Abstract
Natural forms of vitamin E comprise four tocopherols and four tocotrienols. During the last twenty years, there have been breakthroughs in our understanding of vitamin E metabolism and biological activities of vitamin E metabolites. Research has established that tocopherols and tocotrienols are metabolized via ω-hydroxylase (CYP4F2)-initiated side chain oxidation to form 13′-hydroxychromanol and 13′-carobyxychromanol (13′-COOH). 13′-COOHs are further metabolized via β-oxidation and sulfation to intermediate carboxychromanols, terminal metabolite carboxyethyl-hydroxychroman (CEHC), and sulfated analogs. Animal and human studies show that γ-, δ-tocopherol and tocotrienols are more extensively metabolized than α-tocopherol (αT), as indicated by higher formation of CEHCs and 13′-COOHs from non-αT forms than those from αT. 13′-COOHs are shown to be inhibitors of cyclooxygenase-1/−2 and 5-lipoxygenase and much stronger than CEHCs for these activities. 13′-COOHs inhibit cancer cell growth, modulate cellular lipids and activate peroxisome proliferator-activated receptor-γ and pregnane X receptor. Consistent with mechanistic findings, αT-13′-COOH or δTE-13′-COOH, respective metabolites of αT or δ-tocotrienol, show anti-inflammatory and cancer-preventive effects, modulates the gut microbiota and prevents β-amyloid formation in mice. Therefore, 13′-COOHs are a new class of bioactive compounds with anti-inflammatory and anti-cancer activities and potentially capable of modulating lipid and drug metabolism. Based on the existing evidence, this author proposes that metabolites may contribute to disease-preventing effects of γ-, δ-tocopherol and tocotrienols. The role of metabolites in αT’s actions may be somewhat limited considering controlled metabolism of αT because of its association with tocopherol-transport protein and less catabolism by CYP4F2 than other vitamin E forms.
Keywords: Vitamin E, Metabolism, Carboxychromanols, Tocopherol, Tocotrienol, Cytochrome P-450, Inflammation, Cancer, Cyclooxygenase, 13′-carboxychromanol, 5-Lipoxygenase, Natriuretic, CEHC, PXR, PPAR, Gut microbiota
Graphical Abstract
1. Introduction
The vitamin E family has eight lipophilic antioxidants including α-, β-, γ-, δ-tocopherol (αT, βT, γT, δT) and α-, β-, γ-, δ-tocotrienol (αTE, βTE, γTE, δTE). Tocopherols and tocotrienols have a chromanol ring, on which the phenolic group can donate electrons to scavenge lipid peroxyl radicals. Tocopherols have a saturated 16-carbon side chain and tocotrienols have three double bonds in the side chain (Figs. 1 and 2). Within each category, α-, β-, γ-, and δ-isoform differ at the 5- or 7-position on the chromanol ring with hydrogen (−H) or methyl (−CH3) group. Naturally occurring tocopherols have the R,R,R-configuration at the 2, 4′ and 8′-position, and tocotrienols have the R configuration at the 2-position, although synthetic αT contains a mixture of R or S-configuration analogs at the 2, 4′ and 8′-position.
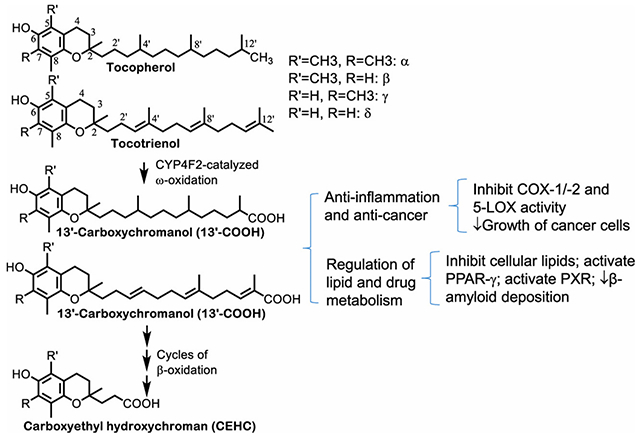
Fig. 1. Metabolism of tocopherols -.
Tocopherols are metabolized via CYP4F2-mediated ω-hydroxylation and oxidation to generate 13′-OHs and 13′-COOHs. 13′-COOHs are further metabolized via β-oxidation that removes two- or three-carbon moieties each cycle and generate intermediate carboxychromanols and terminal CEHCs. Under the condition of supplementation, sulfation of carboxychromanols may take place in parallel with β-oxidation.
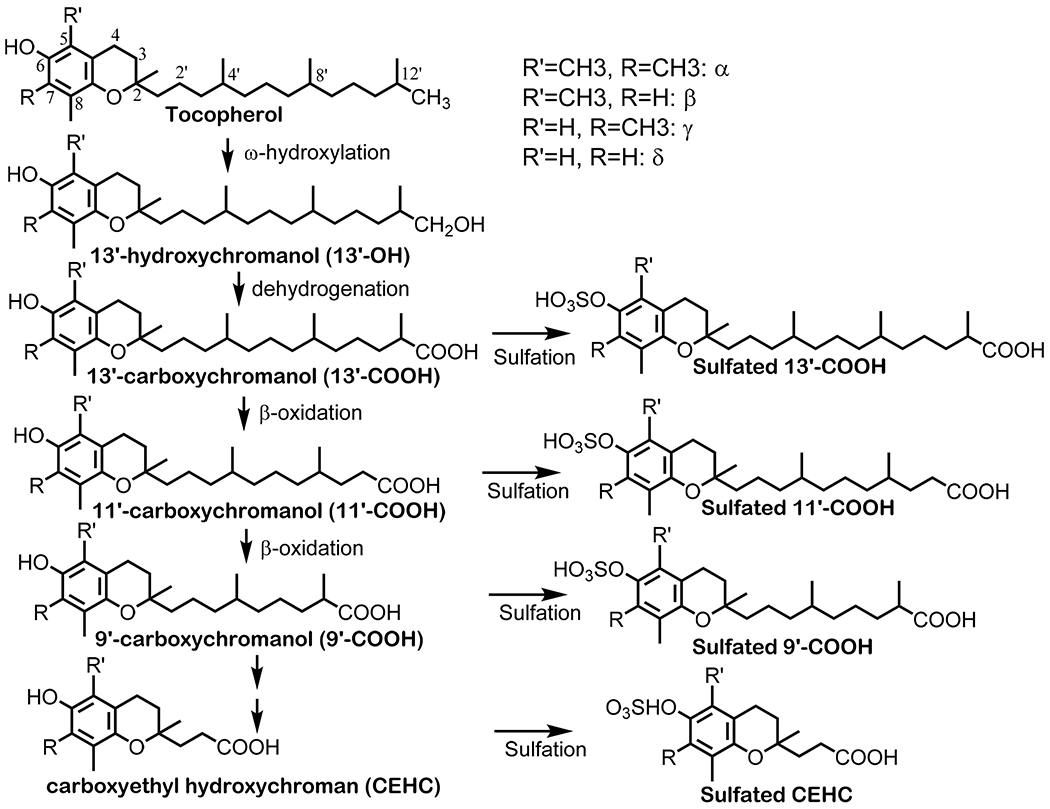
Fig. 2. Metabolism of tocotrienols –
Tocotrienols are metabolized via CYP4F2-mediated ω-oxidation to generate 13′-COOHs with three double bonds (3DB), which may be reduced by reductases to the analogs with two double bonds (2DB) in the side chain. 13′-COOHs are further metabolized via β-oxidation to generate shorter-chain carboxychromanols and CEHCs. Under the condition of supplementation, sulfation of carboxychromanols may take place in parallel with β-oxidation.
While tocopherols and tocotrienols are believed to have similar antioxidant activities, these vitamin E forms have markedly different bioavailability [1]. In particularly, αT is the predominant form of vitamin E in tissues and has much higher bioavailability than other vitamin E forms. During the last twenty years, research has revealed that differential bioavailability of vitamin E forms is at least in part rooted in different extent of catabolism of tocopherols and tocotrienols. Two proteins play critical roles in controlling body retention of vitamin E and formation of metabolites, including tocopherol transport protein (TTP) and vitamin E ω-hydroxylase (CYP4F2). Accumulating evidence indicate that specific metabolites, including (2′-carboxyethyl)-6-hydroxychromans (CEHCs) and 13′-carboxychromanol (13′-COOH), have unique biological activities compared to the precursors. This review will discuss the mechanism of how tocopherols and tocotrienols are metabolized, key proteins involved in the metabolism, bioavailability and bio-activities of the metabolites including short-chain and long-chain carboxychromanols and the role of metabolites in vitamin E-related beneficial effects.
2. Metabolism of natural forms of vitamin E
In the gastrointestinal track, tocopherols and tocotrienols are released from food matrix and incorporated into mixed micelles along with dietary fats. The vitamin E forms are absorbed into enterocytes by micelle diffusion and membrane proteins-facilitated transport [2]. These fat-soluble compounds are then packed into chylomicron particles together with triacylglycerol, phospholipids and cholesterol [3–6]. Recently, high-density lipoprotein (HDL) and ATP-binding cassette A1 (ABCA1) have also been suggested to be involved in the absorption of vitamin E [7,8]. Based on the content of different forms of vitamin E in chylomicron particles posterior intake, tocopherols and tocotrienols appear to be similarly absorbed in the intestine [9]. The chylomicron-bound tocopherols and tocotrienols are then transported through lymphatic system to peripheral tissues including bone marrow, adipose, muscle, skin and possibly brain. While the mechanism of tissue uptake of vitamin E forms is not fully understood, it is believed that these fat soluble vitamins are taken into tissue cells via lipoprotein receptor- and lipoprotein lipase-mediated process [3,6,10]. It is noteworthy that chylomicron-associated tissue uptake of vitamin E may play a role in tissue retention of αT and more so for non-αT forms of vitamin E, which are more readily metabolized than αT in the liver. In particular, γT is found in human skin, adipose and muscle at unexpectedly high concentrations despite its low levels in the plasma [11]. Subsequently, the resulting chylomicron remnants are taken up by the liver where vitamin E forms are transported and metabolized.
In the liver, while all tocopherols and tocotrienols are metabolized, higher portions of non-αT forms of vitamin E than αT are catabolized via cytochrome P450 (CYP4F2) initiated ω-hydroxylation and oxidation, which generate 13′-hydroxychromanol (13′-OH) and 13′-carboxychromanol (13′-COOH). Subsequently, 13′-COOHs are catabolized via a series of β-oxidation that leads to form terminal metabolite 3′-carboxychromanol (3′-COOH) or (2′-carboxyethyl)-6-hydroxychromans (CEHCs) (Figs. 1 and 2). Based on observations in human cells and animals [12–14], sulfation of the phenolic group on the chromanol occur in parallel with β-oxidation especially when there is relatively high intakes of vitamin E forms (Figs. 1 and 2).
2.1. Elucidating how vitamin E is metabolized via identification of the metabolites of vitamin E
To celebrate the centennial of vitamin E discovery, I summarize key research milestones related to the discovery of vitamin E metabolism and metabolites in Table 1. In 1922, the existence of a dietary factor (vitamin E) essential for reproduction was proposed, the landmark of vitamin E discovery [15]. In 1956, Simon and coworkers proposed α-tocopheronic acid and α-tocopheronolactone, which have an opened and oxidized chromanol and shortened side chain, as the major urinary metabolites in rats and humans given large doses of αT [16]. However, these so-called Simon metabolites turned out to be in vitro (artificial) oxidative products of α-CEHC [17]. In 1984, Chiku et al. [18] gave rats radiolabeled d-3,4-[3H2]-δT intravenously. By following radiolabeled materials in the urine and after sulfatase digestion, these investigators found that the major metabolite from δT was 2,8-dimethyl-2-(2′-carboxyethyl)-6-chromanol (δ-CEHC), rather than the tocopheronolactone analog. Since δ-CEHC has an unchanged chromanol ring with shortened and carboxylated side chain compared to the parental δT, Chiku et al. [18] proposed that δT is likely metabolized by ω-oxidation and β-oxidation of the hydrophobic side chain of the precursor. In 1995, α-CEHC was detected as the major urinary metabolite of αT in humans [17]. Subsequently, α-CEHC and γ-CEHC were detected in the urine and serum of humans [17,19–22].
Table 1.
