Abstract
Prolonged drought due to climate change has negatively impacted amphibians in southern California, U.S.A. Due to the severity and length of the current drought, agencies and researchers had growing concern for the persistence of the arroyo toad (Anaxyrus californicus), an endangered endemic amphibian in this region. Range‐wide surveys for this species had not been conducted for at least 20 years. In 2017–2020, we conducted collaborative surveys for arroyo toads at historical locations. We surveyed 88 of the 115 total sites having historical records and confirmed that the arroyo toad is currently extant in at least 61 of 88 sites and 20 of 25 historically occupied watersheds. We did not detect toads at almost a third of the surveyed sites but did detect toads at 18 of 19 specific sites delineated in the 1999 Recovery Plan to meet one of four downlisting criteria. Arroyo toads are estimated to live 7–8 years, making populations susceptible to prolonged drought. Drought is estimated to increase in frequency and duration with climate change. Mitigation strategies for drought impacts, invasive aquatic species, altered flow regimes, and other anthropogenic effects could be the most beneficial strategies for toad conservation and may also provide simultaneous benefits to several other native species that share the same habitat.
Keywords: amphibian decline, California, climate change, endangered species, riparian habitat
U.S. range‐wide surveys for arroyo toads were conducted at 100% of the historical watersheds and ~76% of the historical sites following prolonged drought. Arroyo toads were detected at most sites delineated in the 1999 Recovery Plan for a downlisting consideration, yet toads could not be detected at 31% of sites surveyed indicating further declines. These are the most current and comprehensive data on number of extant arroyo toad populations since 1999 and can be used as a baseline for reevaluation of the Recovery Plan.

1. INTRODUCTION
Climate can be a major driver of amphibian health and persistence, and survival strategies influenced by climate change could contribute to population extinctions (Bucciarelli et al., 2020). For example, global warming and severe drought decrease body size and body condition in many amphibian species, which consequently decreases survivorship and fecundity (Caruso et al., 2014; Cayuela et al., 2016; Reading, 2007; Stanley et al., 2020). Climate‐induced habitat changes, such as decreased availability of surface water for breeding, can also cause amphibian populations to decline (Miller et al., 2018). Decline and extinction rates from climate change could be exacerbated by anomalous catastrophic events and anthropogenic threats. Ultimately, the rate of environmental change resulting from warming and drought, along with compounding effects of anthropogenic threats may exceed the rate of adaptation or resiliency for many amphibians. This is especially a concern in southern California where freshwater is extremely limited to begin with, and drought might cause limited surface water to dry up in some years.
California, U.S.A., has experienced notable changes in climate over the last several decades. For example, LaDochy et al. (2007) report that the mean temperature in the state has risen by approximately 0.99°C since 1950, and Goss et al. (2020) report a similar increase in California's mean temperature since 1980. The southern California region has experienced numerous extreme wildfire events (Goss et al., 2020; Keeley et al., 2009; Nauslar et al., 2018; Tracey et al., 2017) and unprecedented drought over the last decade (Fisher et al., 2018; Griffin & Anchukaitis, 2014; Swain et al., 2014). In addition, the rapid growth rate of the human population and urban development in southern California (Kindlmann et al., 2005) conflicts with preserving habitat for the region's extraordinary biodiversity, which holds a high level of endemism resulting in part from the region's diverse geomorphology and climate (Dobson et al., 1997; Howard et al., 2013; Myers et al., 2000; Wilson, 1992). The effects of climate change, wildfire, drought, disease, invasive species, and other anthropogenic impacts are threatening amphibian persistence in southern California and adjacent northern Baja California (Bucciarelli et al., 2020; Diffenbaugh et al., 2015; Griffin & Anchukaitis, 2014; Jones et al., 2017; Miller et al., 2012; Peralta‐Garcia et al., 2016; Richmond et al., 2021; Russell et al., 2019).
The arroyo toad (Anaxyrus californicus; Figure 1) is endemic to southwestern California and northern Baja California and is federally listed as endangered in the United States and by México (Hammerson & Santos‐Barrera, 2004; Poder Ejecutivo Federal, 2008; Sweet & Sullivan, 2005; Thomson et al., 2016; USFWS, 1994). This species was listed under the Endangered Species Act in 1994 after reported declines from approximately 75% of formerly occupied habitat across its range in California (Jennings & Hayes, 1994; Sweet, 1992; USFWS, 1994). Its status was retained upon reevaluation in 2014 (USFWS, 2015). Anthropogenic threats identified by various authors include off‐highway vehicle (OHV) use, dam/hydrological operations, disease, and invasive species (Ervin et al., 2006; Funk et al., 2014; Madden‐Smith et al., 2003; Miller et al., 2012; Ramirez, 2003; Robeson, 2015; Sweet, 1992). In the United States, the arroyo toad historically occupied 25 watersheds along mostly coastal and a few desert drainages from Monterey County to San Diego County (Ervin et al., 2013; USFWS, 2015). The 1999 USFWS Recovery Plan for the arroyo toad lists 20 (but actually 19 due to a misidentification; see Ervin et al., 2013) populations at specific locations that must be self‐sustaining for a downlisting consideration (USFWS, 1999, pp. 75–76). According to the Recovery Plan, self‐sustaining populations are defined as “having successful recruitment equal to 20% or more of the average number of breeding individuals in seven of ten years of average to above‐average rainfall amounts with normal rainfall patterns” (USFWS, 1999, p. 76).
FIGURE 1.

Arroyo toad (Anaxyrus californicus)
The arroyo toad is a habitat specialist, requiring low‐gradient intermittent streams and rivers with sandy terraces and banks, as well as gravel and sand bars (Cunningham, 1962; Sweet, 1992; Sweet & Sullivan, 2005). Reproduction is dependent upon the availability of shallow and slow‐moving streams typical of a natural flood‐disturbed environment in which breeding, egg laying, and larval development occur (Sweet, 1992; Sweet & Sullivan, 2005; Thomson et al., 2016; USFWS, 1999). These habitat features are largely dependent on natural hydrological cycles and scouring events (Jennings & Hayes, 1994). A recent study on longevity estimates that this species lives approximately 7–8 years on average (Fisher et al., 2018). The drought in southern California peaked in 2012–2016 (Diffenbaugh et al., 2015; Griffin & Anchukaitis, 2014) and has continued through 2022 despite some occasional wet years (https://www.ncdc.noaa.gov/cag/divisional/time‐series/0406/pcp/12/12/2000‐2022?trend=true&trend_base=10&begtrendyear=2000&endtrendyear=2022). Given this prolonged period of drought, there has been growing concern that the number of consecutive years of drought may have surpassed the lifespan of the species (Fisher et al., 2018), and recruitment may have been severely diminished due to lack of surface water (especially in ephemeral watersheds), resulting in possible population declines and local extirpations. Additionally, evidence of direct mortality of toads due to drought was reported during a telemetry study that included observations of desiccated toads found under the sand in which they had burrowed (Gallegos, 2011–2013, 2016 unpublished data). These concerns prompted collaborative, range‐wide surveys for the arroyo toad in 2017 that continued with several additional surveys through 2020.
We investigated population status by surveying known historical arroyo toad locations within the United States and compared the locations where toads were detected/not detected to locations where they were extant in 1999 (the time the recovery plan was written; USFWS, 1999) and in 2014 (the time of the last reevaluation of their status; USFWS, 2014, 2015). To cover the extent of the historical locations within the United States, we formed a collaboration of researchers to comprehensively survey as many historical sites as possible from Monterey County to San Diego County from 2017 to 2020 and combined our detection/non‐detection findings.
1.1. Study area
Our study area included all the watersheds in the United States within the known range of the arroyo toad that were delineated in the 1999 Recovery Plan (USFWS, 1999). A few locations have been updated and revised from the original Recovery Plan to account for corrections made after publication (Ervin et al., 2013; USFWS, 2014). The revised total includes 25 watersheds spanning Monterey to San Diego counties. Multiple sites within these watersheds were surveyed to determine presence (Figure 2). All sites described in the 1999 Recovery Plan, 2014 Species Report (USFWS, 2014), or other literature and databases were treated as separate locations within shared watersheds, and all had arroyo toads historically. Rainfall and temperatures are highly variable throughout the broad geographic range of this species, with several different ecoregions inhabited, including both desert and coastal drainages. According to the National Oceanic and Atmospheric Administration (NOAA), annual rainfall since 1950 varied from 50 to 325 millimeters (mm) in the desert basins (https://www.ncdc.noaa.gov/cag/divisional/time‐series/0407/pcp/12/12/1950‐2022?trend=true&trend_base=10&begtrendyear=1950 &endtrendyear=2022) and 135–900 mm in the south coast drainages (https://www.ncdc.noaa.gov/cag/divisional/time‐series/0406/pcp/12/12/1950‐2022?trend=true&trend_base=10&begtrendyear=1950&endtrendyear=2022); in general, the southern California climate is hot and dry in summer with cooler temperatures and low to moderate rainfall in winter. Air temperatures at many arroyo toad sites can briefly drop below freezing at times during winter but can also reach in excess of 40°C in summer.
