Abstract
The fast growth rate of broiler chickens is a welfare concern that has increased consumer interest in chicken from slower growing (SG) broilers. Replacing conventional (CONV) broilers with SG broilers will reduce chicken supply and SG broilers require different management practices than CONV. This study evaluated the effects of 2 stocking densities on the carcass composition and meat quality of CONV broilers that reach market weight at 42 d and SG broilers that reach market weight at 63 d. Male broilers from each strain were exclusively stocked into 16 pens at a density of either 29 kg/m2 or 37 kg/m2. Live body, carcass without giblets (WOG), and part weights were recorded and used to calculate yield. Initial and 24-hour pH, color (L*, a*, and b*), cooking loss, and Warner Bratzler shear force of the breast and thigh muscles were measured. Birds from both strains reached similar live body and carcass WOG weights. CONV had 3.4%, 13.0%, and 2.8% greater (P ≤ 0.002), carcass WOG, breast, and tenderloin yields, while SG had 2.7%, 5.0%, 7.5%, and 1.2% greater (P < 0.0001) wings, leg quarters, frame, and skin yields, respectively. CONV breast 24-h pH and cooking loss were greater (P ≤ 0.04) than SG. While SG thigh shear force was greater than CONV (P = 0.008), breast shear force was the lowest for CONV stocked at 29 kg/m2 and the greatest for SG stocked at 37 kg/m2 (P = 0.04). SG had a paler breast than CONV, while CONV had a yellower breast than SG (P < 0.0001). While SG had a redder thigh than CONV (P = 0.002), SG stocked at 29 kg/m2 had a redder breast than SG stocked at 37 kg/m2, with both CONV groups intermediate (P = 0.04). These results indicate that differences in male broiler meat quality were affected more by strain than by stocking density. Compared with CONV, SG broiler meat quality was more affected by stocking density in this study.
Key words: broiler, strain, stocking density, performance, welfare
INTRODUCTION
The increasing global population is fueling the demand for animal protein. Chicken meat consumption is growing in popularity, especially in countries with growing economies (Godfray et al., 2018). The U.S. is the global leader in chicken meat production, producing over 22 million tons of chicken meat, which is 5.7 times more than 50 yr ago (NCC, 2021). Key drivers of this remarkable increase in production are attributed to the scientific achievements in genetic selection, nutrition, disease control, equipment, and management strategies. Improvements in broiler growth rate and carcass yield have been driven by increases in breast muscle and reductions in abdominal fat in younger birds (Le Bihan-Duval et al., 2001). However, the detrimental impacts of fast growth on the welfare of today's conventional broiler chicken is at the forefront of public concern (Meluzzi and Sirri, 2009; You et al., 2014; Vizzier-Thaxton et al., 2016; Broom, 2017) and producers of slower growing broilers can capitalize on marketing their chicken meat as a higher welfare product than conventional broilers (Singh et al., 2021).
The production costs of raising slow-growing broilers are greater and they yield less meat than conventional broilers, decreasing the affordability of chicken. From 1957 to 2005, the breast (pectoralis major) and tenderloin (pectoralis minor) muscle yields of the conventional broiler have increased by over 80% and 30%, respectively (Zuidhof et al., 2014). This is why slower growing broilers have lower carcass, breast, and tenderloin yields and greater thigh, drumstick, and wing yields than conventional broilers (Sarsenbek et al., 2013; Singh et al., 2021). Previous meat quality work focusing on broilers with divergent growth rates have found differences in breast meat pH, color, drip and cooking loss, and texture (Le Bihan-Duval et al., 1998, 1999, 2001; Castellini et al., 2002; Berri et al., 2005; Fanatico et al., 2005; Wen et al., 2017; Singh et al., 2021). However, these differences are often heavily influenced by the age of the birds at processing.
Genetics and environment can affect muscle composition and meat quality. Fast growth rate has been identified as the leading cause of emergent meat quality issues in broilers, and the most prevalent are white striping and woody breast muscle myopathies (Kuttappan et al., 2016). However, management may play a larger role in the prevalence of these myopathies. For example, the heritability of woody breast has been reported to be low (0.02–0.1) and moderate (0.19–0.34) for white striping (Bailey et al., 2015). In the same heritability study, the authors concluded that environmental and management factors contributed to 65% of the variance in the incidence of white striping and 90% of the incidence of woody breast (Bailey et al., 2015).
Genetics affect the bird's response to its environment. For example, one study found that slow-growing broilers with access to the outdoors had yellower breast meat compared with the redder breast meat of slow-growing birds raised indoors, yet this effect was not observed in the conventional birds (Fanatico et al., 2005). Debut and colleagues (2005) found that slow-growing broilers exhibited more wing flapping (stress) during shackling compared with 2 faster-growing broiler strains. In the same aforementioned study, the slow-growing broiler breast muscle rate of pH decline was greater, the meat was darker, yellow, redder, and had greater drip loss compared with birds from two faster growing broiler strains (Berri et al., 2005). Thus, environmental conditions and the animal's response to the environment can affect meat quality. Most importantly, the stress response to the same environments is not consistent across broiler strains.
Slower growing broilers are typically raised at lower stocking densities than conventional strains and oftentimes in alternative organic or pasture-based systems. Although research has been conducted comparing the meat characteristics of slow-growing and conventional broilers, the strains of birds and housing systems used vary greatly by geographical region and very little research has been conducted in the U.S. High stocking density can negatively affect broiler production, health and welfare (Dawkins et al., 2004; Estevez, 2007). Given that genetics and environment influence meat quality, the objective of this study was to evaluate the effect of stocking density on the carcass composition and meat quality of conventional and slow-growing broilers raised indoors.
MATERIALS AND METHODS
Experimental Design and Husbandry
The current study was conducted on the same birds from a previous report on the effect of stocking density on conventional and slow-growing broiler performance, body conformation, and welfare (Weimer et al., 2020). A 2 × 2 complete randomized design was used. Male day-of-hatch conventional (CONV) broiler chicks (N = 284 chicks), denoted as a typical broiler strain raised the U.S., were obtained from a commercial hatchery and placed on the day the study began. Male day-of-hatch slow-growing (SG) chicks (N = 284 chicks), denoted as a slower growing strain than a typical CONV broiler strain raised in the U.S., were shipped from a commercial hatchery, and were placed one day after the CONV chicks had been placed. Chicks from each strain were exclusively and randomly placed into 16 pens (1.5 m x 2.4 m pens; N = 4 pens per strain-stocking density combination) and subjected to a stocking density (defined as final body weight [BW] per square meter (m2)), of either 29 kg/m2 (N = 31 birds/pen) or 37 kg/m2 (N = 40 chicks/pen). The study was conducted at the Purdue University Poultry Research Unit in West Lafayette, Indiana from July to September 2018. The Purdue Animal Care and Use Committee approved all experimental methods and procedures (PACUC#1803001706). Chicks were unvaccinated and hatched on the same day. Birds from both strains were raised to the age when a target market live body weight of 2.8 kg was predicted to be achieved, which was at 42 days for CONV (Aviagen, LLC) and 63 days for SG (Hubbard, LLC). Animal and environmental management details have been reported previously (Weimer et al., 2020).
