Abstract
Fava bean (Vicia faba L.) is a high-protein crop consumed worldwide and is an exceptional plant-based protein source for human consumption. The present study evaluated in vitro nutritional properties of four different protein flours of fava bean: minimal processed flour (MPF), cooked flour (CF), non-polyphenol protein concentrate (NPP), and polyphenol-protein concentrate (PP). NPP showed the highest protein concentration of 94.39 ± 0.76%. The heat treatment significantly increased the in vitro protein digestibility in CF (94.15 ± 2.45%). NPP and PP showed the highest bioaccessibility, 29.85 ± 1.88 and 33.19 ± 1.65%, respectively, no significant differences. SDS-PAGE analysis revealed bioaccessible low molecular weight peptides (<15 kDa) and legumin and vicilin presence. In silico analysis of bioactive peptides of legumin and vicilin presented high occurrence frequencies of bioactivities, as angiotensin-converting enzyme-inhibitor and dipeptidyl peptidase III/IV inhibitor peptides. This study supports the use and further investigation of fava bean proteins for human nutrition.
Keywords: Fava bean, Vicia faba, Fava bean flour, Food proteins, Bioactive peptides, In vitro protein digestibility, In vitro protein bioaccesibility
1. Introduction
There is a significant increase in the demand for animal-based protein due to the increase in population and a higher standard of living in developing countries, expected to duplicate by 2050 (United Nations, 2015). Therefore, it is relevant to evaluate alternative protein sources and their functional and nutritional qualities. Vegetable proteins, especially legume proteins, are considered viable options to substitute animal-based proteins (Alves & Tavares, 2019). Furthermore, diversifying protein sources in dietary intake and increasing the consumption of plant proteins could reduce the health and environmental risks associated with the production and excessive consumption of animal-based protein (Berrazaga et al., 2020). In addition, various legume seed proteins and peptides can now be included in the category of nutraceuticals or functional components due to biofunctionality (Carbonaro et al., 2015).
Fava bean (Vicia faba L.) is one of the oldest and most valued crops for human nutrition, occupying the fifth place of production among different legumes in the world. Its relevance in the diet lies in its high content of proteins and bioactive compounds, demonstrating its potential to maintain human health and prevent diseases (FAO, 2016). Compared with cereals, dry fava bean seeds have high content of lysine (19.8 g / kg of dry matter), and low content of methionine, cysteine, and tryptophan (2.6, 3.7, and 2.7 g / kg of matter dry, respectively) (Vioque et al., 2012). Therefore, the study of fava bean proteins has interest due to their nutritional and functional value. In addition, the protein extract from the fava bean can be used as a powder in different food products (Alpizar-Reyes et al., 2018). Hence, fava bean extract would generate protein hydrolysates by producing bioactive peptides, creating new food applications by modifying their biological, nutritional, and functional properties.
Bioactive peptides in fava beans present interesting properties. Reports include in vitro antiproliferative activity towards Hep G2 hepatoma cells from the VFTI-G1 polypeptide isolated from fava beans with trypsin inhibitory activity (Fei Fang et al., 2011), improvement of in vivo metabolic alterations induced by feeding with a hypercholesterolemic diet in rats (Macarulla et al., 2001), and antioxidant capacity of the peptides released from the hydrolyzed fava bean protein isolate due to the ferrous chelating activity, as well as inhibitory activity of tyrosinase enzyme (Karkouch et al., 2017). In addition, they can also be considered a natural source of L-3,4-dihydroxyphenylalanine (l-DOPA), the precursor to the neurotransmitter catecholamine and a drug used in Parkinson's disease (Turco et al., 2016). However, to explain the mechanisms regulated by food-derived bioactive peptides it is necessary to understand the functional role they can assume, thus, representing a promising area for future research (Martínez-Sánchez et al., 2020). This aim of this study was to provide scientific information on the digestibility and bioaccessibility of protein flours of fava bean seeds following in silico and in vitro methodologies to validate their functional properties as human food.
2. Material and methods
2.1. Chemical and reagents
Ultrapure (type 1) water obtained from Simplicity® Water Purification System (Millipore Corporation, Milford) was used for the preparation of all reagent solutions, buffers, and sample preparations. High purity analytical enzyme preparations, including pepsin (P7125, from porcine gastric mucosa), pancreatin (P1750, 4 × USP, porcine pancreas), bile (B8631, extract porcine), as well as the tubular cellulose membrane for dialysis, were purchased from Sigma-Aldrich Co. LLC. Electrophoresis purity reagents including Acrylamide, Sodium dodecyl sulfate (SDS), 2-Mercaptoethanol, Bromophenol Blue, Bis (N,N'-Methylen–bis-acrylamide), Coomassie Brilliant Blue G-250 protein stain powder, N,N,N'N'-Tetra-methylenediamine, and Tris bas were supplied by BIO-RAD (Bio-Rad Laboratories, Inc.). All the experimental analyzes were carried out in triplicate.
2.2. Materials
The mature fava bean seeds were provided by producers of the rural area of Puebla, Mexico. The seeds were washed and disinfected, and the damaged seeds were discarded. The dried fava beans were stored at 4° C and protected from light until they were processed.
2.2.1. Cooked (CF) and minimal processed (MPF) fava bean flour
The obtention of cooked fava bean (CF) was followed according to the methodology proposed by Khalil (2001) with modifications. First, fava bean seeds were soaked in distilled water (1:5 w/v) and kept under refrigeration at 4° C for 12 h. Once the soaking time was completed, they were rinsed three times with distilled water and allowed to drain, then placed in 1 L beakers adding 100 mL of distilled water for autoclaving (Yamato Scientific America Inc., SM300) at 121° C for 15 min. Once cooked, the seeds were dried in an electric oven (Felisa FE-292D) for 48 h at 50° C and were constantly stirred to ensure uniform drying. For the obtention of flour, firstly, the cooked and dried seeds were crushed (Oster BEST02-E01) and grounded (IKA® A 11 basic analytical mill). The resulting flour was sifted through a mesh sieve (850 μm) to obtain a uniform flour. Defatting of flour was carried out according to Serrano-Sandoval et al. (2019). Hexane (1:4 w/v) was added to the samples followed by constant stirring at 250 rpm for 4 h at room temperature. Suspension was filtered under vacuum through a glass microfiber filter (Whatman 1827–047 model) and dried for 24 h in a level 2 laminar flow hood (AirClean® Systems). The sample was stored in polyethylene bags at room temperature. For the minimal processed (MPF) fava bean flour preparation, seeds were dried in an electric oven at 70° C for 24 h, then crushed and grounded and finally were sifted through a mesh sieve (850 μm) to obtain a uniform flour.
