Abstract
A mild visible-light-induced Pd-catalyzed one-pot three-component alkyl-carbamoylation and cyanation of alkenes was developed. This general transformation, which proceeds via the in situ formation of a reactive ketenimine intermediate, allows for a rapid construction of a broad range of valuable amides and nitriles from readily available alkenes, alkyl iodides, and isocyanides. An efficient synthesis of tetrazole and amidine via this approach was also demonstrated.
Keywords: difunctionalization, isocyanide, radical, palladium, light-induced
Graphical Abstract
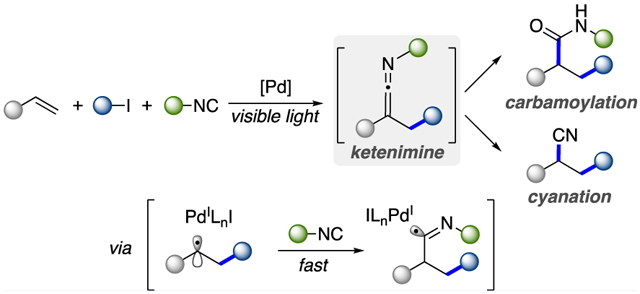
The multicomponent 1,2-difunctionalization of alkenes is a valuable synthetic strategy that allows for an efficient construction of complex molecules in a single step from easily accessible and commercially available starting materials (Scheme 1a).1 Particularly attractive could be the 1,2-alkyl carbamoylation of alkenes, which would enable access to synthetically important amides, the prevalent structural motifs found in pharmaceuticals, biological molecules, and polymeric materials.2 Along this line, the two-component intramolecular annulative approach of alkyl-carbamoylation has been reported,3 but the process is substantially limited to carbamoyl-tethered alkenes, furnishing cyclic amides (Scheme 1b). To the best of our knowledge, no general methodology for the overall three-component alkyl carbamoylation has been developed. Herein, we report a one-pot mild visible-light-induced Pd-catalyzed three-component 1,2-alkyl carbamoylation and cyanation of alkenes, which proceeds via the in situ generation of ketenimines A (Scheme 1c).
Scheme 1.

1,2-Difunctionalization of Alkenesa
a(a) Three-component 1,2-difunationalization of alkenes. (b) Intramolecular annulative carbocarbamoylation of alkenes. (c) This work: three-component alkyl carbamoylation/cyanation of alkenes.
On the basis of the highly reactive nature of ketenimine functionality and its facile subsequent transformations,4 we envisioned a strategy for the in situ generation/hydrolysis of ketenimine intermediate to achieve the formal carbamoylation of alkenes. Traditionally, ketenimine can be prepared through cross coupling between isocyanides and carbenes or metal-locarbenes,5 α-halophosphonates,6 allyl carbonates,7 α-haloketones,8 and diazo compounds.9 Moreover, the synthesis of ketenimines via a radical addition to isocyanides was also demonstrated.10
Recently, visible-light-induced palladium catalysis has become an emerging field of study.11 We and others demonstrated that hybrid palladium C(sp3)-centered radical species, generated through the cleavage of C–X (X = halide, CO2NPhth) bonds in the presence of a photoexcited Pd(0) complex, enable desaturation,12 alkyl Heck,13 and other transformations.14
Inspired by the synthesis of ketenimines via free radical additions to isocyanides10 and a new mild and general visible-light-induced method for generation of radical species,11 we hypothesized an approach to ketenimines via an addition of hybrid palladium C(sp3)-centered radical species to isocyanides.
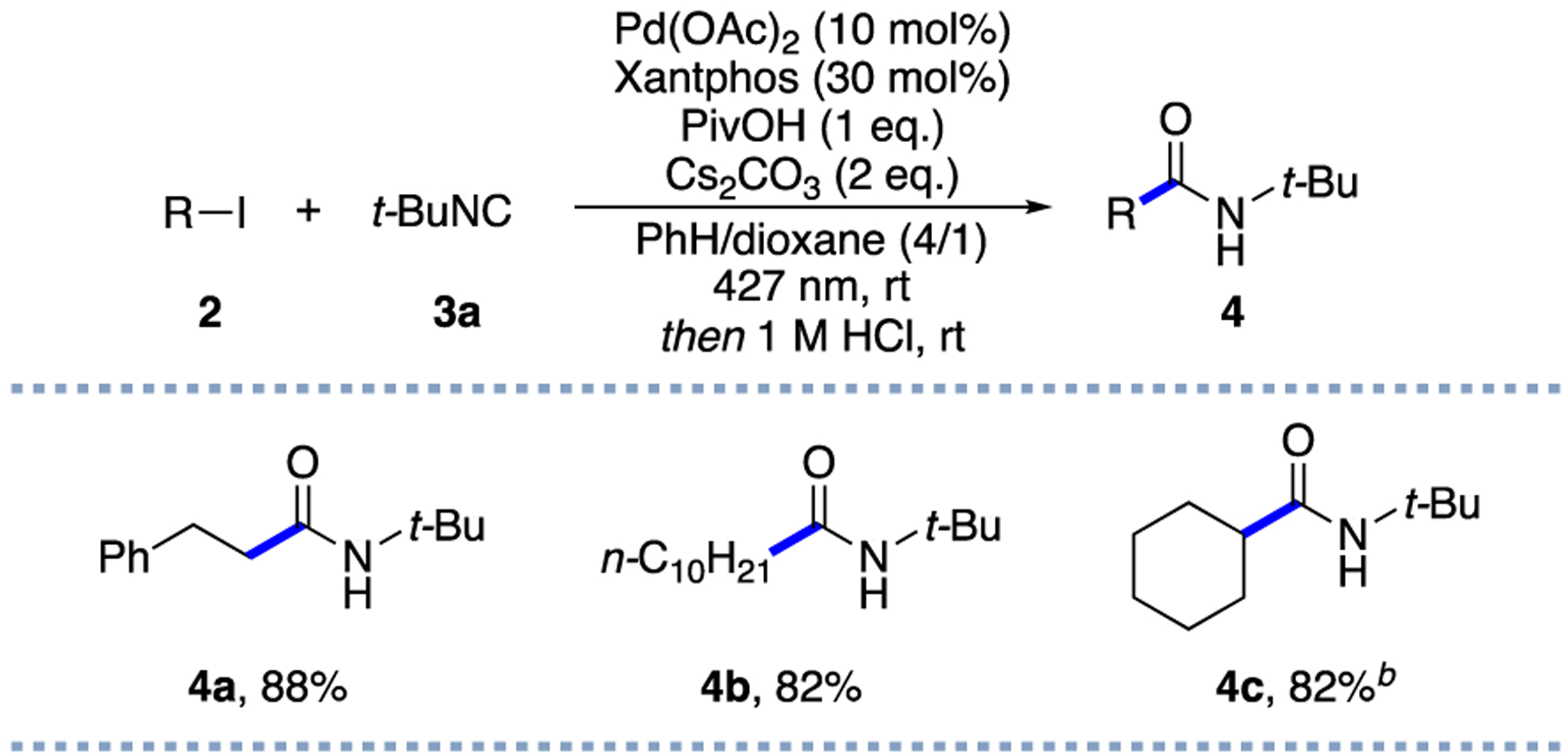
The initial two-component proof-of-concept experiments were performed between unactivated alkyl iodides and tert-butyl isocyanide (t-BuNC), under our standard palladium(II) acetate/Xantphos system.13a,c,d The following acidic hydrolysis of the in situ generated ketenimines (Scheme 2) was expected to provide the alkyl carbamoylation product. To our delight, the intended amide products 4a–4c were obtained in good yields, thus supporting the feasibility of the proposed strategy.
