Abstract
EmrR negatively regulates the transcription of the multidrug resistance pump-encoding operon, emrRAB, by binding to its regulatory region. The binding site spans the promoter and the downstream sequence up to the transcriptional start site of the operon. Structurally unrelated drugs that induce the pump interfere with this binding.
Bacterial antibiotic resistance is a well-recognized threat to public health. Particularly serious aspects of this phenomenon are the multidrug resistance (MDR) mechanisms that enable bacteria to extrude structurally unrelated antimicrobial agents. Several classes of MDR pumps are known. They appear to have evolved independently several times by recruiting, in some cases at least, preexisting pumps with unrelated functions. As this recruitment can result from relatively minor alterations in the amino acid sequence (5, 11), MDR has the potential of quickly compromising the effectiveness also of new antimicrobial agents.
We have been studying the emrRAB operon-encoded MDR mechanism that can extrude, using proton motive force, several structurally unrelated drugs from Escherichia coli, e.g., protonophores and the antibiotics thiolactomycin and nalidixic acid (NA). The EmrB protein pumps the drugs across the cytoplasmic membrane; EmrA probably facilitates passage through the periplasm; and a third, as yet unidentified protein transports it through the outer membrane (7, 8, 10, 11). The pump is induced by a variety of structurally unrelated drugs, many of which are also its substrates (7, 8).
The emrRAB operon, which has a ς70-type promoter, is negatively regulated by the product of one of its own genes, namely, emrR (8). We postulated that EmrR repression is due to its binding to the promoter of the operon and that this binding is antagonized by drugs that induce the EmrAB pump (6, 8). Here we present direct evidence for this postulate, using gel mobility shift (GMS) assays; further, using footprinting, we have delineated the region of the emrRAB promoter to which EmrR binds.
The regulatory region of the emrRAB operon and the emrR gene were cloned by PCR, using the E. coli K12 Δlac genomic DNA as template and appropriate primers based on previously determined sequences (8). The sequence of the PCR products was determined to ensure lack of mismatches. The cloned regulatory region consisted of 244 nucleotides (nt), spanning 152 nt upstream and 74 nt downstream of the emrR translational start site. It was cloned in pUC18 plasmid (12) and is heretofore referred to as the probe DNA. For labeling, [α-33P]dCTP and the Klenow fragment filling-in reaction were used. The emrR gene was subcloned in pET-28a(+) vector (Novagen, Madison, Wis.), and EmrR was overexpressed in E. coli B834(DE3) by isopropyl-β-d-thiogalactopyranoside (1 mM) induction. The protein was purified according to the manufacturer's protocol, using an Ni2+ column and elution with imidazole buffer.
GMS was performed as described (9) using the following reaction mixture (total volume, 20 μl): 1 μg of bovine serum albumin, 1 μg of sheared calf thymus DNA, and various concentrations of EmrR protein (Fig. 1) in 1× GMS buffer [20 mM HEPES (pH 7.6), 1 mM EDTA, 10 mM (NH4)2SO4, 1 mM dithiothreitol, 30 mM KCl, and 0.2% Tween 20]. After 15 min of incubation (25°C), 0.2 ng of labeled probe DNA was added, and the mixture was further incubated for 20 min. Ten microliters of the individual mixtures along with loading buffer (9) were applied to nondenaturing polyacrylamide gels. Following electrophoresis, the gels were dried and the DNA-protein complexes were visualized by autoradiography. Excess calf thymus DNA was included in reaction mixtures to detect specific interaction with the probe DNA.
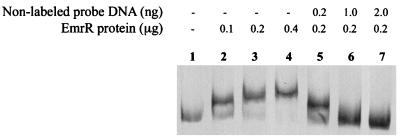
FIG. 1.
GMS analysis to determine interaction of the EmrR protein with the regulatory region (probe) of the emrRAB operon. Lanes 2 to 4, labeled probe, plus specified EmrR concentrations; lanes 5 to 7, labeled probe plus indicated concentrations of nonlabeled probe DNA and EmrR; lane 1, control (labeled probe without EmrR).
To determine the effect of EmrAB pump inducers on the probe DNA GMS (Fig. 2), 0.4 μg of EmrR was used. Reaction mixtures showing EmrR binding to the promoter region (Fig. 1) were incubated in the presence of different indicated inducers at different concentrations (Fig. 2) prior to electrophoresis.
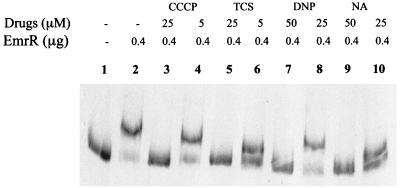
FIG. 2.
