Abstract
Early in vertebrate evolution, a single homeobox (Hox) cluster in basal chordates was quadrupled to generate the Hox gene clusters present in extant vertebrates. Here we ask how this expanded gene pool may have influenced the evolution of the visual system. We suggest that a single neurosensory cell type split into ciliated sensory cells (photoreceptors, which transduce light) and retinal ganglion cells (RGC, which project to the brain). In vertebrates, development of photoreceptors is regulated by the basic helix-loop-helix (bHLH) transcription factor Neurod1 whereas RGC development depends on Atoh7 and related bHLH genes. Lancelet (a basal chordate) does not express Neurod or Atoh7 and possesses a few neurosensory cells with cilia that reach out of the opening of the neural tube. Sea-squirts (Ascidians) do not express Neurod and express a different bHLH gene, Atoh8, that is likely expressed in the anterior vesicle. Recent data indicate the neurosensory cells in lancelets may correspond to three distinct eye fields in ascidians, which in turn may be the basis of the vertebrate retina, pineal and parapineal. In this review we contrast the genetic control of visual structure development in these chordates with that of basal vertebrates such as lampreys and hagfish, and jawed vertebrates. We propose an evolutionary sequence linking whole-genome duplications, initially to a split between photoreceptor and projection neurons (RGC) and subsequently between pineal and lateral eye structures.
Keywords: Opsin, Retina, Neuropore, Pineal, Retinal ganglion cell, Eye
1. How to make an eye from scratch
Opsins are central to phototransduction as well as to understanding evolution of the visual system (Arendt et al., 2016, Nilsson, 2021, Schwab, 2018). Opsins evolved together with the regulatory gene Pax6 that interacts with several other genes during eye development (Fritzsch and Piatigorsky, 2005, Schlosser, 2021, Schwab, 2018). A second and largely independent control mechanism comprises retinoic acids (RA) and their synthesizing Raldh enzymes (Crandall et al., 2011, Neitz et al., 2021). A third and equally important aspect of vertebrate eye development is the process of lateral evagination from the neural tube; each evagination interacts with a lens placode to develop a retina and central projections from retinal ganglion cells (Fuhrmann, 2010, Lamb, 2013, Schwab, 2018). In addition to retinal ganglion cells, the developing vertebrate retina needs to generate, in sequence, horizontal, cone, amacrine, rod, bipolar and Müller cells (Lamb, 2013). Finally, additional molecular, development and neuronal control mechanisms work to transform some retinal ganglion cells to yield a melanopsin-based phototransduction cell family with unique properties and brain connections (Lucas et al., 2020).
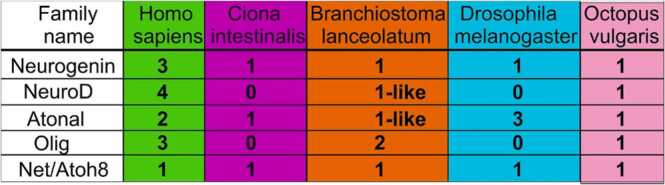
In this review we contrast the sensory cell types and genetic control of cell diversity in the evolution of chordates. We propose a parallel duplication of certain basic Helix-Loop-Helix genes (bHLH) that are expressed near the brainstem and spinal cord (Fig. 1, Fig. 2) as well as the eyes (Fritzsch et al., 2015, Murre, 2019, Simionato et al., 2007). A single atonal/lin-32/Atoh bHLH gene is known in Protostomia (fly, D. melanogaster; nematode, C elegans) and Deuterostomia but is unclear in the lancelet (Holland, 2020, Holland et al., 2008, Simionato et al., 2007, Zhao et al., 2020). Emergence of the genes Atoh in sensory cells and Neurog in spinal cord has been demonstrated for ascidians (Cao et al., 2019, Stolfi et al., 2015, Tang et al., 2013). Furthermore, a duplication of Neurog1/2 and formation of a bHLH gene, Neurod1, is associated with the emergence of distinct sensory cells (under control of Atoh1) and neurons (under control of Neurog1/2; Neurod1) in vertebrates (Fritzsch, 2021). In addition, we highlight the numbers of Neurod1 (4 in vertebrates), Neurog1 (3 in vertebrates), Atoh/atonal/lin-32 (2 in vertebrates,3 in flies) and Olig’s (3 in vertebrates, 2 in the lancelet; Fig. 2). The absence of Neurod1 and Olig (C. intestinalis; D. melanogaster) stands in contrast to to Octopus vulgaris, which has at least one bHLH gene [Fig. 2; (Deryckere et al., 2021; Simionato, et al., 2007)].
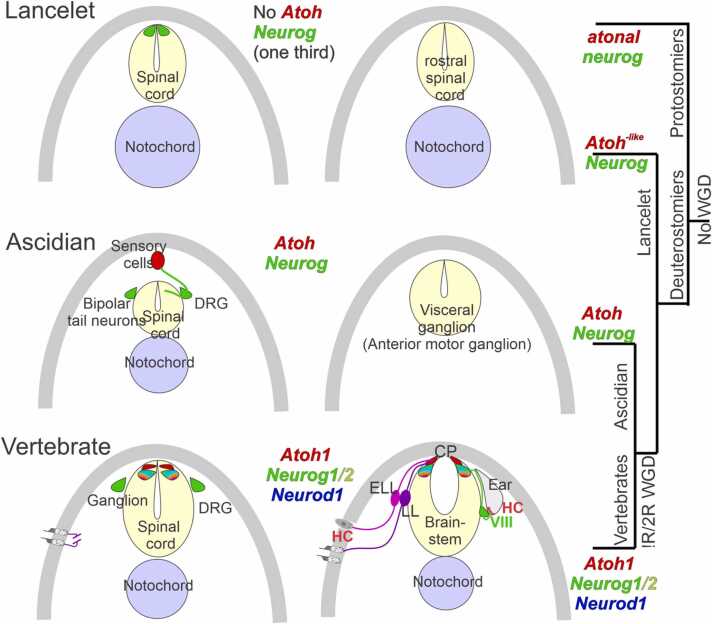
Fig. 1.
Comparing lancelet, ascidians, and lamprey in the spinal cord/brainstem. Lancelets show a limited expression of the bHLH Neurog and have no Atoh-like gene (top). Ciona has sensory cells that require Atoh and are innervated by bipolar tail neurons expressing Neurog which extend to reach the visceral ganglion (middle, DRG). Vertebrates have dorsal root ganglia that depend on Neurog1/2, which is also expressed in Atoh1, Neurog1/2 and Neurod1 in the spinal cord and the brainstem (bottom). The brainstem is innervated by electroreceptor (ELL) and lateral line fibers (LL) that extend to innervate migration populations of LL and some ELL. The ear is unique to vertebrates, giving rise to the VIII ganglia that innervate more ventral nuclei compared to LL and ELL projections to depend on Atoh1 for hair cells, Neurog1 and Neurod1 for neurons. Note, the fly uses atonal for eyes and ears that is known as lin-23 in C. elegans. CP, choroid plexus; DRG, dorsal root ganglia; WGD, whole genome duplication. Modified after (Elliott et al., 2021, 2022; Fritzsch, 2021; Holland, 2020; Holland and Daza, 2018; Stolfi, et al., 2015; Tang, et al., 2013; Zhao, et al., 2020).
Fig. 2.
. basic Helix-Loop-Helic (bHLH) genes are found in all animals and plants and are shown here for only a small set of bHLH genes and species. Note that the total of bHLH genes of 13 (Hs), 3 (Ci), 6 (Bl), 5 (Dm) and 5 (Ov) are documented in various gene analysis. Atoh-like and NeuroD-like are incompletely understood in the lancelet. Note that the largest number of bHLH genes are known among vertebrates (Hs) which is reduced to just 3 bHLH genes in the ascidians (Ci). Modified after (Chen et al., 2011, Deryckere et al., 2021, Holland et al., 2008, Simionato et al., 2007).
We propose that the two rounds of whole-genome duplications [WGD; (Holland and Daza, 2018)] which occurred near the base of the vertebrate lineage (around 580–530 million years ago; (Holland and Holland, 2022)), initially allowed a divergence between photoreceptor and projection neurons (RGCs) and subsequently between pineal and lateral eye structures to evolve Neurod1 and Atoh7 (Fritzsch et al., 2010).
1.1. Amphioxus (Lancelet)
Among chordates, lancelets share with vertebrates a notochord, spinal cord, a neuropore and associated brain-like structures (Fig. 1, Fig. 2, Fig. 3). Lancelets did not experience the two whole genome duplications that underly the genetic makeup of vertebrates, and they possess fewer bHLH genes than humans do (Holland and Daza, 2018, Holland and Holland, 2022). Four different photoreceptive regions are present among lancelets: these are the frontal eye next to the neuropore, the lamellar body, the Joseph cells and the dorsal ocelli (Holland, 2020, Lacalli et al., 1994). The frontal eye has similarities to the retina, with specific gene expression domains having counterparts in the vertebrate retina (Pergner and Kozmik, 2017). To date it has not been confirmed that the ciliary cells of the frontal eye and lamellar body are light-responsive (Lamb, 2013). Moreover, the frontal eye receptors lack several other features of ciliary neurons. The Joseph cells and the dorsal ocelli cells have some features of protostome microvillar photoreceptors, and also express the enzymatic machinery of the transduction cascade, have established photoreceptor function with excitation maximum near 470 nm (del Pilar Gomez et al., 2009) and are connected with the frontal eye fibers (Lacalli, 2018). Immediately anterior to the neuropore are pigmented cells that express the opsin-related transcription factors Pax2/5/8, Otx, Mitf as well as melanin synthesis genes (Fig. 3; (Lamb et al., 2007)). Further from the pigmented cells are sensory cells positive for Pax4/6 as well as Otx, Six3/6, and c-Opsins (Vopalensky et al., 2012). Each sensory cell has a ciliary process that extends toward and out of the neuropore. A central projection through an axon is forming which reaches a central target (Lacalli et al., 1994, Pergner and Kozmik, 2017) that may connect to the primary motor output (Lacalli, 2018, Schlosser, 2021).
