Abstract
We sought to elucidate the acute effects of high-intensity interval training (HIIT) among college students with and without attention-deficit/hyperactivity disorder (ADHD). Participants were age- and sex-matched across ADHD (n = 18) and non-ADHD groups (n = 18) and both groups completed baseline (non-HIIT) and experimental sessions (HIIT). We examined within- and between-subject effects on a continuous performance task (CPT) and self-reported ADHD and internalizing symptomatology. We found that the degree of improvement on ADHD and depressive symptomatology, as well as processing speed and response variability following HIIT was significantly greater for the ADHD group than the comparison group. Further investigations such as randomized controlled trials focusing on the chronic effects of sustained HIIT interventions are needed to substantiate the potential feasibility and efficacy of HIIT as an intervention. HIIT may be a useful adjunct to psychosocial and/or pharmacological treatments for college students with ADHD because it: (a) yields immediate, acute improvements in executive functioning, ADHD, and mood; (b) promotes improved physical and mental health; (c) poses a relatively low risk of deleterious effects in apparently healthy college students. Even with the need for additional research, current data suggest a single, brief, high-intensity bout of aerobic exercise can yield immediate significant short-term improvements. These improvements may enhance functioning and improve outcomes for college students with ADHD.
Keywords: attention-deficit/hyperactivity disorder, internalizing symptomatology, cognition, high-intensity interval training, college students
Attention-deficit/hyperactivity disorder (ADHD) is characterized by developmentally inappropriate levels of inattention (IA) and hyperactivity/impulsivity (HI) that result in significant impairment in cross-domain daily functioning (APA, 2013). Although ADHD has traditionally been viewed as a disorder of childhood, empirical studies estimate that approximately two-thirds of children with ADHD experience clinically significant impairment and symptomatology into emerging adulthood (ages 18–25; Arnett, 2000) and adulthood with the prevalence among adults estimated to be at 3 to 4% (Kessler et al., 2006). ADHD is associated with various adverse health outcomes, such as increased risks of obesity (Nigg, 2013).
Individuals with ADHD often show deficits in executive functioning (EF), a set of top-down cognitive processes used to regulate behavior toward long-term goals (Barkley, 1997). Specific EF factors implicated in ADHD include the ability to inhibit impulses (i.e., inhibition), sustain attention over time (i.e., sustained attention), hold and manipulate information across a short delay (i.e., working memory), quickly respond to stimuli (i.e., processing speed), and consistently respond to stimuli (i.e., response variability; Willcutt et al., 2012). The neurological structures underlying self-regulation are among the latest to mature with development continuing until the end of emerging adulthood as demonstrated in neuro-imaging studies of population samples (Giedd, 2004) and ADHD samples (Roman-Urrestarazu et al., 2016). EF deficits in ADHD, however, are not solely the result of delayed development, but rather a core deficit that often persists and manifests in impairments for several important outcomes, such as academic achievement and socioeconomic status (Biederman & Faraone, 2006).
Emerging adulthood coincides with considerable developmental and environmental changes which yield a distinct set of challenges, especially for those with ADHD. The transition to emerging adulthood including attending college brings dramatic changes in lifestyle, autonomy, and responsibility, often including a marked decrease of parental oversight and structure, and corresponding increases in independence (Fleming & McMahon, 2012). An increasing number of individuals with ADHD are pursuing postsecondary education (Kuriyan et al., 2013). The base rate for ADHD among college students has been estimated to be between 2 and 8% (DuPaul et al., 2009), and up to 25% of students receiving university disability support services reported a diagnosis of ADHD (Wolf, 2001).
The confluence of an abrupt loss of structure and parental support, and a neurological system maturing at a delayed rate, often results in significant impairment for college students with ADHD (Fleming & McMahon, 2012). Thus, these individuals experience increased demands on executive control at a time when they experience a “double-deficit” (ADHD- and maturational-related deficits) in self-regulation (Fleming & McMahon, 2012). Compared to their peers, students with ADHD tend to report more academic concerns (Lewandowski et al., 2008), have higher rates of academic probation (Frazier et al., 2007), have lower grade point averages (GPAs; Frazier et al., 2007) and lower graduation rates (Wolf, 2001). In addition to academic difficulties, college students with ADHD have higher rates of tobacco and risky alcohol use (Rooney et al, 2012), internalizing symptoms (Rabiner et al., 2008), overall psychological distress (Richards et al., 1999), and poorer quality of life (Grenwald-Mayes, 2002).
Despite the well-documented effectiveness of psychosocial and pharmacological interventions for ADHD in children, there are limitations to their use in emerging adults. Although both interventions are efficacious during active treatment, engagement in these treatments diminishes precipitously in emerging adulthood (McCarthy et al., 2009). Some of the known reasons for the decline in treatment use include intolerable side effects of medication (e.g., decreased appetite, poor sleep), cognitive-behavioral therapies not being available on all college campuses, and development of comorbid disorders (e.g., anxiety, depression) that may interfere with the effectiveness of ADHD treatments.
Current limitations of behavioral and stimulant treatments highlight the need for novel, adjunctive treatments given the unique context of college and transitions of emerging adulthood (LaCount et al., 2019; Vasko et al., 2020). Physical exercise (PE) has received increasing attention because of its beneficial acute and chronic effects on physical and mental health-related outcomes. A growing body of research among children and adults has suggested that PE has powerful effects on cognitive and neurobiological functioning (Hillman et al., 2008). Acute PE increases levels of certain neurotransmitters (e.g., endorphins, dopamine, norepinephrine, serotonin), increases blood flow to the brain, and increases levels of brain derived neurotrophic factor (BDNF) (Basso & Suzuki, 2017). BDNF is involved in brain plasticity and increased levels of BDNF following exercise are associated with improved cognitive function (Basso & Suzuki, 2017). A meta-analysis by Moreau and Chou (2019) found that high-intensity PE results in significant improvements in EF for adults. Developing from this burgeoning evidence, researchers have begun to explore physical exercise as an avenue to improve mental health.
The benefits of PE may be larger for individuals with ADHD due to a higher likelihood of EF deficits and poorer mental and physical health outcomes. Indeed, PE has been hypothesized to impact EF among individuals with ADHD in ways similar to psychostimulants by increasing the catecholamines (e,g., dopamine, norepinephrine) believed to influence cognitive functioning and mood (Wigal et al., 2012). A recent review of the literature for children and adolescents with ADHD concluded that there are numerous benefits of both acute (i.e., a single bout) and chronic PE (i.e., repeated bouts; Neudecker et al., 2019). Regarding acute effects, efficacy studies found improvements following aerobic exercise in EF, behavioral, and academic performance. Chronic effects of various forms of exercise in youth with ADHD included improvements in ADHD symptomatology, motor skills, and physical fitness (Neudecker et al., 2019). Despite only one published study in emerging adults with ADHD, preliminary results are promising. Specifically, Gapin and colleagues (2015) examined the acute effects of moderate-intensity aerobic exercise and found improvements in EF (i.e., behavioral inhibition) following exercise for college students with ADHD.