FIGURE 2.
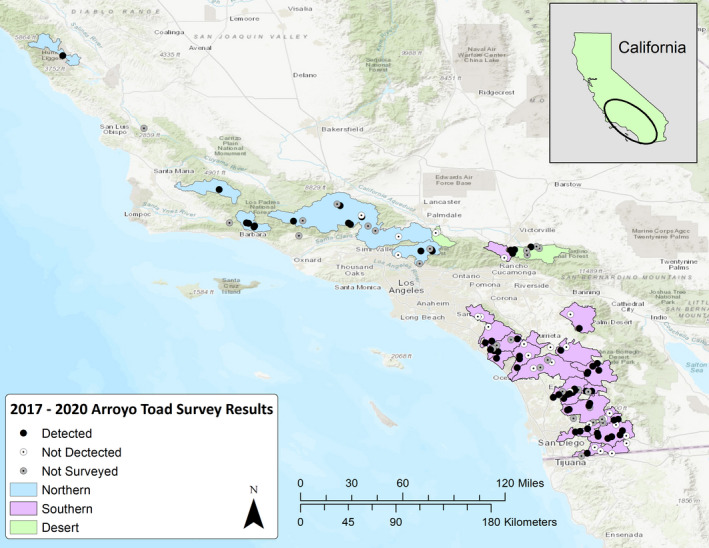
Of the 115 known sites in the United States, 88 were surveyed as part of this effort and arroyo toads were detected at 61 (see also Appendix)
2. METHODS
We collaborated as 37 partners from 19 various state and government agencies, consulting groups, universities, and independent researchers, to survey for arroyo toads at historical locations. We compiled range‐wide comprehensive and current data for the detection/non‐detection of the species at as many sites as possible that were listed in the 1999 Recovery Plan and other literature or databases (mostly the USFWS 2014 Species Report). Most of our surveys were conducted during 2017, but several sites initially skipped for logistical reasons were surveyed 2018–2020. Incidentally, the winter of 2018–2019 had more rainfall compared to the surrounding years; therefore, toads were expected to be more easily detectable during that year. Location descriptions and Global Positioning System (GPS) coordinates from the Recovery Plan, grey literature, and from biologists who had been to the sites were used to determine the precise multiple locations to survey within the 25 watersheds. Because arroyo toads have been documented as having an average dispersal distance of ~3 km (USFWS, 1999), long swaths of habitat were surveyed within each documented location to account for movement of arroyo toads up and down waterways even if some of the habitat was marginal. A location within a watershed was regarded as a “site” if it had historical records of arroyo toads described for that specific location within the watershed. We also considered the ~3 km average dispersal distance (USFWS, 1999) and any geographic barriers (i.e., mountains, urban development) to establish which locations we could regard as being a single “site” versus more than one “site” within a watershed. Our team of collaborators developed a spreadsheet of arroyo toad sites to survey, categorized by watershed and recovery unit. The spreadsheet included the following: (1) a list of all known arroyo toad historical sites based on literature (mostly from the 1999 Recovery Plan and USFWS 2014 Species Report), and (2) fields for participants to provide date surveyed, specific location, and age class observed. Collaborators throughout southern California conducted surveys at as many locations as possible, mostly according to their proximity to nearby sites. Participants conducted daytime and/or night surveys during the breeding and active season of the toad (generally April–July depending on elevation, latitude, and local climate). Surveyors walked the creeks at historical locations surveying visually and dip‐netting for tadpoles during the day. There was no minimum or maximum number of linear meters walked; the presence of suitable (and even marginal) arroyo toad habitat dictated the length of creek surveyed. If no toads or larvae were detected during the day, most surveys were continued at nighttime along the same length of creek and in the same manner by looking but also listening for calling adults. One survey (day or day/night) per site was made, although occasionally different participants happened to overlap the same site. Data from all participants were compiled and number of locations where toads were detected/not detected were compared to number of locations where toads were recorded as extant in the 1999 Recovery Plan. Years that toads were last documented from all sites were also compiled (Appendix).
We also examined weather data, reports, unpublished data, and gray literature from past surveys conducted by U.S. Geological Survey (USGS), U.S. Forest Service (USFS), U.S. Fish and Wildlife Service (USFWS), and other partners to assess whether any anomalous events may have affected population presence or detectability (i.e., major local weather events or anthropogenic changes to the habitat) at any sites. To gain perspective on climate change in the region, we compiled literature and online climate data (https://www.ncdc.noaa.gov/cag/divisional/time‐series/0406/tavg/12/12/1950‐2022?trend=true&trend_base=10&begtrendyear=1950&endtrendyear=2022, and https://www.ncdc.noaa.gov/cag/divisional/time‐series/0406/pcp/12/12/1950‐2022?trend=true&trend_base=10&begtrendyear=1950&endtrendyear=2022, accessed March 2022; NOAA, 2022) and plotted average annual temperatures and precipitation from 1950–2020 for California's South Coast Drainage. Our precipitation and temperature profiles were produced from data on the NOAA website by selecting the “divisional” tab at the top, then “time series,” then parameters from the drop‐down menus for temperature or precipitation, annual average, bounding years (1950–2021), “state” (California) and “division” (south coast drainage). Precipitation measurements were converted to millimeters and temperature was converted to degrees Celsius. These data were graphed in Microsoft Excel™ to show the difference in temperature and precipitation from the mean over time.
3. RESULTS
Of the more than 70 individuals asked to participate in surveys, we received data and input from 37 researchers and citizen scientists. Collectively, we were able to survey within all 25 (~100%) historical watersheds and at 88 of the 115 (~76.5%) individual sites assembled mainly from the 1999 Recovery Plan and USFWS 2014 Species Report. Due to logistical and time constraints, 27 sites were not surveyed. At the watershed scale, arroyo toads were detected in 20 of 25 (80%) watersheds surveyed (Table 1). At the site scale, arroyo toads were detected at 61 of the 88 (~69%) sites surveyed (Figure 2; Appendix). Diurnal surveys detected toads at 52 of 61 extant sites. Nocturnal surveys detected toads at the remaining nine extant locations. Our diurnal surveys detected toads during ~85% of the total surveys conducted.
TABLE 1.
Summary of detection/non‐detection results for the 25 watersheds historically occupied by arroyo toads in the U.S. (USFWS, 1999)
| Recovery unit | # Watersheds in recovery unit | Watershed names | a Detected Yes or No |
|---|---|---|---|
| Northern | 5 | Los Angeles River Basin | Y |
| Salinas River Basin | Y | ||
| Santa Clara River Basin | Y | ||
| Santa Maria River Basin | Y | ||
| Santa Ynez River Basin | Y | ||
| Southern | 18 | Cottonwood Creek Basin (lower) | Y |
| Cottonwood Creek Basin (upper) | Y | ||
| Murrieta Creek Basin | N | ||
| San Diego River Basin (upper) | Y | ||
| San Jacinto River Basin | Y | ||
| San Juan Creek Basin | Y | ||
| San Luis Rey River Basin (lower and middle) | N | ||
| San Luis Rey River Basin (upper) | Y | ||
| San Mateo Creek Basin | Y | ||
| San Onofre Creek Basin | Y | ||
| Santa Ana River Basin (lower) | N | ||
| Santa Ana River Basin (upper) | N | ||
| Santa Margarita River Basin (upper) | Y | ||
| Santa Margarita River Basin (lower) | Y | ||
| Santa Ysabel Creek Basin (lower) | Y | ||
| Santa Ysabel Creek Basin (upper) | Y | ||
| Sweetwater River Basin (lower) | Y | ||
| Sweetwater River Basin (upper) | Y | ||
| Desert | 2 | Antelope‐Fremont River Basin | N |
| Mojave River Basin | Y |
Y = yes, N = no.
Our review of published and gray literature, and unpublished data, did not uncover any localized novel impacts that might suggest causes for a population crash or extirpation (besides the known drought). Potential threats such as OHV use, hiking, camping, bathing, trash, and exotic species were recorded at nearly all sites and all years surveyed. Also, several sites with known toad populations had been closed to public use for a prolonged period to protect the species from direct anthropogenic impacts (USFS, personal communication). We did not quantify prevalence of disturbances or collect data on disturbances over time.