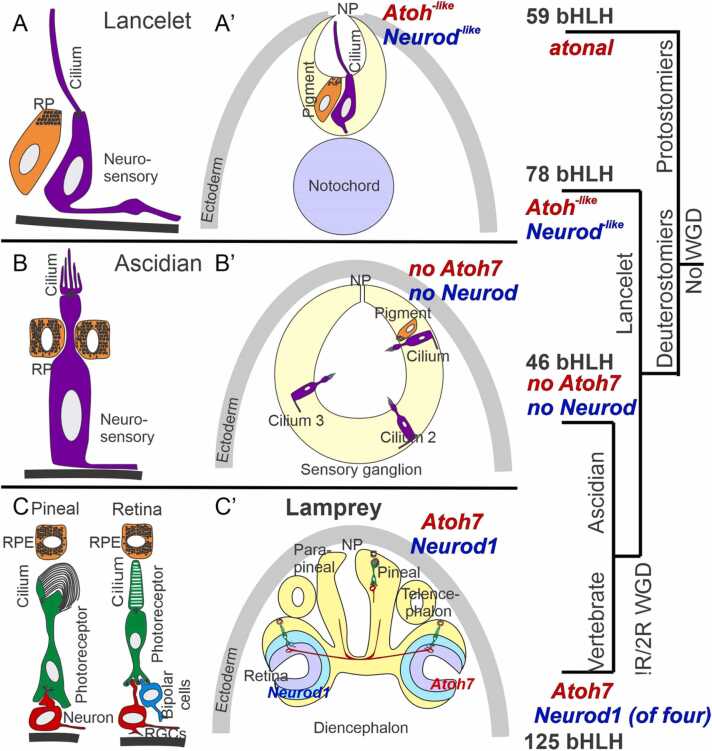
Fig. 3.
Comparing lancelet, ascidians, and lamprey eyes. (A) Lancelet has cilia without lamellae in a frontal eye (A’) that protrudes through the neuropore and has neurosensory cells that project rostrally (A’’). Note that the notochord (lilac) extends beyond the rostrum (section view) and contains pigment cells. (B) Ascidians have a short (or absent) notochord (lilac) and a neuropore which in adults will open through the mouth and possess single or complex cilia (B’) with an outer segment of lamellae. The outer segments are next to or separate from pigment cells (B’’) and project to the neural ganglion that may exhibit a ‘spinal cord’ in derived ascidians. (C) Lampreys have asymmetric pineal dorsal photoreceptor cells that connect with ganglion cells and form synapses. The eye is moved by three sets of extraocular muscles (inset). A transient neuropore opens anterior to the pineal (C’) and expands into a bilateral retina (C’’) containing rods and cones that interconnect with bipolar, horizontal, amacrine and ganglion cells, some of which extend to the outer plexiform layer. Modified after (Barnes, 1971, Braun and Stach, 2017, Braun and Stach, 2019, Collin et al., 2009, Eakin , 1973, Eakin and Kuda, 1970, Fritzsch and Collin, 1990, Fritzsch et al., 1990, Lacalli et al., 1994, Martin and Fritzsch, 2022, Suzuki and Grillner, 2018).
Current data suggest the pigment regeneration chemistry in lancelets is homologous that in vertebrates (Albalat et al., 2012, Poliakov et al., 2012, Vopalensky et al., 2012). The chromophore covalently binds at position Lys 296 in all opsins (Lamb, 2013, Pasqualetto et al., 2020). Molecular fingerprinting of lancelets has identified a total of 21 opsin genes (Pergner and Kozmik, 2017) which produce highly variable opsin proteins: this diversity is comparable to that in vertebrates. The opsin gene family structure thus provides information on duplications and gene loss events in lancelet (Pantzartzi et al., 2018). Also in common with vertebrates, lancelets have genes for melanopsin that could be comparable to vertebrate melanopsin (Koyanagi et al., 2005, Lucas et al., 2020, Pantzartzi et al., 2017).
From the point of view of eye evolution, the central projections of vertebrate retina arise from ganglion cells that express the basic helix-loop-helix (bHLH) gene Atoh7 (Brzezinski and Reh, 2015, Chan et al., 2020, Zhao and Peng, 2021). The Atoh 7 gene is not present in the lancelet (Holland, 2020, Holland et al., 2008). A single atonal-like gene does resemble the net genes of Drosophila but is considered an Orphan bHLH gene rather than indicating an ancient origin of Atoh 7 (Cao et al., 2019, Chen et al., 2011). Further, as shown in following sections, the ciliary sensory cells of vertebrates end in a synaptic ribbon which contacts secondary sensory cells and is also under control of Atoh7 (Zhao and Peng, 2021). Gene expression that resembles certain aspects of other chordates is known among lancelets (Holland, 2020). For example, Foxg1, Emx, Otx, and Gbx genes are common to lancelets and vertebrates (Cao et al., 2019, Glover et al., 2018). However, in vertebrates the transcription factor Foxg1 has an early interaction with Sox2 to control retina formation (Cao et al., 2019, Dvorakova et al., 2020) downstream of Eya1/Six1 (Moody and LaMantia, 2015, Zhang et al., 2021).
In summary, beyond the early steps that produce a ‘frontal eye’ under control of certain primordial genes (Pax2/5/6, Pax4/6, Six3/6, Otx, c-opsins) in lancelets, the evolution of vertebrate eyes required major steps to transform a ciliated sensory neuron (lancelet) into the split arrangement of a ciliated sensory cell and a central projection neuron (RGCs in vertebrates). We suggest at least two or more additional genes evolved in vertebrates (Atoh7, Neurod1) to convert a single neurosensory, neuropil-projecting cell into a ciliary sensory cell connected with a neuropil-projecting ganglion cell.
1.2. Ascidians (Tunicates)
Over 3000 species of ascidians have been characterized (Braun et al., 2020, Winkley et al., 2020). Ascidians have a reduced genome of 70–170 million base pairs (Mbp) compared to lancelet (~ 520 million base pairs) and humans [~ 3 billion base pairs; (Fodor et al., 2021, Holland, 2020)]. Adults in two of five major ascidian groups possess a nerve cord, which is reduced compared to the lancelet [there are ~89 neurons in the near adult sea-squirt Ciona compared to ~20,000 neurons in the lancelet; (Cao et al., 2019, Holland, 2020, Ryan et al., 2018)]. We know that the telencephalon is absent and has no olfactory connections (Cao, et al., 2019). Ciliated sensory cells with adjacent pigment cells are present in nearly all ascidians, at least during early development (Braun et al., 2020, Konno et al., 2010, Kourakis et al., 2019, Winkley et al., 2020). The sensory cells have an outer segment with multiple lamellae (Fig. 3), and each sensory cell connects directly to the nearby neuronal ganglion (Braun and Stach, 2019, Konno et al., 2010, Lacalli and Holland, 1998, Lacalli et al., 1994, Ryan et al., 2018). Detailed analysis shows the origin of the neuropore in Ciona (Veeman and Reeves, 2015) and confirmed the independent connection of the neuropores with the opening of the gut (Veeman et al., 2010). The neuropore is fused with the mouth orifice, an arrangement that is prevented by the rostrally extended notochord of lancelets [Fig. 2; (Ota and Kuratani, 2006)].
In the free-floating ascidian salp Thalia, oocytes develop into a blastozooid stage within a few hours (Winkley et al., 2020). The oocyte has a single horseshoe-shaped set of pigmented cells that are adjacent to the receptor cells (Lacalli and Holland, 1998). However, in the blastozooid stage, the receptor cells form three distinct pigment-associated cups, each one pointing in a different direction (Braun and Stach, 2017). The cups have transparent transient lens cells (Barnes, 1971) and have three discrete groups of sensory cells of which only one is associated with melanin (Konno, et al., 2010). The simple brain of all larval ascidians is connected by multiple branches to these ‘eyes’ but the connections have not been analysed at the level of individual terminals (Braun and Stach, 2019, Ryan et al., 2018).
Among the two Atoh-like genes in ascidians, one is clearly related to Atoh1 and develops with Neurog to innervate the sensory cells and reach out to the anterior motor ganglion (Cao et al., 2019, Fritzsch, 2021, Stolfi et al., 2015, Tang et al., 2013). Another gene, Foxg1, that is expressed in the olfactory system in vertebrates (Dvorakova, et al., 2020) is also expressed in the brain, which is housed in the anterior neural plate (C. intestinalis; Fig. 2; (Liu and Satou, 2019) and likely incorporated with the ectoderm to contribute to the vertebrate forebrain (Cao, et al., 2019). A gene expressed in the brain is currently equated with Atoh8 (Negrón-Piñeiro et al., 2020) that is identified as an Orphan bHLH gene by others (Cao, et al., 2019). Moreover, Atoh8 is split into three parts in chordates that make it difficult to align the genes (Chen, et al., 2011). Atoh8 is expressed broadly in earliest mammals, is broadly expressed outside the brain in vertebrates (Rawnsley et al., 2013) and exerts only a limited effect on eye (Divvela et al., 2022) and ear development (Tang et al., 2021). Moreover, there is no expression of Atoh-like genes in the forebrain and Orphan-bHLH genes are present, suggesting no Atoh is present in Ciona [for example, Pax2/5/8. Pou, Isl; (Cao et al., 2019, Fritzsch and Piatigorsky, 2005)]. These data are consistent with the proposal that duplication of several bHLH genes through WGD enabled evolution of Atoh1/Atoh7 in vertebrates (Fritzsch et al., 2010, Holland and Daza, 2018).
