Abstract
Reactive oxygen species (ROS)-mediated reductions in nitric oxide (NO)-dependent dilation are evident in adults with major depressive disorder (MDD); however, the upstream mechanisms remain unclear. Here, we hypothesized that nuclear factor-κB (NF-κB) activation-induced ROS production contributes to microvascular endothelial dysfunction in MDD. Thirteen treatment-naive adults with MDD (6 women; 19–23 yr) and 10 healthy nondepressed adults (HAs; 5 women; 20–25 yr) were tested before and after (open-label design) systemic NF-κB knockdown (nonacetylated salicylate; 3,000–4,500 mg/day × 4 days). Red cell flux (laser Doppler flowmetry) was measured during graded intradermal microdialysis perfusion of the endothelium-dependent agonist acetylcholine (ACh), alone and in combination with NO synthase inhibition [NG-nitro-l-arginine methyl ester (l-NAME)] or ROS scavenging (apocynin). Serum salicylate concentrations following treatment were not different between groups (22.8 ± 7.4 HAs vs. 20.8 ± 4.3 mg/dL MDD; P = 0.46). When compared with HAs, the NO-dependent component of ACh-induced dilation was blunted in adults with MDD before (P = 0.023), but not after (P = 0.27), salsalate treatment. In adults with MDD, the magnitude of improvement in endothelium-dependent dilation following salsalate treatment was inversely related to the degree of functional impairment at baseline (R2 = 0.43; P = 0.025). Localized ROS scavenging improved NO-dependent dilation before (P < 0.01), but not after (P > 0.05), salsalate treatment. Salsalate did not alter systemic concentrations of pro- or anti-inflammatory cytokines (all P > 0.05). These data suggest that NF-κB activation, via increased vascular ROS production, contributes to blunted NO-dependent dilation in young adults with MDD but otherwise free of clinical disease. These data provide the first direct evidence for a mechanistic role of vascular inflammation-associated endothelial dysfunction in human depression.
NEW & NOTEWORTHY Our data indicate that short-term treatment with therapeutic doses of the nuclear factor-κB (NF-κB) inhibitor salsalate improved nitric oxide (NO)-mediated endothelium-dependent dilation in adults with major depressive disorder (MDD). In adults with MDD, acute localized scavenging of reactive oxygen species (ROS) with apocynin improved NO-dependent dilation before, but not after, salsalate administration. These data suggest that activation of NF-κB, in part via stimulation of vascular ROS production, contributes to blunted NO-mediated endothelium-dependent dilation in young adults with MDD.
Keywords: inflammation, microdialysis, nitric oxide, salsalate, superoxide
INTRODUCTION
Major depressive disorder (MDD), defined as a persistently depressed mood and/or anhedonia that causes significant functional impairment in everyday life (1), is an episodic and highly recurrent mood disorder that manifests in ∼10%–15% of adults across their lifespan (2). Extensive evidence links MDD to a substantial increase in the risk of developing future cardiovascular disease, an association that is independent of both traditional cardiovascular risk factors and the remission of depressive symptoms (3–6). Although the mechanisms underlying this link are undoubtedly multifactorial and complex, impairments in vascular endothelial function are evident in adults with MDD and likely contribute (7–11). Therefore, understanding the specific molecular mediators of vascular dysregulation in adults with MDD but otherwise free of clinical disease has clear relevance for the development of novel therapeutic interventions to prevent or slow cardiovascular disease progression.
Evidence from rodent models of depression demonstrates that increased vascular oxidative stress and subsequent reductions in nitric oxide (NO) bioavailability contribute to depression-induced endothelial dysfunction (12–16). Using a targeted in vivo pharmacological approach, our laboratory recently translated these preclinical findings in rodents to human depression, demonstrating blunted NO-dependent dilation in the microvasculature of young adults with MDD (9). Furthermore, acute localized antioxidant administration, using either a scavenger of superoxide or an inhibitor of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, improved NO-dependent dilation (9). Collectively, these data suggest that increased reactive oxygen species (ROS), specifically superoxide, functionally contribute to blunted NO-mediated endothelium-dependent dilation in adults with MDD.
Depression is also associated with sustained activation of the innate immune system, leading to increased production of proinflammatory cytokines [e.g., interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α] and acute phase proteins [e.g., C-reactive protein (CRP)] (17–19). The inducible transcription factor nuclear factor-κB (NF-κB) is a critical upstream regulator of both the inflammatory and redox state of vascular endothelial cells (20, 21). In adults with subclinical cardiovascular disease, NF-κB activation has been directly linked to oxidative stress-mediated reductions in NO bioavailability and subsequent arterial dysfunction (22–25). Although the upstream mechanisms are not clear, NF-κB activity appears to be upregulated in adults with MDD, in turn increasing proinflammatory cytokine production (26, 27), and there is indirect evidence supporting a role for inflammatory cytokines in mediating endothelial dysfunction in adults with depressive disorders (28). However, no studies have mechanistically examined a potential role of inflammation in contributing to oxidative stress-induced detriments in NO-mediated endothelium-dependent dilation in adults with MDD. Given this background, we conducted a small, open-label, proof-of-concept evaluation of the effect of short-term administration of the anti-inflammatory nonacetylated salicylate [e.g., oral salsalate, an inhibitor of NF-κB activation (24, 29–32)] on the mechanisms contributing to blunted endothelium-dependent dilation in young adults with MDD. We hypothesized that salsalate administration would improve NO-dependent dilation in young adults with MDD, in part, by suppressing ROS (Fig. 1).
Figure 1.
