Abstract
Pathogenic INS gene mutations are causative for mutant INS-gene-induced diabetes of youth (MIDY). We characterize a novel de novo heterozygous INS gene mutation (c.289A>C, p.T97P) that presented in an autoantibody-negative 5-month-old male infant with severe diabetic ketoacidosis. In silico pathogenicity prediction tools provided contradictory interpretations, while structural modeling indicated a deleterious effect on proinsulin folding. Transfection of wildtype and INS p.T97P expression and luciferase reporter constructs demonstrated elevated intracellular mutant proinsulin levels and dramatically impaired proinsulin/insulin and luciferase secretion. Notably, proteasome inhibition partially and selectively rescued INS p.T97P-derived luciferase secretion. Additionally, expression of INS p.T97P caused increased intracellular proinsulin aggregate formation and XBP-1s protein levels, consistent with induction of endoplasmic reticulum stress. We conclude that INS p.T97P is a newly identified pathogenic A-chain variant that is causative for MIDY via disruption of proinsulin folding and processing with induction of the endoplasmic reticulum stress response.
Keywords: insulin, proinsulin, insulin gene, human gene mutation, de novo mutation, permanent neonatal diabetes
Neonatal diabetes presents in the first 6 months of life and may be transient or permanent (1). Most cases are monogenic and 80% to 90% of effected individuals have a pathogenic variant in 1 of ~20 genes. The etiology of diabetes from these gene variants include altered insulin synthesis or secretion (KCNJ11, ABCC8, INS, GCK, SLC2A2, SLC19A2, RFX6), pancreatic dysgenesis (PDX1, PTF1A, HNF1B, RFX6, GATA4, GATA6, GLIS3, NEUROG3, NEUROD1, PAX6, NKX2-2, MNX1), and/or beta-cell damage (INS, EIF2, AK3, IER3IPI, FOXP3, WFS1) (2), with a minority of neonatal diabetes remaining idiopathic (3). Liu et al suggested that individuals harboring a pathogenic INS variant should be diagnosed with mutant INS-gene-induced diabetes of youth (MIDY) (4).
Normally, INS messenger RNA is translated into preproinsulin, consisting of the signal peptide, B-chain, C-peptide, and A-chain. Preproinsulin is cotranslationally or posttranslationally transported into the endoplasmic reticulum (ER) where the signal peptide is rapidly cleaved, producing proinsulin. After 3 intramolecular disulfide bonds are formed, proinsulin transits through the Golgi into secretory granules where it is cleaved by prohormone convertases to release C-peptide and mature insulin (5).
There are several potential pathogenic mechanisms of dominantly inherited insulin gene variants including noncovalent proinsulin dimerization (6), formation of disruptive intermolecular disulfide bonds (5), insulin misfolding, and induction of ER stress response pathways (5,7,8). Overall, pathogenicity is primarily believed to result from beta-cell cytotoxicity associated with proinsulin misfolding that impair synthesis, secretion, and processing of wildtype proinsulin (4,5,9-14). Herein we expand the repertoire and mechanistic understanding of MIDY-associated INS variants with characterization of a novel noncysteine insulin A-chain missense mutation.
Methods
Case Report
The patient was identified at Lucille Packard Children’s Hospital; his guardians consented to this reporting. He underwent testing with a targeted neonatal diabetes panel offered by the University of Chicago. The test includes complete sequence analysis of the coding regions of the following genes: ABCC8, EIF2AK3, FOXP3, GATA4, GATA6, GCK, INS, KCNJ11, MNX1, NKX2-2, PDX1, and ZFP57.
Molecular Modeling
Molecular modeling with the lowest-energy conformer from a nuclear magnetic resonance (NMR) solution of proinsulin (PDB 2KQP) was used to clarify the effect of the INS p.T97P mutation on secondary structure and folding of proinsulin. Using Maestro (Schrodinger), wildtype proinsulin was prepared by running the protein preparation wizard [use CCD database, add hydrogens, create zero-order bonds to metals, create disulfide bonds, delete waters beyond 5 Å, generate het states using Epik pH 7.0 ± 2.0, and assign H-bonds and proton acidity (PROPKA pH 7.0)], and then threonine was mutated in silico to proline to generate proinsulin p.T97P. A homology model was constructed of wildtype and p.T97P with comparative modeling and PDB 2KQP as a template with the Robetta structure prediction tool (15). Finally, the RosETTAFold de novo folding prediction tool (16) was employed to generate 5 models each of wildtype and p.T97P proinsulin. All structures were minimized in Maestro (Schrodinger) using the OPLS_2005 force field. Each p.T97P structure was aligned to its similarly modeled wildtype structure using the QuickAlign tool in Maestro, and final energies (MacroModel) and root mean-squared deviation () between backbone carbons were calculated (averaged over 5 models for RosETTA).
Cloning
Human wildtype and INS p.T97P were cloned into pTwist CMV BetaGlobin WPRE Neo expression construct.