The sea squirt Ciona expresses an opsin-1 gene, which is closely related to the vertebrate visual opsins (Kusakabe et al., 2001, Pisani et al., 2020, Terakita, 2005). As intimated above for the lancelet, the ascidians are unable to transform all-trans to 11-cis retinal, relying instead on photoisomerization (Albalat et al., 2012, Poliakov et al., 2012). Interestingly, certain species do not bind RA while others use conventional RA signaling (Fujiwara and Cañestro, 2018). Further, the ascidian arrestin binds to opsin-based pigments, a function similar to the vertebrate non-visual arrestin, β-arrestin (Kawano-Yamashita et al., 2011, Nakagawa et al., 2002).
In summary, although certain similarities are clear with respect to opsins in the lancelet, ascidians and vertebrates, we lack a model of how to transform the simple frontal eye of lancelet to the tunicate eye (Lamb, 2013). The main difference is the lack of certain genes (Pax4/6, Eya/Six, Mitf) which are required for eye development at the level of vertebrates. On the other hand, studies of gene regulatory networks have revealed evolutionarily conserved gene circuits between ascidians and vertebrates (Cao et al., 2019, Liu and Satou, 2020, Ryan et al., 2018). Moreover, ascidians have neurosensory cells that are associated with pigment cells and likely depend on Mitf signals from the mesenchyme as is the case in lancelets and vertebrates. These similarities let us to propose a split into independent populations of sensory and projection cells in the ascidians must have happened at or near the base of the vertebrate lineage some 530 million years ago in line with the quadrupling of Hox clusters (Holland and Daza, 2018).
2. From lampreys and hagfish to jawed fishes: Retina development and evolution
As summarized above, from an evolutionary perspective, eye development starts with neuropore-associated sensory cells. We next consider eye and retina development as exemplified in the extant cyclostomes (lampreys and hagfish) that first appeared ~525 million years ago and have evolved into 35 lamprey and 79 hagfish species [OneZoom: Jawless vertebrates].
2.1. Lampreys
In lampreys, retina formation begins in the late embryos with a few receptors and retinal ganglion cells (RGC) that receive a prominent efferent input from the brain (Anadon et al., 1998, Barreiro‐Iglesias et al., 2017, De Miguel et al., 1989, Fain, 2019, Fritzsch, 1991, Fritzsch and Collin, 1990, Lamb, 2013, Pombal and Megías, 2019, Suzuki and Grillner, 2018, Villar-Cheda et al., 2006). Opsin-expressing photoreceptor cells are reported to be present early in larvae and to make contact with RGCs and bipolar cells (Suzuki and Grillner, 2018). If confirmed, this result means the developmental sequence of RGC>horizontal>cone>amacrine>rod>bipolar (Lamb, 2013 ), which is characteristic of jawed vertebrates (as described in detail below), is different to that in lampreys where there is simultaneous formation of rods>RGC>bipolar followed by horizontal and amacrine cells (Suzuki and Grillner, 2018). Lampreys (Fig. 3) express up to five different sensory cells (Collin et al., 2009, Warrington et al., 2021) with morphology resembling that of jawed vertebrates (Lamb, 2013, Lamb et al., 2007). One sensory cell type expresses rhodopsin and shows rod-like transduction (Lamb, 2013) yet also shows cone-like light saturation dynamics (Fain, 2019, Morshedian and Fain, 2017). In jawed vertebrates the retinal ganglion cells form a distinct layer but in lampreys the majority (~74%) of RGC somas are in the inner nuclear layer (INL) (Fritzsch and Collin, 1990, Jones et al., 2009) and, unlike the arrangement in jawed vertebrates, in lampreys the optic fibers run scleral to the inner plexiform layer (Fritzsch and Collin, 1990). Further, some ganglion cells make direct contact with photoreceptors: a situation otherwise unprecedented in gnathostomes (Fain, 2019, Jones et al., 2009). The different position of RGCs (IPL versus GCL) combined with branches that reach photoreceptors may indicate a unique and direct input of cones to RGCs in lampreys (Fritzsch and Collin, 1990, Jones et al., 2009, Suzuki and Grillner, 2018). On the other hand, recent electrophysiological data (Morshedian et al., 2021) shows the classical vertebrate split into On-type and Off-type light response polarity is present in lamprey retina.
Recent data confirmed earlier interaction in the cerebellum between Atoh1 and Neurod1 (Kersigo et al., 2021, Sugahara et al., 2021). Unfortunately, Atoh7 is known to be present but has not been analysed in the lamprey (Smith et al., 2013) in which there is expression of Neurod1 in the eye and the pineal is needed.
2.2. Hagfish
Available evidence suggests that unlike lamprey eye, the hagfish eye does not have a lens (Baden et al., 2020, Collin and Fritzsch, 1993, Dong and Allison, 2021, Gustafsson et al., 2008, Song and Kim, 2020), which is now clarified as playing a major role in eye development (Fain, 2020, Fuhrmann, 2010). If confirmed, the hagfish eye would be the only extant eye without a lens (Miyashita, 2020, Schwab, 2018). The ciliated sensory cells in hagfish require rhodopsin and are likely rod cells (Lamb, 2013): a suggestion consistent with recent evidence for true rods and cones in lampreys (Fain, 2019, Fain, 2020). The retinal ganglion cell disposition resembles that of lampreys (Fritzsch and Collin, 1990, Suzuki and Grillner, 2018), likely correlated with the absence of myelin (Fritzsch and Glover, 2007), and there may be a direct input from ciliated sensory cells to RGCs via ribbons (Holmberg, 1971, Holmberg, 1977, Locket and Jørgensen, 1998). Its similarity with the brainstem expression in the hagfish (Higuchi et al., 2019) makes it likely to be positive for Atoh7 and Neurod1 in the eye expression.
2.3. Gnathostomes
The sequence of development of different retinal cell types in gnathostomes (jawed vertebrates) has been established following the initial discovery of proliferation of a column of retinal cells (Cepko et al., 1996). The earliest stage is the formation of RGCs: this process depends on the transcription factor Atoh7 in all vertebrates (Baker and Brown, 2018, Wu et al., 2021, Zhao and Peng, 2021). Downstream of Atoh7 are Brn3b/Pou4f2 and Isl1 and the distalless homolog (Dlx2) which is not regulated by Atoh7 (Brzezinski and Reh, 2015, Cao et al., 2019). The next stage is generation of cone and amacrine cells, followed by horizontal cells. Rod and cone cells depend on the transcription factor Neurod1 (Dennis et al., 2019, Fritzsch et al., 2010, Kersigo et al., 2021, Neitz et al., 2021) and interactions with other genes to allow their differentiation as distinct sensory cells of cone and rods such as Crc (Brzezinski and Reh, 2015, Wu et al., 2021). The best-studied species are zebrafish, Xenopus, chicken and (predominantly) mouse (Baker and Brown, 2018, Hutcheson et al., 2005).
A detailed description of retinal development is provided by Fuhrmann (2010), highlighting multiple steps involved in retina expansion and interaction with the lens (Fig. 3). The eye field in the neural plate first splits into two parts which each interact with the lens placode to form a retina. As the retina expands it induces development of retinal pigment epithelium (RPE). The next steps are driven by mesenchymal induction of the eye field transcription factors Mitf, Pax6, Rax, Otx2, Six3 and Lhx2 (Fuhrmann, 2010). In addition, the fibroblast growth factor Fgf is secreted from the lens ectoderm (Fuhrmann, 2010) and upregulates pERK signal transduction genes as well as transcriptional regulators Vsx2 and Sox2. Expression of Sox2 drives the neuronal part of the retina development, requiring Vsx2-mediated suppression of Mitf (Fuhrmann, 2010).
Once the lens begins forming, three distinct areas (RPE, neural retina, optic stalk) require additional interactions under wingless / integrated (Wnt) signal transduction pathways to retain RPE under Mitf, BMP, RA and β-catenin control (Fig. 2), and interaction with Shh to define the optic stalk (Buono and Martinez‐Morales, 2020, Fuhrmann, 2010). These complex interactions have been detailed in Xenopus with emphasis on the role of noggin as an antagonist for BMPs involving tbx3, Pax6, Pax2, Vsx2, Shh and RA (Sasagawa et al., 2002). Comparable information exists for early ear development in the zebrafish for which interesting effects of the same controlling genes can be studied in context of visual development, anophthalmia, retinitis pigmentosa, cyclopia and coloboma (Cavodeassi et al., 2005, Richardson et al., 2017). Unfortunately, much work on cyclostomes is still required to fill in the gaps in our understanding.
In summary, a set of intrinsic and extracellular factors control eye organogenesis but there are gaps in knowledge of these interactions, in particular the control of evagination of the retina and the specific roles of RA and other genes (Fuhrmann, 2010, Lamb, 2013, Manns and Fritzsch, 1991).
