Abstract
In this investigation, the incidence and intensity of gastrointestinal parasites of domestic pigeons were evaluated, additionally, in vitro and in vivo evaluation of the antiparasitic activity of chitosan nanoparticles against the most predominant gut parasite. Therefore, 240 domestic pigeons (160 adults and 80 squabs) obtained from different localities in Giza governorate, Egypt, from February to July 2021, were subjected to parasitological and postmortem examination. The results revealed that 97% of pigeons were vulnerable to single or mixed gastrointestinal parasites. The detected helminths were identified as Capillaria columbae (C. columbae) with a total incidence of (12.5%), Ascaridia columbae (A. columbae) (83.3.%), Heterakis gallinarum (H. gallinarum) (18.7%), Raillietina cesticillus (R. cesticillus) (7.5%), Raillietina echinobothrida (R. echinobothrida) (29%), Choanotaenia infundibulum (C. infundibulum) (22.9%), Davainea proglottina (D. proglottina) (26.6%), and Cotugnia proglottina (C. proglottina) (14.5%). At the same time, the identified protozoan parasites were Trichomonas gallinae (T. gallinae), and Eimeria columbae (E. columbae), with a total incidence of 25 and 79%, respectively. Helminths and Eimeria infections were higher in adults than squabs, while T. gallinae infection was reported with a higher incidence in squabs (62.5%) than adults (6.2%). From our findings, A. columbae was the most predominant gut parasite in the examined pigeons. Thus, it was subjected to in vitro and in vivo treatment with chitosan nanoparticles. Serum and tissue samples were collected from the birds which have been used in the in vitro study to evaluate the oxidative stress markers as malondialdehyde (MDA), Nitric oxide levels and Tumor Necrosis Factor-alpha (TNF-α) and Interleukin-1β activity also, chitosan nanoparticles- treated worm ultrastructure were determined using scanning electron microscopy (SEM). Finally, we recommend periodic monitoring of pigeon's farm to detect the parasitic infestation, and from our results, we recommend chitosan nanoparticles as a potent nematocidal agent.
Key words: antiparasitic, cestode, coccidia, chitosan nanoparticles, nematode, pigeons, trichomoniasis
INTRODUCTION
Pigeons cohabit with many avian and animal species and humans (Attia et al., 2021). They can be infected with various pathogens and act as a reservoir for many parasitic infections. Their droppings pollute the surrounding environment, enhancing the danger of parasitic infestation spread between different animal and avian species (Attia and Salem, 2022). Parasitism is a debilitating condition that harms pigeon's health (Salem et al., 2022a). The gastrointestinal parasites infecting pigeons can be classified into protozoon parasites as Trichomonas gallinae, and Eimeria Columbiformes (McDougald, 2003; Lawson et al., 2006) while, metazoan gastrointestinal parasites can be classified into nematode helminths as Capillaria species, Syngamous species, Ascaridia columbae, Ascaridia longecirrata (Senlik et al., 2005) and cestode helminths as Raillietina tetragona, Raillietina echinobothrida (Msoffe et al., 2010).
These gastrointestinal parasites produce a variety of clinical signs and postmortem lesions as; depression, diarrhea, loss of condition, drop in egg production, nervous signs, ruffled feathers, anorexia, different degrees of enteritis with nodular formation, intestinal impaction, and liver lesions (Qamar et al., 2017). In addition, a gastrointestinal parasite in pigeons resulted in a significant elevation in Nitric oxide and MDA levels and reduced serum zinc. Moreover, infected pigeons showed a significant increase in gene expression for IL1-β and TNF-α (Salem et al., 2022).
To overcome and control microbial infections and parasitism, many approaches could be applied such as; using biological control (Hegazy et al., 2022), application of antiparasitic chemical drugs (Attia et al., 2022; Soliman et al., 2022), or using natural plant extract (Abd El-Hack et al., 2021, 2022, 2022a; El-Saadony et al., 2022; Saad et al., 2022).
Many types of anthelmintics used to treat and prevent worms’ infection in pigeons as; piperazine citrate, levamisole, ivermectin, albendazole, and fenbendazole (Simon and Emeritus, 2005; Tanveer et al., 2011; Rahman et al., 2019). Also, amprolium, sulfonamides, clazuril, diclazuril, and toltrazuril are common drugs used to treat coccidiosis in pigeons while diclazuril and toltrazuril have been considered as more potent chemical drugs in the treatment of avian coccidiosis (Krautwald-Junghanns et al., 2009; Salem et al., 2022b).