Milestones of discovery of vitamin E metabolism and bioactivities of metabolites.
| Year | The key events of discovering vitamin E metabolism and metabolites | Investigators and references |
|---|---|---|
| 1984 | Identification of δ-CEHC in rats’ urine after supplementation of radiolabeled δT | Chiku, S. [18] |
| 1995 and onward | α-CEHC and γ-CEHC are detected in the plasm and urine in humans | Schultz et al. [17,19–22] |
| 1996, 2000 | Early study of CEHCs bioactivities: γ-CEHC has natriuretic activities; γ-CEHC inhibits COX-2 activity and ↓PGE2 | Wechter, W.J. [19]; Jiang, Q [64] |
| 2002 | Identifying 13’-OH, 13’-COOH and intermediate carboxychromanols in cultured HepG2 cells, which provides evidence of ω-oxidation and β-oxidation; CYP4F2 is identified as vitamin E ω-hydroxylase | Sontag, T.J. and Parker, R.S. [23]; Birringer, M. [24] |
| 2007 | Identifying sulfated long-chain carboxychromanols in human cells and in rats supplemented with γT or δT; First evidence of long-chain carboxychromanols in vivo | Jiang, Q. [12] |
| 2008, 2021 | Identifying 1st target of 13’-COOH: δT-13’-COOH inhibits COX-1 and COX-2 activity, and is stronger than 9’-COOH or CEHC for these actions; 13’-COOHs reduced COX-1 catalyzed thromboxane in platelets. | Jiang, Q. [65]; Park, N. [69] |
| 2009, 2010, 2016, 2020 | αT-13’-COOH, δT-13’-COOH, δTE-13’-COOH have anticancer effects in vitro and in animal models. | Mazzini, F. [77]; Jang, Y. [68]; Birringer, M. [78]; Yang, C. [25] |
| 2010, 2015, 2021 | LC-MS/MS was used to measure vitamin E metabolites (vitamin E metabolome) in animals and humans | Zhao, Y. [101]; Jiang, Q. [43]; Bartolini, D. [51] |
| 2011, 2016, 2018, 2021 | 13’-COOHs inhibit 5-LOX activity without affecting 5-LOX translocation, ↓LTB4 in neutrophils and show anti-inflammatory effects in vivo | Jiang, Z. [75]; Jang, Y. [68]; Pein [54]; Park, N. [69] |
| 2012 | The study in CYP4f14 KO mice indicates that ω-hydroxylase accounts for 70% metabolism of vitamin E | Bardowell, S. A. [29,31] |
| 2014, 2015, 2017 | αT-13’-COOH and δTE-13’-COOH inhibit NO2−, iNOS, cytokines and lipid accumulation in macrophages | Wallert, M. [79,82]; Schmolz, L. [80] |
| 2017, 2020, 2021 | δT-13’-COOH, δTE-13’-COOH, αT-13’-COOH activate PXR and PPARs, which are relevant to drug and lipid metabolism, respectively. | Podszun, M.C [39]; Bartolini, D. [87]; Willems, S. [98] |
In 2002, specific reactions involved in vitamin E catabolism was unequivocally elucidated via identifying key intermediate and terminal metabolites in cell-based studies. Sontag and Parker [23] and Birringer et al. [24] showed that incubation of γT, δT and γTE with hepatic HepG2 cells resulted in formation of 13′-hydroxychromanol (13′-OH), long-chain carboxychromanols including 13’-, 11′ and 9′-carboxychromanol (13′-COOH, 11′-COOH, 9′-COOH) and shorter side chain carboxychromanols (7′-COOH, 5′-COOH) as well as terminal metabolite 3′-COOH (also called CEHC) (Figs. 1 and 2). The identification of these metabolites in liver cell culture media provides direct evidence that vitamin E forms are metabolized via cytochrome P-450 mediated ω-hydroxylation and oxidation of the 13′-carbon, followed by stepwise β-oxidation to remove a two- or three carbon moiety each cycle from the side chain. For tocotrienols, reductases are likely involved in reducing double bonds because γTE or δTE-derived 13′-COOHs are found to have three and two double bonds in the side chain based on cell-based and animal studies [13,24–26] (Fig. 2). Furthermore, Mustacich et al. [27] observed that upon injection of mega doses of αT, αT and αT-13′-OH were predominantly found in the liver microsomes which contain endoplasmic reticulum membranes, whereas the terminal metabolite α-CEHC was mainly detected in the mitochondria. These data indicate that like other CYP enzymes, ω-hydroxylation and oxidation of 13′-carbon (by CYP4F2) likely takes place in hepatic endoplasmic reticulum, and subsequent β-oxidation occurs in the mitochondria and possibly peroxisomes [27].
In 2007, Jiang et al. [12] identified sulfated long-chain carboxychromanols from metabolism of γT and δT in cultured human cells and in the rat’s plasma. Similar sulfated long-chain metabolites were also derived from γTE in cells and rats [13]. These data indicate that sulfation of long-chain metabolites involves in vitamin E metabolism and provide the first evidence of formation of long-chain carboxychromanols in the whole body environment. at that time, identification of sulfated long-chain carboxychromanols was unexpected as only sulfated CEHCs were previously reported. Specifically, in human lung epithelial A549 cells, γT and δT appear to be catabolized to sulfated-13′-COOH, sulfated-11′-COOH and sulfated-9′-COOH, in addition to the unconjugated analogs (Fig. 1) [12]. Similarly, γTE was also metabolized to sulfated and unconjugated long-chain carboxychromanols in A549 cells, and the portion of sulfated metabolites increased while incubation prolonged, indicating sulfation took place after β-oxidation [13]. Furthermore, sulfated 13′-COOH, 11′-COOH and 9′-COOH as well as unconjugated 13′-OH and 13′-COOH were detected in the plasma of rats supplemented with γT, δT or γTE [12–14]. Indeed, the majority of these carboxychromanols in the plasma were found to be in the conjugated form in rats supplemented with γTE [13]. In 2011, Hashiguchi et al. [28] showed that sulfotransferases in SULT1 family may be responsible for sulfation of carboxychromanols. These observations indicate that under supplementation condition, sulfation takes place in parallel with β-oxidation (Figs. 1 and 2) [12,13]. The detection of long-chain carboxychromanols in vivo further validates that vitamin E forms are metabolized via ω-hydroxylation and β-oxidation as well as sulfation in the whole body environment.
2.2. Cytochrome P-450 4F2 (CYP4F2) is the major vitamin E ω-hydroxylase in the liver, while ω-hydroxylase activity was also observed in the kidney and intestine; potential ω-1 and ω-2 hydroxylases remain to be identified
In 2002, the seminal work by Sontag and Parker [23] demonstrated that tocopherol-ω-hydroxylase activity is mainly associated with cytochrome P450 4F2 (CYP4F2). Specifically, they found that γT and αT were metabolized to 13′-OH and 13′-COOH in insect microsomes expressing recombinant human liver CYP4F2 via NADPH-dependent ω-oxidation. In contrast, these investigators did not observe ω-oxidation activity in microsomes expressing CYP3A, CYP1A1/2, CYP4F3A and various CYP2 isoforms [23]. CYP4F3B showed tocopherol-ω-hydroxylase activity at the level of less than 1% of CYP4F2. In addition to the liver enzyme, tocopherol ω-hydroxylase activity was also observed in rat kidney homogenates and microsomes, suggesting that kidney may be a site of vitamin E metabolism [23].
In 2012, Dr. Parker and coworkers [29] provided unambiguous evidence that CYP4F2 plays a key role in vitamin E metabolism in mice with knockout of Cyp4f14, a murine ortholog of human CYP4F2. These investigators found that mice with knockout of Cyp4f14 have greatly decreased urinary and fecal excretion of metabolites and enhanced tissue concentrations of γT and δT compared with wild-type mice. These investigators estimated that CYP4F2-initiated ω-oxidation metabolism accounts for formation of 70–80% of whole body vitamin E metabolites [29]. Nevertheless, knockout of Cyp4f14 did not completely abolish omega-series metabolites, suggesting potential involvement of other enzymes that cause ω-oxidation of vitamin E in mice [29]. In this regard, CYP3A4 has also been suggested to be involved in metabolism of vitamin E based on the observation that ketoconazole or sesame oil, inhibitors of CYP3A family, inhibited formation of CEHCs in hepatocytes, animals and humans [30]. However, since these inhibitors are not strictly specific to CYP3A, further studies are needed to validate the role of CYP3A in metabolism of vitamin E including examining formation of ω-oxidation metabolites in CYP3A knockout cells or animals.
In addition to ω-oxidation, significant amounts of 12′- and 11′-hydroxychromanol (12′-OH, 11′-OH) were detected in mouse feces, suggesting ω-1 and ω-2 hydroxylase-catalyzed reactions [29,31]. However, the identity of cytochrome P450 enzymes with ω-1 and ω-2 hydroxylase activities remains to be determined. In addition, these investigators found that mice with liver-specific knockout of cytochrome P-450 reductase and thus lack of cytochrome P-450 activity have reduced body metabolic capacity by 70%, indicating that 30% metabolites may be generated in extra-hepatic tissues [31]. To this end, rat’s kidney and human intestine microsomes appear to have ω-hydroxylase activity, but seem to have lowered activity compared with the enzyme in the liver [23,31].
2.3. The catalytic activity of CYP4F2 shows substrate specificity: Tocotrienols are better substrates than tocopherol counterparts and γT and δT are more readily metabolized than αT
Studies have been conducted to characterize the influence of key structural features in tocopherols and tocotrienols on CYP4F2-catalyzed metabolite formation. In cultured A549 cells, we observe that metabolite formation from δT is quicker and higher than that of γT and metabolites from either δT or γT are higher than αT [12]. In cultured A549 cells and rats, γTE is more extensively metabolized than γT, as indicated by quicker formation and higher amounts of metabolites from γTE than those from γT [13]. Consistently, Sontag and Parker [32] observed more metabolites generated from tocotrienols than those from tocopherols in cultured liver cells. These observations suggest that CYP4F2-catalyzed ω-oxidation has higher activity toward substrates with double bonds in the side chain than those with saturated side chain. Among tocopherols, the relative metabolites formed are δT > γT ≫ αT, indicating that methylation at the C5 position of the chromanol ring appear to be important for regulating CYP4F2 ω-hydroxylase activity.
In agreement with these cell-based studies, kinetic studies with microsomes expressing CYP4F2 revealed that tocotrienols has higher Vmax and lower apparent Km than tocopherols, indicating better activity and affinity of the enzyme toward tocotrienols than tocopherols [32]. Among tocopherols, apparent Km values increased with the number of methyl groups and thus validate the relative affinity of δT > γT≈βT ≫αT. The enzyme shows lower Vmax toward αT and βT than γT and δT. Interestingly, αT appears to serve as a positive effector of ω-hydroxylation as it stimulates metabolism of other vitamers [32]. Overall, these enzyme and cell-based studies indicate that the relative catabolic rates by CYP4F2 are higher for tocotrienols than tocopherols, and δT and γT are more readily metabolized than αT.
2.4. Formation of vitamin E metabolites are controlled by tocopherol-transfer protein (TTP) and ω-hydroxylase (cytochrome P450 4F2)
While CYP4F2 is responsible for catabolizing vitamin E forms, TTP protects αT and to less extent other vitamin E forms from being metabolized (Fig. 3) [1]. Specifically, in the liver, TTP preferentially binds to αT over other forms of vitamin E and functions to transport these lipophilic compounds from endosomes or lysosomes to the plasma membrane of hepatocytes [33,34]. Subsequently, TTP interacts with phosphoinositides to release αT to the plasma membrane [34] where ABCA1 helps vitamin E forms to be incorporated into lipoproteins [33, 35,36]. Lipoproteins then transport vitamin E forms in the circulation to other tissues. Meanwhile, unbound vitamin E forms are catabolized by vitamin E ω-hydroxylase (Fig. 3). Because of higher affinity of TTP and relatively lower metabolic activity by CYP4F2 toward αT, most tissues have much higher retention of αT than other vitamin E forms and thus αT is the most abundant vitamin E in the plasma, liver and other tissues. In contrast to αT, other vitamin E forms are less bound to α-TTP and preferentially metabolized by CYP4F2 to hydroxycarboxychromanol, carboxychromanols and their conjugated counterparts
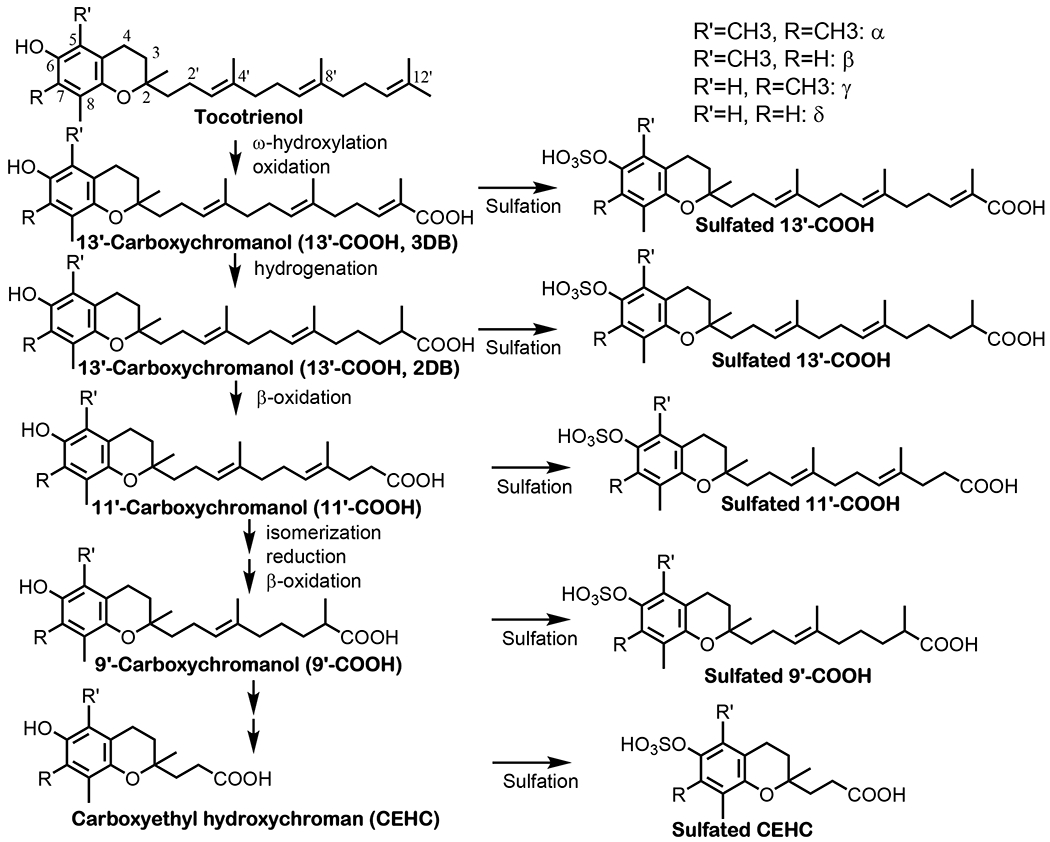
Fig. 3. Metabolism of vitamin E forms in the liver and possibly other tissues -.