3. Pineal sensory system
A parallel sensory system to the retina is the pineal and parapineal of lampreys and gnathostomes; these structures are also known as parietal or pineal eyes (de Vlaming and Olcese, 2020, Koyanagi et al., 2004, Meléndez‐Ferro et al., 2002, Pu and Dowling, 1981, Reiter, 1980, Roberts, 1978, Simonneaux and Ribelayga, 2003, Vigh-Teichmann et al., 1983). Pineal eyes are absent in hagfish (Lamb, 2013). A different pattern of connections is present in mammals compared to non-mammalian vertebrates (Reiter, 1980, Simonneaux and Ribelayga, 2003). Melatonin is a hormone that is secreted by the pineal and plays a role in the biorhythms and reproductive activity in mammals (Kim et al., 2022).
The pineal forms adjacent to the neuropore and represents a dorsal expansion of the diencephalon. In the pineal, ciliated sensory cells connect to ganglion cells with ribbon synapses (Lamb, 2013, Pu and Dowling, 1981). The pineal receptors have rod-like function and use rhodopsin (Lamb, 2013). The presence of two cell types (ciliated sensory and ganglion cells) sets the pineal apart from the direct neurosensory output in lancelet and ascidians (neurosensory cells), and suggests that pineal eyes evolved together with or following the evolution of lateral eyes with RGCs (Fain, 2019, Jones et al., 2009). The left and right pineal and parapineal in lampreys and several gnathostomes have different sizes (de Vlaming and Olcese, 2020, Pu and Dowling, 1981, Smith et al., 2018). The pineal is connected with the right whereas the parapineal is connected to the left, forming an asymmetric habenula (Meléndez‐Ferro et al., 2002, Puzdrowski and Northcutt, 1989). The presence of three distinct receptor clusters in tunicates (Braun and Stach, 2019) could be the base of the pineal that has been equated with ascidians split into three eye clusters (Horie et al., 2008, Negrón-Piñeiro et al., 2020, Schlosser, 2021).
Several genes are associated with the mammalian pineal including Lhx9, Pax6 and Otx2 (Yamazaki et al., 2015, Yang et al., 2019). The expression of Neurod1 is documented (Coon et al., 2019, Ge et al., 2020, Munoz et al., 2007) but the pineal appears histologically normal after Neurod1 deletion (Ochocinska et al., 2012). A limited expression of Atoh7 in Xenopus pineal has been shown (Hutcheson, et al., 2005); resolving these differences will require a detailed analysis in some of the fully developed vertebrates and mammals (which show a different organization of the pineal).
4. Neuropore formation is unique among chordates
A common feature in chordates is the formation of the neuropore that transiently connects with the rostral neural tube and the external skin (Fig. 3, Fig. 4, Fig. 5). Formation of the neural tube starts in the hindbrain and fusion progresses anteriorly, leaving the neuropore at the last step of closure the dorsal part of the brain in gnthostomes (Maniou et al., 2021). Likewise, he ‘frontal eye’ in lancelets exhibits a few sensory cells which reach out of the neuropore (Holland, 2020, Lacalli et al., 1994). A detailed description in ascidians shows the formation of a neuropore that opens to the mouth directly (Veeman and Reeves, 2015, Veeman et al., 2010). In lampreys the neuropore starts as a keel that forms into the neural tube in later development (Richardson et al., 2010, Sauka-Spengler and Bronner-Fraser, 2008). A neuropore has been described for hagfish but not much further information is available due to limited knowledge of hagfish development (Higuchi et al., 2019, Ota and Kuratani, 2006, Wicht, 1996). Overall, this early step is comparable to the keel of zebrafish neuropore formation and similar factors may influence closure of the neuropore (Shinotsuka et al., 2018) in amniotes (Copp and Greene, 2010, Maniou et al., 2021, Müller and O'Rahilly, 1987, Rolo et al., 2019).
Fig. 4.
Molecular control of retina development. Retina formation starts ventral to the neuropore, adjacent to the IIIrd ventricle and shows expression of the eye field (A). Right panels display sequential upregulation of transcription factors Mitf and Rax to interact with Pax6, Six1, Otx2 and Lhx2 (B) followed by Fgf expression collaborating with Sox2 to suppress Mitf (C). Several genes interact to upregulate the lens placode during eye formation (D). Shh defines the optic stalk, Mitf develops pigments whereas Sox2 and Pax6 induce neuronal precursors in the retina (E). (A’) shows the effect of high doses of RA causing the evagination to be suppressed but a normal retina develops adjacent to the pigment. Modified after (Buono and Martinez‐Morales, 2020, Fuhrmann, 2010, Manns and Fritzsch, 1991).
Fig. 5.
Comparison of sensory cilia formations in chordates. Lancelet has a simple cilium (A) that is located adjacent to pigment cells (A’). The central lumen of the terminal sac extends cilia into the neuropore (NP). The ‘sensory ganglion’ comprises one cilium associated with pigment and two ciliary sensory cells that are not associated with pigments. Ascidians have cilium branches (B), tightly associated pigment cells, and projections to the neuropore comprising three groups of pigment cells in certain ascidians (B’). Lampreys have two photoreceptive organs: the pineal/parapineal and the retina (C). Photoreceptor cells in both organs terminate in ribbons (C). Brain projections arise either directly (pineal) or from ganglion cells (RGCs) in a retina that also has bipolar cells, horizontal and amacrine cells to process the visual input and transmit it to the brain. Note that vertebrates have distinct bHLH genes (Atoh7, Neurod1, C’) that are not found in the lancelet (A’) nor the ascidians (B’). atonal is needed for eye development in flies and possibly evolved into 4 distinct genes in vertebrates. Modified after (Cao et al., 2019, Elliott et al., 2021, Fritzsch et al., 2015, Holland, 2020, Martin and Fritzsch, 2022, Suzuki and Grillner, 2018).
The genes Opb (open brain) and Zic2 interact with Shh to regulate closure of the neural tube and reduced eye development is one consequence of failure of neural tube closure (Eggenschwiler et al., 2001, Gunther et al., 1994, Guo et al., 2006, Rolo et al., 2019). In part, the absence of Msx1 and Wnt3a underlies the effects seen in Opb and Zic2 null mice, in which the brain remains open without any closure (Copp and Greene, 2010). Downstream to Opb/Zic2 are Fgfs, Wnts, Retinoic acid (RA), Nodal and BMPs that provide the signals that will, eventually, close the neuropore (Cao et al., 2019, Wilson and Houart, 2004) and are involved with Shh in mutants (Eggenschwiler et al., 2001). Triazole interferes with RA signaling and produces numerous defects in retinal and anterior neuropore formation as well as otic defects (Shinotsuka, et al., 2018). Wnt/Frizzled signaling involves a set of the planar cell-polarity genes (Vang, Scrb1, Crash, Disheveled) that cause the craniorachisis in mutant mice, implying these signal pathways in closure of the neuropore (Rolo, et al., 2019). In summary, the proximity of the neuropore to the pineal/parapineal in lancelet, ascidians, mice and man can affect the pineal and retina in extreme cases after loss of Opb to connect with craniorachisis.
5. A plausible sequence for retina development and evolution
A new hypothesis of retina evolution is proposed (Fig. 5). Building on previous work describing the formation of two distinct cell types through whole genome duplication (Fritzsch and Elliott, 2017, Holland and Daza, 2018), we can envision the same process permitting eye development through retina multiplication (Fig. 5). The vertebrate genome duplicated twice prior to divergence of the lineage that became vertebrates from other chordates (Fig. 2): this whole-genome duplication permitted the splitting of sensory cells from brain-projecting neurons in lampreys as well as gnathostomes (Holland and Daza, 2018, Smith et al., 2013). The alternative hypothesis that multiple cell types existed in ancestral organisms and were then lost in lancelets and tunicates cannot be ruled out, but the weight of evidence summarized in the foregoing sections is in favor of the splitting hypothesis.
We now propose that the increased gene pool also allowed divergence of more complex visual structures, that is, lateral eyes and pineal that develop next to the neuropore. Neurosensory cells have dual function of light transduction and central projection, represented by cells that have a ciliary process with its own axon in the lancelet and ascidians. These cell types form three distinct clusters in the blastocyst life stage of the tunicate sea squirt Thalia. We propose two different sized clusters in Thalia and Ciona evolved into pineal/parapineal and the third, largest, cluster evolved into the lateral eyes of vertebrates. By analogy with a split into distinct cell types (sensory cells and ganglion cells) from a single cell type (neurosensory cells) in lancelet and ascidians (Fritzsch, 2021, Fritzsch and Elliott, 2017, Holland and Daza, 2018), we suggest a split into distinct retina-like structures that is shown by the presence in lampreys of the pineal, parapineal, and bilateral retinas proper. An intermediate stage of this process may be represented by the direct neuropil projections of sensory cells in certain lampreys and hagfish. The divergence in lamprey of two kinds of ciliated sensory cells (under control of Neurod1) and a set of neurons (under control of Atoh7) is proposed to parallel that in gnathostomes, which subsequently have developed many more basic neuron types (Lamb, 2013). Further work is needed to detail the three vertebrate Atoh (Atoh1, Atoh7, Atoh8) and four Neurod (Neurod1, Neurod2, Neurod4, Neurod6) genes that will allow to establish the bHLH genes in chordates.