Data Collection
At the beginning of the study, 8 focal birds from each pen were randomly selected and uniquely identified (N = 32 birds/treatment combination, 128 birds total). After fasting for 10 h, on day 41 CONV (N = 60 birds) were processed and on day 62 SG (N = 59 birds) were processed at the Purdue University Boilermaker Butcher Block (West Lafayette, IN).
Live body weight (BW, g) was collected, and birds were stunned and exsanguinated with an electrical stunning knife in a restraining cone. Automated equipment was used for scalding and feather picking. After the heads and hocks were removed, the initial acidity of the breast and thigh muscle was measured with a pH meter (HANNA HI 99163, Hanna Instrument, Inc., Warner, NH) calibrated to standard 4.0, 7.0, and 10.0 buffers. Carcasses were manually eviscerated and the weight (g) of the heart, liver, small intestine (duodenum, ileum, and jejunum), ceca, and large intestine (rectum and cloaca) were recorded and used to calculate relative organ weights (yield, %). The ceca, small and large intestine lengths (cm) were measured with a ruler.
After 2 h in the air blast chiller, carcass with neck and without giblets (WOG) weight (g) was collected and carcasses were deboned into wings, leg quarters, breast, tenderloins, frame, and skin with weights (g) recorded and used to calculate carcass WOG and part yields (%). Carcass WOG yield was calculated as a percentage of BW and part yields were calculated as a percentage of carcass WOG weight. The color of the liver and right breast and thigh muscles were measured with a Minolta colorimeter (Konica Minolta Cr-400, Minolta Corp., Ramsey, NJ) with an 8 mm aperture, 2° observer, measuring illuminant D65, and calibrated using a standard white tile (No. 14833165; Y = 84.40, x = 0.3179, y = 0.3340). In this method, greater L* values are lighter, greater a* values are redder, and greater b* values are yellower. An average of 3 color measures taken on the ventral surface of each liver, breast, and thigh was used for analysis.
Breast and thigh muscle samples were stored at 4°C overnight and pH was measured again 24 h postmortem. Breast and thigh muscles were stored at −62°C until they were thawed overnight at 4°C to determine cooking loss and Warner-Bratzler shear force. To calculate cooking loss (%), breasts and thigh muscle samples were weighed, cooked in plastic bags submerged in a water bath set to 80°C until the internal temperature reached 71°C measured with a T-type thermocouple (Omega Engineering, Stamford, CT) connected to a data logger (OctTemp 2000, Madge Tech, Inc., Warner, NH) inserted into the center, and reweighed. Cooking loss was calculated as the weight loss during cooking as a percentage of the weight before cooking. After cooking, breast muscle and thigh muscle Warner-Braztler shear force (Newtons) was measured on six 1 cm x 1 cm cores per sample on a texture analyzer (TA-XT Plus Texture Analyzer, Stable Micro Systems, Ltd., Godalming, UK) with the Warner-Bratzler shear attachment, at a test speed of 2 mm/s, and sample cores averaged for analysis.
Statistical Analysis
Statistical analysis was performed using JMP Pro (version 14.2, SAS Institute Inc., Cary, NC). Data was tested for normality using the Distribution platform. The pen was the experimental unit. A 2-way ANOVA included the main effects of strain (CONV and SG), stocking density (29 kg/m2 and 37 kg/m2), and the strain-stocking density interaction and the random effect of pen for all measures. Significant LS means were separated post hoc with Tukey's HSD. Data were considered significant at P ≤ 0.05.
RESULTS
Independent of strain, there were minimal effects of stocking density on part and organ weights and yields. The slow-growing (SG) broilers were 21 days older than conventional (CONV) broilers and reached a similar body weight, ranging between 2,426 g and 2,884 g at processing (Table 1).
Table 1.
Part and organ weight (g) of male broilers from 2 strains (CONV and SG) raised at 2 stocking densities (29 kg/m2 and 37 kg/m2).
| CONV1 |
SG |
P-value2 |
||||||
|---|---|---|---|---|---|---|---|---|
| Weight (g) | 29 kg/m2 | 37 kg/m2 | 29 kg/m2 | 37 kg/m2 | SEM | S | D | S*D |
| Live BW | 2856 | 2704 | 2426 | 2884 | 154 | 0.07 | 0.34 | 0.43 |
| Carcass (WOG)3 | 2152 | 2055 | 1747 | 2068 | 118 | 0.10 | 0.12 | 0.36 |
| Breast | 589a | 588a | 263b | 318b | 30 | 0.0001 | 0.39 | 0.36 |
| Tenderloins | 87 | 82 | 70 | 78 | 6 | 0.13 | 0.78 | 0.29 |
| Wings | 202b | 196b | 220a | 246a | 12 | 0.01 | 0.19 | 0.43 |
| Leg Quarters | 599b | 569b | 566ab | 697a | 37 | 0.21 | 0.20 | 0.05 |
| Frame | 551b | 525b | 590a | 680a | 34 | 0.01 | 0.36 | 0.11 |
| Skin | 20b | 19b | 41a | 43a | 5 | 0.0007 | 0.91 | 0.74 |
| Heart | 12.8 | 12.1 | 11.3 | 14.6 | 0.9 | 0.62 | 0.18 | 0.06 |
| Liver | 45.0a | 48.1a | 31.2b | 43.1a | 2.4 | 0.002 | 0.09 | 0.009 |
| Small intestine | 58.4b | 57.6b | 53.4b | 67.4a | 2.4 | 0.39 | 0.02 | 0.01 |
| Ceca | 14.2b | 13.9b | 18.2a | 18.8a | 0.8 | 0.0001 | 0.88 | 0.55 |
| Large intestine | 2.1b | 2.1b | 2.8a | 3.0a | 0.3 | 0.02 | 0.83 | 0.81 |
Conventional (CONV) broilers were processed on d 41 and slow-growing (SG) broilers were processed on d 62.
S = Strain, D = Density, S*D = Strain*Density interaction.