Despite only one published study examining the acute effects of PE for college students with ADHD, there are numerous reasons that PE may be a uniquely powerful adjunct to psychosocial and/or pharmacological treatments (LaCount & Hartung, 2018). First, the existing literature in children and adolescents with ADHD provides preliminary evidence to suggest that PE can produce acute and chronic improvements in cognitive and behavioral functioning that may generalize to college students. Second, regardless of ADHD status, PE is recommended for all adults to improve overall health and reduce the likelihood of future diseases (U.S. Department of Health and Human Services, 2008) and is arguably more critical for individuals with ADHD, who are a greater risk for many poor health outcomes (Nigg, 2013). Third, PE helps ameliorate depressive and anxiety symptoms (De Moor et al., 2006), which commonly co-occur with ADHD, especially among emerging adults (Meinzer et al., 2013). Lastly, for college students without medical contraindications to PE, safe levels of PE are a “do no harm” intervention tool with no known persistent adverse effects (Hoza et al., 2015).
To elucidate the acute benefits of PE, university students with ADHD and age-and sex-matched non-ADHD peers were recruited for two in-lab sessions spaced approximately one week apart. Across two counterbalanced sessions, participants in both groups completed outcome measures without any exercise and following a single bout of high-intensity interval training (HIIT). The primary outcome measures included EF performance indices (i.e., sustained attention, inhibition, processing speed, and response variability) from a computerized task and ratings of depression, anxiety, stress, IA symptoms, and HI symptoms from questionnaires. To evaluate the effect of a single bout of high-intensity aerobic exercise on EF and related symptomatology in college students with ADHD, we hypothesized: (a) all participants, regardless of ADHD status, would demonstrate significant acute improvements on all EF performance indices (i.e., sustained attention, inhibition, processing speed, and response variability) following HIIT, relative to non-HIIT: (b) the degree of improvement between HIIT and non-HIIT sessions on all EF performance indices would be significantly greater among those with ADHD than their non-ADHD peers; (c) all participants, regardless of ADHD status, would demonstrate significant acute improvements on depression, anxiety, stress, inattention, and hyperactivity/impulsivity following HIIT, relative to non-HIIT, from ratings that were obtained the following day; and (d) the degree of improvement between HIIT and non-HIIT sessions on ratings of depression, anxiety, stress, IA, and HI would be significantly greater among those with ADHD than their non-ADHD peers.
Method
A power analysis conducted using G-Power 3.1.9.7 (Faul et al., 2007) indicated that 36 total participants (18 participants for the ADHD group and 18 for the comparison group) would be necessary to achieve adequate power (0.80) to detect a medium effect size (f = 0.25) using a repeated measure, within-between interaction ANOVA. Participants were recruited via multiple means (e.g., flyers, department participant pool pre-screener, referrals from the University Psychology Clinic).
Participants included students at the university who were between 18- to 25-years-old, were determined to be at low risk for PE contraindications based on an extensive health history obtained through an online health screener, were not taking medications that could confound results (e.g., SSRIs, sedatives), and were not taking non-stimulant ADHD medication (e.g., Strattera). Participants were eligible for the ADHD group if they met Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM-5) criteria for either the combined (ADHD-C; n = 12) or predominantly inattentive presentation of ADHD (ADHD-IA; n = 8) via a clinical interview (Adult ADHD Diagnostic Interview; ACDS; Adler & Cohen, 2004) administered by clinical psychology doctoral students. The audio/video recorded interviews were scored by another interviewer to evaluate inter-rater reliability, which was good to excellent (Cohen’s Kappa ranged from .81 to .96). Most ADHD participants reported using stimulants to manage their ADHD symptoms (n = 11).
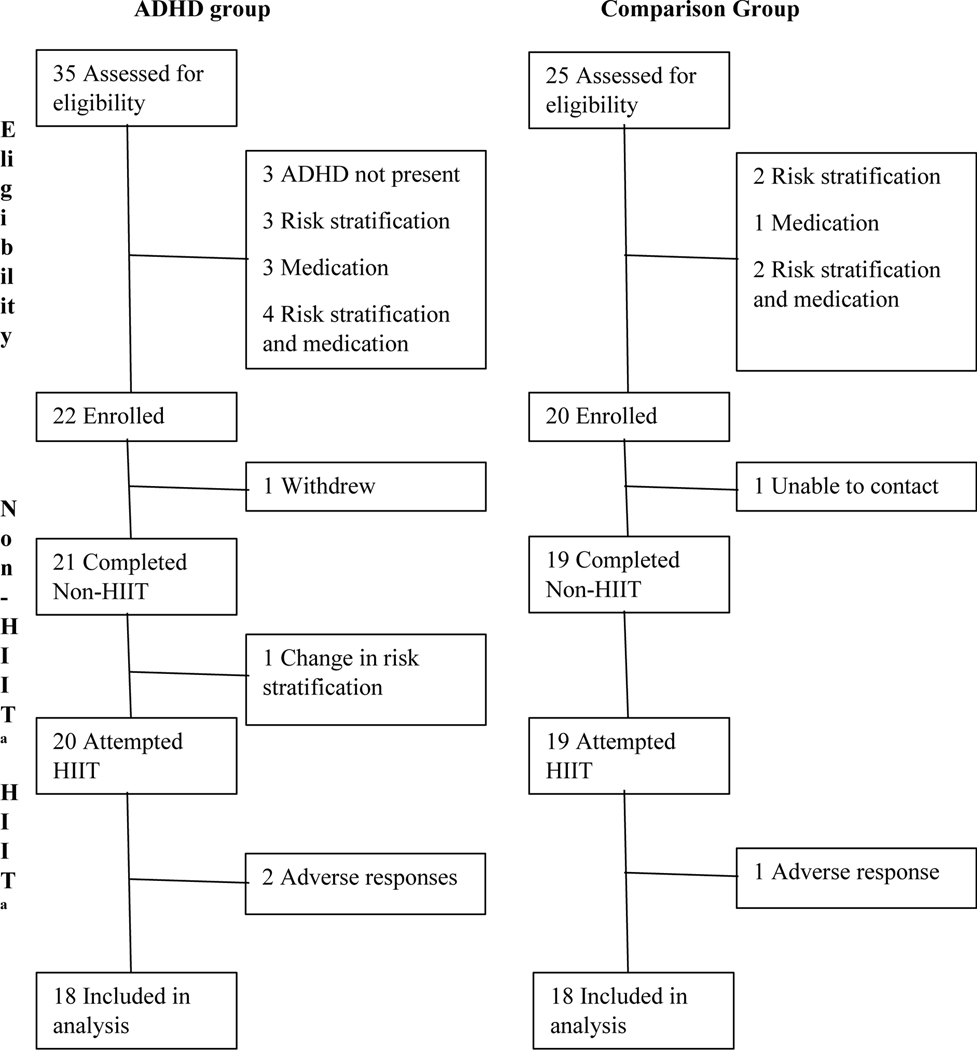
Non-ADHD group participants were eligible if they disavowed ever being diagnosed with ADHD, endorsed sub-clinical levels of ADHD symptoms on a questionnaire (i.e., fewer than three symptoms of IA or HI; Hartung et al., 2019), and were an age and sex match for an ADHD group participant. Figure 1 outlines the participant recruitment process. The characteristics of the final sample (N = 36) are provided in Table 1. Participants completed a three-minute validated step test (Queens College/McArdle Step Test; Liguori, 2021) in which HR was measured to estimate and compare aerobic fitness between ADHD and non-ADHD participants. Notably, the baseline aerobic fitness step test revealed that ADHD and non-ADHD groups had similar estimated aerobic fitness.
Figure 1.
Participation flowchart. ADHD = attention-deficit/hyperactivity disorder; Non-HIIT = in-lab appointment without high-intensity interval training; HIIT = in-lab appointment with high-intensity interval training. Adverse response = experienced lightheadedness and queasiness
a The Non-HIIT and HIIT sessions were counterbalanced, while also keeping the day of the week and time consistent across appointments.