In the liver, large portions of γT, δ;T, γTE and δTE are metabolized by CYP4F2-initiated ω-hydroxylation and oxidation to generate 13′-OHs and 13′-COOH, which is further metabolized to terminal metabolite CEHCs. In contrast, most αT and small amounts of other vitamin E forms are bound and transported by TTP (tocopherol transport protein) in hepatocytes and then incorporated into lipoproteins with the help of ABCA1 (ATP-binding cassette transporter A1). Vitamin E bound to lipoproteins are transported to other tissues via circulation. The crisscross arrows (light blue) indicate relatively minor events taking place for αT (catabolism) and other forms of vitamin E (binding to TTP) in the liver. It is estimated that over 70% metabolism of vitamin E takes place in the liver, while the kidney and intestine are found to have CYP4F2 activity and may therefore involve in vitamin E metabolism. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.5. Other factors affecting metabolism
Many factors may play a role in regulation of the metabolism of vitamin E forms. First, supplementation of αT appears to decrease γT and enhance γCEHC formation [37]. This accelerated metabolism of γT may be caused by αT’s competing to the binding of TTP. Further, αT has also been known to enhance CYP4F2 activity [32] and increase the expression of CYP via interaction with PXR [38]. However, αT or γT was recently reported to fail to activate PXR in LS180 cells, whereas αT-13′-COOH and γTE-13′-COOH activated PXR and induced P-glycoprotein, suggesting these metabolites may promote metabolism of vitamin E and other drugs [39]. Secondly, smoking may be a factor affecting vitamin E metabolism. In particular, Bruno et al. [40] observed that smokers had decreased α-CEHC in the plasma compared to non-smokers after supplementation of labeled αT, suggesting decreased vitamin E metabolism in smokers. In addition, potential gender difference in metabolism of vitamin E has been reported. In particular, women appear to have greater metabolic rates of γT than men, as indicated by greater maximal plasma γ-CEHC and higher excretion of γ-CEHC [41]. Nevertheless, age may not play a key role in vitamin E metabolism [42].
2.6. Summary of metabolism of different forms of vitamin E
Over 70% metabolism of vitamin E occurs in the liver where all the vitamin E forms are metabolized by CYP4F2-initiated formation of 13′-OHs and 13′-COOHs, followed by stepwise β-oxidation to generate terminal metabolite CEHCs. Because of less-bound to TTP and more readil-catabolized by CYP4F2, γT, δT, γTE and δTE are preferentially catabolized compared with αT that is the most abundant vitamin E in tissues. Besides liver, other tissues like kidney and intestine may involve in vitamin E metabolism (Fig. 3).
3. The bioavailability of metabolites of different forms of vitamin E
While Chiku et al. [18] identified δ-CEHC using 3H-labeled δT in the rat’s urine in 1984, it was not until 1995–1996 that α-CEHC and γ-CEHC were reported as the major urinary metabolite of αT and γT, respectively, in humans [17,19]. Subsequently, many studies reported quantification of these CEHCs [17,19–22], including method development and quantification of CEHCs in the urine and plasma. Relatively recently, LC-MS/MS and GC-MS/MS have been developed to quantify the whole spectrum of vitamin E metabolomics, including long-chain and sulfated metabolites [29,43,44].
3.1. Comparison of relative metabolite formation between αT and γT, or natural RRR-αT vs. synthetic αT via monitoring CEHCs in the plasma and urinary excretion in humans
Utilizing isotope-labeled αT and γT, researchers compared relative metabolite formation between αT and γT or synthetic αT vs. the natural RRR form. For instance, Traber et al. [20] showed that urinary excretion of α-CEHC after dosing with 150 mg deuterium-labeled synthetic αT (d (6)-all rac αT) acetate was much higher than that from d(3)-RRR-αT acetate, indicating that synthetic αT is preferentially metabolized and excreted compared with natural RRR-αT. Furthermore, Brigelius-Flohe et al. [42] showed that <1% of 30 mg deuterium-labeled αT (d(6)-αT acetate) was excreted as urinary α-CEHC in humans, whereas ~7.5% of deuterated d(2)-γT acetate appeared as γ-CEHC in the urine, indicating that a higher portion of γT is metabolized than αT. In addition, Leonard et al. [41] also used deuterated tocopherols (50 mg each) and showed that labeled γT disappeared faster than that of αT in the plasma. While deuterium-labeled α-CEHC was too low to be detected, urinary excretion of γ-CEHC peaked at 12 h after dosing and returned to baseline by 48 h. Interestingly, women compared with men had a greater rate of labeled γT fractional disappearance, greater maximal plasma γ-CEHC and higher excretion of γ-CEHC. These data demonstrate that γT is metabolized to a greater degree in women than in men and support the notion that γT is more extensively metabolized than αT in humans.
3.2. Quantification of CEHCs after intakes of pharmaceutical doses of vitamin E forms in clinical studies
We have documented the bioavailability of γ-CEHCs in the plasma after supplementation of γT at pharmacological doses in phase I and II studies. Wiser et al. [45] observed that supplementation of γT-rich tocopherols including 623 mg of γT and 61 mg of αT in a tablet, resulted in time- and dose-dependent increase of serum γ-CEHC in healthy and mild asthmatic participants. Specifically, when blood samples were collected 6 h post intake, serum γ-CEHC reached ~0.8 ¼M after the first dosing, >2.3 μM after daily supplementation for 8 days, and >3.7 μM after 8-day supplementation of two tablets a day [45]. Burbank et al. [46] conducted a study where ten participants consumed γT-enriched geltabs containing γT, αT, βT and δT at 612, 7, 28 and 8 mg, respectively, every 12 h for 3 doses. They observed that there were huge interpersonal differences in elevation of plasma γ-CEHC ranging from 3 to >10 μM, and γ-CEHC remained elevated even 12 h after the last dosing. Interestingly, the changes of plasma γ-CEHC negatively correlated with those of IL-8 induced by LPS in PBMCs [46]. In a follow-up study, Burbank et al. [47] conducted a randomized, placebo controlled crossover study investigating the effect of supplementation of γT at ~612 mg twice a day for two weeks on inhaled LPS-induced acute inflammatory responses including sputum neutrophilia, mucins and cytokines in subjects with mild asthma. These investigators observed that γT supplementation reduced inflammatory features of asthma and resulted in an increase of γ-CEHC to ~3 μM in the plasma compared to 0.17 μM in placebo (700 mg of safflower oil). It is noteworthy that in these studies, no sulfatase or glucoronidase was used for pre-digestion, and therefore, γ-CEHCs are in the unconjugated form, unlike those found in rodents that have sulfated metabolites. These data indicate that γ-CEHC in the unconjugated form can reach from 1 to 3 to up to 15 μM in the serum and maintained at elevated levels within 12 h post supplementation of γT.
Plasma concentrations and urinary excretion of CEHCs have also been evaluated in response to supplementation of tocotrienols. Mahipal et al. [48] observed that supplementations of δTE at escalating doses of 100–1600 mg for 8–14 days resulted in the increase of δ-CEHC in the plasma and urine to 3–6 and 60–90 μM, respectively, in healthy adults. It is noteworthy that plasma concentrations of δ-CEHC at 12 h after the last intake of δTE are comparable to the Cmax of δTE (Tmax at 4–7 h), while δTE concentrations almost decreased to the baseline at 12 h post intake, indicating short half-life of δTE and its continuous metabolism after dosing. Drotleff et al. [49] compared the bioavailability and pharmacokinetics of a single oral intake of 450 mg total tocotrienols from αTE-rich barley oil (αTE, βTE, γTE and δTE at 324, 28, 55 and 43 mg) or from γTE-rich palm oil (αTE, βTE, γTE and δTE at 131, 13, 218 and 85 mg) in 7 healthy male subjects. These investigators also recorded 24 h urinary excretion of major metabolites including CEHCs and α-carboxymethylbutyl-hydroxychromans (α-CMBHC or 5′-COOH). For these metabolites, the highest proportions were excreted during the period 6–12 h post intake, while excretion of these metabolites continued beyond 24 h. Subjects receiving the barley oil increased excretion of α-CEHC (the major metabolite), α-5′-COOH, γ-CEHC and δ-CEHC, and the total urinary metabolites accounted for ~15% of total intake. Subjects receiving the palm oil formulation mainly excreted γ-CEHC, and the total metabolites accounted for ~13% of intake.
In summary, these studies have shown that more metabolites from tocotrienols (accounting for 13–15% of intake) [49] were formed than those from γT (7.5%), the latter of which are higher than those from αT (1% or less) [42]. Furthermore, supplementation of pharmacological doses of γT or tocotrienols leads to increase of CEHCs to several μM to >10 μM in the plasma. In addition, there are large interpersonal differences of metabolite formation and the mechanisms underlying this observation remain to be determined.
3.3. The bioavailability of long-chain metabolites in humans
In 2012, Bardowell et al. [31] observed long-chain carboxychromanols and hydroxychromanols in feces from an individual supplemented with γT, but the concentrations of these metabolites were not reported. Ciffolilli et al. [50] reported increase of αT-13′-OH to 15–50 nM in the plasma compared to baseline at 7.5 nM in humans supplemented with 671 mg of αT. Giusepponi et al. [44] detected an increase of α-CEHC and αT-13′-OH as well as possibly αT-13′-COOH after supplementation of αT at 1000 IU in healthy humans, although the concentrations of αT-13′-COOH could not be quantified due to low concentrations and interferences. Recently, Bartolini et al. [51] measured plasma concentrations of αT metabolites in humans after oral intake of daily 800 U RRR-αT for a week. They used the term “vitamin E metabolome”, although similar concept and approaches have been used to measure vitamin E metabolites in many studies where LC-MS/MS was used to simultaneously measure short-, medium- and long-chain carboxychromanols as well as hydroxychromanols [43]. Bartolini et al. [51] reported that αT supplementation resulted in increases of plasma α-CEHC (3′-COOH), α-CMBHC (5′-COOH), αT-13′-OH, αT-13′-COOH from baselines of 19.0, 11.6, 1.7, and 3,1 nM to post-supplement concentrations of 371.0, 84.8, 16.8 and 5.6 nM, respectively. These investigators observed large interpersonal variances in formation of these metabolites, which was also previously documented for γ-CEHC as a result of γT supplementation [46]. Besides these metabolites, they observed that αT oxidation product α-tocopheryl quinone, but not other metabolites, significantly correlated with the post-supplementation levels of αT. Overall, these studies documented the existence of low concentrations of αT-derived long-chain metabolites (in nM range) in the plasma, although there is still lacking of quantitative measures of long-chain metabolties from other vitamin E forms.
3.4. Quantification of long-chain metabolites including 13′-COOHs in animals
Despite limited data in humans, the bioavailability of long-chain metabolites of vitamin E has been reported in animals. Our early studies show that sulfated long-chain carboxychromanols including sulfated 11′-COOH are detected in the plasma of rats supplemented with γT or γTE [12,13]. Specifically, a single gavage of γTE resulted in appearance of γCEHCs and long-chain metabolites in the plasma, including sulfated 9′-COOH, sulfated 11′-COOH and sulfated 13′-COOH as well as unconjugated 13′-COOH [13,14]. Among the long-chain metabolites, sulfated 11′-COOH is the most abundant reaching 1.2 μM at 6 h after supplementation [13]. Recently, we conducted a pharmacokinetic study characterizing time-dependent metabolite formation in rats given a single dose of γT-rich tocopherols (50 mg/kg) containing γT/δT/αT at 57.7, 21.9 and 10.9% or δTE-rich tocotrienols (35 mg/kg) containing δTE/γTE at 8:1 [26]. We observed that sulfated γ- and δ-CEHC and sulfated γ- and δ-11′-COOH are the major metabolites in the plasma and reached Cmax of 0.6 and 0.4–0.5 μM, respectively. Conjugated CEHCs are the major metabolite in the urine, while unconjugated long-chain carboxychromanols including 13′-COOHs, 11′-COOHs and 9′-COOHs are excreted to feces with 13′-COOHs as the predominant metabolite accounting for over 45–60% of total excreted metabolites in feces. Overall, >70% metabolites from γT, δT, γTE or δTE appear to be excreted via feces as unconjugated long-chain metabolites. These observations are consistent with those by Bardowell et al. [29,31] who detected high levels of long-chain carboxychromanols including 13′-COOHs found in feces of mice supplemented with γT, δT or mixed tocopherols. In addition, comparison of the concentrations of long-chain metabolites from tocopherols and tocotrienols revealed higher formation of δE-13′-COOHs (two- and three-double bond analogs combined) than γT-13′-COOH in feces, consistent with the idea that tocotrienols are more extensively metabolized than tocopherol counterparts.
In addition to the study in healthy rodents, supplementation of γT, δT or δTE/γTE led to substantial fecal excretion of unconjugated carboxychromaols in feces with 13′-COOHs as the predominant metabolites in colitis and colon cancer models [25,43,52]. In agreement with higher metabolism of γT than αT, we observed higher amounts of long-chain metabolites from γT in feces than those from αT under colitis condition, which correlated with more excretion of intact αT than γT in feces [53].