2.2.2. Protein (NPP, PP) fava bean concentrates
For the fava bean protein extraction, the method proposed by Sair (1959) was used with some modifications (Hernández-García et al., 2016, Vioque et al., 2012). Elimination of non-protein compounds was carried out using an alkaline solution and the obtention of the protein fraction was achieved by acid precipitation (pH 4) with the isoelectric point of the fava bean protein. Two treatments were evaluated: a) non-polyphenols protein concentrate (NPP) and b) polyphenol protein concentrate (PP). The extraction of polyphenols in NPP was followed by stirring the defatted flour (10% w/v) with 75% acetone in an orbital shaker (Sheldon Manufacturing, Inc., 1365 PC) at 280 rpm at room temperature for 24 h. The suspension was centrifuged at 13,520 g at 22° C for 20 min, the supernatant was discarded, and the pellet was recovered. After this, the pellet was subjected to the alkaline phase (10% w/v) with 0.25% Na2SO3, adjusting the pH to 10.5 with 1 M NaOH and was left stirring at 280 rpm at room temperature for 24 h. The suspension was centrifuged at 13,520 g at 4 °C for 20 min. The soluble proteins in the supernatant were precipitated with the isoelectric point (pH 4 with 12 M HCl) of the fava bean protein. Suspension was centrifuged under the same previous conditions to recover the pellet and discard the supernatant. The protein pellet was dried in the oven at 50 °C for 16 h. For PP, the procedure was the same, omitting the extraction of phenolic compounds.
2.3. Chemical and nutritional characteristics
2.3.1. Proximate composition
Moisture, fat, ash, crude fiber, and protein were determined using AOAC International (2019) methods 14.003, 7.056, 14.006, 962.09, 992.23, respectively. The N-free extract was abstained by subtracting the percentages calculated for each nutrient from 100 (FAO, 1993). The determination of the amino acid score of the samples (CF, MPF, NPP and PP) was carried out through the method of Vázquez-Ortiz et al. (1995).
2.3.2. Amino acid score (AAS)
The lowest essential amino acid in the sample represented the limiting essential amino acid. The AAS was determined as the ratio between the limiting essential amino acid content of the samples and that of the reference protein established by FAO / WHO using the amino acid requirement for children 3 to 10 years of age (FAO, 2013).
According to FAO (2013), the AAS calculation was made with slight modifications proposed by Le Roux et al., (2020). The equation was the following:
2.3.3. Estimated protein efficiency ratio (E-PER)
The estimated protein efficiency ratio (E-PER) values were calculated from the amino acid composition of the samples based on the following equation developed by Alsmeyer et al., (1974).
2.3.4. In vitro protein digestibility (IVPD)
According to the standardized international consensus protocol INFOGEST (Brodkorb et al., 2019) with slight modifications. Solutions were prepared to simulate the electrolyte concentration in the physiological fluids of human digestion for simulated salivary fluid (SSF), simulated gastric fluid (SGF), and simulated intestinal fluid (SIF) (Supplementary material Table S1 indicates the concentrations and phases). In vitro protein digestibility (IVPD) was expressed as the percentage of the protein content before digestion and the protein content in the supernatant after digestion according to the method proposed by Li et al., (2017) with slight modifications. First, 1 mL was taken from each digested sample (intestinal phase) and was transferred to a 1.5 mL Eppendorf™ microtube to be centrifuged at 15,000 rpm for 10 min at room temperature. The precipitate was considered as the insoluble part that is potentially non-absorbable, while the supernatant contains the soluble potentially absorbable proteins. For protein quantification in the supernatant, the previously described method was proposed by Scopes (1974).
The degree of digestibility was calculated as follows:
Where is the protein content of the sample without treatment before digestion; is the protein content in the sample precipitate after intestinal digestion.
2.3.5. In vitro protein digestibility-corrected amino acid score (IV-PDCAAS)
The in vitro protein digestibility-corrected amino acid score (IV-PDCAAS) was calculated by adapting the FAO (2013) methodology:
2.3.6. In vitro protein bioaccessibility (IVPB)
The absorption processes were simulated employing a static dialysis procedure with a cellulose membrane following the method described by Managa et al. (2021) with slight modifications. First, the digested intestinal phase samples (8 mL) were poured into a tubular cellulose membrane for dialysis (D9652, Sigma-Aldrich), previously hydrated in distilled water for 10 min, as a simplified model of the epithelial barrier. Then, each dialysis bag was placed inside a 125 mL flask and were totally immersed with 40 mL of SIF. This mixture was kept covered at 37° C in a water bath for 120 min with gentle manual shaking every 15 min. The formula used to calculate the bioaccessibility of the digested protein was (Liu et al., 2021):
Where is the bioaccessible fraction in percentage; is the dialyzed protein of the digested samples; is the protein content in the initial undigested sample.
2.3.7. In vitro kinetics release of protein
The protein release kinetics of the digested samples was determined according to the method proposed by Perales-Vázquez et al. (2020) with slight modifications. Aliquots of 5 µL were collected every 15 min from the SIF with the proteins released from dialysis bags into the flask for later analysis. The protein quantification of the collected samples was according to Scopes (1974).
To determine the release kinetics of the digested protein in the intestinal phase of in vitro digestion, the following equation was used:
Where is the final release rate of the digested protein during release kinetics (mg/mL per min); is the difference of the initial protein concentration and the final protein concentration; is the difference of specific time with the initial time.
2.4. Electrophoresis (SDS–PAGE)
Protein from samples was analyzed with one-dimensional denaturing SDS-PAGE with a discontinuous buffer system as Laemmli (1970) described with slight modifications (Xing et al., 2017) using Mini-PROTEAN® 3 Cell system. The reagents and stock solutions for SDS-PAGE (12% resolving and 4% stacking) were prepared according to the Bio-Rad Laboratories manual (2014). 25 µL of each sample were taken and diluted 1:2 with sample buffer with 2-mercaptoethanol and heated at 95° C for 4 min to load the samples (14 µL) on the gel using BenchMark Protein Ladder (15 µL) as a molecular weight marker. Electrophoresis was performed at 60 V and 120 V for stacking and resolution, respectively. The gel was stained overnight with Coomassie solution (0.1% Coomassie blue, 45% methane, and 45% glacial acetic acid), then faded with a methanol solution (5% glacial acetic acid, 45% distilled water, and 50% methanol). Image analysis of the SDS-PAGE gels was carried out using GelAnalyzer 19.1 software (Istvan, 2021).
2.5. Predicted profiles of peptides in fava bean with biological activities
The analysis of the potential bioactivity of peptides from two protein of fava bean were following Montoya-Rodríguez et al. (2015) method with some modifications. The reviewed protein sequences of legumin type B (P05190) and vicilin (P08438) (Supplementary table 2) were obtained from UniProt database (http://www.uniprot.org). To cut and to evaluate the protein sequences, the software Peptide Cutter (https://web.expasy.org/peptide_cutter/) and BIOPEP database (http://www.uwm.edu.pl/biochemia/index.php/en/biopep) were used.
2.6. Statistical analysis
The samples analysis and experiments were realized in triplicate. The results are expressed as mean ± standard deviation (SD). For the statistical analysis, the results were analyzed with the statistical software IBM SPSS Statistics 25.0 (IBM Inc., Armonk, NY, USA). To verify the normality of the data, the Shapiro-Wilk test was used. Once the homogeneity of variances (homoscedasticity) was confirmed with the Levene test, a one-way analysis of variance (ANOVA) was performed to verify differences between groups. The Tukey's post hoc test confirmed statistically significant differences between groups with a significance value ρ < 0.05.
