Abbreviations
- AGC
advanced gastric cancer
- DIC
disseminated intravascular coagulation
- BMM
bone marrow metastasis
- HAGC
highly aggressive gastric cancer
- NAGC
normal advanced gastric cancer
- AST
glutamic oxalacetic transaminase
- ALT
glutamic‐pyruvic transaminase
- AKP
alkaline phosphatase
- CKMB
creatine phosphokinase isoenzyme
- LDH
lactate dehydrogenase
- CA199
carbohydrate antigen 199
- CEA
carcinoembryonic antigen
- WBC
white blood cells
- NLR
neutrophil to lymphocyte ratio
- CRP
C‐reactive protein
- TIME
tumor immune environment
- JAK‐STAT3
Janus Kinase‐ Signal Transducers and Activators of Transcription 3
- OS
overall survival
- BSC
best supportive care
- DFT
DIC free time
Dear Editor,
Gastric cancer (GC) is the fifth most commonly diagnosed cancer and the fourth cause of cancer‐related death worldwide [1, 2]. It has a greater prevalence in Eastern Asia, compared to other parts of the world, whereby more than two‐thirds of the cases are diagnosed as advanced gastric cancer (AGC) [3]. Disseminated intravascular coagulation (DIC) is a clinicopathologic syndrome characterized by laboratory evidence of platelets and clotting factors consumption, and proteolytic degradation. The occurrence of DIC in advanced stage diseases has been reported in several types of solid carcinomas but rarely reported in GC (∼1.6% in previous studies) [4]. AGC frequently presents with visceral metastasis to the liver and lung and less than 10% of the patients have bone metastasis [5].
The double occurrence of multiple bone marrow metastasis (BMM) and DIC in AGC is extremely rare. This type of AGC can be characterized as highly aggressive GC (HAGC) due to its highly aggressive biological behavior and very poor prognosis. Such cases are being increasingly recognized in clinical practice. During the past several decades, HAGC has been reported mainly in the form of case reports and mostly by Japanese researchers. Except for simultaneous DIC and systemic BMM of AGC, various clinical features have been scarcely described and a standard treatment has not yet been established.
In this study, from a cohort of 964 AGC patients, 36 patients (3.73%) were observed to have double occurrence of diffuse BMM and DIC, and were classified as HAGC. The remaining patients were classified as normal AGC (NAGC). The proportion of women (52.8%) was higher in the HAGC group. The median age of HAGC was 4 years younger than NAGC, but HAGC patients had poorer physical condition (Supplementary Table S1). Other than bone marrow, HAGC patients also had greater extent of distant metastasis (Supplementary Table S2). Significant difference in laboratory data between HAGC and NAGC was also observed, i.e., the level of serum glutamic oxalacetic transaminase (AST), glutamic‐pyruvic transaminase (ALT), alkaline phosphatase (AKP), creatine phosphokinase isoenzyme (CKMB), lactate dehydrogenase (LDH) levels were obviously increased in the HAGC group. Carbohydrate antigen 199 (CA199) and carcinoembryonic antigen (CEA) levels were remarkably elevated in HAGC patients (Supplementary Table S3). Poorly differentiated or undifferentiated adenocarcinoma was observed to be predominant in HAGC, which is consistent with a much higher distribution of diffuse‐type GC than intestinal type according to Lauren's classification. Differences in clinicopathological features between HAGC and NAGC are described in Supplementary Table S4.
In addition to DIC and diffuse BMM, another distinctive feature of HAGC was hyperinflammation which was displayed in many aspects. Firstly, inflammatory markers, such as white blood cells (WBC) counts, neutrophil counts, neutrophil to lymphocyte ratio (NLR) and C‐reactive protein (CRP) level were increased in HAGC when effects of colony‐stimulating factor and infection were excluded (Supplementary Table S3). Secondly, the ratio of pro‐inflammatory M1 and anti‐inflammatory M2 was elevated in the HAGC tumor immune environment (TIME), indicating an inflammatory environment [6] (Figure 1A, Supplementary Figure S1). Thirdly, the levels of pro‐inflammatory cytokines, including IL‐8, RANTES, IP‐10 and MCP‐1, were higher in the blood serum of HAGC patients (Figure 1B, Supplementary Figure S2). Finally, RNA‐seq results demonstrated inflammatory response and IL‐6‐JAK‐STAT3 signaling activation in HAGC patients (Figure 1C, Supplementary Figure S2). As compared to NAGC, three dominant clinical features of HAGC included DIC, BMM and a hyperinflammatory status, which were confirmed by bone marrow biopsy of HAGC (Supplementary Figure S3).
FIGURE 1.