As a proxy for insulin production, we utilized a construct where the C-peptide portion of proinsulin is replaced with Gaussia luciferase (17). Insulin gene blocks (wildtype and INS c.289A>C) were synthesized by Integrated DNA Technologies and cloned into the pJET1.2 intermediate vector (CloneJET PCR Cloning Kit, ThermoFisher Scientific, Waltham, MA, USA) before transfer into the proinsulin-NanoLuc reporter vector [Addgene, #62057 (17)]. All plasmids were sequence-confirmed (MCLAB, South San Francisco, CA, USA).
Cell Culture and Transfection
R7T1 (18-21) and HEK293T Cells were maintained at 37°C, 5% CO2, and 21% O2 in Dulbecco’s Modified Eagle Medium High Glucose (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum, 2 mM GlutaMAX (ThermoFisher Scientific 35050061, Waltham, MA, USA), 1 IU/mL penicillin, 1 µg/mL streptomycin, 1 mM sodium pyruvate (Hyclone, Logan, UT, USA) and 1 µg/mL doxycycline (R7T1 cells only, Sigma, St. Louis, MO, USA). R7T1 cells were authenticated by characteristically exhibiting doxycycline-dependent growth and utilized in experiments at passage number 15-34. HEK 293T cells were authenticated by performing short tandem repeat analysis (ATCC, Manassas, VA, USA). Mycoplasma screening was not performed.
Twenty-four hours before transfection, cells were plated in 96-, 12-, or 6-well adherent culture plates (ThermoFisher Scientific, Waltham, MA, USA). Cells were Lipofectamine 2000- (R7T1, Fisher 11668027, Waltham, MA, USA) or polyethylenimine- (HEK293T) transfected at approximately 80% (R7T1) or 30% (HEK293T) confluence using Opti-MEM Reduced Serum Media (Gibco, Waltham, MA, USA) according to manufacturer’s specifications. For dual luciferase assays, cells were cotransfected with pmaxGFP (Lonza, Basel, Switzerland; 50 ng/96-well), wildtype or mutant proinsulin-NanoLuc (100 ng/96-well), and CMV-Firefly (Addgene #18964; 50 ng/96-well).
Insulin Secretion and Luciferase Reporter Assays
Conditioned media (1 h in fresh media) was collected 48 h posttransfection. Cells were lysed in radioimmunoprecipitation assay buffer [25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulphate (SDS)] supplemented with a protease inhibitor cocktail (Fisher PI87785, Waltham, MA, USA). Mature insulin or proinsulin levels were assessed by ELISA (ALPCO Diagnostics, Cat# 80-INSHU-CH01, RRID:AB_2894946 and ALPCO Diagnostics, Cat# 80-PINHUT-CH01, RRID:AB_2894945). Lysate protein content was measured by Lowry assay (Biorad, Hercules, CA, USA).
Conditioned media for luciferase reporter assays was collected 48 h posttransfection following 24 h in fresh media, or 6 hours in proteasome inhibiting media [MG-132 (5 μM)]. Cells were lysed in 25 mM Tris/phosphate, 4 mM EGTA, 1% Triton X-100, 10% glycerol, 1mM dithiothreitol and luminescence assessed (Molecular Devices, San Jose, CA, USA; SpectraMax iD3).
Immunostaining and Imaging
Forty-eight hours posttransfection, HEK293T cells were fixed (4% paraformaldehyde/phosphate-buffered saline) and stained (anti-insulin/proinsulin, does not distinguish: Cell Signaling Technology Cat# 3014, RRID:AB_2126503, 1:200; Cy5-anti-rabbit: Jackson ImmunoResearch Labs Cat# 711-175-152, RRID:AB_2340607, 1:200; Hoechst, ThermoFisher Scientific 62249, Waltham, MA, USA). Aggregates were imaged 48 h posttransfection following 6 h MG-132 (5 μM) treatment. Insulin intensity and aggregate formation were quantified with Cellomics software (ArrayScan VTI, Waltham, MA, USA) (22). Quantification was normalized to number of green fluorescent protein–positive cells. For high magnification images, cells were grown on slides (Tissue culture chamber slide, Celltreat, Pepperell, MA, USA; 4′,6-diamidino-2-phenylindole mounting media, Vector Labs, H-1500).
Western Blotting
Transfected HEK293T cells were lysed (48 h posttransfection) in protease inhibitor cocktail-supplemented (Fisher PI87785, Waltham, MA, USA) radioimmunoprecipitation assay buffer (25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS). Lysates (35 μg protein/lane) were subjected to reducing or non-reducing SDS-polyacrylamide gel electrophoresis using a 4% to 15% gradient gel (Bio-Rad). The following primary antibodies were used: human proinsulin monoclonal antibodies (DSHB Cat# GS-9A8, RRID:AB_532383, 0.5 ug/mL), anti-Xbp1s antibody (Cell Signaling, #12782S RRID:AB_2687943), 1:1000), and anti-beta actin antibody (Sigma-Aldrich Cat# A5316, RRID:AB_476743, 1:10000). Antibodies were detected with IRDyes® and scanned on the Odyssey® CLX (LI-COR Biosciences). Relative band intensity was quantified using Odyssey Image Studio 2.0.
