Abstract
Primary adrenal insufficiency (PAI) is often the first clinical sign of X-linked adrenoleukodystrophy (X-ALD), a rare genetic disorder that can present with various clinical phenotypes. A subset of boys with X-ALD develop cerebral ALD (cALD), characterized by progressive central demyelination, neurocognitive decline, and ultimately death. Timely intervention with hematopoietic cell transplant (HCT) can be a life-saving therapy by stopping progression of cerebral disease. We report the case of an 11-year-old boy with type 1 diabetes mellitus who presented with PAI, growth delay, and symptoms of attention deficit hyperactivity disorder. Given his history of T1DM, his PAI was presumed to be autoimmune and he was started on hydrocortisone and fludrocortisone. Eleven months later brain magnetic resonance imaging revealed white matter hyperintensity consistent with advanced cALD. The degree of disease progression at the time of diagnosis rendered the patient ineligible for transplant and he has continued to experience progressive neurologic decline. Initial symptoms of cALD are often subtle but should not be overlooked, as early identification of X-ALD is critical to allow early intervention with lifesaving HCT. PAI typically presents prior to the onset of neurologic symptoms. All boys who present with PAI should undergo workup for X-ALD with plasma very long chain fatty acid testing, even in the setting of underlying autoimmune disease.
Keywords: primary adrenal insufficiency, X-linked adrenoleukodystrophy, cerebral ALD, type I diabetes, autoimmune polyglandular syndrome
Primary adrenal insufficiency (PAI) is the rare, life-threatening failure of the adrenal glands to produce sufficient glucocorticoids and mineralocorticoids, presenting with fatigue, weakness, weight loss, hyperpigmentation, hypoglycemia, and hypotension.
Pediatric PAI is most commonly due to congenital adrenal hyperplasia (CAH), with the classical type presenting in infancy and the nonclassical type presenting later in childhood [1-3]. Laboratory testing in CAH reveals increased levels of steroid precursors, distinguishing it from other causes of PAI [3]. Most cases of pediatric PAI not associated with CAH were previously assumed to be autoimmune, but autoimmune PAI is rare in children. Developments in molecular testing have made it possible to efficiently identify specific genetic variants associated with PAI. Underlying rare genetic diseases may make up a larger proportion of cases of PAI than was previously understood [2].
PAI is often the first documented clinical finding in boys with X-linked adrenoleukodystrophy (X-ALD). X-ALD is a rare (1/14 700-20 000 live male births) genetic disorder in which deficiency of a peroxisomal membrane protein (ALDP) leads to accumulation of very long chain fatty acid (VLCFA; ≥C22) in the plasma, white matter, spinal cord, and adrenal cortex [3-5]. It presents more severely in males and is often categorized into 3 main clinical phenotypes. Males with X-ALD may remain asymptomatic into late adulthood, or they may develop any combination of the following disease processes: (1) primary adrenal insufficiency; (2) cerebral ALD (cALD), characterized by progressive white matter demyelination, neurodegeneration, and ultimately death if untreated; and (3) adrenomyeloneuropathy, associated with spastic paresis, gait instability, and incontinence [6, 7]. There is wide phenotypic variability, even among affected individuals of the same kindred or sibship. Approximately 35% of males with X-ALD develop cALD in childhood, with a peak incidence between 3 and 10 years of age [8]. A majority of boys with cALD will demonstrate biochemical or clinical evidence of PAI prior to the onset of neurologic symptoms [7, 9]. Recognizing that PAI is due to X-ALD has important clinical implications as delayed diagnosis of X-ALD after onset of PAI is associated with greater clinical impairment and higher mortality [10]. Timely diagnosis of cALD allows for earlier intervention with lifesaving hematopoietic cell transplant (HCT).
Here we present the case of a boy with a history of type 1 diabetes mellitus (T1DM) diagnosed with PAI who was ultimately found to have X-ALD.
