Abstract
Background
Selective serotonergic reuptake inhibitors, including fluoxetine (FLX), are the most commonly used for the treatment of major depression. However, they are effective for remission in only 30% of patients. Recently, we observed that Galanin (1-15) [GAL(1-15)] enhanced the antidepressant effects of FLX in naïve animals, suggesting a new augmentation strategy in depression.
Methods
We have analyzed in an animal model of depression, the olfactory bulbectomy (OBX) rats, the effect of GAL(1-15) on FLX-mediated responses in the forced swimming test and the sucrose preference test and the involvement of GAL receptor 2 with its antagonist, M871. We have also studied the corticosterone levels in OBX after the coadministration of GAL(1-15) with FLX. Moreover, we studied whether the effects of GAL(1-15) on FLX actions were mediated via auto- and heteroreceptor 5-HT1A (5-HT1AR), analyzing the binding characteristics, mRNA levels, and functionality of 5-HT1AR in the dorsal hippocampus.
Results
GAL(1-15) enhances the antidepressant-like effects induced by FLX in OBX animals in the forced swimming test and the sucrose preference test. The involvement of the GALR2 was demonstrated with M871. Importantly, the mechanism underlying the GAL(1-15)/FLX interactions in the OBX animals involves the 5-HT1AR in the hippocampus at the plasma membrane (increase of affinity and density of 5HT1AR in the DG) and transcriptional (increase of 5HT1AR mRNA levels in DG and CA1) levels. Besides, the coadministration of GAL(1-15) and FLX also reduced OBX-increased corticosterone levels.
Conclusions
The results open the possibility to use GAL(1-15) in combination with FLX as a novel strategy for the treatment of depression.
Keywords: Depression, fluoxetine, galanin(1-15), olfactory bulbectomy rats
Significance Statement.
Selective serotonergic reuptake inhibitors, including fluoxetine (FLX), are the most commonly used for the treatment of major depression. However, they are effective for remission in only 30% of patients. Recent studies indicate that several neuropeptides, including Galanin(1-15) [GAL(1-15)], are involved in the pathophysiology of depression and suggest the possibility to use them as a combined treatment with antidepressant drugs. Our results indicate that GAL(1-15) enhances the antidepressant-like effects induced by FLX in an experimental model of depression, the olfactory bulbectomy (OBX) rats, in behavioral tests related to despair and anhedonic behavior. Importantly, the mechanism underlying the GAL(1-15)/FLX interactions involves the 5-HT1AR in the hippocampus (changes at plasma membrane and transcriptional levels) as well as the regulation of the hypothalamic-pituitary-adrenocortical system. The results open the possibility to use GAL(1-15) in combination with FLX as a novel strategy for treatment of depression.
Introduction
Major depression is the largest contributor to global disability by years lived with disability (World Health Organization 2017), with an annual overall prevalence of 4.4%: 3.6% in men and 5.1% in women (Baxter et al., 2014; World Health Organization, 2017). This disorder is characterized with several symptoms, such as hopelessness, anhedonia, and exacerbated guilt (Castaneda et al., 2008), that could result in suicidal thoughts and attempts.
Several neurotransmitter systems are associated with this pathology; however, the pharmacological treatment is mainly based on drugs elevating serotonergic (5-HT) activity (Blier and de Montigny 1994; Jans et al., 2007), and fluoxetine (FLX) is the oldest and best-studied selective 5-HT reuptake inhibitor (SSRI) (Artigas 2013; Edinoff et al., 2021). Notwithstanding the above, only 30% of patients adequately respond to this treatment (Rush et al., 2009). Moreover, the treatment effect appears 3 to 7 weeks after the beginning of the treatment, and during this delay, patients remain symptomatic and at risk of self-harm (Rush et al., 2009). There is a need to develop novel treatments providing effective, more rapid-acting or long-term relief of depressive symptoms, especially in the patient with treatment-resistant depression.
Recent studies indicate that several neuropeptides, including Galanin (GAL), are involved in the pathophysiology of depression and suggest the possibility to use them as a combined treatment with antidepressant drugs.
GAL is a neuropeptide widely distributed in the CNS, and 3 GAL receptor (GALR) subtypes have been cloned (Mitsukawa et al., 2008).
GAL participates in mood regulation and depression in rodents (Bellido et al., 2002; Juhasz et al., 2014; Wang et al., 2016; Demsie et al., 2020). Thus, the activation of GALR1 and GALR3 receptors results in a depression-like behavior, while stimulation of GALR2 leads to anti-depressant-like effects (Kuteeva et al., 2008). In addition, it was recently described that in brains of patients suffering major depression disorder in a region- and sex-specific manner, GAL and GALR3 genes were differentially methylated and expressed (Barde et al., 2016).
Importantly, GAL modulates auto- and heteroreceptor 5-HT1A (5-HT1AR) functions by an antagonistic interaction especially in the limbic forebrain regions and the dorsal raphe (DR), brain areas that have been suggested to be of high relevance for depression (Misane et al., 1998; Razani et al., 2001; Borroto-Escuela et al., 2018).
Not only GAL but also the N-terminal fragments like GAL(1-15) are active in the CNS (Hedlund and Fuxe 1996; Díaz-Cabiale et al., 2010, 2011; Millón et al., 2019). We have described that GAL(1-15) induces strong depression and anxiogenic-like effects (Millón et al., 2014, 2017) and also a strong anhedonia-like phenotype, a key symptom of depression (Millón et al., 2019), acting through GALR1-GALR2 heteroreceptor complexes in the CNS, especially in the dorsal hippocampus and DR (Borroto-Escuela et al., 2014; Millón et al., 2014). However, GAL(1-15) is able to enhance the antidepressant effects induced by the 5-HT1AR agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) in the forced swimming test (FST) (Millón et al., 2016), which involves alterations in both the binding characteristics and mRNA levels of 5-HT1AR in the dorsal hippocampus and DR (Millón et al., 2016).
Although there is still no evidence of the viability of GAL(1-15) systemic administration, we have considered the possibility of using GAL(1-15) as a combined treatment with SSRIs. We observed that GAL(1-15) enhanced the antidepressant effects of FLX in the FST (Flores-Burgess et al., 2017; 2018) and reversed the memory impairment induced by FLX in the novel object recognition test (Flores-Burgess et al., 2019), being involved the 5-HT1AR in the hippocampus and mPFC, respectively.
Although all these results suggest a new augmentation strategy in depression, a further detailed analysis of the effects of the co-administration of GAL(1-15) and FLX in an animal model of depression is needed. Therefore, for this work we selected a widely used experimental model of depression, the olfactory bulbectomy (OBX) rats. The bilateral destruction of the olfactory bulbs caused complex alterations in behavior and biochemical and cellular mechanisms, such as an anhedonia-like state in sucrose preference, increased hyperactivity in a novel environment, reduced sexual activity, and elevated corticosterone levels (Romeas et al., 2009; Jiménez-Sánchez et al., 2016). Thus, these animals mimic several symptoms observed in patients with major depression (Morales-Medina et al., 2017), resulting in an optimal animal model (Morales-Medina et al., 2017).
To assess the ability of GAL(1-15) to modulate the behavioral effects mediated by FLX in OBX rats, we have analyzed the effect of GAL(1-15) on FLX-mediated responses in behavioral tests related with behavioral despair and anhedonic behavior and the involvement of GALR2 with the GALR2 antagonist M871. We have also studied the corticosterone levels in OBX animals after the coadministration of GAL(1-15) with FLX. Moreover, we studied whether the effects of GAL(1-15) on FLX actions were mediated via 5-HT1AR analyzing the binding characteristics, mRNA levels, and functionality of 5-HT1AR in the dorsal hippocampus.