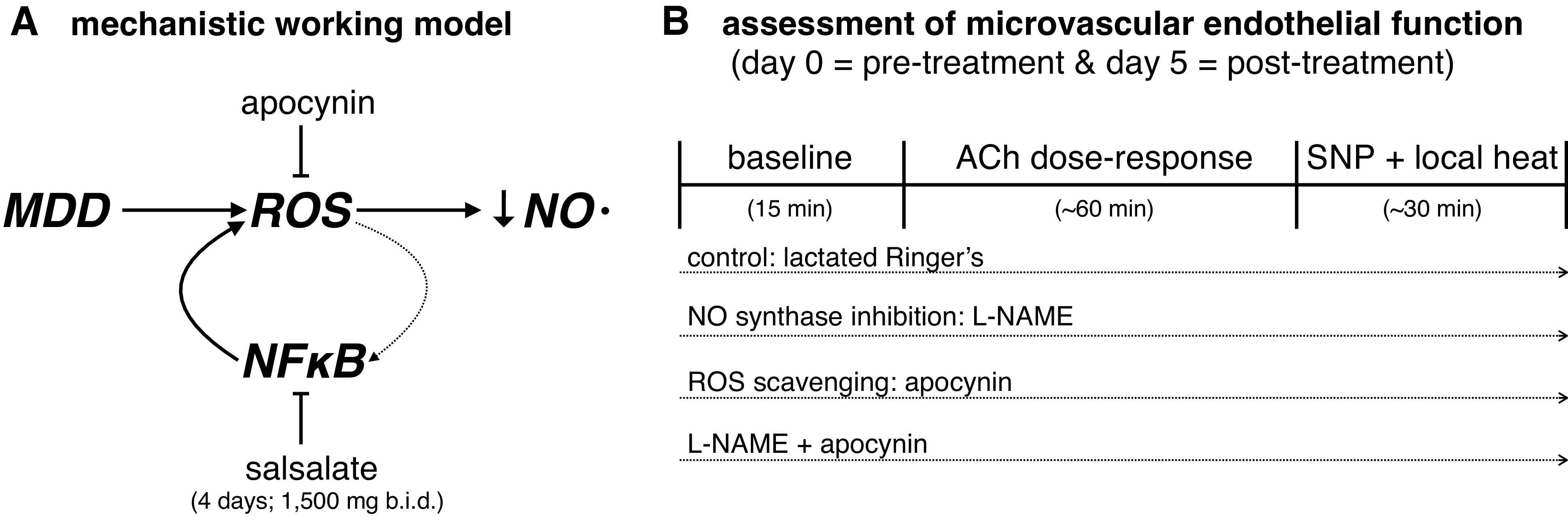
A: increased reactive oxygen species (ROS) contribute to reductions in nitric oxide (NO) bioavailability and associated reductions in endothelium-dependent dilation. The inducible transcription factor nuclear factor-κB (NF-κB) is a critical upstream regulator of both the inflammatory and redox state of vascular endothelial cells and its activation has been directly linked to oxidative stress-mediated reductions in NO bioavailability and subsequent arterial dysfunction. Here, we evaluated the effect of short-term administration of the anti-inflammatory nonacetylated salicylate (e.g., salsalate, an inhibitor of NF-κB activation) on the mechanisms contributing to blunted endothelium-dependent dilation in young adults with major depressive disorder (MDD). We hypothesized that salsalate administration would improve NO-dependent dilation in young adults with MDD, in part, by suppressing ROS. Please note the interconnected linkage between oxidative stress and inflammation, and the directionality of signaling pathway activation, is complex and discussed in depth in several excellent review articles (33, 34). B: experimental protocol for the assessment of microvascular endothelial function; this testing occurred before (day 0) and immediately after (day 5) short-term salsalate administration. Each dotted arrow reflects the perfusion of selected pharmacological agents via intradermal microdialysis. See text for additional detail. ACh, acetylcholine; l-NAME, NG-nitro-l-arginine methyl ester; SNP, sodium nitroprusside.
METHODS
All experimental procedures and protocols were approved by the Institutional Review Board at The Pennsylvania State University and the Food and Drug Administration (IND 125,944). The investigation was conducted in accordance with the Declaration of Helsinki. The nature, risks, and benefits were explained, and verbal and written informed consent was obtained before voluntary participation. Data collection was completed before the effective date of compliance with the updated definitions and requirements for registration of clinical trials. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Diagnostic Assessment of Major Depressive Disorder
All participants underwent the Mini-International Neuropsychiatric Interview (35) (administered by J.L.G.), a short structured clinical interview used to assess the presence or absence of major psychiatric illness defined by standard Diagnostic and Statistical Manual of Mental Disorders (DSM-5) diagnostic criteria (1). Results were reviewed with a psychiatrist (E.F.H.S.) as part of a best estimate diagnostic process (36), consistent with our previous studies (9, 37–39). Participants were excluded for comorbid current psychiatric disorders (e.g., psychosis, schizophrenia, bipolar disorder, panic disorder, posttraumatic stress disorder, etc.), the use of psychoactive or psychopharmacological drugs within 1 year, and active suicidal ideation. Fifteen otherwise healthy nonmedicated adults with MDD were enrolled. A reference group of 10 healthy nondepressed adults (HAs) without any history or evidence of major psychiatric illness was also included.
At each of the experimental visits, depressive symptom severity was evaluated using the self-administered Patient Health Questionnaire-9 (PHQ-9), a valid and sensitive index of symptomology based on the diagnostic criteria for depressive disorders (40). The PHQ-9 rates the nine symptoms of depression on a 4-point Likert scale, ranging from 0 = “not at all” to 3 = “nearly every day.” Response options are used to calculate a total score (maximum = 27), and symptom severity is quantified as none (0–4), mild (5–9), moderate (10–14), moderately severe (15–19), or severe (20–27).
In addition to the psychiatric assessment, all participants completed a medical health history, including anthropometric, seated blood pressure, and resting heart rate measurements (Connex Spot Monitor; Welch Allyn, Skaneateles Falls, NY), as well as basic blood biochemistry and a lipid profile (Quest Diagnostics; Pittsburgh, PA). All participants were free of cardiovascular, renal, and metabolic disease; were recreationally active; were nonobese (body mass index, <30 kg/m2); did not use tobacco products or illicit drugs; and were not taking prescription medications except for hormonal contraception (HAs, n = 1; adults with MDD, n = 2; Table 1). Because adults with MDD were not currently treated for their depressive symptoms, eligible participants were enrolled and tested as soon as possible (with ∼1 wk of enrollment) to facilitate expedient follow-up with a mental healthcare provider. This decision regarding the methodological approach was made in consultation with our collaborating psychiatrist. Thus, the timing of the experimental visits was not controlled for the menstrual cycle phase, which is consistent with our laboratory’s previous studies in adults with depression (9, 37–39). Importantly, a growing body of literature spanning multiple vascular beds and vascular function outcome measures reports limited or no variability across the menstrual cycle phase (41–45). A urine pregnancy test confirmed the absence of pregnancy at each experimental visit.
Table 1.
Participant characteristics
| HA | MDD | |
|---|---|---|
| Characteristic | ||
| n (men/women) | 10 (5/5) | 13 (7/6) |
| Age, yr | 22 ± 1 | 21 ± 1* |
| Height, cm | 173 ± 9 | 172 ± 13 |
| Mass, kg | 75 ± 19 | 67 ± 15 |
| Body mass index, kg/m2 | 25.6 ± 4.4 | 22.5 ± 2.9 |
| Heart rate, beats/min | 64 ± 3 | 66 ± 7 |
| Systolic BP, mmHg | 121 ± 13 | 111 ± 13 |
| Diastolic BP, mmHg | 74 ± 5 | 71 ± 7 |
| Blood biochemistry | ||
| HbA1c, % | 5.3 ± 0.16 | 5.3 ± 0.15 |
| Fasting total cholesterol, mg/dL | 165 ± 36 | 146 ± 31 |
| Fasting HDL, mg/dL | 60 ± 22 | 55 ± 17 |
| Fasting LDL, mg/dL | 84 ± 16 | 73 ± 24 |
| Fasting triglycerides, mg/dL | 82 ± 27 | 94 ± 52 |
Values are means ± SD. BP, blood pressure; HA, healthy nondepressed adult; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MDD, major depressive disorder. *P < 0.05 vs. HA.
Salsalate Administration
This was a small open-label trial designed to probe the mechanistic control of microvascular function, and as such, a placebo treatment arm was not used. Instead, microvascular endothelial function was assessed before (day 0) and after 4 days of oral salsalate administration (1,500 mg twice a day; total daily dose 3,000 mg); the final dose of salsalate (1,500 mg) occurred on the morning of the postsalsalate treatment experimental visit (day 5). This short-term treatment approach elicits increases in serum salicylate concentrations to the therapeutic range and reduces total and nuclear NF-κB in vascular endothelial cells (22, 24). Serum salicylate concentrations were measured by the Mount Nittany Medical Center Laboratory using a colorimetric assay on day 3 of salsalate treatment to ensure concentrations were not toxic (<30 mg/100 mL). If serum salicylate concentrations were not within the therapeutic range (10–30 mg/100 mL) on day 3 of treatment, the total daily dosage was increased to 3,500–4,500 mg (n = 3 HAs, n = 1 MDD). This dosing regimen has been used clinically and experimentally to produce steady-state salicylate concentrations sufficient to inhibit NF-κB and improve indices of vascular function, while minimizing untoward side effects (22–25, 29, 46, 47). Participants were monitored for any signs or symptoms of toxicity (e.g., tinnitus, nausea). No subjects had serum salicylate concentrations in the toxic range. Two patients with MDD withdrew from the study on day 3 (tinnitus, n = 1; development of a rash, n = 1). Only data from the 13 adults with MDD who completed both pre- and posttreatment experimental visits are included.
