Abstract
Background:
Glucosaminidase (Gmd) is known to be a protective antigen in animal models of Staphylococcus aureus osteomyelitis. We compared the endogenous anti-Gmd antibody levels in sera of patients with culture-confirmed S. aureus bone infections to their sera at 1 year after operative treatment of the infection.
Methods:
A novel global biospecimen registry of 297 patients with deep-wound culture-confirmed S. aureus osteomyelitis was analyzed to assess relationships between baseline anti-Gmd serum titers (via custom Luminex assay), known host risk factors for infection, and 1-year postoperative clinical outcomes (e.g., infection control, inconclusive, refracture, persistent infection, septic nonunion, amputation, and septic death).
Results:
All patients had measurable humoral immunity against some S. aureus antigens, but only 20 patients (6.7%; p < 0.0001) had high levels of anti-Gmd antibodies (>10 ng/mL) in serum at baseline. A subset of 194 patients (65.3%) who completed 1 year of follow-up was divided into groups based on anti-Gmd level: low (<1 ng/mL, 54 patients; 27.8%), intermediate (<10 ng/mL, 122 patients; 62.9%), and high (>10 ng/mL, 18 patients; 9.3%), and infection control rates were 40.7%, 50.0%, and 66.7%, respectively. The incidence of adverse outcomes in these groups was 33.3%, 16.4%, and 11.1%, respectively. Assessing anti-Gmd level as a continuous variable showed a 60% reduction in adverse-event odds (p = 0.04) for every tenfold increase in concentration. No differences in patient demographics, body mass index of >40 kg/m2, diabetes status, age of ≥70 years, male sex, Charlson Comorbidity Index of >1, or Cierny-Mader host type were observed between groups, and these risk factors were not associated with adverse events. Patients with low anti-Gmd titer demonstrated a significant 2.68-fold increased odds of adverse outcomes (p = 0.008).
Conclusions:
Deficiency in circulating anti-Gmd antibodies was associated serious adverse outcomes following operative treatment of S. aureus osteomyelitis. At 1 year, high levels of anti-Gmd antibodies were associated with a nearly 3-fold increase in infection-control odds. Additional prospective studies clarifying Gmd immunization for osteomyelitis are needed.
Level of Evidence:
Prognostic Level IV. See Instructions for Authors for a complete description of levels of evidence.
Osteomyelitis remains the bane of orthopaedic surgery, and a great need exists for novel interventions1. Osteomyelitis can affect otherwise healthy hosts as well as those who are less healthy. The vast majority of severe cases involve Staphylococcus aureus2, primarily methicillin-resistant S. aureus in some regions3, and strains with pan-resistance are emerging4. An urgent need exists for non-antibiotic, immune-based approaches to treat resistant infections, as antibiotic resistance is a serious public-health threat5. Infection rates following total joint replacement and trauma surgical procedures have remained largely unchanged over the last 50 years1. Adherence to rigorous prophylactic and surgical protocols (e.g., Surgical Care Improvement Project6) cannot reduce infection rates for elective procedures below 1% to 2%7. These findings strongly suggest that host factors play an essential role in orthopaedic infections. S. aureus bone infections are caused by pathogenic mechanisms that have developed to achieve immune evasion8,9. Mechanisms include (1) biofilm formation on the implant10 and necrotic bone11,12, (2) generation of staphylococcal abscess communities in soft tissues and bone marrow13-15, (3) intracellular infection8, and (4) ability to colonize the osteocytic-canalicular network of live cortical bone16,17. Persistence of infection following surgical treatment of S. aureus osteomyelitis is common (15% to 40%) and often requires multiple surgeries18-22.
To date, 19 S. aureus immunizations have been evaluated in U.S. Food and Drug Administration registration trials, and all failed to demonstrate efficacy5,23. Expert opinion on the root cause of these failures has focused on the inability to predict the protective role of staphylococcal immune responses in humans based on animal data5,23. Thus, we departed from this traditional vaccine development approach and aimed to develop an immunotherapy that was based on monoclonal antibodies that have dual-acting mechanisms: (1) direct inhibition of critical S. aureus enzymes, and (2) immunomodulatory activity to stimulate host response and bacterial clearance14,24. Utilizing a murine tibial osteomyelitis model with an infected transosseous pin that faithfully recapitulates the salient features of implant-associated osteomyelitis25, we identified the glucosaminidase (Gmd) protein subunit of S. aureus autolysin as our lead target14,15,24,26. An important validation of this discovery was that other groups also identified autolysin as an immunodominant antigen27-29, including studies of tibial osteomyelitis in a rabbit model28. Autolysin is critical for cell-wall biosynthesis and degradation during binary fission30-32 and functions as an adhesin33, a biofilm enzyme34, and a facilitator of host-cellular internalization and immune evasion35. Most importantly, anti-Gmd passive immunization has been shown to synergize with vancomycin therapy in rabbit and murine tibial osteomyelitis and murine peritoneal infection models15,28,36. Our clinical studies of patients with osteomyelitis from periprosthetic joint infection, trauma, and diabetic foot ulcers have found anti-Gmd antibodies in patients who have recovered from these serious infections26,37,38.