Chitosan is a cationic polysaccharide derived naturally from chitin and chitosan as well as its derivatives have been used in a variety of fields because they are potent, inexpensive, simple to use, and suited for a variety of medical applications, such as wound dressings and bandages as they could enhance wound healing plus its antibacterial, antifungal and antiparasitic activities (AbdElKader, et al., 2021; Attia et al., 2021). Recently, many parasites showed antiparasitic resistance against commonly used chemical antiparasitic preapations and as an alternative to chemically manufactured pesticides, the use of nanoparticles as a pesticide agent has become more common as technological advances have made their products more eco-friendly (Alfy et al., 2020). The unique properties of chitosan nanoparticles, such as their small size and quantum size impact, may make chitosan nanoparticles have better antibacterial properties (Divya and Jisha, 2018). In the same way, chitosan nanoparticles are natural materials with exceptional physicochemical, antibacterial, and biological properties, making them ideal environmentally friendly materials with bioactivity that is very safe for humans (Divya and Jisha, 2018).
Therefore, the current study aimed to detect the incidence of the most common gastrointestinal parasites infecting domestic pigeons in Egypt with particular reference to the efficacy trials of chitosan nanoparticles as a biodegradable agent against the most common detectable helminths in the pigeon with the evaluation of the in vitro study using scanning electron microscopic study and the in vivo trials assessment through evaluation of the oxidative stress and genetic markers.
MATERIAL AND METHODS
Collection and Examination of Pigeon
The present study was carried out for six months, from February to July 2021. A total of 240 pigeons (160 adults and 80 squabs) showed signs of general depression and weakness were collected from different poultry clinics and purchased from poultry markets as well as a farm in which birds were maintained in the free-range system from different localities in Giza governorate, Egypt which located in 29.9870oN 31.2118oE with a hot desert climate.
Ethical Statement
The study design and all bird handling procedures followed the Ethical Committee, Faculty of Veterinary Medicine, regulations.
Postmortem Investigations and Samples Collection
The alimentary tract of freshly dead and ethically slaughtered birds was removed intact and labeled. The esophagus, crop, proventriculus, gizzard, small intestine, and large intestine were separated and placed into separate Petri dishes. A longitudinal incision was made out in all parts to expose their contents, and then direct intestinal smears were prepared and examined from each part. All unattached worms were gently harvested by forceps.
Parasitological Examination of the Pigeon
Fresh droppings were collected from each bird and examined (macroscopically and microscopically) as well as concentration techniques using the concentrated salt solution for the presence of any parasitic eggs, oocysts, whole worms and or gravid segments, according to Soulsby (1986), Saad and Attia (2021) and positive birds were further ethically slaughtered and exposed for postmortem examination as well as, freshly dead birds were opened for postmortem following Swayne (2020). The parasitological positive pigeon for any internal parasitic disease was identified for infection type.
Identification of the Parasites
All the collected parasites were identified according to Soulsby (1986). The helminths were removed from the intestine then washed many times with phosphate buffer saline (PBS) (pH 7.2), kept in 70% glycerin alcohol and preserved in a separate jar for further parasitological investigations according to Attia et al. (2019), Salem and Attia (2021).
Chitosan Nanoparticles
Chitosan was purchased from nanotech Egypt The characterization of the particles was spherical and ranged from 15 to 25 nm.
Determination of the Chitosan Nanoparticles lethal Concentration 50 (LC50) on A. columbae as in vitro Study
The chitosan nanoparticles were diluted in PBS as 25, 12.5, 6.25, 3, and 2 ppm. One hundred and twenty A. columbae worms were used for each dilution which further subdivided into 6 replicated (20 worm/ replicate).
Only freshly harvested intact, highly active nematodes were in vitro tested for the chitosan nanoparticles' anthelmintic activity. During the in vitro study, careful observation on the nematodes' movement was adopted during 1 h, 2 h till 4 h post chitosan nanoparticle treatment (ingestion assay). The A. columbae was carefully examined under a stereoscopic microscope for movement and the mortality rate.