Carcass without giblets (WOG) and part weights were recorded after 2 hours in the air blast chiller.
Rows not sharing the same letter are significantly different at P ≤ 0.05.
Part Weight and Yields
Live body, carcass, part, and organ weights are in Table 1 and yields are in Table 2. Carcass WOG weights were similar, ranging from 1,747 to 2,152 g, but CONV broilers had 3.4% greater (P = 0.0001) carcass yield than SG broilers. The CONV bird breast muscle weight (588.5 g) and yield (28.1%) were about twice (P < 0.0001) that of SG birds (290.5 g and 15.1%, respectively). While tenderloin weights were similar (average 79.3 g), CONV birds had 2.8% greater tenderloin yield than SG birds (P = 0.0002). SG broilers stocked at 37 kg/m2 had the heaviest (P = 0.05) leg quarters that were at least 113 g heavier than CONV broilers (Table 1). The leg quarters yield of CONV broilers was 5.0% lower (P < 0.0001) than SG broilers (Table 2). The wing, frame, and skin weights of SG were 34 g, 97 g, and 22 g heavier (P ≤ 0.01) than CONV, respectively. Similarly, SG wing (12.3%), frame (33.3%), and skin (2.2%) yields were greater (P ≤ 0.0002) than CONV (9.6% wing, 25.8% frame, and 1.0% skin, respectively; Table 2).
Table 2.
Part and organ yield (%) of male broilers from 2 strains (CONV and SG) raised at 2 stocking densities (29 kg/m2 and 37 kg/m2).
| CONV1 |
SG |
P-value2 |
||||||
|---|---|---|---|---|---|---|---|---|
| Yield (%)3 | 29 kg/m2 | 37 kg/m2 | 29 kg/m2 | 37 kg/m2 | SEM | S | D | S*D |
| Carcass (WOG) | 75.03a | 75.78a | 72.31b | 71.71b | 0.54 | 0.0001 | 0.88 | 0.21 |
| Breast | 27.71a | 28.50a | 14.93b | 15.22b | 0.61 | 0.0001 | 0.38 | 0.69 |
| Tenderloins | 14.46a | 14.37a | 12.05b | 11.16b | 0.54 | 0.0002 | 0.37 | 0.47 |
| Wings | 9.58b | 9.57b | 12.64a | 11.93a | 0.19 | 0.0001 | 0.08 | 0.09 |
| Leg Quarters | 28.44c | 27.78c | 32.42b | 33.79a | 0.31 | 0.0001 | 0.27 | 0.006 |
| Frame | 26.02b | 25.63b | 33.79a | 32.86a | 0.41 | 0.0001 | 0.13 | 0.51 |
| Skin | 0.98b | 0.94b | 2.29a | 2.00a | 0.22 | 0.0002 | 0.46 | 0.59 |
| Heart | 0.42 | 0.45 | 0.46 | 0.50 | 0.25 | 0.09 | 0.20 | 0.84 |
| Liver | 1.54b | 1.78a | 1.29c | 1.49b | 0.05 | 0.0001 | 0.001 | 0.03 |
| Small intestine | 2.08 | 2.14 | 2.21 | 2.35 | 0.08 | 0.06 | 0.23 | 0.57 |
| Ceca | 0.67b | 0.68b | 1.04a | 0.92a | 0.04 | 0.0001 | 0.16 | 0.13 |
| Large intestine | 0.51b | 0.51b | 0.75a | 0.66a | 0.03 | 0.0001 | 0.07 | 0.13 |
Conventional (CONV) broilers were processed on d 41 and slow-growing (SG) broilers were processed on d 62.
S = Strain, D = Density, S*D = Strain*Density interaction.
Carcass yield was calculated as a proportion of live body weight and part yields were calculated as a proportion of carcass yield.
Rows not sharing the same letter are significantly different at P ≤ 0.05.
Organ Yields and Lengths
Heart weights and yields were similar, but SG birds stocked at 29 kg/m2 had the lowest liver weight (31.2 g) and the lowest liver yield (1.29%) compared with all other treatments (P ≤ 0.03; Tables 1 and 2). The SG broilers stocked at 37 kg/m2 had the heaviest (P = 0.01) small intestines (67.4 g) compared with all other treatment combinations (Table 1). The SG birds had heavier ceca and large intestines and correspondingly greater yields than CONV (P ≤ 0.05; Tables 1 and 2). The SG birds had longer (P < 0.0001) ceca (19.2 cm) than CONV (17.5 cm), while CONV had longer (P = 0.05) large intestines (6.9 cm) than SG (5.6 cm; Table 3). The SG broilers stocked at 29 kg/m2 had shorter small intestines (139.7 cm; P ≤ 0.05) compared with other treatment combinations (Table 3).
Table 3.
Intestine lengths (cm) of male broilers from 2 strains (CONV and SG) raised at 2 stocking densities (29 kg/m2 and 37 kg/m2).
| CONV1 |
SG |
P-value2 |
||||||
|---|---|---|---|---|---|---|---|---|
| Length (cm) | 29 kg/m2 | 37 kg/m2 | 29 kg/m2 | 37 kg/m2 | SEM | S | D | S*D |
| Small intestine | 148.13a | 150.31a | 139.73b | 146.50a | 2.09 | 0.01 | 0.05 | 0.29 |
| Ceca | 17.60b | 17.47b | 19.00a | 19.45a | 0.24 | 0.0001 | 0.50 | 0.23 |
| Large Intestine | 6.47a | 7.25a | 5.80b | 5.33b | 0.61 | 0.05 | 0.32 | 0.80 |
Conventional (CONV) broilers were processed on d 41 and slow-growing (SG) broilers were processed on d 62.
S = Strain, D = Density, S*D = Strain*Density interaction.
Rows not sharing the same letter are significantly different at P ≤ 0.05.
Meat Quality
pH
The initial breast and thigh and 24-hour thigh muscle pH did not differ. The 24-hr breast muscle pH of CONV (6.12) was greater (P < 0.0001) than SG (5.97; Table 4).
Table 4.