Data Analysis
Data were analyzed using GraphPad Prism and Microsoft Excel; significance testing is described in figure legends.
Results
Neonatal diabetes occurred in a 5-month-old Hispanic male born premature at 34 weeks gestation (birth weight 2060 g), with a history of prenatal hydrops fetalis and left inguinal and umbilical hernia repaired at 2 months of age. During the neonatal period and hernia repair hospitalization, point-of-care glucose varied between 56 and 153 mg/dL (3.1-8.5 mmol/L). Maternal grandmother had a history of adult-onset diabetes. He presented with severe diabetic ketoacidosis (pH 6.93, bicarbonate 6 mmol/L, anion gap 23 mmol/L, glucose 669 mg/dL or 37.2 mmol/L, and hemoglobin A1c 14.2%). His clinical condition improved with administration of intravenous insulin, fluids, and electrolytes. Sulfonylurea treatment initially resulted in improved glycemic control, but hyperglycemia recurred after ~48 h, and he was transitioned to insulin pump therapy. Autoimmune type 1a diabetes was excluded with a negative autoantibody screen (insulin, GAD-65, and ICA-512). A neonatal diabetes sequencing panel performed on the proband and biologic parents (University of Chicago Genetic Services Laboratories) identified a novel heterozygous de novo insulin gene sequence variant c.289A>C (p.T97P) of unknown significance (Fig. 1). In silico pathogenicity prediction tools (23-27) provided contradictory results (Table 1). At 25 months of age, the patient’s hemoglobin A1c was 5.7% with a total daily insulin of 0.9 units/kg/day and 9 boluses per day indicative of endogenous insulin deficiency. Developmentally, he was age appropriate for gross and fine motor skills but had expressive language delay requiring speech therapy.
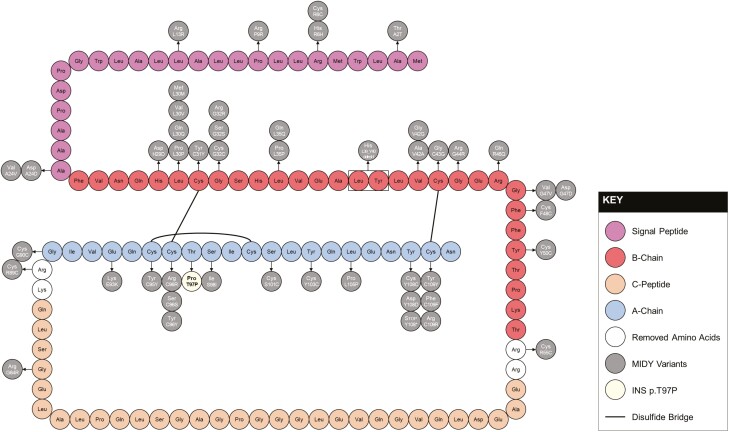
Figure 1.
Schematic of preproinsulin and mutant INS-gene-induced diabetes of youth (MIDY)-causing missense mutations. Preproinsulin consists of a signal peptide (purple), B-chain (red), C-peptide (orange), and A-chain (blue). Missense variants causing MIDY are indicated with arrows and INS p.T97P is highlighted in yellow.
Table 1.
In silico pathogenicity prediction results for INS c.289A>C
| Tool | Result |
|---|---|
| SIFT (23) Sorting Intolerant From Tolerant |
Nonpathogenic (Score = 0.15) |
| A-GVGD (24) Align Grantham Variation and Grantham Deviation |
Class 0 (Less likely) |
| PolyPhen-2 (25, 26) Polymorphism Phenotyping v2 |
Pathogenic (Score = 0.986) |
| REVEL (27) Rare Exome Variant Ensemble Learner |
Indeterminate (Score = 0.528) |
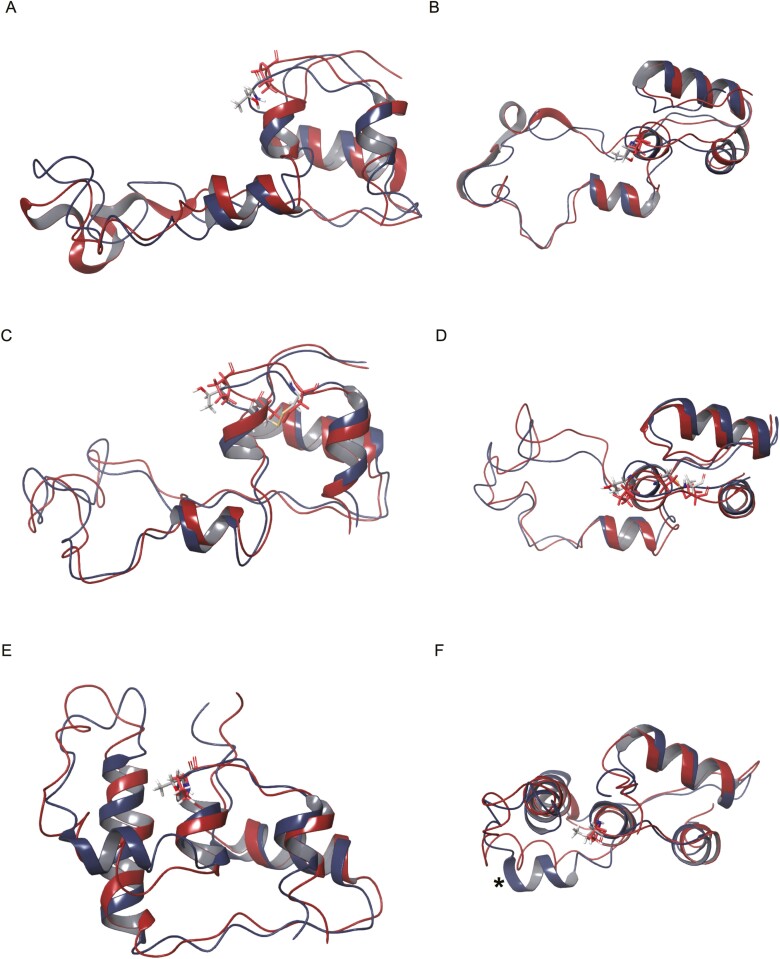
