
139 (133–144) (n = 57) |
135 (130–141) (n = 59) |
|
| 3 months § |
135 (131–138) (n = 45) |
137 (133–140) (n = 41) |
2.2 (−3.0 to 7.3) |
| 6 months § |
134 (130–137) (n = 42) |
138 (134–141) (n = 47) |
3.8 (−0.9 to 8.5) |
| 12 months § |
132 (130–135) (n = 50) |
138 (135–141) (n = 38) |
5.6 (1.5–9.7) |
The number of available data are shown in parenthesis.
The data at 3, 6, and 12 months were adjusted for baseline hemoglobin levels.
For users of sodium–glucose cotransporter 2 inhibitors (SGLT2i), hemoglobin levels before and the closest to the initiation of SGLT2i were used, and for non‐users of SGLT2i, the first measurement of hemoglobin levels during the study period were used.
For users of SGLT2i, the time from initiation of SGLT2i was used and for non‐users of SGLT2i, the time from the first measurement of hemoglobin levels was used. The data within 1 month were allowed.
Variables associated with an increase in hemoglobin levels among SGLT2i users
Among SGLT2i users, 196 participants had hemoglobin measurements at 6 months after initiation of SGLT2i. The mean increase in hemoglobin levels at 6 months was 3.0 g/L from baseline. Six months after initiation of SGLT2i, the increase in hemoglobin seemed to plateau. Associations between variables and an increase in hemoglobin >3.0 g/L at 6 months after initiation of SGLT2i are shown in Table 4. Older age, higher baseline hemoglobin levels and eGFR ≤15 mL/min/1.73 m2 compared with eGFR >60 mL/min/1.73 m2 were associated with a lower incidence of increased hemoglobin. Serum albumin levels, CRP levels and RDW were not associated with an increase in hemoglobin after initiation of SGLT2i. There were no significant differences in the odds of an increase in hemoglobin levels between different SGLT2 inhibitors. The association between baseline hemoglobin and increase in hemoglobin levels at 6 months is shown in Figure S6.
Table 4.
Variables associated with the increase in hemoglobin levels (increase in hemoglobin levels >3.0 g/L) at 6 months after initiation of sodium–glucose cotransporter 2 inhibitors
| Odds ratio (95 % confidence interval) | |
|---|---|
| Age (per year) | 0.94 (0.89–0.98) |
| Sex (male vs female) | 1.51 (0.56–4.09) |
| Baseline hemoglobin (per 10 g/L) | 0.58 (0.43–0.77) |
| Serum albumin (per 10 g/L) | 1.05 (0.48–2.28) |
| CRP, log‐transformed (per 10 mg/L) | 0.96 (0.71–1.31) |
| Estimated glomerular filtration rate | |
| >60 mL/min/1.73 m2 | 1 (Reference) |
| >30 and ≤60 mL/min/1.73 m2 | 1.21 (0.46–3.16) |
| >15 and ≤30 mL/min/1.73 m2 | 0.84 (0.14–4.92) |
| ≤15 mL/min/1.73 m2 | 0.04 (0.00–0.93) |
| Canagliflozin | 1 (Reference) |
| Empagliflozin | 1.59 (0.20–12.55) |
| Dapagliflozin | 0.38 (0.13–1.16) |
| Tofogliflozin | 0.39 (0.09–1.71) |
| Others | 1.06 (0.29–3.95) |
| Red cell distribution width (%) | 1.06 (0.96–1.16) |
The bold values indicate variables with significant association with increase in hemoglobin.
CRP, C‐reactive protein; SGLT2 inhibitors, sodium‐glucose cotransporter 2 inhibitors.
DISCUSSION
In the present study, we showed that the use of SGLT2i was associated with higher hemoglobin at an eGFR above approximately 15 mL/min/1.73 m2, and was also associated with a lower prevalence of anemia in real clinical practice. The novelty of this study is twofold: (i) the use of SGLT2i was associated with higher hemoglobin among those with advanced kidney diseases; and (ii) the use of SGLT2i was associated with a lower prevalence of anemia in real clinical practice, including in patients with active malignancy or acute illness.