Additional blood samples were obtained in sodium heparin-coated vacutainers at each experimental visit, and plasma was stored at –80°C for batched analysis of inflammatory biomarkers. For the current analyses, we selected specific inflammatory biomarkers (a priori) that have been linked to MDD (26, 27): the proinflammatory cytokines IL-1β, IL-6, and TNF-α, and the chemokine for neutrophils IL-8. Plasma concentration was measured via multiplex arrays using commercially available assay kits (Meso Scale Diagnostics; Rockville, MD). The lower detection limit for each cytokine was ≤0.69 pg/mL. The intra-assay coefficients of variation ranged from 2.8% to 5.8%, and the inter-assay coefficients of variation ranged from 6.4% to 9.7%.
Assessment of Microvascular Endothelial Function
Intradermal microdialysis probes (CMA Linear 30 probe, 6 kDa; Harvard Apparatus, Holliston, MA) were inserted into the dermal layer of the ventral forearm using sterile technique for the local delivery of pharmacological agents, as previously described in detail (9, 38, 39, 48). Pharmacological agents were prepared immediately before use, dissolved in lactated Ringer solution, filtered using sterile syringe microfilters (Acrodisc; Pall, Ann Arbor, MI), wrapped in foil to prevent photodegradation, and subsequently perfused through the microdialysis probes (2 μL/min; Bee Hive controller and Baby Bee microinfusion pump; BASi, West Lafayette, IN). Intradermal microdialysis probes were perfused with lactated Ringer solution (control) or the nonselective NO synthase inhibitor NG-nitro-l-arginine methyl ester to inhibit the production of NO (l-NAME, 15 mmol/L; Calbiochem, EMD Millipore, Billerica, MA). This study was part of a larger ongoing project in the laboratory and, as such, in a subset of adults with MDD (n = 7), additional microdialysis probes were perfused with the ROS scavenger apocynin (100 μmol/L; Bio-Techne Corporation, Minneapolis, MN) or apocynin + l-NAME to determine the effect of ROS scavenging on NO production (49, 50). Red cell flux, an index of cutaneous blood flow (51), was continuously measured directly over each microdialysis probe with an integrated laser Doppler flowmeter probe secured in a local heating unit (VP12 and VHP2; Moor Instruments, Wilmington, DE) set to thermoneutrality (33°C). The experimental protocol is depicted in Fig. 1.
After an initial hyperemia-resolution period (∼60 min), during which the site-specific pharmacological agents were perfused, baseline red cell flux was measured for 15 min. Thereafter, increasing concentrations of acetylcholine (ACh; 10−10–10−1 mol/L; United States Pharmacopeia), an endothelium-dependent agonist, were coperfused with the site-specific pharmacological agent sequentially for 5 min each. At the conclusion of the dose-response protocol, the NO donor sodium nitroprusside (SNP; 28 mmol/L; United States Pharmacopeia, Rockville, MD) was perfused through each probe and the local temperature was increased to 43°C to elicit maximal dilation (∼30 min) (9, 38, 39, 48). Automated brachial blood pressure (Connex Spot Monitor; Welch Allyn, Skaneateles Falls, NY) was measured every 5 min throughout the protocol.
Data and Statistical Analysis
All data collection and analysis procedures were standardized before testing. Red cell flux was recorded at 40 Hz (PowerLab and LabChart; AD Instruments, Bella Vista, NSW, Australia). Vascular conductance was calculated as laser Doppler flux (perfusion units) divided by mean arterial pressure, normalized as a percentage of the maximum (%max) (9, 38, 39, 48), and averaged during 5 min of baseline and during the last minute of each ACh dose. Analysis of vascular conductance was confirmed by an outside investigator from our laboratory blinded to group and treatment. Area under the dose-response curve was calculated using the trapezoid rule (Prism, v 8.1; GraphPad Software, La Jolla, CA). Based on our previous findings (9, 38), an a priori power analysis indicated that 10 subjects per group would be sufficient to detect a meaningful physiological difference of 15% (10% standard deviation of the residuals) between groups and within treatments (α = 0.05, β = 0.80).
Student’s unpaired t tests were used to compare participant characteristics. Vascular conductance was analyzed using two- or three-way, mixed-model, repeated-measures analysis of covariance (SAS, v. 9.4; Cary, NC), with post hoc Bonferroni corrections applied for specific planned comparisons when appropriate. Vascular conductance values are presented as means ± SD, effect sizes are reported as partial eta squared (; small effect = 0.01, medium effect = 0.06, large effect = 0.14), and significance was set at α < 0.05.
RESULTS
The groups were well matched for anthropometric characteristics, resting hemodynamics, and blood biochemistry, and all parameters were within optimal clinical ranges in both groups (Table 1; all P > 0.05). Although all participants were young adults (range: 19–25 yr), adults with MDD were slightly younger than HA (Table 1; P = 0.004). Adults with MDD were experiencing depressive symptoms of mild-moderate severity, and depressive symptomology was not affected by salsalate administration [PHQ-9: 11 ± 6 pre vs. 10 ± 5 arbitrary units (AU) post; P = 0.87]. HAs were not experiencing depressive symptoms at either visit (PHQ-9: 1 ± 2 pre vs. 1 ± 2 AU post; P = 0.44).
Short-term salsalate treatment increased serum salicylate concentrations to the therapeutic range (>10 mg/dL) for all subjects, and concentrations were not different between groups (22.8 ± 7.4 HAs vs. 20.8 ± 4.3 mg/dL MDD; P = 0.46). Salsalate administration did not affect systolic blood pressure (HAs: 123 ± 9 pre vs. 117 ± 9 mmHg post; MDD: 117 ± 9 pre vs. 117 ± 10 mmHg post, Group × Condition: P = 0.49) or diastolic blood pressure (HAs: 78 ± 5 pre vs. 75 ± 5 mmHg post; MDD: 76 ± 6 pre vs. 73 ± 6 mmHg post, Group × Condition: P = 0.99) in either group. Circulating inflammatory biomarkers were not different between groups pretreatment (Table 2; all P > 0.05) and were unaffected by salsalate administration (Table 2; all P > 0.05).
Table 2.