3. Results and discussion
3.1. Chemical and nutritional characteristics
The results in Table 1 showed no statistically significant differences between samples (ρ > 0.05) regarding ash content, while in the rest of the results, there were significant differences (ρ < 0.05). NPP showed higher protein concentration compared to PP, with 94.4 and 84.4%, respectively. In both samples, it was not possible to quantify the fiber content. The parameters evaluated in the MPF in this study are like those reported by the U.S.D.A. (2019). Colca (2014) compared the fiber of three different fava bean flours: unprocessed flour, another one subjected to 120° C for 20 min, and the last one subjected to 130° C for 15 min, reporting 1.33, 1.22, and 1.29%, respectively, which is consistent with our results. NPP showed a protein concentration of 94.39 ± 0.76%, which is comparable to those reported for other legumes such as soybean (89.7%), red bean (89.3%), red kidney bean (92.2%), mung bean (93.9%), black bean (88.21%) and chickpea (89.9–94.4%) (Kaur and Singh, 2006, Kudre et al., 2013, Tang, 2008). With respect to the above, Vogelsang-O’Dwyer et al. (2020) compared enriched bean flour and a protein isolated bean; the protein concentration was 64.1 and 90.1%, respectively. These data support the results obtained in our NPP and PP based on the same isoelectric point extraction technique. The variety of fava bean can influence the variation of protein concentration. The analysis of protein isolates of 7 different bean genotypes with over 21 samples from different regions carried out by Singhal et al., (2016), reported a variation up to 9% in the protein concentration of the samples. PP showed a lower concentration of protein attributed to polyphenols. The interactions of polyphenols with proteins can result in soluble or insoluble complexes, this phenomenon can affect protein extraction and the concentration obtained (Kosińska et al., 2011). Finally, concerning CF, it is noteworthy to mention the slight increase in moisture content due to the seed soaking period and subsequent cooking with moist heat. Protein content increased after subjecting the fava beans to heat treatment. This result is due to the application of thermal energy causing the unfolding of proteins, as well as that of other non-protein compounds (such as glycosides) that may interfere with protein quantification, such as convicine and vicine, present in the fava bean, which can be significantly removed after heat treatment (Rizzello et al., 2016).
Table 1.
Proximal composition of minimal processed flour, cooked flour, non-polyphenol protein flour and protein flour with polyphenols of fava bean (Vicia faba L).
| Samples/Proximal chemical composition | Moisture | Ash | Protein | Lipids | Dietary fiber |
Carbohydrates by difference |
|---|---|---|---|---|---|---|
| MPF | 3.34 ± 0.70a | 3.17 ± 0.40a | 26.47 ± 0.26a | 2.00 ± 0.20c | 1.89 ± 0.02a | 63.13 ± 1.07a |
| CF | 5.79 ± 0.98bc | 3.26 ± 0.02a | 27.98 ± 0.25b | 1.66 ± 0.29bc | 1.78 ± 0.12a | 57.86 ± 3.16b |
| NPP | 4.08 ± 0.15ab | 2.66 ± 0.08a | 94.39 ± 0.76c | 0.41 ± 0.06a | <0.1b | <0.1c |
| PP | 7.55 ± 0.85c | 2.90 ± 0.43a | 84.37 ± 0.30d | 1.43 ± 0.15b | <0.1b | 3.29 ± 0.50d |
MPF = minimal processed flour, CF = cooked flour, NPP = non-polyphenol protein flour, PP = polyphenol protein flour.
Data expressed in % (w/w) dry basis, mean ± SD (n = 3).
Means with different superscripts in columns differ significantly (p < 0.05).
Table 2 shows results regarding the amino acid profile and the in vitro nutritional evaluation. Of the 15 AA evaluated, CF presented higher concentration in 7 AA, followed by NPP in 5 AA, 2 AA in PP, and finally MPF, only showed higher quantity in one of the quantified AAs. In CF, 6 of the 7 AA (ALA, PHE + TYR, ILE, MET + CYS, TYR, and VAL) with the highest concentration corresponded to hydrophobic AAs. While in NPP, 3 of the 5 AA (ASP, GLU, and THR) with higher concentrations belong to the category of hydrophilic, in addition, two of these contain carboxylic acids in their side chains. Among the determined AAs, the limiting essential amino acid in the four samples belongs to the sulfur AAs (MET + CYS). The nutritional quality of legume proteins depends to a large extent on essential AAs. Depending on the species, TRP or sulfur-containing AA (MET-CYS) are found to be deficient in legumes. It is known that the application of heat modifies the AA content, in addition, during cooking, the food matrix is in contact with water which could facilitate the leaching of water-soluble AA. This phenomenon could explain the higher concentration of hydrophobic AA in cooked bean flour. Nosworthy et al., (2018) reported an increase in sulfur AA after a cooking process. Another study on the protein quality of fava bean (Le Roux et al., 2020) reported an essential AA score higher than in our study (83); however, the limiting amino acid was tryptophan.
Table 2.
Comparison of the amino acid profile and nutritional parameters of minimal processed flour, cooked flour, non-polyphenol protein flour and polyphenol protein flour.
|
Amino acid (g / 100 g protein) a |
MPF | CF | NPP | PP | FAO, 2013b |
|---|---|---|---|---|---|
| Ala | 3.25 | 3.54 | 3.19 | 3.26 | – |
| Arg | 2.82 | 2.96 | 2.21 | 2.72 | – |
| Asp | 5.78 | 6.17 | 6.70 | 6.20 | – |
| Phe + Tyr | 8.60 | 10.46 | 8.68 | 8.61 | 4.1 |
| Gly | 5.38 | 5.57 | 6.11 | 6.07 | – |
| Glu | 11.53 | 14.06 | 14.96 | 13.02 | – |
| His | 18.44 | 15.81 | 13.95 | 11.90 | 1.6 |
| Ile | 2.67 | 3.19 | 2.96 | 2.99 | 3.0 |
| Leu | 5.36 | 6.32 | 6.81 | 6.72 | 6.1 |
| Lys | 6.04 | 6.01 | 5.98 | 6.43 | 4.8 |
| Met + Cys | 1.41 | 1.81 | 1.02 | 1.21 | 2.3 |
| Ser | 2.80 | 2.77 | 3.19 | 3.27 | – |
| Thr | 15.29 | 9.16 | 16.60 | 15.87 | 2.5 |
| Trp | – | – | – | – | 0.66 |
| Val | 2.72 | 3.26 | 3.08 | 3.19 | 4.0 |
| Total protein (%) c | 26.47 | 27.98 | 94.39 | 84.37 | |
| Limiting essential amino acid | Met + Cys | Met + Cys | Met + Cys | Met + Cys | |
| AAS | 61 | 78 | 44 | 53 | 100 |
| IVPD (%) | 31.34 ± 1.40a | 94.15 ± 2.45d | 77.73 ± 2.26c | 71.09 ± 1.69b | |
| IVPB (%) | 16.43 ± 0.40a | 20.47 ± 1.78b | 29.85 ± 1.88c | 33.19 ± 1.65c | |
| IV-PDCAAS(%)d | 19.12 ± 0.86a | 73.44 ± 1.92d | 34.20 ± 0.99b | 37.68 ± 0.90c | |
| E-PER | 1.43 | 1.75 | 2.15 | 2.11 |
MPF = minimal processed flour, CF = cooked flour, NPP = non-polyphenol protein flour, PP = polyphenol protein flour, IV-PD(%) = In vitro Protein Digestibility, IV-PB (%) = In vitro Protein Bioaccessibility, PDCAAS-IV = Amino Acid Score Corrected for In vitro Protein Digestibility. aComposition on a dry basis; bAmino acid requirement for children 3 to 10 years of age according to FAO, 2013; cDetermination of free amino acids with high pressure liquid chromatography performed; dIV-PDCAAS(%) = IVPD(%) × AAS, E-PER = Estimated protein efficiency ratio. Different superscript letters in the same row indicate significant statistical difference (ρ < 0.05).