The present study showed that the use of SGLT2i was associated with higher hemoglobin among diabetes patients with advanced CKD. Although multiple previous clinical trials have shown that the use of SGLT2i increases hemoglobin levels 7 , 8 , 9 , 10 , 11 , most of these studies excluded patients with eGFR <30 mL/min/1.73 m2. One study of stage 3–4 CKD 15 included just 12 patients with eGFR <30 mL/min/1.73 m2. SGLT2i were shown to increase hemoglobin levels by increasing erythropoietin synthesis 16 , 17 . Another study showed that after dapagliflozin treatment, hepcidin and ferritin levels, and transferrin saturation were reduced 17 , suggesting that SGLT2i might improve iron utilization. However, these studies were of patients with preserved renal function. In advanced kidney diseases, erythropoietin synthesis is impaired, and hepcidin levels are elevated due to chronic inflammation. It is unknown whether SGLT2i have any effect on hemoglobin among those with advanced kidney disease. The present study suggests that SGLT2i still improve anemia among those with an eGFR of 15–30 mL/min/1.73 m2. However, hemoglobin levels were less likely to increase among those with eGFR ≤15 mL/min/1.73 m2 than those with eGFR >60 mL/min/1.73 m2. This might be due to severely impaired erythropoietin synthesis. Additionally, lower MCV and MCH among SGLT2i users might suggest improved iron utilization. The results of the present study raise the possibility that SGLT2i might be used to treat anemia in CKD and might decrease the dose or use of ESAs. This might help reduce the cost for ESAs.
The present study also showed that the use of SGLT2i was associated with a lower prevalence of anemia among those with various comorbidities, including malignancy and acute illness. Clinical studies showing an increase in hemoglobin levels by SGLT2i excluded those with active malignancy within 5 years or recent cardiac events 7 , 8 , 9 , 10 , 11 . In the present case–control analysis, approximately 25% had active malignancy, and approximately 10% were receiving chemotherapy. Those with acute illness were not excluded. The mean difference in hemoglobin level between SGLT2i users and non‐users in the present study was 7.0 g/L (interquartile range 3.0–10.0 g/L), which was comparable to the difference in hemoglobin levels of 7.0–9.0 g/L between those taking SGLT2i and placebo in previous clinical trials 7 , 8 , 9 , 10 , 11 .
The present study shows the external validity of previous clinical trials showing improvement of anemia by SGLT2i. Additionally, among SGLT2i users, serum albumin, CRP and RDW were not associated with an increase in hemoglobin levels, suggesting that the use of SGLT2i is associated with an increase in hemoglobin levels irrespective of inflammation or nutritional status. Inflammation is one of the causes of ESA hyporesponsiveness 18 , 19 . SGLT2i might improve anemia in patients with ESA hyporesponsiveness due to inflammation. Concerns have been raised that HIF‐PH inhibitors might promote the progression of malignancy 20 , and that high‐dose ESAs might be associated with an increased risk of death due to malignancy 21 , 22 . However, multiple large‐scale clinical trials of SGLT2i have not reported an increase in the incidence of malignancy 1 , 2 , 3 , 4 , 5 , 6 . Several basic research studies have suggested that SGLT2i might prevent carcinogenesis or slow tumor growth 23 , 24 , 25 . Recently, the dapagliflozin and prevention of adverse outcomes in chronic kidney disease trial showed that malignancy‐related mortality was significantly lower among those taking dapagliflozin than among those taking a placebo (hazard ratio 0.42, 95% CI 0.19–0.97) 26 . These data suggest that SGLT2i could be safe for those with malignancy. The results of the present study showed that SGLT2i might improve anemia of CKD in patients with active malignancy, which would be a great help for patients with anemia of CKD or malignancy in whom the use of ESA and HIF‐PH inhibitors is restricted.
The present study had several strengths. We included a large number of diabetes patients (n = 2,063) from real‐world clinical practice. The population was representative of diabetes patients in clinical practice with a high burden of cardiovascular morbidities, as well as active malignancy or acute illness.