Circulating proinflammatory markers before and after salsalate treatment
| HA |
MDD |
Group () | Condition () | Sex () | Group × Condition () | |||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||||
| CRP, mg/L | 0.7 ± 0.3 [0.2–2.5] | 1.8 ± 0.6 [0.2–3.8] | 2.0 ± 1.3 [0.2–12.1] | 0.7 ± 0.2 [0.2–2.1] | 0.19 (0.15) |
0.06 (0.28) |
0.93 (<0.01) |
0.06 (0.30) |
| IL-1β, pg/mL | 0.06 ± 0.07 [0–0.18] | 0.08 ± 0.08 [0–0.22] | 0.12 ± 0.13 [0–0.4] | 0.11 ± 0.16 [0–0.56] | 0.18 (0.08) |
0.88 (<0.01) |
0.715 (0.10) |
0.76 (<0.01) |
| IL-6, pg/mL | 0.52 ± 0.41 [0.16–1.70] | 0.66 ± 0.65 [0.10–1.84] | 0.51 ± 0.23 [0.18–1.16] | 0.36 ± 0.16 [0.14–0.70] | 0.20 (0.08) |
0.95 (0.0) |
0.44 (0.03) |
0.22 (0.07) |
| IL-8, pg/mL | 3.37 ± 0.98 [2.22–5.56] | 3.90 ± 0.1.93 [2.18–6.46] | 4.00 ± 1.24 [1.64–9.24] | 4.25 ± 1.52 [1.90–7.34] | 0.47 (0.03) |
0.14 (0.11) |
0.07 (0.15) |
0.74 (<0.01) |
| TNFα, pg/mL | 1.82 ± 0.54 [0.98–2.74] | 2.04 ± 0.60 [1.30–3.26] | 2.05 ± 0.47 [1.40–2.74] | 2.06 ± 0.60 [1.18–3.04] | 0.48 (0.03) |
0.32 (0.05) |
0.95 (0.0) |
0.47 (0.03) |
Values are means ± SD [ranges]. Data were analyzed using three-way, mixed-model, repeated-measures analysis of covariance; n = 10 HAs (5 women), and n = 13 MDD (6 women). CRP, C-reactive protein; HA, healthy nondepressed adult; IL, interleukin; MDD, major depressive disorder; TNF, tumor necrosis factor.
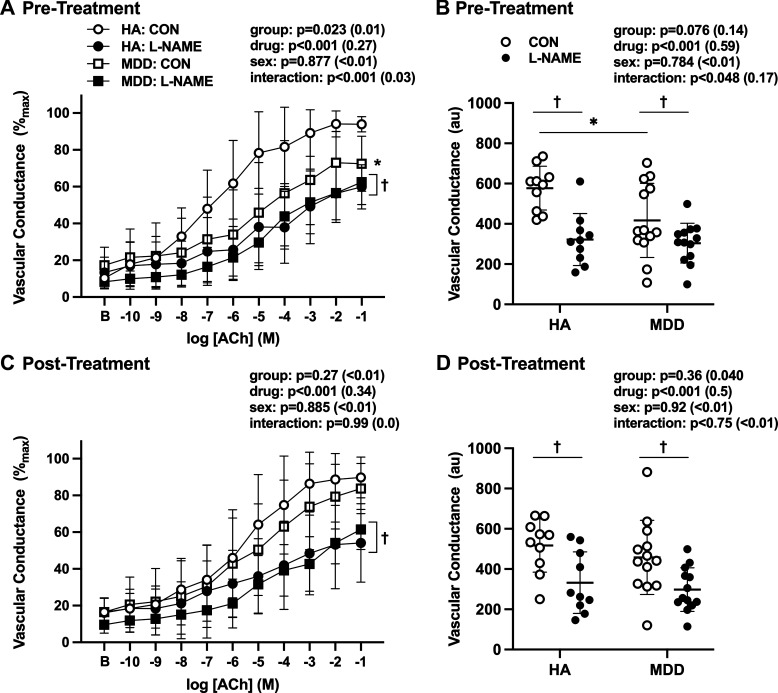
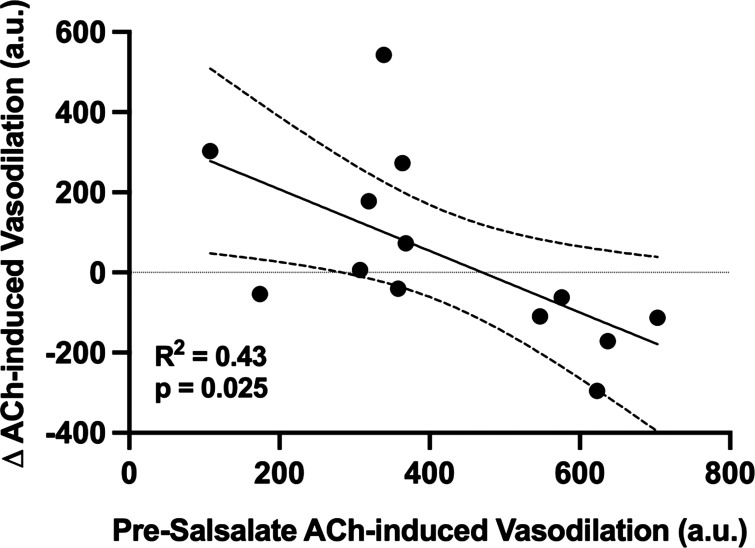
Neither baseline nor maximal vascular conductance was different between groups, pharmacological treatments, or conditions (Table 3; all P > 0.05). Presalsalate treatment, ACh-induced dilation was attenuated in adults with MDD compared with HA (Fig. 2, A and B) and l-NAME-induced NOS inhibition blunted ACh-induced dilation to the same extent in both groups (Fig. 2, A and B), suggesting a relative loss of NO-dependent dilation in adults with MDD. Following acute salsalate treatment, there were no group differences in ACh-induced dilation, alone or during concurrent NOS inhibition (Fig. 2, C and D). In adults with MDD, there was a significant linear relation between ACh-induced dilation presalsalate treatment and the salsalate-induced improvement in endothelium-dependent dilation (Fig. 3; R2 = 0.38, P = 0.024), demonstrating that the adults with MDD having the most severe reductions in endothelium-dependent dilation at baseline exhibited the greatest magnitude of improvement following short-term salsalate treatment.
Table 3.
Baseline and maximum vascular conductance
| Pharmacological Treatment | HC |
MDD |
Group () | Condition () | Drug () | Sex () | Group × Condition × Drug () | ||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||||
| Baseline | 0.30 (0.01) |
0.34 (<0.01) |
<0.01 (0.14) |
0.98 (0.0) |
0.58 (0.02) |
||||
| Control | 0.21 ± 0.09 | 0.26 ± 0.15 | 0.26 ± 0.11 | 0.25 ± 0.10 | |||||
| l-NAME | 0.19 ± 0.11 | 0.22 ± 0.08 | 0.12 ± 0.04 | 0.15 ± 0.07 | |||||
| Apocynin | 0.16 ± 0.07 | 0.20 ± 0.07 | |||||||
| l-NAME + apocynin | 0.12 ± 0.04 | 0.19 ± 0.10 | |||||||
| Maximum | 0.19 (0.02) |
0.35 (<0.01) |
0.12 (0.06) |
0.32 (0.03) |
0.47 (0.03) |
||||
| Control | 1.96 ± 0.30 | 1.69 ± 0.91 | 1.75 ± 0.57 | 1.71 ± 0.50 | |||||
| l-NAME | 1.50 ± 0.63 | 1.45 ± 0.49 | 1.48 ± 0.48 | 1.77 ± 0.64 | |||||
| Apocynin | 1.46 ± 0.58 | 1.61 ± 0.30 | |||||||
| l-NAME + apocynin | 1.65 ± 0.69 | 1.81 ± 0.51 | |||||||
Values are means ± SD. Vascular conductance = flux/mean arterial pressure (perfusion units/mmHg). Data were analyzed using three-way, mixed-model, repeated-measures analysis of covariance; n = 10 HA (5 women), and n = 13 MDD (6 women). HA, healthy nondepressed adult; l-NAME, NG-nitro-l-arginine methyl ester; MDD, major depressive disorder.