Recently, we reported on the safety and pharmacokinetics of a candidate anti-Gmd monoclonal antibody (1C11) passive immunization in a sheep model39. In addition to reporting the favorable profile of 1C11, we described the behavior of endogenous human anti-Gmd antibodies analyzed from sera collected in a unique biospecimen registry (AO Trauma Clinical Priority Program [CPP] Bone Infection Registry) of 297 patients with culture-confirmed S. aureus osteomyelitis40. The results of that study demonstrated that anti-Gmd antibody levels ranged from undetectable (<1 ng/mL) to 300 μg/mL, and the mean concentration was 21.7 μg/mL39. We estimated that the circulating half-life of endogenous anti-Gmd antibodies was 120.4 days, which is roughly equivalent to ~4 passive immunizations over a 1-year course of treatment. However, as critical questions regarding the relationships between endogenous anti-Gmd antibodies in these patients and the clinical outcome following standard-of-care surgical and postoperative treatment remained open, we performed a post-hoc analysis of the AO Trauma CPP Bone Infection Registry to test 3 hypotheses: (1) most osteomyelitis patients with normal immune status (commonly referred to as “type-A hosts”41) do not have high levels of anti-Gmd antibodies (>10 ng/mL) in their serum, (2) osteomyelitis patients with low levels of anti-Gmd antibodies (<1 ng/mL) at the time of the surgical procedure have an increased risk of an adverse event during the first postoperative year, and (3) high levels of anti-Gmd antibody are associated with a high rate of “infection controlled” outcomes at 1 year postoperatively. In the present study, we describe our biostatistical analyses of the AO Trauma CPP Bone Infection Registry data and the results supporting the aforementioned hypotheses. To our knowledge, this is the first clinical evidence directly associating the humoral immune response of a patient with osteomyelitis against a specific S. aureus bacterial surface antigen with clinical outcomes following surgical treatment of the infection.
Materials and Methods
AO Trauma CPP Bone Infection Registry
This study was part of an international, prospective, observational case series of patients with long-bone S. aureus infection, conducted according to ISO (International Organization for Standardization) 9001 guidelines and registered at ClinicalTrial.gov (NCT01677000). Details on patient enrollment, data and sample collection, clinical outcome measures, and end points have previously been published40; additional results are pending publication. In brief, patients ≥18 years old with culture-confirmed S. aureus infection of a long bone (i.e., femur, tibia, fibula, humerus, radius, ulna, or clavicle) following fracture fixation, arthroplasty, or injury were enrolled into the registry prior to the surgical treatment (i.e., debridement or 1-stage or 2-stage revision). Patient demographic data (i.e., age, sex, race, place of residence at admission, and body mass index [BMI]) and medical information (i.e., comorbidities and prior treatment) were collected prior to the surgical procedure (baseline). Patients were categorized as A, B, or C-type hosts according to the methodology previously described by Lazzarini et al.41. Charlson Comorbidity Index scores were also calculated for each patient42.
Serology
Although clinical blood-laboratory data were collected in the registry at baseline, 6 months, and 12 months postoperatively40, the present study only analyzed data on baseline immunoglobulin G (IgG) antibodies against 8 immunodominant S. aureus antigens (Gmd, aminidase [Amd], iron scavenging determinant A [IsdA], iron scavenging determinant B [IsdB], iron scavenging determinant H [IsdH], chemotaxis inhibitory protein of staphylococci [CHIPS], staphylococcal complement inhibitor [SCIN], and staphylococcal hemolysin [Hla]) with use of a custom multiplex Luminex assay that reports titers as median fluorescent intensity (MFI) in arbitrary units, as previously described37,38. Quantification of antibody concentration (ng/mL) was only performed for anti-Gmd in the baseline sera, determined by interpolation to a standard curve generated with mouse:human chimeric 1C11 anti-Gmd monoclonal antibodies as previously described43. This analysis determined that the lower limit of detection of the custom Luminex assay is 1 ng/mL43. We considered a tenfold increase over this limit of detection (10 ng/mL) to be the high threshold of anti-Gmd antibodies in human serum. Using thresholds for the assay level of detection (MFI = 1,550, 1 ng/mL)43 and using a 10 ng/mL high level of circulating anti-Gmd antibody (MFI = 9,000) as prospectively defined43, we stratified the cohort into 3 anti-Gmd groups: low (MFI <1,550), intermediate (MFI = 1,550 to 9,000), and high (MFI >9,000). All patients were studied who had informed consent and complete baseline demographic and serology data (297 patients, including 292 from the AO Registry40 and 5 who were excluded from the AO Trauma CPP Bone Infection Registry because they did not have a final positive wound culture for S. aureus, 297 total). The inclusion criteria were age of ≥18 years and deep culture-confirmed S. aureus infection (methicillin-resistant or sensitive). Infections involved a long bone (i.e., femur, tibia, fibula, humerus, radius, ulna, clavicle) and followed fracture fixation or arthroplasty. Prisoners, pregnant patients, patients <18 years old, patients with a culture negative for S. aureus, and those unwilling or unable to consent were excluded.