Breast and thigh pH and cooking loss of male broilers from 2 strains (CONV and SG) raised at 2 stocking densities (29 kg/m2 and 37 kg/m2).
| CONV1 |
SG |
P-value2 |
||||||
|---|---|---|---|---|---|---|---|---|
| 29 kg/m2 | 37 kg/m2 | 29 kg/m2 | 37 kg/m2 | SEM | S | D | S*D | |
| Breast initial pH3 | 6.38 | 6.51 | 6.34 | 6.35 | 0.06 | 0.17 | 0.20 | 0.32 |
| Breast 24h pH4 | 6.15a | 6.09a | 5.95b | 5.99b | 0.02 | 0.0001 | 0.65 | 0.03 |
| Thigh initial pH | 6.24 | 6.28 | 6.31 | 6.40 | 0.05 | 0.07 | 0.24 | 0.63 |
| Thigh 24h pH | 6.24 | 6.25 | 6.30 | 6.29 | 0.04 | 0.20 | 0.96 | 0.84 |
| Breast cooking loss (%)5 | 8.79ab | 9.85a | 7.14b | 8.40ab | 0.52 | 0.01 | 0.04 | 0.85 |
| Thigh cooking loss (%) | 7.68b | 7.26b | 8.27b | 10.11a | 0.54 | 0.007 | 0.21 | 0.05 |
Conventional (CONV) broilers were processed on d 41 and slow-growing (SG) broilers were processed on d 62.
S = Strain, D = Density, S*D = Strain*Density interaction.
Initial pH was measured at slaughter, prior to chilling.
24h pH was measured 24 hours after processing and after chilling overnight at 4°C.
Cooking loss was calculated as the weight loss during cooking as a percentage of the weight before cooking.
Rows not sharing the same letter are significantly different at P ≤ 0.05.
Cooking loss
The cooking loss of breast muscle from SG broilers stocked at 29 kg/m2 (7.14%) was less than CONV broilers stocked at 37 kg/m2 (9.85%), with intermediate values for the other two treatments (P = 0.04; Table 4). Slow-growing birds stocked at 37 kg/m2 had greater thigh muscle cooking loss (10.11%) compared with birds from the other treatment groups (P = 0.05; Table 4).
Color
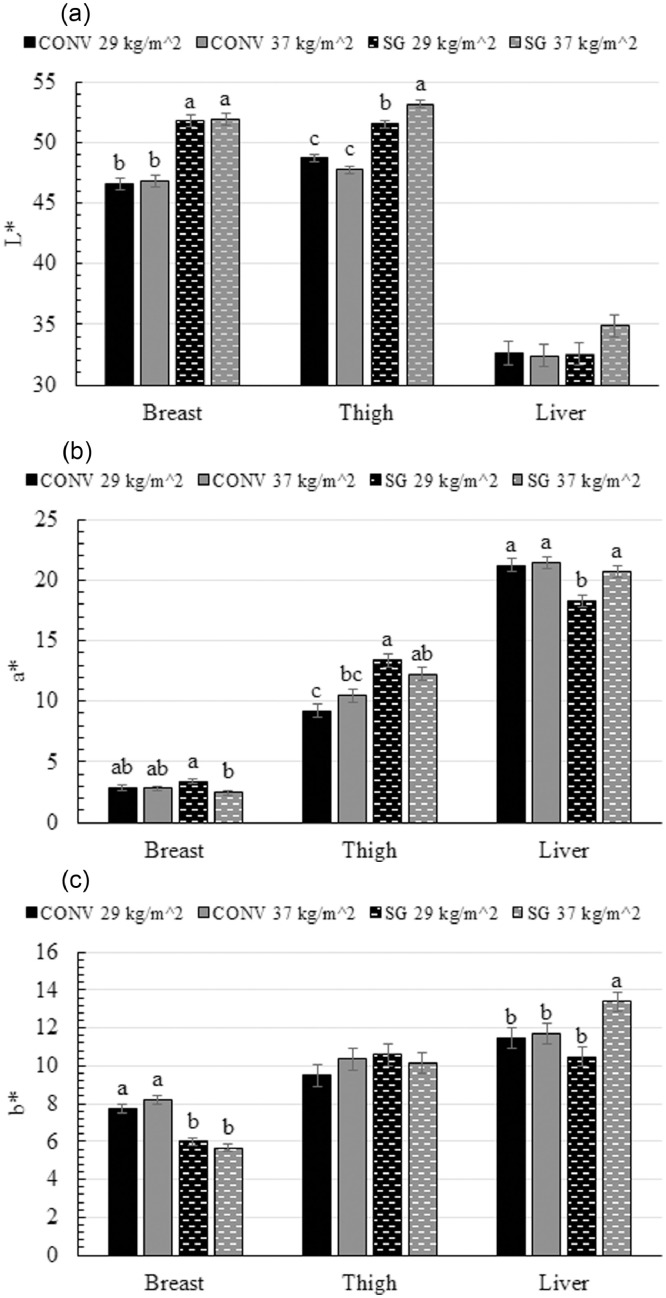
SG broilers had a greater (lighter) breast muscle L* value (51.9) than CONV broilers (46.7), while CONV broilers had a greater (yellower) b* value (8.0) than SG broilers (5.8; P < 0.0001; Figure 1 a,c). Slow-growing broilers stocked at 29 kg/m2 a greater (redder) breast muscle a* value (3.4) than SG stocked at 37 kg/m2 (2.5), while both CONV birds stocked at both densities were intermediate (2.9; P= 0.04; Figure 1b).
Figure 1.
Color values of the breast, thigh, and liver of male broilers from 2 strains (CONV and SG) raised at 2 stocking densities (29 kg/m2 and 37 kg/m2) for a) paleness [L*], b) redness [a*] and c) yellowness [b*]. abcBars within the same tissue type not sharing the same letter are significantly different at P ≤ 0.05.
Thigh muscle b* values did not differ (Figure 1c), but SG broilers stocked at 37 kg/m2 had the greatest (palest) thigh muscle L* value (53.2), followed by SG broilers stocked at 29 kg/m2 (51.5), and CONV broilers had the least pale thigh muscle (48.2; P = 0.001; Figure 1a). SG broilers had a greater (redder) thigh muscle a* value (12.8) than CONV broilers (9.9; P = 0.002; Figure 1b).
While liver L*values did not differ (Figure 1a), SG broilers stocked at 29 kg/m2 had the lowest (least red) liver a* value (18.3) by at least 2.4 compared with other treatments (P = 0.05; Figure 1b). The liver b* value of SG broilers stocked at 37 kg/m2 was greater (yellower; 13.4) than SG broilers stocked at 29 kg/m2 (10.5), with both CONV birds stocked at both densities were intermediate (11.6; P = 0.05; Figure 1c).