Our graph of annual temperature data for California's South Coast Drainage is consistent with LaDochy et al. (2007) in that average annual temperatures between 1950 and 2020 increased almost 2°C during this span of time (Figure 3), which is greater than what is reported for the state of California (increased 0.99°C since 1950; LaDochy et al., 2007). Average annual precipitation for southern California was highly variable from year to year but was 41 mm lower for the last 20 years compared to the last 70 years; between 1955 and 2020 the average annual precipitation was 430 mm whereas between 2000 and 2020 the average annual precipitation was 389 mm (Figure 3; https://www.ncdc.noaa.gov/cag/divisional/time‐series/0406/pcp/12/12/1950‐2021?trend=true&trend_base=10&begtrendyear=1950&endtrendyear=2022, accessed March 2022; NOAA, 2022).
FIGURE 3.
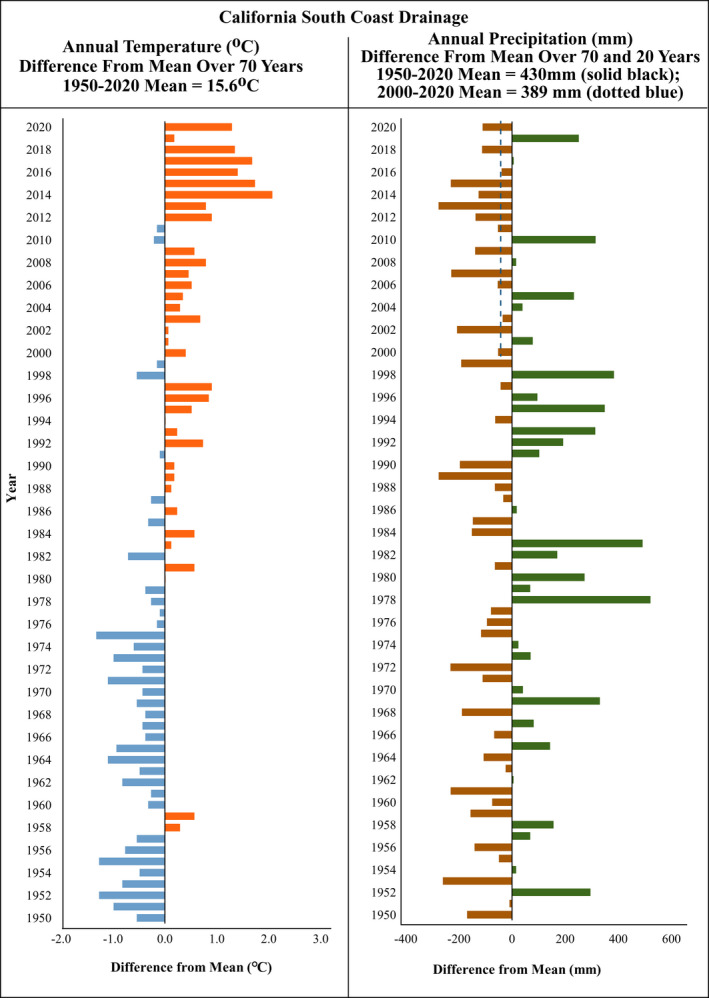
California South Coast Drainage annual temperatures (left) and precipitation (right) showing difference from mean over time. Mean precipitation has declined 40 mm in the last 20 years. Data are from NOAA (2022)
4. DISCUSSION
We compiled results for 2017–2020 arroyo toad surveys conducted at all historical watersheds and most of the historical sites, hypothesizing that we would document numerous extirpations due to prolonged periods of drought. Given the lifespan of the toad (average 7–8 years; Fisher et al., 2018), the prolonged duration of the drought, and the comparison to extant sites from 1999 (20+ year duration), we expected to find fewer extant populations than we did. The short duration of time for our surveys (2017–2020) could have also produced an underestimation the number of extant populations. Toads were not detected at about 31% of the sites surveyed. Our data show that over the past 20+ years, this species has persisted in ~80% of the watersheds and ~69% of the sites surveyed, but has possibly disappeared from ~31% of these locations. Though this may imply arroyo toad persistence at the majority (~69%) of sites, we do not know if arroyo toad populations are stable or self‐sustaining at these sites, which is one of the Recovery Plan metrics (see USFWS, 1999, p. 76). Additional surveys are needed to determine if these extant populations are declining or at risk of extirpation. Overall, we consider the 61 extant sites to be a minimum estimate of extant populations because we did not survey 27 of 115 sites and because it is possible to have missed detection at some sites.
Though this species is known to be adapted to the generally hot, dry climate of southern California, increased drought severity and length may eventually surpass the limits of this species’ tolerance. Toads are more terrestrial than frogs and are known to have physiological adaptations for water retention, such as storing water in their bladder or metabolically producing water from their diet (Bundy & Tracy, 1977; McClanahan & Baldwin, 1969). Schmajuk and Segura (1982) show that toads in the Bufo boreas group specifically store more water in their bladder when deprived of it, and Jørgensen (1994) reports that the common toad (B. bufo) can retain up to 20% of its mass as water in the bladder when water deprived. Therefore, the xeric‐adapted arroyo toad likely uses this strategy to retain water through prolonged drought. Furthermore, arroyo toads may benefit from moderate drought because suitable conditions for breeding and metamorphosis generally occur in the form of slow‐moving braided streams when water levels are low. Though without the typical cycle of flooding and scouring events, habitat that is ordinarily lightly or moderately vegetated can fill in with riparian vegetation—including both native species such as mulefat (Baccharis salicifolia), cattail (Typha spp.), willow (Salix spp.), and invasive species such as giant reed (Arundo donax)—which can overtake areas formerly suitable for arroyo toad breeding (Brehme et al., 2006; Griffin & Case, 2001). While arroyo toads have persisted at most sites despite variable precipitation, our inability to detect the species at approximately 27 sites at which they were previously found suggests that the species is likely continuing to decline. In addition, desiccated adults documented by telemetry during drought years (Gallegos, 2011–2013, 2016, unpublished data), suggest that estivating toads are not impervious to drought effects on soil moisture.
Negative impacts from recreation, non‐native species, and altered hydrological regimes were documented at several locations (Ervin et al., 2006; Madden‐Smith et al., 2003; Matsuda et al., 2018; Miller et al., 2012) and may exacerbate the environmental challenges being experienced by these toads. More data are needed to quantify threats such as OHVs, habitat conversions, hydrological changes from dams, disease (i.e., chytrid fungus (Batrachochytrium dendrobatidis); Sweet & Sullivan, 2005), and predation, competition, or habitat manipulation from non‐native species (Richmond et al., 2021). Anthropogenic threats may also impact other native species associated with arroyo toads; therefore, addressing these threats may be a tractable and effective way to protect a suite of native species. For example, arroyo toads share or have historically shared habitat with several native common or special status species including western toad (Anaxyrus boreas), two‐striped garter snake (Thamnophis hammondii), red‐sided gartersnake (Thamnophis sirtalis infernalis), Santa Ana sucker (Catostomus santaanae), unarmored threespine stickleback (Gasterosteus aculeatus williamsoni), California red‐legged frog (Rana draytonii), western spadefoot (Spea hammondii), and western pond turtle (Actinemys pallida) (Richmond et al., 2013, 2014; Richmond, Jacobs, et al., 2014; Sweet & Sullivan, 2005). Anthropogenic alteration of habitat for waterplay (e.g., damming to create pools) and releasing unwanted pets (e.g., turtles, aquarium fish) or game fish for fishing often makes areas incompatible for native species and can promote persistence of non‐native species (Miller et al., 2012). Introduced species such as bullfrogs (Lithobates catesbeianus), rainbow trout (Oncorhynchus mykiss), several centrarchid, cyprinid, and ictalurid fish species, sliders (Trachemys sp.), and crayfish (Procambarus sp.) prefer, tend to be found, and can persist in areas where the habitat has been altered to contain areas with deeper pools, sometimes via non‐indigenous beaver (Castor canadesis) (Fisher & Shaffer, 1996; Miller et al., 2012; Richmond et al., 2021; Riley et al., 2005). These introduced species can negatively impact native species via direct predation or competition for resources (Bucciarelli et al., 2014; Matsuda et al., 2018; Miller et al., 2012). Maintaining natural shallow braided aquatic systems with sandy substrates and periodic drying may prevent many invasive predatory species from establishing by eliminating the pooled areas in which they are able to persist (Miller et al., 2012). Unfortunately, shallow braided streams and terraces with sandy substrate are also favored as locations for OHV use, which can be especially damaging to toad populations during the breeding and post‐breeding season when eggs, larvae, and metamorphs are reliant on surface water (Ervin et al., 2006; Griffin & Case, 2001). OHV use can cause direct mortality by crushing individuals burrowed under the soil or have indirect effects by habitat modification (e.g., soil compaction), thus reducing or preventing friable sands in which they burrow (Griffin & Case, 2001; Sweet, 1992). Driving in creekbeds also causes the collapse of berms and flattening of sand bars, which can drain occupied pools in braided sections (Sweet pers obs.). OHV and other recreational activity (e.g., mountain biking or equestrian use) within active streams and pools can dislodge sediments and harm both eggs and larvae (Ervin et al., 2006; Griffin & Case, 2001). Overall, protecting arroyo toads and their habitat from anthropogenic impacts could also help protect a host of other native aquatic‐associated species in southern California.