Scheme 2.
Aminocarbonylation of Unactivated Alkyl Iodidesa
a2 (0.2 mmol), 3a (0.4 mmol); isolated yields. bNMR yield.
Encouraged by these promising results and the previously developed alkyl Heck-type reactions,13a,c,d,j we turned our attention to more desirable three-component couplings of alkenes, alkyl iodides, and isocyanides. We hypothesized that the radical intermediate B formed via an addition of hybrid alkyl Pd radical species across an alkene would undergo a fast trapping with isocyanide, thus outcompeting an undesired premature β-H elimination, to produce the imidoyl radical C (Scheme 1c). The anticipated ketenimine intermediate would be delivered upon a β-H elimination from the latter.
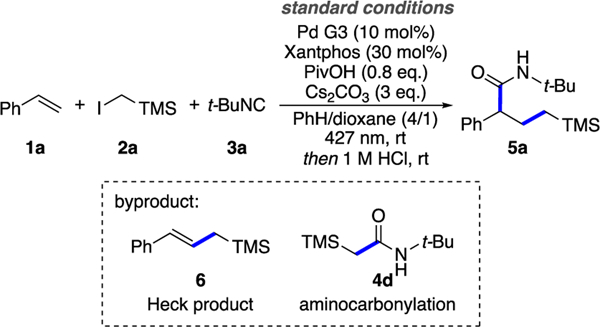
To this end, we examined a model reaction between styrene 1a, (trimethylsilyl)methyl iodide 2a, and t-BuNC 3a, followed by a subsequent acidic hydrolysis of the in situ forming ketenimine (Table 1). We realized that the success of this three-component coupling reaction hinges on suppressing an undesired premature Heck reaction leading to product 6 and a direct trapping of an alkyl radical with isocyanide to give 4d. After an extensive screening of reaction parameters,15 we found conditions that allowed for obtaining the desired alkyl-carbamoylation product 5a in 65% yield, using Pd G3 as the precatalyst, Xantphos as the ligand, pivalic acid as an additive, and benzene/1,4-dioxane as the cosolvent (Table 1, entry 1). The reaction was less efficient when Pd(OAc)2 was used (entry 2). Pivalic acid further promoted the reaction efficiency, which was consistent with the reported literature (entries 1 & 3).16 Control experiments proved the importance of Pd catalyst and Xantphos (entry 3) and light (entries 4 & 5) for this transformation.
Table 1.
Reaction Optimizationa
| |||
|---|---|---|---|
| entry | deviation from standard conditions | 5a:6:4db | yield of 5a, % |
| 1 | none | 90:9:1 | 65(61c) |
| 2 | Pd(OAc)2 instead of Pd G3 | 88:10:2 | 47 |
| 3 | without PivOH | 88:11:1 | 40 |
| 4 | without Pd/Xantphos | 0:0:0 | 0 |
| 5 | rt, dark | 0:0:0 | 0 |
| 6 | 80 °C, dark | 0:0:100 | 0 |
Yields of 1a (0.1 mmol), 2a (0.3 mmol), and 3a (0.2 mmol) were determined by gas GC/MS using pentadecane as an internal standard. Pd G3: [(4,5-bis(diphenylphosphino)-9,9-dimethylxanthene)-2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate.
The ratios were determined by GC/MS.
0.3 mmol scale, isolated yield.
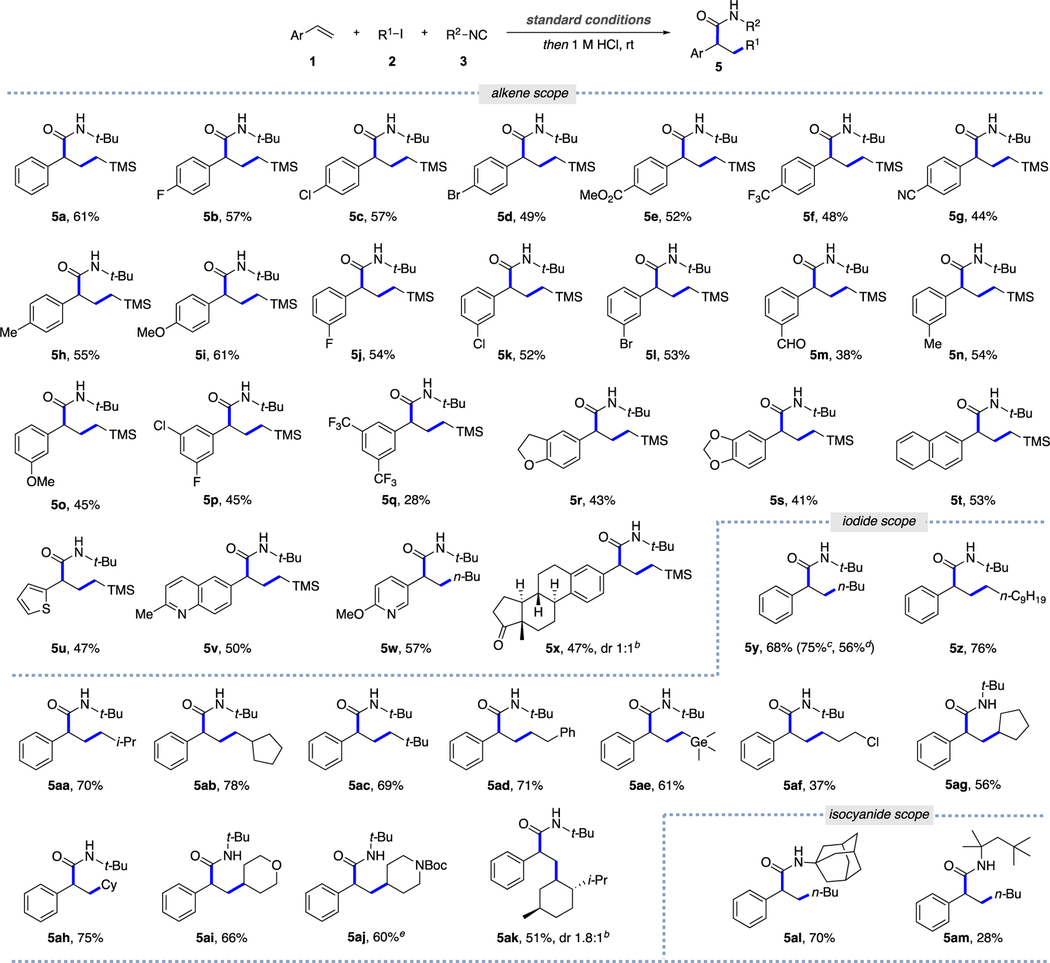
With the optimized conditions in hand, the scope of alkenes was examined first (Table 2). A diverse array of styrenes, possessing electron-withdrawing and -donating groups at the para- or meta-positions, smoothly reacted with alkyl iodide and isocyanide to give the desired products 5a–5s in moderate yields. Notably, 2-vinylnaphthalene and vinyl heteroarenes were fully compatible with this protocol, affording products 5t–5w. We were pleased to find that this reaction can work in a more complex setting, producing estrone derivative 5x, thus highlighting the broad applicability of this multicomponent coupling protocol.
