Abstract
BACKGROUND
Wound healing is a critical clinical concept. We aimed to evaluate the effects of topical Mentha piperita essence on cutaneous wound healing.
METHODS
This randomized controlled trial was conducted in Tehran University of Medical Sciences, Tehran, Iran in 2019. Square-shaped 1.5×1.5 cm wounds were made on the neck of 60 male Wistar rats in a sterile condition. Samples were randomly divided into a control group and three experimental groups. Group A treated with M. piperita essence and Vaseline. The second group received the M. piperita essence, and the third group received Vaseline. Histological specimens were obtained in 4th, 7th, and 14th days and were explored for fibroblasts, epithelial cells, inflammatory cells, and vessels. RT-PCR was performed for molecular and gene expression evaluation of TGF-β.
RESULTS
The M. piperita essence increases TGF-β gene expression as an important factor in wound healing. After 14 d, group A, who received M. piperita and Vaseline, had 99.73% of wound healing with the mean wound size of 0.006 cm2 while wound healing in the control group was only 52%. Samples treated with M. piperita have 74.58% wound healing following by group treated with Vaseline, which was 67.02% (P<0.05), respectively.
CONCLUSION
The application of the M. piperita essence for wound healing accelerates the process and improves outcomes.
Key Words: Mentha piperita, Peppermint, Wound healing, Herbal medicine, TGF- β
INTRODUCTION
One of the most important issues in medical science is tissue repair after devastations due to trauma. Physicians have been searching for an effective way of wound healing in the shortest possible time with the least complications1, 2.
Synthetic drugs are shown to have more adverse effects and limitations. Hence, researchers are looking for a natural combination to accelerate wound healing3, 4. In the past, natural ingredients, especially medicinal plants, were the basic and even the only treatment in some conditions. Their raw materials were used in the pharmaceutical industry5. Mankind has been using honey, mint, garlic, aloe vera, fish oil, and similar materials as medication in wound healing for many years3. A new tendency to use medicinal plants is spreading around the world3, 6, 7.
Various growth factors, such as transforming growth factor- ß (TGF-ß), affect the healing process. TGF-ß, the most powerful factor in tissue repair, is releases after degranulation of platelets in the wound area8. This factor is also a crucial fibrogenic moderator of connective tissue9.
Mentha piperita (peppermint) belongs to the Lamiaceae family and is one of the most widely used herbal medicine. This perennial herbaceous has quadrangular stems and light purple flowers in the form of compound clusters. Peppermint contains a considerable amount of menthol (40.7%). Dried mint has 0.3%-0.4% menthol. Moreover, the oil contains menthone (23.4%), menthyl esters, especially menthyl acetate, limonin, pulegone, caryophyllene, and pinene. There are also different flavonoids in this plant, such as Eriocitrin, hesperidin, and kaempferol9.
Peppermint leaves are useful for intestinal inflammation, buccal mucosa inflammation, coryza, and respiratory problems, due to their antispasmodic features. The topical use of peppermint oil helps reduce neuromuscular pains. This oil is also known for fungicidal, antimicrobial, antiseptic, antipyretic, and anti-aging properties9.
This study aimed to evaluate the effects of topical M. piperita essence on wound healing considering critical variables such as clinical outcome, amount of fibroblast, epithelial cells, inflammatory cells and vessels, and TGF- β gene expression.
MATERIALS AND METHODS
Essence Preparation
Fresh M. piperita leaves were collected from gardens of the medicinal plants of Kashan (Iran) in June 2019. They were washed, dried, and powdered at room temperature after the pharmacognosy approval. The amount of 100 gr of the powder was mixed in the Clevenger balloon (Hei-VAP Platinum 3 Rotary Evaporator, Heidolph, Germany) with 400 milliliters of water. The essence was prepared by the Clevenger after 5 hours. We kept this essence in the dark glass container in the refrigerator after dehydration with sodium anhydride sulfate (Merck, Germany). The main constituents of this essence were menthol (39.8%), menthone (19.5%), neomenthol (8.83%), and acetyl menthol (8.64%).
Surgical method
The ethics committee of Tehran University of Medical Sciences, School of Medicine confirmed the method of this study with the ethics code: IR.TUMS.MEDICINE.REC.1397.322.
Sixty adult healthy male Wistar rats were included in this study. The samples were kept in cages individually with 12 h of light each day. Proper food and water were available for each sample.
On the surgery day (day 0), rats were anesthetized with 5 mg/100 g Ketamine Hydrochloride (Ketalar, Gedeonrichter, Germany) as the primary anesthetic and 0.04 mg/100 g Pentazocine (Pentazocine, Toliddaru, Iran) as the pre-anesthetic drug. Diazepam (Valium, Chemidarou, Iran) with a dose of 0.45 mg/100 g was added to relax the muscles6, 10. Samples received all these drugs by intramuscular injection.