Clinical Outcomes
Although a diverse array of clinical, patient-reported, and functional outcome data were collected in the registry40, the present study focused only on the 1-year clinical outcome of the baseline surgical procedure, which was categorized as either “infection control,” “adverse outcome,” or “inconclusive.” “Infection control” was determined according to a note from the treating surgeon at the 1-year postoperative visit, which specifically cited “infection control” and/or the lack of any signs or symptoms of infection at that time. “Adverse outcome” was defined prior to study initiation as documentation of refracture or infection related to the initial surgical procedure at 1 year, septic nonunion, amputation, or septic death. Outcomes were recorded by the treating surgeons, who were blinded to antibody levels. Patients who had a complete data set for the 1-year outcome but could not be categorized as “infection control” or “adverse outcome” were defined as “inconclusive.” Patients who did not complete the study or had insufficient documentation of outcomes at 1 year postoperatively were excluded (103 patients; 34.7%).
Statistics
Exact binomial tests were utilized to compare relative frequencies of anti-Gmd antibody titer groups. Fisher exact tests and Cochran-Armitage exact trend tests were utilized to compare event rates and categorical characteristics across these groups. Continuous variables were compared across groups with use of Wilcoxon rank-sum tests. The odds of adverse events and controlled infections was modeled with use of univariate logistic regression, with goodness-of-fit assessed with use of the Hosmer-Lemeshow test. Odds ratios (ORs), 95% confidence intervals (CIs), and p values were calculated for each risk factor. When modeled as a continuous predictor, a log-transformation was applied to anti-Gmd antibody titer values to reduce skewness and improve model fit. Predicted probabilities of adverse events and controlled infections were calculated with use of these logistic models. Analyses were conducted with use of SAS (version 9.4; SAS Institute). Significance was set at p = 0.05.
Twelve patients were missing Charlson Comorbidity Index data, 1 was missing BMI data, and 28 were missing data regarding diabetes status. The n value was reduced accordingly when performing statistical analyses.
Results
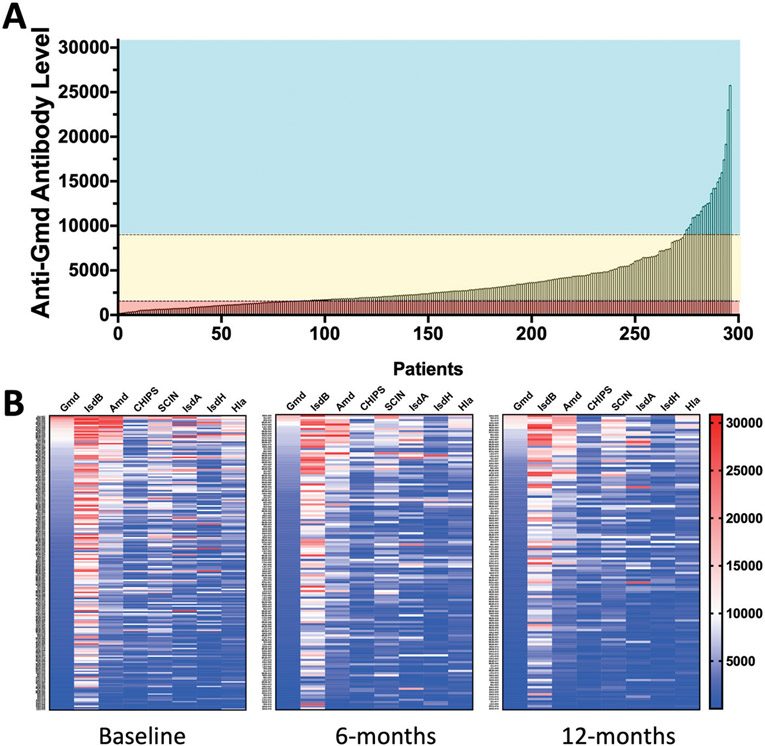
The anti-Gmd antibody titers in baseline sera from 297 patients with osteomyelitis are presented in Figure 1-A and Appendix Supplemental Table 1. Consistent with the asymmetric graphical illustration of anti-Gmd titers in this cohort, an exact binomial test confirmed that the number of patients with high levels of anti-Gmd antibodies in their serum at the time of the baseline surgical procedure was significantly low (20 of 297; 6.7%; p < 0.0001). This result was not the result of immunosuppression, as all patients had detectable titers against at least 1 of the S. aureus antigens tested (Fig. 1-B).
Fig. 1.
Figs. 1-A and 1-B Anti-S. aureus antibody IgG titers in patients with orthopaedic infections. The data in Figure 1-A are of all 297 patients. Antibody titers are represented as the MFI. Fig. 1-A The anti-Gmd IgG titers are presented in rank, ordered from lowest to highest, and are stratified into 3 groups: undetectable or low anti-Gmd (MFI <1,550, cyan), intermediate anti-Gmd (MFI 1,550 to 9,000, yellow), and high anti-Gmd (MFI >9,000, red). Note that 29% of osteomyelitis patients have undetectable levels of anti-Gmd titers. Fig. 1-B Heat maps of serum IgG levels of antibodies against the 8 antigens are presented for the 194 patients who completed the study out to 1 year postoperatively. Baseline and available 6-month and 12-month measurements illustrate that detectable anti-S. aureus antibodies against at least 1 antigen were evident over the course of therapy. Also of note is that IsdB is by far the most immunogenic antigen among these patients, whereas Gmd is one of the lowest (IsdB > Amd > SCIN > IsdA > Hla > IsdH = CHIPS = Gmd).