In silico modeling of wildtype and mutant proinsulin structures obtained by minimization of the NMR structure, Robetta CM, or RosETTA showed that wildtype proinsulin structures were more energetically stable by 197, 113, and 301 kJ/mol, respectively (Table 2); the positional difference of mutant backbone carbons (root mean-squared deviation) was altered by 3.8, 3.8, and 5.8 Å, respectively. Structural modeling of the proinsulin p.T97P mutant based upon the wildtype structure (Fig. 2A-2D) predicted reduced structural impact compared with de novo modeling (Fig. 2E and 2F), which predicted an alpha helix (A81-L86) that was absent in the p.T97P mutant. Notably, since both the mutant NMR solutions and Robetta CM use a folded, disulfide-linked proinsulin structure as a template, only the RosETTA approach lacks the structural constraint of the disulfide bond (C31-C96) that borders the mutated T97. Overall, modeling indicated that the p.T97P mutation impaired proinsulin folding prior to disulfide bond formation, which enforced structural rigidity, conferred an energetic penalty, and limited the structural impact.
Table 2.
In silico modeling of wildtype and mutant (T97P) proinsulin
| Modeling type | RMSD (Å), WT vs T97P |
Minimized potential energy (kJ/mol) | ∆energy (WT-T97P) (kJ/mol) | |
|---|---|---|---|---|
| WT | T97P | |||
| NMR structure minimization | 3.7835 | −17963 | −17766 | −197 |
| Robetta CM homology | 3.8537 | −18004 | −17891 | −113 |
| RosETTA folding | 5.85476 | −18310 | −18009 | −301 |
Abbreviations: NMR, nuclear magnetic resonance; RMSD, root mean-squared deviation; WT, wildtype.
Figure 2.
Molecular modeling of INS p.T97P proinsulin. (A-B) Lowest-energy conformer from an nuclear magnetic resonance solution of wildtype (blue) proinsulin (PDB 2KQP) compared to in silico T97P mutated proinsulin (red); (A) side view and (B) edge-on view. (C-D) Comparative homology modeling of wildtype (blue) and T97P (red) proinsulin with the Robetta structure prediction tool using PDB 2KQP as a template; (C) side view and (D) edge-on view. (E-F) Representative images of 5 models generated by the RosETTAFold de novo folding prediction tool for wildtype (blue) and T97P (red) proinsulin; (E) side view and (F) edge-on view. *Alpha helix from A81 to L86 predicted in wildtype models.
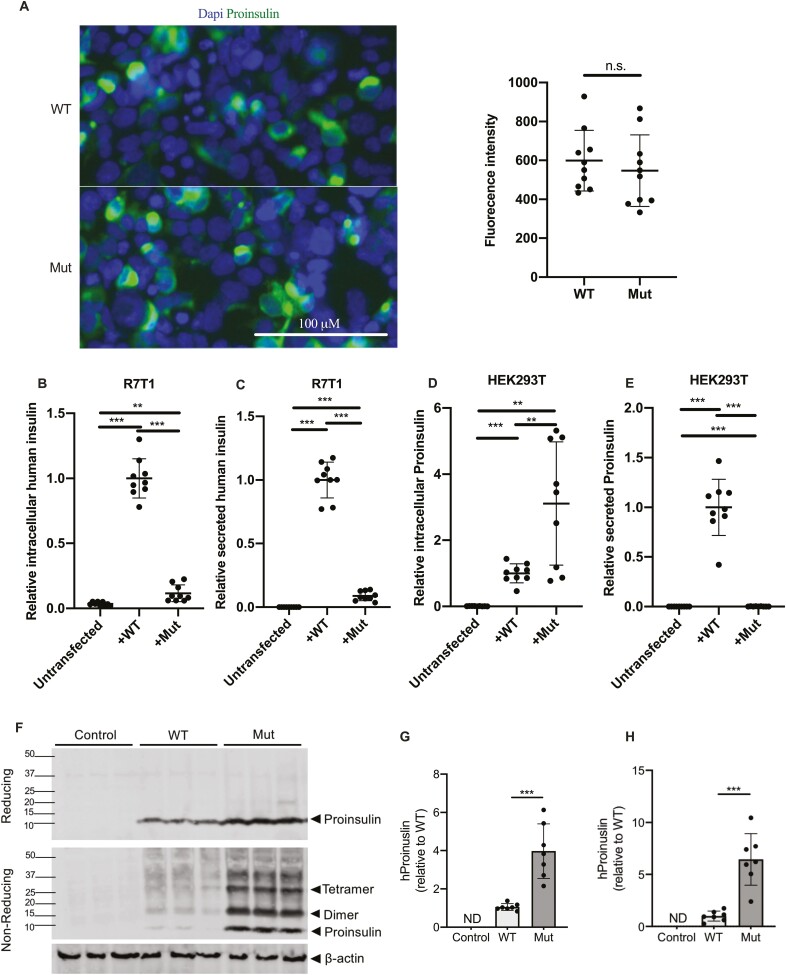