Table 1.
Baseline Demographics and Characteristics by Group
| ADHD group (N = 18) | Non-ADHD group (N = 18) | |||
|---|---|---|---|---|
|
|
|
|||
| Variable | M (SD) | M (SD) | t a | P |
| Age in years | 20.8 (1.7) | 20.8 (1.7) | .00 | 1.0 |
| Year in college | 3.0 (1.5) | 2.8 (1.3) | .36 | .72 |
| ACT composite | 26.2 (3.6) | 24.9 (4.1) | .92 | .37 |
| WFIRS | ||||
| Family | 0.57 (.46) | 0.17 (.16) | 3.5 | <.01 |
| Work | 0.80 (.53) | 0.11 (.19) | 5.2 | <.01 |
| School | 1.32 (.70) | 0.26 (.26) | 6.0 | <.01 |
| Life skills | 1.17 (.54) | 0.45 (.56) | 3.9 | <.01 |
| Self-concept | 1.51 (.77) | 0.53 (.52) | 4.5 | <.01 |
| Social | 0.91 (.55) | 0.24 (.25) | 4.7 | <.01 |
| Risk | 0.42 (.26) | 0.21 (.22) | 2.6 | .02 |
| BAARS | ||||
| IA symptoms | 6.17 (2.66) | 0.50 (1.15) | 8.29 | <.01 |
| HI symptoms | 3.28 (2.37) | 0.11 (.32) | 5.61 | <.01 |
| DASS | ||||
| Depression | 3.94 (3.62) | 1.22 (2.02) | 2.8 | .01 |
| Anxiety | 4.56 (4.16) | 1.72 (1.53) | 2.7 | .01 |
| Stress | 6.33 (4.10) | 2.94 (3.17) | 2.8 | .01 |
| Step Test (ml/kg/min) | 40.49 (8.74) | 40.82 (7.39) | .12 | .91 |
| n (%) | n (%) | χ2ab | p | |
|
|
||||
| Male | 9 (50.0) | 9 (50.0) | .00 | 1.0 |
| European American | 13 (72.2) | 15 (83.3) | 2.81 | .42 |
| Active lifestyle | 5 (35.7) | 9 (50.0) | 1.87 | .17 |
Note. ADHD = attention-deficit/hyperactivity disorder; ACT = American College Test; Active Lifestyle = self-reported competitive athlete or has job that requires being physically active (e.g., athletic trainer, ranch hand); DASS = Depression Anxiety Stress Scale; HI = ADHD hyperactivity/impulsivity symptoms present; IA = ADHD inattention symptoms present; WFIRS = Weiss Functional Impairment Rating Scale.
p-values are 2-tailed
Fisher’s exact test was employed when the frequency count within cells was < 5 (Fisher, 1922).
Due to the inclusion of human subjects, this study was reviewed and approved by the local institutional review board (IRB). This included IRB review and approval of procedures for screening participants as low risk for high-intensity PE and addressing complications during or after PE. All participants provided verbal and written informed consent.
Cognitive Functioning Measures
AX-Continuous Performance Test (AX-CPT).
This standardized computer-administered version of the CPT (Cohen et al., 1999) consists of 360 letter presentations that appear on the screen for 250ms and are 1-inch in size. Participants were instructed to press the spacebar when they saw the letter “X” after seeing the letter “A” (creating an A-X sequence). Four performance indices were derived from the CPT: omission errors (no response when presented target), commission errors (response when target was not presented), reaction time, and reaction time variability. Omission errors were used as a measure of sustained attention; commission errors were used as a measure of inhibition; reaction time was used as a measure of processing speed; and reaction time variability was used as a measure of response variability. While psychometric data is limited for the AX-CPT, in both community and clinical samples spanning from 8 to 60+ years of age, the Conners CPT (a similar version of the AX-CPT) has demonstrated excellent internal consistency (α > .90) and robust test-retest reliability (r = .67; Conners, 2008). The AX-CPT was chosen for this study over the Conners because it has been shown to be effective at detecting within-participant changes (e.g., Gonthier et al., 2016); whereas the Conners CPT is typically used to detect the presence of deficits associated with ADHD.
Psychological Functioning Measures
Barkley Adult ADHD Rating Scale - Modified (BAARS-M).
The BAARS includes 18 ADHD symptom items (9 IA, 9 HI) that closely follow the DSM-5 criteria for ADHD (Barkley, 2011). Participants rated their off-medication behavior (if applicable) from 0 (Never or Rarely) to 3 (Very Often). The BAARS has demonstrated acceptable (α = .78) to excellent (α = .91) internal consistency, adequate reliability over a 2- to 3-week period (r = .66 to r = .75) and has been supported by factor analysis in adult samples (Barkley, 2011). For the current study, the time frame of reporting for the BAARS-M was modified from being based on behavior “during the past six months” to being based on behavior “yesterday (from the time after your lab appointment until you went to bed).” The sum of item responses was used to quantify IA and HI severity for days in which participants attended an in-lab session. In the present study, Cronbach’s α for IA and HI scores were .94 and .93, respectively.
Depression Anxiety Stress Scale - Modified (DASS-M).
The DASS includes 21 items measuring current mood, anxiety, and stress levels including a choice of four responses from 0 to 3 (Lovibond & Lovibond, 1995). The DASS items yield three subscales: depression, anxiety, and stress. The subscales correlate with similar measures, such as the Beck Depression Inventory (BDI-II; r = .79), Beck Anxiety Inventory (BAI; r = .85), and State-Trait Anxiety Inventory (STAI-T; r = .68), respectively. The DASS subscales demonstrate good (α = .87) to excellent (α = .94) internal consistency among college students (Lovibond & Lovibond, 1995). The time frame of reporting for the DASS-M was modified from being based on behavior “during the past week” to being based on behavior “yesterday (from the time after your lab appointment until you went to bed).” Subscale scores on the DASS-M were used to quantify depressive, anxiety, and stress on days in which participants attended an in-lab session. In the present study, Cronbach’s α for depression, anxiety, and stress subscales were .90, .74, and .89, respectively.
Exercise Measures
Borg’s Rating of Perceived Exertion (RPE).
The RPE scale (Borg, 1998) was used to quantify participant’s perceived exertion during HIIT. Participants were introduced to the scale prior to exercise and reported their exertion from 6 (No Exertion) to 20 (Maximal Exertion) after each exercise interval. RPE has been validated in studies of steady-state exercise and in studies typically using non-ADHD specific samples. RPE is significantly associated with heart rate (r = .62), blood lactate (r = .57), %VO2max (r = .64), VO2 (r = .63), ventilation (r = .61), and respiratory rate (r = .72; Chen et al., 2002). RPE was used to verify that participants were reaching the target perceived exertion of 17 (Very Hard).
Heart rate (HR).
HR was measured with a Polar (chest strap) HR monitor (Mode S 610i; Polar Electro, Finland). This data was used to verify that participants reached 85% of their age-predicted max HR (HRmax; 220 – age), corresponding to high intensity, and that they recovered before starting the cognitive tasks.