Table 2.
Alkyl Carbamoylation of Alkenesa
|
0.3 mmol scale; isolated yields. bDiastereomeric ratio was determined by 1H NMR of the crude reaction mixture. c1 mmol scale using 10 mol % Pd G3 and 30 mol % Xantphos. d1 mmol scale using 5 mol % Pd G3 and 15 mol % Xantphos. e0.2 mmol scale.
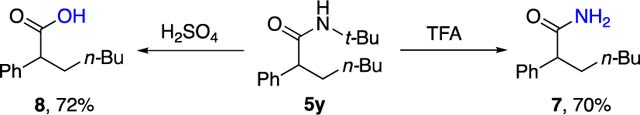
Next, the scope of alkyl iodides was evaluated. In general, primary and secondary alkyl iodides proceeded smoothly to give products 5y–5z and 5aa–5ah in moderate to good yields. Importantly, primary alkyl iodides bearing α-germyl and γ-chloride functionalities were also amenable to this process. Moreover, saturated heterocyclic derivatives, such as tetrahy-dropyran (5ai) and piperidine (5aj) as well as (−)-menthol-derivative (5ak) can also be employed. Among isocyanides, all tested tertiary isocyanides, such as 1-adamantyl and 1,1,3,3-tetramethylbutyl isocyanides, readily participated in this reaction, furnishing diverse alkylated amides 5al and 5am. Finally, it was shown that the synthesized N-tert-butyl amide 5y can routinely be transformed into unprotected amide17 7 and carboxylic acid18 8 in respectable yields (eq 1).
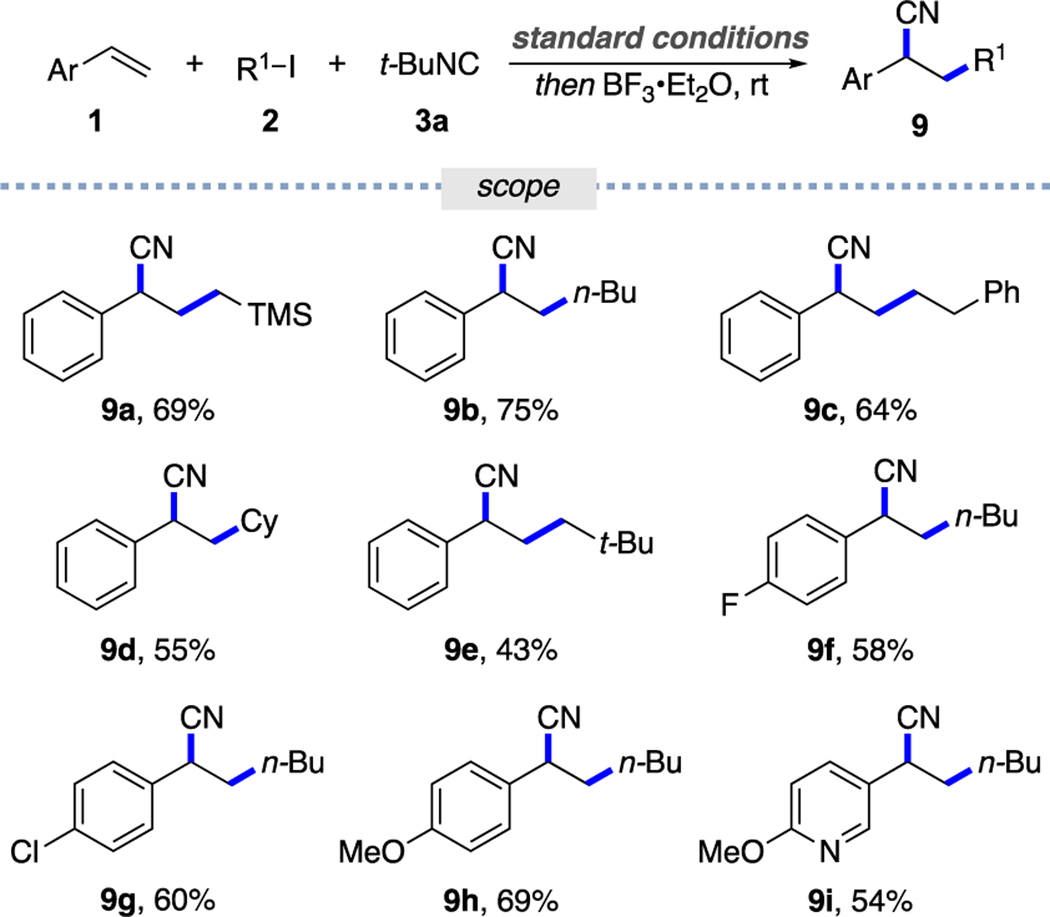
Motivated by the successfully developed alkyl-carbamoylation of alkenes, we sought to expand this protocol to an alkyl cyanation, which would provide one-pot access to organonitriles, versatile synthetic synthons,19 and common functional groups found in natural products and bioactive molecules.20 Thus, we tested the cleavage of the N–C bond in the formed ketenimine by BF3·Et2O (Table 3).9b It was found that this protocol allows for the synthesis of a range of benzyl nitriles that possess primary and secondary alkyl substituents at a side chain (9a–9e), differently substituted styrenes (9f–9h), and m-vinyl pyridine (9i).
Table 3.
Alkyl Cyanation of Alkenesa
|
0.3 mmol scale, isolated yields.
It was also shown that other nitrogen-containing compounds, such as unprotected benzyl tetrazole 10 and amidine 11, can easily be synthesized by combining this three-component coupling strategy coupled with a hydroazidation/[3 + 2] cycloaddition with tetramethylsuccinonitrile (TMSN3) (Scheme 3, eq 1) or a nucleophilic addition of aniline (Scheme 3, eq 2).
Scheme 3.