To determine the effect of INS p.T97P on proinsulin production, maturation, and secretion, we overexpressed wildtype and p.T97P proinsulin in HEK293T cells (Fig. 3A) and R7T1 beta cells. Transfected R7T1 cells demonstrated impaired generation (~8-fold) and secretion (~13-fold) of mature insulin from the mutant construct (Fig. 3B and 3C). Furthermore, mutant-transfected HEK293T cells, which lack prohormone convertases, exhibited increased intracellular proinsulin accumulation (~3-fold) but dramatically reduced proinsulin secretion (~500-fold) (Fig. 3D and 3E). Taken together, these data indicated that INS p.T97P, although expressed, was inefficiently processed, and intracellularly retained, likely because of proinsulin misfolding. To test this hypothesis, we analyzed intracellular wildtype and mutant proinsulin from transfected HEK293T cells by Western blot (Fig. 3F-3H). Indeed, these studies confirmed increased intracellular accumulation of mutant proinsulin, which, under nonreducing conditions, exhibited increased high molecular weight complex formation. These data indicate disrupted processing, secretion, and folding of the INS p.T97P mutant.
Figure 3.
The INS p.T97P mutant exhibits impaired proinsulin processing and secretion. (A) Proinsulin immunostaining (left) and quantification (right) of wildtype (WT) and INS p.T97P (Mut) transfected HEK293T cells (P > 0.05, n = 3 independent experiments). (B) Relative intracellular insulin content of untransfected, WT-, or INS p.T97P-transfected R7T1 cells measured by human-specific enzyme-linked immunosorbent assay (ELISA; normalized to total cellular protein; n = 3 independent experiments). (C) Relative secreted insulin from untransfected, WT-, or INS p.T97P-transfected R7T1 cells measured by human-specific ELISA (normalized to total cellular protein; n = 3 independent experiments). (D) Relative intracellular proinsulin content of untransfected, WT-, or INS p.T97P-transfected HEK293T cells measured by proinsulin-specific ELISA (normalized to total cellular protein; n = 3 independent experiments). (E) Relative secreted proinsulin of untransfected, WT-, or INS p.T97P-transfected HEK293T cells measured by proinsulin-specific ELISA (normalized to total cellular protein; n = 3 independent experiments). (F) Representative reducing (top) and nonreducing (middle) immunoblots of proinsulin (predicted 9.4 kDa) in mock (Control), WT-, and INS p.T97P (Mut)-transfected HEK293T cells. Loading normalized to β-actin (bottom). (G, H) Quantification of (G) proinsulin under reducing conditions and (H) total reactivity under nonreducing conditions; data are normalized to β-actin (n = 3 independent experiments). Data are expressed as means, and error bars are SDs. *P ≤ 0.05; **P < 0.01; ***P ≤ 0.001, unpaired t-tests.