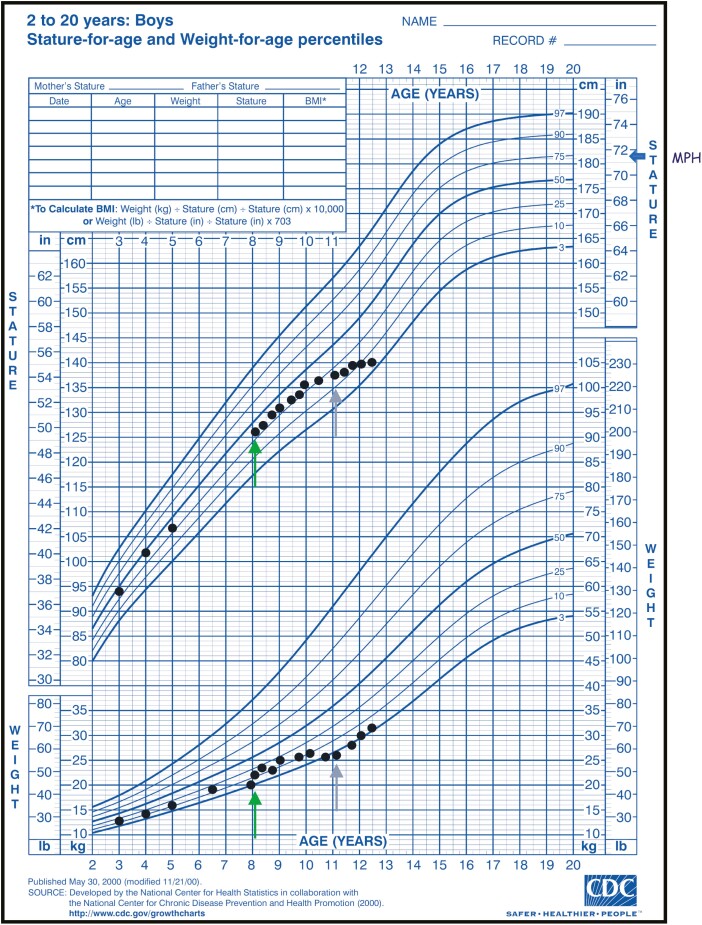
Case Report
An 11-year-old male with a history of T1DM was diagnosed with PAI after presenting with poor school performance, growth delay, and skin hyperpigmentation. He was tracking between the 10th and 25th percentile for weight until 8 years old when he showed deceleration of weight gain and was diagnosed with T1DM (Fig. 1). Following diagnosis and management of T1DM he was tracking between the 10th and 25th percentile for weight and between the 25th and 50th percentile for height. Between 10 and 11 years of age he demonstrated persistent growth deceleration of both height and weight despite good diabetes control (HbA1c 7.2-8.5%). Screening for celiac disease performed at the time of diagnosis of T1DM and annually was consistently negative (tissue transglutaminase < 2 IU/mL, normal IgA). Annual screening of his thyroid axis was performed and was normal and he remained clinically euthyroid.
Figure 1.
Growth chart. Green arrows indicate the time of diagnosis of type 1 diabetes mellitus. purple arrows indicate the time of diagnosis of primary adrenal insufficiency. Midparental height (MPH) is indicated with a blue arrow.
Laboratory testing was significant for elevated adrenocorticotropin (ACTH) (>2000 pg/mL, ref 7.2-63.3) and cortisol within normal limits (10.1 μg/dL, ref 6.2-19.4) with a normal plasma renin level (0.950 ng/mL/hour, ref 0.500-3.300). Adrenal androgens were undetectable. At this time he reportedly did not have focal hyperpigmentation; however, he was noted to have skin that was “tanner” than other members of his family. Routine blood pressures were normal. He was diagnosed with PAI and started on hydrocortisone (5 mg 3 times daily). He was also started on fludrocortisone (0.1 mg daily) empirically despite a lack of salt cravings and normal plasma renin activity at the time of diagnosis of PAI. Despite repeat labs indicating good control of his PAI, his linear growth did not improve (Fig. 1 and Table 1). This was initially thought to be due to insufficient adrenal replacement and his hydrocortisone was increased from 10 mg/m2/day to 17.5 mg/m2/day. He also demonstrated symptoms of attention deficit hyperactivity disorder (ADHD) beginning prior to his diagnosis of PAI which did not improve despite the use of stimulant medication, and he required services through an Individualized Education Program in school. His family history was significant for an older brother with ADHD which did not improve despite the use of multiple stimulants as well as academic difficulties with loss of skills over time. His past medical history was not suggestive of hypoglycemia at birth or during childhood.
