Abstract
In the present study we found that after a single oral dose of 1,800 mg of praziquantel, following a high-lipid diet and a high-carbohydrate diet, the maximum levels in plasma increased 243 and 515% and the area under the plasma concentration curve from 0 to 8 h increased 180 and 271%, respectively.
Nine healthy volunteers participated in the study. The mean age was 33.44 years (range, 26 to 47 years), and the mean weight was 72.22 ± 11.29 kg. The protocol was approved by the local ethics committee, and informed written consent was obtained from each subject after detailed explanation of the purpose and risks of the study. Subjects did not take any other medication or alcohol for at least 15 days prior to the study.
Volunteers were randomly separated into three groups of three subjects each. Group I received three tablets of 600 mg of praziquantel (1,800 mg) after 10 h of fasting; group II received the same dose of praziquantel immediately after administration of a high-fat diet, and group III received the same dose of praziquantel after a high-carbohydrate diet. Volunteers received a standard lunch 4 h after drug ingestion. The study was repeated in a crossover design allowing 1 week of washout between treatments. Blood samples were obtained through an indwelling catheter placed in the antecubital vein 0.0, 0.25, 0.5, 0.75, 1.0, 1.5, 2.0, 2.5, 3.0, 4.0, 6.0, and 8.0 h after the drug administration. Samples were centrifuged; the plasma was separated and stored at −4°C until analysis.
The high-fat diet consisted of two fried eggs, one slice of ham, orange juice, and milk (200 ml) (protein, 19.63%; fat, 32.44%; and carbohydrate, 47.91%; 656 cal); the high-carbohydrate diet consisted of four tortillas, tomato, chicken (100 g), a slice of white bread, and a glass (200 ml) of orange juice (protein, 15.30%; fat, 10.54%, and carbohydrate, 74.15%; 674.5 cal).
Praziquantel in plasma was determined using a high-performance liquid chromatography assay as previously reported (1). The method was linear from 15.6 to 8,000 ng/ml. The limit of quantitation was 15.6 ng/ml. The maximum within-day coefficient of variation was 7.9% at 15.6 ng/ml, and the mean value was 4.9% in the concentration range. Interday precision measured over four consecutive days produced coefficients of variation between 4.49 and 7.49% in the range of 15.6 to 8,000 ng/ml. The recovery ranged between 95 and 100%.
The maximum concentration of drug in plasma (Cmax) and time to attain maximum concentration in plasma (Tmax) were obtained directly from the individual plasma concentration profiles. The area under the plasma concentration curve from 0 to 8 h (AUC0–8) was calculated by applying the linear trapezoidal rule. The terminal first-order rate constant was estimated by the least-squares fit of the terminal concentration using the program Pkanalyst MicroMath Scientific Software for Windows (Salt Lake City, Utah). The mean residence time (MRT) was calculated according to the method of Yamaoka et al. (10).
The pharmacokinetic parameters were analyzed by the analysis of variance test, including effects due to sequences, subjects, periods, and treatments. The 90% confidence intervals were calculated using the BIOPAK program (version 4.0), taking fasting conditions as the reference.
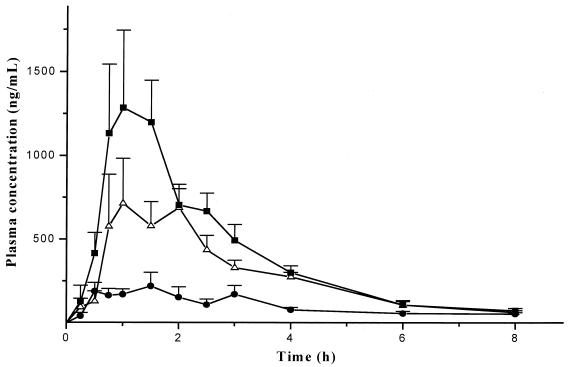
Figure 1 shows the mean plasma concentration-versus-time profiles obtained after a single oral dose of 1,800 mg of praziquantel under fasting and nonfasting conditions. Pharmacokinetic parameters for each treatment are presented in Table 1. The values for Cmax after treatments ranged from 1,544 to 4,426 ng/ml with a high-carbohydrate diet, 697 to 4,291 ng/ml with a high-lipid meal, and 407 to 1,581 ng/ml under fasting conditions. The values of MRT of fed groups were lower than the values obtained in the fasting state; however, the mean values were similar between treatments. The AUC0–8 increased in the food treatments; the relative bioavailability of the praziquantel was increased by a factor of 2.72 and 3.98 when the drug was administered with the high-lipid and high-carbohydrate diets, respectively.
FIG. 1.
Mean concentration in plasma (+standard error of the mean) of praziquantel in healthy volunteers treated with a single oral dose of 1,800 mg (three tablets of 600 mg) during fasting (●) or immediately after a high-fat (▵) or a high-carbohydrate (■) breakfast.
TABLE 1.