Neck hair shaved and surgery cites sterilized with Povidone Iodine (Betadine, Chemidarou, Iran). We used a 1.5×1.5 cm square-shaped stencil to make wounds on the back of their necks. The wound was made in a sterile condition and contained the full thickness of the skin. After that, wounds were washed with normal saline and determined treatment started.
The rats were randomly divided into one control group and three experimental groups. The first experimental group (group A) was treated with 5mg of M. piperita essence and Vaseline once a day. Group B received 5mg of M. piperita essence each day, and group C received Vaseline. Samples in the control group did not receive any treatment.
Wound healing assay
Wound sizes and healing percentage were measured on 1st, 4th, 7th, 10th, and 14th d after starting the treatment. For this purpose, a photograph was obtained from the wounds with a ruler beside it. Then the exact wound size was measured with Image J software (Digital Image Processing for Medical Applications Image J 1.46 r, Java 1.6.0-20 Cambridge University). At the end of each course, the rats were sacrificed by inhaling carbon dioxide (CO2). Wound healing percentage calculated with the following formula:
*100
Histological study
On the 4th, 7th and 14th d after starting the treatment, histological specimens were obtained under general anesthesia. Specimens were divided into two parts, one soaked in 10% formalin for tissue processing, and the other part was used to evaluate gene expression using reverse transcription-polymerase chain reaction (RT-PCR) technique. For tissue processing, specimens were stained with specific Masson’s trichrome to assess the density of the collagen fibers and Hematoxylin and Eosin (H&E) staining to assess other cells. Using a light microscope (CX31-OLYMPUS, Japan) and a magnification field of 40×, specimens were investigated to explore 10 areas of each wound surface. ImageJ software version 1.45 (imagej.nih.gov/ij/UK) was used to count fibroblasts, neutrophils, macrophages, and vessels.
Quantitative Reverse transcription PCR (RT-PCR)
RT-PCR was performed for molecular and gene expression evaluations in each group. First, the primer was designed (Table 1), and the RNA was extracted from the tissue using RNXTM (PLUS) kit (CinnaGen, Iran)11. Electrophoresis of agarose gel and ultraviolet spectrophotometry was performed to check the quality and the quantity of the extracted RNA. The RNA was converted to cDNA and proliferated with the PCR technique and was evaluated for TGF-β gene expression. Reverse transcription of the extracted RNA was performed using the AccuPower® RocketScript™ RT PreMix kit (Bioneer Company). The produced cDNAs were used as transcription patterns for performing PCR.
Table 1.
TGF-ß gene-specific primers designed to evaluate the TGF-ß gene expression
| Template strand | Length | Start | Stop | Tm | GC% | |
|---|---|---|---|---|---|---|
| Forward primer | AGGAGACGGAATACAGGGCT | 20 (plus strand) | 775 | 794 | 60.03 | 55.00 |
| Reverse primer | GGATCCACTTCCAACCCAGG | 20 (minus strand) | 1079 | 1060 | 60.03 | 60.00 |
RT-PCR product electrophoresis on agarose gel
Electrophoresis was done on the PCR product on agarose gel 1% after performing PCR. The electrophoresis was done on the samples with a voltage of 150 volts for 25 minutes. After performing electrophoresis, the produced bands were observed and interpreted by projecting ultraviolet rays using a UV transilluminator device.
Data Analysis
The data of this study analyzed using SPSS software version 20 (IBM corporation, New York, US), and all the graphs were prepared with Prism software version 6 (GraphPad Inc., California, US).
A modified histologic scoring system (Abramov’s scoring system) was introduced for wound healing 12. Quantitative data were reported by mean ± standard deviation (SD). We used the Shapiro-Wilk test of normality to check if the distribution is normal. Mann-Whitney U test was performed to compare data between groups. All the tests were 2-tailed. The Significance level was considered P<0.05.
RESULTS
Sixty healthy adult male Wistar rats were used in this study. The first experimental group (group A) treated with 5 mg of M. piperita essence and Vaseline once a day. Group B received 5mg of M. piperita essence each day, and group C received Vaseline. Samples in the control group did not receive any treatment. The wound healing process was investigated based on both microscopic and macroscopic findings.