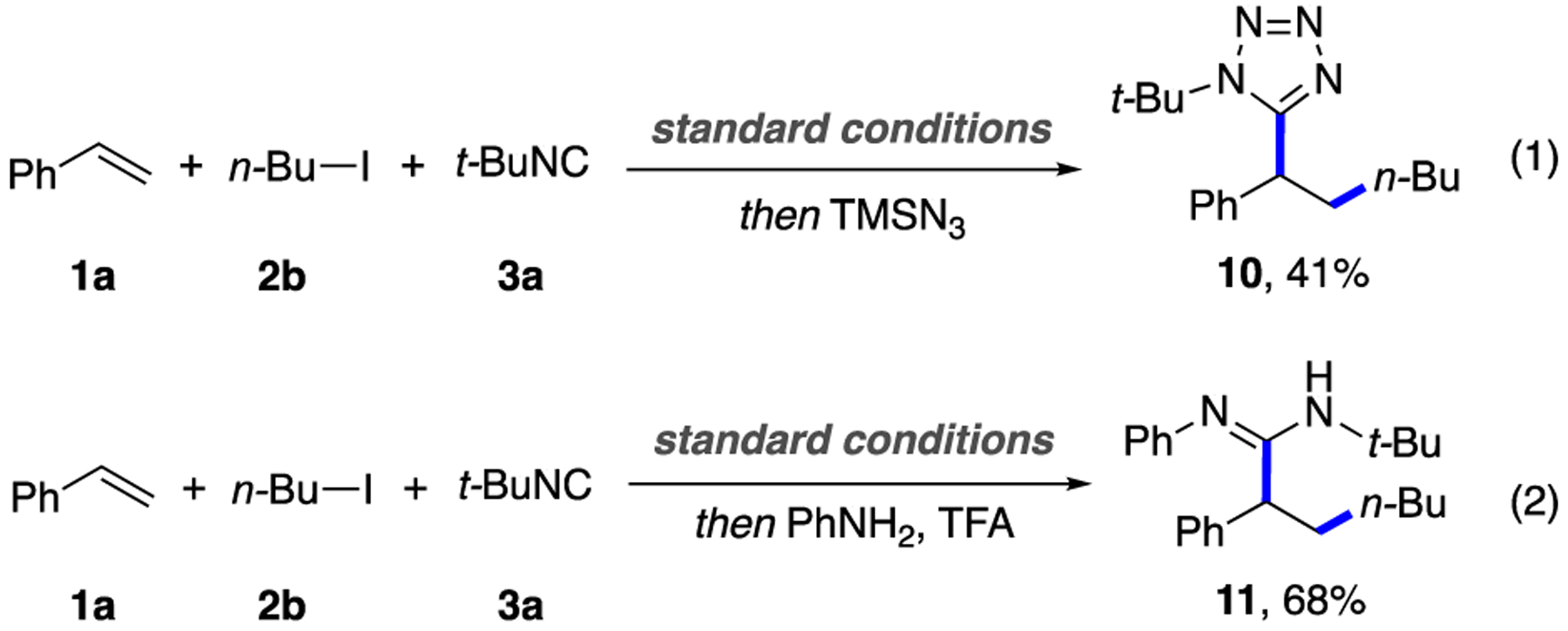
Synthesis of Tetrazole and Amidine
 |
(1) |
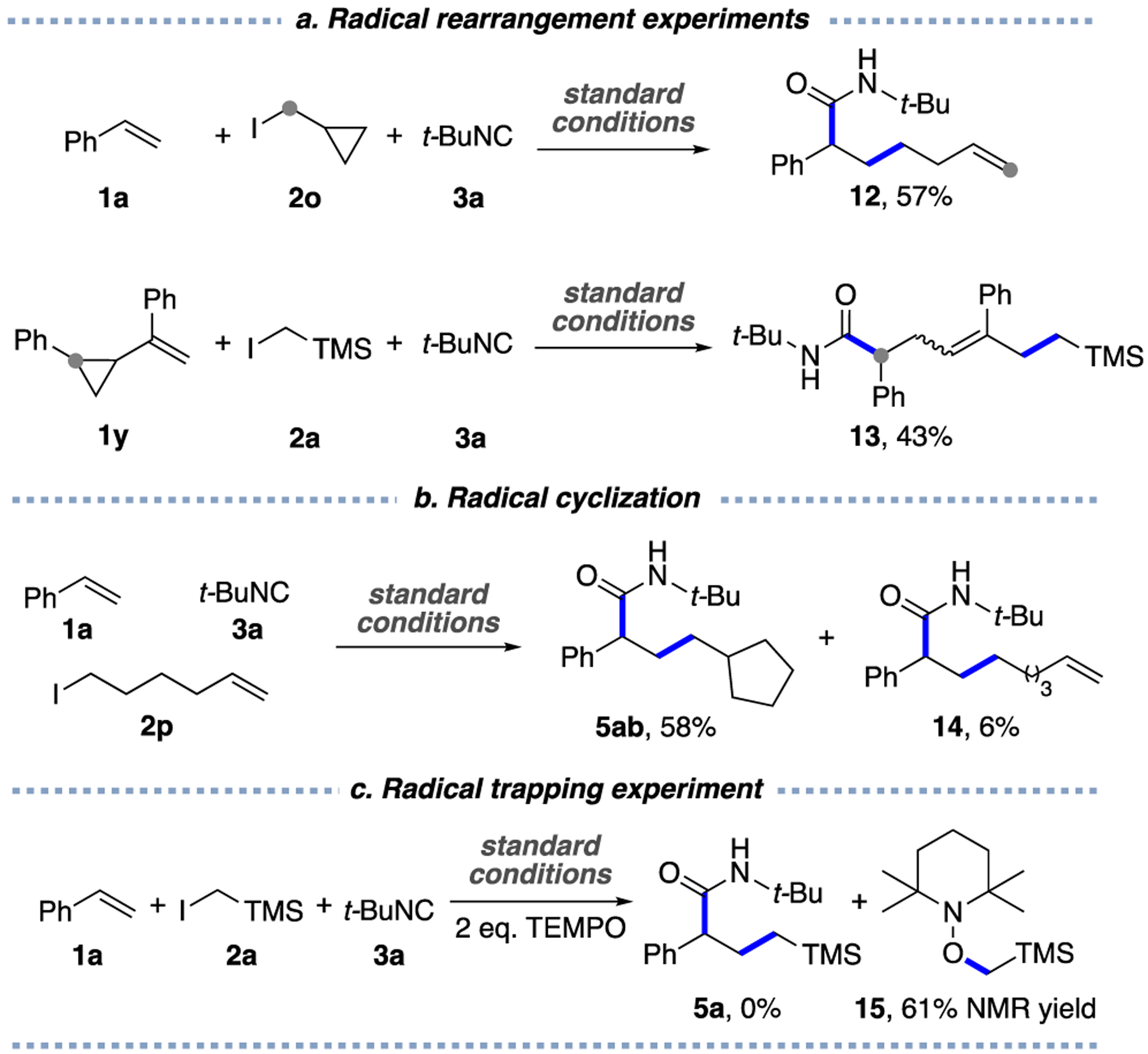
Next, a series of mechanistic tests were performed. The radical nature of this transformation was unambiguously confirmed by radical clock and radical trap experiments (Scheme 4). Thus, the reaction of styrene 1a and t-BuNC with alkyl iodide 2o, possessing a cyclopropyl substituent,21 produced terminal alkene 12, as a result of the ring opening of methylenecyclopropyl radical (Scheme 4a). Likewise, the latter, generated from a radical addition onto alkene 1y, regioselectively rearranged into a benzylic radical, which was trapped by t-BuNC, producing a stereoisomeric mixture of trisubstituted alkene 13 in 43% yield. Moreover, the employment of alkenyl halide 2p led to the product 5ab, possessing a cyclic substituent, which resulted from an initial 5-exo-trig radical cyclization (Scheme 4b). Finally, the reaction in the presence of radical scavengers such as TEMPO, led to trapping product 15 in 61% yield (Scheme 4c).