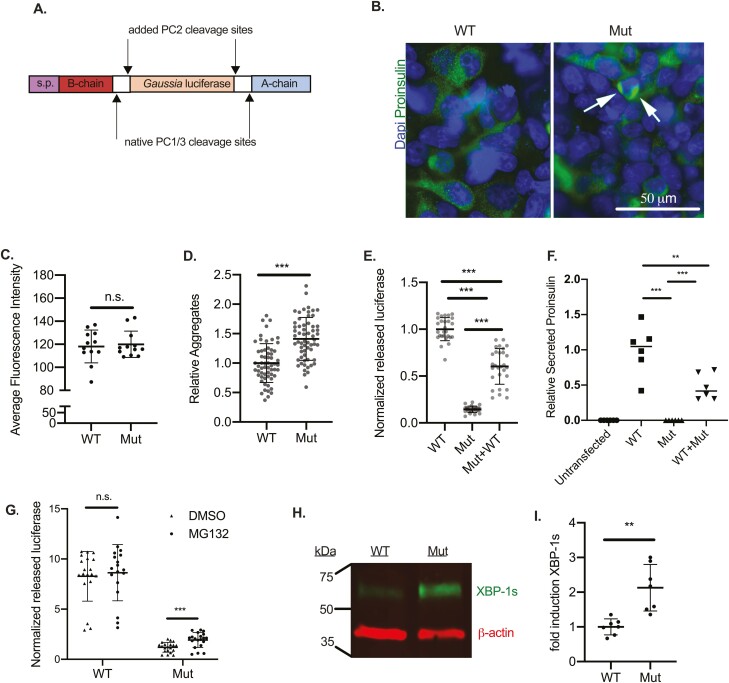
To further evaluate the pathogenic mechanism of the INS p.T97P variant, we transfected HEK293T cells with Gaussia luciferase reporter constructs encoding wildtype or INS p.T97P and performed proinsulin immunofluorescent staining (Fig. 4A and 4B). In contrast to the ELISA and Western blot findings, quantification of wildtype and variant protein by immunofluorescence were similar (Fig. 4C), perhaps reflecting epitope masking in the INS p.T97P luciferase construct that reduced immunofluorescence. Notably, visual examination and quantitative analysis of the INS p.T97P construct protein revealed a ~50% increase in proinsulin aggregates (Fig. 4B and 4D), consistent with an increased propensity for misfolding. Consistent with native insulin results, when transfected with wildtype, INS p.T97P, or equimolar amounts of both reporter constructs, the variant reduced secreted Gaussia luciferase activity by ~10-fold relative to wildtype in R7T1 cells (Fig. 4E). Notably, HEK293T cotransfection of INS p.T97P and wildtype reporter constructs (each at half-dose) or native insulin-based constructs (Fig. 4F), led to an intermediate (~50%) level of Gaussia luciferase and proinsulin secretion, respectively. Hence, INS p.T97P did not demonstrate a dominant negative intermolecular interaction.
Figure 4.
Impaired INS p.T97P processing causes endoplasmic-reticulum-associated protein degradation activation and β-cell endoplasmic reticulum stress induction. (A) Schematic of the proinsulin-luciferase reporter construct. (B) Proinsulin immunostaining of HEK293T cells transfected with the wildtype (WT) or INS p.T97P (Mut) reporter construct and (C) quantification of intracellular immunofluorescence (not significant, P =0.73). White arrows indicate proinsulin-luciferase aggregates, quantified in (D) (n = 3 independent experiments). (E) Dual luciferase assay with transfected R7T1 cells. Extracellular luciferase-insulin activity normalized to lysate CMV-firefly activity. To combine independent experiments, values were first normalized to the average dimethylsulfoxide (DMSO) value for a given experiment (n = 3 independent experiments). (F) Relative secreted proinsulin content of untransfected, WT-transfected, INSp.T97P-transfected, or WT + INSp.T97p-transfected HEK293T cells measured by proinsulin-specific enzyme-linked immunosorbent assay (normalized to total cellular protein). (G) Activity of secreted luciferase-insulin in extracellular media normalized to CMV-firefly expression in cell lysates after treatment with DMSO or MG-132 (n = 3 independent experiments). (H) Representative image of XBP-1s Western blot of HEK293T cells transfected with WT or INS p.T97P (Mut) constructs. (I) Quantification of 7 transfections (XBP-1s/actin, n = 3 independent experiments). Data are expressed as means, and error bars are SDs. *P ≤ 0.05; **P < 0.01; ***P ≤ 0.001, unpaired t-tests.
We hypothesized that the variant proinsulin was misfolded and targeted for degradation by the endoplasmic-reticulum-associated protein degradation response. To test this hypothesis, we measured Gaussia luciferase secretion from INS p.T97P or wildtype reporter-transfected R7T1 beta-cells treated with the proteasome inhibitor MG132 (Fig. 4G). Strikingly, proteasome inhibition increased variant (1.6-fold) but not wildtype insulin secretion, supporting the hypothesis that misfolded INS p.T97P variant activated the endoplasmic-reticulum-associated protein degradation pathway. To investigate upstream mechanisms of proteasome activation, we examined the ER stress response. When misfolded proteins are present in the ER, they bind and activate IRE1, which promotes XBP-1 messenger RNA splicing, XBP-1s production, and induction of a transcriptional stress response. Forced expression of the INS p.T97P reporter construct increased XBP-1s levels (Fig. 4H and 4I). Collectively, these data support the hypothesis that the INS p.T97P mutation disrupts proinsulin folding and processing, leading to intracellular accumulation and induction of ER stress, providing a mechanism for the development of MIDY in our patient.
Discussion
We report a novel pathogenic INS missense variant, c.289A>C (p.T97P) that presented in an autoantibody negative 5-month-old male infant with diabetic ketoacidosis. When expressed in cell lines, the variant exhibited elevated intracellular proinsulin levels with impaired maturation and secretion that led to increased aggregation and ER stress, a mechanism similar to that observed in the KINGS variant (7). Modeling suggested that INS p.T97P altered proinsulin secondary structure likely at a stage of folding prior to the formation of disulfide bonding constraints. Notably, INS p.T97P secretion was partially rescued with proteasome inhibition, providing a novel approach for mitigating the MIDY phenotype.