Figure 2.
Vascular conductance pre (A and B)- and post (C and D)-salsalate administration in healthy nondepressed adults (HAs, n = 10 total, n = 5 female) and in those with major depressive disorder (MDD, n = 13 total, n = 6 female). Data are presented in response to increased doses of acetylcholine (ACh) alone [control (Con)] and during concurrent nitric oxide synthase inhibition [NG-nitro-l-arginine methyl ester (l-NAME)] in A and C and as areas under the dose-response curve in B and D. When compared with HA, endothelium-dependent dilation was blunted in adults with MDD before (A and B), but not after (C and D), short-term salsalate administration. B, baseline. Data are means ± SD () and were analyzed using three-way, mixed-model, repeated-measures analysis of covariance. *P < 0.05 vs. HA; †P < 0.05 vs. Con.
Figure 3.
The relation between acetylcholine (ACh)-induced dilation before treatment and the magnitude of the change in ACh-induced dilation post-salsalate administration in adults with major depressive disorder (MDD). Data are presented as areas under the dose-response curve, with 95% confidence intervals, and were analyzed by linear regression, covarying for sex (P = 0.366).
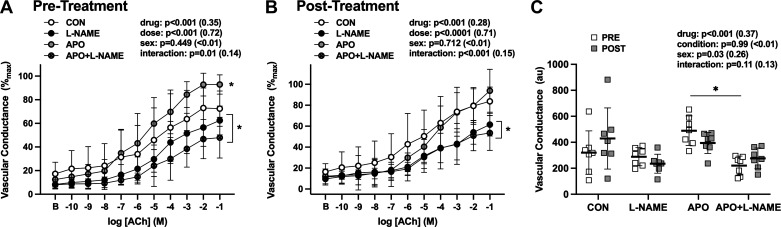
In adults with MDD, before salsalate treatment, apocynin significantly improved ACh-induced dilation, via increases in the NO-dependent component of this response (Fig. 4, A and C). In contrast, after salsalate treatment, acute apocynin administration did not further improve endothelium-dependent dilation in adults with MDD (Fig. 4, B and C), suggesting that salsalate reduced the ROS-associated suppression of endothelium-dependent dilation in young adults with MDD.
Figure 4.
Vascular conductance pre (A)- and post (B)-salsalate administration in adults with major depressive disorder (MDD, n = 7 total, n = 3 women). Data are presented in response to increased doses of acetylcholine (ACh) alone [control (Con)] and during concurrent nitric oxide synthase inhibition [NG-nitro-l-arginine methyl ester (l-NAME)], NADPH oxidase inhibition with apocynin (Apo), or both (Apo + l-NAME) in A and B and as areas under the dose-response curve in C. Before salsalate treatment, acute administration of Apo improved ACh-induced dilation via increases in the NO-dependent contribution of the response. Following short-term salsalate treatment, Apo-induced improvements in ACh-induced dilation were no longer evident. B, baseline. Data are means ± SD (ηp2) and were analyzed using three-way, mixed-model, repeated-measures analysis of covariance. *P < 0.05 vs. Con.
DISCUSSION
This was a small, open-label study designed to examine the potential effect of short-term anti-inflammatory treatment on mechanisms contributing to blunted endothelium-dependent dilation in young adults with MDD but otherwise free of clinical disease. Consistent with our previous reports (9, 39), adults with MDD demonstrated blunted ACh-induced dilation, mediated by reductions in the NO-dependent component. Short-term treatment with therapeutic doses of the NF-κB inhibitor salsalate improved NO-mediated endothelium-dependent dilation in adults with MDD. Furthermore, in adults with MDD, acute administration of the ROS scavenger apocynin directly to the microvasculature improved NO-dependent dilation before, but not after, salsalate administration. Taken together, these data suggest that activation of NF-κB, in part via stimulation of vascular ROS production, contributes to blunted NO-mediated endothelium-dependent dilation in young adults with MDD. These proof-of-concept data provide support for larger placebo-controlled clinical trials to examine the efficacy of chronic adjunctive anti-inflammatory administration to mitigate the pathophysiological sequelae of vascular inflammation in MDD.
In rodent models of depression, increased expression of proinflammatory cytokines in the vasculature has been reported and long-term anti-inflammatory treatment increased NO bioavailability and secondarily improved endothelium-dependent dilation (16, 52). Despite the large body of evidence linking depression and chronic inflammation (19, 53–56), limited studies have directly examined the effect of inflammation on vascular function in adults with MDD. Using plasma concentrations of soluble vascular cell adhesion molecule-1, soluble E-selectin, and Von Willebrand factor as indirect surrogates of endothelial function, results from The Maastricht Study suggest a crude association between low-grade inflammation, endothelial dysfunction, and depressive disorder (28).
To begin to mechanistically probe the potential role of inflammation in contributing to blunted endothelium-dependent dilation in adults with depression, we modeled this small intervention study on those pioneered by Seals and colleagues (22–25), who have extensively examined the influence of NF-κB activation on the mechanistic mediators of age-related vascular dysfunction. This approach has since been adopted by others (57). In the present study, we demonstrated that short-term salsalate treatment improved the NO-dependent component of ACh-induced dilation in adults with MDD, findings consistent with the beneficial vascular effects reported in middle-aged and older adults (22–25, 29). Interestingly, two recent studies report that long-term high-dose salsalate treatment either failed to improve, or actually worsened, brachial artery flow-mediated dilation in adults with metabolic or atherosclerotic disease (58, 59). The reason(s) for these disparate findings is not readily apparent but may be related to differing dosing paradigms, concurrent medication use, or varied etiologies of endothelial dysfunction. This is an important consideration for future clinical trials, particularly given that the beneficial effects of anti-inflammatory treatment to improve depressive symptoms appear evident only in the subset of adults with MDD who have clinically apparent inflammation (56).
Of note, short-term salsalate treatment did not affect the systemic inflammatory biomarkers measured in the present study, despite clear increases in serum salicylate concentrations. A failure to observe reductions in circulating biomarkers of inflammation was also evident in the aforementioned investigations, none of which detected altered systemic concentrations of inflammatory cytokines following short-term salsalate administration (22–25, 59). High-sensitivity CRP (hsCRP) is an inflammatory biomarker associated with a marked increase in the risk of a future vascular event, even in apparently healthy individuals, and concentrations ≥2 mg/L are clinically indicative of enhanced inflammation (60). In this regard, despite substantial variability, salsalate treatment reduced hsCRP concentrations below this clinical threshold in adults with MDD, perhaps indicative of a mild anti-inflammatory effect. Nevertheless, abundant in vitro data demonstrate that nonacetylated salicylic acids (e.g., salsalate) inhibit phosphorylation of the inhibitor of NF-κB (IκB) protein, thereby preventing the liberation and migration of the NF-κB p65/p50 and p50/p50 dimers to the cell nucleus and the transcriptional regulation of target genes (30–32). Furthermore, our dosing regimen for oral salsalate has been shown to decrease the expression of both whole cell and nuclear NF-κB p65 and concurrently increase the expression of IκBα in venous endothelial cells (22, 24, 25), despite the absence of a clear reduction in circulating markers of inflammation, thereby validating the efficacy of this approach in reducing vascular NF-κB activation in patients with MDD. Consistent with this, although short-term NF-κB inhibition also did not reduce circulating markers of inflammation in the present study, it nevertheless elicited improvements in NO-dependent dilation in young, otherwise healthy, adults with MDD, suggesting a possible role for vascular inflammation in contributing to MDD-associated microvascular endothelial dysfunction.