Scheme 4.
Mechanistic Studiesa
a(a) Radical rearrangement experiments. (b) Radical cyclization. (c) Radical trapping experiment.
On the basis of the above mechanistic studies and literature reports,13a,c,j the following plausible mechanism was proposed (Scheme 5). First, the hybrid alkyl Pd(I)-radical species D is generated from the single electron transfer (SET) of photoexcited Pd(0) complex to alkyl iodide. Next, a radical addition to alkene takes place, leading to the hybrid benzylic radical B, which is rapidly trapped by isocyanide to give imidoyl radical C. A subsequent β-H elimination from the latter furnishes the reactive ketenimine intermediate A with the concomitant regeneration of Pd catalyst. With a quench, the ketenimine A under acidic conditions is hydrolyzed into amide 5 or, upon treatment with Lewis acid, is transformed into nitrile 9.
Scheme 5.
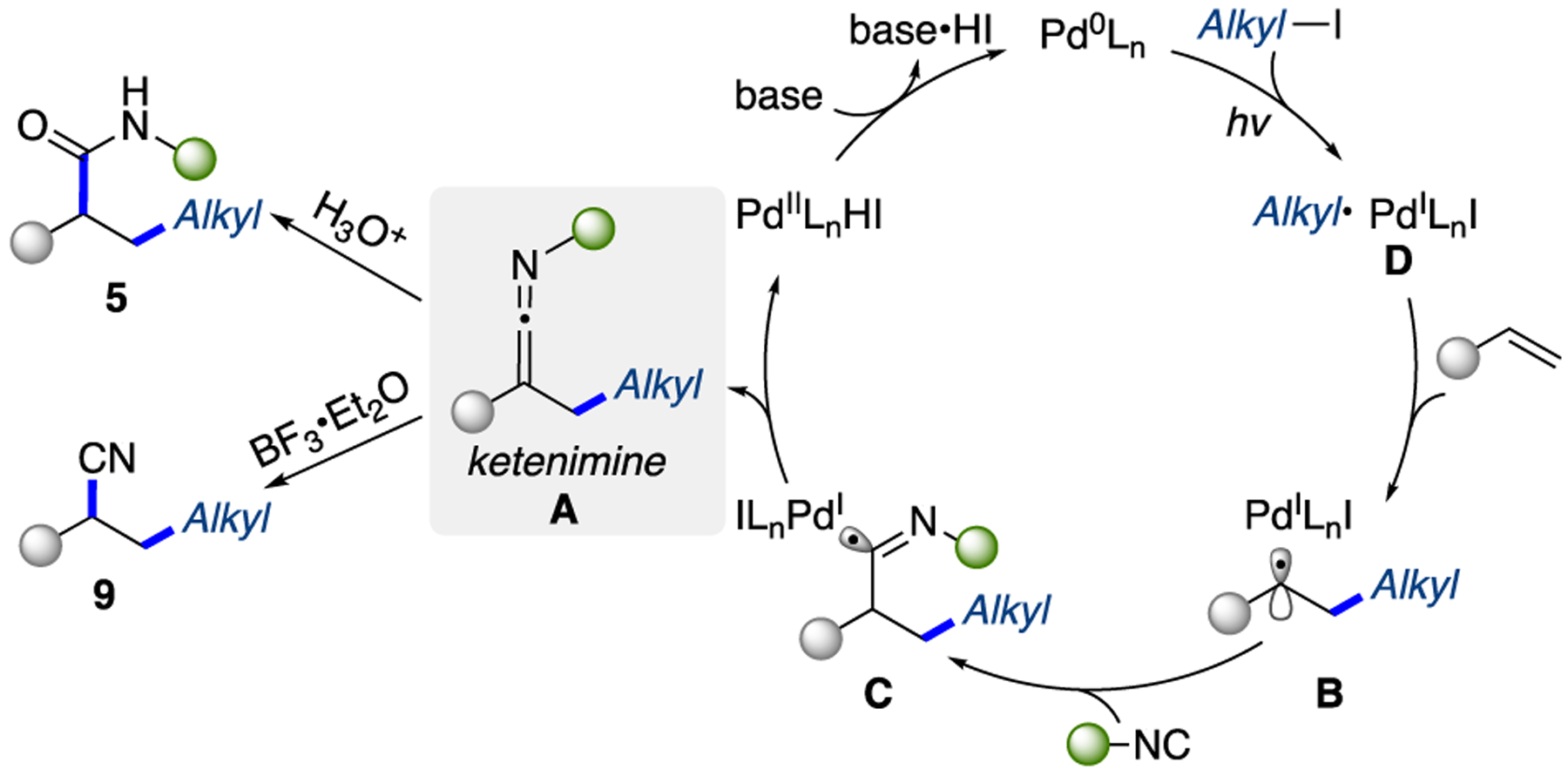
Proposed Mechanism
In conclusion, we developed a visible-light-induced Pd-catalyzed protocol for three-component 1,2-alkyl functionalization of alkenes with alkyl iodides and isocyanides. It features the formation of a reactive ketenimine intermediate, which under one-pot conditions is transformed into various useful synthetic synthons, such as amides, nitriles, tetrazoles, and amidines. This mild multicomponent coupling reaction, which utilizes easily accessible reaction partners, exhibits a wide functional group tolerance. It is anticipated that this mild visible-light-induced approach will find applications in synthesis and will stimulate the development of new 1,2-difunctionalization methods.
ACKNOWLEDGMENTS
We thank the National Institutes of Health (GM120281), National Science Foundation (CHE-1955663), and Welch Foundation (Chair, AT-0041) for financial support.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.1c04183.
Experimental procedures, analytical data for all new compounds (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acscatal.1c04183
The authors declare no competing financial interest.
Contributor Information
Xiangqing Jia, Department of Chemistry and Biochemistry, The University of Texas at Dallas, Richardson, Texas 75080-3021, United States.
Ziyan Zhang, Department of Chemistry and Biochemistry, The University of Texas at Dallas, Richardson, Texas 75080-3021, United States.
Vladimir Gevorgyan, Department of Chemistry and Biochemistry, The University of Texas at Dallas, Richardson, Texas 75080-3021, United States.