Some of the recovery tasks in the 1999 Recovery Plan have been studied and addressed to varying degrees. These include developing management plans, developing protocols for monitoring and surveying, managing dam releases in some areas, active research on exotic species interactions, toad movements, habitat analyses, and surveying areas within the potential range of the species (Brehme et al., 2006; Ervin et al., 2006, 2013; Fisher et al., 2018; Gallegos, 2011–2013, 2016, unpublished data; Madden‐Smith et al., 2003; Matsuda et al., 2018; Miller et al., 2012; Ramirez, 2003). This study contributes to the recovery tasks by providing the most comprehensive and up‐to‐date information on extant arroyo toad populations throughout their range in the United States. However, this study also had several limitations. Trying to cover the entire United States range while collecting and reporting data in a consistent manner was challenging due to the engagement of so many participants. A more stringent study design with fewer participants may have increased consistency of data collection methods and allowed for more rigorous analyses on occupancy; however, with fewer participants we may not have been able to survey as many sites. Population trend data and multiple visits over multiple years to all sites could also have improved our ability to accurately determine occupancy over time and help investigate one of the Recovery Plan's metrics (to document self‐sustaining populations “…equal to 20% or more of the average number of breeding individuals in seven of ten years…” see USFWS, 1999, p. 76). However, our main objective was to try and detect arroyo toads at as many of the historical sites as possible to provide a comprehensive understanding of which populations were still on the landscape and provide a baseline for future studies. This study provides information on which sites still need to be verified for toad persistence, and it may help identify additional sites within the range of the arroyo toad that could be explored for yet‐unknown populations (another metric of the Recovery Plan; USFWS, 1999). By identifying currently occupied sites, the study also could lead to new assessments of management at those sites.
These baseline data documenting the current occupancy status of the species, which had not been explored comprehensively or consistently for the past 20+ years, may help managers understand the current recovery status of arroyo toad. Currently, the Recovery Plan states that 20 (actually 19, see Ervin et al., 2013) self‐sustaining populations at specific locations are required for downlisting consideration. Though our data show that 20 of the 25 delineated watersheds in the Recovery Plan currently have extant populations (Table 1) and 18 of the 19 specific sites named within these watersheds have verified toad populations, data to assess whether or not populations are self‐sustaining are lacking. Detailed spatial and demographic data are needed to understand whether the Recovery Plan's definition of “self‐sustaining” has been met. This study may also inform the USFWS’ recovery planning into the future, including the number of sites that might constitute recovery. Defining such sites could involve considering factors such as proximity to other sites and types of negative impacts that may need to be mitigated within specific locations. This may also involve conducting repeated surveys during optimal years at the 27 non‐detection sites and 27 sites not surveyed.
Our comprehensive surveys confirmed that toads are extant at ~69% of sites; toads were not detected at ~31% of sites. Detection at the majority of sites suggests that arroyo toads may be better evolutionarily suited to the effects of drought cycle changes than previously understood. However, we emphasize that any tolerance to drought is not well‐studied. We suggest that minimizing anthropogenic impacts (including introduced aquatic invasive species) to historically and currently occupied sites may be the most effective strategy for arroyo toad conservation; this approach can also have positive implications for native species sharing the same habitat. The results of this study can inform recovery planning for the arroyo toad.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Cynthia J. Hitchcock: Data curation (supporting); Formal analysis (equal); Methodology (equal); Project administration (equal); Supervision (equal); Writing – original draft (lead); Writing – review & editing (lead). Elizabeth A. Gallegos: Conceptualization (equal); Data curation (lead); Formal analysis (equal); Methodology (equal); Project administration (equal); Supervision (equal); Writing – original draft (supporting); Writing – review & editing (supporting). Adam R. Backlin: Conceptualization (equal); Data curation (supporting); Project administration (supporting); Supervision (supporting); Writing – original draft (supporting); Writing – review & editing (supporting). Russell Barabe: Data curation (supporting); Investigation (supporting). Peter H. Bloom: Data curation (supporting); Investigation (supporting). Kimberly Boss: Data curation (supporting); Investigation (supporting). Cheryl S. Brehme: Conceptualization (equal); Data curation (supporting); Investigation (supporting); Writing – review & editing (supporting). Christopher W. Brown: Data curation (supporting); Investigation (supporting). Denise R. Clark: Data curation (supporting); Investigation (supporting). Elizabeth R. Clark: Data curation (supporting); Investigation (supporting). Kevin Cooper: Conceptualization (supporting); Data curation (supporting). Julie Donnell: Data curation (supporting); Investigation (supporting). Edward Ervin: Data curation (supporting); Investigation (supporting). Peter Famorlaro: Data curation (supporting); Writing – review & editing (supporting). Kim M. Guilliam: Data curation (supporting); Investigation (supporting). Jaquelyn J. Hancock: Data curation (supporting); Investigation (supporting). Nicholas Hess: Data curation (supporting); Investigation (supporting). Steven Howard: Data curation (supporting); Investigation (supporting). Valerie Hubbartt: Data curation (supporting); Investigation (supporting); Writing – review & editing (supporting). Patrick Lieske: Data curation (supporting); Investigation (supporting). Robert Lovich: Conceptualization (equal); Data curation (supporting); Investigation (supporting); Writing – review & editing (supporting). Tritia Matsuda: Data curation (supporting); Investigation (supporting). Katherin Meyer‐Wilkins: Data curation (supporting); Writing – review & editing (supporting). Kamarul Muri: Data curation (supporting); Investigation (supporting). Barry Nerhus: Data curation (supporting); Investigation (supporting). Jeff Nordland: Data curation (supporting); Investigation (supporting). Brock Ortega: Data curation (supporting); Investigation (supporting). Robert Packard: Data curation (supporting); Investigation (supporting). Ruben Ramirez: Data curation (supporting); Investigation (supporting). Sam C. Stewart: Data curation (supporting); Investigation (supporting). Samuel Sweet: Data curation (supporting); Investigation (supporting). Manna Warburton: Data curation (supporting); Investigation (supporting). Jeffrey Wells: Data curation (supporting); Investigation (supporting). Ryan Winkleman: Data curation (supporting); Investigation (supporting); Writing – review & editing (supporting). Kirsten Winter: Data curation (supporting); Investigation (supporting). Brian Zitt: Data curation (supporting); Investigation (supporting). Robert N. Fisher: Conceptualization (equal); Data curation (supporting); Formal analysis (supporting); Funding acquisition (lead); Investigation (supporting); Methodology (equal); Project administration (equal); Supervision (equal); Writing – original draft (supporting); Writing – review & editing (supporting).
ACKNOWLEDGMENTS
We are grateful to the many people who contributed to the development of this project. Without the discussions, efforts and collaborations of this large group, this project would not have been possible. We also thank Carlton Rochester, Jeremy Sebes, Jordyn Ochoa, James Molden, Andrew Borcher, James Hickman, Monica Jacinto, Will Kohn, Ryan Layden, Marty Lewis, Phil Richards, Paul Schwartz, Andrew Underwood, and Christy Wolf who helped conduct surveys and contributed in numerous ways to this dataset. We are grateful to Chris Dellith, Joseph Brandt, Chris Kofron, Cat Darst, Jenny Marek, Collette Thogerson, Dou‐Shuan Yang, and Laura Patterson, for their involvement in the original development and support of this project. We thank Brian Halstead, Chris Dellith, Robert Espinoza and Jonathan Richmond for their suggestions with our first draft of the manuscript. Funding was provided by U.S. Fish and Wildlife Service and Ecosystems Mission Area of the U.S. Geological Survey. This is Contribution Number 837 of the U.S. Geological Survey Amphibian Research and Monitoring Initiative (ARMI). The views presented in the article are those of authors and do not necessarily represent the views of the Department of Defense or NAVFAC Southwest. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
1.