3.5. Bioavailability of 13′-COOHs and metabolites in rodents supplemented with αT-13′-COOH or δTE-13′-COOH
Recently, potential bioavailability of αT-13′-COOH and δTE-13′-COOH as well as their metabolites was documented in animal studies where these bioactive compounds were directly administered. For instance, shortly after i.p. injection, αT-13′-COOH can reach to μM in the plasma and exudate [54]. Supplementation of δTE-13′-COOH in diet resulted in an increase of hydrogenated metabolite, i.e., δTE-13′-COOH that has two double bonds in the sulfated form and shorter chain carboxychromanols including sulfated 11′-COOH and δ-CEHC in the plasma, whereas the supplementation had no effect on the concentrations of vitamin E forms [25]. In feces, supplementation of δTE-13′-COOH in diets resulted in excretion of the parental compound, the hydrogenated metabolite of δTE-13′-COOH with two double bonds in the side chain and β-oxidation metabolite 11′-COOH [25]. Among these compounds in feces, the most abundant compound is hydrogenated metabolite of δTE-13′-COOH with two double bonds in the side chain. Interestingly, total 13′-COOHs (including both three- and two double bonds at the side chain) was positively correlated with Lactococcus and Lachnospiraeae NK4A136 [25].
3.6. Summary of the bioavailability of vitamin E metabolites
Studies in humans and animals have confirmed that tocotrienols are more extensively metabolized than their tocopherol counterparts, and γT or δT are more readily metabolized than αT. Animal studies indicate that more metabolites are excreted as unconjugated long-chain carboxychromanols with 13′-COOHs as the predominant metabolites in feces than those found in the urine where conjugated CEHCs are the major metabolites. Supplementation of high doses of tocopherols and tocotrienols leads to formation of CEHCs at μM in the unconjugated form in the plasma of humans, while sulfated CEHCs and sulfated 11′-COOHs are found in the plasma of rodents.
4. Biological activities and molecular targets of vitamin E metabolites in mechanistic studies
This section focuses on biological actions and mechanisms of various metabolites of different forms of vitamin E based on in vitro biochemical and cell-based studies (Fig. 4). The timeline of these discoveries are summarized in Table 1.
Fig. 4. Biological activities of 13′-COOHs -.
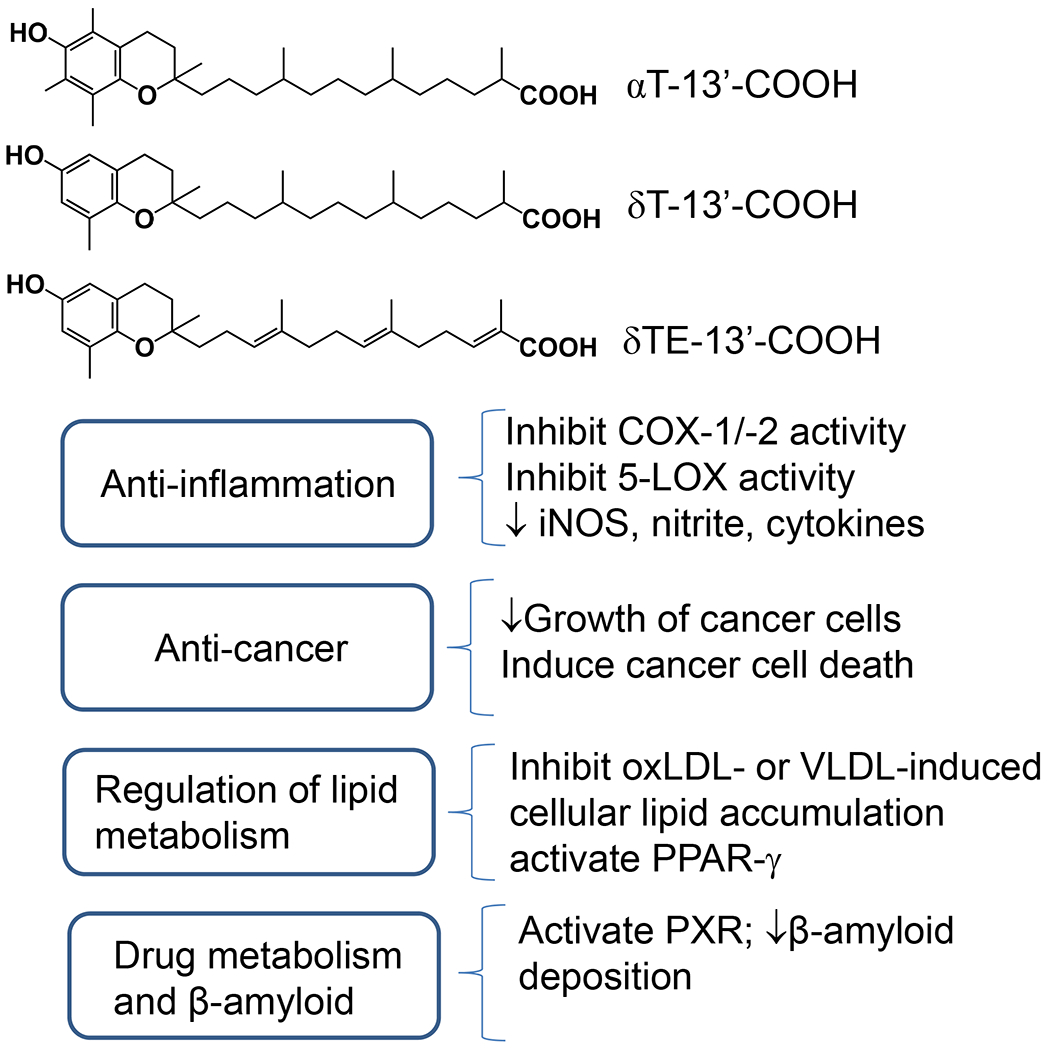
αT-13′-COOH, δ-13′-COOH and δTE-13′-COOH derived from αT, δT and δTE have been shown to have bioactivities including anti-inflammation, anti-cancer, and modulation of lipid accumulation and drug metabolism.
4.1. Antioxidant effects
Carboxychromanols and hydroxychromanols, like their precursors, have a phenolic group on the chromanol ring. Therefore, these vitamin E metabolites are expected to have antioxidant property by donating electrons to scavenge reactive free radicals. In contrast, sulfated metabolites, which have sulfation of the phenolic group, should not have antioxidant activities. With the carboxyl group added to the side chain, carboxychromanols are more hydrophilic than their vitamin precursors, and the hydrophilicity enhances with shorter sidechain metabolites. Consistently, δT-13′-OH, δT-13′-COOH and δTE-13′-COOH have been shown to have potent antioxidant activities by preventing lipid peroxidation in vitro. For instance, compared with δTE, δTE-13′-COOH, which is also called garcinoic acid isolated from Garcinia kola, exhibit similar radical scavenging activity. Similarly, δT-13′-COOH showed comparable antioxidant activity to δT or αT [55].
4.2. Natriuretic activities of γ-CEHC
In 1996, Wechter et al. [19] reported natriuretic activity of γCEHC. In contrast, γ-CEHC did not show this effect, indicating high specificity for γ-CEHC. Further mechanistic studies revealed that the natriuretic activity of γ-CEHC appeared to stem from reversible inhibition of 70 pS K+ channel in the apical membrane of the thick ascending limb of the kidney [19]. Consistently, it was subsequently shown that γTE supplementation, which should lead to increased formation and excretion of γ-CEHC in the urine, elevated urinary excretion of sodium in rats fed high sodium diet but not in animals with normal sodium intake [56]. In a subsequent human study, Yoshikawa et al. [57] report that supplementation of γT for 4 weeks non-significantly increased sodium excretion and significantly enhanced sodium excretion one week after the cessation of γT administration compared to the placebos.
4.3. 13′-COOHs are competitive inhibitors of cyclooxygenase-1 (COX-1) and COX-2, and much stronger than short-chain analogs including CEHCs in inhibition of COXs
COX-1 and COX-2 catalyze oxidation of arachidonate to form prostaglandin H, which is subsequently converted to prostaglandin E2 (PGE2), thromboxane A2 (TxA2) and other eicosanoids. These eicosanoids are bioactive “tissue hormones” regulating many physiological and pathological process [58]. COX-1 is constitutively expressed in many tissues including the gastrointestinal track and platelets. TXA2, which is synthesized by COX-1-mediated reaction in platelets, is known to stimulate platelet aggregation. Overproduction of TxA2 increases the risk of cardiovascular diseases [59]. Moreover, COX-2 is often induced in response to inflammatory stimuli in immune cells. at the site of inflammation, elevated PGE2 is believed to cause pain and fever [60,61] and activate cytokine formation [62]. Additionally, PGE2 is elevated in cancer tissues and promotes cell proliferation, resistance to apoptosis and even metastasis. Consistently, COX inhibitors including aspirin are anti-inflammatory drugs and effective for chemoprevention of colon cancer [63].
In 2000, we studied the impact of γ-CEHC on COX-mediated PGE2 formation in macrophages and epithelial cells, and this study represents an early work on non-antioxidant activities of vitamin E metabolites. Specifically, we show that γ-CEHC inhibits COX-2-mediated PGE2 formation and COX-2 activity with the IC50 of ~30 μM in LPS-stimulated macrophages and IL-1β-activated A549 epithelial cells [64]. On the other hand, γT, when incubated with cells for prolonged time, can also decrease PGE2 but does not affect the expression of COX-2 or its activity in purified enzyme assays [64]. In retrospect, the observation that γT inhibited pre-induced COX-2 activity in A549 cells may stem from formation of long-chain carboxychromanols in the conditioned media as a result of metabolism of γT by A549 cells [64,65]. Subsequently, Grammas et al. [50] reported that both α-CEHC and γ-CEHC inhibited TNFα- or LPS-stimulated PGE2 formation in rat aortic endothelial cells and mouse microglial cells [66].
In 2008, we documented that 13′-COOHs inhibit COX-1 and COX-2 and elucidated the mechanism underlying this action including structural characteristics required for inhibiting COXs among different carboxychromanols. This study is the first to show anti-inflammatory activities and identifies a molecular target of long-chain carboxychromanols [65]. Specifically, we show that the conditioned media enriched with 13′-COOHs, 11′-COOHs and 9′-COOHs from δT, γT or γTE inhibited COX-2 activities and PGE2 formation. Consistently, δT-13′-COOH, which was purified from conditioned media derived from metabolism of δT by A549 cells, inhibits COX-1/COX-2 activity with IC50s of ~4 μM [65]. In contrast, vitamin E forms do not inhibit COX-2 enzyme activity, despite modestly suppressing PGE2 formation in cellular environment after prolonged incubation and in animals [67]. Further, for inhibition of COX activities, δT-13′-COOH (IC50s at 4–5 μM) is much stronger than 9′-COOH, 5′-COOH and 3′-COOH, which show IC50 of ~6–20, 140–160 μM, 30–70 μM (in cell-based assays) to >300 μM (in assays with purified enzymes), respectively [65]. Our enzyme kinetic data reveal that δT-13′-COOH is a competitive inhibitor of COX-1 and COX-2 with Ki at 3.9 and 10.7 μM, respectively [65]. Computer simulation confirms that δT-13′-COOH is capable of binding to the substrate-binding site of COX and forms strong interaction with Tyr355 and Arg120 via the −COOH group at the 13′-position. Consistent with the enzyme kinetic data, the estimated binding energy indicates that δT-13′-COOH can bind stronger than arachidonic acid (the substrate) to COX-1 via potentially interacting with Phe209, Phe381 and His226 as these interactions may provide extra contacts with the chromanol moiety of 13′-COOH via hydrophobic interaction and hydrogen bond formation [65]. Unlike unconjugated carboxychromanols, conditioned media enriched with sulfated γTE-13′-COOH or sulfated γTE-11′-COOH failed to inhibit COX-2 [65], suggesting that sulfated carboxychromanols are not inhibitors of this enzyme.
In addition to δT-13′-COOH purified conditioned cell culture media, we show that chemically synthesized δT-13′-COOH exhibits similar inhibitory potency against COX-2 [68]. Interestingly, δTE-13′-COOH, which has three double bonds in the side chain and is chemically synthesized, is weaker than δT-13′-COOH (IC50 ~4–5 μM) in inhibition of COX-2 with IC50 of ~10 μM [68]. This observation indicates that double bonds in the side chain may weaken the interaction of 13′-COOH with the enzyme. Moreover, αt-13′-OH, after 24 h incubation with cells, was found to decrease LPS-stimulated PGE2, cytokines or the expression of COX-2 and iNOS in RAW264.7 murine macrophages [50].
In agreement with the observation that δT-13′-COOH competitively inhibits COX-1 activity [65], there is evidence showing that αt-13′-COOH and δTE-13′-COOH inhibit COX-1 activity with estimated IC50s of 6.9 and ~1 μM, respectively, as indicated in experiments with platelets [54,69]. Furthermore, δT-13′-COOH and δTE-13′-COOH potently blocked collagen- or ionophore-stimulated COX-1 catalyzed formation of thromboxane B2 (TXB2), the metabolite of TxA2, in rats’ platelets with IC50s of 1–2.5 μM [69].
Collectively, for inhibition of COX-2, the relative inhibitory potency follows δT-13′-COOH > δTE-13′-COOH or αt-13′-COOH in the enzyme assays, while δT-13′-COOH and δTE-13′-COOH appear to similarly inhibit COX-1 with stronger inhibitory potency in platelets than that in enzyme assays.