Pharmacokinetic parameters of praziquantel obtained after different treatmentsa
| Treatment | Parameter | Cmax (ng/ml) | AUC0–8 (ng · h ml−1) | Tmax (h) | t1/2b (h) | MRT (h) |
|---|---|---|---|---|---|---|
| Fasting | Mean (SD) | 318.81 (227.19) | 882.33 (416.79) | 1.39 (0.98) | 2.03 (0.24) | 4.39 (0.89) |
| High-fat diet | Mean (SD) | 1,095.44 (779.91) | 2,474.59 (1,165.99) | 1.94 (1.095) | 1.72 (0.18) | 3.25 (0.54) |
| Statistical comparison | P < 0.05 | P < 0.05 | NS | NS | P < 0.05 | |
| Ratio (90% CI) | 3.12 (1.36–4.88) (NE) | 2.72 (1.86–3.58) (NE) | ||||
| High-carbohydrate diet | Mean (SD) | 1,962.18 (779.76) | 3,276.20 (969.73) | 1.47 (0.64) | 1.66 (0.32) | 2.91 (0.81) |
| Statistical comparison | P < 0.05 | P < 0.05 | NS | NS | P < 0.05 | |
| Ratio (90% CI) | 5.84 (4.08–7.61) (NE) | 3.98 (3.12–4.85) (NE) |
NE, not equivalent; NS, no statistically significant difference; CI, confidence interval.
t1/2, half-life.
Our results showed that the bioavailability of praziquantel was significantly influenced by concomitant food intake. Statistically significant differences were found in Cmax and AUC0–8 between treatments, as well as in the MRT (P < 0.05); however, plasma elimination half-lives were similar to each other and comparable to those obtained previously (3, 9). When preprandial and postprandial data were compared, there were no statistically significant differences with respect to Tmax or to elimination half-lives. It therefore appears that food does not affect the elimination rate but enhances the bioavailability of praziquantel. When the 90% confidence intervals were calculated, we found that the diet exerted a significant effect. The influence was greater with carbohydrates than with lipids. In previous studies it has been suggested that food components influence the bioavailability of praziquantel. Mandour et al. found that levels in plasma increased by factors of 1.60 and 2.14 when the drug was administered with a high-oil and low-oil Sudanese meal, respectively (4). Homeida also reported a 1.7-fold increase in the Cmax when the drug was given with a standardized fatty meal (2). The increase in our subjects' plasma drug levels was greater than those obtained previously, which might be due to the differences in the composition of the diets.
The present study shows that food significantly increases the bioavailability of praziquantel. The mechanism by which food increases the bioavailability of praziquantel remains to be demonstrated. The effect could be related to tablet disintegration, better drug dissolution, or other factors, such as changes in hepatic blood flow or in the metabolism of the drug during the first passage through the liver (5–8). Considering that both treatments had a high carbohydrate content, it is possible that carbohydrates had the major influence on the bioavailability of praziquantel.
REFERENCES
- 1.González-Esquivel D, Morano C O, Sánchez R M, Sotelo J, Jung H. Sensitive high-performance liquid chromatographic assay for praziquantel in plasma, urine and liver homogenates. J Chromatogr. 1993;613:174–178. doi: 10.1016/0378-4347(93)80213-n. [DOI] [PubMed] [Google Scholar]
- 2.Homeida M, Copeland W L S, Ali M M M, Harron D W G. Pharmacokinetic interaction between praziquantel and albendazole in Sudanese men. Ann Trop Med Parasitol. 1994;88:551–559. doi: 10.1080/00034983.1994.11812903. [DOI] [PubMed] [Google Scholar]
- 3.Jung H, Medina R, Castro N, Corona T, Sotelo J. Pharmacokinetic study of praziquantel administered alone and in combination with cimetidine in a single-day therapeutic regimen. Antimicrob Agents Chemother. 1997;41:1256–1259. doi: 10.1128/aac.41.6.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandour M M, Turabi H, Homeida M M A, Sadig T, Ali H M, Bennett J L, Leahey W J, Harron D W G. Pharmacokinetics of praziquantel in healthy volunteers and patients with schistosomiasis. Trans R Soc Trop Med Hyg. 1990;84:389–393. doi: 10.1016/0035-9203(90)90333-a. [DOI] [PubMed] [Google Scholar]
- 5.Olanoff L S, Walle T, Cowart T D, Walle U K, Oexmann M J, Conradi E C. Food effects on propanolol systemic and oral clearance: support for a blood flow hypothesis. Clin Pharmacol Ther. 1986;40:408–414. doi: 10.1038/clpt.1986.198. [DOI] [PubMed] [Google Scholar]
- 6.Silberbauer C J, Jacober B, Langhans W. Dietary fat level and short-term effects of a high-fat meal on food intake and metabolism. Ann Nutr Metab. 1997;42:75–89. doi: 10.1159/000012721. [DOI] [PubMed] [Google Scholar]
- 7.Svensson C K, Edwards D J, Mauriello P M, Barde S H, Foster A C, Lanc R A, Middleton E, Lalka D. Effect of food on hepatic blood flow: implications in the “food effect” phenomenon. Clin Pharmacol Ther. 1983;34:316–323. doi: 10.1038/clpt.1983.174. [DOI] [PubMed] [Google Scholar]
- 8.Svensson C K, Mauriello P M, Barde S H, Middleton E, Lalka D. Effect of carbohydrates on estimated hepatic blood flow. Clin Pharmacol Ther. 1984;35:660–665. doi: 10.1038/clpt.1984.91. [DOI] [PubMed] [Google Scholar]
- 9.Vazquez M L, Jung H, Sotelo J. Plasma levels of praziquantel decrease when dexamethasone is given simultaneously. Neurology. 1987;37:1561–1562. doi: 10.1212/wnl.37.9.1561. [DOI] [PubMed] [Google Scholar]
- 10.Yamaoka K, Nakagawa T. Statistical moments in pharmacokinetics. J Pharmacokinet Biopharm. 1978;6:547–558. doi: 10.1007/BF01062109. [DOI] [PubMed] [Google Scholar]