| Recovery Unit | Watersheds | Site Name/Description | Detected 2017, 18, 19, or 20 (Y, N, N/S a ) | 2017–2020 Surveyed by b | Age Class Observed between 2017–2020 (A, J, M, T, E c ) | Reported as Extant in 1999 Recovery Plan (Y, N, N/A, UN, PE, PX a ) | Date(s) Reported Extant |
|---|---|---|---|---|---|---|---|
| Northern | 1. Salinas River Basin (Monterey and San Luis Obispo Counties) | Salinas River, near city of Santa Margarita | N/S | – | – | PX | 1936 |
| San Antonio River, above Lake San Antonio | Y | DOD | A, E | Y | 1996, 2005, 2008, 2017 | ||
| 2. Santa Maria River Basin (Santa Barbara County) | Sisquoc River, from Manzana Creek junction to Sycamore Campground | Y | USFS | T | PE | 1991, 1993–4, 1999, 2000, 2007, 2017 | |
| 3. Santa Ynez River Basin (Santa Barbara County) | Agua Caliente Creek, from confluence w/Santa Ynez River upstream 2.5km | Y | USGS | T | N/A | 2020 | |
| Indian Creek, from confluence w/ Mono Creek upstream 1.5km | Y | USGS | M, T | Y | 1989, 1992, 1993, 1999, 2000, 2020 | ||
| Mono Creek, from confluence w/Santa Ynez River upstream 1.5km | Y | USGS | M, T | Y | 1989, 1992, 1993, 1999, 2000, 2020 | ||
| Santa Ynez River (upper), above Gibraltor Reservoir in scattered locations along 13km | N/S | – | – | Y | 1989, 1992–3, 1999, 2000 | ||
| Santa Ynez River, at confluence with Agua Caliente Creek | Y | USGS | A | N/A | 2020 | ||
| 4. Santa Clara River Basin (Ventura and Los Angeles Counties) | Agua Blanca Creek, from confluence w/Piru Creek upstream ~2km | Y | ESA | A, T | Y | 1992, 1999, 2000, 2010–11, 2017, 2020 | |
| Castaic Creek, at Basin 3 above Castaic Dam | N | USGS | – | Y | 2009, 2011, 2020 | ||
| Castaic Creek, below Castaic Dam for 4km | N/S | – | – | Y | 1992, 1996, 2001, 2009, 2011 | ||
| Castaic Creek, between power plant and Fish Canyon | Y | USGS | T | Y | 1996, 2001, 2020 | ||
| Lion Creek, at old Lion Campground (closed), at confluence w/Santa Clara River | N/S | – | – | Y | 2010, 2011 | ||
| Piru Creek (lower), Blue Point Campground upstream to Lower Piru Gorge | Y | ESA | T | Y | 1992, 2000, 2010–11, 2017 | ||
| Piru Creek (upper), from headwaters of Pyramid Lake upstream to Bear Gulch | N/S | – | – | Y | 1989–91, 1999 | ||
| Piru Creek (upper), near Hardluck Campground | Y | USGS | A, J, M, T | PE | 2009, 2012, 2020 | ||
| San Francisquito Creek | N/S | – | – | N/A | 1997 | ||
| Santa Clara River, Soledad Canyon, from Hwy 14 to Agua Dulce Road | N | ECORP | – | N/A | 2001 | ||
| 4. Santa Clara River Basin (Ventura and Los Angeles Counties) | Sespe Creek, at Beaver Campground downstream to a large pool | Y | ESA; USFS; R2 Resource Consultants Inc.; USGS | A, M, T, E | Y | 2011, 2018, 2019, 2020 | |
| Sespe Creek, from Hot Springs Canyon upstream to mouth of Tule Creek | N/S | – | – | Y | 1980s−90s, 1999, 2000 | ||
| 5. Los Angeles River Basin (Los Angeles County) | Alder Creek, ~150m upstream w/ confluence of Big Tujunga Creek | Y | IND | A | Y | 1999, 2011, 2017, 2018 | |
| Arroyo Seco, just above Devil's Gate Reservoir | N/S | – | – | Y | 1996, 1997, 1998 | ||
| Big Tujunga Creek (upper), 3N27 crossing and upstream of Big Tujunga Reservoir | Y | PSOMAS | A | Y | 2011, 2018 | ||
| Big Tujunga Creek, ~1km south of I−210 crossing | N | USGS | – | PX | 1915–1954 | ||
| Big Tujunga Creek, from confluence w/Alder Creek downstream ~1km | Y | IND | A, T | Y | 1999, 2001, 2011–12, 2017, 2019 | ||
| Lynx Gulch Creek | N/S | – | – | N/A | 2011 | ||
| Southern | 6. Lower Santa Ana River Basin (Orange County) | Santiago Creek | N | ICF | – | PX | 1974 |
| Silverado Creek | N | ICF | – | PX | 70s, 80s, 1998, 2005, 2008–9 | ||
| 7. Upper Santa Ana River Basin (San Bernardino County) | Cajon Wash | N | Endemic Environmental; USGS | – | N/A | 2000, 2005, 2007 | |
| 8. San Jacinto River Basin (Riverside County) | Bautista Creek | Y | MSHCP | A, T, E | PE | 2002–3, 2010, 2017 | |
| San Jacinto River | N | MSHCP | N/A | PE | 2000, 2017 | ||
| 9. San Juan Creek Basin (Orange and Riverside Counties) | Bell Canyon, from confluence w/San Juan Creek to Crow Canyon | Y | Dudek | A (calling) | Y | 1998, 2017 | |
| San Juan Creek, from Antonio Parkway to San Juan Hot Springs | Y | Dudek; USFS; MSHCP | M, T, E | Y | 1974, 1992, 2010, 2017 | ||
| Trabuco Creek | N | ICF | – | Y | 1997 | ||
| 10. San Mateo Creek Basin (Orange, Riverside, and San Diego Counties) | Cristianitos Creek | Y | USGS | A, M, T | Y | 1995, 1998, 2001, 2005, 2010, 2017, 2020 | |
| Gabino Creek | N/S | – | – | Y | 1995, 1998, 2001, 2005, 2010 | ||
| La Paz Creek | N/S | – | – | Y | 1995, 1998, 2001, 2005, 2010 | ||
| 10. San Mateo Creek Basin (Orange, Riverside, and San Diego Counties) | Los Alamos Canyon Creek | Y | USFS | T | N/A | 1991, 1998–9, 2005, 2010, 2017 | |
| San Mateo Creek, from estuaries to northern border of Camp Pendleton | Y | USGS | A, M, T | Y | 1991, 1999, 2005, 2010, 2017, 2020 | ||
| San Mateo Creek, mainstem | N/S | – | – | Y | 1991, 1999, 2005, 2010 | ||
| Talega Creek | Y | USGS | T | Y | 1995, 1998, 2001, 2005, 2010, 2017, 2019, 2020 | ||
| 11. San Onofre Creek Basin (San Diego County) | San Onofre Creek, from mouth to confluence of North and South Forks San Onofre Canyon | Y | USGS | A, M, T | Y | 2010, 2017, 2020 | |
| 12. Lower Santa Margarita River Basin (San Diego County) | DeLuz Creek | Y | USGS | M, T | PE | 2010, 2017, 2020 | |
| Roblar Creek | Y | USGS | T | PE | 2010, 2017, 2019 | ||
| Sandia Creek | N | USGS | – | PE | N/A | ||
| Santa Margarita River, from the airfield to Fallbrook | Y | USGS; DOD | A, M, T | PE | 2010, 2017, 2020 | ||
| 13. Upper Santa Margarita River Basin (Riverside County) | Arroyo Seco Creek, Dripping Springs Campground | Y | USFS; IND | A, M, T, E | PE | 1993, 2000, 2010, 2017, 2020 | |
| Temecula Creek | N | IND | – | PE | 1992, 2001, 2003, 2004 | ||
| Wilson Creek | N | IND | – | N/A | 1998 | ||
| 14. Murrieta Creek Basin (Riverside County) | Cole Creek | N | IND; USGS | N/A | N/A | 2001, 2005 | |
| 15. Lower and Middle San Luis Rey River Basin (San Diego County) | Keys Creek | N/S | – | – | N/A | 1998, 1999, 2001 | |
| Pala Creek | N/S | – | – | Y | 1959, 1998 | ||
| San Luis Rey River (lower), west of I−15 | N | IND; USGS | – | Y | 1991–2, 2010 | ||
| San Luis Rey River (middle), east of I−15 | N | IND; USGS | – | Y | 1928, 1996, 1998, 2000, 2004, 2011 | ||
| 16. Upper San Luis Rey River Basin (San Diego County) | Agua Caliente Creek | Y | USFS; USGS | T | Y | 1992, 1999, 2005, 2017, 2020 | |
| Cañada Aguanga, ~2km upstream of confluence with San Luis Rey River | Y | USFS; USGS | T | N/A | 1989, 1991, 2003, 2006, 2010, 2017 | ||
| San Luis Rey River (upper), Indian Flats | Y | USFS; USGS | T | Y | 1932, 2017, 2020 | ||