A subset of 194 patients with 1 year of follow-up (65.3% of the registry) was divided into the 3 anti-Gmd antibody-level groups defined in Figure 1-A: low (undetectable, 54 patients; 27.8%), intermediate (<10 ng/mL, 122 patients; 62.9%), and high (>10 ng/mL, 18 patients; 9.3%) (Table I). Although no specific outcomes were significantly associated with anti-Gmd titers, the incidences of adverse outcomes were 33.3%, 16.4%, and 11.1%, respectively (trend p = 0.010). Consistently, the observed infection control rates were 40.7%, 50.0%, and 66.7%, respectively. A logistic regression analysis comparing the high-antibody and low-antibody groups showed that high levels of anti-Gmd antibodies were associated with a nearly 3-fold increase in infection-control odds at 1 year postoperatively (see Appendix Supplemental Figure 1; OR, 2.91; 95% CI, 0.95 to 8.92; p = 0.06). Additionally, by assessing anti-Gmd levels as a continuous variable on a logarithmic scale, we found that for every tenfold increase in Gmd antibody concentration, there was a significant 60% reduction in adverse event odds (see Appendix Supplemental Figure 2; OR, 0.40; 95% CI, 0.17 to 0.96; p = 0.04).
TABLE I.
Clinical Outcome Versus Anti-Gmd Antibody Titer
| Outcome | Anti-Gmd Antibody Levels | P Value* | |||
|---|---|---|---|---|---|
| Low (MFI <1,550) (N = 54) |
Intermediate (MFI 1,550-9,000) (N = 122) |
High (MFI >9,000) (N = 18) |
|||
| High Vs. Low | Trend | ||||
| Adverse outcome | 18 (33.3%) | 20 (16.4%) | 2 (11.1%) | 0.078 | 0.010 |
| Fracture present | 2 (3.8%) | 2 (1.6%) | 0 (0%) | ||
| Infection present | 5 (9.4%) | 5 (4.1%) | 1 (5.6%) | ||
| Definitive procedure† | 2 (3.8%) | 7 (5.7%) | 1 (5.6%) | ||
| Amputation | 6 (11.3%) | 4 (3.3%) | 0 (0%) | ||
| Septic death | 3 (5.7%) | 2 (1.6%) | 0 (0%) | ||
| Infection controlled | 22 (40.7%) | 61 (50.0%) | 12 (66.7%) | 0.101 | 0.064 |
| Inconclusive | 14 (26.4%) | 41 (33.3%) | 4 (22.2%) | 0.999 | 0.893 |
Based on Fisher exact test for comparing high versus low groups and the exact Cochran-Armitage test for trend for comparing the ordinal low, intermediate, and high groups.
Outcomes cited as “pseudarthrosis,” “arthrodesis,” “retained spacer,” and “fusion” in the doctor note at 1 year postoperatively.
Given these data suggesting that the absence of circulating anti-Gmd antibodies at the time of debridement for osteomyelitis is a risk factor for an adverse event within 1 year, we performed logistic regression analyses to determine relative odds compared with other known risk factors for surgical site infection and/or periprosthetic joint infection1. These risk factors included BMI of >40 kg/m2, diabetes, age of >70 years, male sex, and Charlson Comorbidity Index of >1. Table II shows the results of this analysis, in which the absence of detectable circulating anti-Gmd antibodies was the only significant risk factor associated with increased odds of an adverse outcome in this cohort (2.68-fold increase; p = 0.008). Of these 194 patients, the AO Trauma CPP Bone Infection Registry only contained sufficient risk factor data on patients with BMI of >40 kg/m2, diabetes, age of >70 years, sex (male), and Charlson Comorbidity Index of >1.
TABLE II.
Relative Odds of an Adverse Event from Lack of Anti-Gmd Monoclonal Antibodies and Known Risk Factors*
| Risk Factors | Risk of Adverse Events | ||||
|---|---|---|---|---|---|
| No. | Incidence | Odds Ratio | 95% CI | P Value | |
| Anti-Gmd <1 ng/mL | 194 | 27.80% | 2.68 | 1.30, 5.54 | 0.008* |
| Charlson Comorbidity Index >1 | 182 | 21.40% | 1.63 | 0.70, 3.76 | 0.255 |
| BMI >40 kg/m2 | 193 | 7.30% | 1.05 | 0.28, 3.95 | 0.946 |
| Diabetes | 166 | 17.50% | 1.71 | 0.68, 4.29 | 0.256 |
| Age >70 yr | 194 | 17.00% | 2.28 | 0.99, 5.21 | 0.052 |
| Female | 194 | 32.50% | 1.52 | 0.74, 3.12 | 0.256 |
Odds ratios, 95% CIs, and p values were calculated based on univariate logistic regression models.