Protein digestibility is an important indicator of nutritional quality, influenced by the AA profile that constitutes the protein fraction and interacts with the food matrix. MPF presented the lowest percentage of protein digestibility (31.34 ± 1.40%), the highest IVPD was found in CF (94.15 ± 2.45%), according to the INFOGEST method. It is well established that in raw or minimal processed beans, protein digestibility is low. Among the factors that influence this we can find the structure and its intermolecular interaction with the food matrix. The interaction of fava bean proteins with other seed components, such as tannins, phytic acid, protease inhibitors, and antigenic proteins, reduces or inhibits their digestibility, and their amounts differ according to the variety of the seed (Ohanenye et al., 2020). Despite the above, a higher digestibility has been reported than that obtained in this study. Luo & Xie, (2013) evaluated raw bean seeds with green or white shells grown in the province of Jiangsu and obtained in vitro protein digestibility of 72.65 ± 0.58 and 73.28 ± 0.52%, respectively.
On the other hand, applying thermal treatments such as ordinary cooking or by autoclave, with previous soaking and peeling, as with CF, NPP and PP, considerably increase the protein digestibility in vitro. For example, Abdel-Aleem et al., (2019) evaluated the protein digestibility of bean in different samples; raw seeds soaked for 14 h with cooking at 100° C, presented digestibility of 70.35 ± 0.13 and 87.29 ± 0.17%, respectively. NPP showed an in vitro digestibility of 77.73 ± 2.26%, while in PP, it was significantly (ρ < 0.05) lower 71.09 ± 1.69%. Legume proteins are found in seed matrices with other chemical components, such as polysaccharides and polyphenols, which hinder the digestibility of proteins (Ohanenye et al., 2020). This effect is consistent with our results, as the extraction of polyphenols in NPP increased significantly (ρ < 0.05) by ∼ 6% digestibility. Le Roux et al., (2020) compared digestibility through in vitro dynamic digestion of two protein concentrates of bean and peas without polyphenols, reporting in vitro protein digestibility of 91.1 ± 3.1 and 74.9 ± 6.7%, respectively. It also has been reported that the digestibility of the fava bean concerning other legumes depends on the albumin content because this protein fraction of plant foods has higher CYS content. The albumin fraction is often highly resistant to heat denaturation and enzymatic digestion with trypsin, chymotrypsin, and pepsin (Warsame et al., 2020). Stability is conferred by a high number of disulfide bonds contained in low molecular weight proteins. In the bean, the albumin fraction is < 20% of the total protein, which justifies its low content of sulfur AA and its protein digestibility superior to some other legumes.
The most significant number of bioaccessible (<14 kDa) and dialysis peptides were obtained from NPP and PP samples, without statistically significant difference (ρ = 0.110). In terms of MPF and CF, CF presented a higher percentage of bioaccessibility; however, this was not significantly different from that of MPF (ρ = 0.05). An in vitro bioaccessibility model is an effective and valid strategy for understanding structural changes in food ingested under simulated physiological conditions in the human gastrointestinal tract. Lin et al., (2019) established the nutraceutical potential of various bioactive compounds of the bean. As for the protein fraction of the fava bean, there are few studies on its bioaccessibility in vitro since most of the available studies focus on the bioaccessibility of phenolic compounds. However, in recent years, interest in bioactive peptides that arise through proteolysis by different methods has increased. Xing et al., (2017) evaluated the effect of the pH of fermentation on the bioaccessibility of the proteins of four soybean curds, with a cut-off point of 10 kDa. In their study, a significant increase in low molecular weight peptides (<10 kDa) from 0.71 mg/mL to 1.44 mg/mL was observed as gastrointestinal digestion progressed. These results were confirmed by electrophoresis (SDS-PAGE) and are similar to those obtained in our study on the dialyzed CF protein.
IV-PDCAAS showed statistically significant differences (ρ < 0.05) in all samples, with MPF being the lowest value (<20%) and CF the highest value (73.44 1.92%), suggesting that the protein fraction of bean after heat treatment significantly increases its nutritional value. This effect is because the proteins in their native state fold into a specific conformation determined by the AA composition that constitutes it, further restricting the access of proteolytic enzymes to their substrates (Joye, 2019).
About E-PER, the highest value was presented in NPP, followed by PP, CF, and finally MPF, with values of 2.15, 2.11, 1.75, and 1.43, respectively. Studies have proposed in vitro methods to correlate with in vivo methods to develop reliable methods for measuring E-PER through regression equations. Nosworthy et al., (2018) reported a value of 0.85 and 0.66 for boiled bean and baked fava bean, respectively. This behavior is also observed in other legumes. López-Ibarra et al., (2021) evaluated the protein fraction of the tepary bean (Phaselous acutifolius) in samples of raw and cooked flour and crude and cooked protein concentrate. The heat treatment favored the increase of E-PER values in flours (0.86 to 1.93) and concentrates (1.34 to 1.81). The fact that E-PER relates the value of digestibility and AA content suggests that the nutritional value increases due to thermal and proteolytic processes causing samples to be more accessible to subsequent digestive processes.
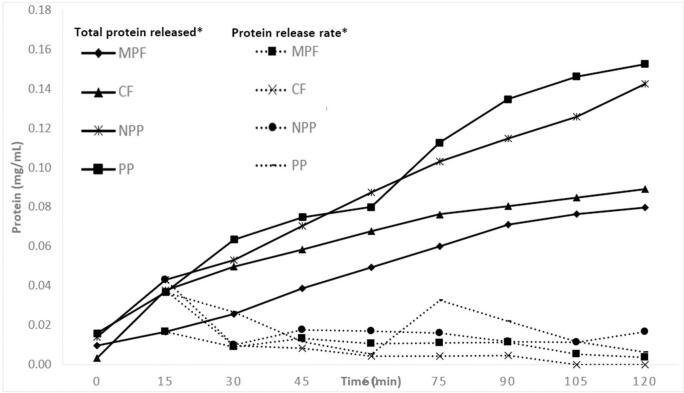
The results of protein release kinetics (Fig. 1), show average velocities of total protein released (mg/mL) in vitro from 0.010 to 0.019 mg/mL of protein per min. MPF and CF showed similar velocities with no significant differences (ρ > 0.05). NPP and PP did not show significant differences in their protein release velocities (ρ > 0.05), in addition to reporting the highest release velocity. The amount of total protein released (mg/mL) in vitro showed significant differences (ρ < 0.05) in all samples over 120 min, however, statistically similar values (ρ > 0.05) were obtained in MPF and CF at min 15, 75, 90, 105 and 120, suggesting that heat treatment does not influence the amount of protein released. On the other hand, NPP and PP did not show statistically different values (ρ > 0.05) except for min 105, which indicates that NPP and PP present the same amount of total protein released. In addition, these samples reported the highest total protein release. On the total protein released, the results could be related to the type of sample and its food matrix, since statistically similar values were observed for MPF and CF and NPP with PP. The greater bioaccessibility and total protein release by NPP and PP could be due to a lower interaction with the food matrix, because of its higher degree of purification, eliminating most of the non-protein compounds, as opposed to MPF and CF that showed a statistically lower (ρ < 0.05) bioaccessibility and total protein release (Kosińska et al., 2011). As mentioned above, antinutritional factors affect protein digestibility; they decrease their digestibility, affecting their bioaccessibility, and finally is reflected in the protein release rate (Perales-Vázquez et al., 2020). Hence, the information concerning this parameter is relevant as it can be used to evaluate potential absorption peaks in additional in vivo tests.