Macroscopic Findings
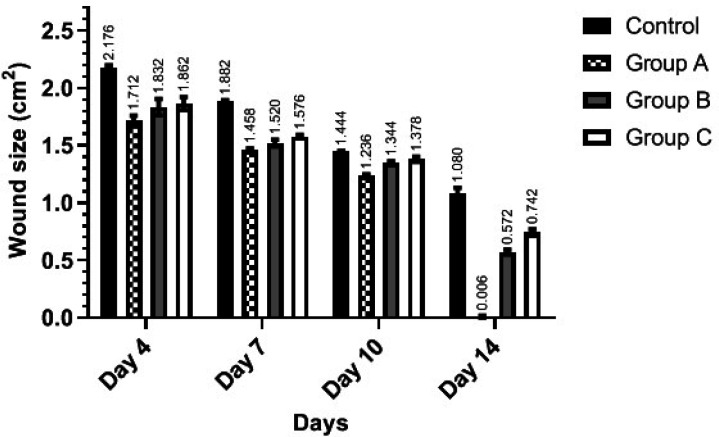
Figure 1 reveals the mean of wound size in each group on different days. At the beginning of the study, all rats had the same 2.25 cm2 wound at the back of their neck. On 4th day, the wound size of group A (1.71±0.037 cm2) was significantly smaller than the control group (2.17±0.017 cm2) (P=0.009). Groups B and C did not show a significant difference with each other (P=0.394). However, both had significantly smaller wound sizes compared to the control group (P<0.001 for both).
Fig. 1.
Wound size of each group in 4, 7, 10, and 14 days after starting the treatment. Group A: Mentha piperita and Vaseline, Group B: Mentha piperita, and Group C: Vaseline
Wound healing progressed until day 14, and the wound size decreased significantly from the beginning until day 14 in all groups (P<0.001).
At the end of the study (day 14), the mean wound size of group A (0.006±0.008 cm2) was significantly smaller than the control group (1.08±0.41 cm2), group B (0.572±0.16 cm2) and group C (0.742±0.023 cm2) (P<0.001). Moreover, the wound size was smaller in group B in comparison with group C and the control group, and the difference was significant (P<0.001).
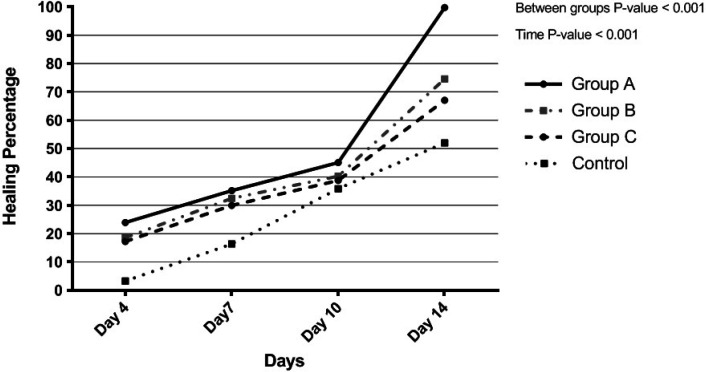
We measured the wound healing percentage by using the mentioned formula in the 4th, 7th, 10th, and 14th days. As Figure 2 demonstrates, the healing percentage was the highest in group A (the one with received both treatments), followed by group B (the one who received M. piperita). The control group had the lowest healing percentage. As explained, the application of both Vaseline and M. piperita essence provided almost 100% (99.734%) healing, whereas without receiving any treatment, the healing percentage reduces to almost half (51.998%).
Fig. 2.
Healing percentage of each group during the study. Group A: Mentha piperita and Vaseline, Group B: Mentha piperita, and Group C: Vaseline
Microscopic Findings
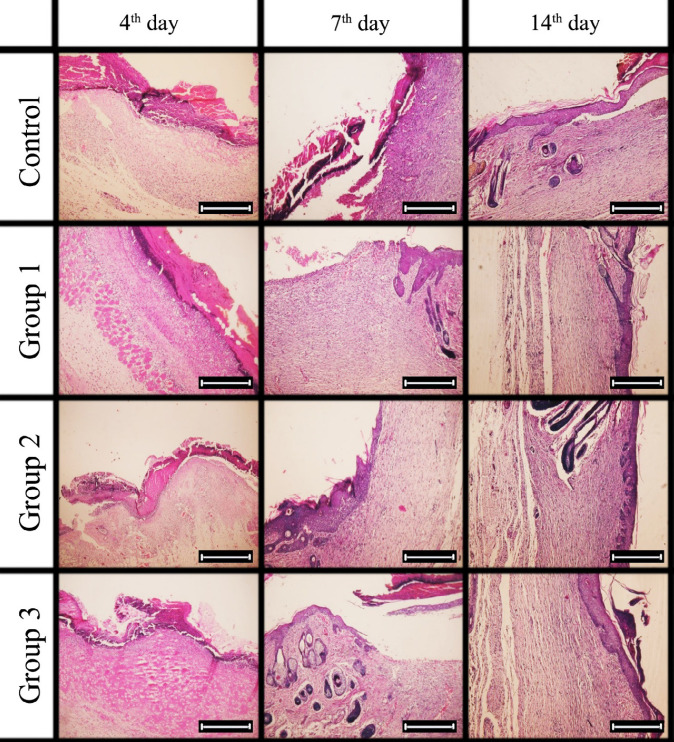
Microscopic specimens were obtained after sacrificing samples from each group on the 4th, 7th, and 14th d of the experiment. Specimens were stained with Hematoxylin and Eosin (H&E) to investigate the fibroblasts, neutrophils, macrophages, and vessels. Specific Masson’s Trichrome staining to assess the density of collagen fibers (Figure 3 & 4).
