Abstract
Primary splenic stromal tumors have rarely been reported in rodents. We report the case of a 90-week-old male WBN/Kob rat with a nodular demarcated mass in the spleen, which was kept as a non-treated animal in a long-term animal study. Histopathology revealed round to short spindle-shaped tumor cells arranged in a solid growth pattern. Invasive growth, anisokaryosis, and high mitotic activity (46 per 10 high-power fields [2.37 mm2]) were observed to be multifocal, but most tumor cells showed mild nuclear pleomorphism. The pattern of silver impregnation corresponded to that of the marginal zone of the red pulp. Immunohistochemistry revealed that the tumor cells were double positive for fascin and desmin and focally positive for Iba-1 and OX-6 expression. These characteristics were similar to those observed in fibroblastic reticular cells and dendritic cells in the marginal zone of the red pulp. These findings suggest that the malignant stromal cell tumor of the spleen in this case had characteristics of both fibroblastic reticular cells and dendritic cells.
Keywords: dendritic cell, fibroblastic reticular cell, rat, spleen, stromal cell tumor
Neoplastic lesions of splenic stromal cells in rodents are classified as fibroma/fibrosarcoma, pleomorphic fibrosarcoma, and histiocytic sarcoma according to the International Harmonization of Nomenclature and Diagnostic Criteria (INHAND)1. Stromal cells of the spleen are composed of fibroblastic reticular cells (FRCs), dendritic cells (DCs), and macrophages2. Thus, in humans, tumors originating from stromal cells of the spleen are classified by cell origin using immunostaining, allowing the diagnosis of FRC tumor, follicular DC sarcoma, and histiocytic sarcoma3, 4. Similarly, in rats, immunostaining for stromal cells (FRC, DC, and macrophages) and silver impregnation may be used to identify tumor cells in the spleen. In this report, we clarify the histological and immunohistochemical characteristics of a malignant tumor originating from splenic stromal cells in a male WBN/Kob rat.
Male WBN/Kob rats supplied by Japan SLC Inc. (Hamamatsu, Japan) were reared in a barrier-sustained animal room maintained at a temperature of 24 ± 2 °C with a relative humidity of 60 ± 20%, a 12 h light/dark cycle, and ventilation given at least 12 times/h. The rats were kept as untreated animals in a long term study, and exhibited severe hyperglycemia (>350 mg/dL) and glucosuria (>500 mg/dL), both of which continued from approximately 45 weeks to 90 weeks of age. No clinical signs except coarse hair were observed until the sacrifice at 90 weeks of age. The rats were euthanized with a high dose of pentobarbital (Nembutal, Sumitomo Dainippon Pharma Co., Ltd., Osaka, Japan). The study was approved by the Committee for Animal Experiments at the Setsunan University.
In one of the non-treated rats, a mass of 30 × 20 × 25 mm in size was observed in the spleen, adhering to the gastrosplenic ligament. The mass was soft, and the cut surface was peach in color (Fig. 1). In addition, mucosal proliferation of the forestomach and involution of the thymus were observed. Specimens were fixed in 10% neutral phosphorylated formalin (pH 7.4), dehydrated in a graded series of ethanol, and embedded in paraffin. Sections (4 µm) were stained with hematoxylin, eosin, and silver impregnation stains. Immunohistochemical staining using a labeled polymer method was performed using N-Histofine MAX PO rat (M or R) reagent (Nichirei, Tokyo, Japan). The following primary antibodies were used: anti-Ki-67 (1:400, rabbit polyclonal antibody, Abcam, Cambridge, UK), anti-vimentin (1:800, rabbit polyclonal antibody, DAKO, Tokyo, Japan), anti-Iba-1 (1:4000, rabbit polyclonal antibody, Wako, Tokyo, Japan), anti-OX6 (1:500, mouse monoclonal antibody, Bio-Rad, Hercules, CA, USA), anti-fascin (1:800, rabbit polyclonal antibody, Abcam), anti-αSMA (1:100, rabbit polyclonal antibody, Abcam), and anti-desmin (1: 200, mouse monoclonal antibody, DAKO). In addition, immunofluorescence double staining was performed for anti-fastin and anti-desmin with Alexa Fluor 594- and 488-conjugated secondary antibodies (Thermo Fisher Scientific, Waltham, MA, USA)5.
Fig. 1.

Gross appearance of the splenic mass. The cut surface was solid, and peach in color. Scale bar: 2.5 cm.