MATERIALS AND METHODS
Animals
Male Sprague Dawley rats (CRIFFA, Barcelona, Spain) weighing approximately 250 g at the time of the surgery were housed individually under standard laboratory conditions (12-hour-dark/-light cycle, humidity 55%–60%, and 22 ± 2ºC) with free access to food pellets and tap water. All experimental procedures were approved by the Institutional Animal Ethics Committee of the University of Málaga, Spain. All methods were performed in accordance with the relevant guidelines and regulations.
After 1 week of adaptation period, animals were randomly assigned to undergo either OBX or sham surgery. The OBX procedure and the stereotaxically implanted guide cannula has been described previously (Díaz-Cabiale et al., 2011) (Supplement 1).
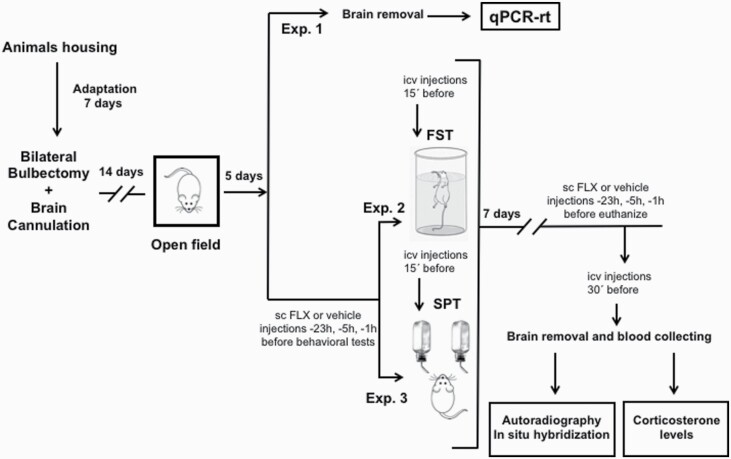
On day 14 post-surgery, we confirmed validity for the lesions by assessing increased activity in the open field. A pictogram of the entire protocol is represented in Figure 1.
Figure 1.
Diagram of the complete experimental schedule. After bilateral olfactory bulbectomy, all animals had a recovery period of 14 days, and then we determine their hyperactivity using the open field test. (A) In the first set of animals, we analyzed the mRNA levels of the receptors involved in our studies. (B). In a second set of animals, we evaluated the effects of different pharmacological treatments in the forced swimming test (FST) or in the sucrose preference test (SPT). One week after the behavioral tests, we collected the blood and brain of the animals to perform the experiments of autoradiography and in situ hybridization and to determine the blood levels of corticosterone.
Five days after the open field, animals were randomly divided in 3 experiments. In the first experiment, we analyzed the relative mRNA expression of GALR1, GALR2, 5-HT1AR, and brain-derived neurotrophic factor (BDNF) in a set of OBX and Sham-operated rats. In the other experiments, groups of rats were assessed in the FST or the sucrose preference test (SPT). Sample size was estimated based on our previous work, where both behavioral tasks (FST and SPT) in Sprague-Dawley rats were used (Millón et al., 2014, 2016, 2019; Flores-Burgess et al., 2017). We have evaluated the effects of the administration of FLX (10 mg/kg) and GAL(1-15) (1 nmol) alone or in combination in both tests. FLX (10 mg/kg) or vehicle was injected 3 times s.c. 23, 5 and 1 hour before the beginning of the tests. We also determined the involvement of GALR2 in the effect of GAL(1-15) on FLX-mediated action in rats that received 3 injections of s.c. FLX (10 mg/kg) and a single i.c.v. injection of GAL(1-15) (1 nmol) and M871 (3 nmol), a preferential GALR2 antagonist (Ki for GalR1 420 nM, for GalR2 13.1 nM) (Mitsukawa et al., 2010), in combination. OBX animals were randomly divided in groups to assess the treatments.
Immediately after the last behavioral test, animals were randomized in 2 different sets: one was used for autoradiography, [35S]GTPγS autoradiography and in situ hybridization histochemistry and the other for corticosterone assay. The sample size for autoradiography and in situ hybridization experiments is based on previous works (Millón et al., 2016; Flores-Burgess et al., 2017, 2019).
Detailed descriptions on animals, surgical procedures, and administration of substances and drugs are available in Supplement 1.
Behavioral Assessment
Open Field Test
Rats were individually placed in the center of the arena and allowed to freely explore. Their activity was recorded over a 5-minute period using the video tracking software EthovisionXT 13.0 (Noldus, Wageningen, Netherlands) (Millón et al., 2014) (Supplement 1).
Forced Swimming Test
Two swimming sessions were conducted: a 15-minute pretest followed 24 hours later by a 5-minute test. The total duration of immobility behavior and swimming was recorded during the second 5 minutes. The administration of drugs was performed between sessions (Millón et al., 2014; Flores-Burgess et al., 2017) (Supplement 1).
Sucrose Preference Test
SPT was performed as described previously with minor modifications (Millón et al., 2019). Briefly, on the testing day, rats were allowed free access to 2 bottles: 1 containing 1% (w/v) sucrose solution and the other containing tap water. After 2 hours, the bottles were weighed to calculate the sucrose intake and sucrose preference. Treatments were administered considering the beginning of the test 2 hours before weighing the bottles (Supplement 1).
Quantitative Reverse Transcriptase PCR
The relative mRNA expression of GALR1, GALR2, 5-HT1AR, and BDNF was performed by quantitative reverse transcriptase PCR as previously described (Millón et al., 2014; Flores-Burgess et al., 2017) (Supplement 1).
Autoradiography, [35S]GTPγS Autoradiography, and In Situ Hybridization Histochemistry
Coronal sections were obtained at the dorsal hippocampus. The procedure to perform receptor autoradiography, functional autoradiography, and in situ hybridization histochemistry was previously described (Castro et al., 2008; Flores-Burgess et al., 2017) (Supplement 1).
Quantitative Autoradiography
Saturation experiments were performed using [3H]-8-OH-DPAT as previously described (Flores-Burgess et al., 2017) (Supplement 1). Briefly, the sections were preincubated for 30 minutes at room temperature in 50 mM Tris-HCl buffer (pH 7.6) containing 4 mM CaCl2, 0.01% ascorbic acid, and 10 mM pargyline. The sections were then incubated for 60 minutes at room temperature with [3H]-8-OH-DPAT in the same solution as above. Film exposure time for sections was 6 weeks.
[35S]GTPγS Autoradiography
Labeling of brain sections with [35S]GTPγS was carried out as previously described (Castro et al., 2008) with some modifications (Supplement 1). Slide-mounted sections were preincubated for 20 minutes at room temperature in a buffer containing 50 mM Tris–HCl, 0.2 mM EGTA, 3 mM MgCl2, 100 mM NaCl, and 2 mM GDP at pH 7.4. Slides were subsequently incubated, for 2 hours, in the same buffer containing adenosine deaminase (3 mU/mL) with [35S]GTPγS (0.05 nM), and consecutive sections were also co-incubated with 8-OH-DPAT (10μM).
In Situ Hybridization Histochemistry
Oligonucleotide labeling, in situ hybridization histochemistry, and specificity controls have been described elsewhere (Tomiyama et al., 1997) (Supplement 1). The oligonucleotides complementary to the mRNA coding for 5-HT1AR are found in the supplemental Information. Hybridized sections were exposed to Kodak Biomax-MR (Sigma-Aldrich, St. Louis, MO, USA) for 10 days.
Image Analysis
Measurements were made in the dentate gyrus (DG) and the CA1 area of the hippocampus (0.15 mm2) (Supplement 1).
Corticosterone Assay
Trunk blood samples from animals killed by beheading was used to perform determination of corticosterone plasma levels with corticosterone ELISA kit (ArborAssay, Ann Arbor, MI, USA) (Supplement 1).
The study was carried out in compliance with the ARRIVE guidelines.