Determination of the in vivo Activity of Chitosan Nanoparticles as Nematocidal Agents Against A. columbae
Thirty pigeons were divided into 3 groups, 10 birds each as follow group 1) Ten naturally infected pigeon with A. columbae which have been proved to be single infected with A. columbae by dropping examination of each bird for 7 successive days, postmortem, and parasitological examination of the intestine of freshly died birds from the same farms, were treated with 6.25 ppm of the chitosan nanoparticles as lethal concentration 50 suspended in 1 cm physiological saline and have been administered to the bird orally by crop gavage for 3 successive days; group 2) Ten control positive single infected pigeon with A. columbae and group 3).
Ten apparently healthy parasitologically negative were obtained from a private pigeon farm where pigeons reared in captivity under controlled hygienic measures kept negative control. Each group was subdivided into 5 replicates. Two birds were kept in thoroughly clean wooden cages with wire nets (90 cm width × 90 cm length × 90 cm height) under the same environmental temperature and humidity conditions. All birds were supplied with a balanced seed mix and clean water ad libitum.
The pigeons were monitored for 2 successive weeks. Then, 2 pigeons from the treated group were ethically slaughtered at 5, 8, 11, and 15 d post-treatment, while birds in both controls negative and control positive groups were slaughtered at the end of the observation period.
Blood, sera, and intestinal samples were collected from ethically slaughtered birds then labeled with the time of slaughtering and kept at −20°C for further analysis.
Estimation of Tumor Necrosis Factor-Alpha (TNF-α) and Interleukin-1β Activity
Intestinal tissue samples from A. columbae infected, treated and controlled un-infected pigeons were dissected and aseptically preserved at −20°C for further examination.
RNA Extraction
RNA was extracted from 100 mg of pigeon intestine using the total RNA kit (Ambion, Applied Biosystems), following the manufacturer's guide.
The intestinal tissue was homogenized and subjected to Lysing Matrix D tubes (MP Biomedicals) using a FastPrep-24 homogenizer (MP Biomedicals, 2 cycles of 30 s at 6 m/s).
Nanodrop (Thermo Scientific) was used to evaluate the RNA purity and quantity. Following the manufacturer guide, a five hundred ng of RNA resulted from DNaseI amplification grade (Invitrogen). The reverse transcription of treated RNA was gained by High-Capacity cDNA Archive Kit (Applied Biosystems) following Liu et al. (2014), Salem et al. (2022).
Quantitative Real-Time PCR Protocol (qRT-PCR)
PCR primers specific for tumor necrosis factor-alpha (TNF-α), and Interleukin-1β specific for pigeon were designed and based on the sequences submitted in the GenBank by Liu et al. (2014) as seen in Table 1.
Table 1.
Primers designs for qRT-PCR.
Quantitative Real-time PCR Cycling Conditions
Amplification was established for 40 cycles as a denaturation for 30 s at 94°C, annealing for 30 s at 60°C and extension for 45 s at 72°C and repeated for three successive times (Attia et al., 2021).
Ultrastructure Determination of the Effects of Treatment With Chitosan Nanoparticle Collected From Pigeon After Treatment Using Scanning Electron Microscopy (SEM) Study
Adult A. columbae were collected from the treated pigeon's droppings and/ or intestine at the end of the experiment. Then worms were washed using 0.9% saline (Attia and Salem, 2022). The collected worms were fixed in 2.5% Glutaraldehyde following to Attia and Salaeh (2020), Abdelsalam et al. (2020), then the worms were dehydrated using ascending ethanol series, then the nematodes were dried in a CO2 critical point drier (Autosamdri-815, Germany).
The adult was coated with 20 nm gold (Abu-Elala et al., 2018) in a sputter coater (Spi-Module sputter Coater, UK). All the specimens were photographed with a scanning electron microscope (JSM 5200, Electron prob); Microanalyzer Jeol, Japan; at Faculty of Agriculture, Cairo University.
Assessment of the Oxidative Stress Markers
Oxidative stress markers were evaluated in sera samples as malondialdehyde (MDA). Nitric oxide levels were assessed according to Attia et al. (2021).