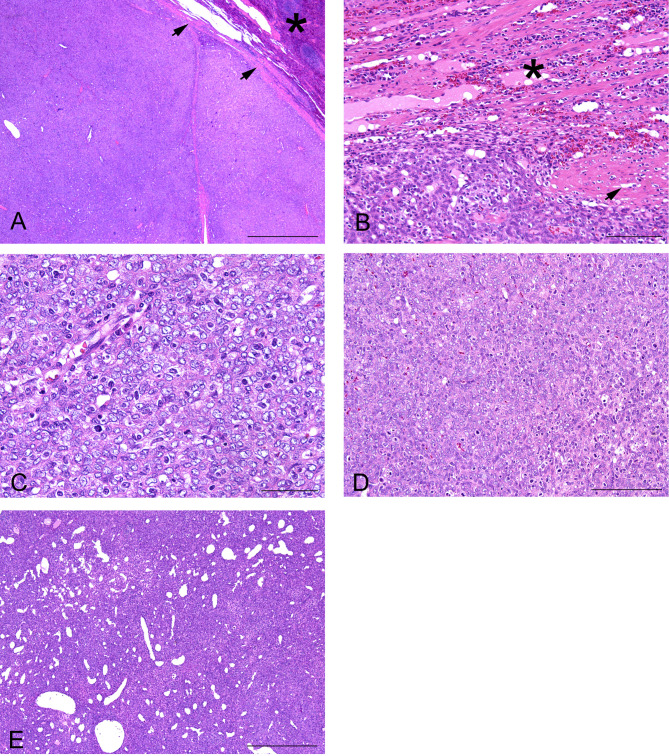
Histopathologically, the mass was distinctly delineated by the capsule structure (Fig. 2A). In certain places, the capsule structure partially disrupted, enabling the tumor cells to infiltrate the existing splenic parenchyma (Fig. 2B). The mass was composed of round to short spindle-shaped tumor cells arranged in a solid growth pattern. The sinus of the blood vessel resembling the splenic sinus was conspicuous in the tumor (Fig. 2B), and fragments of the splenic trabeculae and white pulp were occasionally retained in the mass. The tumor cells had round to short spindle nuclei, with small nucleoli and finely stippled chromatin (Fig. 2C). Slight nuclear pleomorphism was observed in most tumor cells, and the mitotic count was 3 per 10 high-power fields (HPF, 2.37 mm2). However, increased anisokaryosis was multifocally observed and the mitotic count in these regions was 46 per 10 HPF (2.37 mm2) (Fig. 2D). Almost all the tumor cells had scarce eosinophilic cytoplasms, but there were some exceptions where tumor cells had abundant eosinophilic cytoplasms. The cell borders were unclear, and the tumor cells were wrapped with delicate collagen fibers. The silver impregnation staining revealed that black-stained reticular fibers or red-stained collagen fibers surrounded individual cells or nests consisting of several neoplastic cells (Fig. 3A). In the normal spleen the reticular fibers showed a stratiform pattern in the periarterial lymphoid sheath (PALS), were sparsely distributed in the lymphoid follicle (LF), and were arranged in a mech structure in the marginal zone (MZ) and red pulp (RP) (Fig 3B). The staining pattern of silver impregnation in our case resembled that of the MZ and RP in the normal spleen (Fig 3B).
Fig. 2.
(A) The border between tumor and the spleen parenchyma (asterisk) was almost clearly delineated by the capsule structure (arrows) (HE stain. Scale bar: 1 mm). (B) The capsule structure (arrows) partially disappeared, and the tumor cells infiltrated into the existing spleen tissue (asterisk) (HE stain. Scale bar: 100 µm). (C) The nuclei shape in the tumor cells were round to short spindle, with small nucleoli and finely stippled chromatin (HE stain. Scale bar: 50 µm). (D) Increased anisokaryosis and high mitotic activity were observed (HE stain. Scale bar: 50 µm). (E) The sinus structure of the blood vessel resembled the normal splenic sinus of red pulp (HE stain. Scale bar: 500 µm).
Fig. 3.
(A) Black-stained reticular fibers surrounded individual tumor cells or tumor cell nests (silver impregnation. Scale bar: 100 µm). (B) In the normal spleen, reticular fibers were arranged in a stratiform pattern in the periarterial lymphoid sheath (PALS), were sparse in the lymphoid follicle (LF), and arranged in a mech structure in the marginal zone (MZ) and red pulp (RP) (silver impregnation. Scale bar: 100 µm). (C) The majority of the tumor cells stained positive for fascin (scale bar: 100 µm). (D) The tumor cells stained positive for desmin (scale bar: 100 µm). (E) Many non-tumor stellate-shaped cells were positive for Iba-1 (scale bar: 100 µm). (F) Double staining for fascin (red) and desmin (green). Numerous double positive tumor cells (yellow) were observed.
Immunohistochemical staining showed that almost all tumor cells exhibited diffuse staining for fascin (Fig. 3C), perinuclear staining for desmin (Fig. 3D), and negative staining for cytokeratin AE1/AE3. Some tumor cells were positive for vimentin, Iba-1, and MHC class II (OX-6). Many non-tumor stellate-shaped cells that existed between the tumor cells had different nuclear morphologies and were positive for Iba-1 and MHC class II (OX-6) (Fig. 3E). Immunofluorescence staining showed that the majority of tumor cells were positive for both fascin and desmin (yellow color), whereas in other areas the tumor cells showed positive staining for just one of the stains (red or green color) (Fig. 3F).