Increased circulating markers of oxidative stress have been reported in adults with MDD (61). Extending these findings, we recently demonstrated increased abundance of nitrotyrosine (a generalized marker of oxidative damage), increased expression of NADPH oxidase, and increased production of superoxide in cutaneous tissue homogenates isolated from young adults with MDD (9). Moreover, increased vascular NADPH oxidase-derived superoxide contributed to reductions in NO-dependent dilation in adults with MDD (9), the first direct evidence that oxidative stress mediates microvascular endothelial dysfunction in human depression. Relatively few studies, however, have examined the specific mechanism(s) underlying upregulated ROS production in MDD. In this regard, NF-κB increases the expression of NADPH oxidase contributing to increased generation of superoxide (62). In human venous endothelial cells collected via biopsy, NF-κB inhibition with oral salsalate reduced NADPH oxidase expression and mediated improvements in endothelial function via a reduction in vascular oxidative stress (24, 25). To test this mechanism in the current study, we examined the effect of apocynin [a nonspecific scavenger of free radicals (50)] on NO-dependent dilation before and after short-term oral salsalate treatment in a subset of adults with MDD. Before treatment, and similar to our earlier report (9), acute localized apocynin administration improved NO-mediated dilation in adults with MDD, indicative of a role for increased vascular ROS production in mediating endothelial dysfunction. However, following salsalate treatment, apocynin did not affect NO-dependent dilation. Collectively, these data suggest that NF-κB activation-induced increases in vascular oxidative stress contribute, at least in part, to blunted endothelium-dependent dilation in MDD. However, it is important to note that reactive oxygen species themselves are activators of NF-κB (20, 21). The interconnected linkage between free radicals and NF-κB is complex and determining the directionality of NO signaling pathway activation, though beyond the scope of this study, merits future targeted investigation.
Several experimental considerations warrant discussion. First, the present study included both males and females in both HA and MDD groups. Females are twice as likely as males to have a single MDD episode and four times as likely to have recurrent MDD (2, 63), yet have lower CVD risk (64), suggesting potential sex differences in the mechanisms contributing to MDD-CVD comorbidity. However, earlier work from our laboratory demonstrated no differences in ACh-induced dilation alone or during concurrent NOS inhibition in males with MDD compared with females with MDD (9), suggesting that the NO-dependent component of ACh-induced dilation is not different between males and females with MDD. Therefore, because NO-dependent dilation was the primary outcome variable, the present study was neither designed nor powered to detect sex differences. However, we tested for a main effect of sex in all statistical models and did not observe any sex differences. Given the small sample sizes and lack of power, these data should be interpreted cautiously. Prospective studies designed to specifically examine sex differences and incorporate experimental controls for female sex hormones in larger cohorts are necessary. Second, this study did not include a placebo control. Although there are inherent limitations to this design, this was a small proof-of-concept study to determine the potential effect of short-term anti-inflammatory treatment on the mechanistic regulation of vascular regulation in adults with MDD. The results provide the basis for designing more rigorous placebo-controlled clinical trials. Moreover, pre- and posttreatment testing approaches have routinely been used by our laboratory and others as a strategy to assess mechanistic control of microvascular function in health and in disease (65–70). Furthermore, because a placebo arm would have further delayed participants with MDD from treatment, this approach maximized patient safety.
Conclusions and Perspectives
In conclusion, these data demonstrate that NF-κB activation may contribute to blunted NO-mediated endothelium-dependent dilation in young adults with MDD, in part by increased vascular oxidative stress. The results from this small proof-of-concept investigation provide an experimental basis for larger-scale clinical trials designed to assess the efficacy of longer-term adjunctive anti-inflammatory treatment to prevent, slow, or reverse microvascular endothelial dysfunction in patients with MDD. Given the large body of literature linking chronic psychosocial stress to the pathogenesis of mood disorders (71), coupled with the compelling evidence that stress-induced overactivation of inflammatory signaling pathways is linked to the development of depressive symptoms (27, 53, 72), the mechanistic regulation of the potential beneficial anti-inflammatory effects on the link between psychological and vascular health represents an exciting line of future inquiry.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL093238 (to L.M.A.) and HL133414 (to J.L.G.) and American Heart Association Grant 16SDG30240006 (to J.L.G.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
J.L.G., E.F.H.S., and L.M.A. conceived and designed research; J.L.G. performed experiments; J.L.G. analyzed data; J.L.G., E.F.H.S., and L.M.A. interpreted results of experiments; J.L.G. prepared figures; J.L.G. drafted manuscript; J.L.G., E.F.H.S., and L.M.A. edited and revised manuscript; J.L.G., E.F.H.S., and L.M.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate the effort expended by the volunteer participants. We thank Susan Slimak, Jane Pierzga, Gabrielle Dillon, and Dr. Chris Engeland and the Biomarker Core Laboratory (Penn State) for assistance. We also thank Dr. Jacqueline Mogle for statistical consultation.