REFERENCES
- (1).For selected reviews, see:; (a) McDonald RI; Liu G; Stahl SS Palladium(II)-Catalyzed Alkene Functionalization via Nucleopalladation: Stereochemical Pathways and Enantioselective Catalytic Applications. Chem. Rev 2011, 111, 2981–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yin G; Mu X; Liu G Palladium(II)-Catalyzed Oxidative Difunctionalization of Alkenes: Bond Forming at a High-Valent Palladium Center. Acc. Chem. Res 2016, 49, 2413–2423. [DOI] [PubMed] [Google Scholar]; (c) Giri R; Kc S Strategies toward Dicarbofunctionalization of Unactivated Olefins by Combined Heck Carbometalation and Cross-Coupling. J. Org. Chem 2018, 83, 3013–3022. [DOI] [PubMed] [Google Scholar]; (d) Zhang J-S; Liu L; Chen T; Han L-B Transition-Metal-Catalyzed Three-Component Difunctionalizations of Alkenes. Chem. - Asian J 2018, 13, 2277–2291. [DOI] [PubMed] [Google Scholar]; (e) Dhungana RK; KC S; Basnet P; Giri R Transition Metal-Catalyzed Dicarbofunctionalization of Unactivated Olefins. Chem. Rec 2018, 18, 1314–1340. [DOI] [PubMed] [Google Scholar]; (f) Lin J; Song R-J; Hu M; Li J-H Recent Advances in the Intermolecular Oxidative Difunctionalization of Alkenes. Chem. Rec 2019, 19, 440–451. [DOI] [PubMed] [Google Scholar]; (g) Huang H-M; Garduño-Castro MH; Morrill C; Procter DJ Catalytic Cascade Reactions by Radical Relay. Chem. Soc. Rev 2019, 48, 4626–4638. [DOI] [PubMed] [Google Scholar]; (h) Li Z-L; Fang G-C; Gu Q-S; Liu X-Y Recent Advances in Copper-Catalysed Radical-Involved Asymmetric 1,2-Difunctionalization of Alkenes. Chem. Soc. Rev 2020, 49, 32–48. [DOI] [PubMed] [Google Scholar]; (i) Luo Y-C; Xu C; Zhang X Nickel-Catalyzed Dicarbofunctionalization of Alkenes. Chin. J. Chem 2020, 38, 1371–1394. [Google Scholar]; (j) Derosa J; Apolinar O; Kang T; Tran VT; Engle KM Recent Developments in Nickel-Catalyzed Intermolecular Dicarbofunctionalization of Alkenes. Chem. Sci 2020, 11, 4287–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Qi X; Diao T Nickel-Catalyzed Dicarbofunctionalization of Alkenes. ACS Catal. 2020, 10, 8542–8556. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Jiang H; Studer A Intermolecular Radical Carboamination of Alkenes. Chem. Soc. Rev 2020, 49, 1790–1811. [DOI] [PubMed] [Google Scholar]; (m) Shi W-Y; Ding Y-N; Liu C; Zheng N; Gou X-Y; Li M; Zhang Z; Liu H-C; Niu Z-J; Liang Y-M Three-Component Ruthenium-Catalyzed Remote C–H Functionalization of 8-Aminoquinoline Amides. Chem. Commun 2020, 56, 12729–12732. [DOI] [PubMed] [Google Scholar]; (n) Wickham LM; Giri R Transition Metal (Ni, Cu, Pd)-Catalyzed Alkene Dicarbofunctionalization Reactions. Acc. Chem. Res 2021, 54, 3415–3437. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Gou X-Y; Li Y; Luan Y-Y; Shi W-Y; Wang C-T; An Y; Zhang B-S; Liang Y-M Ruthenium-Catalyzed Radical Cyclization/meta-Selective C–H Alkylation of Arenes via σ-Activation Strategy. ACS Catal. 2021, 11, 4263–4270. [Google Scholar]; (p) Liang Y-Y; Huang J; Ouyang X-H; Qin J-H; Song R-J; Li J-H Radical-Mediated Alkoxypolyhaloalkylation of Styrenes with Polyhaloalkanes and Alcohols via C(sp3)–H bond cleavage. Chem. Commun 2021, 57, 3684–3687. [DOI] [PubMed] [Google Scholar]; (q) Zhu S; Zhao X; Li H; Chu L Catalytic Three-component Dicarbofunctionalization Reactions Involving Radical Capture by Nickel. Chem. Soc. Rev 2021, 50, 10836. [DOI] [PubMed] [Google Scholar]
- (2).(a) Greenberg A; Breneman CM; Liebman JF The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science; Wiley-Interscience: New York, 2002. [Google Scholar]; (b) Carey JS; Laffan D; Thomson C; Williams MT Analysis of the Reactions Used for the Preparation of Drug Candidate Molecules. Org. Biomol. Chem 2006, 4, 2337–2347. [DOI] [PubMed] [Google Scholar]
- (3).For selected recent examples, see:; (a) Chen C; Hu J; Su J; Tong X Synthesis of Substituted γ-Lactam via Pd(0)-Catalyzed Cyclization of Alkene-Tethered Carbamoyl Chloride. Tetrahedron Lett. 2014, 55, 3229–3231. [Google Scholar]; (b) Whyte A; Burton KI; Zhang J; Lautens M Enantioselective Intramolecular Copper-Catalyzed Borylacylation. Angew. Chem., Int. Ed 2018, 57, 13927–13930. [DOI] [PubMed] [Google Scholar]; (c) Li Y; Zhang F-P; Wang R-H; Qi S-L; Luan Y-X; Ye M Carbamoyl Fluoride-Enabled Enantioselective Ni-Catalyzed Carbocarbamoylation of Unactivated Alkenes. J. Am. Chem. Soc 2020, 142, 19844–19849. [DOI] [PubMed] [Google Scholar]; (d) Wu X; Qu J; Chen Y Quinim: A New Ligand Scaffold Enables Nickel-Catalyzed Enantioselective Synthesis of α-Alkylated γ-Lactam. J. Am. Chem. Soc 2020, 142, 15654–15660. [DOI] [PubMed] [Google Scholar]; (e) Wu X; Tang Z; Zhang C; Wang C; Wu L; Qu J; Chen Y Pd-Catalyzed Regiodivergent Synthesis of Diverse Oxindoles Enabled by the Versatile Heck Reaction of Carbamoyl Chlorides. Org. Lett 2020, 22, 3915–3921. [DOI] [PubMed] [Google Scholar]; (f) Fan P; Lan Y; Zhang C; Wang C Nickel/Photo-Cocatalyzed Asymmetric Acyl-Carbamoylation of Alkenes. J. Am. Chem. Soc 2020, 142, 2180–2186. [DOI] [PubMed] [Google Scholar]; (g) Lan Y; Wang C Nickel-Catalyzed Enantioselective Reductive Carbo-Acylation of Alkenes. Commun. Chem 2020, 3, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Marchese AD; Wollenburg M; Mirabi B; Abel-Snape X; Whyte A; Glorius F; Lautens M Nickel-Catalyzed Enantioselective Carbamoyl Iodination: A Surrogate for Carbamoyl Iodides. ACS Catal. 2020, 10, 4780–4785. [Google Scholar]; (i) Wu XQ; Shrestha M; Chen YF Asymmetric Synthesis of α-Alkylated γ-Lactam via Nickel/8-Quinim-Catalyzed Reductive Alkyl-Carbamoylation of Unactivated Alkene. Synlett 2021, 32, 955–961. [Google Scholar]; (j) Chen C; Liu L; Sun W; Zhu B Palladium-Catalyzed Aryl-Carbamoylation of Alkene-Tethered Carbamoyl Chlorides: Access to Diverse Aryl-Functionalized Oxindoles. ChemistrySelect 2021, 6, 6464–6467. [Google Scholar]