6. Summary and conclusion
Central projection of visual information in vertebrates has its evolutionary origin in a single neurosensory, central projecting, cell type. This single cell type split into a sensory ciliated cell (Neurod1) connecting via ribbons to the Atoh7-regulated cell families that send the visual information to the brain. The three different branches of vertebrate eyes (retina, pineal, parapineal) could have originated in the three retinal outputs present in ascidians that all develop near the neuropore.
Author Contributions
BF and PM wrote the manuscript. All authors read and approved the final manuscript.
Funding
BF was supported by NIH/NIA (R01 AG060504).
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Albalat R., Martí-Solans J., Cañestro C. DNA methylation in amphioxus: from ancestral functions to new roles in vertebrates. Brief. Funct. Genom. 2012;11:142–155. doi: 10.1093/bfgp/els009. [DOI] [PubMed] [Google Scholar]
- Anadon R., Melendez-Ferro M., Perez-Costas E., Pombal M.A., Rodicio M.C. Centrifugal fibers are the only GABAergic structures of the retina of the larval sea lamprey: an immunocytochemical study. Brain Res. 1998;782:297–302. doi: 10.1016/s0006-8993(97)01330-9. [DOI] [PubMed] [Google Scholar]
- Arendt D., Musser J.M., Baker C.V., Bergman A., Cepko C., Erwin D.H., Pavlicev M., Schlosser G., et al. The origin and evolution of cell types. Nat. Rev. Genet. 2016;17:744–757. doi: 10.1038/nrg.2016.127. [DOI] [PubMed] [Google Scholar]
- Baden T., Euler T., Berens P. Understanding the retinal basis of vision across species. Nat. Rev. Neurosci. 2020;21:5–20. doi: 10.1038/s41583-019-0242-1. [DOI] [PubMed] [Google Scholar]
- Baker N.E., Brown N.L. All in the family: proneural bHLH genes and neuronal diversity. Dev. 2018;145(9) doi: 10.1242/dev.159426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S.N. Fine structure of the photoreceptor and cerebral ganglion of the tadpole larva of Amaroucium constellatum (Verrill)(Subphylum: Urochordata; Class: Ascidiacea). Z. für Zellforsch. mikroskopische Anat. 1971;117:1–16. doi: 10.1007/BF00331097. [DOI] [PubMed] [Google Scholar]
- Barreiro‐Iglesias A., Fernández‐López B., Sobrido‐Cameán D., Anadón R. Organization of alpha‐transducin immunoreactive system in the brain and retina of larval and young adult Sea Lamprey (Petromyzon marinus), and their relationship with other neural systems. J. Comp. Neurol. 2017;525:3683–3704. doi: 10.1002/cne.24296. [DOI] [PubMed] [Google Scholar]
- Braun K., Leubner F., Stach T. Phylogenetic analysis of phenotypic characters of Tunicata supports basal Appendicularia and monophyletic Ascidiacea. Cladistics. 2020;36:259–300. doi: 10.1111/cla.12405. [DOI] [PubMed] [Google Scholar]
- Braun K., Stach T. Structure and ultrastructure of eyes and brains of Thalia democratica (Thaliacea, Tunicata, Chordata) J. Morphol. 2017;278:1421–1437. doi: 10.1002/jmor.20722. [DOI] [PubMed] [Google Scholar]
- Braun K., Stach T. Morphology and evolution of the central nervous system in adult tunicates. J. Zool. Syst. Evolut. Res. 2019;57:323–344. [Google Scholar]
- Brzezinski J.A., Reh T.A. Photoreceptor cell fate specification in vertebrates. Development. 2015;142:3263–3273. doi: 10.1242/dev.127043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buono L., Martinez‐Morales J.R. Retina development in vertebrates: systems biology approaches to understanding genetic programs: on the contribution of next‐generation sequencing methods to the characterization of the regulatory networks controlling vertebrate eye development. Bioessays. 2020;42 doi: 10.1002/bies.201900187. [DOI] [PubMed] [Google Scholar]
- Cao C., Lemaire L.A., Wang W., Yoon P.H., Choi Y.A., Parsons L.R., Matese J.C., Levine M., et al. Comprehensive single-cell transcriptome lineages of a proto-vertebrate. Nature. 2019;571:349–354. doi: 10.1038/s41586-019-1385-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavodeassi F., Carreira-Barbosa F., Young R.M., Concha M.L., Allende M.L., Houart C., Tada M., Wilson S.W. Early stages of zebrafish eye formation require the coordinated activity of Wnt11, Fz5, and the Wnt/β-catenin pathway. Neuron. 2005;47:43–56. doi: 10.1016/j.neuron.2005.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C.L., Austin C.P., Yang X., Alexiades M., Ezzeddine D. Cell fate determination in the vertebrate retina. Proc. Natl. Acad. Sci. USA. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C.S., Lonfat N., Zhao R., Davis A.E., Li L., Wu M.-R., Lin C.-H., Ji Z., et al. Cell type-and stage-specific expression of Otx2 is regulated by multiple transcription factors and cis-regulatory modules in the retina. Development. 2020:147. doi: 10.1242/dev.187922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Dai F., Balakrishnan-Renuka A., Leese F., Schempp W., Schaller F., Hoffmann M.M., Morosan-Puopolo G., et al. Diversification and molecular evolution of ATOH8, a gene encoding a bHLH transcription factor. PLOS One. 2011;6 doi: 10.1371/journal.pone.0023005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin S.P., Davies W.L., Hart N.S., Hunt D.M. The evolution of early vertebrate photoreceptors. Philos. Trans. R. Soc. B: Biol. Sci. 2009;364:2925–2940. doi: 10.1098/rstb.2009.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin S.P., Fritzsch B. Observations on the shape of the lens in the eye of the silver lamprey, Ichthyomyzon unicuspis. Can. J. Zool. 1993;71:34–41. [Google Scholar]
- Coon S.L., Fu C., Hartley S.W., Holtzclaw L., Mays J.C., Kelly M.C., Kelley M.W., Mullikin J.C., et al. Single cell sequencing of the pineal gland: the next chapter. Front. Endocrinol. 2019;10:590. doi: 10.3389/fendo.2019.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp A.J., Greene N.D. Genetics and development of neural tube defects. J. Pathol. 2010;220:217–230. doi: 10.1002/path.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall J.E., Goodman T., McCarthy D.M., Duester G., Bhide P.G., Dräger U.C., McCaffery P. Retinoic acid influences neuronal migration from the ganglionic eminence to the cerebral cortex. J. Neurochem. 2011;119:723–735. doi: 10.1111/j.1471-4159.2011.07471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miguel E., Rodicio M.C., Anadón R. Ganglion cells and retinopetal fibers of the larval lamprey retina: an HRP ultrastructural study. Neurosci. Lett. 1989;106:1–6. doi: 10.1016/0304-3940(89)90192-4. [DOI] [PubMed] [Google Scholar]
- de Vlaming V., Olcese J. The Pineal Gland. CRC press; 2020. The pineal and reproduction in fish, amphibians, and reptiles; pp. 1–29. [Google Scholar]
- del Pilar Gomez M., Angueyra J.M., Nasi E. Light-transduction in melanopsin-expressing photoreceptors of Amphioxus. Proc. Natl. Acad. Sci. USA. 2009;106:9081–9086. doi: 10.1073/pnas.0900708106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis D.J., Han S., Schuurmans C. bHLH transcription factors in neural development, disease, and reprogramming. Brain Res. 2019;1705:48–65. doi: 10.1016/j.brainres.2018.03.013. [DOI] [PubMed] [Google Scholar]
- Deryckere A., Styfhals R., Elagoz A.M., Maes G.E., Seuntjens E. Identification of neural progenitor cells and their progeny reveals long distance migration in the developing octopus brain. eLife. 2021;10 doi: 10.7554/eLife.69161. e69161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divvela S.S.K., Saberi D., Brand-Saberi B. Atoh8 in development and disease. Biology. 2022;11:136. doi: 10.3390/biology11010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E.M., Allison W.T. Vertebrate features revealed in the rudimentary eye of the Pacific hagfish (Eptatretus stoutii) Proc. R. Soc. B Biol. Sci. 2021;288 doi: 10.1098/rspb.2020.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorakova M., Macova I., Bohuslavova R., Anderova M., Fritzsch B., Pavlinkova G. Early ear neuronal development, but not olfactory or lens development, can proceed without SOX2. Dev. Biol. 2020;457:43–56. doi: 10.1016/j.ydbio.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakin R.M. Univ of California Press; 1973. The Third Eye. [Google Scholar]
- Eakin R.M., Kuda A. Ultrastructure of sensory receptors in ascidian tadpoles. Z. für Zellforsch. mikroskopische Anat. 1970;112:287–312. doi: 10.1007/BF02584045. [DOI] [PubMed] [Google Scholar]
- Eggenschwiler J.T., Espinoza E., Anderson K.V. Rab23 is an essential negative regulator of the mouse Sonic hedgehog signalling pathway. Nature. 2001;412:194–198. doi: 10.1038/35084089. [DOI] [PubMed] [Google Scholar]
- Elliott K.L., Pavlínková G., Chizhikov V.V., Yamoah E.N., Fritzsch B. Development in the mammalian auditory system depends on transcription factors. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22084189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K.L., Sokolowski B., Yamoah E.N., Fritzsch B. In: The Evolution of Sensory System Revealed: Gene Regulation, Cellular Networks and Processes. Fritzsch B., Elliott K., editors. Wiley; 2022. An integrated perspective of communalities and difference across sensory receptors and their distinct central input; pp. 255–290. [Google Scholar]
- Fain G.L. Lamprey vision: photoreceptors and organization of the retina. Semin. Cell Dev. Biol. 2019 doi: 10.1016/j.semcdb.2019.10.008. [DOI] [PubMed] [Google Scholar]
- Fain G.L. Lamprey vision: photoreceptors and organization of the retina. Semin. Cell Dev. Biol. 2020;105:5–11. doi: 10.1016/j.semcdb.2019.10.008. [DOI] [PubMed] [Google Scholar]
- Fodor A., Liu J., Turner L., Swalla B.J. Transitional chordates and vertebrate origins: Tunicates. Curr. Top. Dev. Biol. 2021;141:149–171. doi: 10.1016/bs.ctdb.2020.10.001. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. The Changing Visual System. Springer; 1991. Ontogenetic clues to the phylogeny of the visual system; pp. 33–49. [Google Scholar]
- Fritzsch B. An integrated perspective of evolution and development: from genes to function to ear, lateral line and electroreception. Diversity. 2021;13:364. doi: 10.3390/d13080364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B., Collin S.P. Dendritic distribution of two populations of ganglion cells and the retinopetal fibers in the retina of the silver lamprey (Ichthyomyzon unicuspis) Vis. Neurosci. 1990;4:533–545. doi: 10.1017/s0952523800005745. [DOI] [PubMed] [Google Scholar]
- Fritzsch B., Eberl D.F., Beisel K.W. The role of bHLH genes in ear development and evolution: revisiting a 10-year-old hypothesis. Cell. Mol. Life Sci. 2010;67:3089–3099. doi: 10.1007/s00018-010-0403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B., Elliott K.L. Gene, cell, and organ multiplication drives inner ear evolution. Dev. Biol. 2017;431:3–15. doi: 10.1016/j.ydbio.2017.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B., Glover J. , 2007. Evolution of the deuterostome central nervous system: an intercalation of developmental patterning processes with cellular specification processes. 1-24.