Statistical Analysis
Data are presented as the mean ± SEM, and sample numbers (n) are indicated in figure legends. All data were analyzed using GraphPad PRISM 8.0 (GraphPad Software, Inc., San Diego, CA, USA). For comparing 2 experimental conditions, 2-tailed Student’s unpaired t tests were performed. For comparing more than 2 groups, 1-way ANOVA was performed. Fisher’s least significant difference (LSD) comparison post-test was performed only when the F ratio in the 1-ANOVA was statistically significant. Differences were considered statistically significant at P < .05 (*P < .05, **P < .01, ***P < .001).
In the saturation experiments, the dissociation constant (Kd) and the maximal number of binding sites (Bmax) values were determined by fitting the data to the equation Y = Bmax*X/(X+Kd) by nonlinear regression using the Prism program, version 8 (GraphPad Software).
RESULTS
First, we used the open-field test to confirm that bulbectomized rats displayed the expected behavioral changes. At 14 days after the bilateral olfactory bulbectomy surgery, OBX rats displayed increased locomotor activity in the open field relative to sham-operated rats (t12 = 4.643; P = .0006) (supplemental Figure 1). Moreover, as previously shown (Freitas et al., 2013), OBX rats exhibited increased BDNF expression in the hippocampus (t8 = 2.309; P = .0438) (supplemental Table 1).
Behavioral Effects
We have evaluated the effects of GAL(1-15) in the FLX-mediated effects in OBX animals in the FST and SPT.
GAL(1-15) Enhanced the Antidepressant-Like Effects of FLX in the FST in OBX Rats
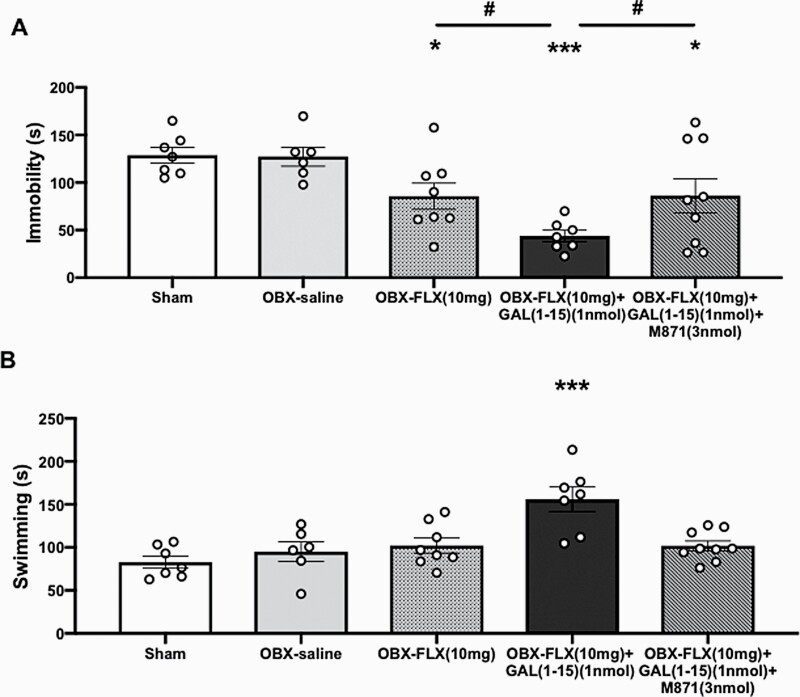
In FST, the threshold dose of GAL(1-15) (1 nmol) enhanced the antidepressant-like effects mediated by FLX (10 mg/kg) in OBX animals. The i.c.v. GAL(1-15) statistically significant decreased the immobility time by 50% (1-way ANOVA, F4,32 = 6.524, P = .0006; Fisher’s LSD post hoc: P < .05) (Figure 2A) and increased the swimming time (1-way ANOVA, F4,32 = 8.448, P < .0001; Fisher’s LSD post hoc: P < .001) by approximately 30% (Figure 2B) induced by FLX.
Figure 2.
Behavioral effects of the coadministration of fluoxetine (FLX) (10 mg/kg) alone (n = 8) or in combination with Galanin(1-15) [GAL(1-15)] (1 nmol) (n = 7) or GAL(1-15) (1 nmol)+M871 (3 nmol) (n = 9) in the forced swimming test (FST). FLX was administered s.c. 23, 5 and 1 hour before the test. Cerebrospinal fluid, GAL(1-15), or GAL(1-15) (1 nmol)+M871 (3 nmol) was injected i.c.v. 15 minutes before the test. Vertical bars represent the mean ± SEM of the immobility (A) or swimming time (B) during the 5-minute test period. In (A) *P < .05, ***P < .001 vs sham (n = 7) and OBX-saline groups (n = 6); #P < .05 vs OBX-Flx (10 mg/kg)+Gal(1-15) (1 nmol); in (B) ***P < .001 vs rest of the groups according to 1-way ANOVA followed by Fisher’s least significance difference test.
FLX induced an antidepressant-like effect in OBX rats since the immobility time was statistically significant reduced vs the OBX-saline group (Fisher’s LSD post hoc: P < .05) as previously described (Zhou et al., 2019) (Figure 2A).
GAL (1-15) (1 nmol) or M871 (3 nmol) alone in OBX animals lacked effects in the FST (supplemental Table 2).
We also tested the involvement of the GALR2 in the GAL(1-15)–FLX interaction with the GALR2 antagonist M871 in the FST. M871 (3 nmol) blocked the GAL(1-15)-induced reduction of the immobility time (Figure 2A) and the GAL(1-15)-induced increase in the swimming time (Fisher’s LSD post hoc: P < .001) (Figure 2B) found after the coadministration of GAL(1-15) and FLX in the FST.
Coadministration of GAL(1-15) and FLX Reversed OBX-Mediated Effects in the SPT
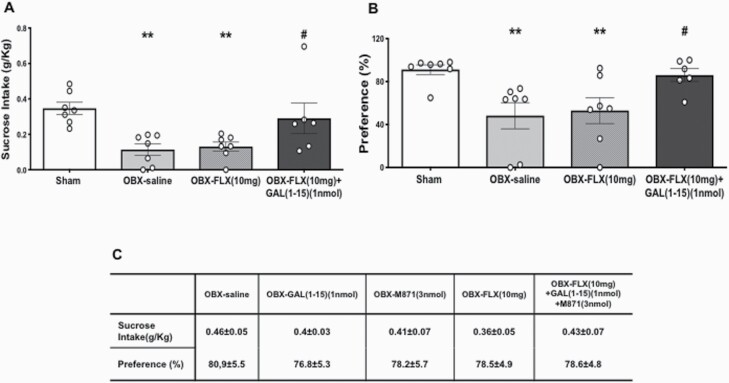
OBX animals exhibited a reduction in sucrose intake (1-way ANOVA, F3,23 = 6.121, P = .0032; Fisher’s LSD post hoc: P < .01) (Figure 3A) and a decrease in sucrose preference (1-way ANOVA, F3,23 = 5.362, P = .006; Fisher’s LSD post hoc: P < .01) (Figure 3B) compared with sham animals as previously described (Romeas et al., 2009; Jiménez-Sánchez et al., 2016).
Figure 3.
Behavioral effects of the coadministration of fluoxetine (FLX) (10 mg/kg) alone (n = 7) or in combination with Galanin(1-15) [GAL(1-15)] (1 nmol) (n = 6) or GAL(1-15) (1 nmol)+M871 (3 nmol) (n = 6–7) with 1% the sucrose concentration in the sucrose preference test (SPT). FLX was administered s.c. 23, 5 and 1 hour before the start of the test. Cerebrospinal fluid, GAL(1-15), M871 (3 nmol), or GAL(1-15) (1 nmol)+M871 (3 nmol) was injected i.c.v. 15 minutes before the beginning of the test. Vertical bars represent the mean ± SEM of (A) sucrose intake (g/kg) and (B) preference (percentage respect water) 2 hours after the beginning of the test. **P < .01 vs sham (n = 7); #P < .05 vs OBX-Saline (n = 7) and OBX-Flx (10 mg/kg) groups according to 1-way ANOVA followed by Fisher’s least significance difference test. (C) Values represent the mean ± SEM.