- (4).(a) Lu P; Wang Y The Thriving Chemistry of Ketenimines. Chem. Soc. Rev 2012, 41, 5687–5705. [DOI] [PubMed] [Google Scholar]; (b) Alajarin M; Marin-Luna M; Vidal A Recent Highlights in Ketenimine Chemistry. Eur. J. Org. Chem 2012, 2012, 5637–5653. [Google Scholar]
- (5).(a) Aumann R Ketenimine Complexes from Carbene Complexes and Isocyanides: Versatile Building Blocks for Carbocycles and N-Heterocycles. Angew. Chem., Int. Ed. Engl 1988, 27, 1456–1467. [Google Scholar]; (b) Solé S; Gornitzka H; Schoeller WW; Bourissou D; Bertrand G Amino)(Aryl)Carbenes: Stable Singlet Carbenes Featuring a Spectator Substituent. Science 2001, 292, 1901–1903. [DOI] [PubMed] [Google Scholar]; (c) Canac Y; Conejero S; Donnadieu B; Schoeller WW; Bertrand G Persistent (Amino)(Silyl)Carbenes. J. Am. Chem. Soc 2005, 127, 7312–7313. [DOI] [PubMed] [Google Scholar]; (d) Maity AK; Zeller M; Uyeda C Carbene Formation and Transfer at a Dinickel Active Site. Organometallics 2018, 37, 2437–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Grass A; Dewey NS; Lord RL; Groysman S Ketenimine Formation Catalyzed by a High-Valent Cobalt Carbene in Bulky Alkoxide Ligand Environment. Organometallics 2019, 38, 962–972. [Google Scholar]
- (6).Yang Q; Li C; Cheng M-X; Yang S-D Palladium-Catalyzed Migratory Insertion of Isocyanides for Synthesis of C-Phosphonoketenimines. ACS Catal. 2016, 6, 4715–4719. [Google Scholar]
- (7).(a) Ito Y; Hirao T; Ohta N; Saegusa T Synthesis of Ketenimine via (N-Alkylimino)Acylpalladium Complex Intermediate. Tetrahedron Lett. 1977, 18, 1009–1012. [Google Scholar]; (b) Qiu G; Mamboury M; Wang Q; Zhu J Ketenimines from Isocyanides and Allyl Carbonates: Palladium-Catalyzed Synthesis of β,γ-Unsaturated Amides and Tetrazoles. Angew. Chem., Int. Ed 2016, 55, 15377–15381. [DOI] [PubMed] [Google Scholar]
- (8).Mamboury M; Wang Q; Zhu J α-Oxo-Ketenimines from Isocyanides and α-Haloketones: Synthesis and Divergent Reactivity. Chem. - Eur. J 2017, 23, 12744–12748. [DOI] [PubMed] [Google Scholar]
- (9).(a) Zhou F; Ding K; Cai Q Palladium-Catalyzed Amidation of N-Tosylhydrazones with Isocyanides. Chem. - Eur. J 2011, 17, 12268–12271. [DOI] [PubMed] [Google Scholar]; (b) Liu Z; Cao S; Wu J; Zanoni G; Sivaguru P; Bi X Palladium(II)-Catalyzed Cross-Coupling of Diazo Compounds and Isocyanides to Access Ketenimines. ACS Catal. 2020, 10, 12881–12887. [Google Scholar]
- (10).(a) Rohe S; McCallum T; Morris AO; Barriault L Transformations of Isonitriles with Bromoalkanes Using Photoredox Gold Catalysis. J. Org. Chem 2018, 83, 10015–10024. [DOI] [PubMed] [Google Scholar]; (b) Gomes G. d. P.; Loginova Y; Vatsadze SZ; Alabugin IV Isonitriles as Stereoelectronic Chameleons: The Donor–Acceptor Dichotomy in Radical Additions. J. Am. Chem. Soc 2018, 140, 14272–14288. [DOI] [PubMed] [Google Scholar]; (c) Cannalire R; Amato J; Summa V; Novellino E; Tron GC; Giustiniano M Visible-Light Photocatalytic Functionalization of Isocyanides for the Synthesis of Secondary Amides and Ketene Aminals. J. Org. Chem 2020, 85, 14077–14086. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Huang W; Wang Y; Weng Y; Shrestha M; Qu J; Chen Y Nickel-Catalyzed Formal Aminocarbonylation of Unactivated Alkyl Iodides with Isocyanides. Org. Lett 2020, 22, 3245–3250. [DOI] [PubMed] [Google Scholar]; (e) Li Q; Jin H; Liu Y; Zhou B Nickel-Catalyzed Multicomponent Coupling Reaction of Alkyl Halides, Isocyanides and H2O: An Expedient Way to Access Alkyl Amides. Synthesis 2020, 52, 3466–3472. [Google Scholar]
- (11).For selected reviews, see:; (a) Chuentragool P; Kurandina D; Gevorgyan V Catalysis with Palladium Complexes Photoexcited by Visible Light. Angew. Chem., Int. Ed 2019, 58, 11586–11598. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kurandina D; Chuentragool P; Gevorgyan V Transition-Metal-Catalyzed Alkyl Heck-Type Reactions. Synthesis 2019, 51, 985–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhou W-J; Cao G-M; Zhang Z-P; Yu D-G Visible Light-induced Palladium-catalysis in Organic Synthesis. Chem. Lett 2019, 48, 181–191. [Google Scholar]; (d) Kancherla R; Muralirajan K; Sagadevan A; Rueping M Visible Light-Induced Excited-State Transition-Metal Catalysis. Trends Chem. 2019, 1, 510–523. [Google Scholar]; (e) Cheng W-M; Shang R Transition Metal-Catalyzed Organic Reactions under Visible Light: Recent Developments and Future Perspectives. ACS Catal. 2020, 10, 9170–9196. [Google Scholar]; (f) Sarkar S; Cheung KPS; Gevorgyan V C–H. Functionalization Reactions Enabled by Hydrogen Atom Transfer to Carbon-Centered Radicals. Chem. Sci 2020, 11, 12974–12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).(a) Parasram M; Chuentragool P; Sarkar D; Gevorgyan V Photoinduced Formation of Hybrid Aryl Pd-Radical Species Capable of 1,5-HAT: Selective Catalytic Oxidation of Silyl Ethers into Silyl Enol Ethers. J. Am. Chem. Soc 2016, 138, 6340–6343. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Parasram M; Chuentragool P; Wang Y; Shi Y; Gevorgyan V General, Auxiliary-Enabled Photoinduced Pd-Catalyzed Remote Desaturation of Aliphatic Alcohols. J. Am. Chem. Soc 2017, 139, 14857–14860. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Cheng W-M; Shang R; Fu Y Irradiation-Induced Palladium-Catalyzed Decarboxylative Desaturation Enabled by A Dual Ligand System. Nat. Commun 2018, 9, 5215. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Chuentragool P; Parasram M; Shi Y; Gevorgyan V General, Mild, and Selective Method for Desaturation of Aliphatic Amines. J. Am. Chem. Soc 2018, 140, 2465–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).(a) Kurandina D; Parasram M; Gevorgyan V Visible Light-Induced Room-Temperature Heck Reaction of Functionalized Alkyl Halides with Vinyl Arenes/Heteroarenes. Angew. Chem., Int. Ed 2017, 56, 14212–14216. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang G-Z; Shang R; Cheng W-M; Fu Y Irradiation-Induced Heck Reaction of Unactivated Alkyl Halides at Room Temperature. J. Am. Chem. Soc 2017, 139, 18307–18312. [DOI] [PubMed] [Google Scholar]; (c) Kurandina D; Rivas M; Radzhabov M; Gevorgyan V Heck Reaction of Electronically Diverse Tertiary Alkyl Halides. Org. Lett 2018, 20, 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Chuentragool P; Yadagiri D; Morita T; Sarkar S; Parasram M; Wang Y; Gevorgyan V Aliphatic Radical Relay Heck Reaction at Unactivated C(sp3)-H Sites of Alcohols. Angew. Chem., Int. Ed 2019, 58, 1794–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Xing W-L; Shang R; Wang G-Z; Fu Y Visible Light-Induced Palladium-Catalyzed Ring Opening β-H Elimination and Addition of Cyclobutanone Oxime Esters. Chem. Commun 2019, 55, 14291–14294. [DOI] [PubMed] [Google Scholar]; (f) Zhao B; Shang R; Wang G-Z; Wang S; Chen H; Fu Y Palladium-Catalyzed Dual Ligand-Enabled Alkylation of Silyl Enol Ether and Enamide under Irradiation: Scope, Mechanism, and Theoretical Elucidation of Hybrid Alkyl Pd(I)-Radical Species. ACS Catal. 2020, 10, 1334–1343. [Google Scholar]; (g) Zhang Z; Rogers CR; Weiss EA Energy Transfer from CdS QDs to a Photogenerated Pd Complex Enhances the Rate and Selectivity of a Pd-Photocatalyzed Heck Reaction. J. Am. Chem. Soc 2020, 142, 495–501. [DOI] [PubMed] [Google Scholar]; (h) Koy M; Bellotti P; Katzenburg F; Daniliuc CG; Glorius F Synthesis of All-Carbon Quaternary Centers by Palladium-Catalyzed Olefin Dicarbofunctionalization. Angew. Chem., Int. Ed 2020, 59, 2375–2379. [DOI] [PubMed] [Google Scholar]; (i) Lee GS; Kim D; Hong SH Pd-Catalyzed Formal Mizoroki–Heck Coupling of Unactivated Alkyl Chlorides. Nat. Commun 2021, 12, 991. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Kvasovs N; Iziumchenko V; Palchykov V; Gevorgyan V Visible Light-Induced Pd-Catalyzed Alkyl-Heck Reaction of Oximes. ACS Catal. 2021, 11, 3749–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).(a) Zhou W-J; Cao G-M; Shen G; Zhu X-Y; Gui Y-Y; Ye J-H; Sun L; Liao L-L; Li J; Yu D-G Visible-Light-Driven Palladium-Catalyzed Radical Alkylation of C–H Bonds with Unactivated Alkyl Bromides. Angew. Chem., Int. Ed 2017, 56, 15683–15687. [DOI] [PubMed] [Google Scholar]; (b) Wang G-Z; Shang R; Fu Y Irradiation-Induced Palladium-Catalyzed Direct C–H Alkylation of Heteroarenes with Tertiary and Secondary Alkyl Bromides. Synthesis 2018, 50, 2908–2914. [Google Scholar]; (c) Huang H-M; Koy M; Serrano E; Pflüger PM; Schwarz JL; Glorius F Catalytic Radical Generation of π-Allylpalladium Complexes. Nat. Catal 2020, 3, 393–400. [Google Scholar]; (d) Huang H-M; Bellotti P; Pflueger PM; Schwarz JL; Heidrich B; Glorius F A Three-Component, Interrupted Radical Heck/Allylic Substitution Cascade Involving Unactivated Alkyl Bromides. J. Am. Chem. Soc 2020, 142, 10173–10183. [DOI] [PubMed] [Google Scholar]; (e) Cheung KPS; Kurandina D; Yata T; Gevorgyan V Photoinduced Palladium-Catalyzed Carbofunctionalization of Conjugated Dienes Proceeding via Radical-Polar Crossover Scenario: 1,2-Aminoalkylation and Beyond. J. Am. Chem. Soc 2020, 142, 9932–9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).See the Supporting Information for details.
- (16).Wang J; Liu Y; Xiong Z; Zhong L; Ding S; Li L; Zhao H; Chen C; Shang Y Palladium-Catalysed Dearomative Aryl/Cycloimidoylation of Indoles. Chem. Commun 2020, 56, 3249–3252. [DOI] [PubMed] [Google Scholar]
- (17).Schleicher KD; Jamison TF Nickel-Catalyzed Synthesis of Acrylamides from α-Olefins and Isocyanates. Org. Lett 2007, 9, 875–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Zhao D; Luo H; Chen B; Chen W; Zhang G; Yu Y Palladium-Catalyzed H/D Exchange Reaction with 8-Aminoquinoline as the Directing Group: Access to ortho-Selective Deuterated Aromatic Acids and β-Selective Deuterated Aliphatic Acids. J. Org. Chem 2018, 83, 7860–7866. [DOI] [PubMed] [Google Scholar]
- (19).(a) Rappoport Z Chemistry of Cyano-Group; Wiley-Interscience: London, UK, 1970. [Google Scholar]; (b) Larock RC Comprenhensive Organic Transformations: A Guide to Functional Group Preparations, 2nd ed.; VCH: New York, 1999. [Google Scholar]
- (20).(a) Fleming F Nitrile-Containing Natural Products. Nat. Prod. Rep 1999, 16, 597–606. [Google Scholar]; (b) Fleming FF; Yao L; Ravikumar PC; Funk L; Shook BC Nitrile-Containing Pharmaceuticals: Efficacious Roles of the Nitrile Pharmacophore. J. Med. Chem 2010, 53, 7902–7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).(a) Newcomb M; Toy PH Hypersensitive Radical Probes and the Mechanisms of Cytochrome P450-Catalyzed Hydroxylation Reactions. Acc. Chem. Res 2000, 33, 449–455. [DOI] [PubMed] [Google Scholar]; (b) Baldwin JE Thermal Rearrangements of Vinylcyclopropanes to Cyclopentenes. Chem. Rev 2003, 103, 1197–1212. [DOI] [PubMed] [Google Scholar]