4.4. 13′-COOHs inhibit 5-lipoxygenae (5-LOX) and are much stronger than short-chain analogs for this activity, but do not affect 5-LOX translocation
5-LOX is a pro-inflammatory enzyme catalyzing leukotriene formation in neutrophils, eosinophils and mast cells [70]. Under resting condition, 5-LOX stays in the cytosol. Upon stimulation by receptor-mediated or ionophore-caused increase of intracellular calcium, 5-LOX translocates to the nucleus membrane where it interacts with 5-lipoxygenase-activating protein (FLAP) to convert arachidonic acid to 5-hydroperoxyeicosatetraenoic acid (5-HPETE) and then leukotriene A4, which is subsequently converted to leukotriene B4 (LTB4), C4 or D4 in different cells [70]. LTB4 is considered one of the most potent chemotactic agents [71], and LTC4 and D4 play key roles in allergic inflammatory diseases and asthma [72]. Therefore, 5-LOX inhibitor Zileuton is clinically used to treat asthma [73]. In addition, 5-LOX appears to be elevated in tumor tissues including colon cancer patients. 5-LOX knock out or 5-LOX inhibitors have been shown to block tumorigenesis in animal models [74].
My laboratory first observed that δT-13′-COOH inhibits 5-LOX and subsequently that δTE-13′-COOH appeared to be even stronger than δT-13′-COOH for this activity. Specifically, in 2011, we reported that δT-13′-COOH, which is derived from δT after metabolism by A549 cells, blocked ionophore-stimulated LTB4 in differentiated HL-60 cells and freshly isolated human neutrophils with IC50 of 4–7 μM and inhibited 5-LOX activity showing IC50 at ~1 μM [75]. In 2016, we reported that δTE-13′-COOH inhibits 5-LOX activity (IC50 of ~0.5–1.5 μM) and appears to be slightly stronger than δT-13′-COOH (1.5–2.5 μM) for this activity [68]. It should be mentioned that both δTE-13′-COOH and δT-13′-COOH were chemically synthesized in this study, while our former study in 2011 reported inhibition of 5-LOX by δT-13′-COOH that was purified from conditioned media. In 2018, Pein et al. [54] reported that αt-13′-COOH and δTE-13′-COOH inhibit 5-LOX with IC50s of 0.27 and 0.035 μM, respectively, whereas these compounds are not good inhibitors of 12- or 15-lipoxygenase. Mechanistically, it was proposed that αT-13′-COOH and δTE-13′-COOH may allosterically inhibit 5-LOX, but no enzyme kinetics was conducted which is considered vital for characterization of the mechanism of enzyme inhibition [76]. Recently our enzyme kinetic studies show that δT-13′-COOH and δTE-13′-COOH competitively inhibit human recombinant 5-LOX with Ki of 1.6 and 0.8 μM, respectively. In contrast, these compounds showed no impact on ionophore- or thapsigargin-induced 5-LOX translocation from cytosol to the nucleus, a key event for activation of 5-LOX and mediated LTB4 formation [69]. In addition, while Pein et al. observed similar IC50 of δT-13′-COOH compared with that reported by our earlier studies (above), they reported stronger inhibition of 5-LOX by δTE-13′-COOH than that reported by Jang et al. [68], i.e., IC50 of ~0.035 μM vs. 0.5–1 μM. The reason for the discrepancy may be resultant from different compound sources as Jang et al. [68] used synthetic compound, while Pein et al. [54] used naturally occurring δTE-13′-COOH purified from Garcinia amplexicaulis. Further, other factors may contribute to the difference, including enzyme concentrations and endpoint difference such as evaluation of peroxyl radical formation vs. assessment of specific enzyme products.
4.5. Anti-proliferation and induction of death in cancer cells
13′-Carboxychromanols have been shown to have anti-proliferation effects in cell-based studies. In 2009, a preliminary study reported anti-proliferation effects of αT-13′-COOH, αT-13′-OH, δT-13′-COOH and δTE-13′-COOH in glioma C6 cancer cells [77]. In 2010, δT-13′-COOH and αT-13′-COOH were shown to inhibit the growth of liver HepG2 cancer cells with IC50s of 6.5 and 13.5 μM, respectively. In contrast, δT-13′-OH or αT-13′-OH are much less effective for this activity (IC50 > 50 μM) [78]. Further, δT-13′-COOH and αT-13′-COOH appeared to induce apoptosis and mitochondrial dysfunction, and increase formation of reactive oxygen species in these cells [78]. In another study, we show that δT-13′-COOH and δTE-13′-COOH suppressed the growth of human colon HCT116 and HT29 cancer cells with IC50s of 8.6–8.9 and 16–17 μM, respectively, whereas normal colon epithelial cells are much less sensitive to these compounds [68]. δT-13′-COOH and δTE-13′-COOH induced apoptosis and autophagy in cancer cells, as indicated by increased PARP cleavage and LC-3II, respectively. While δT-13′-COOH and δTE-13′-COOH inhibit COX-2 and 5-LOX, their anti-proliferation effects can only be modestly reversed by addition of arachidonate, indicating that anticancer effects are independent of inhibiting these enzymes. Using lipodomic approaches with liquid chromatography tandem mass spectrometry (LC-MS/MS), we show that 13′-COOHs increased intracellular dihydroceramides and dihydrosphingosine after short-time treatment, and enhanced ceramides during prolonged treatment. These changes of sphingolipids took place before manifestation of cell death. In addition, with further mechanistic studies, we conclude that 13′-COOHs inhibit dihydroceramide desaturase (DES-1) activity without affecting the protein expression of this enzyme [68]. Taken together, these results indicate that 13′-COOHs but not 13′-OHs are capable of blocking cancer cell proliferation potentially via interaction with sphingolipids and impairing mitochondrial functions.
4.6. Effects on pro-inflammatory cytokines and iNOS in cells
In addition to inhibition of COX and 5-LOX, the vitamin E metabolites have been found to exert other anti-inflammatory activities. For instance, both α-CEHC and γ-CEHC inhibit TNFα- or LPS-induced increase of nitrite and iNOS in rat aortic endothelial cells (RAEC) and EOC-20 microglial cells [66]. αT-13′-OH at 10 μM after 24-h pre-incubation, inhibited LPS-stimulated increase of IL-6, IL-1α, nitrite and PGE2 and upregulation of inducible nitric oxide synthase (iNOS) and COX-2 in RAW264.7 macrophages [50]. In the same cellular system, αT-13′-COOH and δT-13′-COOH (at 5 μM) showed stronger anti-inflammatory effects than αT-13′-OH or δT-13′-OH [79,80]. These observations suggest potential anti-inflammatory effects of these long-chain metabolites via blocking cytokines and reactive nitrogen species.
4.7. The effect of αt-13′-COOH and δTE-13′-COOH on cellular lipid accumulation and peroxisome proliferator-activated receptor-γ (PPAR-γ)
Formation of foam cells contribute to all stages of atherosclerosis development. Macrophages differentiated from monocytes that enter the arterial intima in response to endothelial cell damage internalize modified low-density lipoproteins (LDL) including oxidized LDL (oxLDL) via scavenger receptors including CD36. The subsequent and gradual accumulation of lipids including cholesterol esters in macrophages and thus their transformation into foam cells in the sub-endothelial space results in fatty streak formation, a hallmark of atherosclerosis [81]. Given the key role of foam cell formation, targeting lipid accumulation in macrophages may lead to prevention or therapy of cardiovascular diseases.
Despite controversial results from clinical studies concerning the use of αT supplementation in prevention of cardiovascular diseases, the role of αT-13′-OH and αT-13′-COOH in foam cell formation and lipid accumulation has been examined in cell-based studies. For instance, Wallert et al. [82] show that αT-13′-OH (10 μM) and αT-13′-COOH (5 μM) decreased cellular uptake of oxLDL and oxLDL-induced accumulation of neutral lipids in THP-1 macrophages via inhibition of phagocytosis. Surprisingly, these compounds themselves enhanced CD36 mRNA and protein expression. In another study, Kluge S et al. [83] report that αT-13′-COOH decreased ver-low density lipoprotein (VLDL)-induced neutral lipid accumulation and inhibited lipoprotein lipase (LPL) activity. These investigators also showed that αT-13′-COOH upregulated angiopoietin-like 4 (ANGPTL4) at the transcriptional level and enhance processed ANGPTL4 that is known to antagonize LPL catalytic activity [83]. Although these authors concluded that upregulation of ANGPTL4 was not caused by activation of PPAR-δ [83], a recent study shows that 13′-COOHs appear to activate PPARs with EC50 s at μM concentrations [98]. In particular, αT-13-COOH and δTE-13′-COOH activated PPAR-γ with EC50s of 6 and 1.7 μM, respectively. δTE-13′-COOH also showed co-regulatory activities of PPAR-γ [98]. These findings may explain the observation that αT-13′-COOH alone induced neutral lipid accumulation and increased lipid droplet associated protein PLIN2 in THP-1 macrophages [84].
4.8. The effect of vitamin E metabolites on pregnane X receptor (PXR) and drug metabolism
Intestinal P-glycoprotein (P-gp) is a member of the atP binding cassette transporter family (ABC transporters) and functions as a drug efflux pump. Therefore, P-gp plays a key role in limiting the bioavailability of drugs and phytochemicals. P-gp expression is regulated by multiple mechanisms including posttranslational modification or at the transcriptional level. One of the transcriptional factors is PXR [85]. In one study, tocotrienols but not tocopherols were found to activate PXR and xenobiotic receptor [86]. However, in another study, αT was reported to increase the expression of CYP3a11 (the murine analog to human CYP3A4) via interaction with PXR [38]. Consistent with the impact of αT on PXR, a recent study showed that αT supplementation activated PXR in leukocytes, but αT did not affect its target CYP4F2 expression [51], which is somewhat different from previous observation [38]. These observations suggest that the role of αT in PXR may vary with different systems.
13′-COOHs appear to be potent activators of PXR. Podszun MC et al. [38] showed that αT-13′-COOH and γTE activated PXR activity and induced protein expression and transporter activity of P-gp. Bartolini et al. [87] reported that δTE-13′-COOH and other 13′-COOHs are agonist of PXR with EC50s at 1.5–3.3 μM, but only δTE-13′-COOH exhibited 100% efficacy. The subsequent binding assays with calorimetric titrations revealed that δTE-13′-COOH binds to a single site in ligand binding domain (LBD) of PXR with Kd = 0.3 μM, whereas δTE or αT-13′-COOH do not show any detectable binding of the PXR LBD in this assay. Moreover, crystal structure of PXR-δTE-13′-COOH complex confirmed that δTE-13′-COOH binds to the PXR ligand binding pocket of LBD. These data demonstrate that PXR specifically recognizes δTE-13′-COOH. Consistent with in vitro data, mice treated with δTE-13′-COOH had increased expression of PXR, CYP3A4 and multidrug resistance protein (MDR1) [87].
Overall, the interaction of 13′-COOHs and tocotrienols as well as αT with PXR may have impact on drug metabolism, e.g., limiting the bioavailability of the concurrent intake of drugs including these compounds themselves. This possibility should be considered when combinatory drugs are developed.
4.9. Summary of the bioactivities of vitamin E metabolites
The existing literature indicate that 13′-COOHs have anti-inflammatory effects via competitively inhibiting COXs- and 5-LOX-catalyzed eicosanoids, and may modulate drug and lipid metabolism via activation of PXR and PPARs, respectively. These actions do not require long-time incubation with cells or enzymes, and may therefore exert bioactivities in vivo. In contrast, studies with relatively long-incubation with cells have revealed that 13′-COOHs have anti-proliferation and pro-apoptotic effects on cancer cells and decrease iNOS, nitrite or lipid accumulation in cells (Figure. 4).
5. Disease-preventing effects by γ-CEHC and 13′-COOHs in animal models
Based on anti-inflammatory and anticancer effects observed in enzyme- and cell-based research, studies have been carried out to delineate potential in vivo bioactivities of CEHC and 13′-COOHs in animal models. In 2003, we showed that consistent with inhibition of COX-2 activity in cells, γ-CEHC decreased carrageenan-induced increase of PGE2 in the air pouch inflammation model in rats [67]. Since δTE-13′-COOH inhibits both COXs and 5-LOX and shows anti-proliferation of colon cancer cells, we examined potential cancer preventive effects of this compound in mice. We observe that δTE-13′-COOH at 0.022% in diet (220 ppm or ~25 mg/kg) significantly suppressed azoxymethane (AOM) and dextran sulfate sodium (DSS)-induced colitis-associated colon cancer in mice, as indicated by decreased the multiplicity of total and large-size tumors [68]. This study represents the first in vivo evidence that a long-chain carboxychromanol can block tumor development. In a subsequent study, we found that δTE-13′-COOH at 0.04% diet blocked tumor development and showed anti-inflammatory effects by decreasing pro-inflammatory cytokines in the same murine colon cancer model induced by AOM/DSS [25]. Moreover, δTE-13′-COOH modulated the gut microbiota including separating gut microbial composition (β-diversity) from that in mice fed the control diet, and increased Lactococcus, which include species shown to have anti-cancer effects [25]. Interestingly, the changes of Lactococcus and Lachnospiraceae NK4A136 group uncultured bacterium positively correlate with fecal concentrations of δTE-13′-COOH. The impact of δTE-13′-COOH on the gut microbiota shows new activity of this compound and warrants further investigation.