Table 1.
Laboratory values at time of diagnosis (11 years, 2 months) of PAI and throughout treatment course
| Age | Cortisol (μg/dL) |
ACTH (pg/mL) |
Renin, plasma (ng/mL/hour) |
|---|---|---|---|
| ref: 6.2-19.4 | ref: 7.2-63.3 | ref: 0.500-3.300 | |
| 11 years 2 months | 10.1 | >2000 | 0.950 |
| 11 years 3 months | 1.063 | ||
| 11 years 9 months | 1.791 | ||
| ref: 4-22 | ref: <47 | ref: 1.2-2.4 | |
| 12 years 2 months | 6.9 | <10 | 1.0 |
| 12 years 4 months | 2.6 | 22 | 1.1 |
| 12 years 8 months | 27.2 | 10 | 2.4 |
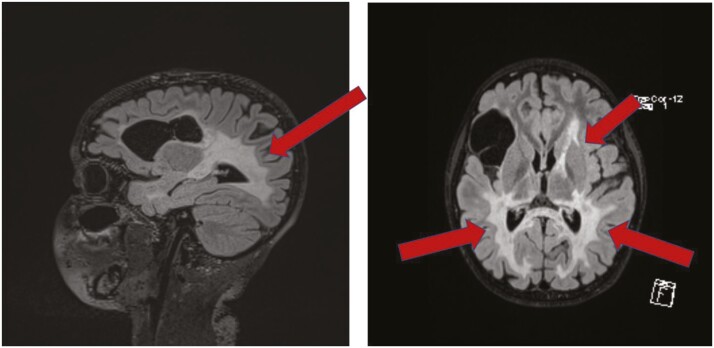
Given his history of T1DM, his adrenal insufficiency was presumed to be autoimmune and further diagnostic testing was not performed. Eleven months later a brain magnetic resonance imaging (MRI) performed for complaints of visual disturbances and chronic headaches revealed extensive white matter T2 hyperintensity suggestive of cerebral demyelination with gadolinium enhancement (Fig. 2). Findings were consistent with advanced cALD with an MRI severity score of 17 on the Loes scale [11]. Elevated plasma VLCFAs and a pathogenic variant in the ABCD1 gene confirmed the diagnosis of X-ALD. The patient was transferred to our institution for further workup and management of his disease.
Figure 2.
(A) Sagittal and (B) axial T2 FLAIR showing abnormal T2 hyperintensity. Incidental cystic lesion within the right inferior frontal lobe. Gadolinium enhancement is not shown here.
Following the patient’s diagnosis, his brother was also found to have X-ALD based on plasma VLCFA testing. MRI demonstrated cerebral involvement. The patient’s brother was also found to have elevated ACTH (>1250 pg/mL, ref < 47) and a low normal cortisol level (6.8 µg/dL, ref 4-22) consistent with primary adrenal insufficiency.
Further workup of the patient’s growth failure included assessment of growth hormone (GH)/insulin-like growth factor (IGF) 1 axis function. IGF-binding protein was just below average (5.4 µg/mL, ref 2.8-9.3). IGF-1 was significantly low for his chronologic age (134 ng/mL, ref 146-541), but normal for a prepubertal child (134 ng/mL, Tanner 1: 109-368). In addition, his oral intake had been negatively impacted by his progressive neurologic decline which may have led to a state of partial GH resistance and low IGF-1 concentration. Some degree of excessive glucocorticoid replacement may have also contributed to his growth failure. For these reasons, growth hormone stimulation testing was not performed with the plan to monitor for further growth deceleration as puberty progressed.