Data Analysis
Data of stress markers and gene expression were statistically analyzed using SPSS Version 18.0 software (Inc., Chicago, IL). A P-value was considered significant when P < 0.05. The lethal concentration (LC50) of the tested nanoparticles was statistically analyzed using probity analysis by Finney (1971).
RESULTS
Clinical Signs and Postmortem Findings
Most of the examined pigeons showed signs of general depression, emaciation, ruffled feathers with variable degrees of diarrhea, as shown in Figure 1, Figure 2. T. gallinae positive birds showed signs of severe emaciation, vomiting, drooling of offensive odor fluid from bird mouth with the presence of white caseated membrane in the buccal cavity, crop impaction with doughy material and navel inflammation was recorded in some squabs as shown in Figure 2.
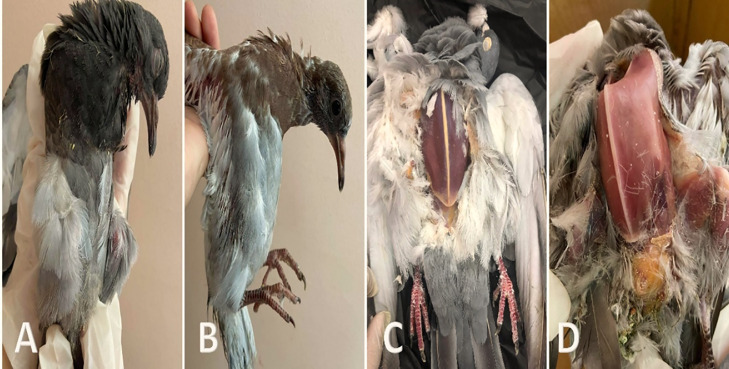
Figure 1.
(A) Squab showing general weakness, sleepy appearance, ruffled feathers and emaciation. (B) Squab showing ruffled feathers with emaciation. (C and D) Postmortem of freshly dead pigeons showing severe emaciation with protrusion of keel bone.
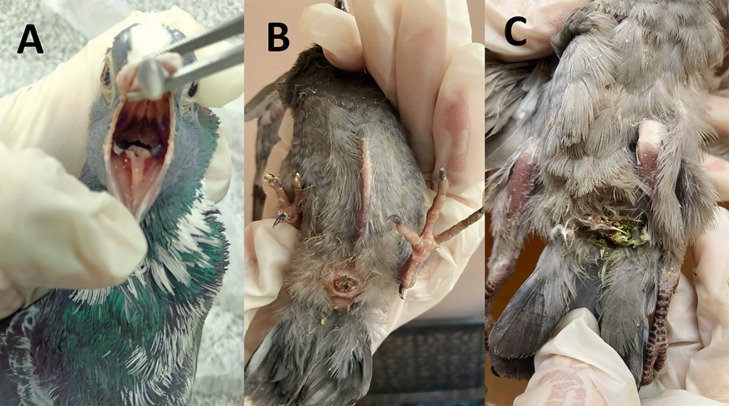
Figure 2.
(A) Opened buccal cavity of adult pigeon showing Prescence of white caseated membrane. (B) Squab showing severe emaciation with protrusion of keel bone and navel inflammation (omphalitis), (C) Squab showing greenish diarrhea with ruffled feathers.
Postmortem examination of freshly dead birds or ethically slaughtered ones revealed protrusion of the keel bone Figure 1. The liver showed different degrees of inflammation, subcapsular hemorrhage, and in some cases, fatty degeneration was reported, as shown in Figure 3. Intestine in postmortem examination showed different degrees of enteritis, ballooning, impaction with worms, presence of undigested feed particles mixed with frothy fluid with variable colors (yellow, orange, and greenish contents) as shown in Figures 4, 5, and 6.
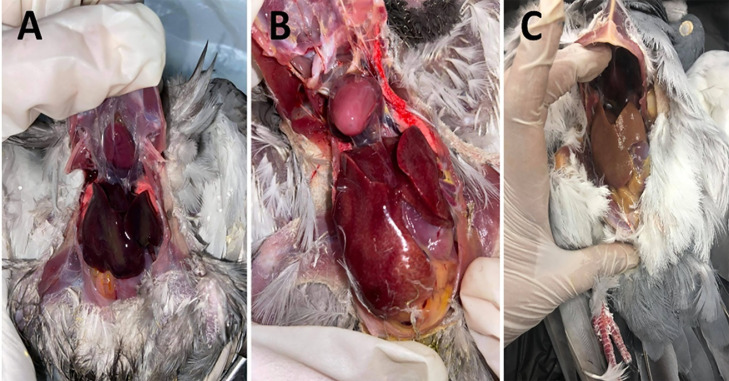
Figure 3.