- Fritzsch B., Jahan I., Pan N., Elliott K.L. Evolving gene regulatory networks into cellular networks guiding adaptive behavior: an outline how single cells could have evolved into a centralized neurosensory system. Cell Tissue Res. 2015;359:295–313. doi: 10.1007/s00441-014-2043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B., Piatigorsky J. Ancestry of photic and mechanic sensation? Science. 2005;308:1113–1115. [PubMed] [Google Scholar]
- Fritzsch B., Sonntag R., Dubuc R., Ohta Y., Grillner S. Organization of the six motor nuclei innervating the ocular muscles in lamprey. J. Comp. Neurol. 1990;294:491–506. doi: 10.1002/cne.902940402. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S. Eye morphogenesis and patterning of the optic vesicle. Curr. Top. Dev. Biol. 2010;93:61–84. doi: 10.1016/B978-0-12-385044-7.00003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S., Cañestro C. Transgenic Ascidians. Springer; 2018. Reporter analyses reveal redundant enhancers that confer robustness on cis-regulatory mechanisms; pp. 69–79. [DOI] [PubMed] [Google Scholar]
- Ge L.-J., Yang F.-H., Li W., Wang T., Lin Y., Feng J., Chen N.-H., Jiang M., et al. In vivo neuroregeneration to treat ischemic stroke through NeuroD1 AAV-based gene therapy in adult non-human primates. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.590008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover J.C., Elliott K.L., Erives A., Chizhikov V.V., Fritzsch B. Wilhelm His’ lasting insights into hindbrain and cranial ganglia development and evolution. Dev. Biol. 2018;444:S14–S24. doi: 10.1016/j.ydbio.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther T., Struwe M., Aguzzi A., Schughart K. Open brain, a new mouse mutant with severe neural tube defects, shows altered gene expression patterns in the developing spinal cord. Development. 1994;120:3119–3130. doi: 10.1242/dev.120.11.3119. [DOI] [PubMed] [Google Scholar]
- Guo A., Wang T., Ng E.L., Aulia S., Chong K.H., Teng F.Y.H., Wang Y., Tang B.L. Open brain gene product Rab23: expression pattern in the adult mouse brain and functional characterization. J. Neurosci. Res. 2006;83:1118–1127. doi: 10.1002/jnr.20788. [DOI] [PubMed] [Google Scholar]
- Gustafsson O., Collin S., Kröger R. Early evolution of multifocal optics for well-focused colour vision in vertebrates. J. Exp. Biol. 2008;211:1559–1564. doi: 10.1242/jeb.016048. [DOI] [PubMed] [Google Scholar]
- Higuchi S., Sugahara F., Pascual-Anaya J., Takagi W., Oisi Y., Kuratani S. Inner ear development in cyclostomes and evolution of the vertebrate semicircular canals. Nature. 2019;565:347–350. doi: 10.1038/s41586-018-0782-y. [DOI] [PubMed] [Google Scholar]
- Holland L. Evolutionary Neuroscience. Elsevier; 2020. Invertebrate origins of vertebrate nervous systems; pp. 51–73. [Google Scholar]
- Holland L.Z., Albalat R., Azumi K., Benito-Gutiérrez È., Blow M.J., Bronner-Fraser M., Brunet F., Butts T., et al. The amphioxus genome illuminates vertebrate origins and cephalochordate biology. Genome Res. 2008;18:1100–1111. doi: 10.1101/gr.073676.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland L.Z., Daza D.O. A new look at an old question: when did the second whole genome duplication occur in vertebrate evolution? Genome Biol. 2018;19:1–4. doi: 10.1186/s13059-018-1592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland L.Z., Holland N.D. The invertebrate chordate amphioxus gives clues to vertebrate origins. Curr. Tope. Dev. Biol. 2022;147:563–594. doi: 10.1016/bs.ctdb.2021.12.011. [DOI] [PubMed] [Google Scholar]
- Holmberg K. The hagfish retina: electron microscopic study comparing receptor and epithelial cells in the Pacific hagfish, Polistotrema stouti, with those in the Atlantic hagfish, Myxine glutinosa. Z. für Zellforsch. mikroskopische Anatomie. 1971;121:249–269. doi: 10.1007/BF00340676. [DOI] [PubMed] [Google Scholar]
- Holmberg K. The Visual System in Vertebrates. Springer; 1977. The cyclostome retina; pp. 47–66. [Google Scholar]
- Horie T., Sakurai D., Ohtsuki H., Terakita A., Shichida Y., Usukura J., Kusakabe T., Tsuda M. Pigmented and nonpigmented ocelli in the brain vesicle of the ascidian larva. J. Comp. Neurol. 2008;509:88–102. doi: 10.1002/cne.21733. [DOI] [PubMed] [Google Scholar]
- Hutcheson D.A., Hanson M.I., Moore K.B., Le T.T., Brown N.L., Vetter M.L. bHLH-dependent and-independent modes of Ath5 gene regulation during retinal development. Dev. 2005;132:829–839. doi: 10.1242/dev.01653. [DOI] [PubMed] [Google Scholar]
- Jones M.R., Grillner S., Robertson B. Selective projection patterns from subtypes of retinal ganglion cells to tectum and pretectum: distribution and relation to behavior. J.Comp. Neurol. 2009;517:257–275. doi: 10.1002/cne.22203. [DOI] [PubMed] [Google Scholar]
- Kawano-Yamashita E., Koyanagi M., Shichida Y., Oishi T., Tamotsu S., Terakita A. Beta-arrestin functionally regulates the non-bleaching pigment parapinopsin in lamprey pineal. PLOS One. 2011;6 doi: 10.1371/journal.pone.0016402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersigo J., Gu L., Xu L., Pan N., Vijayakuma S., Jones T., Shibata S.B., Fritzsch B., et al. Effects of Neurod1 expression on mouse and human schwannoma cells. Laryngoscope. 2021;131:E259–E270. doi: 10.1002/lary.28671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.S., Jeong S.P., Park B.S., Kim I.R. Melatonin attenuates RANKL-induced osteoclastogenesis via inhibition of Atp6v0d2 and DC-STAMP through MAPK and NFATc1 signaling pathways. Molecules. 2022;27 doi: 10.3390/molecules27020501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno A., Kaizu M., Hotta K., Horie T., Sasakura Y., Ikeo K., Inaba K. Distribution and structural diversity of cilia in tadpole larvae of the ascidian Ciona intestinalis. Dev. Biol. 2010;337:42–62. doi: 10.1016/j.ydbio.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Kourakis M.J., Borba C., Zhang A., Newman-Smith E., Salas P., Manjunath B., Smith W.C. Parallel visual circuitry in a basal chordate. eLife. 2019;8 doi: 10.7554/eLife.44753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M., Kawano E., Kinugawa Y., Oishi T., Shichida Y., Tamotsu S., Terakita A. Bistable UV pigment in the lamprey pineal. Proc. Natl. Acad. Sci. USA. 2004;101:6687–6691. doi: 10.1073/pnas.0400819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M., Kubokawa K., Tsukamoto H., Shichida Y., Terakita A. Cephalochordate melanopsin: evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr. Biol. 2005;15:1065–1069. doi: 10.1016/j.cub.2005.04.063. [DOI] [PubMed] [Google Scholar]
- Kusakabe T., Kusakabe R., Kawakami I., Satou Y., Satoh N., Tsuda M. Ci-opsin1, a vertebrate-type opsin gene, expressed in the larval ocellus of the ascidian Ciona intestinalis. FEBS Lett. 2001;506:69–72. doi: 10.1016/s0014-5793(01)02877-0. [DOI] [PubMed] [Google Scholar]
- Lacalli T. Amphioxus, motion detection, and the evolutionary origin of the vertebrate retinotectal map. EvoDevo. 2018;9:1–5. doi: 10.1186/s13227-018-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacalli T., Holland L. The developing dorsal ganglion of the salp Thalia democratica, and the nature of the ancestral chordate brain. Philos. Trans. R. Soc. Lond. Ser. B: Biol. Sci. 1998;353:1943–1967. [Google Scholar]
- Lacalli T.C., Holland N., West J. Landmarks in the anterior central nervous system of amphioxus larvae. Philos. Trans. R. Soc. Lond. Ser. B: Biol. Sci. 1994;344:165–185. [Google Scholar]
- Lamb T.D. Evolution of phototransduction, vertebrate photoreceptors and retina. Prog. Retinal Eye Res. 2013;36:52–119. doi: 10.1016/j.preteyeres.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Lamb T.D., Collin S.P., Pugh E.N. Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nat. Rev. Neurosci. 2007;8:960–976. doi: 10.1038/nrn2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Satou Y. Foxg specifies sensory neurons in the anterior neural plate border of the ascidian embryo. Nat. Commun. 2019;10:1–10. doi: 10.1038/s41467-019-12839-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Satou Y. The genetic program to specify ectodermal cells in ascidian embryos. Dev. Growth Differ. 2020;62:301–310. doi: 10.1111/dgd.12660. [DOI] [PubMed] [Google Scholar]