Fig. 1.
Fava bean in vitro kinetics of Total protein released and Protein release rate *(mg/mL per minute) of MPF, CF, NPP and PP. MPF = minimal processed flour; CF = cooked flour NPP = non-polyphenol protein flour; PP = polyphenol protein flour.
It is important to emphasize, according to previous studies, that bioaccessible low molecular weight peptides resulting from in vitro digestion reported in this study are potentially effective in inhibiting the pathogenesis of metabolic syndrome (Jakubczyk et al., 2019), and even peptides with molecular weight < 3 kDa have antioxidant and antitumor activity in different cell lines (Kuerban et al., 2020). Furthermore, according to the literature, these peptides are released from food proteins in the gastrointestinal tract, which are absorbed and reach the systemic circulation to perform specific activities at the systemic level (Samaei et al., 2020).
3.2. Electrophoresis
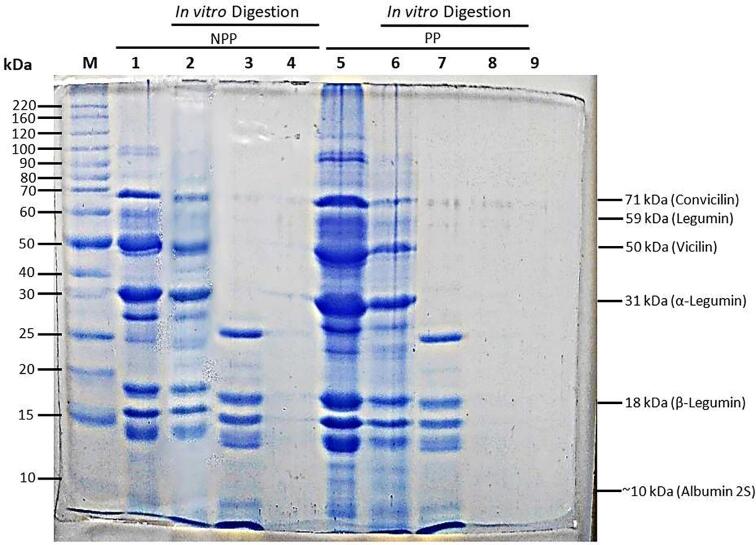
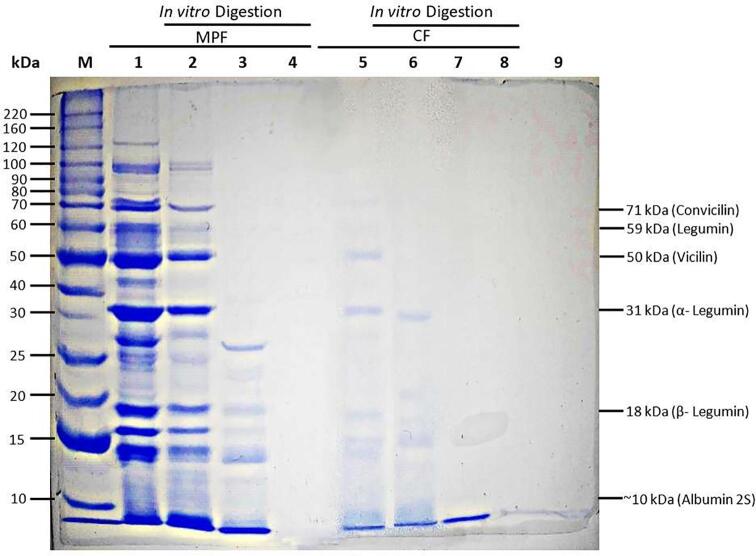
In Fig. 2, Fig. 3 the electrophoretic profiles of the treatments through in vitro digestion are shown. In the treatments, predominant proteins with a molecular weight between 50 and 30 kDa were observed, followed by proteins between 15 and 20 kDa, and finally those of 70 kDa. In addition, faint bands with molecular weight of 60, 40, 27, and 17 kDa were observed. The electrophoretic profile of MPF showed the presence of 12 proteins, CF 4 proteins, NPP 12, and PP 11. It was observed that the action of proteolytic enzymes hydrolyses proteins of higher molecular weight through the process of in vitro digestion. In MPF and NPP, potentially bioaccessible proteins < 15 kDa were observed, while in PP, only a faint band below 10 kDa was observed. CF did not show any bands. Most of the proteins found in the fava bean seed are globulins (convicillin, legumin and vicillin), about 70 to 78%, albumin with 10 to 20%, 15% are glutelins, and<5% prolamines. Proteins have different conformational characteristics; as a result, they show different solubility. The electrophoretic profile of MPF before in vitro digestion corresponds to subunits of the globulins convicilin, legumin, and vicillin, respectively. These bands were also observed in the NPP and PP, which shows that the results obtained in this study are consistent with other studies (Bühler et al., 2020, Vogelsang-O’Dwyer et al., 2020). However, in CF, well-defined bands were not found, probably due to the exhaustive heat treatment, which affects the solubility and concentration of protein fractions (Căpriţă et al., (2010). Regarding the bioaccessibility of the protein fraction after in vitro digestion (Fig. 2; rails 4 and 8), NPP and PP showed bioaccessible protein bands < 15 kDa. It is well established that the most bioaccessible proteins are peptides with a low molecular weight close to 10 kDa, especially peptides with a short length of 2 to 10 AA. This has been reported in other studies conducted by Rui et al., (2016) and Xing et al., (2017), where they evaluated the protein bioaccessibility of soy through in vitro digestion and dialysis methods, proving that peptides below < 10 kDa have greater bioaccessibility, clearly visible through SDS-PAGE analysis. However, it is remarkable that slight discrepancies with other studies have been found because there are variations between the genotypes of bean seed (Tahir, 2015), which diversifies the proteins of the bean qualitatively and quantitatively, thus, there are proteins that are not yet well identified or studied in the scientific literature as reported by Warsame et al., (2020).
Fig. 2.