There are several limitations to the present study. First, cell lines and primary cells exhibit distinct susceptibility to cellular stress. Second, transfection-based expression differs from endogenous gene expression, which could affect protein folding, processing, and secretion. Third, a subtle dominant negative effect of INS p.T97P on wildtype insulin secretion may have been detected by independently tracking these species with distinct reporters or epitopes. Finally, although the patient’s clinical presentation (antibody negative infantile diabetes and a de novo insulin variant) and robust in vitro evidence of INS p.T97P dysfunction strongly suggest pathogenicity of this variant, contribution of additional, unidentified mutations cannot be excluded.
While myriad MIDY related B-chain variants are known, few noncysteine A-chain variants have been described [p.L105P (28), p.E93K (3), and p.S98I (29)]. Amino acid 97 is not conserved among mammalian insulins, unlike most MIDY loci (4,30,31). Diverse substitutions at this site are tolerated by the insulin receptor (30), suggesting that most substitutions at this locus are not causative for MIDY. However, our data indicate that a T97P substitution perturbs proinsulin secondary structure, disulfide bonding, folding, processing, and secretion in a manner similar to a B-chain proline substitution (6) and causes MIDY.
Acknowledgments
The authors thank O. K. Smith (Stanford University, Chemical and Systems Biology) who assisted with designing constructs and cloning strategy.
Funding
R.A.L. is supported by NIH NIDDK grants (T32DK007217, 1K12DK122550, 1K23DK122017), the Stanford Diabetes Research Center (P30DK116074) and has additional research support from the Stanford Maternal and Child Health Research Institute. H.P.M was supported by funding from the Stanford Molecular Pharmacology Training Grant 5T32GM113854, Stanford Graduate Fellowship, and the Stanford Bio-X Interdisciplinary Initiatives Seed Grants Program (IIP) (R10-56) (J.P.A., A.P.). E.A.T. is supported by the Stanford Graduate Fellowship and Stanford Bio-X Bowes Fellowship. T.M.H was supported by funding from the Stanford ChEM-H Chemistry/Biology Interface Predoctoral Training Program (T32GM120007) and the Bio-X Interdisciplinary Graduate Fellowship (GM120007). S.L. received funding from Endocrinology Training grant (T32DK007217). P.P. has research support from the Stanford Maternal and Child Health Research Institute. A.P. is a Chan Zuckerberg Biohub investigator, and has research support from the Stanford Bio-X Interdisciplinary Initiatives Seed Grants Program (IIP) and the Stanford Precision Health and Integrated Diagnostics Center (PHIND). J.P.A. is supported by NIH NIDDK grants (R01DK119955, R01DK101530), the Stanford Diabetes Research Center (P30DK116074) and the Stanford Bio-X Interdisciplinary Initiatives Seed Grants Program (IIP) (R10-56).
Contribution Statement
R.A.L. provided inpatient care for the described infant, wrote the manuscript, and provided final approval for publishing. H.P.M. wrote the manuscript, performed the bench research, and provided final approval for publishing. E.A.T assisted in bench research and writing the manuscript and provided final approval for publishing. T.M.H. performed the molecular modeling and assisted with writing the manuscript and provided final approval for publishing. S.L. performed the Western blot, edited the manuscript, and provided final approval for publishing. R.F. assisted in bench research and writing the manuscript and provided final approval for publishing. P.P. provided outpatient care for the described case, contributed to the manuscript, and provided final approval for publishing. A.P. provided personnel, edited the manuscript, and provided final approval for publishing. J.P.A. wrote the manuscript, assisted with experimental design, and supervised the bench research. He is the guarantor of the study.
Disclosures
R.A.L. has consulted for GlySens Incorporated, Abbott Diabetes Care, Biolinq, Capillary Biomedical, Deep Valley Labs, Morgan Stanley, Provention Bio and Tidepool. H.P.M. is employed by Quantum Leap Healthcare Collaborative. E.A.T., T.M.H., S.L., R.F., P.P., A.P., and J.P.A. have no conflicts of interest or disclosures.
Data Availability
The data sets and expression constructs generated during the current study are available from the corresponding authors upon reasonable request.
Previous Presentation
Initial findings were presented at the American Diabetes Association 80th Scientific Sessions (1801-P), held virtually in June 2020.