| San Luis Rey River, above Lake Henshaw | Y | IND; USGS | A, M, T | Y | 2019, 2020 | ||
| 16. Upper San Luis Rey River Basin (San Diego County) | San Luis Rey River, West Fork | Y | USGS; IND | A, M, T | Y | 1991–2, 1999, 2010, 2017, 2020 | |
| 17. Lower Santa Ysabel Creek Basin (San Diego County) | Boden Canyon, up to 3km north of confluence with Santa Ysabel Creek | N | USGS | – | N/A | 2003–4, 2017 | |
| Guejito Creek | N/S | – | – | Y | 1937, 2005–2008 | ||
| San Dieguito River (upper), above Lake Hodges | Y | USGS | A, M, T | PE | 2005, 2012, 2017, 2020 | ||
| Santa Maria Creek (lower), from confluence of Santa Ysabel Creek upstream 3km | N | USGS | – | PE | 2001, 2005, 2008, 2010, 2016 | ||
| Santa Maria Creek (middle), near gaging station (5km upstream of confluence with Santa Ysabel Creek) | Y | USGS; IND | A, J, M, T | PE | 2001, 2005, 2008, 2017, 2020 | ||
| Santa Ysabel Creek, at confluence with Boden Canyon | Y | USGS; CDFW; Merkel & Assoc. | A, M, T | N/A | 2003–4, 2017, 2019, 2020 | ||
| Santa Ysabel Creek, at confluence with Temescal Creek | Y | USGS | T | PE | 1996, 2020 | ||
| Santa Ysabel Creek, between Boden and Tim's Canyon | Y | USGS; CDFW; Merkel & Assoc. | A, M, T | N/A | 2005, 2012, 2017, 2019, 2020 | ||
| Santa Ysabel Creek, between Sutherland Lake and Pamo Road | N/S | – | – | PE | – | ||
| Santa Ysabel Creek, between Temescal Creek and Boden Canyon | Y | USFS; USGS; CDFW; Merkel & Assoc. | J, M, T | N/A | 1991, 2005, 2008, 2017, 2020 | ||
| Santa Ysabel Creek, near confluence with Santa Maria Creek | Y | USGS | A | N/A | 2017, 2020 | ||
| Temescal Creek, in Pamo Valley | Y | USFS; USGS | A, T | PE | 1937, 1993, 2012, 2017, 2020 | ||
| 18. Upper Santa Ysabel Creek Basin (San Diego County) | Santa Ysabel Creek and Tributary, west of Santa Ysabel Open Space Preserve | Y | USGS | M | N/A | 2010, 2020 | |
| Santa Ysabel Creek, above Sutherland Lake | Y | USGS | T | PE | 2020 | ||
| Santa Ysabel Creek, at Witch Creek | N/S | – | – | PE | 1991, 2005, 2008 | ||
| 19. Upper San Diego River Basin (San Diego County) | Boulder Creek (lower) | N/S | – | – | N/A | N/A | |
| Cedar Creek, below falls | Y | USFS; USGS; Dudek | M, T | Y | 2017 | ||
| San Diego River, below El Capitan Reservoir | N/S | – | – | Y | 1993, 1997, 2002, 2008, 2016 | ||
| 19. Upper San Diego River Basin (San Diego County) | San Diego River, between El Capitan Reservoir and Temescal Creek | Y | USFS; USGS; Dudek | A, M, T | Y | 1993, 1997, 2002, 2008, 2017, 2020 | |
| San Vicente Creek, ~1km south of Poole Ranch | Y | USGS; IND | T | Y | 2017, 2020 | ||
| San Vicente Creek, West Branch | Y | IND | A | Y | 1992, 1997, 2008, 2017, 2018 | ||
| 20. Lower Sweetwater River Basin (San Diego County) | Sweetwater River (lower), Sycuan Peak Ecological Reserve | Y | USGS | J | Y | 1999, 2000–1, 2005, 2008, 2010, 2017, 2019 | |
| Sweetwater River, Sloane Canyon | Y | USGS; Sweetwater Authority | A, M, T | Y | 2000–1, 2005, 2008, 2010, 2017 | ||
| 21. Upper Sweetwater River Basin (San Diego County) | Peterson Creek/Canyon | Y | Sweetwater Authority | M, T | Y | 1998–9, 2017 | |
| Sweetwater River, above Hwy 79, Green Valley | N | USGS | – | Y | 1990s, 2000–1, 2005, 2008, 2010 | ||
| Sweetwater River, along Merigan Fire Road | N | USGS | – | Y | 1990s, 2000–1, 2005, 2008–9, 2010, 2012 | ||
| Sweetwater River, at Hulburd Grove | N/S | – | – | Y | 1990s, 2000–1, 2005, 2008, 2010 | ||
| Sweetwater River, below Descanso Junction | N/S | – | – | Y | 1990s, 2000–1, 2005, 2008, 2010 | ||
| Viejas Creek | N/S | – | – | UN | 1996 | ||
| Viejas Creek, east of Alpine, near I−8 bridge | N | USFS | – | Y | 1996 | ||
| 22. Lower Cottonwood Creek Basin (San Diego County, Baja Caifornia, México ‐ not surveyed) | Campo Creek | N | USGS | – | PE | 1923, 2008 | |
| Cottonwood Creek (lower), below Barrett Reservoir | N | USGS | – | PE | 1998, 2002–3, 2008, 2015 | ||
| Cottonwood Creek (lower), near Marron Valley | Y | USGS | A | Y | 1998, 2002, 2003, 2008, 2017, 2020 | ||
| Potrero Creek | N | Merkel & Assoc. | – | PE | 1923, 2010 | ||
| Tijuana River | N/S | – | – | UN | 1998 | ||
| 23. Upper Cottonwood Creek Basin (San Diego County) | Corral Creek | Y | USFS | M, T | N/A | 2017 | |
| Cottonwood Creek (upper), above Lake Morena | Y | Merkel & Assoc.; USFS | A, T | Y | 1990–2, 1999, 2005, 2011, 2017, 2020 | ||
| Horsethief Canyon, for 2km (+) above Pine Valley Creek | Y | USFS | M, T | Y | 1923, 1992, 2000, 2001, 2010, 2017 | ||
| 23. Upper Cottonwood Creek Basin (San Diego County) | Kitchen Creek | Y | USFS; Merkel & Assoc. | T | PE | 1923, 1990–2, 1999, 2005, 2011, 2017 | |
| La Posta Creek | N | USFS | – | N/A | 2005 | ||
| Miller Creek, Clover Flat | N | Merkel & Assoc. | – | N/A | – | ||
| Morena Creek | Y | Merkel & Assoc.; USFS | A, T | PE | 1923, 1993, 1999, 2017 | ||
| Noble Canyon | Y | USFS | A, M, T | PE | 2017 | ||
| Pine Valley Creek | Y | USFS; USGS | A, M, T | Y | 1923, 1991–2, 1998, 1999, 2001, 2009, 2017, 2020 | ||
| Pine Valley Creek, between Barrett Lake and Horsethief Canyon | Y | USFS | M, T | PE | 1923, 1992, 2000–1, 2010, 2017 | ||
| Scove Canyon and Tributary | N | USFS | – | PE | 1923 | ||
| Desert | 24. Antelope‐ Fremont River Basin (Los Angeles County) | Little Rock Creek, from the reservoir upstream | N | IND; USGS | – | Y | 1996, 2001, 2011 |
| Santiago Canyon | N | USGS | – | N/A | 1999, 2010 | ||
| 25. Mojave River Basin (San Bernardino County) | Deep Creek, Devil's Hole | N/S | – | – | Y | 1999, 2003 | |
| Deep Creek, Hot Springs | N/S | – | – | Y | 1999, 2005 | ||
| Grass Valley Creek | N/S | – | – | Y | 1999, 2005–6 | ||
| Horsethief Creek, Summit Valley | Y | Endemic Environmental | M, T | N/A | 2017 | ||
| Little Horsethief Canyon | Y | USFS | A | Y | 1999, 2004, 2007, 2017 | ||
| Miller Canyon | N/S | – | – | Y | 1999 ‐ common since 1930 | ||
| Mojave River (West Fork), Lake Silverwood | Y | Endemic Environmental | M, T | Y | 1999, 2002–3, 2006–7, 2010, 2013, 2017 | ||
| Mojave River, vicinity of Mojave Forks Dam, in Mojave River, West Fork and Deep Creek | Y | USGS | A, J, M, T, E | Y | 1999, 2001, 2008, 2010, 2020 |
Y, N, N/A, N/S, UN, PE, PX: Y = yes, N = no, N/A = no data available, N/S = not surveyed for the study, UN = unknown, PE = presumed extant, PX = presumed extinct, – = not observed.
Acronyms: USGS = U.S. Geological Survey, USFS = U.S. Forest Service, DOD = Department of Defense, CDFW = California Department of Fish and Wildlife, IND = independent consultant or researcher, ESA = Environmental Science Associates, ECORP = ECORP Consulting, Inc., ICF = ICF International, Inc., MSHCP = Multiple Species Habitat Conservation Plan.