Discussion
Recent studies have shown no changes in the rates of periprosthetic joint infection, the primary pathogen, treatment algorithm, or prevalence of poor outcomes since the original revision surgical standards of care were established half a century ago1,44,45. These experts concluded that development of effective immunotherapy against S. aureus is among the highest priorities in orthopaedics1. All active and passive vaccine trials to date have failed46. Of note, these trials were based on protective efficacy in small animals, and antibody opsonophagocytic activity was the primary biomarker of immunity in human volunteers and patients. Retrospectively, failure of opsonophagocytic antibodies to protect humans is not surprising when considering that patients with agammaglobulinemia show no increase in the incidence of S. aureus infection46. Additionally, traditional rodent models have not been predictive of human responses to staphylococcal infections for either protective efficacy47,48 or human inflammatory responses to sepsis49.
Knowing that these failed attempts to develop vaccines neglected translational research with in vivo models containing face and construct validity of surgical site infection48,50, we identified Gmd as a validated target for immunotherapy and developed an anti-Gmd monoclonal antibody (1C11) via an unbiased antigen discovery screen14,24,26,37,38. Recently, we showed that (1) 1C11 synergizes with the standard-of-care antibiotic therapy (vancomycin) in the 1-stage-exchange murine model of methicillin-resistant S. aureus implant-associated osteomyelitis15; (2) 1C11 passive immunization of a clinically relevant sheep model is feasible, is safe, and has favorable pharmacokinetics43; and (3) humans who recover from methicillin-resistant S. aureus osteomyelitis have high titers of circulating anti-Gmd antibodies38,43.
Another historical weakness of staphylococcal vaccine development has been a rush to clinical trials prior to clinical-validation studies that assessed the relationship between host immune response against the target antigen and patient outcomes5,23. Therefore, we utilized the AO Trauma CPP Bone Infection Registry to assess the relationship between circulating anti-Gmd antibody levels in patients with S. aureus osteomyelitis and clinical outcomes. Consistent with our theory of specific anti-Gmd antibody deficiency in the rare ~1% of elective surgical patients who develop a surgical site infection8, we found that all osteomyelitis patients studied had detectable humoral immunity against some S. aureus antigens, but only 20 patients (6.7%; p < 0.0001) had high levels of anti-Gmd antibodies (>10 ng/mL) in their serum prior to surgical treatment of the infection (Fig. 1). Moreover, patients with undetectable anti-Gmd titers demonstrated a trend of increased adverse outcomes compared with those with high anti-Gmd titers (Table I), as well as having significantly (2.68-fold) higher odds of adverse outcomes overall, which was greater than all of the other known host risk factors assessed in the present study (Table II). In terms of protection, we found a trend of higher “infection control” rates in patients with high versus low anti-Gmd titers (Table I), and for every tenfold increase in concentration of endogenous anti-Gmd antibody in serum, a 60% reduction in adverse event odds was observed (see Appendix Supplemental Figure 2; OR, 0.40; p = 0.04). These results imply that endogenous anti-Gmd antibody deficiency is common in osteomyelitis patients and that those with undetectable levels prior to a surgical procedure might benefit from passive immunization with an immunomodulatory neutralizing anti-Gmd monoclonal antibody.
Although these findings represent the first clinical evidence directly associating the humoral immune response of a patient with osteomyelitis against a specific S. aureus bacterial surface antigen with their clinical outcome, several important limitations need to be considered. Registry data analyzed retrospectively cannot formally establish a cause-and-effect relationship between the presence or absence of an antibody response and clinical outcome at 1 year postoperatively. We are unable to correlate 3 types of surgical procedures (fracture repair, arthroplasty, or osteomyelitis debridement) and outcomes (194 patients in 27 subgroups) because the subgroup n values were too small to be meaningful. Additionally, we had missing data, possible reporting errors, potential selection bias of included patients, and patients lost to follow-up. Thus, the present data merely establish significant associations between anti-Gmd antibody levels and S. aureus bone infection outcomes and require randomized controlled trials to prove that anti-Gmd antibodies are efficacious in S. aureus osteomyelitis patients. Second, no data were collected on other known risk factors for infection such as alcohol consumption, drug use, or smoking, and the number of patients with the other known demographic risk factors for surgical site infection and/or periprosthetic joint infection were too low to study. Although the very low numbers of patients with high levels of anti-Gmd titers established a significant association between antibody deficiency and S. aureus osteomyelitis (p < 0.0001), this high anti-Gmd group with validated clinical outcomes proved to be too small (n = 18) for statistical analyses (Table I). Thus, the trends for reduced adverse events and increased infection control in the high anti-Gmd versus low anti-Gmd groups need to be tested in a prospective study with 2.4 times more osteomyelitis patients in each group for 80% power, and 3 times more patients for 90% power.
Conclusions
Deficiency in circulating anti-Gmd antibodies was associated with S. aureus osteomyelitis incidence in type-A hosts and serious adverse clinical outcomes. At 1 year postoperatively, high levels of anti-Gmd antibodies were associated with a nearly 3-fold increase in infection-control odds. Prospective studies to validate these findings in order to enable an anti-Gmd passive immunization therapy for osteomyelitis are needed.