REFERENCES
- 1.Diagnostic and Statistical Manual of Mental Disorders. Washington, D.C.: American Psychiatric Publishing, 2013. [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry 62: 593–602, 2005. [Erratum in Arch Gen Psychiatry 62:768, 2005]. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.Gilsanz P, Walter S, Tchetgen EJ, Patton KK, Moon JR, Capistrant BD, Marden JR, Kubzansky LD, Kawachi I, Glymour MM. Changes in depressive symptoms and incidence of first stroke among middle-aged and older US adults. JAHA 4: e001923, 2015. doi: 10.1161/JAHA.115.001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein BI, Carnethon MR, Matthews KA, McIntyre RS, Miller GE, Raghuveer G, Stoney CM, Wasiak H, McCrindle BW; American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young. Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease: a scientific statement from the American Heart Association. Circulation 132: 965–986, 2015. doi: 10.1161/CIR.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 5.Lett HS, Blumenthal JA, Babyak MA, Sherwood A, Strauman T, Robins C, Newman MF. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med 66: 305–315, 2004. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- 6.Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry 55: 580–592, 1998. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- 7.Cooper DC, Milic MS, Tafur JR, Mills PJ, Bardwell WA, Ziegler MG, Dimsdale JE. Adverse impact of mood on flow-mediated dilation. Psychosom Med 72: 122–127, 2010. doi: 10.1097/PSY.0b013e3181cdbfc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper DC, Tomfohr LM, Milic MS, Natarajan L, Bardwell WA, Ziegler MG, Dimsdale JE. Depressed mood and flow-mediated dilation. Psychosom Med 73: 360–369, 2011. doi: 10.1097/PSY.0b013e31821db79a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greaney JL, Saunders EFH, Santhanam L, Alexander LM. Oxidative stress contributes to microvascular endothelial dysfunction in men and women with major depressive disorder. Circ Res 124: 564–574, 2019. doi: 10.1161/CIRCRESAHA.118.313764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paranthaman R, Greenstein AS, Burns AS, Cruickshank JK, Heagerty AM, Jackson A, Malik RA, Scott ML, Baldwin RC. Vascular function in older adults with depressive disorder. Biol Psychiatry 68: 133–139, 2010. doi: 10.1016/j.biopsych.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Rajagopalan S, Brook R, Rubenfire M, Pitt E, Young E, Pitt B. Abnormal brachial artery flow-mediated vasodilation in young adults with major depression. Am J Cardiol 88: 196–198, 2001. [Erratum in Am J Cardiol 88: 722, 2001]. doi: 10.1016/s0002-9149(01)01623-x. [DOI] [PubMed] [Google Scholar]
- 12.Brooks SD, Hileman SM, Chantler PD, Milde SA, Lemaster KA, Frisbee SJ, Shoemaker JK, Jackson DN, Frisbee JC. Protection from chronic stress- and depressive symptom-induced vascular endothelial dysfunction in female rats is abolished by preexisting metabolic disease. Am J Physiol Heart Circ Physiol 314: H1085–H1097, 2018. doi: 10.1152/ajpheart.00648.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks SD, Hileman SM, Chantler PD, Milde SA, Lemaster KA, Frisbee SJ, Shoemaker JK, Jackson DN, Frisbee JC. Protection from vascular dysfunction in female rats with chronic stress and depressive symptoms. Am J Physiol Heart Circ Physiol 314: H1070–H1084, 2018. doi: 10.1152/ajpheart.00647.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.d’Audiffret AC, Frisbee SJ, Stapleton PA, Goodwill AG, Isingrini E, Frisbee JC. Depressive behavior and vascular dysfunction: a link between clinical depression and vascular disease? J Appl Physiol (1985) 108: 1041–1051, 2010. doi: 10.1152/japplphysiol.01440.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matchkov VV, Kravtsova VV, Wiborg O, Aalkjaer C, Bouzinova EV. Chronic selective serotonin reuptake inhibition modulates endothelial dysfunction and oxidative state in rat chronic mild stress model of depression. Am J Physiol Regul Integr Comp Physiol 309: R814–R823, 2015. doi: 10.1152/ajpregu.00337.2014. [DOI] [PubMed] [Google Scholar]
- 16.Stanley SC, Brooks SD, Butcher JT, d’Audiffret AC, Frisbee SJ, Frisbee JC. Protective effect of sex on chronic stress- and depressive behavior-induced vascular dysfunction in BALB/cJ mice. J Appl Physiol 117: 959–970, 2014. doi: 10.1152/japplphysiol.00537.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun 49: 206–215, 2015. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiles SA, Baker AL, de Malmanche T, Attia J. A meta-analysis of differences in IL-6 and IL-10 between people with and without depression: exploring the causes of heterogeneity. Brain Behav Immun 26: 1180–1188, 2012. doi: 10.1016/j.bbi.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65: 732–741, 2009. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol 25: 904–914, 2005. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- 21.Read MA, Whitley MZ, Williams AJ, Collins T. NF-kappa B and I kappa B alpha: an inducible regulatory system in endothelial activation. J Exp Med 179: 503–512, 1994. doi: 10.1084/jem.179.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension 57: 63–69, 2011. doi: 10.1161/HYPERTENSIONAHA.110.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jablonski KL, Donato AJ, Fleenor BS, Nowlan MJ, Walker AE, Kaplon RE, Ballak DB, Seals DR. Reduced large elastic artery stiffness with regular aerobic exercise in middle-aged and older adults: potential role of suppressed nuclear factor kappa B signalling. J Hypertens 33: 2477–2482, 2015. doi: 10.1097/HJH.0000000000000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-{kappa}B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation 119: 1284–1292, 2009. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker AE, Kaplon RE, Pierce GL, Nowlan MJ, Seals DR. Prevention of age-related endothelial dysfunction by habitual aerobic exercise in healthy humans: possible role of nuclear factor kappaB. Clin Sci 127: 645–654, 2014. doi: 10.1042/CS20140030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pace TW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM. Increased peripheral NF-kappaB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav Immun 26: 13–17, 2012. doi: 10.1016/j.bbi.2011.07.232. [DOI] [PubMed] [Google Scholar]
- 27.Pace TWW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry 163: 1630–1633, 2006. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 28.Van Dooren FEP, Schram MT, Schalkwijk CG, Stehouwer CDA, Henry RMA, Dagnelie PC, Schaper NC, Van Der Kallen CJH, Koster A, Sep SJS, Denollet J, Verhey FRJ, Pouwer F. Associations of low grade inflammation and endothelial dysfunction with depression – The Maastricht Study. Brain Behav Immun 56: 390–396, 2016. doi: 10.1016/j.bbi.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Alba BK, Greaney JL, Ferguson SB, Alexander LM. Inhibition of nuclear factor kappa B improves nitric oxide-dependent dilation in the cutaneous microvasculature of psoriatic adults. FASEB J 33: 696.16, 2019. doi: 10.1096/fasebj.2019.33.1_supplement.696.16.30044923 [DOI] [Google Scholar]
- 30.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science 265: 956–959, 1994. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 31.Pierce JW, Read MA, Ding H, Luscinskas FW, Collins T. Salicylates inhibit I kappa B-alpha phosphorylation, endothelial-leukocyte adhesion molecule expression, and neutrophil transmigration. J Immunol 156: 3961–3969, 1996. https://pubmed.ncbi.nlm.nih.gov/8621937/. [PubMed] [Google Scholar]
- 32.Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature 396: 77–80, 1998. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 33.Rossman MJ, LaRocca TJ, Martens CR, Seals DR. Healthy lifestyle-based approaches for successful vascular aging. J Appl Physiol 125: 1888–1900, 2018. doi: 10.1152/japplphysiol.00521.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wadley AJ, Veldhuijzen van Zanten JJ, Aldred S. The interactions of oxidative stress and inflammation with vascular dysfunction in ageing: the vascular health triad. Age 35: 705–718, 2013. doi: 10.1007/s11357-012-9402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59, Suppl 20: 22–33, 1998. https://pubmed.ncbi.nlm.nih.gov/9881538/. [PubMed] [Google Scholar]
- 36.Kosten TA, Rounsaville BJ. Sensitivity of psychiatric diagnosis based on the best estimate procedure. Am J Psychiatry 149: 1225–1227, 1992. doi: 10.1176/ajp.149.9.1225. [DOI] [PubMed] [Google Scholar]