This study clarified the histopathological and immunohistochemical features of a rat diagnosed with a malignant stromal tumor of the spleen. The present case involved nodular formation and homogeneous tumor cell invasive proliferation around the splenic parenchyma and retention of white pulp and splenic trabeculae. Tumors can originate from stromal cells of the spleen because the cell border of tumor cells is unclear, and individual cells are surrounded by reticular and collagen fibers. Furthermore, since the tumor showed anisokaryosis, cell atypia, and high mitotic activity in some areas, the tumor was diagnosed as a malignant tumor originating from the splenic stroma.
In INHAND, the proliferative lesions of rat splenic stromal cells are classified as non-neoplastic lesions, which include fibrosis and stromal cell hyperplasia, and neoplastic lesions, which include fibroma/fibrosarcoma, pleomorphic fibrosarcoma, and histiocytic sarcoma2, 6. Fibrosis is not well-circumscribed and localized proliferative fibroblasts have marked collagen deposition. Stromal cell hyperplasia is characterized by the focal/diffuse proliferation of stromal cells in the RP. The morphological characteristics and staining pattern of the silver impregnation are similar to this case, but stromal cell hyperplasia has rare mitosis and no nodular lesions2, 7. Fibroma/fibrosarcoma is composed of fibrocytes/fibroblasts, with bands of dense mature collagen as the major components. Pleomorphic fibrosarcoma contains abundant collagen and pleomorphic atypical cells arranged in a storiform or cartwheel growth pattern. Histiocytic sarcoma is composed of large tumor cells with abundant cytoplasm and cell atypia. The mesenchymal tumors described in INHAND are different from the tumor in the present case study, as shown by the cell and tissue morphology and growth pattern6.
The stromal cells of the spleen are composed of FRC, DC, and macrophages, which are present in RP, MZ, and WP2. In humans, tumors originating from stromal cells of the spleen are classified as fibroblastic reticular cell tumor, follicular dendritic cell sarcoma, and histiocytic sarcoma by the identification of tumor cells using immunohistochemical analysis3, 4. Although antibodies that can be used in rats are limited, we attempted to identify the origin of the tumor cells. In previous reports, expression of Iba-1 and OX-6 was positive in macrophages and some DCs, fascin expression was positive in DCs, and desmin and αSMA expression was positive in FRC8, 9. In this case, many tumor cells were double-positive for fascin and desmin. Since the immunohistochemical characteristics were similar to those of fibroblastic reticular cells and dendritic cells, we recognized that the tumor cells had differentiated into FRC and DC. In addition, the pattern of silver impregnation corresponded to that of MZ to RP in normal spleen tissue, suggesting that tumor cells may have originated from the MZ and RP. Based on these findings, the present case was diagnosed as a malignant stromal cell tumor of the spleen with the characteristics of both FRC and DC.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
: We would like to thank Takeshi Izawa at Osaka Prefecture University for his kind help with immunohistochemistry.
References
- 1.Willard-Mack CL, Elmore SA, Hall WC, Harleman J, Kuper CF, Losco P, Rehg JE, Rühl-Fehlert C, Ward JM, Weinstock D, Bradley A, Hosokawa S, Pearse G, Mahler BW, Herbert RA, and Keenan CM. Nonproliferative and proliferative lesions of the rat and mouse hematolymphoid system. Toxicol Pathol. 47: 665–783. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katakai T. Marginal reticular cells: a stromal subset directly descended from the lymphoid tissue organizer. Front Immunol. 3: 200. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalia S, Shao H, Sagatys E, Cualing H, and Sokol L. Dendritic cell and histiocytic neoplasms: biology, diagnosis, and treatment. Cancer Contr. 21: 290–300. 2014. [DOI] [PubMed] [Google Scholar]
- 4.Martel M, Sarli D, Colecchia M, Coppa J, Romito R, Schiavo M, Mazzaferro V, and Rosai J. Fibroblastic reticular cell tumor of the spleen: report of a case and review of the entity. Hum Pathol. 34: 954–957. 2003. [DOI] [PubMed] [Google Scholar]
- 5.Moroki T, Matsuo S, Hatakeyama H, Hayashi S, Matsumoto I, Suzuki S, Kotera T, Kumagai K, and Ozaki K. Databases for technical aspects of immunohistochemistry: 2021 update. J Toxicol Pathol. 34: 161–180. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elmore SA. Enhanced histopathology of the spleen. Toxicol Pathol. 34: 648–655. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruehl-Fehlert C, Hartmann E, and Rinke M. Reactive and proliferative changes of splenic reticulum cells of rats investigated with special staining methods and immunohistochemistry. Exp Toxicol Pathol. 59: 281–290. 2008. [DOI] [PubMed] [Google Scholar]
- 8.Mueller SN, and Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. 9: 618–629. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lokmic Z, Lämmermann T, Sixt M, Cardell S, Hallmann R, and Sorokin L. The extracellular matrix of the spleen as a potential organizer of immune cell compartments. Semin Immunol. 20: 4–13. 2008. [DOI] [PubMed] [Google Scholar]