Given that αT-13′-COOH inhibits 5-LOX, Pein et al. [54] conducted in vivo studies investigating the effect αT-13′-COOH on inflammation. Specifically, αT-13′-COOH at 10 mg/kg via intraperitoneal injection (i.p.) attenuated zymosan-induced murine peritonitis, as indicated by decreases of LTB4 and immune cell infiltration. Furthermore, αT-13′-COOH (10 mg/kg, i.p.) inhibited bronchial hyper-reactivity and LTC4 in ovalbumin-sensitized mice [54]. Moreover, the impact of δTE-13′-COOH on atherosclerosis was tested in the ApoE−/− mice fed high-fat diet. δTE-13′-COOH did not significantly affect atherosclerotic plaque morphology including lesion area, lipid accumulation and vascular cell adhesion molecule 1, but significantly attenuated 3-nitro-tyrosine, a marker of reactive nitrogen species [88]. Recently, Hoff et al. [89] reported that topical application of αT-13′-COOH and δTE-13′-COOH to wounds accelerated wound healing and improved the quality of the newly formed tissues.
Since δTE-13′-COOH is a specific ligand for PXR [87] that is believed to play a role in β-amyloid (Aβ) clearance through P-glycoprotein dependent transport at the blood brain barrier [90], Marinelli et al. [91] investigated potential impact of this compound on Aβ deposition in the mouse brain. Specifically, TgCRND8 mice with double mutant genes of APP695 were given 200 mg/kg δTE-13′-COOH in olive oil. δTE-13′-COOH treatment prevented the formation and reduced the size of Aβ plaques throughout the brain, and this effect was more pronounced in older mice (14–15 months old) than younger mice (5-month old), suggesting potential benefits for preventing Alzheimer’s diseases. In addition to these observations in animals, δTE-13′-COOH reduced Aβ aggregation and accumulation in mouse cortical astrocytes, increased expression of PPAR-γ and PXR, and enhanced apolipoprotein E (ApoE) efflux. Interestingly, δTE-13′-COOH-induced restoration of ApoE efflux was not affected by PPAR-γ antagonist, whereas δTE-13′-COOH activated PXR activity and upregulated PXR-downstream genes including MDR1, suggesting that δTE-13′-COOH provided protective effects via activation of PXR-mediated mechanism [91].
In addition to above-mentioned animal studies, potential link between metabolic dysregulation in vitamin E with human frailty was recently proposed. Rattray et al. [92] analyzed serum metabolomics from 1191 individuals (aged between 56 and 84 years old) and performed subsequent longitudinal validation. Through multivariate regression and network modeling, these investigators identified 12 significant metabolites, including three 13′-COOHs derived from tocotrienols, that differentiate frail and non-frail phenotypes. This observation suggests that dysregulation of vitamin E metabolism may play a role in the risk of frailty [92].
6. Summary and conclusion remarks
During the last 20 years, there has been great advance in our understanding of vitamin E metabolism and new functions of metabolites. First, based on the identified structures of initial, intermediate and terminal metabolites, it is now recognized that vitamin E forms are metabolized via ω-hydroxylation to form 13′-OH and 13′-COOH, the latter of which is further catabolized via β-oxidation to various carboxychromanols including the terminal metabolite CEHCs and conjugated carboxychromanols. Studies in animals indicate that 70% metabolites are excreted in feces as hydroxychromanols and carboxychromanols with 13′-COOHs being the most abundant, while 30% metabolites are excreted in the urine as conjugated CEHCs. Second, CYP4F2 has been identified as the key ω-hydroxylase initiating the metabolism of vitamin E forms. Studies in CYP4f14 knockout mice demonstrate that ω-hydroxylase-initiated metabolism is responsible for over 70% of formation of ω-series metabolites of vitamin E. Both CYP4F2 and TTP play important roles in differential metabolism of different forms of vitamin E, e.g., metabolites from tocotrienols > those from tocopherols, and within tocopherols, metabolites from γT or δT δT ≫ those from αT. In addition, mechanistic and animal studies have revealed biological activities and targets of 13′-COOHs. In particular, 13′-COOHs are inhibitors of COX-1/-2 and 5-LOX, and thus have anti-inflammatory effects. 13′-COOHs show anticancer actions, modulate cellular lipid accumulation, and activate nuclear receptors including PPARs and PXR, which are known to regulate lipid and drug metabolism, respectively (Fig. 4).
Given interesting bioactivities of these metabolites, an important question is what roles of the metabolites play in vitamin E’s functions. Previously, this author proposed that anti-inflammatory and anticancer activities by metabolites including 13′-COOHs may contribute to the beneficial effects of non-αT forms of vitamin E [1]. Subsequently, others proposed that formation of active metabolites via cytochrome P-450 mediated reactions is responsible for the activities of vitamin E, like vitamin A and D [93]. Considering contemporary knowledge of vitamin E metabolism and metabolites, this author proposes that metabolite formation plays distinct roles in αT vs. other forms of vitamin E mediated actions. Specifically, formation of metabolites likely contributes to disease-preventing effects of γT, δT and tocotrienols. However, metabolites from αT are minimal when its intake is below 50 mg. Even when the intake of αT exceeds 50 mg, metabolites’ contribution to αT’s function is still debatable. These points are elaborated below.
Vitamin E is essential for protecting membrane integrity because of its ability to scavenge radicals including lipid peroxyl radicals. For the lipophilic antioxidant activity in vivo, αT is the most important vitamin E because αT is much more bioavailable than other forms of vitamin E in tissues. In the membrane environment, hydroxychromanols or carboxychromanols would not likely fulfil lipophilic antioxidant function as well as αT due to their increased hydrophilicity. Indeed, tissue retention of αT is ensured by its high binding affinity to TTP and low catabolic activity by CYP4F2. Vitamin E deficiency leads to muscle weakness, anemia, vitamin E deficienc-associated ataxia and infertility. To prevent the deficiency, the vitamin E dietary reference intake (DRI) has been recommended with an estimated daily allowance (RDA) of 15 mg (for >14 y age) [94]. When αT intake is αT intake is at the RDA level or below 30–50 mg, metabolites formed have been reported to be minimal [17] and will therefore be insignificant to αT’s functions. Under relatively high supplemental doses (50 mg or more), metabolites from αT are detected in the plasma and urine. However, metabolites may contribute less to αT’s actions than other vitamin E forms because of relatively lower bioavailability due to poorer CYP4F2 activity toward αT. Many cited human studies in this review observe less metabolites formed from αT than those from γT. In a recent study where mice were supplemented with αT or γT in diets, αT-derived metabolites including αT-13′-COOH are much lower than those from γT and higher quantities of αT than γT were excreted as the intact form in feces [53]. This observation confirms relatively limited metabolism of αT compared with γT. Having said these, the metabolites may contribute to αT’s in vivo effects if they can be generated in tissues, which warrants investigation.
Metabolites likely play roles in γT, δT and tocotrienols mediated anti-inflammatory and disease-preventing effects in vivo. γT, δT and tocotrienols are preferentially metabolized compared with αT as a result of their poor binding to TTP and preferentially being catabolized by CYP4F2. Consistently, studies in animals and humans show that γT, δT and tocotrienols have much lower tissue retention and more metabolites formed than αT [1] (see section 3). Interestingly, Goodin et al. [95] recently showed that γ- or δ-CEHC and γT- or δT-5′-COOH were significantly elevated in prostate tissues as a result of supplementation of γT-rich vitamin E mixture in men diagnosed with localized prostate cancer. These data demonstrate that the metabolites are bioavailable in tissues in addition to the plasma or urine excretion in humans. Equally importantly, metabolites including 13′-COOHs have anti-inflammatory and anticancer effects. Further evidence supporting the idea that metabolites likely contribute to beneficial effects of γT, δT or tocotrienols is that supplementation of these vitamin E forms has been shown to attenuate diseases more effectively than αT in preclinical animal models, despite their poorer bioavailability than αT. For instance, γT and δT inhibit colon and breast cancer more effectively than αT in rodents [96,97]. We recently show that δTE-13′-COOH is slightly stronger than δTE in suppressing colitis-associated colon tumorigenesis and attenuating cytokines in mice [25]. In this study, δTE-13′-COOH and δTE similarly separated gut microbial composition from control diet and increased Lactococcus, suggesting that these effects are not dependent on δTE and may at least in part stem from δTE-13′-COOH and related metabolites.
Despite recent advance in our understanding of vitamin E metabolism, there are remaining questions concerning metabolites and their roles in vitamin E’s functions. First, although amber literature have documented metabolite formation in the plasma and urine excretion (fecal excretion in animals) as a result of supplementation of vitamin E forms, few studies measured the concentrations of metabolites in tissues and there is limited information of long-chain metabolites in humans. This knowledge gap should be addressed using LC-MS/MS to quantify vitamin E metabolomics [43,51]. Second, although mechanistic studies have identified bioactivities of 13′-COOHs, research should be conducted to further examine disease preventing effects of these compounds in animal models. Further, the role of metabolites in tocopherols and tocotrienols’ mediated protective effects should be further delineated. Third, our recently published data suggest potential interaction of δTE-13′-COOH with the gut microbiota [25], which should be further explored. In addition, metabolites and their precursors appear to have different activities. For instance, 13′-COOHs inhibit COXs and 5-LOX activity, whereas tocopherols do not directly inhibit 5-LOX or COX-2. Tocotrienols including δTE and γTE are known to inhibit activation of nuclear factor (NF)-κB and appear to be stronger than δTE-13′-COOH in blocking cancer cell growth [99,100]. Because of these distinct activities, the possibility that metabolites and vitamin E forms complement each other to exert health benefits should be evaluated.
Funding
The author gratefully acknowledges the support of USDA Hatch 1022869 and Research Awards from the Purdue Center for Cancer Research, NIH grant P30 CA023168.