The limitations of the present study should also be noted. As this was an observational study, there might be residual confounding factors. Although we adjusted for potential confounders as much as possible in the case–control study, there might be an indication bias for SGLT2i prescription. Additionally, this was a single‐center study. Just 9% or 8% of patients had data on ferritin or transferrin saturation, respectively. Although we adjusted for MCV, MCH, MCHC and RDW, we might not have completely accounted for the difference in the iron status of SGLT2i users and non‐users.
In conclusion, the use of SGLT2i was associated with higher hemoglobin levels and a lower prevalence of anemia in real‐world clinical practice, including those with advanced CKD, active malignancy or acute illness. The results suggest that SGLT2i might be used for the treatment of anemia of CKD, including those with malignancy, and might reduce the use of high‐dose ESAs or HIF‐PH inhibitors.
DISCLOSURE
TH has received honorariums from Ono Pharmaceutical Co., Ltd., and grants and honorarium from Kyowa Hakko Kirin Co., Ltd., and does consultancy for Kyowa Hakko Kirin Co. Ltd. TT has received honoraria from Mitsubishi Tanabe Pharma, Daiichi Sankyo Co., Ltd., AstraZeneca, Ono Pharmaceutical Co., Ltd., Taisho Pharmaceutical Co., Ltd., Kowa Pharmaceutical Co., Ltd., Boehringer Ingelheim Japan, Eli Lilly Japan K.K. and Astellas Pharma Inc., and obtained base powder of canagliflozin from Mitsubishi Tanabe Pharma. The other authors declare no conflict of interest. Part of this work was supported by a research grant from Aichi Kidney Foundation and a grant from the Kidney Foundation, Japan (grant No. JKFB20‐5).
Approval of the research protocol: The study protocol and waiver of consent were approved by Nagoya City University Graduate School of Medical Sciences and Nagoya City University Hospital Institutional Review Board (approval number 60‐20‐0118), and the study was conducted in accordance with the Helsinki Declaration.
Informed consent: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Supporting information
Table S1 | Demographics of cases and matched controls (case control analyses).
Table S2 | Demographics of propensity score‐matched cohort (propensity score‐matched analyses).
Figure S1 | Flow chart of patient inclusion.
Figure S2 | The proportion of sodium‐glucose cotransporter 2 inhibitors used in the subjects.
Figure S3 | Relationship between estimated glomerular filtration rate and hemoglobin levels modeled by restricted cubic splines (cross‐sectional analyses) among those with and without history of malignancy.
Figure S4 | Relationship between estimated glomerular filtration rate and hemoglobin levels modeled by restricted cubic splines (cross‐sectional analyses) among those with and without recent hospitalization.
Figure S5 | Relationship between estimated glomerular filtration rate and hemoglobin levels modeled by restricted cubic splines (cross‐sectional analyses).
Figure S6 | The association between baseline hemoglobin levels and increase in hemoglobin levels.