A, J, M,T, E: A = adult, J = juvenile, M = metamorph, T = tadpole, E = egg string.
Hitchcock, C. J. , Gallegos, E. A. , Backlin, A. R. , Barabe, R. , Bloom, P. H. , Boss, K. , Brehme, C. S. , Brown, C. W. , Clark, D. R. , Clark, E. R. , Cooper, K. , Donnell, J. , Ervin, E. , Famolaro, P. , Guilliam, K. M. , Hancock, J. J. , Hess, N. , Howard, S. , Hubbartt, V. , … Fisher, R. N. (2022). Range‐wide persistence of the endangered arroyo toad (Anaxyrus californicus) for 20+ years following a prolonged drought. Ecology and Evolution, 12, e8796. 10.1002/ece3.8796
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available within this article (Table 1 and Appendix). Authors may be contacted if more information is needed.
REFERENCES
- Brehme, C. S. , Schuster, S. L. , Rochester, C. J. , Hathaway, S. A. , & Fisher, R. N. (2006). MCBCP arroyo toad monitoring program: 3‐year trend analyses for 2003–2005 (102 pp.). U.S. Geological Survey Data Summary prepared for Marine Corps Base Camp Pendleton. [Google Scholar]
- Bucciarelli, G. M. , Blaustein, A. R. , Garcia, T. S. , & Kats, L. B. (2014). Invasion complexities: The diverse impacts of nonnative species on amphibians. Copeia, 2014(4), 611–632. 10.1643/OT-14-014 [DOI] [Google Scholar]
- Bucciarelli, G. M. , Clark, M. A. , Delaney, K. S. , Riley, S. P. D. , Shaffer, H. B. , Fisher, R. N. , Honeycutt, R. L. , & Kats, L. B. (2020). Amphibian responses in the aftermath of extreme climate events. Scientific Reports, 10, 3409. 10.1038/s41598-020-60122-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy, D. , & Tracy, C. R. (1977). Behavioral response of American toads (Bufo americanus) to stressful thermal and hydric environments. Herpetologica, 33(4), 455–458. [Google Scholar]
- Caruso, N. M. , Sears, M. W. , Adams, D. C. , & Lips, K. R. (2014). Widespread rapid reductions in body size of adult salamanders in response to climate change. Global Change Biology, 20(6), 1751–1759. 10.1111/gcb.12550 [DOI] [PubMed] [Google Scholar]
- Cayuela, H. , Arsovski, D. , Bonnaire, E. , Duguet, R. , Joly, P. , & Besnard, A. (2016). The impact of severe drought on survival, fecundity, and population persistence in an endangered amphibian. Ecosphere, 7(2), e01246. [Google Scholar]
- Cunningham, J. D. (1962). Observations on the natural history of the California toad, Bufo californicus Camp. Herpetologica, 17(4), 255–260. [Google Scholar]
- Diffenbaugh, N. S. , Swain, D. L. , & Touma, D. (2015). Anthropogenic warming has increased drought risk in California. Proceedings of the National Academy of Sciences of the United States of America, 112(13), 3931–3936. 10.1073/pnas.1422385112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, A. P. , Rodriguez, J. P. , Roberts, W. M. , & Wilcove, D. S. (1997). Geographic distribution of endangered species in the United States. Science, 275, 550–553. 10.1126/science.275.5299.550 [DOI] [PubMed] [Google Scholar]
- Ervin, E. L. , Beaman, K. R. , & Fisher, R. N. (2013). Correction of locality records for the endangered arroyo toad (Anaxyrus californicus) from the desert region of southern California. Bulletin of the Southern California Academy of Sciences, 112(3), 197–205. [Google Scholar]
- Ervin, E. , Kisner, D. , & Fisher, R. N. (2006). Bufo californicus (Arroyo Toad). Mortality. [now Anaxyrus californicus; killed by crushing by off road recreational quad (ORV)]. Herpetological Review, 37, 199. [Google Scholar]
- Fisher, R. N. , Brehme, C. S. , Hathaway, S. A. , Hovey, T. E. , Warburton, M. L. , & Stokes, D. C. (2018). Longevity and population age structure of the arroyo southwestern toad (Anaxyrus californicus) with drought implications. Ecology and Evolution, 8, 6124–6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, R. N. , & Shaffer, H. B. (1996). The decline of amphibians in California’s Great Central Valley. Conservation Biology, 10(5), 1387–1397. 10.1046/j.1523-1739.1996.10051387.x [DOI] [Google Scholar]
- Funk, C. , Hoell, A. , & Stone, D. (2014). Examining the contribution of the observed global warming trend to the California droughts of 2012/13 and 2013/14. Bulletin of the American Meteorological Society, 95(9), S11–S15. [Google Scholar]
- Goss, M. , Swain, D. L. , Abatzoglou, J. T. , Sarhadi, A. , Kolden, C. A. , Williams, A. P. , & Diffenbaugh, N. S. (2020). Climate change is increasing the likelihood of extreme autumn wildfire conditions across California. Environmental Research Letters, 15, 094016. 10.1088/1748-9326/ab83a7 [DOI] [Google Scholar]
- Griffin, D. , & Anchukaitis, K. J. (2014). How unusual is the 2012–2014 California drought? Geophysical Research Letters, 41, 9017–9023. 10.1002/2014GL062433 [DOI] [Google Scholar]
- Griffin, P. C. , & Case, T. J. (2001). Terrestrial habitat preferences of adult arroyo southwestern toads. The Journal of Wildlife Management, 65(4), 633–644. 10.2307/3803014 [DOI] [Google Scholar]
- Hammerson, G. , & Santos‐Barrera, G. (2004). Anaxyrus californicus . In IUCN 2011 . IUCN red list of threatened species (Version 2011.2). [Google Scholar]
- Howard, J. , Klausmeyer, K. , & Fesenmyer, K. (2013). Below the surface: California’s freshwater biodiversity (p. 20). The Nature Conservancy of California. [Google Scholar]
- Jennings, M. R. , & Hayes, M. P. (1994). Amphibian and reptile species of special concern in California (255 pp.). Report to the California Department of Fish and Game, Inland Fisheries Division, Rancho Cordova, California. [Google Scholar]
- Jones, M. T. , Milligan, W. R. , Kats, L. B. , Vandergon, T. L. , Honeycutt, R. L. , Fisher, R. N. , Davis, C. L. , & Lucas, T. A. (2017). A discrete stage‐structured model of California newt population dynamics during a period of drought. Journal of Theoretical Biology, 414(2017), 245–253. 10.1016/j.jtbi.2016.11.011 [DOI] [PubMed] [Google Scholar]
- Jørgensen, C. B. (1994). Water economy in a terrestrial toad (Bufo bufo), with special reference to cutaneous drinking and urinary bladder function. Comparative Biochemistry and Physiology Part A: Physiology, 109(2), 311–324. 10.1016/0300-9629(94)90134-1 [DOI] [Google Scholar]
- Keeley, J. E. , Safford, H. , Fotheringham, C. J. , Franklin, J. , & Moritz, M. (2009). The 2007 southern California wildfires: Lessons in complexity. Journal of Forestry, 107, 287–296. [Google Scholar]
- Kindlmann, P. , Aviron, S. , & Burel, F. (2005). When is landscape matrix important for determining animal fluxes between resource patches? Ecological Complexity, 2(2), 150–158. 10.1016/j.ecocom.2004.11.007 [DOI] [Google Scholar]
- LaDochy, S. , Medina, R. , & Patzert, W. (2007). Recent California climate variability: Spatial and temporal patterns in temperature trends. Climate Research, 33, 159–169. 10.3354/cr033159 [DOI] [Google Scholar]
- Madden‐Smith, M. C. , Atkinson, A. J. , Fisher, R. N. , Danskin, W. R. , & Mendez, G. O. (2003). Assessing the risk of Loveland Dam operations to the arroyo toad (Bufo californicus) in the Sweetwater River Channel, San Diego County, California (58 pp.). USGS Final Report. Prepared for Sweetwater Authority. [Google Scholar]
- Matsuda, T. A. , Brehme, C. S. , & Fisher, R. N. (2018). MCBCP arroyo toad monitoring results for 2017 with trend analysis from 2003–2017 (71 pp.). U.S. Geological Survey Report Prepared for AC/S Environmental Security, Marine Corps Base Pendleton. [Google Scholar]
- McClanahan, L. Jr , & Baldwin, R. (1969). Rate of water uptake through the integument of the desert toad, Bufo punctatus . Comparative Biochemical Physiology, 28(1), 381–389. 10.1016/0010-406X(69)91351-6 [DOI] [PubMed] [Google Scholar]