The coadministration of GAL(1-15) (1 nmol) and FLX (10 mg/kg) reversed the effects of the OBX procedure as these animals cotreated with GAL(1-15)+FLX showed a statistically significant increase in both sucrose intake (Fisher’s LSD post hoc: P < .05) (Figure 3A) and preference (Fisher’s LSD post hoc: P < .05) (Figure 3B) compared with the OBX-saline group.
No statistically significant differences were observed in the total fluid drinking in animals between groups (supplemental Table 5).
In the second set of experiments, we also tested the involvement of the GALR2 in the GAL(1-15)–FLX interaction (Figure 3C). The presence of the GALR2 antagonist M871 (3 nmol) blocked the GAL(1-15)-induced effect since no differences were observed between the FLX+GAL(1-15)+M871 and the OBX-saline group or in sucrose intake or preference (Figure 3C).
Plasma Corticosterone Levels
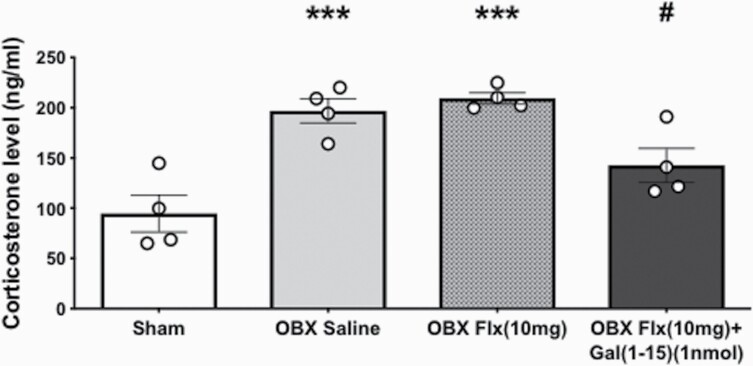
OBX animals had statistically significant higher circulating corticosterone concentrations (1-way ANOVA, F3,12 = 13.79, P = .0003; Fisher’s LSD post hoc: P < .001) than sham animals as previously described (Morales-Medina et al., 2017) (Figure 4).
Figure 4.
Plasma levels of corticosterone (ng/mL) after the administration of fluoxetine (FLX) (10 mg/kg) alone or in combination with Galanin(1-15) [GAL(1-15)] (1 nmol). FLX was administered s.c. 23, 5 and 1.25h before collecting blood. Cerebrospinal fluid or GAL(1-15) were injected i.c.v. 30 minutes before the blood collecting. Data are represented as mean ± SEM (n = 4 rats per group). ***P < .001 vs Sham group and #P < .05 vs rest of the groups according to 1-way ANOVA followed by Fisher’s least significance difference test.
The coadministration of GAL(1-15) (1 nmol) and FLX (10 mg/kg) reduced statistically significant OBX-increased corticosterone levels (Fisher’s LSD post hoc: P < .05) by approximately 50% compared with OBX-saline (Figure 4).
FLX treatment had no effect on basal corticosterone levels in OBX animals as previously described by other authors (Marcilhac et al., 1999).
GAL(1-15) (1 nmol) injected in OBX animals also lacked effect in corticosterone levels (supplemental Table 3).
Neurochemical Effects
The bilateral olfactory bulbectomy lacked effect in the mRNA expression levels of GALR1, GALR2, and 5-HT1A receptors in the hippocampus of the OBX animals compared with sham animals 19 days after the surgery (supplemental Table 1).
Moreover, the binding characteristics of 5-HT1AR agonist [3H]-8-OHDPAT, the 5-HT1AR mRNA levels, and the specific [35S]GTPγS binding in the DG and CA1 of the hippocampus was not modified in the OBX saline vs sham animals (supplemental Table 4; Table 1).
Table 1.
Effects of FLX (10 mg/kg) alone or in combination with GAL(1-15) (1 nmol) on the specific [35S]GTPγS binding in the DG and CA1 of the Hippocampus
| Basal values nCi/g tissue | Net stimulation induced by 10 μM 8-OH-DPAT (nCi/g tissue) | ||
|---|---|---|---|
| DG | Sham | 2.12 ± 0.14 | 0.37 ± 0.07 |
| OBX saline | 2.18 ± 0.12 | 0.29 ± 0.04 | |
| OBX FLX (10 mg) | 1.32 ± 0.1*** | 0.48 ± 0.1 | |
| OBX FLX (10 mg)+Gal(1-15) (1 nmol) | 1.35 ± 0.08*** | 0.37 ± 0.13 | |
| CA1 | Sham | 1.84 ± 0.17 | 0.43 ± 0.01 |
| OBX saline | 2.15 ± 1.14 | 0.68 ± 0.17 | |
| OBX FLX (10 mg) | 1.41 ± 0.12 | 0.43 ± 0.11 | |
| OBX FLX (10 mg)+Gal(1-15) (1 nmol) | 1.5 ± 0.11a | 0.42 ± 0.13 |
Abbreviations: 8-OH-DPAT, 8-hydroxy-2-(di-n-propylamino)tetralin; FLX, fluoxetine; GAL(1-15), Galanin(1-15); OBX, olfactory bulbectomy rat.
Data are represented as mean ± SEM of the basal binding and the net 8-OH-DPAT stimulated [35S]GTPγS binding (n = 6). *P < .05, ***P < .001 vs sham and OBX-saline groups.
a P < .01 vs OBX-saline group according to 1-way ANOVA followed by Fisher’s least significance difference test.
We have analyzed the characteristics, functionality, and mRNA expression of the 5-HT1AR after pharmacological treatments using autoradiographic techniques, in situ hybridization, and specific [35S]GTPγS binding.
In this work, we have focused on the dorsal hippocampus since, in rats, a key role for 5-HT1AR in the dorsal but not ventral hippocampus has been shown in resilience to the chronic mild stress model (Wu and Hen, 2014). However, since 5-HT1AR in the ventral hippocampus in mice is necessary for FLX antidepressant actions, the importance of dorsal vs ventral hippocampus in depression-like behaviors may depend on the type of rodent.
GAL(1-15)+FLX Modifies mRNA Levels and Binding Characteristics of 5-HT1AR in Dorsal Hippocampus in OBX Animals
1. 5-HT1A agonist radioligand binding. Saturation curves
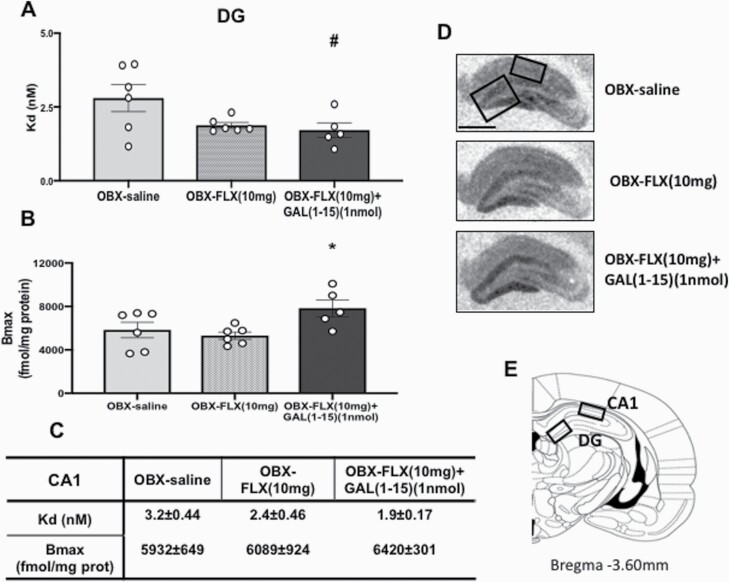
The coadministration of FLX(10 mg/kg) and GAL(1-15)(1 nmol) produced a decrease in the Kd value compared with the OBX-saline group (Figure 5A) and a statistically significant increase in the Bmax value compared with the OBX-saline and -FLX injected group (1-way ANOVA, F2,14 = 4.287, P = .0353; Fisher’s LSD post hoc: P < .05) (Figure 5B) of the agonist radioligand [3H]-8-OH-DPAT in the DG of the dorsal hippocampus in OBX animals.