Electrophoretic profile by molecular weight of NPP and PP of fava bean after in vitro digestion determined by SDS-PAGE. Lanes: M: Molecular weight marker; 1: NPP; 2: NPP gastric phase, 3: NPP intestinal phase, 4: NPP bioaccessible, 5: PP, 6: PP gastric phase, 7: PP intestinal phase, 8: PP bioaccessible and 9: digestive enzyme blank. NPP = non-polyphenol protein flour; PP = polyphenol protein flour.
Fig. 3.
Electrophoretic profile by molecular weight of fava bean MPF and fava bean CF after in vitro digestion determined by SDS-PAGE. Lanes: M: Molecular weight marker; 1: MPF; 2: MPF gastric phase, 3: MPF intestinal phase, 4: MPF bioaccessible, 5: CF, 6: CF gastric phase, 7: CF intestinal phase, 8 CF bioaccessible and 9: digestive enzyme blank. MPF = minimal processed flour; CF = cooked flour.
3.3. Predicted profiles of peptides in fava bean with biological activities
The results of Table 3 showed different bioactivities for the in silico digestion of legumin type B and vicillin to obtain peptides between 2 and 5 AA, which are bioaccessible due to particle size. Both proteins showed high occurrence frequencies of angiotensin-converting enzyme-inhibitor peptides (ACE inhibitor), with A = 0.2500–0.7500, and A = 0.2000–0.6667 for legumin and vicilin, respectively. These results in both proteins are similar to dipeptidyl peptidase III and IV inhibitor, antioxidant activity, glucose uptake stimulating peptide, and renin inhibitor. Other potential bioactivities present are alpha-glucosidase inhibitor, neuropeptides, CaMPDE inhibitor, this one for legumin type B, and anti-thrombotic, anti-inflammatory, and activating ubiquitin-mediated proteolysis, for vicilin. It is well documented that legumes generated bioactive sequences with high potential to inhibit dipeptidyl peptidase IV, angiotensin-converting enzyme inhibition and antioxidant capacity with diverse bioactivities (Mojica & Gonzalez de Mejia, 2018). For all the above, more research about the potential of the fava bean is needed since the proteins present in this legume have been slightly reviewed by the databases and its bioactive potential may be underestimated.
Table 3.
Predicted profiles of peptides (2–5 AA) in fava bean flours with biological activities. *.
| Protein | Predictive bioactivity | Sequence of potential bioactive peptide | Occurrence frequency ratio (A) |
|---|---|---|---|
| Legumin type B | ACE inhibitor |
MSKP, TSTC, TET, CAGVS, IR, HL, IIQGK, GVIG, QEPR, QGSR, FR, YW, LVAIS, DSTPR, FY, HQQK, DGNSV, SG, AHT, LR, SPR, NQIVR, VEGG, SEQGR, NGL, YNPR, PIL, VR, NGI, YAPHW, VIR, GEGR, DNK, GQL, QVTE, SGNR | 0.2500–0.7500 |
| Antioxidative | MSKP, IR, HL, AHT, YAPHW, VIR, SGNR | 0.2500–0.6000 | |
| Dipeptidyl peptidase III/IV inhibitor | MSKP, FL, TSTC, ATSSE, NQCR, DNINA, TET, CAGVS, IR, HL, IIQGK, GVIG, TL, QEPR, SSQSR, QGSR, FR, YW, LVAIS, DSTPR, HQQK, DGNSV, AHT, LR SPR, NQIVR, VEGG, SEQGR, NGL, ADL, YNPR, TL, PIL, SAE, VR, YR, NGI, YAPHW, VIR, GEGR, DNK, VTK, GQL, TNDR, ANA, QVTE, SGNR |
0.2000–0.7500 | |
| Glucose uptake-stimulating peptide | ATSSE, IIQGK, LVAIS, SSE, NQIVR, SEQGR, PIL | 0.2000–0.3333 | |
| CaMPDE inhibitor | VIR, IR | 0.3333–0.5000 | |
| Renin inhibitor | IR, LR, VIR, SGNR | 0.3333–0.5000 |
|
| Alpha-glucosidase inhibitor | VEGG, ADL | 0.2500–0.3333 |
|
| Neuropeptide | YR, GQL s | 0.3333–0.5000 |
|
| Vicilin | ACE inhibitor |
MAATT, DSF, GIA, LQK, SGK, AIL, NSF, ER, GDTIK, SG, NIL, EAS, EIEK, EEHGK, EK, GLK, QIEE, NK, EPIY, SNK, GK, NPQL, PHY, GDF, VGQR, EEY, DEEK, PVAIK, VG, LAF, ENQK, ER, DHL, GS | 0.2000–0.6667 |
| Antioxidative | GLK, EPIY, PHY, DHL | 0.3333 | |
| Dipeptidyl peptidase III/IV inhibitor | MAATT, DSF, LL, GIA, ASVC, ESNR, QT, DQHSK, QN, VV, AIL, TV, LL, PNDR, NSF, GDTIK, VNR, SK, NIL, EAS, NTD, EIEK, LL, EEHGK, EK, HR, GLK, DR, QIEE, NAK, SSSK, FN, EPIY, NPQL, QD, VN, LL, PHY, VGQR, EEY, DEEK, QVQN, PVAIK, ASSN, VG, FL, LAF, ENQK, QSH, DHL, YSI | 0.2000–0.8000 | |
| Glucose uptake-stimulating peptide | LL, AIL, LL, DDEED, NIL, LL, EEHGK, QIEE, SSSK, LL, EEY, DEEK | 0.2000–0.5000 |
|
| Renin inhibitor | DSF, ESNR, NSF, VNR | 0.2500–0.3333 | |
| Alpha-glucosidase inhibitor | EAS | 0.3333 | |
| Neuropeptide | VGQR | 0.2500 | |
| Anti-inflammatory | PHY | 0.3333 | |
| Antithrombotic | DDEED, DEEK | 0.2000–0.2500 | |
| Activating ubiquitin-mediated proteolysis | LAF | 0.3333 |
* Protein sequence is reviewed for UniProt database (http://www.uniprot.org). Peptide sequences obtained from Peptide Cutter https://web.expasy.org/peptide_cutter/. Potential bioactivities and occurrence frequency obtained from BIOPEP http://www.uwm.edu.pl/biochemia/index.php/en/biopep Amino acid nomenclature: A: Ala, R: Arg, N: Asn, D: Asp, C: Cys, Q: Gln, E: Glu, G: Gly, H: His, I: Ile, L: Leu, K: Lys, M: Met, F: Phe, P: Pro, S: Ser, T: Thr, W: Trp, Y: Tyr and V: Val.
4. Conclusions
The nutritional quality of protein from four different fava bean protein flours were analyzed through in vitro and in silico methodologies in this study. The sulfur amino acids (MET + CYS) were found as limiting essential amino acids for four treatments, so fava bean flours should be combined with rich sulfur amino acid foods to increase the digestibility. The conditions of protein extraction have an influence on the bioaccesibility of low molecular weight peptides released from protein during in vitro digestion and depends on the food matrix in which they are embedded. SDS-PAGE indicated that the four fava bean flours were rich in legumin and vicilin, depicted by the presence of low molecular weight protein and peptides (<20 kDa), showing the putative presence of peptides with potential bioactivity. The nutritional properties were altered mainly by the heat treatment and the presence of polyphenols, where cooked flour and non-polyphenol protein flour presented higher digestibility, bioaccesibility, and estimated protein efficiency ratio. Therefore, fava bean protein flours, mainly cooked and polyphenol-free, could be used as a good source of plant protein for human nutrition.