- Miller, D. A. , Brehme, C. S. , Hines, J. E. , Nichols, J. D. , & Fisher, R. N. (2012). Joint estimation of habitat dynamics and species interactions: Disturbance reduces co‐occurrence of non‐native predators with an endangered toad. Journal of Animal Ecology, 81(6), 1288–1297. 10.1111/j.1365-2656.2012.02001.x [DOI] [PubMed] [Google Scholar]
- Miller, D. A. W. , Grant, E. H. C. , Muths, E. , Amburgey, S. M. , Adams, M. J. , Joseph, M. B. , Waddle, J. H. , Johnson, P. T. J. , Ryan, M. E. , Schmidt, B. R. , Calhoun, D. L. , Davis, C. L. , Fisher, R. N. , Green, D. M. , Hossack, B. R. , Rittenhouse, T. A. G. , Walls, S. C. , Bailey, L. L. , Cruickshank, S. S. , … Sigafus, B. H. (2018). Quantifying climate sensitivity and climate‐driven change in North American amphibian communities. Nature. Communications, 9(1), 15. 10.1038/s41467-018-06157-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, N. , Mittermeier, R. A. , Mittermeier, C. G. , da Fonseca, G. A. B. , & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403, 853–858. 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- National Centers for Environmental information (NOAA) . (2022). Climate at a glance: Divisional time series. Retrieved from https://www.ncdc.noaa.gov/cag/ [Google Scholar]
- Nauslar, N. J. , Abatzoglou, J. T. , & Marsh, P. T. (2018). The 2017 North Bay and southern California fires: A case study. Fire, 1, 18. 10.3390/fire1010018 [DOI] [Google Scholar]
- Peralta‐Garcia, A. , Hollingsworth, B. D. , Richmond, J. Q. , Valdez‐Villavicenciao, J. H. , Ruiz‐Campos, G. , Fisher, R. N. , Cruz‐Hernandez, P. , & Galina‐Tessaro, P. (2016). Status of the California red‐legged frog (Rana draytonii) in the state of Baja California, México. Herpetological Conservation and Biology, 11(1), 168–180. [Google Scholar]
- Poder Ejecutivo Federal . (2008). Protección ambiental‐especies nativas de México de flora y fauna silvestres‐Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio‐Lista de especies en riesgo. Diario Oficial de la Federación (Viernes 5 de Diciembre de 2008). NORMA Oficial Mexicana NOM‐059‐ECOL‐2008. [Google Scholar]
- Ramirez, R. S. Jr (2003). Arroyo toad (Bufo californicus) hydrogeomorphic habitat baseline analysis/radio telemetry study ‐ Rancho Las Flores San Bernardino County, California. Final report to Rancho Las Flores Limited Partnership by Cadre Environmental, Carlsbad, California. vi +101 pp. [Google Scholar]
- Reading, C. (2007). Linking global warming to amphibian declines through its effect on female body condition and survivorship. Oecologia, 151, 125–131. [DOI] [PubMed] [Google Scholar]
- Richmond, J. Q. , Backlin, A. R. , Tatarian, P. J. , Solvesky, B. G. , & Fisher, R. N. (2014). Population declines lead to replicate patterns of internal range structure at the tips of the distribution of the California red‐legged frog (Rana draytonii). Biological Conservation, 172, 128–137. 10.1016/j.biocon.2014.02.026 [DOI] [Google Scholar]
- Richmond, J. Q. , Barr, K. R. , Backlin, A. R. , Vandergast, A. G. , & Fisher, R. N. (2013). Evolutionary dynamics of a rapidly receding southern range boundary in the threatened California red‐legged frog (Rana draytonii). Evolutionary Applications, 6(5), 808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond, J. Q. , Jacobs, D. K. , Backlin, A. R. , Swift, C. C. , Dellith, C. , & Fisher, R. N. (2014). Ephemeral stream reaches preserve the evolutionary and distributional history of threespine stickleback in the Santa Clara and Ventura River watersheds of southern California. Conservation Genetics, 16(1), 85–101. 10.1007/s10592-014-0643-7 [DOI] [Google Scholar]
- Richmond, J. Q. , Swift, C. C. , Wake, T. A. , Brehme, C. S. , Preston, K. L. , Kus, B. E. , Ervin, E. L. , Tremor, S. , Matsuda, T. , & Fisher, R. N. (2021). Impacts of a non‐indigenous ecosystem engineer, the American beaver (Castor canadensis), in a biodiversity hotspot. Frontiers Conservation Science, 2, 752400. [Google Scholar]
- Riley, S. P. D. , Busteed, G. T. , Kats, L. B. , Vandergon, T. L. , Lee, L. F. S. , Dagit, R. G. , Kerby, J. L. , Fisher, R. N. , & Sauvagot, R. M. (2005). Effects of urbanization on the distribution and abundance of amphibians and invasive species in southern California streams. Conservation Biology, 19, 1894–1907. 10.1111/j.1523-1739.2005.00295.x [DOI] [Google Scholar]
- Robeson, S. M. (2015). Revisiting the recent California drought as an extreme value. Geophysical Research Letters, 42, 6771–6779. 10.1002/2015GL064593 [DOI] [Google Scholar]
- Russell, R. E. , Halstead, B. J. , Mosher, B. A. , Muths, E. , Adams, M. J. , Grant, E. H. C. , Fisher, R. N. , Kleeman, P. M. , Backlin, A. R. , Pearl, C. A. , Honeycutt, R. K. , & Hossack, B. R. (2019). Effect of amphibian chytrid fungus (Batrachochytrium dendrobatidis) on apparent survival of frogs and toads in the western U.S.A. Biological Conservation, 236(2019), 296–304. 10.1016/j.biocon.2019.05.017 [DOI] [Google Scholar]
- Schmajuk, N. , & Segura, E. (1982). Behavioral regulation of water balance in the toad Bufo arenarum . Herpetologica, 38(2), 296–301. [Google Scholar]
- Stanley, T. R. , Clark, R. W. , Fisher, R. N. , Rochester, C. J. , Root, S. A. , Lombardo, K. J. , & Osterman‐Kelm, S. D. (2020). Changes in capture rates and body size among vertebrate species occupying an insular urban habitat reserve. Conservation Science and Practice, 2020, e245, 1–15. 10.1111/csp2.245 [DOI] [Google Scholar]
- Swain, D. L. , Tsiang, M. , Haugen, M. , Singh, D. , Charland, A. , Rajaratnam, B. , & Diffenbaugh, N. S. (2014). The extraordinary California drought of 2013–2014: Character, context, and the role of climate change. Bulletin of the American Meteorological Society, 95(9), S3–S7. [Google Scholar]
- Sweet, S. S. (1992). Initial report on the ecology and status of the arroyo toad (Bufo microscaphus californicus) on the Los Padres National Forest of southern California, with management recommendations. Report to United States Department of Agriculture, Forest Service, Los Padres National Forest, Goleta, California. ii +198 pp. [Google Scholar]
- Sweet, S. S. , & Sullivan, B. K. (2005). Bufo californicus Camp, 1915. In Lannoo M. (Ed.), Amphibian declines (pp. 396–400). University of California Press. [Google Scholar]
- Thomson, R. C. , Wright, A. N. , & Shaffer, H. B. (2016). California amphibian and reptile species of special concern (390 pp.). University of California Press. [Google Scholar]
- Tracey, J. A. , Rochester, C. J. , Hathaway, S. A. , Preston, K. L. , Syphard, A. D. , Vandergast, A. G. , Diffendorfer, J. E. , Franklin, J. , MacKenzie, J. B. , Oberbauer, T. A. , Tremor, S. , Winchell, C. S. , & Fisher, R. N. (2017). Prioritizing conserved areas threatened by wildfire and fragmentation for monitoring and management. PLoS One, 13(9), e0200203. 10.1371/journal.pone.0200203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Fish and Wildlife Service (USFWS) (1994). Endangered and threatened wildlife and plants; determination of endangered status for the arroyo southwestern toad. Federal Register, 59, 64859–64866. [Google Scholar]
- USFWS . (1999). Arroyo southwestern toad (Bufo microscaphus californicus) recovery plan (119 pp.). Portland, Oregon. [Google Scholar]
- USFWS . (2014). Arroyo toad (Anaxyrus californicus) species report. U.S. Fish and Wildlife Service, Ventura Fish and Wildlife Office, Ventura, CA. March 24, 2014 – Final. 114 pp. [Google Scholar]
- USFWS . (2015). Endangered and threatened wildlife and plants; withdrawal of proposed rule to reclassify the arroyo toad as threatened. Federal Register, 80, 79805–79816. [Google Scholar]
- Wilson, E. O. (1992). The diversity of life. Belknap Press of Harvard University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available within this article (Table 1 and Appendix). Authors may be contacted if more information is needed.