Due to extensive cerebral involvement he was deemed ineligible for HCT as the risk of rapid deterioration outweighed any potential benefit. The patient has since experienced progressive vision loss and is expected to experience continued neurologic decline which is likely to ultimately be fatal.
Discussion
Primary adrenal insufficiency is often the first clinical sign of X-ALD. A study of 49 patients with known X-ALD found that all patients developed biochemical evidence of adrenal insufficiency prior to developing abnormalities on neurologic examination or brain MRI, indicating that proper workup of PAI can facilitate an early diagnosis of X-ALD, in some cases prior to the development of cerebral disease [9]. Elevated plasma VLCFAs have 90.9% to 97.0% sensitivity and 94.1% to 95.0% specificity for the diagnosis of X-ALD and can reliably diagnose or rule out disease [12]. The use of plasma VLCFAs to screen for X-ALD in newborns was initiated in the state of New York in 2013 [7], added to the Recommended Uniform Screening Panel (RUSP) in 2016 [7] and currently is part of newborn screening in 23 states and the District of Columbia [13].
This case highlights key features of the history and examination that should prompt consideration of X-ALD. Initial symptoms of cALD can be subtle or nonspecific, and often involve behavioral problems or worsening school performance. Since these presenting symptoms of cALD often mimic psychiatric or developmental disorders of childhood, especially ADHD, the potential for misdiagnosis is considerable [14, 15]. The patient in this case had been diagnosed with ADHD, but did not show a response to treatment with stimulant medication. Although pharmacotherapy to treat behavioral symptoms such as hyperactivity may initially appear successful, these treatments typically decline in effectiveness as cALD advances [16]. Additional features of our patient’s history suggestive of cALD were his growth deceleration despite good control of his T1DM and the presence of similar behavioral and academic difficulties in his older brother. In patients with PAI, the presence of medication-resistant ADHD, learning or behavioral concerns, persistent growth failure, and/or similar symptoms in male siblings should prompt consideration of X-ALD and further diagnostic workup with plasma VLCFAs.
Although current guidelines recommend diagnostic workup for X-ALD in all male pediatric patients presenting with PAI, our report underscores that PAI due to X-ALD can be co-existent in children with autoimmune diseases. As the patient in this case had T1DM, it was assumed that his PAI was caused by autoimmune adrenalitis, most likely due to autoimmune polyglandular syndrome (APS-2). Autoimmune adrenalitis is the most common cause of PAI in adults presenting either independently or in conjunction with thyroiditis and/or T1DM as a component of APS-2. Affected individuals may also present with primary hypogonadism, myasthenia gravis, vitiligo, and celiac disease. APS-2 is a sporadic, rare syndrome (prevalence 1/20 000) with high familial clustering [17]. APS-2 is 3 times more common in females, has a peak incidence between 20 and 60 years, and very rarely presents in childhood [18, 19]. Patients typically present with T1DM prior to the onset of PAI. However, APS-2 is actually less prevalent than X-ALD. Only 0.5% of patients with T1DM will later develop autoimmune PAI over the course of their lifetime [18].