Fig. 3.
Photomicrographs of histopathological sections representing skin wounds of rat groups following treatment for 14 days. (Staining, H&E ×10)
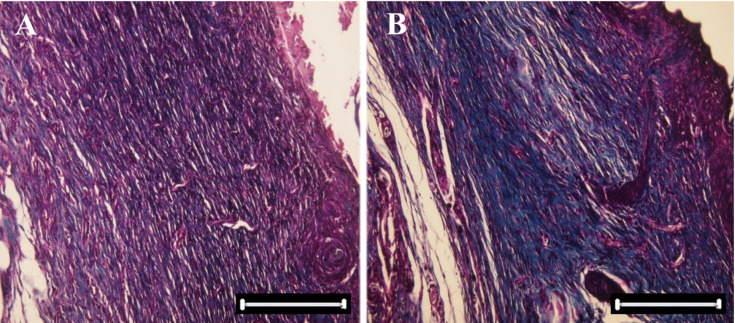
Fig. 4.
Microscopic view of open cutaneous wounds: (a) control group: collagen fibers are lesser than the experimental group in this photomicrograph. (b) The experimental group (Mentha piperita), collagen fibers are more than the control group in this photomicrograph (specific staining, Masson's trichrome ×10)
The number of neutrophils, macrophages, fibroblasts, and wound thickness of group A that received mint essence and Vaseline is significantly different from the control group (P<0.009).
As shown, the highest level of fibroblasts and vessels was found on day 4, which is the proliferative phase of wound healing. The number of vessels and fibroblasts was always the highest in group A and the lowest in the control group. The number of inflammatory cells had a downward trend in all groups, but the lowest level always belonged to group A. Meanwhile, the control group had the highest number of these cells during the whole period (Table 2).
Table 2.
Mean ± SD of the fibroblasts, vessels, neutrophils, and macrophages of the wounds bed in 4th, 7th, and 14th day of the experiment. The highest number of each cell group highlighted
| Groups | Days | Day 4 | Day 7 | Day 14 | P -value |
|---|---|---|---|---|---|
| Parameters | |||||
| Control | Fibroblasts | 54.6 ± 0.548 | 83.20 ± 0.83 | 81.00 ±0.70 | <0.001 |
| Vessels | 4 ± 0.70 | 9.4 ± 0.54 | 6.8 ± 0.83 | <0.001 | |
| Inflammatory cells | 22 ± 2 | 17.4 ± 0.54 | 7.2 ± 0.44 | <0.001 | |
| Group-A | Fibroblasts | 68.2 ± 0.83 | 114.4 ± 3.05 | 111.80 ± 1.48 | <0.001 |
| Vessels | 8 ± 1 | 15.4 ± 0.89 | 11.6 ± 1.89 | <0.001 | |
| Inflammatory cells | 13.4 ± 0.54 | 10.40 ± 0.54 | 1.40 ± 0.54 | <0.001 | |
| Group-B | Fibroblasts | 65 ± 1.22 | 102.6 ± 2.40 | 100.8 ± 0.83 | <0.001 |
| Vessels | 6.6 ± 0.54 | 12.6 ± 1.14 | 9.6 ± 1.14 | <0.001 | |
| Inflammatory cells | 15.2 ± 0.83 | 11.4 ± 1.34 | 2.40 ± 0.54 | <0.001 | |
| Group-C | Fibroblasts | 64 ± 1.22 | 101.8 ± 2.16 | 97.2 ± 1.78 | <0.001 |
| Vessels | 5.8 ± 0.83 | 11.8 ± 0.83 | 7.80 ± 1.09 | <0.001 | |
| Inflammatory cells | 16.4 ± 0.89 | 13.8 ± 0.83 | 3.40 ± 0.54 | <0.001 |
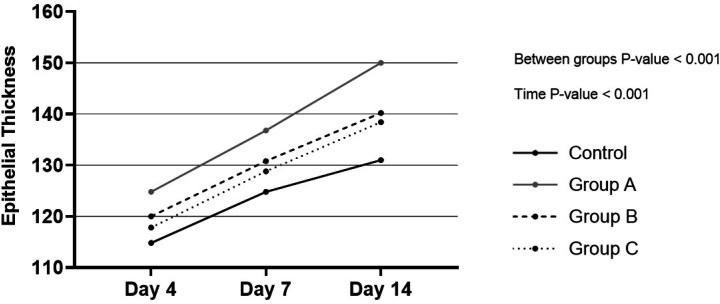
Figure 5 illustrates the epithelial width of each study group in days 4, 7, and 14, which reveals an ascending trend in all the groups, but group A always had the most values.