GMS analysis of the effect of the EmrAB inducers on EmrR binding to the emrRAB promoter region. Controls containing the labeled probe DNA without EmrR (lane 1) or with 0.4 μg of EmrR, without the inducers (lane 2), are also shown.
To determine the binding site of EmrR on the probe DNA, footprinting was performed, using the DNase I protection assay (4). The labeled probe was incubated (in GMS buffer [total volume, 20 μL; 25°C]) with different EmrR concentrations (Fig. 3). After 20 min, 1 U of DNase I was added, and incubation continued for 1 min.
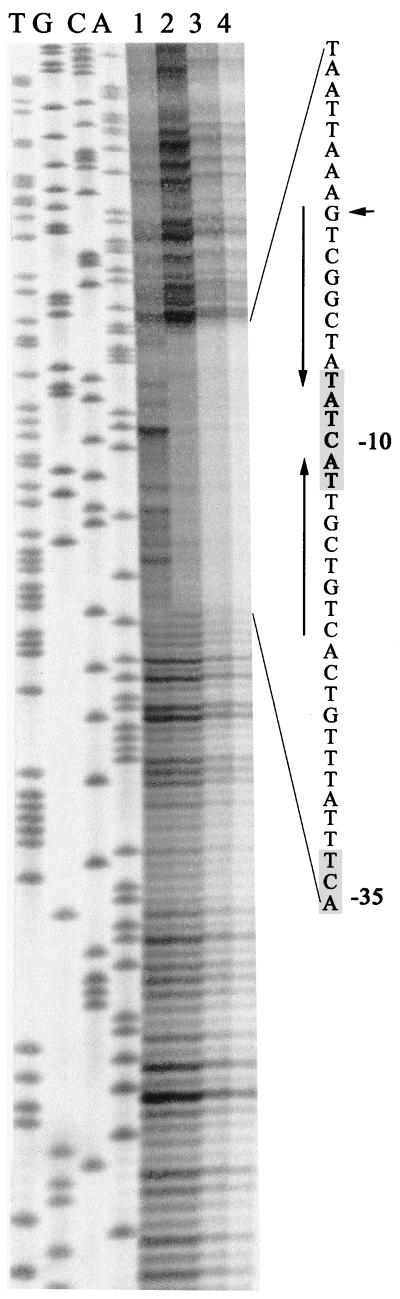
FIG. 3.
Footprinting of the EmrR binding site on the emrRAB promoter region. Lanes T, G, C, and A, represent the dideoxy sequencing ladder of the emrRAB promoter region used in the assay. Lane 1, probe DNA without EmrR; lanes 2 to 4, same as lane 1, but with 0.1, 0.2, or 0.4 μg of EmrR protein, respectively. The protected sequence is shown, and the following are indicated: the −35 (partial) and −10 regions (8) (shaded), the transcriptional start site (horizontal arrow), and the imperfect 9-3-9 inverted repeat (vertical arrows).
EmrR binds specifically to the emrRAB promoter region.
Purification of EmrR resulted in a single band of 24 kDa on sodium dodecyl sulfate-polyacrylamide gels (not shown). This is in good agreement with the calculated molecular mass of EmrR (20.6 kDa [8]) plus the histidine tag. EmrR retarded migration of the probe in a concentration-dependent manner, indicating that it binds to this DNA (Fig. 1, lanes 2 to 4). To determine specificity of the binding, the above reaction was performed in the presence of a 1-, 5-, or 10-fold excess of unlabeled probe; it competed with the labeled probe for EmrR binding (Fig. 1, lanes 5 to 7). Thus, EmrR binds specifically to the probe DNA containing the emrRAB regulatory region. EmrR contains a helix-turn-helix motif (in the region of amino acids 87 to 121), which is consistent with this result. GMS experiments using crude extracts of E. coli B834(DE3) overproducing EmrR without the histidine tag gave similar results (data not shown).
Inducers of the EmrAB pump antagonize EmrR binding.
The effect of the EmrAB pump inducers (7, 8) carbonyl cyanide m-chlorophenyldrazone (CCCP), 2,4-dinitrophenol, tetrachlorosalicylanilide (TCS), and NA on EmrR binding to the probe was determined by performing GMS in the presence of the individual inducers. All interfered with the binding (Fig. 2, lanes 3 to 10). CCCP and TCS were more effective than 2,4-dinitrophenol and NA, as they completely inhibited the promoter-repressor interaction at 25 μM concentration, as opposed to 50 μM concentrations required by the latter drugs. This is consistent with the in vivo results (8) that CCCP and TCS are more-effective inducers of the pump.