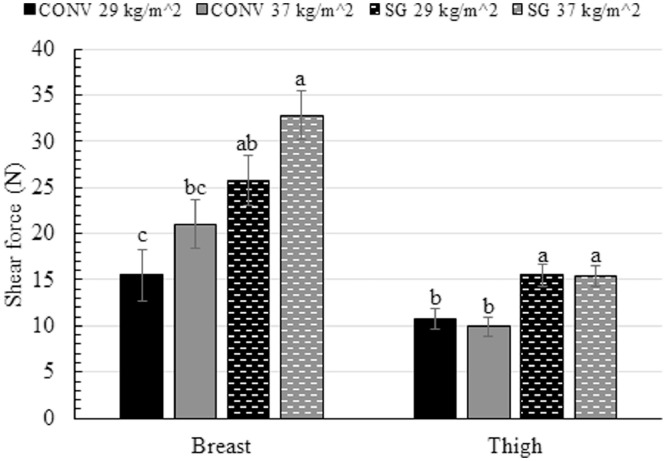
Shear force
CONV broilers stocked at 29 kg/m2 had the lowest breast muscle shear force (15.5 N), followed by CONV broilers stocked at 37 kg/m2 (21.0 N), followed by SG broilers stocked at 29 kg/m2 (25.8 N), followed by SG broilers stocked at 37 kg/m2 (32.8; P = 0.04; Figure 2). Thigh muscle shear force was 33% greater for SG broilers (15.5 N) than CONV broilers (10.4 N; P = 0.008; Figure 2).
Figure 2.
Shear force (N) of the breast, thigh, and liver of male broilers from 2 strains (CONV and SG) raised at 2 stocking densities (29 kg/m2 and 37 kg/m2). abcBars within the same tissue type not sharing the same letter are significantly different at P ≤ 0.05.
DISCUSSION
The present study investigated the impact of raising male broilers with two divergent growth rates at two stocking densities on carcass composition and meat quality. It has been well established that high stocking densities can negatively impact broiler production performance and welfare on-farm (Shanawany, 1988; Feddes et al., 2002; Sørensen et al., 2002; Dawkins et al., 2004; Thomas et al., 2004; Dozier et al., 2005; Bessei, 2006; Buijs et al., 2009). However, limited research has compared the impact of stocking density on broiler meat yield and quality, especially on broilers from different strains.
Part Weight and Yields
Modern broilers are the product of many generations of selection for increased body weight and breast meat yield. Genetics contribute an estimated 85% to 90% of the differences in carcass part weights and yields (Havenstein et al., 2003). In the current study, even though birds from both strains had similar live body and carcass WOG weights, conventional broiler breast muscle weight and yield was about double that of the slow-growing strain, while slow-growing broilers had greater quarters, wings, frame, and skin weights and yields. Previous conventional and slower-growing broiler strain comparison studies have also reported greater breast muscle weights and yields of conventional broilers (Le Bihan-Duval et al., 1998; Fanatico et al., 2005, 2008; Sarsenbek et al., 2013; Singh et al., 2021) and greater weights and yields of slow-growing broiler wings, frame (Fanatico et al., 2008) and leg quarters (Sarsenbek et al., 2013; Singh et al., 2021).
Age likely contributed to the greater slow-growing broiler frame and skin yields because they were older at processing. Slow-growing broilers have longer legs (Essary et al., 1951; Kokoszyński et al., 2017; Weimer et al., 2020) and are more active and mobile than conventional broilers (Savory, 1975; Castellini et al., 2002; Bessei, 2006). Lewis and colleagues (1997) raised Ross and ISA ‘Label Rouge’ broilers at two stocking densities and found that birds stocked at 4.25 birds/m2 had greater breast meat yield and larger frames than birds stocked at 17.0 birds/m2, and slow-growing broilers had greater wings and bone yields than conventional broilers. Increased walking and wing flapping can increase bone loading and muscle mass (Lewis et al., 1997), which may explain why the quarters, wings, frame, and skin yields of slow-growing broilers were greater than conventional in the current study.
Faster growth rates are associated with reduced relative weights of the internal organs in broilers (Havenstein et al., 2003). The heart weights and yields were similar, as has been found previously (Singh et al., 2021), and this is not surprising because the birds weighed the same in the current study. However, slow-growing birds had greater small intestine, ceca, and large intestine yields than conventional. Interestingly, the slow-growing broilers stocked at 29 kg/m2 had lighter small intestines than slow-growing broilers stocked at 37 kg/m2 and the shortest small intestine lengths compared with all other birds. When feed conversion ratio (FCR) was previously measured, it was found that the FCR of conventional broilers stocked at 29 kg/m2 was 20 points greater than at 37 kg/m2 and 220 points greater for the slow-growing broilers stocked at 29 kg/m2 than at 37 kg/m2 (Weimer et al., 2020). Also, although not statistically significant in this study, the live body weight of slow-growing broilers stocked at 29 kg/m2 was 16% less than at 37 kg/m2, while this difference was only 5% for conventional broilers. It was also previously reported that the slow-growing broilers stocked at 29 kg/m2 had the greatest incidence of toe damage (Weimer et al., 2020). The digestive system contains extensive innervation from both the sympathetic and parasympathetic nervous system (Denbow, 2015), which mediate the stress response, and the differences in the slow-growing broilers raised at 29 kg/m2 compared with 37 kg/m2 may have been the result of higher levels of stress.
Meat Quality
Meat quality can be defined as the “composite characteristics that differentiate individual units of a product which have significance in determining the degree of acceptability to the consumer” (Groom, 1990). Measures of meat quality can be categorized into appearance (i.e., color), physical (i.e., cooking loss), and chemical (i.e., fatty acid profile; Zhao et al., 2011). Genetic variation contributes to large differences in meat quality and heritability estimates for meat quality traits in broilers are high (0.35–0.81), making genetic selection a best tool for improvement of broiler meat quality (Mir et al., 2017). However, comparing the meat quality of broilers from different strains is difficult because experimental research conditions limit the number of birds that are processed either at the same age (chronological age) or market body weight (physiological age). Future large-scale studies should compare the chronological and physiological ages when evaluating meat quality of different broiler strains.