Procedure
Participants attended two in-lab morning appointments (non-HIIT and HIIT) separated by approximately one week. The participants began their two appointments at the same time (e.g., both appointments for a participant started at 8AM on a Thursday). Participant pairs were counterbalanced into different session orders to ensure that effects were not related to the order of sessions. For each session, participants were reminded to abstain from PE, alcohol, caffeine, and ADHD medication for the 12 hours prior to their appointment to minimize the influence of confounding variables. Other requirements to attend the sessions included feeling healthy over the past seven days, receiving adequate sleep the prior night (at least six hours), and having eaten within 90 minutes of the session. When obtaining informed consent for the study, researchers told participants the purpose of the study was to evaluate the acute effects of HIIT on cognition and mood. At the beginning of each session, participants were equipped with the HR monitor. After equipping the HR monitor, participants in the HIIT session completed the PE protocol and then the CPT computer task and participants in the non-HIIT condition completed the CPT task. Participants were sent web links to the BAARS-M and DASS-M the mornings after the HIIT and non-HIIT sessions. Participants had until midnight of the day following their sessions to complete the questionnaires and received reminders in the mornings, afternoons, and evenings.
HIIT Protocol.
The researcher led the participant through a set of stretches and 3 minutes of low-intensity cycling on a Schwinn AD2 Airdyne leg-cycling and arm-cranking ergometer to warm up and increase blood flow to active muscles. The participant then completed 16 minutes of HIIT, consisting of eight bouts of 20 seconds of cycling followed by 120 seconds of rest (8×20×120). The experimenter provided encouragement to the participant if their HR was not reaching the target HR (85% HRmax) during the last four sprint intervals. The researcher recorded HR and RPE after every sprint interval. After the last interval, the participant was given 15 minutes to recover (HR < 130bpm) before beginning the cognitive task. Three participants (2 ADHD, 1 non-ADHD) were excluded from the final dataset due to experiencing adverse effects during the HIIT protocol. In these cases, the participants completed at least four HIIT intervals before reporting lightheadedness and queasiness and being told to stop. One of the three participants who experienced adverse effects had not disclosed to researchers about a history of syncope, which resulted in a change in risk stratification such that they were no longer eligible to participate in the study.
Analytic Approach
Data were screened to assess accuracy of entry, presence of outliers, homogeneity of variance, sphericity, and multivariate normality. Bivariate correlations between outcome measures and participant characteristics within groups were performed to assess for possible covariates. No participant characteristics were significantly correlated with the outcome measures (ps <.10). A manipulation check was performed to screen out participants who did not meet our threshold for engaging in high-intensity aerobic exercise during the HIIT session. Specifically, our criteria for high-intensity aerobic exercise were reaching either 85% HRmax or an RPE of 17 (Very Hard) during HIIT. Additionally, independent samples t-tests and chi-square models were used to evaluate differences in HRmax and RPE between groups to examine for any differences between the measures of exercise intensity. Fisher’s exact test was used in place of chi-square when the frequency count within cells was fewer than five (Fisher, 1922).
For primary analyses, relative improvements on objective measures of EF and next-day reports of mood as well as ADHD symptom severity were examined through multiple 2 (Group: ADHD vs. non-ADHD) x 2 (Condition: non-HIIT vs. HIIT) repeated measures ANOVAs. When Group x Condition interactions were significant, paired samples t-tests were used to evaluate differences within groups. Independent sample t-tests were used to evaluate differences between groups on post-intervention measures if interaction effects failed to reach statistical significance.
The magnitude of omnibus effects for repeated measures ANOVAs were calculated using partial eta-square (ηp2). Within-group effects (Cohen’s d) and corresponding confidence intervals for within-group effect sizes were standardized using the variability of non-HIIT scores (Cumming & Finch, 2001).1 Between-group effects were calculated using Hedges g (Hedges, 1988). Values for ηp2 of .01, .06, and .14 were considered small, medium, and large effects, respectively; corresponding values for d and g are .20, .50, and .80 (Cohen, 1988).
Results
Manipulation Check.
All participants reached our threshold for high-intensity aerobic exercise (i.e., 85% HRmax or an RPE of 17) during the HIIT session. There was a significantly larger proportion of ADHD participants who reached both the 85% HRmax and RPE≥ 17 (77.8%) than the non-ADHD group (38.9%, p = .04) during the HIIT session. There was no significant difference in the HRmax reached between the ADHD group (M = 176.61, SD = 8.36) and non-ADHD group (M = 173.83, SD = 7.14, p = .29, g = .35, small effect size). A greater proportion of ADHD participants reached the RPE target (94.4%) than the non-ADHD group (44.4%, p = .003). The ADHD group’s max RPE (M = 17.94, SD = 1.21) was significantly higher than the non-ADHD group’s max RPE (M = 16.56, SD = 1.62, p <.01, g = .94, large effect size).
Executive Functioning
Commission Errors.
There was a non-significant, small Group x Condition interaction effect on commission errors (p = .44, ηp2 = .02, small effect size; see Table 2)2 . There was no significant main effect of Group (p = .85, ηp2 <.01). The main effect of Condition approached statistical significance (p = .052, ηp2 = .11, medium effect size), with both groups committing fewer commission errors following HIIT. Independent samples t-tests found no statistically significant group differences on the rates of commission errors following HIIT (Δ = .28, SEΔ = .50, p = .59, g = .18).
Table 2.
Acute Effects of HIIT by Group
| ADHD (N = 18) | Non-ADHD (N = 18) | Group | Condition | Group × Condition | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| Variable | Non-HIIT M (SD) | HIIT M (SD) | Non-HIIT M (SD) | HIIT M (SD) | P | η p 2 | P | η p 2 | P | η p 2 |
| CPT | ||||||||||
| Commit1 | 2.17 (3.55) | 1.00 (1.09) | 1.83 (1.69) | 1.28 (1.84) | .96 | <.01 | .052 | .11 | .48 | .02 |
| Omit1 | 3.11 (4.67) | 1.44 (1.72) | 2.56 (3.54) | 2.11 (2.95) | .96 | <.01 | .03 | .13 | .20 | .05 |
| RTm1 | 414.33 (79.41) | 370.69 (44.60) | 397.99 (67.82) | 395.02 (49.00) | .83 | <.01 | .01 | .19 | .02 | .15 |
| RTv | 79.89 (26.55) | 63.87 (21.01) | 72.97 (21.44) | 75.56 (24.43) | .73 | <.01 | .08 | .09 | .02 | .15 |
| Mood | ||||||||||
| Depressive | 3.33 (3.45) | 1.72 (2.52) | 0.67 (1.37) | 0.56 (0.71) | .01 | .20 | .03 | .13 | .055 | .10 |
| Anxiety | 2.44 (2.55) | 2.17 (2.04) | 0.78 (1.22) | 0.78 (0.94) | .01 | .20 | .64 | .01 | .64 | .01 |
| Stress | 4.50 (4.00) | 3.22 (2.82) | 1.50 (2.07) | 1.44 (1.16) | .01 | .20 | .14 | .06 | .18 | .05 |
| ADHD | ||||||||||
| IA | 21.06 (5.60) | 15.94 (4.44) | 10.89 (2.08) | 10.78 (2.63) | <.001 | .61 | <.01 | .25 | .01 | .23 |
| HI | 16.17 (5.29) | 14.22 (4.28) | 9.78 (1.31) | 9.89 (1.53) | <.001 | .41 | .03 | .13 | .02 | .16 |
Note. Non-HIIT = in-lab appointment without high-intensity interval training; HIIT = in-lab appointment with high-intensity interval training; CPT = continuous performance task; Commit = commission errors; Omit = omission errors; RTm = average reaction time; RTv = reaction time variability; IA = inattention symptom severity; HI = hyperactivity-impulsivity symptom severity.
The Greenhouse-Geisser correction was applied to the CPT measures that failed the Shapiro-Wilk’s Test of Normality.
Omission Errors.