Abbreviations:
- ABCA1
ATP-binding cassette A1
- AOM
azoxymethane
- αT-, δT-, δTE-13′-COOH
13′ -carboxychromanol derived from αT, δT and δTE, respectively
- αT, βT, γT and δT
α-, β-, γ- and δ-tocopherol
- αTE, βTE, γTE and δTE
α-, β-, γ- and δ-tocotrienol
- CEHC
carboxyethyl-hydroxychroman
- COX-1/-2
cyclooxygenase-1/-2
- CYP4F2
cytochrome P450 omega-hydroxylase encoded by the gene CYP4F2
- DES
dihydroceramide desaturase
- DSS
dextran sulfate sodium
- 13′-OH
13′-hydroxychromanol
- iNOS
inducible nitric oxide synthase
- 5-LOX
5-lipoxygenase
- LTB4
leukotriene B4
- MDR
multidrug resistance protein MDR
- PGE2
prostaglandin E2
- PPAR-γ
Peroxisome proliferator-activated receptor-γ
- PXR
Pregnane X receptor
- TxA2 (B2)
thromboxane A2 (B2)
- TTP
tocopherol transport protein
References
- [1].Jiang Q, Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy, Free Radic. Biol. Med 72 (2014) 76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Reboul E, Vitamin E bioavailability: mechanisms of intestinal absorption in the spotlight, Antioxidants 6 (4) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jiang Q, Christen S, Shigenaga MK, Ames BN, gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention, Am. J. Clin. Nutr 74 (6) (2001) 714–722. [DOI] [PubMed] [Google Scholar]
- [4].Traber MG, Burton GW, Ingold KU, Kayden HJ, RRR- and SRR-alpha-tocopherols are secreted without discrimination in human chylomicrons, but RRR-alpha-tocopherol is preferentially secreted in very low density lipoproteins, J. Lipid Res 31 (4) (1990) 675–685. [PubMed] [Google Scholar]
- [5].Traber MG, Burton GW, Hughes L, Ingold KU, Hidaka H, Malloy M, Kane J, Hyams J, Kayden HJ, Discrimination between forms of vitamin E by humans with and without genetic abnormalities of lipoprotein metabolism, J. Lipid Res 33 (8) (1992) 1171–1182. [PubMed] [Google Scholar]
- [6].Traber MG, Vitamin E regulatory mechanisms, Annu. Rev. Nutr 27 (2007) 347–362. [DOI] [PubMed] [Google Scholar]
- [7].Anwar K, Iqbal J, Hussain MM, Mechanisms involved in vitamin E transport by primary enterocytes and in vivo absorption, J. Lipid Res. 48 (9) (2007) 2028–2038. [DOI] [PubMed] [Google Scholar]
- [8].Reboul E, Vitamin E intestinal absorption: regulation of membrane transport across the enterocyte, IUBMB Life 71 (4) (2019) 416–423. [DOI] [PubMed] [Google Scholar]
- [9].Traber MG, Kayden HJ, Preferential incorporation of alpha-tocopherol vs gamma-tocopherol in human lipoproteins, Am. J. Clin. Nutr 49 (3) (1989) 517–526. [DOI] [PubMed] [Google Scholar]
- [10].Traber MG, Olivecrona T, Kayden HJ, Bovine milk lipoprotein lipase transfers tocopherol to human fibroblasts during triglyceride hydrolysis in vitro, J. Clin. Invest 75 (5) (1985) 1729–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Burton GW, Traber MG, Acuff RV, Walters DN, Kayden H, Hughes L, Ingold KU, Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E [see comments], Am. J. Clin. Nutr 67 (4) (1998) 669–684. [DOI] [PubMed] [Google Scholar]
- [12].Jiang Q, Freiser H, Wood KV, Yin X, Identification and quantitation of novel vitamin E metabolites, sulfated long-chain carboxychromanols, in human A549 cells and in rats, J. Lipid Res 48 (5) (2007) 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Freiser H, Jiang Q, Gamma-tocotrienol and gamma-tocopherol are primarily metabolized to conjugated 2-(beta-carboxyethyl)-6-hydroxy-2,7,8-trimethylchroman and sulfated long-chain carboxychromanols in rats, J. Nutr 139 (5) (2009) 884–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Freiser H, Jiang Q, Optimization of the enzymatic hydrolysis and analysis of plasma conjugated gamma-CEHC and sulfated long-chain carboxychromanols, metabolites of vitamin E, Anal. Biochem 388 (2) (2009) 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Evans HM, Bishop KS, On the existence of a hitherto unrecognized dietary factor essential for reproduction, Science 56 (1922) 650–651. [DOI] [PubMed] [Google Scholar]
- [16].Simon EJ, Eisengart A, Sundheim L, Milhorat AT, The metabolism of vitamin E. II. Purification and characterization of urinary metabolites of alpha-tocopherol, J. Biol. Chem 221 (2) (1956) 807–817 [PubMed] [Google Scholar]
- [17].Schultz M, Leist M, Petrzika M, Gassmann B, Brigelius-Flohe R, Novel urinary metabolite of alpha-tocopherol, 2,5,7,8-tetramethyl-2(2’-carboxyethyl)-6-hydroxychroman, as an indicator of an adequate vitamin E supply? Am. J. Clin. Nutr 62 (6 Suppl) (1995) 1527S–1534S. [DOI] [PubMed] [Google Scholar]
- [18].Chiku S, Hamamura K, Nakamura T, Novel urinary metabolite of d-delta-tocopherol in rats, J. Lipid Res 25 (1) (1984) 40–48. [PubMed] [Google Scholar]
- [19].Wechter WJ, Kantoci D, Murray ED Jr., D’Amico DC, Jung ME, Wang WH, A new endogenous natriuretic factor: LLU-α, in: Proceedings of the National Academy of Sciences of the United States of America 93, 1996, pp. 6002–6007, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Traber MG, Elsner A, Brigelius-Flohe R, Synthetic as compared with natural vitamin E is preferentially excreted as alpha-CEHC in human urine: studies using deuterated alpha-tocopheryl acetates, FEBS Lett. 437 (1-2) (1998) 145–148. [DOI] [PubMed] [Google Scholar]
- [21].Swanson JE, Ben RN, Burton GW, Parker RS, Urinary excretion of 2,7, 8-trimethyl-2-(beta-carboxyethyl)-6-hydroxychroman is a major route of elimination of gamma-tocopherol in humans, J. Lipid Res 40 (4) (1999) 665–671. [PubMed] [Google Scholar]
- [22].Stahl W, Graf P, Brigelius-Flohe R, Wechter W, Sies H, Quantification of the alpha- and gamma-tocopherol metabolites 2,5,7, 8-tetramethyl-2-(2’-carboxyethyl)-6-hydroxychroman and 2,7, 8-trimethyl-2-(2’-carboxyethyl)-6-hydroxychroman in human serum, Anal. Biochem 275 (2) (1999) 254–259. [DOI] [PubMed] [Google Scholar]
- [23].Sontag TJ, Parker RS, Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status, J. Biol. Chem 277 (28) (2002) 25290–25296. [DOI] [PubMed] [Google Scholar]
- [24].Birringer M, Pfluger P, Kluth D, Landes N, Brigelius-Flohe R, Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells, J. Nutr 132 (10) (2002) 3113–3118. [DOI] [PubMed] [Google Scholar]
- [25].Yang C, Zhao Y, Im S, Nakatsu C, Jones-Hall Y, Jiang Q, Vitamin E delta-tocotrienol and metabolite 13’-carboxychromanol inhibit colitis-associated colon tumorigenesis and modulate gut microbiota in mice, J. Nutr. Biochem 89 (2021) 108567. [DOI] [PubMed] [Google Scholar]
- [26].Liu KY, Jiang Q, Tocopherols and tocotrienols are bioavailable in rats and primarily excreted in feces as the intact forms and 13’-carboxychromanol metabolites, J. Nutr 150 (2) (2020) 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mustacich DJ, Leonard SW, Patel NK, Traber MG, Alpha-tocopherol beta-oxidation localized to rat liver mitochondria, Free Radic. Biol. Med 48 (1) (2010) 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hashiguchi T, Kurogi K, Sakakibara Y, Yamasaki M, Nishiyama K, Yasuda S, Liu MC, Suiko M, Enzymatic sulfation of tocopherols and tocopherol metabolites by human cytosolic sulfotransferases, Biosci. Biotechnol. Biochem. 75 (10) (2011) 1951–1956. [DOI] [PubMed] [Google Scholar]
- [29].Bardowell SA, Duan F, Manor D, Swanson JE, Parker RS, Disruption of mouse cytochrome p450 4f14 (Cyp4f14 gene) causes severe perturbations in vitamin E metabolism, J. Biol. Chem 287 (31) (2012) 26077–26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brigelius-Flohe R, Chapter 14: Metabolism of Vitamin E, Food Chemistry, Funtion and Analysis 11. Vitamin E: Chemistry and Nutritional Benefits Ed, Etsuo Niki(The Royal Society of Chemistry; ), 2019, pp. 189–207. [Google Scholar]
- [31].Bardowell SA, Ding X, Parker RS, Disruption of P450-mediated vitamin E hydroxylase activities alters vitamin E status in tocopherol supplemented mice and reveals extra-hepatic vitamin E metabolism, J. Lipid Res 53 (12) (2012) 2667–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sontag TJ, Parker RS, Influence of major structural features of tocopherols and tocotrienols on their {omega}-oxidation by tocopherol-{omega}-hydroxylase, J. Lipid Res 48 (5) (2007) 1090–1098. [DOI] [PubMed] [Google Scholar]
- [33].Qian J, Morley S, Wilson K, Nava P, Atkinson J, Manor D, Intracellular trafficking of vitamin E in hepatocytes: the role of tocopherol transfer protein, J. Lipid Res 46 (10) (2005) 2072–2082. [DOI] [PubMed] [Google Scholar]
- [34].Chung S, Ghelfi M, Atkinson J, Parker R, Qian J, Carlin C, Manor D, Vitamin E and phosphoinositides regulate the intracellular localization of the hepatic alpha-tocopherol transfer protein, J. Biol. Chem 291 (33) (2016) 17028–17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Oram JF, Vaughan AM, Stocker R, ATP-binding cassette transporter A1 mediates cellular secretion of alpha-tocopherol, J. Biol. Chem 276 (43) (2001) 39898–39902. [DOI] [PubMed] [Google Scholar]
- [36].Reboul E, Trompier D, Moussa M, Klein A, Landrier JF, Chimini G, Borel P, ATP-binding cassette transporter A1 is significantly involved in the intestinal absorption of alpha- and gamma-tocopherol but not in that of retinyl palmitate in mice, Am. J. Clin. Nutr 89 (1) (2009) 177–184. [DOI] [PubMed] [Google Scholar]
- [37].Smith KS, Lee CL, Ridlington JW, Leonard SW, Devaraj S, Traber MG, Vitamin E supplementation increases circulating vitamin E metabolites tenfold in end-stage renal disease patients, Lipids 38 (8) (2003) 813–819. [DOI] [PubMed] [Google Scholar]
- [38].Brigelius-Flohe R, Induction of drug metabolizing enzymes by vitamin E, J. Plant Physiol 162 (7) (2005) 797–802. [DOI] [PubMed] [Google Scholar]
- [39].Podszun MC, Jakobi M, Birringer M, Weiss J, Frank J, The long chain alpha-tocopherol metabolite alpha-13’-COOH and gamma-tocotrienol induce P-glycoprotein expression and activity by activation of the pregnane X receptor in the intestinal cell line LS 180, Mol. Nutr. Food Res 61 (3) (2017). [DOI] [PubMed] [Google Scholar]
- [40].Bruno RS, Leonard SW, Li J, Bray TM, Traber MG, Lower plasma alpha-carboxyethyl-hydroxychroman after deuterium-labeled alpha-tocopherol supplementation suggests decreased vitamin E metabolism in smokers, Am. J. Clin. Nutr 81 (5) (2005) 1052–1059 [DOI] [PubMed] [Google Scholar]
- [41].Leonard SW, Paterson E, Atkinson JK, Ramakrishnan R, Cross CE, Traber M, Studies in humans using deuterium-labeled alpha- and gamma-tocopherols demonstrate faster plasma gamma-tocopherol disappearance and greater gamma-metabolite production, Free Radic. Biol. Med 38 (7) (2005) 857–866 [DOI] [PubMed] [Google Scholar]
- [42].Brigelius-Flohe R, Roob JM, Tiran B, Wuga S, Ribalta J, Rock E, Winklhofer-Roob BM, The effect of age on vitamin E status, metabolism, and function: metabolism as assessed by labeled tocopherols, Ann. N. Y. Acad. Sci 1031 (2004) 40–43. [DOI] [PubMed] [Google Scholar]
- [43].Jiang Q, Xu T, Huang J, Jannasch AS, Cooper B, Yang C, Analysis of vitamin E metabolites including carboxychromanols and sulfated derivatives using liquid chromatography tandem mass spectrometry, J. Lipid Res 56 (11) (2015) 2217–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Giusepponi D, Torquato P, Bartolini D, Piroddi M, Birringer M, Lorkowski S, Libetta C, Cruciani G, Moretti S, Saluti G, Galli F, Galarini R, Determination of tocopherols and their metabolites by liquid-chromatography coupled with tandem mass spectrometry in human plasma and serum, Talanta 170 (2017) 552–561. [DOI] [PubMed] [Google Scholar]
- [45].Wiser J, Alexis NE, Jiang Q, Wu W, Robinette C, Roubey R, Peden DB, In vivo gamma-tocopherol supplementation decreases systemic oxidative stress and cytokine responses of human monocytes in normal and asthmatic subjects, Free Radic. Biol. Med 45 (1) (2008) 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Burbank AJ, Duran CG, Almond M, Wells H, Jenkins S, Jiang Q, Yang C, Wang T, Zhou HB, Hernandez ML, Peden DB, A short course of gamma tocopherol mitigates LPS-induced inflammatory responses in humans ex vivo, J. Allergy Clin. Immunol 140 (4) (2017) 1179.(. -+). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Burbank AJ, Duran CG, Pan Y, Burns P, Jones S, Jiang Q, Yang C, Jenkins S, Wells N Alexis, M. Kesimer, W.D. Bennett, H. Zhou, D.B. Peden, M. L. Hernandez, Gamma tocopherol-enriched supplement reduces sputum eosinophilia and endotoxin-induced sputum neutrophilia in volunteers with asthma, J. Allergy Clin. Immunol 141 (4) (2018) 1231–1238 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mahipal A, Klapman J, Vignesh S, Yang CS, Neuger A, Chen DT, Malafa MP, Pharmacokinetics and safety of vitamin E delta-tocotrienol after single and multiple doses in healthy subjects with measurement of vitamin E metabolites, Cancer Chemother. Pharmacol 78 (1) (2016) 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Drotleff AM, Bohnsack C, Schneider I, Hahn A, Ternes W, Human oral bioavailability and pharmacokinetics of tocotrienols from tocotrienol-rich (tocopherol-low) barley oil and palm oil formulations, J Functional Foods 7 (2014) 150–160. [Google Scholar]
- [50].Ciffolilli S, Wallert M, Bartolini D, Krauth V, Werz O, Piroddi M, Sebastiani B, Torquato P, Lorkowski S, Birringer M, Galli F, Human serum determination and in vitro anti-inflammatory activity of the vitamin E metabolite alpha-(13’-hydroxy)-6-hydroxychroman, Free Radic. Biol. Med 89 (2015) 952–962. [DOI] [PubMed] [Google Scholar]