The 24-h breast pH of conventional broilers was greater than slow-growing broilers, and there were no differences in thigh muscle pH. Initial breast muscle pH is reported to have a moderate heritability (0.49; Le Bihan-Duval et al., 2001). Reduced 24-h pH can result in protein denaturation, causing a reduction in water-holding capacity in fresh meat products (Bowker and Zhuang, 2015). Color is a direct result of pH decline as meat goes through rigor mortis with whole muscle meat generally becoming lighter in color (Berri, 2000) and previous research has reported that broiler strains selected for growth had a lower rate of pH decline postmortem than unselected strains (Berri et al., 2001, 2005). The breast muscle of slow-growing broilers was lighter in color than conventional breast muscle in the current study, as in other studies (Singh et al., 2021). However, inconsistent results have been found in similar studies. Others have found the 24-hr pH and lightness of breast muscles to be lower in conventional compared with slow-growing broiler strains (Berri et al., 2001) or no differences between strains (Fanatico et al., 2007; Wen et al., 2017). The breast muscle color of conventional birds was yellower than slow-growing birds in the current study, which could have been related to their more efficient feed conversion and resultant faster carotenoid deposition into intermuscular fat. Also, slow-growing broilers stocked at 29 kg/m2 had a redder breast than SG stocked at 37 kg/m2, while both conventional groups were intermediate. Heme pigments from myoglobin, hemoglobin, and cytochrome C increase with age (Mir et al., 2017) and may explain the differences in the redness of the breast muscle in the current study.
Liver color was measured because it is the primary site of fatty acid synthesis in poultry (Leville, 1969), lipid metabolism is affected by stress (Puvadolpirod and Thaxton, 2000), and to our knowledge, no studies have compared breast, thigh, and liver colors from the same birds. Slow-growing broilers stocked at 29 kg/m2 had the lowest relative liver weight and least red liver color, while slow-growing broilers stocked at 37 kg/m2 had the most yellow livers, with intermediate values for these measures for conventional at both densities. Trampel et al. (2005) compared full-fed and 12-hr fasted conventional broilers and found that the full-fed broilers’ liver color was less pale than fasted broilers. Puvadolpirod and Thaxton (2000) continuously delivered adrenocorticotropic hormone (ACTH) to 5-wk old male Peterson x Arbor Acres broilers for 7 d and found that ACTH administration increased relative liver weight and lipid accumulation and reduced liver moisture. Although we did not measure stress physiology (i.e., corticosterone) and our relative liver weight results are not in agreement with the aforementioned study, it is possible that the low stocking density (29 kg/m2) in this study may have induced some type of stress and stunted the development of the slow-growing broilers.
Texture is an important quality factor associated with consumer satisfaction in the eating quality of poultry products (Mir et al., 2017), of which shear force is a direct measure. The maturity of the connective tissue is a function of chemical cross bonding of the collagen in the muscle, which increases with age, hence tougher meat is found in older birds (Mir et al., 2017) and this was the case with the older slow-growing broilers having greater breast and thigh muscle shear force in the current study. Using an experimental strain and a French commercial strain, Berri and colleagues (2001) selected the progeny from each strain for high body weight, breast yield, and low abdominal fat, and compared meat quality characteristics to their respective unselected controls. The authors found that selection of the commercial strain resulted in greater protein content and lower moisture in the breast muscle and selection of the experimental strain decreased breast heme pigment content, which was linked to the more pale and less red breast meat of selected birds (Berri et al., 2001). Slow-growing broiler thigh muscle was paler and redder than conventional thigh muscle in the current study. As the age at slaughter increases, the myoglobin content of broiler muscle increases (Berri, 2000) and this is likely why slow-growing broilers had more red thigh muscles in the current study.
As broilers age and mature, the fat and protein content of their muscle tissue increase and water content decreases (Grey et al., 1983; Berri et al., 2001). The breast muscle cooking loss of conventional broilers was greater, but lower for thigh muscles compared with slow-growing in the current study. However, slow-growing broilers stocked at 29 kg/m2 had the lowest breast cooking loss while conventional stocked at 37 kg/m2 had the greatest, and slow-growing birds stocked at 37 kg/m2 had the greatest thigh cooking loss. Similarly, others have reported greater cooking loss of breast muscles from slow-growing broilers compared with faster-growing broilers (Fanatico et al., 2005, 2007). Contrarily, Sarsenbek et al. (2013) found greater breast and thigh muscle cooking loss from conventional (Arbor Acres) broilers compared with slow-growing (Baicheng-You). Zhao et al. (2011) found that slow-growing (Beijing-you) broilers had smaller and more dense breast muscle fibers than conventional (Arbor Acres), while Sarsenbek et al. (2013) found no difference in the breast or thigh muscle fiber density of slow-growing (Baicheng-You) and conventional (Arbor Acres) broilers. The greater thigh muscle cooking loss from slow-growing broilers stocked at 37 kg/m2 is likely because these birds also had the largest (heaviest and greatest yielding) quarters.
The ideal environmental conditions for raising broilers from different strains differ from one environment to another because strain-environment interactions exist (Mathur, 2003). Rearing conditions are expected to be even more important under the less intensive conditions generally used for slow-growing broilers (Berri, 2000). For example, Fanatico et al. (2005) conducted an on-farm study where slow-growing and fast-growing broilers were raised indoors and outdoors; the housing conditions were found to have more of an impact on the breast meat quality of the slow-growing strain (outdoor access yielded a more yellow breast) compared with the conventional strain (no effect). Berri and colleagues (2005) compared the breast meat quality from three strains, a slow-growing, fast growing, and high breast meat yield strain, and found that the breast meat of slow-growing broilers had greater rate of postmortem muscle pH decline, and lower L* and b* and higher a* and drip loss, compared with the other two strains selected for growth and yield. In a concurrent study with the aforementioned, the slow-growing broilers exhibited more behavioral indicators of distress (i.e., wing flapping and vocalizations) during shackling compared with the other 2 strains (Debut et al., 2005), which may have induced breast meat quality differences.
CONCLUSION
In the current study, strain had a greater impact on broiler carcass composition and meat quality than stocking density. Compared with conventional broilers, slow-growing broilers had larger wings, quarters, frames, ceca and large intestines, smaller, lighter and tougher breast muscles, and larger, lighter and tougher thigh muscles. Stocking density affected slow-growing broilers more than the conventional broilers. Slow-growing broilers stocked at the lower density had shorter small intestines, smaller legs and livers, lower breast and thigh muscle cooking loss, and darker thigh muscles, compared with the slow-growing broilers stocked at the higher density. We hypothesize that slow-growing broilers are a more reactive animal – meaning that they more readily respond to subtle alterations in their environment than conventional broilers, but further work is needed to examine strain-related differences in reactivity. These results highlight the importance of understanding the effects of management practices, such as stocking density, on the meat quality of different strains of broilers.
ACKNOWLEDGMENTS
This project was supported by the startup funds provided to Darrin Karcher and Marisa Erasmus by the Purdue University Department of Animal Sciences. We would like to thank and acknowledge Blaine Brown, Dr. Brad Kim, Derico Setyabrata, Delaney Hoschstedler, Kyle Reichard, and Kailynn Scoles for their assistance.
Disclosures
The authors declare no conflicts of interest.