Fig. 5.
Epithelial width of each group during the study. Group A: Mentha piperita and Vaseline, Group B: Mentha piperita, and Group C: Vaseline
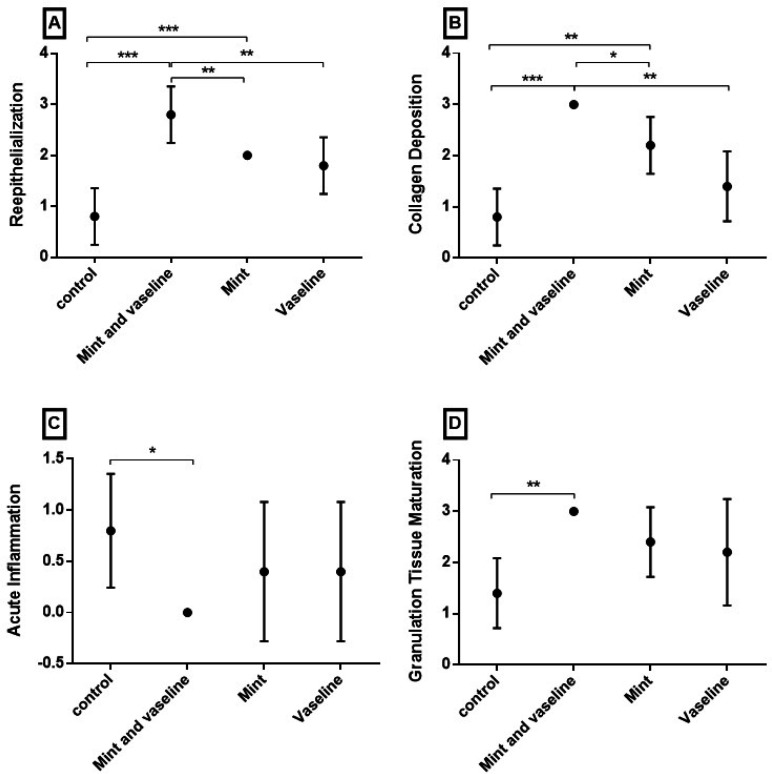
A modified histologic scoring system (Abramov’s scoring system) was introduced for wound healing. Several factors are included in this system. Each parameter receives a score from zero to three independently. We compared re-epithelialization, collagen deposition, acute inflammation, and granulation tissue maturation between our groups using this scoring system (Table 3).
Table 3.
Abramov’s histological scoring system for wound repair
| Parameter | Score | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Acute inflammation | None | Scant | Moderate | Abundant |
| Chronic inflammation | None | Scant | Moderate | Abundant |
| Amount of granulation tissue | None | Scant | Moderate | Abundant |
| Granulation tissue maturation | Immature | Mild maturation | Moderate maturation | Fully matured |
| Collagen deposition | None | Scant | Moderate | Abundant |
| Re-epithelialization | None | Partial | Complete but immature or thin | Complete and mature |
| Neovascularization | None | Up to five vessels per | 6 to 10 vessels per HPF | More than 10 vessels per HPF |
HPF= High Power Field
Figure 6 illustrates Abramov’s score of different study groups on the last day of the experiment. Group-A that received M. piperita essence and Vaseline had a significant difference in re-epithelialization (P<0.001), collagen deposition (P<0.001), acute inflammation (P=0.016) and granulation tissue maturation (P=0.02) compared to control group. However, there was no significant difference between group-A and other experimental groups in acute inflammation and granulation tissue maturation.
Fig. 6.