References
- 1. Aguilar-Bryan L, Bryan J. Neonatal diabetes mellitus. Endocr Rev. 2008;29(3):265-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dahl A, Kumar S. Recent advances in neonatal diabetes. Diabetes Metab Syndr Obes. 2020;13:355-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson SR, McGown I, Oppermann U, Conwell LS, Harris M, Duncan EL. A novel INS mutation in a family with maturity-onset diabetes of the young: variable insulin secretion and putative mechanisms. Pediatr Diabetes. 2018;19(5):905-909. [DOI] [PubMed] [Google Scholar]
- 4. Liu M, Hodish I, Haataja L, et al. Proinsulin misfolding and diabetes: mutant INS gene-induced diabetes of youth. Trends Endocrinol Metab. 2010;21(11):652-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu M, Huang Y, Xu X, et al. Normal and defective pathways in biogenesis and maintenance of the insulin storage pool. J Clin Invest. 2021;131(2):e142240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun J, Xiong Y, Li X, et al. Role of proinsulin self-association in mutant INS gene-induced diabetes of youth. Diabetes. 2020;69(5):954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Austin ALF, Daniels Gatward LF, Cnop M, et al. The KINGS Ins2 +/G32S mouse: a novel model of β-cell endoplasmic reticulum stress and human diabetes. Diabetes. 2020;69(12):2667-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cunningham CN, Williams JM, Knupp J, Arunagiri A, Arvan P, Tsai B. Cells deploy a two-pronged strategy to rectify misfolded proinsulin aggregates. Mol Cell. 2019;75(3):442-456.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Colombo C, Porzio O, Liu M, et al. ; Early Onset Diabetes Study Group of the Italian Society of Pediatric Endocrinology and Diabetes . Seven mutations in the human insulin gene linked to permanent neonatal/infancy-onset diabetes mellitus. J Clin Invest. 2008;118(6):2148-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rajan S, Eames SC, Park SY, et al. In vitro processing and secretion of mutant insulin proteins that cause permanent neonatal diabetes. Am J Physiol Endocrinol Metab. 2010;298(3):E403-E410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park SY, Ye H, Steiner DF, Bell GI. Mutant proinsulin proteins associated with neonatal diabetes are retained in the endoplasmic reticulum and not efficiently secreted. Biochem Biophys Res Commun. 2010;391(3):1449-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hartley T, Siva M, Lai E, Teodoro T, Zhang L, Volchuk A. Endoplasmic reticulum stress response in an INS-1 pancreatic beta-cell line with inducible expression of a folding-deficient proinsulin. BMC Cell Biol. 2010;11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hodish I, Liu M, Rajpal G, et al. Misfolded proinsulin affects bystander proinsulin in neonatal diabetes. J Biol Chem. 2010;285(1):685-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu M, Haataja L, Wright J, et al. Mutant INS-gene induced diabetes of youth: proinsulin cysteine residues impose dominant-negative inhibition on wild-type proinsulin transport. PloS One. 2010;5(10):e13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song Y, DiMaio F, Wang RY, et al. High-resolution comparative modeling with RosettaCM. Structure. 2013;21(10):1735-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baek M, DiMaio F, Anishchenko I, et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science. 2021;373(6557):871-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burns SM, Vetere A, Walpita D, et al. High-throughput luminescent reporter of insulin secretion for discovering regulators of pancreatic beta-cell function. Cell Metab. 2015;21(1):126-137. [DOI] [PubMed] [Google Scholar]
- 18. Wang W, Walker JR, Wang X, et al. Identification of small-molecule inducers of pancreatic beta-cell expansion. Proc Natl Acad Sci U S A. 2009;106(5):1427-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Efrat S, Fusco-DeMane D, Lemberg H, al Emran O, Wang X. Conditional transformation of a pancreatic beta-cell line derived from transgenic mice expressing a tetracycline-regulated oncogene. Proc Natl Acad Sci U S A. 1995;92(8):3576-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Milo-Landesman D, Surana M, Berkovich I, et al. Correction of hyperglycemia in diabetic mice transplanted with reversibly immortalized pancreatic beta cells controlled by the tet-on regulatory system. Cell Transplant. 2001;10(7):645-650. [PubMed] [Google Scholar]
- 21. Fleischer N, Chen C, Surana M, et al. Functional analysis of a conditionally transformed pancreatic beta-cell line. Diabetes. 1998;47(9):1419-1425. [DOI] [PubMed] [Google Scholar]
- 22. Zhao Z, Abdolazimi Y, Armstrong NA, Annes JP. A high-content in vitro pancreatic islet β-cell replication discovery platform. J Vis Exp. 2016; (113): 10.3791/54298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31(13):3812-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tavtigian SV, Deffenbaugh AM, Yin L, et al. Comprehensive statistical study of 452 BRCA1 missense substitutions with classification of eight recurrent substitutions as neutral. J Med Genet. 2006;43(4):295-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet. 2013;Chapter 7:Unit 7.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ioannidis NM, Rothstein JH, Pejaver V, et al. REVEL: an ensemble method for predicting the pathogenicity of rare missense variants. Am J Hum Genet. 2016;99(4):877-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ortolani F, Piccinno E, Grasso V, et al. Diabetes associated with dominant insulin gene mutations: outcome of 24-month, sensor-augmented insulin pump treatment. Acta Diabetol. 2016;53(3):499-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cao B, Gong C, Wu D, et al. Genetic analysis and follow-up of 25 neonatal diabetes mellitus patients in China. J Diabetes Res. 2016;2016:6314368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weiss MA, Wan Z, Zhao M, et al. Non-standard insulin design: structure-activity relationships at the periphery of the insulin receptor. J Mol Biol. 2002;315(2):103-111. [DOI] [PubMed] [Google Scholar]
- 31. Weiss MA, Hua QX, Jia W, et al. Activities of monomeric insulin analogs at position A8 are uncorrelated with their thermodynamic stabilities. J Biol Chem. 2001;276(43):40018-40024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets and expression constructs generated during the current study are available from the corresponding authors upon reasonable request.