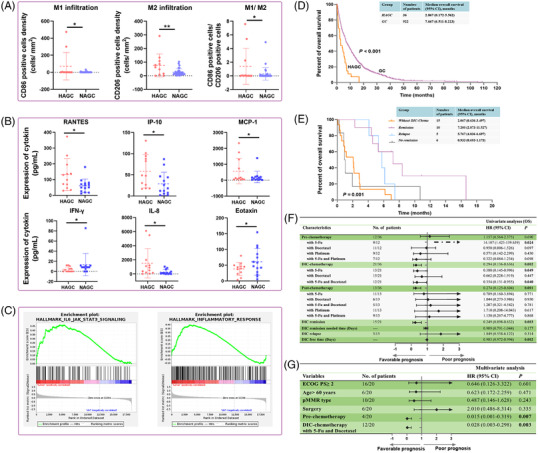
Differences in clinical characteristics between HAGC and NAGC. (A) The density of tumor‐infiltrating CD86+ M1, CD206+ M2, and ratio of M1/M2 in HAGC and NAGC, based on t‐test. (B) Significant release of cytokines between HAGC and NAGC, based on t‐test. (C) Inflammatory signals were active in HAGC by GSEA analysis. (D) OS proportion of patients with HAGC and NAGC, based on Kaplan‐Meier survival analysis. (E) OS in HAGC patients after DIC‐chemotherapy stratification using Kaplan‐Meier curves. (F) Forest plot showing the hazard ratios of treatments of HAGC patients in the Univariate analyses. (G) Multivariate Cox analyses and hazard ratios of treatments as well as clinical features of HAGC patients. P‐values for all survival analyses were calculated using the log‐rank test.
Abbreviations: HAGC: highly aggressive gastric cancer; NAGC: normal advanced gastric cancer; GSEA: Gene Set Enrichment Analysis; OS: overall survival; DIC: disseminated intravascular coagulation
Poor performance status and hematological abnormalities of HAGC perplexed physicians with the tolerability of chemotherapy. HAGC patients demonstrate extremely worse overall survival (OS) than NAGC. The median OS of HAGC patients was 2.867 months (95% CI: 0.172‐5.562) without treatment, while that of NAGC was 7.667 months (95% CI: 6.511‐8.223) (Figure 1D). Of the 36 HAGC patients, 21 (58.3%) underwent chemotherapy during the DIC onset (here, termed as DIC‐chemotherapy). Of them, 61.9% (n = 13), 71.4% (n = 15), and 33.3% (n = 7) received 5‐Fu‐based, taxel‐based, and platinum‐based treatment, respectively. 57.1% (n = 12) were treated with doublet combinations. Fifteen HAGC cases underwent best supportive care (BSC) (Supplementary Figure S4). We observed that patients with a positive DIC status responded sooner to chemotherapy, with a median time to DIC remission of 12 days (4 to 19 days) and the median DIC‐free time (DFT) of 137 days (6 to 457 days), which was much better than previously reported [7], and could significantly prolong the OS of HAGC patients.
Survival of HAGC patients was significantly different based on DIC‐chemotherapy and the DIC status after treatment. Patients without DIC‐chemotherapy had a median OS of 2.067 months (95% CI: 0.636‐3.497). In the DIC‐chemotherapy group, patients who had DIC remission without relapse had a better OS than the DIC relapse group; with a median OS of 7.200 months (95% CI: 2.873‐11.527) in the DIC remission group and 5.767 months (95% CI: 4.836‐6.697) in the DIC relapse group, respectively (Figure 1E). Further, patients who did not have DIC remission had a worse prognosis, with a median OS of 0.933 months (95% CI: 0.693‐1.173). All patients completed DIC‐chemotherapy and related adverse events are shown in Supplementary Table S5.
Clinical treatments consisted of three parts: pre‐chemotherapy, the treatments administered before DIC, DIC‐chemotherapy and post‐chemotherapy which was given after DIC diagnosis and remission. 5‐Fu containing regimen was associated with poor prognosis for HAGC patients in the pre‐chemotherapy stage but was associated with favorable survival in DIC‐chemotherapy patients and post‐chemotherapy stage. Additional 5‐Fu to docetaxel (DF) in DIC‐chemotherapy patients was associated with prolonged survival. DIC remission and DFT were favorable factors of HAGC patients in univariate analysis (Figure 1F). Statistically significant favorable prognostic factors for OS were pre‐chemotherapy and DF combined DIC‐chemotherapy (Figure 1G). Other prognostic factors associated with DFT are presented in Supplementary Figure S5.
Compared to reported studies of AGC with DIC, BMM or bone metastasis, our research comprised of 36 HAGC cases identified from a large cohort of 964 AGC patients, demonstrating an overall prevalence of 3.73%. It has been reported that AGC with DIC had an incidence of 1.2%‐1.6% [4, 8]. In fact, most of these patients only received BSC and did not undergo further examinations; thus, indicating that the incidence of DIC in HAGC could be higher than estimated.