The Group x Condition interaction effect for omission errors was small and not significant (p = .20, ηp2 = .05, small effect size). There was a significant main effect of Condition (p = .03, ηp2 = .13, medium effect size) but not Group (p = .95, ηp2 <.01). Across groups, participants committed fewer omission errors following HIIT. Independent samples ttests found no statistically significant group differences on the rates of omission errors following HIIT (Δ = .67, SEΔ = .81, p = .41, g = .14).
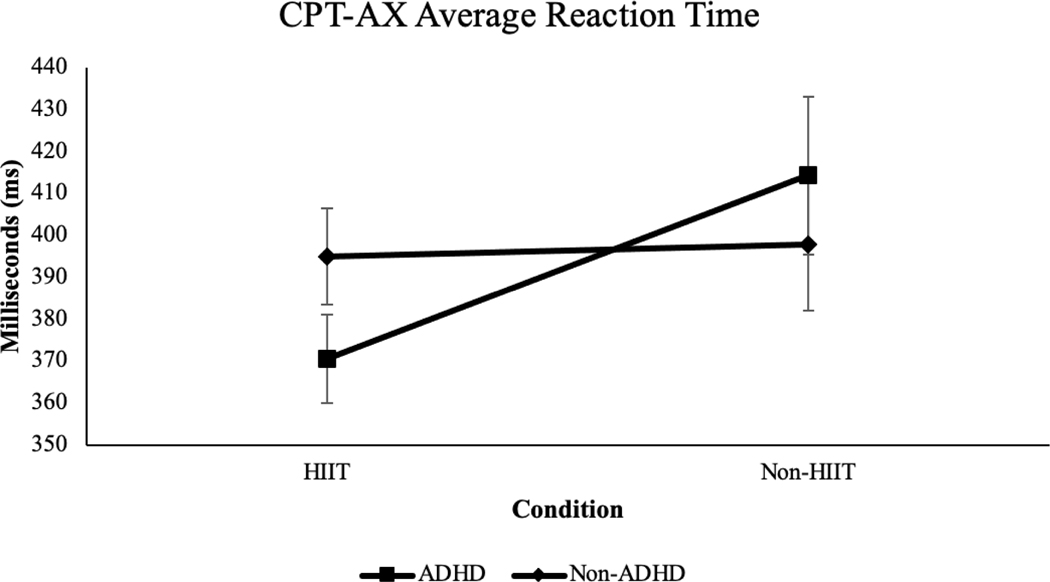
Average Reaction Time.
There was a large Group x Condition effect on average reaction time (p = .02, ηp2 = .15, large effect size; see Figure 2). Within-group pairwise comparisons indicated a moderate-sized beneficial effect of HIIT on reaction time among the ADHD group (Δ = 43.64, SEΔ = 14.29, p = .01, d = .55, medium effect size 95% CI [.18, 1.16]) and no significant change among the non-ADHD group (Δ = 2.97, SEΔ = 8.72, p = .74, d = .04).
Figure 2.
Average reaction time for ADHD and non-ADHD groups across HIIT and non-HIIT conditions, p = .02, ηp2 = .15, large effect size.
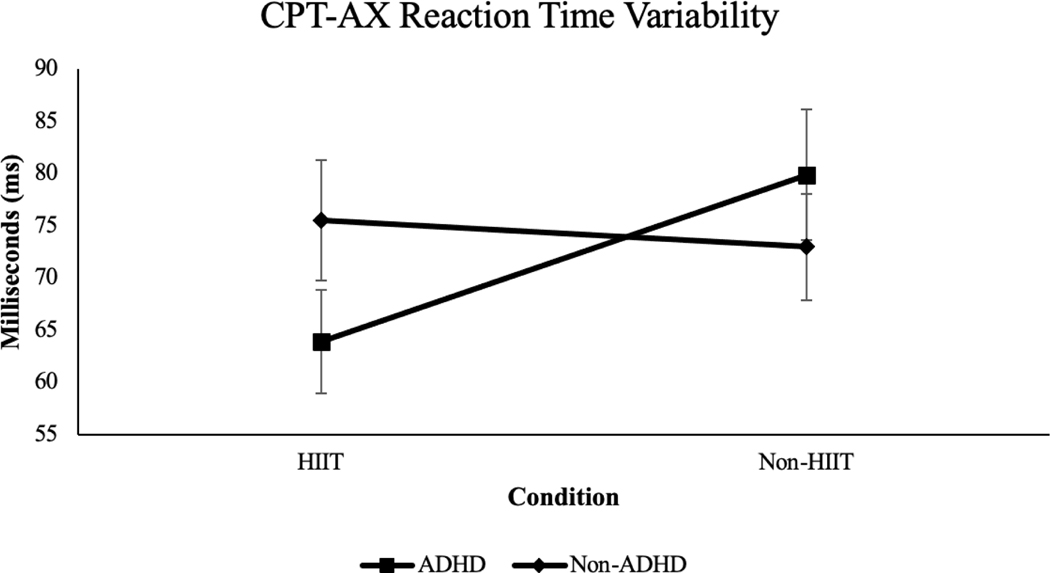
Reaction Time Variability.
The Group x Condition interaction on reaction time variability was large and statistically significant (p = .02, ηp2 = .15, large effect size; see Figure 3). Within-group pairwise comparisons found HIIT was associated with a medium-sized reduction of reaction time variability among those with ADHD (Δ = 16.02, SEΔ = 5.87, p = .01, d = .60, large effect size, 95% CI [.42, .92]) and no significant change for those in the non-ADHD group (Δ = 2.59, SEΔ = 4.70, p = .59, d = .12).
Figure 3.
Reaction time variability for ADHD and non-ADHD groups across HIIT and non-HIIT conditions, p = .02, ηp2 = .15, large effect size.
Ratings of Same-Day ADHD Symptoms
Inattention Symptom Severity.
There was a significant and large Group x Condition effect on IA symptom severity (p = .01, ηp2 = .23, large effect size). Within-group pairwise comparisons found a moderate reduction of IA symptom severity following HIIT for those with ADHD (Δ = 5.11, SEΔ = 5.94, p < .01, d = .78, medium effect size, 95% CI [.30, 1.64]) and no significant change among the non-ADHD group (Δ = .11, SEΔ = 2.93, p = .87, d = .05).
Hyperactive/Impulsive Symptom Severity.
There was a large Group x Condition effect on HI symptom severity (p = .01, ηp2 = .23, large effect size). Regardless of ADHD status, HIIT had a moderate effect on HI symptom severity (p = .03, ηp2 = .13, medium effect size). Within-group pairwise comparisons found HIIT was associated with a small reduction of HI symptom severity among those with ADHD (Δ = 1.94, SEΔ = 3.39, p = .03, d = .37, small effect size, 95% CI [.05, .75]) and no significant change among the non-ADHD group (Δ = .11, SEΔ = .18, p = .54, d = .08).
Ratings of Same-Day Mood and Stress
Depression.
There was a medium Group x Condition effect on depression ratings that approached statistical significance (p = .055, ηp2 = .10, medium effect size). The ADHD group reported medium-sized improvements in depression (Δ = 1.61, SEΔ = .72, p = .04, d = .47, small effect size, 95% CI [.32, .74]) whereas no significant change was detected for the non-ADHD group (Δ = .11, SEΔ = .24, p = .65, d = .08).
Anxiety.