Figure 5.
Effects of the administration of fluoxetine (FLX) (10 mg/kg) alone (n = 6) or in combination with Galanin(1-15) [GAL(1-15)] (1 nmol) (n = 5) on the binding characteristics of 5-HT1AR agonist [3H]-8-OHDPAT in the dentate gyrus (DG) of the dorsal hippocampus. FLX was administered s.c. 23, 5 and 1.25 hours before rats were euthanized. Cerebrospinal fluid or GAL(1-15) was injected i.c.v. 30 minutes before rats were euthanized. Saturation experiments were performed with 10 concentrations of [3H]-8-OHDPAT (0.26–10 nM) in dorsal hippocampus sections. Non-specific binding was defined as the binding in the presence of 10 mM serotonin. Data are represented as mean ± SEM of Kd (A) and Bmax (B) values in DG and in the CA1 (C). In the control group, the Kd (nM) value was 2.8 ± 0.46 and Bmax (fmol/mg prot) was 5822 ± 716 in DG. #P < .05 vs OBX-saline group (n = 6) and *P < .05 vs rest of the groups according to 1-way ANOVA followed by Fisher’s least significance difference test. No statistical differences were obtained in CA1 in either Kd or Bmax values. (D) Representative autoradiograms from the dorsal hippocampus sections of OBX showing the increase of the 5-HT1AR agonist binding in DG with a high concentration of the radioligand (10 nM); scale bar = 1 mm. (E) Schematic drawing showing the areas analyzed in coronal sections of the rat brain at Bregma −3.60 mm.
No effects due to the cotreatment were observed in either the Kd or Bmax of the agonist radioligand [3H]-8-OH-DPAT (Figure 5C) in the CA1 with respect to OBX-saline animals.
GAL (1-15) (1 nmol) alone in OBX animals lacked effects in the binding characteristics of 5-HT1AR agonist [3H]-8-OHDPAT in the DG and CA1 of the hippocampus (supplemental Table 4).
Representative autoradiograms with a high radioligand concentration (10 nM) illustrate the increase of labeling (increase in Bmax) in the DG 30 minutes after the coadministration of FLX (10 mg/kg) and GAL(1-15) (1 nmol) in OBX animals (Figure 5D,E).
2. 5-HT1A mRNA levels
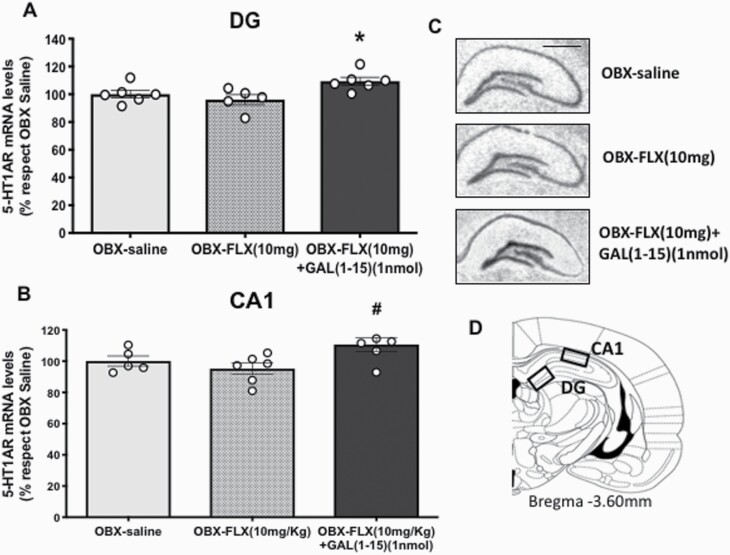
The coadministration of FLX (10 mg/kg) and GAL(1-15) (1 nmol) produced a statistically significant increase in the 5HT1AR mRNA levels in DG (Figure 6A) (1-way ANOVA, F2,14 = 4.934, P = .024; Fisher’s LSD post hoc: P < .05) and in the CA1 (Figure 6B) (1-way ANOVA, F2,14 = 4.345, P = .034 Fisher’s LSD post hoc: P < .05) of the dorsal hippocampus compared with theOBX-FLX group.
Figure 6.
Effects of the administration of fluoxetine (FLX) (10 mg/kg) alone (n = 5) or in combination with Galanin(1-15) [GAL(1-15)] (1 nmol) (n = 6) on 5-HT1AR mRNA levels in the (A) dentate gyrus (DG) and (B) CA1 regions of the dorsal hippocampus of bulbectomy rats (OBX). FLX was administered s.c. 23, 5 and 1.25 hours before euthanized. Cerebrospinal fluid or GAL(1-15) was i.c.v. 30 minutes before being euthanized. Vertical bars represent the mean ± SEM of the percentage of change from the OBX saline group of the optical density (O.D.). *P < .05 vs rest of the group and #P < .05 vs OBX-FLX group according to 1-way ANOVA followed by Fisher’s least significance difference test. (C) Representative autoradiograms from dorsal hippocampus sections of OBX showing the mRNA levels coding for 5-HT1AR; scale bar = 1 mm. (D) Schematic drawing showing the areas analyzed in coronal sections of the rat brain at Bregma −3.60 mm.
GAL(1-15) (1 nmol) alone in OBX animals lacked effects in the 5-HT1AR mRNA levels in the DG and CA1 of the hippocampus (supplemental Table 4).
Representative autoradiograms illustrate the increase of 5HT1AR mRNA levels in both areas, CA1 and DG, after the coadministration of FLX (10 mg/kg) and i.c.v. injection of GAL(1-15) (1 nmol) (Figure 6C,D).
3. -Effects of treatments on 8-OH-DPAT-stimulated [35S]GTPγ binding
Basal [35S]GTPγ binding levels in the DG were statistically significant reduced in the OBX animals treated with FLX (10 mg/kg) (1-way ANOVA, F3,20 = 16.19, P < .0001; Fisher’s LSD post hoc: P < .001) (Table 1) or FLX (10 mg/kg)+GAL(1-15) (1 nmol) (post hoc: P < .001) (Table 1) compared with the OBX-saline group. This reduction was also observed in the CA1 area in OBX animals treated with either FLX (10 mg/kg) (1-way ANOVA, F3,20 = 6.158, P = .0039; Fisher’s LSD post hoc: P < .01) (Table 1) or FLX (10 mg/kg)+GAL(1-15) (1 nmol) (Fisher’s LSD post hoc: P < .01) (Table 1).
No changes among groups were observed in the net specific stimulation of [35S]GTPγ binding induced by 8-OH-DPAT in the DG or the CA1 (Table 1).
These results agree with previous data where the chronic administration of FLX reduced the basal binding of [35S]GTPγ but not the net stimulation by 8-OH-DPAT in hippocampus (Castro et al., 2008).
DISCUSSION
In the current study, we describe for the first time, to our knowledge, that GAL(1-15) enhances the antidepressant-like effects induced by FLX in OBX animals in behavioral tests related to despair and anhedonic behavior. Indications were also obtained for the involvement of GALR2 in these effects since the GALR2 antagonist M871 blocked GAL(1-15) mediated actions in the FST. Importantly, the mechanism underlying the GAL(1-15)–FLX interactions in the OBX animals involves the 5-HT1AR in the hippocampus at the plasma membrane (increase of density of 5HT1AR in the DG) and transcriptional (increase of 5HT1AR mRNA levels in DG and CA1) levels. Besides, the coadministration of GAL(1-15) and FLX also reduced OBX-increased corticosterone levels. All these results support the possible use of GAL(1-15)+FLX to treat several symptoms associated with depression.