Mean ± 95% confidence interval of Scoring of wound healing in different groups at the end of the study based on Abramov's scoring system. (*= P-Value <0.05; **= P-Value <0.01; *** = P-Value <0.001)
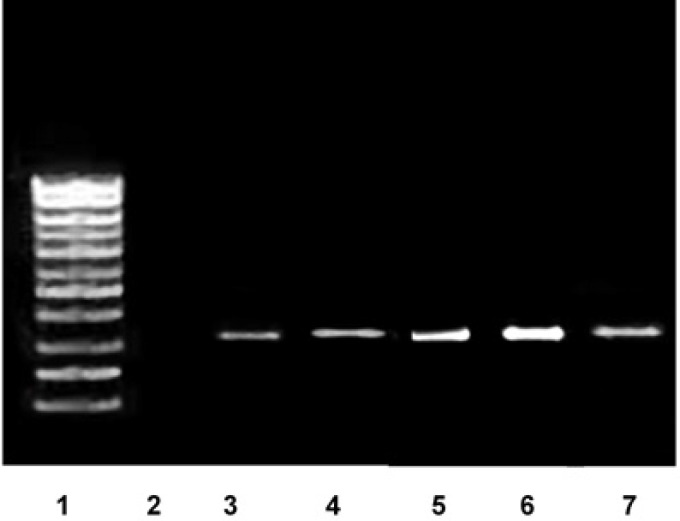
RT-PCR product electrophoresis on agarose gel
After performing PCR, we ran electrophoresis on agarose gel 1% with a voltage of 150 volts for 25 minutes. Then, ultraviolet ray was projected to the gel using a UV transilluminator device and produced bands were observed and interpreted (Figure 7).
Fig. 7.
Evaluation of TGF-ß gene expression (PCR size: 305 bp) in the wound bed at 7th days after injury by RT-PCR technique in the treatment groups. 1: Ladder 100 bp, 2: Negative control (H2O) of PCR, 3: Negative control of TGF-ß, 4: Control, 5: Mentha piperita, 6: Mentha piperita &Vaseline, 7: Vaseline
DISCUSSION
Wound healing is a complicated and organized process divided into three phases, including inflammation, proliferation, and remodeling (reconstructing). Therefore, improving each of these stages can result in the acceleration of wound healing and a decrease in complications.
The M. piperita (Peppermint) essence improves the healing process by preventing infections and microorganisms’ growth. The M. piperita essence had more bactericidal effects on gram-positive batteries than gram-negative ones13, 14. Antimicrobial property of M. piperita essence was studied in food system models; and documented that this feature is a result of M. piperita components including menthol and ketones such as pulegone, iso menthone, piperitone, carvone, and dehydrocarvone. Thymol has a high inhibitory effect on pathogen fungi like Aspergillus and Candida albicans15. Methanol of the peppermint essence has a great antifungal activity on Candida albicans, Sacromices servizieh, and Penicillium nutanum16.
The M. piperita essence helps with wound healing acceleration in several ways. M. piperita essence increases expression of the Transforming growth factor-β (TGF-β) gene as an important factor in wound healing. Platelets release TGF-β1. This isoform attracts neutrophils, macrophages, and other cells that increase the TGF-β1 level17, 18. In the inflammatory phase, the number of neutrophils is maximized. In the next phase, macrophages are dominant cells. Lactate and other mediators released from macrophages cause fibroblasts proliferation and angiogenesis. According to the present study, the topical use of the M. piperita essence shortens the inflammatory period, reduces the inflammatory cells such as neutrophils, and increases the macrophages. These changes cause an increase in angiogenesis and fasten wound healing in experimental groups in comparison to the control group (P<0.05).
The proliferation phase starts after the inflammation. Fibroblasts activate in this phase and produce collagen and glycosaminoglycan. In other words, fibroblasts are builders of new tissue. In large skin injuries, a severe reduction in the number of fibroblasts is the cause of delay or failure of repair. Platelets and macrophages secrete chemotactic materials that induce sprouting of new vessels from the venue. Therefore, angiogenesis can be induced by adding chemotactic materials to the tissue. Mint essence can help with the induction of angiogenesis and epithelialization.
In an open wound, capillaries increase along with the cells’ proliferation. A dense population of macrophages, fibroblasts, and vessels penetrate the collagen and hyaluronic acid matrix. These events create granulated tissue. Every factor that augments vascularization accelerates wound healing.
Peppermint essence speeds up angiogenesis and helps to build granulated tissue. In this research also, the number of vessels was significantly higher in the experimental groups (P<0.009).
Histopathological studies 7 d after creating the wound reveal the beginning of the second step in healing, which is cell proliferation with fibroblast migration and epithelialization (Figure 5). The results of our study indicated that topical use of M. piperita essence increases the migration of fibroblasts to wound area, epithelialization, and acceleration of wound healing in the second phase of repairing in comparison to control group (Figure 1).
The number of fibroblasts increases due to applying peppermint essence in the wound, and this causes growing granulated tissue with higher speed (Table 2). Proliferated fibroblasts secrete collagen and proteoglycans. These results to wound closure. The essence also reduces the inflammation and speeds up fibroblasts transformation19.