- Locket N.A., Jørgensen J.M. The Biology of Hagfishes. Springer; 1998. The eyes of hagfishes; pp. 541–556. [Google Scholar]
- Lucas R.J., Allen A.E., Milosavljevic N., Storchi R., Woelders T. Can we see with melanopsin? Ann. Rev. Vis. Sci. 2020;6 doi: 10.1146/annurev-vision-030320-041239. [DOI] [PubMed] [Google Scholar]
- Maniou E., Staddon M.F., Marshall A.R., Greene N.D., Copp A.J., Banerjee S., Galea G.L. Hindbrain neuropore tissue geometry determines asymmetric cell-mediated closure dynamics in mouse embryos. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2023163118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns M., Fritzsch B. The eye in the brain: retinoic acid effects morphogenesis of the eye and pathway selection of axons but not the differentiation of the retina in Xenopus laevis. Neurosci. Lett. 1991;127:150–154. doi: 10.1016/0304-3940(91)90782-o. [DOI] [PubMed] [Google Scholar]
- Martin P.R., Fritzsch B. In: The Evolution of Sensory System Revealed: Gene Regulation, Cellular Networks and Processes. Fritzsch B., Elliott K.L., editors. Wiley; 2022. Vision and retina information processing: from opsins to the visual cortex; pp. 61–88. [Google Scholar]
- Meléndez‐Ferro M., Villar‐Cheda B., Manoel Abalo X., Pérez‐Costas E., Rodríguez‐Muñoz R., Degrip W.J., Yáñez J., Rodicio M.C., et al. Early development of the retina and pineal complex in the sea lamprey: comparative immunocytochemical study. J. Comp. Neurol. 2002;442:250–265. doi: 10.1002/cne.10090. [DOI] [PubMed] [Google Scholar]
- Miyashita T. A Paleozoic stem hagfish Myxinikela siroka—revised anatomy and implications for evolution of the living jawless vertebrate lineages. Can. J. Zool. 2020;98:850–865. [Google Scholar]
- Moody S.A., LaMantia A.-S. Transcriptional regulation of cranial sensory placode development. Curr. Top. Dev. Biol. 2015;111:301–350. doi: 10.1016/bs.ctdb.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshedian A., Fain G.L. Light adaptation and the evolution of vertebrate photoreceptors. J. Physiol. 2017;595:4947–4960. doi: 10.1113/JP274211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshedian A., Huynh T.H., Frederiksen R., Fain G.L., Sampath A.P. Pupillary light reflex of lamprey Petromyzon marinus. Curr. Biol. 2021;31:R65–R66. doi: 10.1016/j.cub.2020.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F., O’Rahilly R. The development of the human brain, the closure of the caudal neuropore, and the beginning of secondary neurulation at stage 12. Anat. Embryol. 1987;176:413–430. doi: 10.1007/BF00310083. [DOI] [PubMed] [Google Scholar]
- Munoz E.M., Bailey M.J., Rath M.F., Shi Q., Morin F., Coon S.L., Møller M., Klein D.C. NeuroD1: developmental expression and regulated genes in the rodent pineal gland. J. Neurochem. 2007;102:887–899. doi: 10.1111/j.1471-4159.2007.04605.x. [DOI] [PubMed] [Google Scholar]
- Murre C. Helix–loop–helix proteins and the advent of cellular diversity: 30 years of discovery. Genes Dev. 2019;33:6–25. doi: 10.1101/gad.320663.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M., Orii H., Yoshida N., Jojima E., Horie T., Yoshida R., Haga T., Tsuda M. Ascidian arrestin (Ci‐arr), the origin of the visual and nonvisual arrestins of vertebrate. Eur. J. Biochem. 2002;269:5112–5118. doi: 10.1046/j.1432-1033.2002.03240.x. [DOI] [PubMed] [Google Scholar]
- Negrón-Piñeiro L.J., Wu Y., Di Gregorio A. Transcription factors of the bHLH family delineate vertebrate landmarks in the nervous system of a simple chordate. Genes. 2020;11:1262. doi: 10.3390/genes11111262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neitz M., Patterson S.S., Neitz J. In: Fritzsch B., editor. vol. 1. Elsevier; 2021. The genetics of cone opsin based vision disorders; pp. 493–507. (The Senses). [Google Scholar]
- Nilsson D.-E. In: Fritzsch B., editor. vol. 1. Elsevier; 2021. Eye evolution in animals. pp. 96–121. (The Senses). [Google Scholar]
- Ochocinska M.J., Muñoz E.M., Veleri S., Weller J.L., Coon S.L., Pozdeyev N., Iuvone P.M., Goebbels S., et al. NeuroD1 is required for survival of photoreceptors but not pinealocytes: results from targeted gene deletion studies. J. Neurochem. 2012;123:44–59. doi: 10.1111/j.1471-4159.2012.07870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota K.G., Kuratani S. The history of scientific endeavors towards understanding hagfish embryology. Zool. Sci. 2006;23:403–418. doi: 10.2108/zsj.23.403. [DOI] [PubMed] [Google Scholar]
- Pantzartzi C.N., Pergner J., Kozmik Z. The role of transposable elements in functional evolution of amphioxus genome: the case of opsin gene family. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-20683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantzartzi C.N., Pergner J., Kozmikova I., Kozmik Z. The opsin repertoire of the European lancelet: a window into light detection in a basal chordate. Int. J. Dev. Biol. 2017;61:763–772. doi: 10.1387/ijdb.170139zk. [DOI] [PubMed] [Google Scholar]
- Pasqualetto G., Schepelmann M., Varricchio C., Pileggi E., Khogali C., Morgan S.R., Boostrom I., Rozanowska M., et al. Computational studies towards the identification of novel rhodopsin-binding compounds as chemical chaperones for misfolded opsins. Molecules. 2020;25:4904. doi: 10.3390/molecules25214904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergner J., Kozmik Z. Amphioxus photoreceptors-insights into the evolution of vertebrate opsins, vision and circadian rhythmicity. Int. J. Dev. Biol. 2017;61:665–681. doi: 10.1387/ijdb.170230zk. [DOI] [PubMed] [Google Scholar]
- Pisani D., Rota-Stabelli O., Feuda R. Sensory neuroscience: a taste for light and the origin of animal vision. Curr. Biol. 2020;30 doi: 10.1016/j.cub.2020.05.009. R773-r775. [DOI] [PubMed] [Google Scholar]
- Poliakov E., Gubin A.N., Stearn O., Li Y., Campos M.M., Gentleman S., Rogozin I.B., Redmond T.M. Origin and evolution of retinoid isomerization machinery in vertebrate visual cycle: hint from jawless vertebrates. PLOS One. 2012;7 doi: 10.1371/journal.pone.0049975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pombal M.A., Megías M. Development and functional organization of the cranial nerves in lampreys. Anat. Rec. 2019;302:512–539. doi: 10.1002/ar.23821. [DOI] [PubMed] [Google Scholar]
- Pu G.A., Dowling J.E. Anatomical and physiological characteristics of pineal photoreceptor cell in the larval lamprey, Petromyzon marinus. J. Neurophysiol. 1981;46:1018–1038. doi: 10.1152/jn.1981.46.5.1018. [DOI] [PubMed] [Google Scholar]
- Puzdrowski R.L., Northcutt R.G. Central projections of the pineal complex in the silver lamprey Ichthyomyzon unicuspis. Cell Tissue Res. 1989;255:269–274. doi: 10.1007/BF00224108. [DOI] [PubMed] [Google Scholar]
- Rawnsley D.R., Xiao J., Lee J.S., Liu X., Mericko-Ishizuka P., Kumar V., He J., Basu A., et al. The transcription factor Atonal homolog 8 regulates Gata4 and Friend of Gata-2 during vertebrate development. J. Biol. Chem. 2013;288:24429–24440. doi: 10.1074/jbc.M113.463083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter R.J. The pineal and its hormones in the control of reproduction in mammals. Endocr. Rev. 1980;1:109–131. doi: 10.1210/edrv-1-2-109. [DOI] [PubMed] [Google Scholar]
- Richardson M.K., Admiraal J., Wright G.M. Developmental anatomy of lampreys. Biol. Rev. 2010;85:1–33. doi: 10.1111/j.1469-185X.2009.00092.x. [DOI] [PubMed] [Google Scholar]
- Richardson R., Tracey-White D., Webster A., Moosajee M. The zebrafish eye—a paradigm for investigating human ocular genetics. Eye. 2017;31:68–86. doi: 10.1038/eye.2016.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. Pineal eye and behaviour in Xenopus tadpoles. Nature. 1978;273:774–775. doi: 10.1038/273774a0. [DOI] [PubMed] [Google Scholar]