Animal models can help to improve our understanding of mood disorders. In this regard, the OBX model of depression is a valid model of depression (Morales-Medina et al., 2017) since similarly to humans, most of the OBX-induced symptoms are alleviated after chronic, but not acute, antidepressant treatment (Morales-Medina et al., 2017).
In the present study, OBX rats exhibited, as previously described, the characteristic hyperactivity in the open field paradigm, reduction in the sucrose preference, elevated corticosterone levels, increased BDNF mRNA levels, and no changes in the density of 5-HT1A receptors in the hippocampus (Freitas et al., 2013; Morales-Medina et al., 2017; Riad et al., 2017) that validate our model. Besides, we have described for the first time, to our knowledge, that the ablation of the olfactory bulbs lacked effect in the hippocampal mRNA levels of GALR1 or GALR2.
Among behavioral tests, we have selected the FST and the SPT to analyze the effects of the cotreatment of FLX and GAL(1-15). The FST is a test related to despair very useful for antidepressant drug screening since it is quick to perform, reliable across laboratories, and sensitive to the effects of all the main types of antidepressant drugs (Detke et al., 1995). In fact, in the current study, FLX induced a reduction in the immobility in the FST in OBX animals. This result is in agreement with previous FLX chronic studies in OBX rats (Holzmann et al., 2015). However, in OBX animal models, chronic FLX induced both a reduction in immobility and an increase in swimming in the FST (Morales-Medina et al., 2017). In our study, although FLX induced a reduction in the immobility time, no effect was observed in the swimming time. This lack of effect could be explained by the subchronic administration pattern of FLX used, probably indicating that more prolonged FLX treatment should be performed to observe an effect in the swimming parameter. We will need to address in future experiments the effect of GAL(1-15) in chronically administered FLX in OBX rats.
In our OBX model, we did not find any effect in the FST in OBX-saline vs sham rats or in the immobility in swimming. These results suggest that in our study, the hyperactivity observed in the OFT did not affect the FST behavior test. The lack of effect in the FST is consistent with other studies in OBX animals (Zhu et al., 2020), although other authors have found an increase in the immobility in this test (Morales-Medina et al., 2017). The variability in this test indicates that the FST is not a representative behavioral domain in the OBX model (Alcantara et al., 2017).
On the other hand, in the SPT that detects an anhedonia-like condition, which is a core feature of major depression and characteristic of the OBX model (Millón et al., 2019; Zhou et al., 2019), we observed increased anhedonia (Zhu et al., 2020).
Importantly, we demonstrated in the current work that GAL(1-15) enhanced FLX-induced antidepressant effects in the FST and restored the decrease in sucrose preference observed in the OBX animals (Morales-Medina et al., 2017). All these results confirm a potent effect of the combination GAL(1-15)–FLX in reversed depressive symptoms.
Our results suggest the participation of GALR2 in the GAL(1-15)-mediated effects since the GALR2 antagonist M871 blocked in OBX rats the GAL(1-15) enhancement of antidepressant effects of FLX. We have previously described that these pharmacological treatments lacked an effect in the parameters of locomotor activity or time of exploration (Flores-Burgess et al., 2019). These results are in consonance with our previous studies showing that GAL(1-15) preferentially binds to GALR1-GALR2 heteroreceptor complexes (Fuxe et al., 2012; Borroto-Escuela et al., 2014; Millón et al., 2014, 2016).
We have observed in Sprague-Dawley rats that GAL(1-15) enhanced the antidepressant effects of FLX in the FST(Flores-Burgess et al., 2017) and also potentiated a 5-HT1AR agonist 8-OH-DPAT–mediated effect in this test (Millón et al., 2016); we proposed that FLX, as a SSRI, blocked 5-HT uptake and so increased 5-HT volume transmission (Fuxe et al., 2007). This increase of 5-HT managed to activate 5-HT receptor subtypes, including 5-HT1A homo- and heteroreceptor complexes.
Our results show that bulbectomy lacks effect in the density or binding characteristics of the 5HT1AR in the hippocampus as previously described (Gurevich et al., 1993; Riad et al., 2017). However, the coadministration of FLX with GAL(1-15) in OBX animals induced an increase in the mRNA levels of 5-HT1AR in the dorsal hippocampus and a remarkable increase in the Bmax, specifically in the DG, suggesting an important role of hippocampal 5-HT1AR in the GAL(1-15)–FLX interaction. These modifications are in agreement with our previous work in naïve animals (Flores-Burgess et al., 2017) and confirm the key role of 5-HT1A receptor in the hippocampus in the GAL(1-15)–FLX interaction also in the OBX animals.
On the other hand, the results obtained in the functional autoradiography experiment showed that in the OBX animals, there are no modifications in the [35S]GTPγS basal binding, but the administration of FLX, as previously described (Castro et al., 2008), or FLX+GAL(1-15) induced a decreased in the of [35S]GTPγS basal binding in the hippocampus without affecting the net stimulation induced by 8-OH-DPAT, suggesting that the coadministration lacked effect in the G-protein functionality coupled to 5-HT1AR in the hippocampus.
All these data reinforce our previous hypothesis: the existence of a trimeric GALR1-GALR2-5-HT1AR heteroreceptor complex as we have previously suggested in naïve animals (Millón et al., 2016; Flores-Burgess et al., 2017, 2019; Borroto-Escuela et al., 2018) could be the a key point to understand the effects of FLX–GAL(1-15) interaction in the OBX animal depression model. In such complex, altered allosteric receptor-receptor interactions can develop an ability of the GALR1-GALR2 component to enhance the 5-HT1AR protomer signaling (Flores-Burgess et al., 2017).
Because not only 5-HT1AR, but also 5-HT4, 5-HT2A, 5-HT3, and 5-HT7 receptors are involved in modulating the effects of antidepressant treatments, the participation of other 5-HT receptor subtypes in the interaction cannot be excluded.
We also observed in this work that the coadministration of GAL(1-15) and FLX reduced OBX-increased corticosterone blood levels. Preclinical and clinical studies have gathered substantial evidence that hyperactivity of the hypothalamic-pituitary-adrenocortical (HPA) system is one of the major pathophysiological factors for the development of depression (Schüle et al., 2009). The long-term administration of antidepressants may normalize this HPA alteration because this effect is associated with the antidepressant-induced clinical improvement (Holsboer and Barden 1996). In fact, clinical data suggest that normalization of an initial aberrancy in the dexamethasone-CRH tests is predictive of a favorable antidepressant drug treatment response, whereas persistent HPA abnormality correlates with therapy resistance or relapse in major depression (Holsboer 2000).
Our results indicated that elevated corticosterone levels, characteristic of OBX animals, are not counteracted by FLX administration alone (Marcilhac et al., 1999), but the coadministration of GAL(1-15) and FLX induces a decrease in corticosterone blood levels. This result indicates that the interaction between FLX and GAL(1-15) might involve the regulatory elements of the HPA axis and opens the possibility to use this treatment in resistant depression. This possibility remains to be tested.
The decrease in the corticosterone blood levels observed after the coadministration of GAL(1-15) and FLX could be in part responsible for the increase observed in the mRNA levels and Bmax of 5-HT1AR in the dorsal hippocampus. One of the most powerful regulators of 5-HT1AR expression in the rat brain is corticosterone; the expression of 5-HT1AR is decreased by corticosteroid hormones, whereas removal of circulating corticosteroids by adrenalectomy upregulates 5-HT1AR expression. The effect of corticosterone on 5-HT1AR could result from actions of mineralocorticoid and/or glucocorticoid receptors in mediating transcriptional repression (Ou et al., 2001), but modifications in GIRK2 also could be involved (Saenz del Burgo et al., 2013). The effects of GAL(1-15)+FLX on corticosterone levels and 5-HT1AR should be analyzed in future studies.