There was not a significant Group x Condition interaction effect on anxiety ratings (p = .64, ηp2 = .01, small effect size). There was a large, significant main effect of Group on anxiety (p = .01, ηp2 = .20, large effect size), but not Condition (p = .64, ηp2 = .01, small effect size). ADHD participants reported more anxiety across both sessions relative to their non-ADHD peers. Independent samples t-tests found ratings of anxiety were markedly higher among students in the ADHD group than the non-ADHD group following HIIT (Δ = 1.39, SEΔ = .53, p = .02, g = .88, large effect size).
Stress.
The small Group x Condition interaction effect on stress ratings was not significant (p = .18, ηp2 = .05, small effect size). There was a medium significant main effect of Group (p = .01, ηp2 = .20, large effect size) but not Condition (p = .14, ηp2 = .06, medium effect size) on stress ratings. ADHD participants reported higher stress following both sessions relative to their non-ADHD peers. Independent samples t-tests found post-HIIT session ratings of stress were markedly higher among students in the ADHD group than non-ADHD group (Δ = 1.28, SEΔ = .77, p = .03, g = .81, large effect size).
Discussion
We evaluated the acute effects of HIIT for college students with ADHD relative to age- and sex-matched non-ADHD peers. High-intensity physical exercise (PE) has been shown to result in significant and small improvements in executive functioning (EF; Moreau and Chou, 2019), and aerobic exercise in general has been associated with improved mood (Hillman, 2008). Neurobiological mechanisms believed to contribute to these improvements following PE include increased levels of certain neurotransmitters (e.g., endorphins, dopamine, norepinephrine, serotonin), increased blood flow to the brain, and increased levels of brain derived neurotrophic factor (BDNF; Basso & Suzuki, 2017). For these reasons, we expected all college students— regardless of ADHD status—to benefit from a bout of HIIT in EF, cognitive functioning, and mood. However, we expected that the benefits would be significantly larger for students with ADHD due to greater EF deficits and poorer mental health as compared to non-ADHD peers.
The hypothesis that EF performance indices would improve following HIIT for all participants, regardless of ADHD status, was supported for sustained attention (i.e., CPT omission errors) and processing speed (i.e., CPT average reaction time) with medium to large effect sizes. These results are consistent with previous research published on the positive association between PE and EF in general populations including children and adults (Hillman, 2008), childhood ADHD samples (Pontifex et al., 2013), and college student ADHD samples (Gapin et al., 2015).
Additionally, our results partially supported our hypothesis that the degree of improvement across all EF indices would be significantly greater for students with ADHD. Our results for processing speed and response variability were consistent with this hypothesis and prior findings that PE is uniquely beneficial for emerging adults who are already markedly impaired in the cognitive domain being measured (Sibley & Beilock, 2007). It was not surprising that the effect of PE on processing speed and response variability was largely driven by students with ADHD given that individuals with ADHD often experience greater deficits in these areas than their typically developing peers. The results for sustained attention and inhibition failed to reach statistical significance. This suggests that PE may not have an impact on all specific factors of EF such as sustained attention and inhibition that individuals with ADHD may have deficits in. However, in retrospect, another possible explanation is that our chosen EF task was not sensitive to detecting between-group differences. This is evidenced by the lack of between-group differences across outcomes. If the chosen instrument was not sensitive enough to detect differences at baseline between the ADHD and non-ADHD groups that are well established (Solanto, 2015), this suggests that we should use a more challenging version of the CPT in future studies.
The hypothesis that HIIT would be associated with ameliorating self-reported psychological symptoms across college students with and without ADHD was supported for depression, IA, and HI symptom severity with large effect sizes. These results are consistent with the association found between increased PE and decreased depression (Salmon, 2001) and ADHD symptomatology (Neudecker et al., 2019). Conversely, we failed to detect significant improvements in our measures of anxiety and stress across all study participants. This may indicate that one bout of acute HIIT does not impact anxiety and stress. There are also several alternative explanations for these non-significant findings. First, while adapting the DASS allowed us to more easily compare acute ratings of depression, anxiety, and stress, items corresponding to anxiety and stress may not be sensitive to change at the 24-hour time scale. Similarly, there are several DASS items that better correspond to more severe, long-standing anxiety and stress, which may have attenuated the questionnaire’s validity. Lastly, the adapted version of the DASS used for this study, and its psychometric properties have not been evaluated beyond the internal consistency among this sample. These encouraging findings may have important implications for investigating and developing treatments for college students with ADHD, who often present with co-occurring depressive symptomatology (Meinzer et al., 2013).
Limitations.
While our results are encouraging and informative for advancing knowledge of the acute effects of high intensity exercise for college students with ADHD, there are limitations to this preliminary study. First, HIIT effects on underlying neuropsychological deficits of ADHD were limited to proximal, objective measures of EF. Although performance on these tasks have evidenced valid and reliable properties in psychometric investigations, the data obtained through computerized tasks may not generalize to improving EF-related impairment. Therefore, future investigations should incorporate both immediate measures of neuropsychological functioning with more distal outcome measures (e.g., homework completion, exam performance). Along the same lines, our findings demonstrated medium to large statistical effects on reaction time variability and inattention symptoms; however, future studies of the chronic effects of PE on more clinically relevant outcomes will be needed to establish clinical significance. Additionally, ADHD symptoms and mood were measured retrospectively on the day following HIIT and non-HIIT in a non-laboratory setting. Future investigations may want to incorporate additional measures of ADHD symptoms and mood that are completed the same day within a laboratory setting to capture more acutely the impact of HIIT.
Further, participants were not randomly selected, limiting the extent to which they represent the ADHD and non-ADHD college student populations. Our sample may be best defined as college students—both with and without ADHD—who agreed to participate in an exercise study in exchange for compensation, passed a health screener, and safely and comfortably tolerated high-intensity aerobic exercise. This may increase susceptibility for self-selection bias. For example, participants in the current study may have previously experienced acute or chronic benefits of physical exercise in their daily lives. Given this limitation, the results of this study should be considered preliminary, and replication is warranted. Furthermore, there were a few participants who did not feel well during and/or after completing the high-intensity exercise and were excluded from our analyses. It is likely that these participants were not as physically fit as the majority of our participants and would have benefited from gradually working up to 16-minutes of HIIT.
Finally, our sample size attenuated the probability of detecting statistically significant effects on some of our primary outcome measures with small effect sizes and underscores the importance of replicating our findings with larger samples. The small sample also precluded using adequately powered analyses to evaluate possible moderators of HIIT effects (e.g., sex/gender, race/ethnicity). Despite some conclusions limited by sample size and the potential of increased Type I error, it is compelling that we found moderate-to-large improvements in IA and HI symptomatology, depression, processing speed, and reaction time variability—a stable, unique feature of ADHD-related neurocognitive deficits (Kofler et al., 2013).
Future Directions.
Although a single bout of high-intensity aerobic exercise appears to have promise for providing a brief, immediate improvement in aspects of EF, ADHD symptomatology, and depression, much work is needed to develop, evaluate, and disseminate PE interventions. First, moderators of ADHD treatment efficacy have been understudied and constrains the extent to which we can infer effectiveness (e.g., race/ethnicity, sex/gender; Hartung & Lefler, 2019). Additionally, more work is needed to determine the optimal volume, intensity, and modality of PE for college students with ADHD, or how they should engage in PE. Similarly, patients are likely to ask why PE may help them manage ADHD. There is a significant dearth of investigations into the mechanisms through which improved ADHD outcomes manifest after increased PE which warrants scientific study. Large differences in study designs limit the comparability of effects across methods of exercise, and longitudinal studies are needed to investigate the chronic effects of PE.
Conclusion.