Supplementary Material
Disclosure:
This study was supported by National Institutes of Health grants (P30 AR069655, P50 AR72000, CTSA 1UL1TR002649), and the AO Trauma Clinical Priority Program on Bone Infection. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work; “yes” to indicate that the author had a patent and/or copyright, planned, pending, or issued, broadly relevant to this work; and “yes” to indicate that the author had other relationships or activities that could be perceived to influence, or have the potential to influence, what was written in this work (http://links.lww.com/JBJS/G86).
Footnotes
Investigation performed at Virginia Commonwealth University, Richmond, Virginia, and the University of Rochester, Rochester, New York.
Appendix
Supporting material provided by the authors is posted with the online version of this article as a data supplement at jbjs.org (http://links.lww.com/JBJS/G87). ■
References
- 1.Schwarz EM, Parvizi J, Gehrke T, Aiyer A, Battenberg A, Brown SA, Callaghan JJ, Citak M, Egol K, Garrigues GE, Ghert M, Goswami K, Green A, Hammound S, Kates SL, McLaren AC, Mont MA, Namdari S, Obremskey WT, O’Toole R, Raikin S, Restrepo C, Ricciardi B, Saeed K, Sanchez-Sotelo J, Shohat N, Tan T, Thirukumaran CP, Winters B. 2018 International Consensus Meeting on Musculoskeletal Infection: research priorities from the general assembly questions. J Orthop Res. 2019. May;37(5):997–1006. Epub 2019 Apr 25. [DOI] [PubMed] [Google Scholar]
- 2.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004. Apr 1;350(14):1422–9. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan SL. Recent lessons for the management of bone and joint infections. J Infect. 2014. Jan;68(Suppl 1):S51–6. Epub 2013 Oct 9. [DOI] [PubMed] [Google Scholar]
- 4.Assis LM, Nedeljković M, Dessen A. New strategies for targeting and treatment of multi-drug resistant Staphylococcus aureus. Drug Resist Updat. 2017. Mar;31:1–14. Epub 2017 Apr 6. [DOI] [PubMed] [Google Scholar]
- 5.Miller LS, Fowler VG, Shukla SK, Rose WE, Proctor RA. Development of a vaccine against Staphylococcus aureus invasive infections: evidence based on human immunity, genetics and bacterial evasion mechanisms. FEMS Microbiol Rev. 2020. Jan 1;44(1):123–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stulberg JJ, Delaney CP, Neuhauser DV, Aron DC, Fu P, Koroukian SM. Adherence to Surgical Care Improvement Project measures and the association with postoperative infections. JAMA. 2010. Jun 23;303(24):2479–85. [DOI] [PubMed] [Google Scholar]
- 7.Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA. 2012. Sep 26;308(12):1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masters EA, Trombetta RP, de Mesy Bentley KL, Boyce BF, Gill AL, Gill SR, Nishitani K, Ishikawa M, Morita Y, Ito H, Bello-Irizarry SN, Ninomiya M, Brodell JD Jr, Lee CC, Hao SP, Oh I, Xie C, Awad HA, Daiss JL, Owen JR, Kates SL, Schwarz EM, Muthukrishnan G. Evolving concepts in bone infection: redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy”. Bone Res. 2019. Jul 15;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muthukrishnan G, Masters EA, Daiss JL, Schwarz EM. Mechanisms of immune evasion and bone tissue colonization that make Staphylococcus aureus the primary pathogen in osteomyelitis. Curr Osteoporos Rep. 2019. Dec;17(6):395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishitani K, Sutipornpalangkul W, de Mesy Bentley KL, Varrone JJ, Bello-Irizarry SN, Ito H, Matsuda S, Kates SL, Daiss JL, Schwarz EM. Quantifying the natural history of biofilm formation in vivo during the establishment of chronic implant-associated Staphylococcus aureus osteomyelitis in mice to identify critical pathogen and host factors. J Orthop Res. 2015. Sep;33(9):1311–9. Epub 2015 May 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004. Jul 24-30;364(9431):369–79. [DOI] [PubMed] [Google Scholar]
- 12.Birt MC, Anderson DW, Bruce Toby E, Wang J.Osteomyelitis: recent advances in pathophysiology and therapeutic strategies. J Orthop. 2016. Oct 26;14(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009. Oct;23(10):3393–404. Epub 2009 Jun 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varrone JJ, de Mesy Bentley KL, Bello-Irizarry SN, Nishitani K, Mack S, Hunter JG, Kates SL, Daiss JL, Schwarz EM. Passive immunization with anti-glucosaminidase monoclonal antibodies protects mice from implant-associated osteomyelitis by mediating opsonophagocytosis of Staphylococcus aureus mega-clusters. J Orthop Res. 2014. Oct;32(10):1389–96. Epub 2014 Jul 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokogawa N, Ishikawa M, Nishitani K, Beck CA, Tsuchiya H, Mesfin A, Kates SL, Daiss JL, Xie C, Schwarz EM. Immunotherapy synergizes with debridement and antibiotic therapy in a murine 1-stage exchange model of MRSA implant-associated osteomyelitis. J Orthop Res. 2018. Jun;36(6):1590–8. Epub 2018 Feb 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Mesy Bentley KL, MacDonald A, Schwarz EM, Oh I. Chronic osteomyelitis with Staphylococcus aureus deformation in submicron canaliculi of osteocytes: a case report. JBJS Case Connect. 