In conclusion, our results indicate that GAL(1-15) enhances the antidepressant-like effects induced by FLX in OBX animals in behavioral tests related to despair and anhedonic behavior. Importantly, the mechanism underlying the GAL(1-15)–FLX interactions in the OBX animals involves the 5-HT1AR in the hippocampus (changes at plasma membrane and transcriptional levels) as well as the regulation of the HPA system. The results open the possibility to use GAL(1-15) in combination with FLX as a novel strategy for treatment of depression; therefore, it will be necessary to test the effects of intranasal GAL(1-15) administration in future studies.
Supplementary Material
Acknowledgments
This work was supported by grants awarded by Spanish Ministry of Economy SAF2016-79008-P, PSI2017-82604-R, PID2020-114392RB-I00, PDC2021-121566-I00, by UMA18-FEDERJA-008 and by Junta de Andalucia P20_00026, PI-0083-2019 and by Stiftelsen Olle Engkvist Byggmästare to K.F.
Interest Statement
None.
References
- Alcantara LF, Parise EM, Bolaños-Guzmán CA (2017) Animals models of mood disorders. In: Neurobiology of mental illness, 5th ed (Charney DS, Sklar P, Buxbaum JD, eds), pp. 1–15. Oxford: Oxford University Press. [Google Scholar]
- Artigas F (2013) Serotonin receptors involved in antidepressant effects. Pharmacol Ther 137:119–131. [DOI] [PubMed] [Google Scholar]
- Barde S, Rüegg J, Prud’homme J, Ekström TJ, Palkovits M, Turecki G, Bagdy G, Ihnatko R, Theodorsson E, Juhasz G, Diaz-Heijtz R, Mechawar N, Hökfelt TG (2016) Alterations in the neuropeptide galanin system in major depressive disorder involve levels of transcripts, methylation, and peptide. Proc Natl Acad Sci U S A 113:E8472–E8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter AJ, Scott KM, Ferrari AJ, Norman RE, Vos T, Whiteford HA (2014) Challenging the myth of an “epidemic” of common mental disorders: trends in the global prevalence of anxiety and depression between 1990 and 2010. Depress Anxiety 31:506–516. [DOI] [PubMed] [Google Scholar]
- Bellido I, Díaz-Cabiale Z, Jiménez-Vasquez PA, Andbjer B, Mathé AA, Fuxe K (2002) Increased density of galanin binding sites in the dorsal raphe in a genetic rat model of depression. Neurosci Lett 317:101–105. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C (1994) Current advances and trends in the treatment of depression. Trends Pharmacol Sci 15:220–226. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Narvaez M, Di Palma M, Calvo F, Rodriguez D, Millon C, Carlsson J, Agnati LF, Garriga P, Díaz-Cabiale Z, Fuxe K (2014) Preferential activation by galanin 1-15 fragment of the GalR1 protomer of a GalR1-GalR2 heteroreceptor complex. Biochem Biophys Res Commun 452:347–353. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Narváez M, Ambrogini P, Ferraro L, Brito I, Romero-Fernandez W, Andrade-Talavera Y, Flores-Burgess A, Millon C, Gago B, Narvaez JA, Odagaki Y, Palkovits M, Diaz-Cabiale Z, Fuxe K (2018) Receptor-receptor interactions in multiple 5-HT1A heteroreceptor complexes in Raphe-Hippocampal 5-HT transmission and their relevance for depression and its treatment. Molecules 23:1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda AE, Tuulio-Henriksson A, Marttunen M, Suvisaari J, Lönnqvist J (2008) A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord 106:1–27. [DOI] [PubMed] [Google Scholar]
- Castro E, Díaz A, Rodriguez-Gaztelumendi A, Del Olmo E, Pazos A (2008) WAY100635 prevents the changes induced by fluoxetine upon the 5-HT1A receptor functionality. Neuropharmacology 55:1391–1396. [DOI] [PubMed] [Google Scholar]
- Demsie DG, Altaye BM, Weldekidan E, Gebremedhin H, Alema NM, Tefera MM, Bantie AT (2020) Galanin receptors as drug target for novel antidepressants: review. Biologics 14:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I (1995) Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 121:66–72. [DOI] [PubMed] [Google Scholar]
- Díaz-Cabiale Z, Parrado C, Narváez M, Millón C, Puigcerver A, Fuxe K, Narváez JA (2010) Neurochemical modulation of central cardiovascular control: the integrative role of galanin. Exp Suppl 102:113–131. [DOI] [PubMed] [Google Scholar]
- Díaz-Cabiale Z, Parrado C, Narváez M, Puigcerver A, Millón C, Santín L, Fuxe K, Narváez JA (2011) Galanin receptor/Neuropeptide Y receptor interactions in the dorsal raphe nucleus of the rat. Neuropharmacology 61:80–86. [DOI] [PubMed] [Google Scholar]
- Edinoff AN, Akuly HA, Hanna TA, Ochoa CO, Patti SJ, Ghaffar YA, Kaye AD, Viswanath O, Urits I, Boyer AG, Cornett EM, Kaye AM (2021) Selective serotonin reuptake inhibitors and adverse effects: a narrative review. Neurol Int 13:387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Burgess A, Millón C, Gago B, Narváez M, Borroto-Escuela DO, Mengod G, Narváez JA, Fuxe K, Santín L, Díaz-Cabiale Z (2017) Galanin (1-15) enhancement of the behavioral effects of Fluoxetine in the forced swimming test gives a new therapeutic strategy against depression. Neuropharmacology 118:233–241. [DOI] [PubMed] [Google Scholar]
- Flores-Burgess A, Millón C, Gago B, Narváez JA, Fuxe K, Díaz-Cabiale Z (2018) Small interference RNA knockdown rats in behavioral functions: GALR1/GALR2 heteroreceptor in anxiety and depression-like behavior. In: Receptor-receptor interactions in the central nervous system (Fuxe K, Borroto-Escuela D, eds), pp. 133–148. New York: Humana Press (Springer science). [Google Scholar]
- Flores-Burgess A, Millón C, Gago B, García-Durán L, Cantero-García N, Coveñas R, Narváez JA, Fuxe K, Santín L, Díaz-Cabiale Z (2019) Galanin (1-15)-fluoxetine interaction in the novel object recognition test. Involvement of 5-HT1A receptors in the prefrontal cortex of the rats. Neuropharmacology 155:104–112. [DOI] [PubMed] [Google Scholar]
- Freitas AE, Machado DG, Budni J, Neis VB, Balen GO, Lopes MW, de Souza LF, Dafre AL, Leal RB, Rodrigues AL (2013) Fluoxetine modulates hippocampal cell signaling pathways implicated in neuroplasticity in olfactory bulbectomized mice. Behav Brain Res 237:176–184. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Dahlström A, Höistad M, Marcellino D, Jansson A, Rivera A, Diaz-Cabiale Z, Jacobsen K, Tinner-Staines B, Hagman B, Leo G, Staines W, Guidolin D, Kehr J, Genedani S, Belluardo N, Agnati LF (2007) From the Golgi-Cajal mapping to the transmitter-based characterization of the neuronal networks leading to two modes of brain communication: wiring and volume transmission. Brain Res Rev 55:17–54. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Tarakanov AO, Calvo F, Garriga P, Tena M, Narvaez M, Millón C, Parrado C, Ciruela F, Agnati LF, Narvaez JA, Díaz-Cabiale Z (2012) On the existence and function of galanin receptor heteromers in the central nervous system. Front Endocrinol (Lausanne) 3:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich EV, Aleksandrova IA, Otmakhova NA, Katkov YA, Nesterova IV, Bobkova NV (1993) Effects of bulbectomy and subsequent antidepressant treatment on brain 5-HT2 and 5-HT1A receptors in mice. Pharmacol Biochem Behav 45:65–70. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Fuxe K (1996) Galanin and 5-HT1A receptor interactions as an integrative mechanism in 5-HT neurotransmission in the brain. Ann N Y Acad Sci 780:193–212. [DOI] [PubMed] [Google Scholar]