Funding.
This work was supported by PAICYT (Programa de Apoyo a la Investigación Científica y Tecnológica) from Universidad Autónoma de Nuevo León (UANL). Also, we acknowledge CONACYT (Consejo Nacional de Ciencia y Tecnología— México) for the grant (1007010) awarded to Victor Ayala.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100303.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alpizar-Reyes E., Castaño J., Carrillo-Navas H., Alvarez-Ramírez J., Gallardo-Rivera R., Pérez-Alonso C., Guadarrama-Lezama A.Y. Thermodynamic sorption analysis and glass transition temperature of fava bean (Vicia fava L.) protein. Journal of Food Science and Technology. 2018;55(3):935–943. doi: 10.1007/s13197-017-3001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsmeyer R.H., Cunningham A.E., Happich M.L. Equations predict PER from amino acid analysis. Undefined. 1974 https://www.semanticscholar.org/paper/Equations-predict-PER-from-amino-acid-analysis-Alsmeyer-Cunningham/2eaac3e44cac29249254d7ff7a5ba918a7be1960 [Google Scholar]
- Alves A.C., Tavares G.M. Mixing animal and plant proteins: Is this a way to improve protein techno-functionalities? Food Hydrocolloids. 2019;97 doi: 10.1016/j.foodhyd.2019.06.016. [DOI] [Google Scholar]
- AOAC International. (2019). Official Methods of Analysis, 21st Edition (2019). AOAC INTERNATIONAL. https://www.aoac.org/official-methods-of-analysis-21st-edition-2019/.
- Berrazaga I., Bourlieu-Lacanal C., Laleg K., Jardin J., Briard-Bion V., Dupont D., Walrand S., Micard V. Effect of protein aggregation in wheat-legume mixed pasta diets on their in vitro digestion kinetics in comparison to “rapid” and “slow” animal proteins. PLOS ONE. 2020;15(5) doi: 10.1371/journal.pone.0232425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodkorb A., Egger L., Alminger M., Alvito P., Assunção R., Ballance S.…Recio I. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nature Protocols. 2019;14(4):991–1014. doi: 10.1038/s41596-018-0119-1. [DOI] [PubMed] [Google Scholar]
- Bühler J.M., Dekkers B.L., Bruins M.E., van der Goot A.J. Modifying faba bean protein concentrate using dry heat to increase water holding capacity. Foods. 2020;9(8):1077. doi: 10.3390/foods9081077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro M., Maselli P., Nucara A. Structural aspects of legume proteins and nutraceutical properties. Food Research International. 2015;76:19–30. doi: 10.1016/j.foodres.2014.11.007. [DOI] [Google Scholar]
- FAO. (1993). Manual de Técnicas para Laboratorio de Nutrición de Peces y Crustáceos. http://www.fao.org/3/ab489s/ab489s03.htm.
- Food and Agriculture Organization of the United Nations, editor. Dietary protein quality evaluation in human nutrition: Report of an FAO expert consultation, 31 March-2 April, 2011, Auckland. New Zealand; Food and Agriculture Organization of the United Nations: 2013. [Google Scholar]
- Fei Fang E., Hassanien A.E., A., Ho Wong, J., Shui Fern Bah, C., Saad Soliman, S., & Bun Ng, T. Isolation of a New Trypsin Inhibitor from the Fava Bean (Vicia fava cv. Giza 843) with Potential Medicinal Applications. Protein & Peptide Letters. 2011;18(1):64–72. doi: 10.2174/092986611794328726. [DOI] [PubMed] [Google Scholar]
- Hernández-García J.I., Orozco-Villafuerte J., Cuenca-Mendoza F., Pérez-Alonso C., Carrillo-Navas H., Guadarrama-Lezama A.Y. Extracción, caracterización física y térmica de aislados de proteína de haba (vicia. 2016;fava). 1:41–46. [Google Scholar]
- Istvan, L. (2021). GelAnalyzer. http://www.gelanalyzer.com/.
- Jakubczyk A., Karaś M., Złotek U., Szymanowska U., Baraniak B., Bochnak J. Peptides obtained from fermented faba bean seeds (Vicia faba) as potential inhibitors of an enzyme involved in the pathogenesis of metabolic syndrome. LWT. 2019;105:306–313. doi: 10.1016/j.lwt.2019.02.009. [DOI] [Google Scholar]
- Joye I. Protein digestibility of cereal products. Foods. 2019;8(6):199. doi: 10.3390/foods8060199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkouch I., Tabbene O., Gharbi D., Ben Mlouka M.A., Elkahoui S., Rihouey C., Coquet L., Cosette P., Jouenne T., Limam F. Antioxidant, antityrosinase and antibiofilm activities of synthesized peptides derived from Vicia fava protein hydrolysate: A powerful agents in cosmetic application. Industrial Crops and Products. 2017;109:310–319. doi: 10.1016/j.indcrop.2017.08.025. [DOI] [Google Scholar]
- Kaur M., Singh N. Relationships between selected properties of seeds, flours, and starches from different chickpea cultivars. International Journal of Food Properties. 2006;9(4):597–608. doi: 10.1080/10942910600853774. [DOI] [Google Scholar]
- Khalil M.M. Effect of soaking, germination, autoclaving and cooking on chemical and biological value of guar compared with fava bean. 2001;4:5. doi: 10.1002/1521-3803(20010801)45:4<246::AID-FOOD246>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Kosińska A., Karamać M., Penkacik K., Urbalewicz A., Amarowicz R. Interactions between tannins and proteins isolated from broad bean seeds (Vicia faba Major) yield soluble and non-soluble complexes. European Food Research and Technology. 2011;233(2):213–222. doi: 10.1007/s00217-011-1506-9. [DOI] [Google Scholar]
- Kudre T.G., Benjakul S., Kishimura H. Comparative study on chemical compositions and properties of protein isolates from mung bean, black bean and bambara groundnut: Protein isolates from mung bean, black bean and Bambara groundnut. Journal of the Science of Food and Agriculture. 2013;93(10):2429–2436. doi: 10.1002/jsfa.6052. [DOI] [PubMed] [Google Scholar]
- Kuerban A., Al-Ghafari A.B., ALGhamadi S.A., Syed F.Q., Mirza M.B., Mohammed F.A.…Moselhy S.S. Potential antiglycation, antioxidant and antiproliferative activities of Vicia faba peptides. Journal of Food Measurement and Characterization. 2020;14(4):2155–2162. doi: 10.1007/s11694-020-00462-9. [DOI] [Google Scholar]
- Laemmli U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Roux L., Ménard O., Chacon R., Dupont D., Jeantet R., Deglaire A., Nau F. Are Fava Bean and Pea Proteins Potential Whey Protein Substitutes in Infant Formulas? An In vitro Dynamic Digestion Approach. Foods. 2020;9(3):362. doi: 10.3390/foods9030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Liu Y., Zou X., He J., Xu X., Zhou G., Li C. In vitro protein digestibility of pork products is affected by the method of processing. Food Research International. 2017;92:88–94. doi: 10.1016/j.foodres.2016.12.024. [DOI] [PubMed] [Google Scholar]