Although HAGC can present with three distinctive characteristics, acute onset and severe hematologic abnormalities usually stuck physicians in a dilemma. At present, the standard care for DIC has not been established and is surrounded by controversy. Based on our findings, DIC‐chemotherapy could relieve platelet consumption effectively in univariate analysis. To avoid the interaction between different drugs and to find out which cytotoxic agents were better for HAGC, DIC‐chemotherapy was not included in the multivariate analysis, where the application of 5‐Fu was associated with favorable prognosis and additional docetaxel provided a longer DFT and OS. There exist several potential chemotherapy treatments reported in some case reports, including single or double application of chemotherapy drugs [9, 10]. However, due to limited information regarding a standard treatment, we are currently conducting a clinical trial (NCT04547153) to establish an effective DIC‐chemotherapy regimen. In addition, we found that post‐chemotherapy for HAGC could provide durable DFT and favorable prognosis, irrespective of the regimens used.
In summary, we showed that AGC patients presenting with DIC and diffuse BMM could be classified as HAGC as they have unique features regarding clinicopathology, TIME, activated signaling pathway, RNA‐seq data and cytokine release, as compared to NAGC patients. The overall prevalence of HAGC was 3.73% but considering that most of these patients are mostly incompletely diagnosed as they do not undergo deeper examinations, this could be an underestimated number. We also showed that a combination of 5‐Fu and docetaxel as DIC‐chemotherapy was associated with longer DFT and OS in HAGC patients. Further investigations using larger patient cohorts are required to validate our findings.
DECLARATIONS
ETHICS STATEMENT
The study protocol was approved by the Sixth Affiliated Hospital of Sun Yat‐sen University clinical research ethics committee (Number: 2021ZSLYEC‐090).
CONSENT FOR PUBLICATION
The authors declare that they consent for publication.
AVAILABILITY OF DATA AND MATERIALS
The data generated and/or analyzed during the current study are available upon reasonable request to the corresponding author.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
FUNDING
This study was supported by the National Natural Science Foundation of China (grant nos. 81770557 and 82070684), the Guangdong Natural Science Fund for Outstanding Youth Scholars (grant no. 2020B151502067) and the Postdoctoral Start‐up Fund of the Sixth Affiliated Hospital of Sun‐Yat Sen University (grant no. R2021021720212991).
AUTHORS' CONTRIBUTIONS
XHZ, CQW and JX conceptualized the project and designed all experiments. XHZ and CQW conducted most experiments and wrote the manuscript. SSL, TYC, GD, YZ and DKC performed parts of experiments or provided technical support. XHZ and CQW analyzed data and organized figures. XJF, YH, PH and JZ provided histopathology input and reviewed reports and tumor samples. XHZ and CQW did the statistical analyses and data interpretation. PL and JX supervised the project. All authors participated in writing and reviewing the manuscript.
Supporting information
Supporting Information
Figure S1
Figure S2
Figure S3
Figure S4
Figure S5
Xiaohui Zhai and Caiqin Wang contributed equally to this study.
Contributor Information
Xiaohui Zhai, Email: zhaixh@mail.sysu.edu.cn.
Jian Xiao, Email: xiaoj26@mail.sysu.edu.cn.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 2. Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time‐trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond). 2021;41(10):1037–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995‐2009: analysis of individual data for 25,676,887 patients from 279 population‐based registries in 67 countries (CONCORD‐2). Lancet. 2015;385(9972):977–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takashima A, Shirao K, Hirashima Y, Takahari D, Okita NT, Nakajima TE, et al. Sequential chemotherapy with methotrexate and 5‐fluorouracil for chemotherapy‐naive advanced gastric cancer with disseminated intravascular coagulation at initial diagnosis. J Cancer Res Clin Oncol. 2010;136(2):243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Silvestris N, Pantano F, Ibrahim T, Gamucci T, De Vita F, Di Palma T, et al. Natural history of malignant bone disease in gastric cancer: final results of a multicenter bone metastasis survey. PLoS One. 2013;8(10):e74402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gordon S, Plüddemann A. Tissue macrophages: heterogeneity and functions. BMC Biol. 2017;15(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hironaka SI, Boku N, Ohtsu A, Nagashima F, Sano Y, Muto M, et al. Sequential methotrexate and 5‐fluorouracil therapy for gastric cancer patients with bone metastasis. Gastric Cancer. 2000;3(1):19–23. [DOI] [PubMed] [Google Scholar]
- 8. Rhee J, Han SW, Oh DY, Im SA, Kim TY, Bang YJ. Clinicopathologic features and clinical outcomes of gastric cancer that initially presents with disseminated intravascular coagulation: a retrospective study. J Gastroenterol Hepatol. 2010;25(9):1537–42. [DOI] [PubMed] [Google Scholar]
- 9. Takeda H, Nishikawa H, Tsumura T, Sekikawa A, Maruo T, Okabe Y, et al. Prominent hypereosinophilia with disseminated intravascular coagulation as an unusual presentation of advanced gastric cancer. Intern Med. 2014;53(6):563–9. [DOI] [PubMed] [Google Scholar]
- 10. Tokar M, Bobilev D, Ariad S, Geffen DB. Disseminated intravascular coagulation at presentation of advanced gastric cancer. Isr Med Assoc J. 2006;8(12):853–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Figure S1
Figure S2
Figure S3
Figure S4
Figure S5
Data Availability Statement
The data generated and/or analyzed during the current study are available upon reasonable request to the corresponding author.