REFERENCES
- Bailey R.A., Watson K.A., Bilgili S.F., Avendano S. The genetic basis of pectoralis major myopathies in modern broiler chicken lines. Poult. Sci. 2015;94:2870–2879. doi: 10.3382/ps/pev304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berri C. Variability of sensory and processing qualities of poultry meat. Worlds Poult. Sci. J. 2000;56:209–224. [Google Scholar]
- Berri C., Wacrenier W., Millet N., Le Bihan-Duval E. Effect of selection for improved body composition on muscle and meat characteristics of broiler from experimental and commercial lines. Poult. Sci. 2001;80:833–838. doi: 10.1093/ps/80.7.833. [DOI] [PubMed] [Google Scholar]
- Berri C., Debut M., Santé-Lhoutellier V., Arnould C., Boutten B., Sellier N., Baéza E., Jehl N., Jégo Y., Duclos M.J., Le Bihan-Duval E. Variations in chicken breast meat quality: implications of struggle and muscle glycogen content at death. Br. Poult. Sci. 2005;46:572–579. doi: 10.1080/00071660500303099. [DOI] [PubMed] [Google Scholar]
- Bessei W. Welfare of broilers: a review. Worlds Poult. Sci. J. 2006;62:455–466. [Google Scholar]
- Bowker B., Zhuang H. Relationship between water-holding capacity and protein denaturation in broiler breast meat. Poult. Sci. 2015;94:1657–1664. doi: 10.3382/ps/pev120. [DOI] [PubMed] [Google Scholar]
- Broom, D. M. 2017. Animal Welfare in the European Union. European Parliament. Policy Department C: Citizens’ Rights and Constitutional Affairs. Petitions:PE 583.114.
- Buijs S., Keeling L., Rettenbacher S., Van Poucke E., Tuyttens F.A.M. Stocking density effects on broiler welfare: Identifying sensitive ranges for different indicators. Poult. Sci. 2009;88:1536–1543. doi: 10.3382/ps.2009-00007. [DOI] [PubMed] [Google Scholar]
- Castellini C., Dal Bosco A., Mugnai C., Bernardini M. Performance and behaviour of chickens with different growth rate reared according to the organic system. Ital. J. Anim. Sci. 2002;1:291–300. [Google Scholar]
- Dawkins M.S., Donnelly C.A., Jones T.A. Chicken welfare is influenced more by housing conditions than by stocking density. Nature. 2004;427:342–344. doi: 10.1038/nature02226. [DOI] [PubMed] [Google Scholar]
- Debut M., Berri C., Arnould C., Guemeené D., Santé-Lhoutellier V., Sellier N., Baéza E., Jehl N., Jégo Y., Beaumont C., Le Bihan-Duval E. Behavioural and physiological responses of three chicken breeds to pre-slaughter shackling and acute heat stress. British Poult. Sci. 2005;46:527–535. doi: 10.1080/00071660500303032. [DOI] [PubMed] [Google Scholar]
- Denbow D.M. In: Pages 337-342. Sturkie's Avian Physiology. 6th ed. Scanes C.G., editor. Academic Press; New York, NY: 2015. Gastrointestinal anatomy and physiology. [Google Scholar]
- Dozier W.A., III, Thaxton J.P., Branton S.L., Morgan G.W., Miles D.M., Roush W.B., Lott B.D., Vizzier-Thaxton Y. Stocking density effects on growth performance and processing yields of heavy broilers. Poult. Sci. 2005;84:1332–1338. doi: 10.1093/ps/84.8.1332. [DOI] [PubMed] [Google Scholar]
- Essary E.O., Moutney G.J., Goff O.E. Conformation and performance in standardbred and crossbred broilers. Poult. Sci. 1951;30:552–557. [Google Scholar]
- Estevez I. Density allowances for broilers: where to set the limits? Poult. Sci. 2007;86:1265–1272. doi: 10.1093/ps/86.6.1265. [DOI] [PubMed] [Google Scholar]
- Fanatico A.C., Pillai P.B., Cavitt L.C., Owens C.M., Emmert J.L. Evaluation of slower-growing broiler genotypes gown with and without outdoor access: Growth performance and carcass yield. Poult. Sci. 2005;84:1321–1327. doi: 10.1093/ps/84.8.1321. [DOI] [PubMed] [Google Scholar]
- Fanatico A.C., Pillai P.B., Emmert J.L., Owens C.M. Meat quality of slow- and fast-growing chicken genotypes fed low-nutrient or standard diets and raised indoors or with outdoor access. Poult. Sci. 2007;86:2245–2255. doi: 10.1093/ps/86.10.2245. [DOI] [PubMed] [Google Scholar]
- Fanatico A.C., Pillai P.B., Hester P.Y., Falcone C., Mench J.A., Owens C.M., Emmert J.L. Performance, livability, and carcass yield of slow- and fast-growing chicken genotypes fed low-nutrient or standard diets and raised indoors or with outdoor access. Poult. Sci. 2008;87:1012–1021. doi: 10.3382/ps.2006-00424. [DOI] [PubMed] [Google Scholar]
- Feddes J.J.R., Emmanuel E.J., Zuidhof M.J. Broiler performance, bodyweight variance, feed and water intake, and carcass quality at different stocking densities. Poult. Sci. 2002;84:774–779. doi: 10.1093/ps/81.6.774. [DOI] [PubMed] [Google Scholar]
- Grey T.C., Robinson D., Jones J.M., Stock S.W., Thomas N.L. Effect of age and sex on the composition of muscle and skin from a commercial broiler strain. Br. Poult. Sci. 1983;24:219–231. doi: 10.1080/00071668308416733. [DOI] [PubMed] [Google Scholar]
- Godfray H.C.J., Aveyard P., Garnett T., Hall J.W., Key T.J., Lorimer J., Pierrehumbert R.T., Scarborough P., Springmann M., Jebb S.A. Meat consumption, health, and the environment. Science. 2018;361:eaam5324. doi: 10.1126/science.aam5324. [DOI] [PubMed] [Google Scholar]
- Groom G.M. Options me´diterrane´ennes Cambrigde (UK) Agricultural Development and Advisory Service (ADAS), Fisheries and Food; Cambridge,UK: 1990. Factors affecting poultry meat quality. (Se´rie A-L'aviculture en Me´diterrane´e) [Google Scholar]
- Havenstein G.B., Ferket P.R., Qureshi M.A. Carcass composition and yield of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003;82:1509–1518. doi: 10.1093/ps/82.10.1509. [DOI] [PubMed] [Google Scholar]