- Liu F., Kariluoto S., Edelmann M., Piironen V. Bioaccessibility of folate in fava bean, oat, rye and wheat matrices. Food Chemistry. 2021;350 doi: 10.1016/j.foodchem.2021.129259. [DOI] [PubMed] [Google Scholar]
- Macarulla M.T., Medina C., Diego M.A.D., Chávarri M., Zulet M.Á., Martínez J.A., Nöel-Suberville C., Higueret P., Portillo M.P. Effects of the whole seed and a protein isolate of fava bean (Vicia fava) on the cholesterol metabolism of hypercholesterolaemic rats. British Journal of Nutrition. 2001;85(5):607–614. doi: 10.1079/BJN2000330. [DOI] [PubMed] [Google Scholar]
- Managa M.G., Akinola S.A., Remize F., Garcia C., Sivakumar D. Physicochemical Parameters and Bioaccessibility of Lactic Acid Bacteria Fermented Chayote Leaf (Sechium edule) and Pineapple (Ananas comosus) Smoothies. Frontiers in Nutrition. 2021;8 doi: 10.3389/fnut.2021.649189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Sánchez S.M., Gabaldón-Hernández J.A., Montoro-García S. Unravelling the molecular mechanisms associated with the role of food-derived bioactive peptides in promoting cardiovascular health. Journal of Functional Foods. 2020;64 doi: 10.1016/j.jff.2019.103645. [DOI] [Google Scholar]
- Mojica L., de Mejia E.G. Legumes. 2018. CHAPTER 5: Legume Bioactive Peptides; pp. 106–128. [DOI] [Google Scholar]
- Montoya-Rodríguez A., Gómez-Favela M.A., Reyes-Moreno C., Milán-Carrillo J., de Mejía E.G. Identification of Bioactive Peptide Sequences from Amaranth (Amaranthus hypochondriacus) Seed Proteins and Their Potential Role in the Prevention of Chronic Diseases. Comprehensive Reviews in Food Science and Food Safety. 2015;14(2):139–158. doi: 10.1111/1541-4337.12125. [DOI] [PubMed] [Google Scholar]
- Ohanenye I.C., Tsopmo A., Ejike C.E.C.C., Udenigwe C.C. Germination as a bioprocess for enhancing the quality and nutritional prospects of legume proteins. Trends in Food Science & Technology. 2020;101:213–222. doi: 10.1016/j.tifs.2020.05.003. [DOI] [Google Scholar]
- Organización de las Naciones Unidas para la Alimentación y la Agricultura (FAO). (2016). Legumbres, semillas nutritivas para un futuro sostenible. FAO. http://www.fao.org/3/a-i5528s.pdf.
- Perales-Vázquez G.D.C., Mercado-Mercado G., De la Rosa L.A., Sáyago-Ayerdi S.G. Bioaccesibilidad y cinética de liberación in vitro de compuestos fenólicos en algunas salsas de la cocina mexicana. TIP Revista Especializada en Ciencias Químico-Biológicas. 2020;23 doi: 10.22201/fesz.23958723e.2020.0.205. [DOI] [Google Scholar]
- Rizzello C.G., Losito I., Facchini L., Katina K., Palmisano F., Gobbetti M., Coda R. Degradation of vicine, convicine and their aglycones during fermentation of faba bean flour. Scientific Reports. 2016;6(1):32452. doi: 10.1038/srep32452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sair L. Proteinaceous soy composition and method of preparing. (Patent Núm. 1959;2,881,076) [Google Scholar]
- Samaei S.P., Ghorbani M., Tagliazucchi D., Martini S., Gotti R., Themelis T.…Babini E. Functional, nutritional, antioxidant, sensory properties and comparative peptidomic profile of faba bean (Vicia faba, L.) seed protein hydrolysates and fortified apple juice. Food Chemistry. 2020;330 doi: 10.1016/j.foodchem.2020.127120. [DOI] [PubMed] [Google Scholar]
- Scopes R.K. Measurement of protein by spectrophotometry at 205 nm. Analytical Biochemistry. 1974;59(1):277–282. doi: 10.1016/0003-2697(74)90034-7. [DOI] [PubMed] [Google Scholar]
- Serrano-Sandoval S.N., Guardado-Félix D., Gutiérrez-Uribe J.A. Changes in digestibility of proteins from chickpeas (Cicer arietinum L.) germinated in presence of selenium and antioxidant capacity of hydrolysates. Food Chemistry. 2019;285:290–295. doi: 10.1016/j.foodchem.2019.01.137. [DOI] [PubMed] [Google Scholar]
- Tahir N.-A.-R. Identification of genetic variation in some faba bean (Vicia faba L.) genotypes grown in Iraq estimated with RAPD and SDS-PAGE of seed proteins. Indian Journal of Biotechnology. 2015;6 [Google Scholar]
- Tang C.-H. Thermal denaturation and gelation of vicilin-rich protein isolates from three Phaseolus legumes: A comparative study. LWT - Food Science and Technology. 2008;41(8):1380–1388. doi: 10.1016/j.lwt.2007.08.025. [DOI] [Google Scholar]
- Turco I., Ferretti G., Bacchetti T. Review of the health benefits of Fava bean (Vicia fava L.) polyphenols. Journal of Food and Nutrition Research. 2016;55:283–293. [Google Scholar]
- United Nations. (2015). World Population Prospects The 2015 Revision.
- Vioque J., Alaiz M., Girón-Calle J. Nutritional and functional properties of Vicia fava protein isolates and related fractions. Food Chemistry. 2012;132(1):67–72. doi: 10.1016/j.foodchem.2011.10.033. [DOI] [PubMed] [Google Scholar]
- Vogelsang-O’Dwyer M., Petersen I.L., Joehnke M.S., Sørensen J.C., Bez J., Detzel A.…Zannini E. Comparison of Faba Bean Protein Ingredients Produced Using Dry Fractionation and Isoelectric Precipitation: Techno-Functional, Nutritional and Environmental Performance. Foods. 2020;9(3):322. doi: 10.3390/foods9030322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warsame A.O., Michael N., O’Sullivan D.M., Tosi P. Identification and Quantification of Major Faba Bean Seed Proteins. Journal of Agricultural and Food Chemistry. 2020;68(32):8535–8544. doi: 10.1021/acs.jafc.0c02927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing G., Rui X., Wang D., Liu M., Chen X., Dong M. Effect of Fermentation pH on Protein Bioaccessibility of Soymilk Curd with Added Tea Polyphenols As Assessed by in vitro Gastrointestinal Digestion. Journal of Agricultural and Food Chemistry. 2017;65(50):11125–11132. doi: 10.1021/acs.jafc.7b04456. [DOI] [PubMed] [Google Scholar]
Further reading
- Colca Stelman, J. W. (2014). Efecto del tratamiento térmico sobre la solubilidad proteica, el índice de ureasa y la composición química del haba (Vicia Faba L.).
- Scientific T. A205 Custom Method for Protein and Peptide Quantification. Technical Note. 2014;52649:2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.