The amount of wound closure is a pragmatic scale to evaluate wound healing. Wound closure is related to fibroblasts and myofibroblasts activity in granulation tissue. The percentage and speed of wound healing were significantly higher in the experimental group that received the M. piperita essence (Figures 1 & 2). In the present study, day 14 is considered the wound healing process reconstruction phase. On this day, excess of fibroblasts numbers and collagen fibers in the experimental group compared to the control group reveals the healing process continues until the 14th day. Epithelialization and differentiation of the epidermis continue at maximum level as long as the wound area is kept wet. In the experimental group, wound thickness has decreased in a shorter time because of the M. piperita essence. In addition, healing power and wound closure are developed faster than previous groups. TGF-β causes an increase in mitosis power in the skin fibroblasts19. This factor is recognized as a key mediator in the stimulation of fibroblasts and extracellular matrix accumulation20, 21. In this study, a further expression of TGF-β causes an increase in cellular activity, especially fibroblasts (Figure 7).
In addition, the M. piperita essence increases collagen synthesis (Figure 6); therefore, the healing accelerates.
As our macroscopic and microscopic findings demonstrate, the average wound healing seems to be the highest while using both Vaseline and M. piperita essence as the treatment. Application of peppermint as a single treatment ranks as the second effective method, followed by using Vaseline only.
Various studies have studied the M. piperita plant topical use in different areas: cutaneous wound healing acceleration, antimicrobial activity, and analgesic effects. In India, the effectiveness of M. piperita on wound healing acceleration was studied in streptozotocin-induced diabetic rats22. Literature indicates the M. piperita antibacterial potential effect23. Analgesic effects of M. piperita were also studied by literature, and the M. piperita essence was proved to have strong analgesic effects in the experiment24.
A parallel study evaluated the topical application of M. piperita essential oil’s effects on wound healing in the infected mice model. The migration rate of fibroblasts, collagen production and secretion, and regeneration of epithelial tissue was increased in the groups treated with M. piperita19.
Comprehensively, according to the results of the present study and other researchers, t use of M. piperita essence has led to an increase in expression of TGF-β gene in the wound area and has provided wound healing essentials by stimulation of growth factors release and providing a suitable environment in the wound area. The essence has antibacterial effects because of having thymol, menthol, phenol and flavonoid combinations, and oxidants like terpenes. In addition, due to the antioxidant effects of the mentioned chemicals, it has been able to increase angiogenesis, fibroblast proliferation, epithelialization as well as controlling skin infection and acceleration of wound healing.
CONCLUSION
According to histologic measures, gene expression amount wound diameter and healing percentage topical use of M. piperita essence is effective in rats’ cutaneous open wound healing. It shortens the inflammatory phase, deepens the granulated tissue, helps with angiogenesis, causes the proliferative phase to start sooner, and finally accelerates wound healing.
FINANCIAL SOURCE
We received no financial support for this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
References
- 1.Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599–610. doi: 10.1007/s12325-017-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall CD, Hu MS, Leavitt T, Barnes LA, Lorenz HP, Longaker MT. Cutaneous scarring: Basic science, current treatments, and future directions. Adv Wound Care. 2018;7(2):29–45. doi: 10.1089/wound.2016.0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delfan B, Bahmani M, Eftekhari Z, Jelodari M, Saki K, Mohammadi T. Effective herbs on the wound and skin disorders: a ethnobotanical study in Lorestan province, west of Iran. Asian Pacific Journal of Tropical Disease. 2014;4:S938–S42. [Google Scholar]
- 4.Takzaree N, Hadjiakhondi A, Hassanzadeh G, Rouini MR, Manayi A. Synergistic Effect of Honey and Propolis on Cutaneous Wound Healing in Rats. Acta Med Iran. 2016 Apr;54(4):233–9. [PubMed] [Google Scholar]