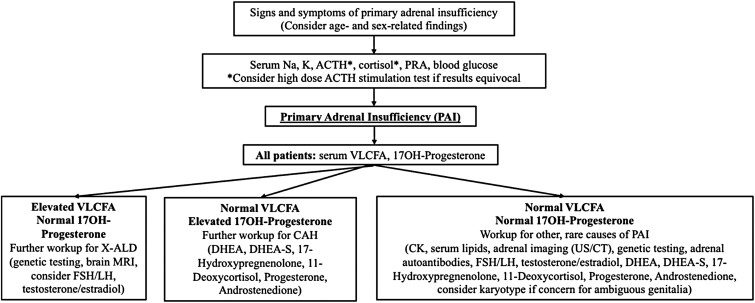
Multiple retrospective studies have aimed to determine the various underlying etiologies of PAI. Perry et al [2] found that 55% of non-CAH PAI was nonautoimmune. Their cohort included 4 boys with X-ALD and 5 boys with autoimmune PAI, showing that in boys autoimmune adrenalitis and X-ALD occurred at similar frequencies. Although there is conflicting data on the relative prevalence of autoimmune vs nonautoimmune PAI and studies are somewhat limited by sample size, these findings indicate that X-ALD is more prevalent than previously understood. Prevalence estimates based on a cohort of infants with X-ALD identified by newborn screening also suggest a higher than expected incidence of X-ALD [20]. This suggests that autoimmune adrenalitis should not be diagnosed without first ruling out other underlying causes of PAI. Positive testing for adrenal autoantibodies or antibodies against steroid 21-hydroxylase with a clinical picture consistent with PAI essentially confirms the diagnosis of autoimmune PAI, whereas individuals with X-ALD typically have absent circulating adrenocortical autoantibodies [21, 22]. We support the diagnostic criteria proposed by Brandão Neto et al, in which exclusion of other causes of PAI is required for the diagnosis of autoimmune adrenalitis. Specifically, they recommend ruling out underlying genetic syndromes, X-ALD, infectious diseases, drugs, adrenal hemorrhage or thrombosis, neoplasias, and infiltrative causes of PAI [21]. Additionally, we recommend the following diagnostic workup (Fig. 3) modified from Kirkgoz et al [3] to ensure that X-ALD does not go undiagnosed in boys presenting with PAI. This new algorithm emphasizes the need to perform plasma VLCFA testing in boys as a part of the initial workup of PAI. VLCFA testing is included in the JCEM guidelines; however, it is recommended only in boys older than 6 months of age and is recommended after testing for 21- hydroxylase antibodies [23]. Autoimmune adrenal insufficiency is rare in children, and since these guidelines were published there have been cases of PAI reported in infants with X-ALD younger than 6 months of age [24]. Therefore earlier testing of plasma VLCFAs is warranted. Also of note is that in the setting of maximal adrenal gland stimulation, as indicated by the single very elevated ACTH value in our patient (>2000 pg/mL), and a suboptimal corresponding cortisol concentration of 10.1 µg/dL, early stage PAI can be diagnosed without performing a high-dose ACTH stimulation test [7]. However, an adrenal stimulation test should be considered to confirm the diagnosis if necessary.
Figure 3.
Proposed diagnostic criteria for boys presenting with PAI. ACTH, adrenocorticotropic hormone; PRA, plasma renin activity; DHEA, dehydroepiandrosterone; DHEA-S, dehydroepiandrosterone sulfate; FSH, follicle stimulating hormone; LH, luteinizing hormone; VLCFA, very long chain fatty acids.
Early diagnosis is critical as timely intervention with HCT can eventually stop the progression of cALD, though it must be done early in the disease course [10]. In children with X-ALD who do not demonstrate radiographic evidence of cALD at the time of diagnosis, serial neurologic and radiographic surveillance is recommended to ensure that HCT can be performed at the earliest evidence of cerebral disease [25]. Delayed diagnosis of X-ALD after diagnosis of PAI has been shown to be associated with significantly worse prognosis [10]. A retrospective study found that boys with a delay in diagnosis of X-ALD after diagnosis of PAI (>1 year) had greater clinical impairment, more evidence of cerebral disease on MRI, and 3.9 times higher mortality. These boys also showed greater progression of clinical and radiographic disease after HCT [10]. This is further supported by studies demonstrating that survival after HCT is better in patients with a lower MRI severity score, fewer neurologic deficits, and better neurocognitive function at the time of transplant [26].
Unfortunately, demyelination was very advanced in our patient (Loes score of 17) at the time of diagnosis, making him ineligible for transplant. This case emphasizes the importance of early diagnosis of X-ALD, outlines pertinent features of the history and physical exam that should raise suspicion for cALD, and demonstrates that it is necessary to consider X-ALD in all boys presenting with PAI, even in those with underlying autoimmune disease.