EmrR binding site includes transcriptional start site of the operon.
Sequencing of the DNA protected in the footprinting assay showed that it extended from half of the −35 sequence of the emrRAB promoter to the transcriptional start site of the operon (Fig. 3). An imperfect inverted repeat (CTGTCGTTA-CTA-TATCGGCTG) was present in this region.
The above results confirm our postulate regarding EmrR and emrRAB regulatory region interaction (6, 8). While this work was in progress, Brooun et al. (2) reported that certain inducers of the EmrAB pump interact with the EmrR protein; the results reported here are consistent with their finding. Thus, EmrR negatively regulates the emrRAB operon by directly binding to its regulatory region. The pump inducers interact with EmrR, rendering it incapable of binding to this region. Other MDR pump regulators, e.g., BmrR (1) and QacR (3), also interact with effectors and regulatory regions. BmrR binds to its effectors by its C-terminal domain, whose high-resolution structure shows that its binding site can accommodate a broad range of substrates (13). The wide substrate range of EmrR probably also results from a similar binding site architecture. This possibility can now be experimentally tested, as EmrR has recently been crystallized (2). Elucidation of mechanisms underlying regulator interactions with MDR-encoding operons and their effectors will help combat MDR.
Acknowledgments
This work was supported by a grant from the Office of Technology Licensing, Stanford University to A.M. M.B., S.P., and A.X. were supported by a Dean's fellowship from the Stanford University School of Medicine; M.B. and S.P. received further support from a NATO postdoctoral fellowship and NIH training grant NRSA 5T32 AI0732801, respectively.
We thank John Cha for assistance in some of the experiments.
REFERENCES
- 1.Ahmed M, Borsch C M, Taylor S S, Vazquez-Laslop N, Neyfakh A A. A protein that activates expression of a multidrug efflux transporter upon binding the transporter substrates. J Biol Chem. 1994;269:28506–28513. [PubMed] [Google Scholar]
- 2.Brooun A, Tomashek J J, Lewis K. Purification and ligand binding of EmrR, a regulator of a multidrug transporter. J Bacteriol. 1999;181:5131–5133. doi: 10.1128/jb.181.16.5131-5133.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grkovic S, Brown M H, Roberts N J, Paulsen I T, Skurray R A. QacR is a repressor protein that regulates expression of the Staphylococcus aureus multidrug efflux pump QacA. J Biol Chem. 1998;273:18665–18673. doi: 10.1074/jbc.273.29.18665. [DOI] [PubMed] [Google Scholar]
- 4.Leblanc B, Moss T. DNase I footprinting. In: Kneale G G, editor. DNA-protein interactions: principles and protocols. 1994. pp. 1–10. Methods in molecular biology. Humana Press, Totawa, N.J. [Google Scholar]
- 5.Lewis K. Multidrug resistance: versatile drug sensors of bacterial cells. Curr Biol. 1999;9:R403–R407. doi: 10.1016/s0960-9822(99)80254-1. [DOI] [PubMed] [Google Scholar]
- 6.Lomovskaya O, Kawai F, Matin A. Differential regulation of the mcb and emr operons of Escherichia coli: role of mcb in multidrug resistance. Antimicrob Agents Chemother. 1996;40:1050–1052. doi: 10.1128/aac.40.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lomovskaya O, Lewis K. Emr, an Escherichia coli locus for multidrug resistance. Proc Natl Acad Sci USA. 1992;89:8938–8942. doi: 10.1073/pnas.89.19.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lomovskaya O, Lewis K, Matin A. EmrR is a negative regulator of the Escherichia coli multidrug resistance pump EmrAB. J Bacteriol. 1995;177:2328–2334. doi: 10.1128/jb.177.9.2328-2334.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin R G, Rosner J L. Binding of purified multiple antibiotic-resistance repressor protein (MarR) to mar operator sequences. Proc Natl Acad Sci USA. 1995;92:5456–5460. doi: 10.1073/pnas.92.12.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pao S S, Paulsen I T, Saier M H., Jr Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62:1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vector. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 13.Zheleznova E E, Markham P N, Neyfakh A A, Brennan R G. Structural basis of multidrug recognition by BmrR, a transcription activator of a multidrug transporter. Cell. 1999;96:353–362. doi: 10.1016/s0092-8674(00)80548-6. [DOI] [PubMed] [Google Scholar]