- Kokoszyński D., Bernacki Z., Saleh M., Stęczny K., Binkowska M. Body conformation and internal organs characteristics of different commercial broiler lines. Braz. J. Poult. Sci. 2017;19:047–052. [Google Scholar]
- Kuttappan V.A., Hargis B.M., Owens C.M. White striping and woody breast myopathies in the modern poultry industry: a review. Poult. Sci. 2016;95:2724–2733. doi: 10.3382/ps/pew216. [DOI] [PubMed] [Google Scholar]
- Le Bihan-Duval F., Mignon-Grasteau S., Millet N., Beaumont C. Genetic analysis of a selection experiment on increased body weight and breast muscle weight as well as on limited abdominal fat. Br. Poult. Sci. 1998;39:346–353. doi: 10.1080/00071669888881. [DOI] [PubMed] [Google Scholar]
- Le Bihan-Duval E., Millet N., Remignon H. Broiler meat quality: effect of selection for increased carcass quality and estimates of genetic parameters. Poult. Sci. 1999;78:822–826. doi: 10.1093/ps/78.6.822. [DOI] [PubMed] [Google Scholar]
- Le Bihan-Duval E., Berri C., Baeza E., Millet N., Beaumont C. Estimation of the genetic parameters of meat characteristics and of their genetic correlations with growth and body composition in an experimental broiler line. Poult. Sci. 2001;80:839–843. doi: 10.1093/ps/80.7.839. [DOI] [PubMed] [Google Scholar]
- Leville G.A. In vitro hepatic lipogenesis in the hen and chick. Comp. Biochem. Physiol. 1969;28:431–435. doi: 10.1016/0010-406x(69)91357-7. [DOI] [PubMed] [Google Scholar]
- Lewis P.D., Perry G.C., Farmer L.J., Patterson R.L.S. Responses of two genotypes of chicken to diets and stocking densities typical of UK and ‘Label Rouge’ production systems: I. Performance, behavior and carcass composition. Meat Sci. 1997;45:501–516. doi: 10.1016/s0309-1740(96)00084-8. [DOI] [PubMed] [Google Scholar]
- Mathur P.K. In: Page 83 in Poultry Genetics, Breeding and Biotechnology. Muir W.M., Aggrey eds S.E., editors. CAB International; Wallingford, UK: 2003. Genotype-Environment Interactions: Problems Associated with Selection for Increased Production. [Google Scholar]
- Meluzzi A., Sirri F. Welfare of broiler chickens. Ital. J. Anim. Sci. 2009;8:161–173. [Google Scholar]
- Mir N.A., Rafiq A., Kumar F., Singh V., Shukla V. Determinants of broiler meat quality and factors affecting them: a review. J. Food Sci. Technol. 2017;54:2997–3009. doi: 10.1007/s13197-017-2789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCC. National Chicken Council. 2021. U.S. Broiler Production. Accessed Aug. 2021. https://www.nationalchickencouncil.org/statistic/us-broiler-production/.
- Puvadolpirod S., Thaxton J.P. Model of physiological stress in chickens 1. Response parameters. Poult. Sci. 2000;79:363–369. doi: 10.1093/ps/79.3.363. [DOI] [PubMed] [Google Scholar]
- Sarsenbek A., Wang T., Zhao J.K., Jiang W. Comparison of carcass yields and meat quality between Baicheng-You chickens and Arbor Acres broilers. Poult. Sci. 2013;92:2776–2782. doi: 10.3382/ps.2012-02841. [DOI] [PubMed] [Google Scholar]
- Savory C.J. A growth study of broiler and layer chicks reared in single-strain and mixed-strain groups. Br. Poult. Sci. 1975;16:315–318. [Google Scholar]
- Shanawany M.M. Broiler performance under high stocking densities. Br. Poult. Sci. 1988;29:43–52. doi: 10.1080/00071668808417025. [DOI] [PubMed] [Google Scholar]
- Singh M., Lim A.J., Muir W.I., Groves P.J. Comparison of performance and carcass composition of a novel slow-growing crossbred broiler with fast-growing broiler for chicken meat in Australia. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen P., Su G., Kestin S.C. Effects of age and stocking density on leg weakness in broiler chickens. Poult. Sci. 2002;79:864–870. doi: 10.1093/ps/79.6.864. [DOI] [PubMed] [Google Scholar]
- Thomas D.G., Ravindran V., Thomas D.V., Camden B.J., Cottam Y.H., Morel P.C.H., Cook C.J. Influence of stocking density on the performance, carcass characteristics and selected welfare indicators of broiler chickens. N. Z. Vet. J. 2004;52:76–81. doi: 10.1080/00480169.2004.36408. [DOI] [PubMed] [Google Scholar]
- Trampel D.W., Sell J.L., Ahn D.U., Sebranek J.G. Preharvest feed withdrawal affects liver lipid and liver color in broiler chickens. Poult. Sci. 2005;84:137–142. doi: 10.1093/ps/84.1.137. [DOI] [PubMed] [Google Scholar]
- Vizzier-Thaxton Y., Christensen K.D., Mench J.A., Rumley E.R., Daugherty C., Feinberg B., Parker M., Siegel P., Scanes C.G. Symposium: animal welfare challenges for today and tomorrow. Poult. Sci. 2016;95:2198–2207. doi: 10.3382/ps/pew099. [DOI] [PubMed] [Google Scholar]
- Weimer S.L., Mauromoustakos A., Karcher D.M., Erasmus M.A. Differences in performance, body conformation, and welfare of conventional and slow-growing broiler chickens raised at 2 stocking densities. Poult. Sci. 2020;99:4398–4407. doi: 10.1016/j.psj.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C., Jiang X.Y., Ding L.R., Wang T., Zhou Y.M. Effects of dietary methionine on growth performance, meat quality, and oxidative status in fast- and slow-growing broilers. Poult. Sci. 2017;96:1707–1714. doi: 10.3382/ps/pew432. [DOI] [PubMed] [Google Scholar]
- You X., Li Y., Zhang M., Yan H., Zhao R. A survey of Chinese citizens’ perceptions on farm animal welfare. Plos One. 2014;9 doi: 10.1371/journal.pone.0109177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G.P., Cui H.X., Liu R.R., Zheng M.Q., Chen J.L., Wen J. Comparison of breast muscle meat quality in 2 broiler breeds. Poult. Sci. 2011;90:2355–2359. doi: 10.3382/ps.2011-01432. [DOI] [PubMed] [Google Scholar]
- Zuidhof M.J., Schneider B.L., Carney V.L., Korver D.R., Robinson F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]