Glossary
Abbreviations
- ACTH
adrenocorticotropin
- ADHD
attention deficit hyperactivity disorder
- APS-2
autoimmune polyglandular syndrome
- cALD
cerebral adrenoleukodystrophy
- CAH
congenital adrenal hyperplasia
- GH
growth hormone
- HCT
hematopoietic cell transplant
- IGF
insulin-like growth factor
- MRI
magnetic resonance imaging
- PAI
primary adrenal insufficiency
- T1DM
type 1 diabetes mellitus
- VLCFA
very long chain fatty acid
- X-ALD
X-linked adrenoleukodystrophy
Financial Support
This study was not supported by grant funding.
Disclosures
K.S. reports research support from the National Institutes of Health National Cancer Institute (R01 CA181024); Office of Orphan Products Development of the Food and Drug Administration (R01FDR0006100); Adrenas Pharmaceutics, Alexion, Spruce Biosciences, and Neurocrine Biosciences; and serves on the scientific advisory board for Eton Pharmaceuticals, Adrenas Pharmaceutics, Alexion, Spruce Biosciences, and Neurocrine Biosciences but does not accept personal income for these activities. B.S.M. is a consultant for AbbVie, Ascendis, BioMarin, EMD Serono, Novo Nordisk, Orchard Therapeutics, Pfizer, Sandoz, Sanofi Genzyme, Tolmar and Vertice and has received research support from Alexion, Abbvie, Amgen, Ascendis, Lumos Pharma, Novo Nordisk, OPKO, and Pfizer. E.I.P. receives research support from the National Institutes of Health National Center for Advancing Translational Sciences (KL2TR002492) and from ALD Alliance. R.E.W., A.O.G., T.C.L., and P.J.O. have no conflicts of interest to declare related to this work.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Wijaya M, Huamei M, Jun Z, et al. Etiology of primary adrenal insufficiency in children: a 29-year single-center experience. J Pediatr Endocrinol Metab. 2019;32(6):615-622. [DOI] [PubMed] [Google Scholar]
- 2. Perry R, Kecha O, Paquette J, Huot C, Van Vliet G, Deal C. Primary adrenal insufficiency in children: twenty years experience at the Sainte-Justine Hospital, Montreal. J Clin Endocrinol Metab. 2005;90(6):3243-3250. [DOI] [PubMed] [Google Scholar]
- 3. Kirkgoz T, Guran T. Primary adrenal insufficiency in children: Diagnosis and management. Best Pract Res Clin Endocrinol Metab. 2018;32(4):397-424. [DOI] [PubMed] [Google Scholar]
- 4. Moser AB, Jones RO, Hubbard WC, et al. Newborn screening for X-linked adrenoleukodystrophy. Int. J. Neonatal Screen 2016;2(4):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bezman L, Moser AB, Raymond GV, et al. Adrenoleukodystrophy: Incidence, new mutation rate, and results of extended family screening. Ann Neurol. 2001;49(4):512-517. [PubMed] [Google Scholar]
- 6. Kemp S, Huffnagel IC, Linthorst GE, Wanders RJ, Engelen M. Adrenoleukodystrophy – neuroendocrine pathogenesis and redefinition of natural history. Nat Rev Endocrinol. 2016;12(10):606-615. [DOI] [PubMed] [Google Scholar]
- 7. Regelmann MO, Kamboj MK, Miller BS, et al. Adrenoleukodystrophy: guidance for adrenal surveillance in males identified by newborn screen. J Clin Endocrinol Metab. 2018;103(11):4324-4331. [DOI] [PubMed] [Google Scholar]
- 8. Moser HW, Mahmood A, Raymond GV. X-linked adrenoleukodystrophy. Nat Clin Pract Neurol. 2007;3(3):140-151. [DOI] [PubMed] [Google Scholar]
- 9. Dubey P, Raymond GV, Moser AB, Kharkar S, Bezman L, Moser HW. Adrenal insufficiency in asymptomatic adrenoleukodystrophy patients identified by very long-chain fatty acid screening. J Pediatr. 2005;146(4):528-532. [DOI] [PubMed] [Google Scholar]