Consistent with previous studies exploring the effectiveness of HIIT for childhood ADHD (Pontifex et al., 2012), the present investigation found evidence that high-intensity aerobic exercise has the potential for significant, acute improvement in inattention, hyperactivity/impulsivity, processing speed, sustained attention, and depressive symptomatology for individuals with ADHD. Further investigations such as randomized controlled trials focusing on the chronic effects of sustained HIIT interventions are needed to substantiate the potential feasibility and efficacy of HIIT. If substantiated, several reasons that HIIT may be a useful adjunct or standalone treatment to psychosocial and/or pharmacological treatments for college students with ADHD include that it: (a) yields immediate, acute improvements in domains of EF and mood; (b) promotes improved physical health and mental well-being; and (c) for college students for whom PE is not contraindicated, poses relatively lower risk of deleterious effects. Future studies of acute and chronic effects of HIIT may provide evidence that physical exercise helps to enhance functioning and improve outcomes for college students with ADHD.
The ADHD group reported small to medium improvements for ADHD symptoms after HIIT
The ADHD group reported medium-sized improvements in depressive symptoms after HIIT
The ADHD group reported medium-sized improvements on two AX-CPT outcomes after HIIT
Results suggest a brief, HIIT session can yield immediate short-term improvements
Future studies should continue to evaluate HIIT and its impact on outcomes
Acknowledgments
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. This project was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under Grant # 2P20GM103432, the Ksir Research Fellowship, and Lillian Portenier Dissertation Award.
Footnotes
The choice to use alternative standardizers (e.g., variance of change scores, pooled variance at non-HIIT and HIIT) is complex, precluding uniform calculations of effect size and corresponding confidence intervals across studies (58). Therefore, raw means and standard deviations are provided to facilitate independent calculations of effect size with alternative standardizers.
Post-hoc, we re-ran analyses with male- and female-only samples and compared their interaction ηp2 to those found in our primary analyses to provide preliminary insight into possible sex differences (69). Relative to the interaction effects found in our primary analysis, effects were approximately similar across men and women on commission errors; larger for men and smaller for women on omission errors, working memory, stress, and HI symptom severity; and smaller for men and larger for women on average reaction time, reaction time variability, depression, anxiety, and IA severity.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler L, & Cohen J. (2004). Diagnosis and evaluation of adults with attention-deficit/hyperactivity disorder. Psychiatric Clinics of North America, 27, 187–201. 10.1016/j.psc.2003.12.003 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (APA) (2013). Diagnostic and statistical manual of mental disorders (5th ed.,) Washington, DC: Author. [Google Scholar]
- Arnett JJ. (2000) Emerging adulthood: A theory of development from the late teens through the twenties. American Psychologist, 55, 469–480. 10.1037/0003066X.55.5.469 [DOI] [PubMed] [Google Scholar]
- Barkley RA (1997). Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin, 121, 65–94. 10.1037//0033-2909.121.1.65 [DOI] [PubMed] [Google Scholar]
- Barkley RA (2011). Barkley Adult ADHD Rating Scale-IV (BAARS-IV). New York, NY: Guilford Press. [Google Scholar]
- Basso JC & Suzuki WA (2017). The effects of acute exercise on mood, cognition, neurophysiology, and neurochemical pathways: A review. Brain Plasticity, 2, 2: 127–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, & Faraone SV (2006). The effects of attention-deficit/hyperactivity disorder on employment and household income. Medscape General Medicine, 8, 12. [PMC free article] [PubMed] [Google Scholar]
- Borg GA (1998). Borg’s perceived exertion and pain scales. Champaign, IL: Human Kinetics. [Google Scholar]
- Chen MJ, Fan X, & Moe ST (2002). Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: A meta-analysis. Journal of Sports Sciences, 20, 873–899. 10.1080/026404102320761787 [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- Cohen JD, Barch DM, Carter C, & Servan-Schreiber D. (1999). Context-processing deficits in schizophrenia: Converging evidence from three theoretically motivated cognitive tasks. Journal of Abnormal Psychology, 108(1), 120–133. 10.1037/0021-843X.108.1.120 [DOI] [PubMed] [Google Scholar]
- Conners CK (2008). Conners manual (3rd ed.). Toronto, Ontario, Canada: Multi-Health Systems. [Google Scholar]
- Cumming G., & Finch S. (2001). A primer on the understanding, use, and calculation of confidence intervals that are based on central and noncentral distributions. Educational and Psychological Measurement, 61, 532–574. 10.1177/00131640121971374 [DOI] [Google Scholar]
- De Moor MHM, Beem AL, Stubbe JH, Boomsma DI, & De Geus EJC (2006). Regular exercise, anxiety, depression and personality: A population-based -study. Preventive Medicine, 42, 273–279. 10.1016/j.ypmed.2005.12.002 [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Weyandt LL, O’Dell SM, & Varejao M. (2009). College students with ADHD: Current status and future directions. Journal of Attention Disorders, 13, 234–250. 10.1177/1087054709340650 [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E. Lang, A., & Buchner A. (2007). GPower 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- Fisher RA (1922). On the interpretation of chi square from contingency tables, and the calculation of P. Journal of the Royal Statistical Society, 85, 87–94. 10.2307/2340521 [DOI] [Google Scholar]
- Fleming AP, & McMahon RJ (2012). Developmental context and treatment principles for ADHD among college students. Clinical Child and Family Psychology Review, 15, 303–329. 10.1007/s10567-012-0121-z [DOI] [PubMed] [Google Scholar]
- Frazier TW., Youngstrom EA., Glutting JJ., & Watkins MW. (2007). ADHD and achievement: Meta-analysis of the child, adolescent, and adult literatures and a concomitant study with college students. Journal of Learning Disabilities, 40, 49–65. 10.1177/00222194070400010401 [DOI] [PubMed] [Google Scholar]
- Gapin JI, Labban JD, & Etnier JL (2011). The effects of physical activity on attention deficit hyperactivity disorder symptoms: The evidence. Preventive Medicine, 52, S70–S74. 10.1016/j.ypmed.2011.01.022 [DOI] [PubMed] [Google Scholar]
- Gapin JI, Labban JD, Bohall SC, Wooten JS, & Chang Y. (2015). Acute exercise is associated with specific executive functions in college students with ADHD: A preliminary study. Journal of Sport and Health Science, 4, 89–96. 10.1016/j.jshs.2014.11.003 [DOI] [Google Scholar]
- Giedd JN (2004). Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences, 1021, 77–85. 10.1196/annals.1308.00 [DOI] [PubMed] [Google Scholar]
- Gonthier C, Mcnamara BN, Chow M, Conway AR, & Braver TS (2016). Inducing proactive control shifts in the AX-CPT. Frontiers in Psychology, 7:1822. doi: 10.3389/fpsyg.2016.01822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenwald-Mayes G. (2002). Relationship between current quality of life and family of origin dynamics for college students with attention-deficit/hyperactivity disorder. Journal of Attention Disorders, 5, 211–222. 10.1177/108705470100500403 [DOI] [PubMed] [Google Scholar]