J Diabetes Investig.2022; 13: 638–646
REFERENCES
- 1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 2. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 3. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 4. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 5. Heerspink HJL, Stefánsson BV, Correa‐Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436–1446. [DOI] [PubMed] [Google Scholar]
- 6. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagloflozin in patients with herat failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 7. Wanner C, Lachin JM, Inzucchi SE, et al. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation 2018; 137: 119–129. [DOI] [PubMed] [Google Scholar]
- 8. Ferrannini E, Ramos SJ, Salsali A, et al. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double‐blind, placebo‐controlled, phase 3 trial. Diabetes Care 2010; 33: 2217–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bailey CJ, Gross JL, Hennicken D, et al. Dapagliflozin add‐on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double‐blind, placebo‐controlled 102‐week trial. BMC Med 2013; 11: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maruyama T, Takashima H, Oguma H, et al. Canagliflozin improves erythropoiesis in diabetes patients with anemia of chronic kidney disease. Diabetes Technol Ther 2019; 21: 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oshima M, Neuen BL, Jardine MJ, et al. Effects of canagliflozin on anaemia in patients with type 2 diabetes and chronic kidney disease: a post‐hoc analysis from the CREDENCE trial. Lancet Diabetes Endocrinol 2020; 8: 903–914. [DOI] [PubMed] [Google Scholar]
- 12. Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA‐REG OUTCOME Trial. Diabetes Care 2018; 41: 356–363. [DOI] [PubMed] [Google Scholar]
- 13. Inoue K, Tsujimoto T, Yamamoto‐Honda R, et al. A newer conversion equation for the correlation between HbA1c and glycated albumin. Endocr J 2014; 61: 553–560. [DOI] [PubMed] [Google Scholar]
- 14. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 15. Dekkers CCJ, Wheeler DC, Sjöström CD, et al. Effects of the sodium‐glucose co‐transporter 2 inhibitor dapagliflozin in patients with type 2 diabetes and stage 3b–4 chronic kidney disease. Nephrol Dial Transplant 2018; 33: 2005–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heerspink HJL, de Zeeuw D, Wie L, et al. Dapagliflozin a glucose‐regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 2013; 15: 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghanim H, Abuaysheh S, Hejna J, et al. Dapagliflozin suppresses hepcidin and increases erythropoiesis. J Clin Endocrinol 2020; 105: e1056–63. [DOI] [PubMed] [Google Scholar]
- 18. Bárány P, Filho JCD, Bergström J. High C‐reactive protein is a strong predictor of resistance to erythropoietin in hemodialysis patients. Am J Kidney Dis 1997; 29: 565–568. [DOI] [PubMed] [Google Scholar]
- 19. Gunnell J, Yeun JY, Depner TA, et al. Acute‐phase response predicts erythropoietin resistance in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis 1999; 33: 63–72. [DOI] [PubMed] [Google Scholar]
- 20. Pezzuto A, Carico E. Role of HIF‐1 in cancer progression: novel insights. A review. Curr Mol Med 2018; 18: 343–351. [DOI] [PubMed] [Google Scholar]
- 21. Pfeffer MA, Burdmann EA, Chen C‐Y, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032. [DOI] [PubMed] [Google Scholar]
- 22. Bennet CL, Becker PS, Kraut EH, et al. Interesting guidelines: administering erythropoiesis‐stimulating agents to chronic kidney disease patients with cancer. Semin Dial 2009; 22: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kuang H, Liao L, Chen H, et al. Therapeutic effect of sodium glucose co‐transporter 2 inhibitor dapagliflozin on renal cell carcinoma. Med Sci Monit 2017; 23: 3737–3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jojima T, Wakamatsu S, Kase M, et al. The SGLT2 inhibitor canagliflozin prevents carcinogenesis in a mouse model of diabetes and non‐alcoholic steatohepatitis‐related hepatocarcinogenesis: association with SGLT2 expression in hepatocellular carcinoma. Int J Mol Sci 2019; 20: 5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nasiri AR, Rodrigues MR, Li Z, et al. SGLT2 inhibition slows tumor growth in mice by reversing hyperinsulinemia. Cancer Metab 2019; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heerspink HJL, Sjöström CD, Jongs N, et al. Effects of dapagliflozin on mortality in patients with chronic kidney disease: a pre‐specified analysis from the DAPA‐CKD randomized controlled trial. Eur Heart J 2021; 42: 1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Demographics of cases and matched controls (case control analyses).
Table S2 | Demographics of propensity score‐matched cohort (propensity score‐matched analyses).
Figure S1 | Flow chart of patient inclusion.
Figure S2 | The proportion of sodium‐glucose cotransporter 2 inhibitors used in the subjects.
Figure S3 | Relationship between estimated glomerular filtration rate and hemoglobin levels modeled by restricted cubic splines (cross‐sectional analyses) among those with and without history of malignancy.
Figure S4 | Relationship between estimated glomerular filtration rate and hemoglobin levels modeled by restricted cubic splines (cross‐sectional analyses) among those with and without recent hospitalization.
Figure S5 | Relationship between estimated glomerular filtration rate and hemoglobin levels modeled by restricted cubic splines (cross‐sectional analyses).
Figure S6 | The association between baseline hemoglobin levels and increase in hemoglobin levels.