2018. Jan-Mar;8(1):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Mesy Bentley KL, Trombetta R, Nishitani K, Bello-Irizarry SN, Ninomiya M, Zhang L, Chung HL, McGrath JL, Daiss JL, Awad HA, Kates SL, Schwarz EM. Evidence of Staphylococcus aureus deformation, proliferation, and migration in canaliculi of live cortical bone in murine models of osteomyelitis. J Bone Miner Res. 2017. May; 32(5):985–90. Epub 2017 Jan 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azzam K, McHale K, Austin M, Purtill JJ, Parvizi J. Outcome of a second two-stage reimplantation for periprosthetic knee infection. Clin Orthop Relat Res. 2009. Jul;467(7):1706–14. Epub 2009 Feb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghanem E, Azzam K, Seeley M, Joshi A, Parvizi J. Staged revision for knee arthroplasty infection: what is the role of serologic tests before reimplantation? Clin Orthop Relat Res. 2009. Jul;467(7):1699–705. Epub 2009 Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parvizi J, Azzam K, Ghanem E, Austin MS, Rothman RH. Periprosthetic infection due to resistant staphylococci: serious problems on the horizon. Clin Orthop Relat Res. 2009. Jul;467(7):1732–9. Epub 2009 May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferry T, Uçkay I,Vaudaux P, François P, Schrenzel J, Harbarth S, Laurent F, Bernard L, Vandenesch F, Etienne J, Hoffmeyer P, Lew D. Risk factors for treatment failure in orthopedic device-related methicillin-resistant Staphylococcus aureus infection. Eur J Clin Microbiol Infect Dis. 2010. Feb;29(2):171–80. Epub 2009 Nov 28. [DOI] [PubMed] [Google Scholar]
- 22.Salgado CD, Dash S, Cantey JR, Marculescu CE. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res. 2007. Aug;461:48–53. [DOI] [PubMed] [Google Scholar]
- 23.Proctor RA. Recent developments for Staphylococcus aureus vaccines: clinical and basic science challenges. Eur Cell Mater. 2015. Dec 2;30:315–26. [DOI] [PubMed] [Google Scholar]
- 24.Varrone JJ, Li D, Daiss JL, Schwarz EM. Anti-glucosaminidase monoclonal antibodies as a passive immunization for methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic infections. Bonekey Osteovision. 2011. Apr 1;8:187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li D, Gromov K, Søballe K, Puzas JE, O’Keefe RJ, Awad H, Drissi H, Schwarz EM. Quantitative mouse model of implant-associated osteomyelitis and the kinetics of microbial growth, osteolysis, and humoral immunity. J Orthop Res. 2008. Jan;26(1):96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gedbjerg N, LaRosa R, Hunter JG, Varrone JJ, Kates SL, Schwarz EM, Daiss JL. Anti-glucosaminidase IgG in sera as a biomarker of host immunity against Staphylococcus aureus in orthopaedic surgery patients. J Bone Joint Surg Am. 2013. Nov 20;95(22):e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holtfreter S, Kolata J, Bröker BM. Towards the immune proteome of Staphylococcus aureus - the anti-S. aureus antibody response. Int J Med Microbiol. 2010. Feb; 300(2-3):176–92. Epub 2009 Nov 3. [DOI] [PubMed] [Google Scholar]
- 28.Brady RA, O’May GA, Leid JG, Prior ML, Costerton JW, Shirtliff ME. Resolution of Staphylococcus aureus biofilm infection using vaccination and antibiotic treatment. Infect Immun. 2011. Apr;79(4):1797–803. Epub 2011 Jan 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Götz F, Heilmann C, Stehle T. Functional and structural analysis of the major amidase (Atl) in Staphylococcus. Int J Med Microbiol. 2014. Mar;304(2):156–63. Epub 2013 Dec 1. [DOI] [PubMed] [Google Scholar]
- 30.Oshida T, Sugai M, Komatsuzawa H, Hong YM, Suginaka H, Tomasz A. A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-L-alanine amidase domain and an endo-beta-N-acetylglucosaminidase domain: cloning, sequence analysis, and characterization. Proc Natl Acad Sci U S A. 1995. Jan 3; 92(1):285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugai M, Komatsuzawa H, Akiyama T, Hong YM, Oshida T, Miyake Y, Yamaguchi T, Suginaka H. Identification of endo-beta-N-acetylglucosaminidase and N-acetylmuramyl-L-alanine amidase as cluster-dispersing enzymes in Staphylococcus aureus. J Bacteriol. 1995. Mar;177(6):1491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada S, Sugai M, Komatsuzawa H, Nakashima S, Oshida T, Matsumoto A, Suginaka H. An autolysin ring associated with cell separation of Staphylococcus aureus. J Bacteriol. 