- 10. Polgreen LE, Chahla S, Miller W, et al. Early diagnosis of cerebral X-linked adrenoleukodystrophy in boys with Addison’s disease improves survival and neurological outcomes. Eur J Pediatr. 2011;170(8):1049-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loes DJ, Hite S, Moser H, et al. Adrenoleukodystrophy: a scoring method for brain MR observations. AJNR Am J Neuroradiol. 1994;15(9):1761-1766. [PMC free article] [PubMed] [Google Scholar]
- 12. Rattay TW, Rautenberg M, Sohn AS, et al. Defining diagnostic cutoffs in neurological patients for serum very long chain fatty acids (VLCFA) in genetically confirmed X-adrenoleukodystrophy. Sci Rep. 2020;10(1):15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Newborn screening status for all disorders: Newsteps. APHL. https://www.newsteps.org/resources/data-visualizations/newborn-screening-status-all-disorders. 2021. Accessed September 16, 2021.
- 14. Güler AS, Fis NP, Berkem M. X-Linked adrenoleukodystrophy in a 7-year-old boy presenting with psychiatric symptoms. Eur Child Adolesc Psychiatry. 2011;20(5):275-276. [DOI] [PubMed] [Google Scholar]
- 15. Ilango TS, Nambi S. X-linked adrenoleukodystrophy presenting as attention deficit hyperactivity disorder. Indian J Psychiatry 2015;57(2):208-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ievers CE, Brown RT, McCandless SE, Devine DE. Case studies: psychological test findings for two children with X-linked adrenoleukodystrophy. J Dev Behav Pediatr. 1999;20(1):31-35. [DOI] [PubMed] [Google Scholar]
- 17. Betterle C, Lazzarotto F, Presotto F. Autoimmune polyglandular syndrome Type 2: the tip of an iceberg? Clin Exp Immunol. 2004;137(2):225-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van den Driessche A, Eenkhoorn V, Van Gaal L, De Block C. Type 1 diabetes and autoimmune polyglandular syndrome: a clinical review. Neth J Med. 2009;67(11):376-387. [PubMed] [Google Scholar]
- 19. Kahaly GJ, Frommer L. Autoimmune polyglandular diseases. Best Pract Res Clin Endocrinol Metab. 2019;33(6):101344. [DOI] [PubMed] [Google Scholar]
- 20. Wiens K, Berry SA, Choi H, et al. A report on state-wide implementation of newborn screening for X-linked Adrenoleukodystrophy. Am J Med Genet A. 2019;179(7):1205-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brandão Neto RA, de Carvalho JF. Diagnosis and classification of Addison’s disease (autoimmune adrenalitis). Autoimmun Rev. 2014;13(4-5):408-411. [DOI] [PubMed] [Google Scholar]
- 22. Laureti S, Falorni A, Volpato M, et al. Absence of circulating adrenal autoantibodies in adult-onset X-linked adrenoleukodystrophy. Horm Metab Res. 1996;28(7):319-322. [DOI] [PubMed] [Google Scholar]
- 23. Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eng L, Regelmann MO. Early onset primary adrenal insufficiency in males with adrenoleukodystrophy: case series and literature review. J Pediatr. 2019;211:211-214. [DOI] [PubMed] [Google Scholar]
- 25. Mallack EJ, Turk BR, Yan H, et al. MRI surveillance of boys with X-linked adrenoleukodystrophy identified by newborn screening: meta-analysis and consensus guidelines. J Inherit Metab Dis. 2021;44(3):728-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller WP, Rothman SM, Nascene D, et al. Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: the largest single-institution cohort report. Blood 2011;118(7):1971-1978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.