- Hartung CM, & Lefler EK (2019). Sex and gender in psychopathology: DSM–5 and beyond. Psychological Bulletin, 145, 390–409. 10.1037/bul0000183 [DOI] [PubMed] [Google Scholar]
- Hartung CM, Lefler EK, Canu WH, Stevens AE, Jaconis M, LaCount PA, Shelton CR, Leopold DR, & Willcutt EG (2019). DSM-5 and other symptom thresholds for ADHD: Which is the best predictor of impairment in college students? Journal of Attention Disorders, 23(13), 1637–1646. 10.1177/1087054716629216 [DOI] [PubMed] [Google Scholar]
- Hedges LV (1982). Fitting categorical models to effect sizes from a series of experiments. Journal of Educational Statistics, 7, 119–37. 10.2307/1164961 [DOI] [Google Scholar]
- Hillman CH, Erickson KI, & Kramer AF (2008). Be smart, exercise your heart: Exercise effects on brain and cognition. Nature Reviews Neuroscience, 9, 58–65. 10.1038/nrn2298 [DOI] [PubMed] [Google Scholar]
- Hoza B., Smith AL., Shoulberg EK., Linnea KS., Dorsch TE., Blazo JA., . . . McCabe GP (2015). A randomized trial examining the effects of aerobic physical activity on attention-deficit/hyperactivity disorder symptoms in young children. Journal of Abnormal Child Psychology, 43, 655–667. 10.1007/s10802-014-9929-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, . . . Zaslavsky AM (2006). The prevalence and correlates of adult ADHD in the United States: Results from the national comorbidity survey replication. The American Journal of Psychiatry, 163, 716–723. 10.1176/ajp.2006.163.4.716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Sarver DE, Raiker JS, Orban SA, Friedman LM, & Kolomeyer EG (2013). Reaction time variability in ADHD: A meta-analytic review of 319 studies. Clinical Psychology Review, 33, 795–811. 10.1016/j.cpr.2013.06.001 [DOI] [PubMed] [Google Scholar]
- Kuriyan AB, Pelham WR, Molina BG, Waschbusch DA, Gnagy EM, Sibley MH, & ... Kent KM (2013). Young adult educational and vocational outcomes of children diagnosed with ADHD. Journal of Abnormal Child Psychology, 41, 27–41. 10.1007/s10802-012-9658-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCount PA, & Hartung CM (2018). Physical exercise interventions for emerging adults with attention-deficit/hyperactivity disorder (ADHD). The ADHD Report, 26(5), 1–11. 10.1521/adhd.2018.26.5.1 [DOI] [Google Scholar]
- LaCount PA., Hartung CM., & Canu WH., & Knouse LE (2019). Interventions for adolescents with ADHD transitioning to emerging adulthood: Developmental context and empirically-supported treatment principles. Evidence-Based Practice in Child & Adolescent Mental Health, 4(2), 170–186. 10.1080/23794925.2018.1518120 [DOI] [Google Scholar]
- Lewandowski LJ, Lovett BJ, Codding RS, & Gordon M. (2008). Symptoms of ADHD and academic concerns in college students with and without ADHD diagnoses. Journal of Attention Disorders, 12, 156–161. 10.1177/1087054707310882 [DOI] [PubMed] [Google Scholar]
- Liguori G (2021). ACSM’s Guidelines for Exercise Testing and Prescription (11th ed.,) Philadelphia, PA: Author. [Google Scholar]
- Lovibond PF, & Lovibond SH (1995). The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behaviour Research and Therapy, 33, 335–343. 10.1016/0005-7967(94)00075-U [DOI] [PubMed] [Google Scholar]
- McCarthy S, Asherson P, Coghill D, Hollis C, Murray M, Potts L, ... & Wong IC (2009). Attention-deficit hyperactivity disorder: Treatment discontinuation in adolescents and young adults. The British Journal of Psychiatry, 194, 273–277. 10.1192/bjp.bp.107.045245 [DOI] [PubMed] [Google Scholar]
- Meinzer MC., Lewinsohn PM., Pettit JW., Seeley JR., Gau JM., Chronis-Tuscano A., & Waxmonsky JG. (2013). Attention–deficit/hyperactivity disorder in adolescence predicts onset of major depressive disorder through early adulthood. Depression and Anxiety, 30, 546–553. 10.1002/da.22082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau D, & Chou E. (2019). The acute effect of high-intensity exercise on executive function: A meta-analysis. Perspectives on Psychological Science, 14(5), 734–764. 10.1177/1745691619850568 [DOI] [PubMed] [Google Scholar]
- Neudecker C, Mewes N, Reimers AK, & Woll A. (2015). Exercise intervention in children and adolescents with ADHD: A systematic review. Journal of Attention Disorders. 10.1177/1087054715584053 [DOI] [PubMed] [Google Scholar]
- Nigg JT (2013). Attention-deficit/hyperactivity disorder and adverse health outcomes. Clinical Psychology Review, 33, 215–228. 10.1016/j.cpr.2012.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontifex MB, Saliba BJ, Raine LB, Picchietti DL, & Hillman CH (2013). Exercise improves behavioral, neurocognitive, and scholastic performance in children with attention-deficit/hyperactivity disorder. The Journal of Pediatrics, 162, 543–551. 10.1016/j.jpeds.2012.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiner DL, Anastopoulos AD, Costello J, Hoyle RH, & Swartzwelder H. (2008). Adjustment to college in students with ADHD. Journal of Attention Disorders, 11, 689–699. 10.1177/1087054707305106 [DOI] [PubMed] [Google Scholar]
- Richards TL, Rosén LA, & Ramirez CA (1999). Psychological functioning differences among college students with confirmed ADHD, ADHD by self-report only, and without ADHD. Journal of College Student Development, 40, 299–304. [Google Scholar]
- Roman-Urrestaraz A., Lindhol P., Moilane I., Kiviniem V., Miettune J., Jääskeläine E., & ... Murray GK (2015). Brain structural deficits and working memory fMRI dysfunction in young adults who were diagnosed with ADHD in adolescence. European Child & Adolescent Psychiatry. 10.1007/s00787-015-0755-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney M, Chronis-Tuscano A, & Yoon Y. (2012). Substance use in college students with ADHD. Journal of Attention Disorders, 16, 221–234. 10.1177/108705471039253 [DOI] [PubMed] [Google Scholar]
- Salmon P. (2001). Effects of physical exercise on anxiety, depression, and sensitivity to stress: A unifying theory. Clinical Psychology Review, 21, 33–61. [DOI] [PubMed] [Google Scholar]
- Solanto MV (2015). Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. In Barkley RA Executive Function Deficits in Adults with ADHD (pp. 256266). Guilford Press. [Google Scholar]
- Sibley BA, & Beilock SL (2007). Exercise and working memory: An individual differences investigation. Journal of Sport & Exercise Psychology, 29, 783–791. 10.1123/jsep.29.6.783 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2008). 2008 Physical Activity Guidelines for Americans. http://www.health.gov/paguidelines/
- Vasko JM, Oddo LE, Meinzer MC, Garner A, & Chronis-Tuscano A. (2020). Psychosocial Interventions for college students with ADHD: Current status and future directions. The ADHD Report, 28(4), 5–12,14. 10.1521/adhd.2020.28.4.5 [DOI] [Google Scholar]
- Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, . . . Lahey BB (2012). Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. Journal of Abnormal Psychology, 121, 991–1010. 10.1037/a0027347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigal SB, Polzonetti CM, Stehli A, & Gratton E. (2012). Phase synchronization of oxygenation waves in the frontal areas of children with attention-deficit hyperactivity disorder detected by optical diffusion spectroscopy correlates with medication. Journal of Biomedical Optics, 17(12). 10.1117/1.JBO.17.12.127002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf LE. (2001). College students with ADHD and other hidden disabilities: Outcomes and interventions. In Wasserstein J, Wolf LE & LeFever FF (Eds.), Adult attention deficit disorder: Brain mechanisms and life outcomes. New York: New York Academy of Sciences. [DOI] [PubMed] [Google Scholar]