1996. Mar;178(6):1565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heilmann C, Hartleib J, Hussain MS, Peters G. The multifunctional Staphylococcus aureus autolysin Aaa mediates adherence to immobilized fibrinogen and fibronectin. Infect Immun. 2005. Aug;73(8):4793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brady RA, Leid JG, Camper AK, Costerton JW, Shirtliff ME. Identification of Staphylococcus aureus proteins recognized by the antibody-mediated immune response to a biofilm infection. Infect Immun. 2006. Jun;74(6):3415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirschhausen N, Schlesier T, Schmidt MA, Götz F, Peters G, Heilmann C. A novel staphylococcal internalization mechanism involves the major autolysin Atl and heat shock cognate protein Hsc70 as host cell receptor. Cell Microbiol. 2010. Dec; 12(12):1746–64. [DOI] [PubMed] [Google Scholar]
- 36.Kalali Y, Haghighat S, Mahdavi M. Passive immunotherapy with specific IgG fraction against autolysin: analogous protectivity in the MRSA infection with antibiotic therapy. Immunol Lett. 2019. Aug;212:125–31. Epub 2018 Nov 26. [DOI] [PubMed] [Google Scholar]
- 37.Nishitani K, Beck CA, Rosenberg AF, Kates SL, Schwarz EM, Daiss JL. A diagnostic serum antibody test for patients with Staphylococcus aureus osteomyelitis. Clin Orthop Relat Res. 2015. Sep;473(9):2735–49. Epub 2015 May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh I, Muthukrishnan G, Ninomiya MJ, Brodell JD Jr, Smith BL, Lee CC, Gill SR, Beck CA, Schwarz EM, Daiss JL. Tracking anti-Staphylococcus aureus antibodies produced in vivo and ex vivo during foot salvage therapy for diabetic foot infections reveals prognostic insights and evidence of diversified humoral immunity. Infect Immun. 2018. Nov 20;86(12):e00629–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee CC, Southgate R, Jiao C, Gersz E, Owen JR, Kates SL, Beck CA, Xie C, Daiss JL, Post V, Moriarty TF, Zeiter S, Schwarz EM, Muthukrishnan G. Deriving a dose and regimen for anti-glucosaminidase antibody passive-immunization for patients with Staphylococcus aureus osteomyelitis. Eur Cell Mater. 2020;39:96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kates SL, Hurni S, Chen MS. Development and challenges in setting up an international bone infection registry. Arch Orthop Trauma Surg. 2020. Jun;140(6): 741–9. Epub 2019 Nov 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazzarini L, Mader JT, Calhoun JH. Osteomyelitis in long bones. J Bone Joint Surg Am. 2004. 86(10):2305–18. [DOI] [PubMed] [Google Scholar]
- 42.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 43.Lee CC, Southgate RD, Jiao C, Gersz E, Owen JR, Kates SL, Beck CA, Xie C, Daiss JL, Post V, Moriarty TF, Zeiter S, Schwarz EM, Muthukrishnan G. Deriving a dose and regimen for anti-glucosaminidase antibody passive-immunisation for patients with Staphylococcus aureus osteomyelitis. Eur Cell Mater. 2020. Jan 31;39: 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saeed K, McLaren AC, Schwarz EM, Antoci V, Arnold WV, Chen AF, Clauss M, Esteban J, Gant V, Hendershot E, Hickok N, Higuera CA, Coraça-Huber DC, Choe H, Jennings JA, Joshi M, Li WT, Noble PC, Phillips KS, Pottinger PS, Restrepo C, Rohde H, Schaer TP, Shen H, Smeltzer M, Stoodley P, Webb JCJ, Witsø E. 2018 International Consensus Meeting on Musculoskeletal Infection: summary from the Biofilm Workgroup and consensus on biofilm related musculoskeletal infections. J Orthop Res. 2019. May;37(5):1007–17. Epub 2019 Feb 12. [DOI] [PubMed] [Google Scholar]
- 45.Parvizi J, Gehrke T, Mont MA, Callaghan JJ. Introduction: proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019. Feb;34(2S): S1–2. Epub 2018 Oct 22. [DOI] [PubMed] [Google Scholar]
- 46.Fowler VG Jr, Proctor RA. Where does a Staphylococcus aureus vaccine stand? Clin Microbiol Infect. 2014. May;20(Suppl 5):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Proctor RA. Is there a future for a Staphylococcus aureus vaccine? Vaccine. 2012. Apr 19;30(19):2921–7. Epub 2011 Nov 21. [DOI] [PubMed] [Google Scholar]
- 48.Salgado-Pabón W, Schlievert PM. Models matter: the search for an effective Staphylococcus aureus vaccine. Nat Rev Microbiol. 2014. Aug;12(8):585–91. Epub 2014 Jul 7. [DOI] [PubMed] [Google Scholar]
- 49.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, López CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG; Inflammation and Host Response to Injury, Large Scale Collaborative Research Program. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013. Feb 26;110(9):3507–12. Epub 2013 Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reizner W, Hunter JG, O’Malley NT, Southgate RD, Schwarz EM, Kates SL. A systematic review of animal models for Staphylococcus aureus osteomyelitis. Eur Cell Mater. 2014. Mar 25;27:196–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.