- Holsboer F (2000) The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23:477–501. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Barden N (1996) Antidepressants and hypothalamic-pituitary-adrenocortical regulation. Endocr Rev 17:187–205. [DOI] [PubMed] [Google Scholar]
- Holzmann I, da Silva LM, Corrêa da Silva JA, Steimbach VM, de Souza MM (2015) Antidepressant-like effect of quercetin in bulbectomized mice and involvement of the antioxidant defenses, and the glutamatergic and oxidonitrergic pathways. Pharmacol Biochem Behav 136:55–63. [DOI] [PubMed] [Google Scholar]
- Jans LA, Riedel WJ, Markus CR, Blokland A (2007) Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry 12:522–543. [DOI] [PubMed] [Google Scholar]
- Jiménez-Sánchez L, Linge R, Campa L, Valdizán EM, Pazos Á, Díaz Á, Adell A (2016) Behavioral, neurochemical and molecular changes after acute deep brain stimulation of the infralimbic prefrontal cortex. Neuropharmacology 108:91–102. [DOI] [PubMed] [Google Scholar]
- Juhasz G, Hullam G, Eszlari N, Gonda X, Antal P, Anderson IM, Hökfelt TG, Deakin JF, Bagdy G (2014) Brain galanin system genes interact with life stresses in depression-related phenotypes. Proc Natl Acad Sci U S A 111:E1666–E1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuteeva E, Hökfelt T, Wardi T, Ogren SO (2008) Galanin, galanin receptor subtypes and depression-like behaviour. Cell Mol Life Sci 65:1854–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcilhac A, Faudon M, Anglade G, Hery F, Siaud P (1999) An investigation of serotonergic involvement in the regulation of ACTH and corticosterone in the olfactory bulbectomized rat. Pharmacol Biochem Behav 63:599–605. [DOI] [PubMed] [Google Scholar]
- Millón C, Flores-Burgess A, Narváez M, Borroto-Escuela DO, Santín L, Parrado C, Narváez JA, Fuxe K, Díaz-Cabiale Z (2014) A role for galanin N-terminal fragment (1-15) in anxiety- and depression-related behaviors in rats. Int J Neuropsychopharmacol 18:pyu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millón C, Flores-Burgess A, Narváez M, Borroto-Escuela DO, Santín L, Gago B, Narváez JA, Fuxe K, Díaz-Cabiale Z (2016) Galanin (1-15) enhances the antidepressant effects of the 5-HT1A receptor agonist 8-OH-DPAT: involvement of the raphe-hippocampal 5-HT neuron system. Brain Struct Funct 221:4491–4504. [DOI] [PubMed] [Google Scholar]
- Millón C, Flores-Burgess A, Narváez M, Borroto-Escuela DO, Gago B, Santín L, Castilla-Ortega E, Narváez JÁ, Fuxe K, Díaz-Cabiale Z (2017) The neuropeptides galanin and galanin(1-15) in depression-like behaviours. Neuropeptides 64:39–45. [DOI] [PubMed] [Google Scholar]
- Millón C, Flores-Burgess A, Gago B, Alén F, Orio L, García-Durán L, Narváez JA, Fuxe K, Santín L, Díaz-Cabiale Z (2019) Role of the galanin N-terminal fragment (1-15) in anhedonia: involvement of the dopaminergic mesolimbic system. J Psychopharmacol 33:737–747. [DOI] [PubMed] [Google Scholar]
- Misane I, Razani H, Wang FH, Jansson A, Fuxe K, Ogren SO (1998) Intraventricular galanin modulates a 5-HT1A receptor-mediated behavioural response in the rat. Eur J Neurosci 10:1230–1240. [DOI] [PubMed] [Google Scholar]
- Mitsukawa K, Lu X, Bartfai T (2008) Galanin, galanin receptors and drug targets. Cell Mol Life Sci 65:1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsukawa K, Lu X, Bartfai T (2010) Galanin, galanin receptors, and drug targets. Exp Suppl 102:7–23. [DOI] [PubMed] [Google Scholar]
- Morales-Medina JC, Iannitti T, Freeman A, Caldwell HK (2017) The olfactory bulbectomized rat as a model of depression: the hippocampal pathway. Behav Brain Res 317:562–575. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2017) Depression and other common mental disorders. Geneva, Switzerland: WHO. [Google Scholar]
- Ou XM, Storring JM, Kushwaha N, Albert PR (2001) Heterodimerization of mineralocorticoid and glucocorticoid receptors at a novel negative response element of the 5-HT1A receptor gene. J Biol Chem 276:14299–14307. [DOI] [PubMed] [Google Scholar]
- Razani H, Díaz-Cabiale Z, Misane I, Wang FH, Fuxe K, Ogren SO (2001) Prolonged effects of intraventricular galanin on a 5-hydroxytryptamine(1A) receptor mediated function in the rat. Neurosci Lett 299:145–149. [DOI] [PubMed] [Google Scholar]
- Riad M, Kobert A, Descarries L, Boye S, Rompré PP, Lacaille JC (2017) Chronic fluoxetine rescues changes in plasma membrane density of 5-HT1A autoreceptors and serotonin transporters in the olfactory bulbectomy rodent model of depression. Neuroscience 356:78–88. [DOI] [PubMed] [Google Scholar]
- Romeas T, Morissette MC, Mnie-Filali O, Piñeyro G, Boye SM (2009) Simultaneous anhedonia and exaggerated locomotor activation in an animal model of depression. Psychopharmacology 205:293–303. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Warden D, Wisniewski SR, Fava M, Trivedi MH, Gaynes BN, Nierenberg AA (2009) STAR*D: revising conventional wisdom. CNS Drugs 23:627–647. [DOI] [PubMed] [Google Scholar]
- Saenz del Burgo L, Cortés R, Mengod G, Montaña M, García del Caño G, Sallés J (2013) Chronic effects of corticosterone on GIRK1-3 subunits and 5-HT1A receptor expression in rat brain and their reversal by concurrent fluoxetine treatment. Eur Neuropsychopharmacol 23:229–239. [DOI] [PubMed] [Google Scholar]
- Schüle C, Baghai TC, Eser D, Rupprecht R (2009) Hypothalamic-pituitary-adrenocortical system dysregulation and new treatment strategies in depression. Expert Rev Neurother 9:1005–1019. [DOI] [PubMed] [Google Scholar]
- Tomiyama M, Palacios JM, Cortés R, Vilaró MT, Mengod G (1997) Distribution of AMPA receptor subunit mRNAs in the human basal ganglia: an in situ hybridization study. Brain Res Mol Brain Res 46:281–289. [DOI] [PubMed] [Google Scholar]
- Wang P, Li H, Barde S, Zhang MD, Sun J, Wang T, Zhang P, Luo H, Wang Y, Yang Y, Wang C, Svenningsson P, Theodorsson E, Hökfelt TG, Xu ZQ (2016) Depression-like behavior in rat: involvement of galanin receptor subtype 1 in the ventral periaqueductal gray. Proc Natl Acad Sci U S A 113:E4726–E4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MV, Hen R (2014) Functional dissociation of adult-born neurons along the dorsoventral axis of the dentate gyrus. Hippocampus 24:751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YF, Feng L, Liu XM, Tao X, Wang LS, Zhang MD, Wang Z, Chen SG, Chang Q (2019) Urinary metabolic disturbance in the olfactory bulbectomized rats and the modulatory effects of fluoxetine. Life Sci 234:116751. [DOI] [PubMed] [Google Scholar]
- Zhu H, Tao Y, Wang T, Zhou J, Yang Y, Cheng L, Zhu H, Zhang W, Huang F, Wu X (2020) Long-term stability and characteristics of behavioral, biochemical, and molecular markers of three different rodent models for depression. Brain Behav 10:e01508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.