- 37.Darling AM, Richey RE, Akins JD, Saunders EFH, Matthew Brothers R, Greaney JL. Cerebrovascular reactivity is blunted in young adults with major depressive disorder: the influence of current depressive symptomology. J Affect Disord 295: 513–521, 2021. doi: 10.1016/j.jad.2021.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greaney JL, Dillon GA, Saunders EFH, Alexander LM. Peripheral microvascular serotoninergic signaling is dysregulated in young adults with major depressive disorder. J Appl Physiol 128: 100–107, 2020. doi: 10.1152/japplphysiol.00603.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greaney JL, Koffer RE, Saunders EFH, Almeida DM, Alexander LM. Self‐reported everyday psychosocial stressors are associated with greater impairments in endothelial function in young adults with major depressive disorder. JAHA 8, 2019. doi: 10.1161/JAHA.118.010825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spitzer RL. Validation and utility of a self-report version of PRIME-MD: the PHQ Primary Care Study. JAMA 282: 1737, 1999. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 41.Ketel IJ, Stehouwer CD, Serne EH, Poel DM, Groot L, Kager C, Hompes PG, Homburg R, Twisk JW, Smulders YM, Lambalk CB. Microvascular function has no menstrual-cycle-dependent variation in healthy ovulatory women. Microcirculation 16: 714–724, 2009. doi: 10.3109/10739680903199186. [DOI] [PubMed] [Google Scholar]
- 42.Rakobowchuk M, Parsloe ER, Gibbins SE, Harris E, Birch KM. Prolonged low flow reduces reactive hyperemia and augments low flow mediated constriction in the brachial artery independent of the menstrual cycle. PLoS One 8: e55385, 2013. doi: 10.1371/journal.pone.0055385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossi M, Di Maria C, Erba P, Galetta F, Carpi A, Santoro G. Study of skin vasomotion during phollicular and luteal phase in young healthy women. Clin Hemorheol Microcirc 42: 107–115, 2009. doi: 10.3233/CH-2009-1189. [DOI] [PubMed] [Google Scholar]
- 44.Shenouda N, Priest SE, Rizzuto VI, MacDonald MJ. Brachial artery endothelial function is stable across a menstrual and oral contraceptive pill cycle but lower in premenopausal women than in age-matched men. Am J Physiol Heart Circ Physiol 315: H366–H374, 2018. doi: 10.1152/ajpheart.00102.2018. [DOI] [PubMed] [Google Scholar]
- 45.Turner CG, Stanhewicz AE, Wong BJ. Female sex hormone effects on the vasculature: considering the validity of restricting study inclusion to low-hormone phases. Front Physiol 11: 596507, 2020. doi: 10.3389/fphys.2020.596507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atkinson MH, Menard HA, Kalish GH. Assessment of salsalate, a nonacetylated salicylate, in the treatment of patients with arthritis. Clin Ther 17: 827–837, 1995. doi: 10.1016/0149-2918(95)80061-1. [DOI] [PubMed] [Google Scholar]
- 47.Wu KK. Aspirin and salicylate: an old remedy with a new twist. Circulation 102: 2022–2023, 2000. doi: 10.1161/01.cir.102.17.2022. [DOI] [PubMed] [Google Scholar]
- 48.Greaney JL, Kutz JL, Shank SW, Jandu S, Santhanam L, Alexander LM. Impaired hydrogen sulfide-mediated vasodilation contributes to microvascular endothelial dysfunction in hypertensive adults. Hypertension 69: 902–909, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, Salvemini D. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol 140: 445–460, 2003. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol 11: 95–102, 1994. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- 51.Ahn H, Johansson K, Lundgren O, Nilsson GE. In vivo evaluation of signal processors for laser Doppler tissue flowmeters. Med Biol Eng Comput 25: 207–211, 1987. doi: 10.1007/BF02442852. [DOI] [PubMed] [Google Scholar]
- 52.Bayramgurler D, Karson A, Yazir Y, Celikyurt IK, Kurnaz S, Utkan T. The effect of etanercept on aortic nitric oxide-dependent vasorelaxation in an unpredictable chronic, mild stress model of depression in rats. Eur J Pharmacol 710: 67–72, 2013. doi: 10.1016/j.ejphar.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA 100: 1920–1925, 2003. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drago A, Crisafulli C, Calabro M, Serretti A. Enrichment pathway analysis. The inflammatory genetic background in bipolar disorder. J Affect Disord 179: 88–94, 2015. doi: 10.1016/j.jad.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 55.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry 66: 407–414, 2009. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression. JAMA Psychiatry 70: 31, 2013. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stanhewicz AE, Dillon GA, Serviente C, Alexander LM. Acute systemic inhibition of inflammation augments endothelium-dependent dilation in women with a history of preeclamptic pregnancy. Pregnancy Hypertens 27: 81–86, 2022. doi: 10.1016/j.preghy.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldfine AB, Buck JS, Desouza C, Fonseca V, Chen YD, Shoelson SE, Jablonski KA, Creager MA; Team T-FAS. Targeting inflammation using salsalate in patients with type 2 diabetes: effects on flow-mediated dilation (TINSAL-FMD). Diabetes Care 36: 4132–4139, 2013. doi: 10.2337/dc13-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nohria A, Kinlay S, Buck JS, Redline W, Copeland-Halperin R, Kim S, Beckman JA. The effect of salsalate therapy on endothelial function in a broad range of subjects. J Am Heart Assoc 3: e000609, 2014. doi: 10.1161/JAHA.113.000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ridker PM; JUPITER Study Group. Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein. Circulation 108: 2292–2297, 2003. doi: 10.1161/01.CIR.0000100688.17280.E6. [DOI] [PubMed] [Google Scholar]
- 61.Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BW. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 51: 164–175, 2015. doi: 10.1016/j.psyneuen.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 62.Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem 281: 5657–5667, 2006. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- 63.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry 60: 929–937, 2003. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 64.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart Disease and Stroke Statistics-2022 Update: a report from the American Heart Association. Circulation 145: e153–e639, 2022. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 65.Durand S, Fromy B, Bouye P, Saumet JL, Abraham P. Vasodilatation in response to repeated anodal current application in the human skin relies on aspirin-sensitive mechanisms. J Physiol 540: 261–269, 2002. doi: 10.1113/jphysiol.2001.013364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holowatz LA, Kenney WL. Acute localized administration of tetrahydrobiopterin and chronic systemic atorvastatin treatment restore cutaneous microvascular function in hypercholesterolaemic humans. J Physiol 589: 4787–4797, 2011. doi: 10.1113/jphysiol.2011.212100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holowatz LA, Santhanam L, Webb A, Berkowitz DE, Kenney WL. Oral atorvastatin therapy restores cutaneous microvascular function by decreasing arginase activity in hypercholesterolaemic humans. J Physiol 589: 2093–2103, 2011. doi: 10.1113/jphysiol.2010.203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wenner MM, Taylor HS, Stachenfeld NS. Androgens influence microvascular dilation in PCOS through ET-A and ET-B receptors. Am J Physiol Endocrinol Metab 305: E818–E825, 2013. doi: 10.1152/ajpendo.00343.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wenner MM, Taylor HS, Stachenfeld NS. Progesterone enhances adrenergic control of skin blood flow in women with high but not low orthostatic tolerance. J Physiol 589: 975–986, 2011. doi: 10.1113/jphysiol.2010.194563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolf ST, Jablonski NG, Ferguson SB, Alexander LM, Kenney WL. Four weeks of vitamin D supplementation improves nitric oxide-mediated microvascular function in college-aged African Americans. Am J Physiol Heart Circ Physiol 319: H906–H914, 2020. doi: 10.1152/ajpheart.00631.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, Takahashi A, Flanigan ME, Aleyasin H, Leclair KB, Janssen WG, Labonté B, Parise EM, Lorsch ZS, Golden SA, Heshmati M, Tamminga C, Turecki G, Campbell M, Fayad ZA, Tang CY, Merad M, Russo SJ. Social stress induces neurovascular pathology promoting depression. Nat Neurosci 20: 1752–1760, 2017. doi: 10.1038/s41593-017-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci USA 107: 2669–2674, 2010. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]