- 5.Mir M, Ali MN, Barakullah A, et al. Synthetic polymeric biomaterials for wound healing: a review. Progress in Biomaterials. 2018;7(1):1–21. doi: 10.1007/s40204-018-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindley LE, Stojadinovic O, Pastar I, Tomic-Canic M. Biology and biomarkers for wound healing. Plast Reconstr Surg. 2016;138(3):18S. doi: 10.1097/PRS.0000000000002682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah A, Amini-Nik S. The role of phytochemicals in the inflammatory phase of wound healing. Int J Molecul Sci. 2017;18(5):1068. doi: 10.3390/ijms18051068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chicharro-Alcántara D, Rubio-Zaragoza M, Damiá-Giménez E, et al. Platelet rich plasma: new insights for cutaneous wound healing management. Journal of Functional Biomaterials. 2018;9(1):10. doi: 10.3390/jfb9010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahendran G, Rahman LU. Ethnomedicinal, phytochemical and pharmacological updates on Peppermint (Mentha piperita L.) A review. Phytother Res . 2020 doi: 10.1002/ptr.6664. [DOI] [PubMed] [Google Scholar]
- 10.Lynch SE, Colvin RB, Antoniades HN. Growth factors in wound healing Single and synergistic effects on partial thickness porcine skin wounds. The Journal of Clinical Investigation. 1989;84(2):640–6. doi: 10.1172/JCI114210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takzaree N, Hadjiakhondi A, Hassanzadeh G, Rouini MR, Manayi A, Zolbin MM. Transforming growth factor-beta (TGF-beta) activation in cutaneous wounds after topical application of aloe vera gel. Can J Physiol Pharmacol. 2016 Dec;94(12):1285–90. doi: 10.1139/cjpp-2015-0460. [DOI] [PubMed] [Google Scholar]
- 12.Abramov Y, Golden B, Sullivan M, et al. Histologic characterization of vaginal vs abdominal surgical wound healing in a rabbit model. Wound Repair Regen. 2007 Jan-Feb;15(1):80–6. doi: 10.1111/j.1524-475X.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- 13.İşcan G, Ki̇ri̇mer N, Kürkcüoǧlu Mn, Başer HC, DEMIrci F. Antimicrobial screening of Mentha piperita essential oils. J Agric Food Chem. 2002;50(14):3943–6. doi: 10.1021/jf011476k. [DOI] [PubMed] [Google Scholar]
- 14.Sivropoulou A, Papanikolaou E, Nikolaou C, Kokkini S, Lanaras T, Arsenakis M. Antimicrobial and cytotoxic activities of Origanum essential oils. J Agric Food Chem. 1996;44(5):1202–5. [Google Scholar]
- 15.Giordani R, Regli P, Kaloustian J, Mikail C, Abou L, Portugal H. Antifungal effect of various essential oils against Candidaalbicans Potentiation of antifungal action of amphotericin B by essential oil from Thymus vulgaris. Phytother Res. 2004;18(12):990–5. doi: 10.1002/ptr.1594. [DOI] [PubMed] [Google Scholar]
- 16.Mathur A, Purohit R, Mathur D, Prasad G, Dua V. Pharmacological investigation of methanol extract of Mentha piperita L roots on the basis of antimicrobial, antioxidant and anti-inflammatory properties. Der Pharmacia Sinica. 2011;2(1):208–16. [Google Scholar]
- 17.Chang Z, Kishimoto Y, Hasan A, Welham NV. TGF-β3 modulates the inflammatory environment and reduces scar formation following vocal fold mucosal injury in rats. Dis Model Mech. 2014;7(1):83–91. doi: 10.1242/dmm.013326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun T, Adra S, Smallwood R, Holcombe M, MacNeil S. Exploring hypotheses of the actions of TGF-β1 in epidermal wound healing using a 3D computational multiscale model of the human epidermis. PLoS One. 2009;4(12):e8515. doi: 10.1371/journal.pone.0008515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Modarresi M, Farahpour M-R, Baradaran B. Topical application of Mentha piperita essential oil accelerates wound healing in infected mice model. Inflammopharmacology. 2019;27(3):531–7. doi: 10.1007/s10787-018-0510-0. [DOI] [PubMed] [Google Scholar]
- 20.Crider BJ, Risinger Jr GM, Haaksma CJ, Howard EW, Tomasek JJ. Myocardin-related transcription factors A and B are key regulators of TGF-β1-induced fibroblast to myofibroblast differentiation. J Invest Dermatol. 2011;131(12):2378–85. doi: 10.1038/jid.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mott GA, Costales JA, Burleigh BA. A soluble factor from Trypanosoma cruzi inhibits transforming growth factor-ß-induced MAP kinase activation and gene expression in dermal fibroblasts. PLoS One. 2011;6(9):e23482. doi: 10.1371/journal.pone.0023482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umasankar K, Nambikkairaj B, Backyavathi M. Wound healing activity of topical Mentha piperita and Cymbopogan citratus essential oil on streptozotocin induced rats. Asian J Pharm Clin Res. 2013:6:180–3. [Google Scholar]
- 23.Sujana P, Sridhar TM, Josthna P, Naidu CV. Antibacterial activity and phytochemical analysis of Mentha piperita L (peppermint)—an important multipurpose medicinal plant. Am J Plant Sci. 2013;4(01) [Google Scholar]
- 24.Taher YA. Antinociceptive activity of Mentha piperita leaf aqueous extract in mice. Libyan J Med. 2012;7(1):16205. doi: 10.3402/ljm.v7i0.16205. [DOI] [PMC free article] [PubMed] [Google Scholar]