- [51].Bartolini D, Marinelli R, Giusepponi D, Galarini R, Barola C, Stabile AM, Sebastiani B, Paoletti F, Betti M, Rende M, Galli F, Alpha-tocopherol metabolites (the vitamin E metabolome) and their interindividual variability during supplementation, Antioxidants 10 (2) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Jiang Q, Jiang Z, Hall YJ, Jang Y, Snyder PW, Bain C, Huang J, Jannasch A, Cooper B, Wang Y, Moreland M, Gamma-tocopherol attenuates moderate but not severe colitis and suppresses moderate colitis-promoted colon tumorigenesis in mice, Free Radic. Biol. Med 65 (2013) 1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Liu KY, Nakatsu CH, Jones-Hall Y, Kozik A, Jiang Q, Vitamin E alpha- and gamma-tocopherol mitigate colitis, protect intestinal barrier function and modulate the gut microbiota in mice, Free Radic. Biol. Med 163 (2021) 180–189. [DOI] [PubMed] [Google Scholar]
- [54].Pein H, Ville A, Pace S, Temml V, Garscha U, Raasch M, Alsabil K, Viault G, Dinh CP, Guilet D, Troisi F, Neukirch K, Konig S, Bilancia R, Waltenberger B, Stuppner H, Wallert M, Lorkowski S, Weinigel C, Rummler S, Birringer M, Roviezzo F, Sautebin L, Helesbeux JJ, Seraphin D, Mosig AS, Schuster D, Rossi A, Richomme P, Werz O, Koeberle A, Endogenous metabolites of vitamin E limit inflammation by targeting 5-lipoxygenase, Nat. Commun 9 (1) (2018) 3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Terashima K, Takaya Y, Niwa M, Powerful antioxidative agents based on garcinoic acid from Garcinia kola, Bioorg. Med. Chem 10 (5) (2002) 1619–1625. [DOI] [PubMed] [Google Scholar]
- [56].Saito H, Kiyose C, Yoshimura H, Ueda T, Kondo K, Igarashi O, Gamma-tocotrienol, a vitamin E homolog, is a natriuretic hormone precursor, J. Lipid Res 44 (8) (2003) 1530–1535. [DOI] [PubMed] [Google Scholar]
- [57].Yoshikawa S, Morinobu T, Hamamura K, Hirahara F, Iwamoto T, Tamai H, The effect of gamma-tocopherol administration on alpha-tocopherol levels and metabolism in humans, Eur. J. Clin. Nutr 59 (8) (2005) 900–905. [DOI] [PubMed] [Google Scholar]
- [58].Funk CD, Prostaglandins and leukotrienes: advances in eicosanoid biology, Science 294 (5548) (2001) 1871–1875. [DOI] [PubMed] [Google Scholar]
- [59].Lebas H, Yahiaoui K, Martos R, Boulaftali Y, Platelets are at the nexus of vascular diseases, Front. Cardiovas. Med 6 (2019) 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ, Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity, Nature 410 (6827) (2001) 471–475. [DOI] [PubMed] [Google Scholar]
- [61].Vane JR, Prostaglandins as mediators of inflammation, Adv. Prostag. Thromboxane Res 2 (1976) 791–801. [PubMed] [Google Scholar]
- [62].Williams JA, Shacter E, Regulation of macrophage cytokine production by prostaglandin E2. Distinct roles of cyclooxygenase-1 and -2, J. Biol. Chem 272 (41) (1997) 25693–25699. [DOI] [PubMed] [Google Scholar]
- [63].Wang D, Dubois RN, Eicosanoids and cancer, Nat. Rev. Cancer 10 (3) (2010) 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN, gamma-tocopherol and its major metabolite, in contrast to alpha- tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells, in: Proceedings of the National Academy of Sciences of the United States of America 97, 2000, pp. 11494–11499, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jiang Q, Yin X, Lill MA, Danielson ML, Freiser H, Huang J, Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases, in: Proceedings of the National Academy of Sciences of the United States of America 105, 2008, pp. 20464–20469, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Grammas P, Hamdheydari L, Benaksas EJ, Mou S, Pye QN, Wechter WJ, Floyd RA, Stewart C, Hensley K, Anti-inflammatory effects of tocopherol metabolites, Biochem. Biophys. Res. Commun 319 (3) (2004) 1047–1052. [DOI] [PubMed] [Google Scholar]
- [67].Jiang Q, Ames BN, Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats, Faseb. J. : off. Publ. Federa. Am. Soc. Exp. Biol 17 (8) (2003) 816–822. [DOI] [PubMed] [Google Scholar]
- [68].Jang Y, Park NY, Rostgaard-Hansen AL, Huang J, Jiang Q, Vitamin E metabolite 13’-carboxychromanols inhibit pro-inflammatory enzymes, induce apoptosis and autophagy in human cancer cells by modulating sphingolipids and suppress colon tumor development in mice, Free Radic. Biol. Med 95 (2016) 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Park N, Im S, Jiang Q, Different forms of vitamin E and metabolite 13’-carboxychromanols inhibit cyclooxygenase-1 and its catalyzed thromboxane in platelets, and tocotrienols and 13’-carboxychromanols are competitive inhibitors of 5-lipoxygenase, J. Nutr. Biochem (2021) 108884, 10.1016/j.jnutbio.2021.108884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Radmark O, Werz O, Steinhilber D, Samuelsson B, 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease, Biochim. Biophys. Acta 1851 (4) (2015) 331–339. [DOI] [PubMed] [Google Scholar]
- [71].Yokomizo T, Izumi T, Shimizu T, Leukotriene B4: metabolism and signal transduction, Arch. Biochem. Biophys 385 (2) (2001) 231–241. [DOI] [PubMed] [Google Scholar]
- [72].Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN, Leukotrienes and lipoxins: structures, biosynthesis, and biological effects, Science 237 (4819) (1987) 1171–1176. [DOI] [PubMed] [Google Scholar]
- [73].Fanning LB, Boyce JA, Lipid mediators and allergic diseases, Ann. Allergy Asthma Immunol. : off. Pub. Am. College. Allergy, Asthma. Immunol 111 (3) (2013) 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Cheon EC, Khazaie K, Khan MW, Strouch MJ, Krantz SB, Phillips J, Blatner NR, Hix LM, Zhang M, Dennis KL, Salabat MR, Heiferman M, Grippo PJ, Munshi HG, Gounaris E, Bentrem DJ, Mast cell 5-lipoxygenase activity promotes intestinal polyposis in APCDelta468 mice, Cancer Res. 71 (5) (2011) 1627–1636. [DOI] [PubMed] [Google Scholar]
- [75].Jiang Z, Yin X, Jiang Q, Natural forms of vitamin E and 13’-carboxychromanol, a long-chain vitamin E metabolite, inhibit leukotriene generation from stimulated neutrophils by blocking calcium influx and suppressing 5-lipoxygenase activity, respectively, J. Immunol 186 (2) (2011) 1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Holdgate GA, Meek TD, Grimley RL, Mechanistic enzymology in drug discovery: a fresh perspective, Nature reviews, Drug Discovery 17 (2) (2018) 115–132. [DOI] [PubMed] [Google Scholar]
- [77].Mazzini F, Betti M, Netscher T, Galli F, Salvadori P, Configuration of the vitamin E analogue garcinoic acid extracted from Garcinia Kola seeds, Chirality 21 (5) (2009) 519–524. [DOI] [PubMed] [Google Scholar]
- [78].Birringer M, Lington D, Vertuani S, Manfredini S, Scharlau D, Glei M, Ristow M, Proapoptotic effects of long-chain vitamin E metabolites in HepG2 cells are mediated by oxidative stress, Free Radic. Biol. Med 49 (8) (2010) 1315–1322. [DOI] [PubMed] [Google Scholar]
- [79].Wallert M, Schmolz L, Koeberle A, Krauth V, Glei M, Galli F, Werz O, Birringer M, Lorkowski S, alpha-Tocopherol long-chain metabolite alpha-13’-COOH affects the inflammatory response of lipopolysaccharide-activated murine RAW264.7 macrophages, Mol. Nutr. Food Res 59 (8) (2015) 1524–1534. [DOI] [PubMed] [Google Scholar]
- [80].Schmolz L, Wallert M, Rozzino N, Cignarella A, Galli F, Glei M, Werz O, Koeberle A, Birringer M, Lorkowski S, Structure-function relationship studies in vitro reveal distinct and specific effects of long-chain metabolites of vitamin E, Mol. Nutr. Food Res 61 (12) (2017). [DOI] [PubMed] [Google Scholar]
- [81].Maguire EM, Pearce SWA, Xiao Q, Foam cell formation: a new target for fighting atherosclerosis and cardiovascular disease, Vase. Pharmacol 112 (2019) 54–71. [DOI] [PubMed] [Google Scholar]
- [82].Wallert M, Mosig S, Rennert K, Funke H, Ristow M, Pellegrino RM, Cruciani G, Galli F, Lorkowski S, Birringer M, Long-chain metabolites of alpha-tocopherol occur in human serum and inhibit macrophage foam cell formation in vitro, Free Radic. Biol. Med 68 (2014) 43–51. [DOI] [PubMed] [Google Scholar]
- [83].Kluge S, Schubert M, Bormel L, Lorkowski S, The vitamin E long-chain metabolite alpha-13’-COOH affects macrophage foam cell formation via modulation of the lipoprotein lipase system, Biochimica et biophysica acta, Mol. Cell. Biol. Lipids 1866 (4) (2021) 158875. [DOI] [PubMed] [Google Scholar]
- [84].Schmolz L, Schubert M, Kirschner J, Kluge S, Galli F, Birringer M, Wallert M, Lorkowski S, Long-chain metabolites of vitamin E: interference with lipotoxicity via lipid droplet associated protein PLIN2, Biochimica et biophysica acta, Mol. Cell. Biol. Lipids 1863 (8) (2018) 919–927. [DOI] [PubMed] [Google Scholar]
- [85].Silva R, Vilas-Boas V, Carmo H, Dinis-Oliveira RJ, Carvalho F, de Lourdes Bastos M, Remiao F, Modulation of P-glycoprotein efflux pump: induction and activation as a therapeutic strategy, Pharmacol. Therapeut 149 (2015) 1–123. [DOI] [PubMed] [Google Scholar]
- [86].Zhou C, Tabb MM, Sadatrafiei A, Grun F, Blumberg B, Tocotrienols activate the steroid and xenobiotic receptor, SXR, and selectively regulate expression of its target genes, Drug Metab. Dispos.: Biol. Fate. Chem 32 (10) (2004) 1075–1082. [DOI] [PubMed] [Google Scholar]
- [87].Bartolini D, De Franco F, Torquato P, Marinelli R, Cerra B, Ronchetti R, Schon A, Fallarino F, De Luca A, Bellezza G, Ferri I, Sidoni A, Walton WG, Pellock SJ, Redinbo MR, Mani S, Pellicciari R, Gioiello A, Galli F, Garcinoic acid is a natural and selective agonist of pregnane X receptor, J. Med. Chem 63 (7) (2020) 3701–3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Wallert M, Bauer J, Kluge S, Schmolz L, Chen YC, Ziegler M, Searle AK, Maxones A, Schubert M, Thurmer M, Pein H, Koeberle A, Werz O, Birringer M, Peter K, Lorkowski S, The vitamin E derivative garcinoic acid from Garcinia kola nut seeds attenuates the inflammatory response, Redox. Biol 24 (2019) 101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hoff J, Karl B, Gerstmeier J, Beekmann U, Schmolz L, Borner F, Kralisch D, Bauer M, Werz O, Fischer D, Lorkowski S, Press AT, Controlled release of the alpha-tocopherol-derived metabolite alpha-13’-carboxychromanol from bacterial nanocellulose wound cover improves wound healing, Nanomaterials 11 (8) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Chai AB, Leung GKF, Callaghan R, Gelissen IC, P-glycoprotein: a role in the export of amyloid-beta in Alzheimer’s disease? FEBS J 287 (4) (2020) 612–625. [DOI] [PubMed] [Google Scholar]
- [91].Marinelli R, Torquato P, Bartolini D, Mas-Bargues C, Bellezza G, Gioiello A, Borras C, De Luca A, Fallarino F, Sebastiani B, Mani S, Sidoni A, Vina J, Leri M, Bucciantini M, Nardiello P, Casamenti F, Galli F, Garcinoic acid prevents beta-amyloid (Abeta) deposition in the mouse brain, J. Biol. Chem 295 (33) (2020) 11866–11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Rattray NJW, Trivedi DK, Xu Y, Chandola T, Johnson CH, Marshall AD, Mekli K, Rattray Z, Tampubolon G, Vanhoutte B, White IR, Wu FCW, Pendleton N, Nazroo J, Goodacre R, Metabolic dysregulation in vitamin E and carnitine shuttle energy mechanisms associate with human frailty, Nat. Commun 10 (1) (2019) 5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Schubert M, Kluge S, Schmolz L, Wallert M, Galli F, Birringer M, Lorkowski S, Long-chain metabolites of vitamin E: metabolic activation as a general concept for lipid-soluble vitamins? Antioxidants 7 (1) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].E. Vitamin, Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/.
- [95].Goodin S, Kim I, Lee MJ, Shih WJ, Orlick M, Zheng X, Yang CS, Plasma, Prostate and Urine Levels of Tocopherols and Metabolites in Men after Supplementation with a Gamma-Tocopherol-Rich Vitamin E Mixture, Nutrition and Cancer, 2020, pp. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Das Gupta S, Patel M, Wahler J, Bak MJ, Wall B, Lee MJ, Lin Y, Shih WJ, Cai L, Yang CS, Suh N, Differential gene regulation and tumor-inhibitory activities of alpha-, delta-, and gamma-tocopherols in estrogen-mediated mammary carcinogenesis, Cancer Prev. Res 10 (12) (2017) 694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Guan F, Li G, Liu AB, Lee MJ, Yang Z, Chen YK, Lin Y, Shih W, Yang CS, delta- and gamma-tocopherols, but not alpha-tocopherol, inhibit colon carcinogenesis in azoxymethane-treated F344 rats, Cancer Prev. Res 5 (4) (2012) 644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Willems S, Gellrich L, Chaikuad A, Kluge S, Werz O, Heering J, Knapp S, Lorkowski S, Schubert-Zsilavecz M, Merk D, Endogenous vitamin E metabolites mediate allosteric PPARgamma activation with unprecedented co-regulatory interactions, Cell. Chem. Biol 28 (10) (2021) 1489–1500, e8. [DOI] [PubMed] [Google Scholar]
- [99].Yang C, Jiang Q, Vitamin E delta-tocotrienol inhibits TNF-alpha-stimulated NF-kappaB activation by up-regulation of anti-inflammatory A20 via modulation of sphingolipid including elevation of intracellular dihydroceramides, J. Nutr. Biochem 64 (2018) 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Jiang Q, Natural forms of vitamin E and metabolites-regulation of cancer cell death and underlying mechanisms, IUBMB Life 71 (4) (2019) 495–506. [DOI] [PubMed] [Google Scholar]
- [101].Zhao Y, Lee MJ, Cheung C, Ju JH, Chen YK, Liu B, Hu LQ, Yang CS, Analysis of multiple metabolites of tocopherols and tocotrienols in mice and humans, J. Agric. Food Chem 58 (8) (2010) 4844–4852. [DOI] [PMC free article] [PubMed] [Google Scholar]