- Rolo A., Galea G.L., Savery D., Greene N.D., Copp A.J. Novel mouse model of encephalocele: post-neurulation origin and relationship to open neural tube defects. Dis. Models Mech. 2019:12. doi: 10.1242/dmm.040683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K., Lu Z., Meinertzhagen I.A. The peripheral nervous system of the ascidian tadpole larva: types of neurons and their synaptic networks. J. Comp. Neurol. 2018;526:583–608. doi: 10.1002/cne.24353. [DOI] [PubMed] [Google Scholar]
- Sasagawa S., Takabatake T., Takabatake Y., Muramatsu T., Takeshima K. Axes establishment during eye morphogenesis in Xenopus by coordinate and antagonistic actions of BMP4, Shh, and RA. Genesis. 2002;33:86–96. doi: 10.1002/gene.10095. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T., Bronner-Fraser M. Insights from a sea lamprey into the evolution of neural crest gene regulatory network. Biol. Bull. 2008;214:303–314. doi: 10.2307/25470671. [DOI] [PubMed] [Google Scholar]
- Schlosser G. CRCPress; 2021. Evolutionary Origin of Sensory Andneurosecretory Cell Types. [Google Scholar]
- Schwab I. The evolution of eyes: major steps. The Keeler lecture 2017: centenary of Keeler Ltd. Eye. 2018;32:302–313. doi: 10.1038/eye.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinotsuka N., Yamaguchi Y., Nakazato K., Matsumoto Y., Mochizuki A., Miura M. Caspases and matrix metalloproteases facilitate collective behavior of non-neural ectoderm after hindbrain neuropore closure. BMC Dev. Biol. 2018;18:1–11. doi: 10.1186/s12861-018-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionato E., Ledent V., Richards G., Thomas-Chollier M., Kerner P., Coornaert D., Degnan B.M., Vervoort M. Origin and diversification of the basic helix-loop-helix gene family in metazoans: insights from comparative genomics. BMC Evolut. Biol. 2007;7:1–18. doi: 10.1186/1471-2148-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonneaux V., Ribelayga C. Generation of the melatonin endocrine message in mammals: a review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol. Rev. 2003;55:325–395. doi: 10.1124/pr.55.2.2. [DOI] [PubMed] [Google Scholar]
- Smith J.J., Kuraku S., Holt C., Sauka-Spengler T., Jiang N., Campbell M.S., Yandell M.D., Manousaki T., et al. Sequencing of the sea lamprey (Petromyzon marinus) genome provides insights into vertebrate evolution. Nat. Genet. 2013;45:415–421. doi: 10.1038/ng.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.T., Bhullar B.-A.S., Köhler G., Habersetzer J. The only known jawed vertebrate with four eyes and the bauplan of the pineal complex. Curr. Biol. 2018;28(1101–1107) doi: 10.1016/j.cub.2018.02.021. [DOI] [PubMed] [Google Scholar]
- Song Y.S., Kim J.-K. Molecular phylogeny and classification of the family Myxinidae (Cyclostomata: Myxiniformes) using the supermatrix method. J. Asia-Pac. Biodivers. 2020;13:533–538. [Google Scholar]
- Stolfi A., Ryan K., Meinertzhagen I.A., Christiaen L. Migratory neuronal progenitors arise from the neural plate borders in tunicates. Nature. 2015;527:371–374. doi: 10.1038/nature15758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara F., Pascual-Anaya J., Kuraku S., Kuratani S., Murakami Y. Genetic mechanism for the cyclostome cerebellar neurons reveals early evolution of the vertebrate cerebellum. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.700860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki D.G., Grillner S. The stepwise development of the lamprey visual system and its evolutionary implications. Biol. Rev. 2018;93:1461–1477. doi: 10.1111/brv.12403. [DOI] [PubMed] [Google Scholar]
- Tang Q., Xie M.Y., Zhang Y.L., Xue R.Y., Zhu X.H., Yang H. Targeted deletion of Atoh8 results in severe hearing loss in mice. Genesis. 2021;59 doi: 10.1002/dvg.23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W.J., Chen J.S., Zeller R.W. Transcriptional regulation of the peripheral nervous system in Ciona intestinalis. Dev. Biol. 2013;378:183–193. doi: 10.1016/j.ydbio.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Terakita A. The opsins. Genome Biol. 2005;6:213. doi: 10.1186/gb-2005-6-3-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman M., Reeves W. Quantitative and in toto imaging in ascidians: working toward an image‐centric systems biology of chordate morphogenesis. Genesis. 2015;53:143–159. doi: 10.1002/dvg.22828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman M.T., Newman-Smith E., El-Nachef D., Smith W.C. The ascidian mouth opening is derived from the anterior neuropore: reassessing the mouth/neural tube relationship in chordate evolution. Dev. Biol. 2010;344:138–149. doi: 10.1016/j.ydbio.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Vigh-Teichmann I., Korf H.-W., Nürnberger F., Oksche A., Vigh B., Olsson R. Opsin-immunoreactive outer segments in the pineal and parapineal organs of the lamprey (Lampetra fluviatilis), the eel (Anguilla anguilla), and the rainbow trout (Salmo gairdneri) Cell Tissue Res. 1983;230:289–307. doi: 10.1007/BF00213806. [DOI] [PubMed] [Google Scholar]
- Villar-Cheda B., Abalo X.M., Anadón R., Rodicio M.C. Calbindin and calretinin immunoreactivity in the retina of adult and larval sea lamprey. Brain Res. 2006;1068:118–130. doi: 10.1016/j.brainres.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Vopalensky P., Pergner J., Liegertova M., Benito-Gutierrez E., Arendt D., Kozmik Z. Molecular analysis of the amphioxus frontal eye unravels the evolutionary origin of the retina and pigment cells of the vertebrate eye. Proc. Natl. Acad. Sci. USA. 2012;109:15383–15388. doi: 10.1073/pnas.1207580109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington R.E., Davies W.I.L., Hemmi J.M., Hart N.S., Potter I.C., Collin S.P., Hunt D.M. Visual opsin expression and morphological characterization of retinal photoreceptors in the pouched lamprey (Geotria australis, Gray). J. Comp. Neurol. 2021;529:2265–2282. doi: 10.1002/cne.25092. [DOI] [PubMed] [Google Scholar]
- Wicht H. The brains of lampreys and hagfishes: characteristics, characters, and comparisons. Brain, Behav. Evol. 1996;48:248–261. doi: 10.1159/000113204. [DOI] [PubMed] [Google Scholar]
- Wilson S.W., Houart C. Early steps in the development of the forebrain. Dev. Cell. 2004;6:167–181. doi: 10.1016/s1534-5807(04)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkley K.M., Kourakis M.J., DeTomaso A.W., Veeman M.T., Smith W.C. Tunicate gastrulation. Curr. Top. Dev. Biol. 2020;136:219–242. doi: 10.1016/bs.ctdb.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Bard J.E., Kann J., Yergeau D., Sapkota D., Ge Y., Hu Z., Wang J., et al. Single cell transcriptomics reveals lineage trajectory of retinal ganglion cells in wild-type and Atoh7-null retinas. Nat. Commun. 2021;12:1–20. doi: 10.1038/s41467-021-21704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki F., Møller M., Fu C., Clokie S.J., Zykovich A., Coon S.L., Klein D.C., Rath M.F. The Lhx9 homeobox gene controls pineal gland development and prevents postnatal hydrocephalus. Brain Struct. Funct. 2015;220:1497–1509. doi: 10.1007/s00429-014-0740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Zhou R., Li W., Liu Y., Zhang Y., Ao H., Li H., Li K. Dynamic transcriptome analysis reveals potential long non-coding RNAs governing postnatal pineal development in pig. Front. Genet. 2019;10:409. doi: 10.3389/fgene.2019.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Xu J., Xu P.X. Eya2 expression during mouse embryonic development revealed by Eya2(lacZ) knockin reporter and homozygous mice show mild hearing loss. Dev. Dyn. 2021;250:1450–1562. doi: 10.1002/dvdy.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D., Chen S., Horie T., Gao Y., Bao H., Liu X. Comparison of differentiation gene batteries for migratory mechanosensory neurons across bilaterians. Evol. Dev. 2020;22:438–450. doi: 10.1111/ede.12331. [DOI] [PubMed] [Google Scholar]
- Zhao M., Peng G.-H. Regulatory mechanisms of retinal photoreceptors development at single cell resolution. Int. J. Mol. Sci. 2021;22:8357. doi: 10.3390/ijms22168357. [DOI] [PMC free article] [PubMed] [Google Scholar]