(A) Postmortem of pigeon showing severe liver congestion. (B) Postmortem of pigeon showing subcapsular hemorrhage in the liver. (C) Postmortem of pigeon liver showing paleness with fatty degeneration.
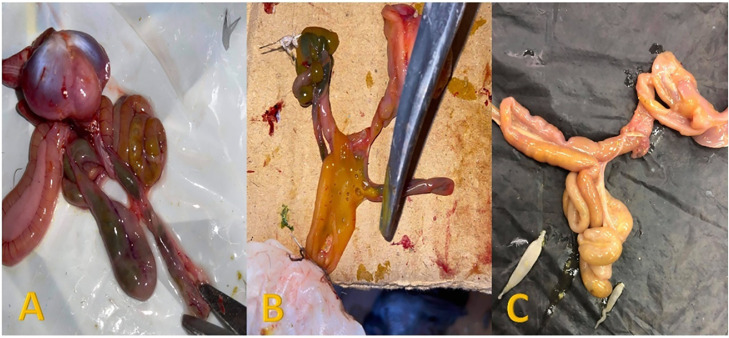
Figure 4.
(A) Intestine of pigeons showing enteritis with greenish and yellowish contents. (B) Opened intestine showing yellowish content mixed with gases. (C) Intestine of pigeons impacted with cestode worms.
Figure 5.
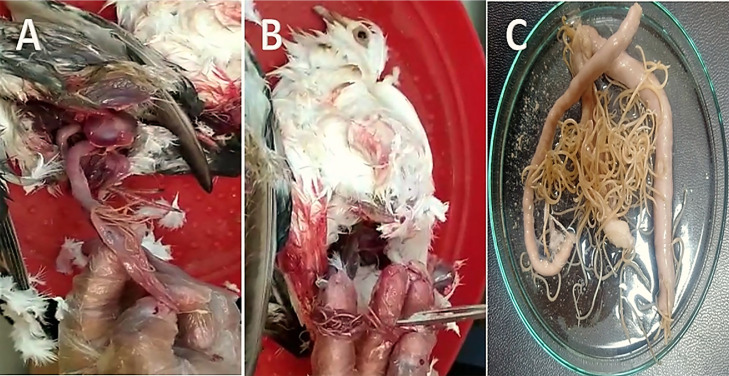
(A and B) Opened pigeon intestine showing presence of Ascaridia worms. (C) Petri-dish containing pigeon small intestine with large number of Ascaridia worms.
Figure 6.
(A, B and C) Opened pigeon intestine showing presence of cestode worms attached with intestinal mucosa.
The present study aimed to estimate the incidence of gastrointestinal parasitic infections in the domestic pigeon with a new treatment trial. The results showed that out of 240 investigated pigeons, 233 were positive for either single or mixed gastrointestinal parasites with an incidence of 97%.
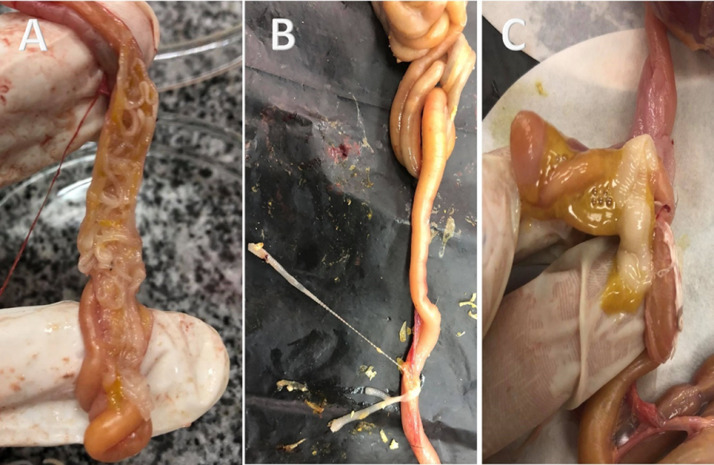
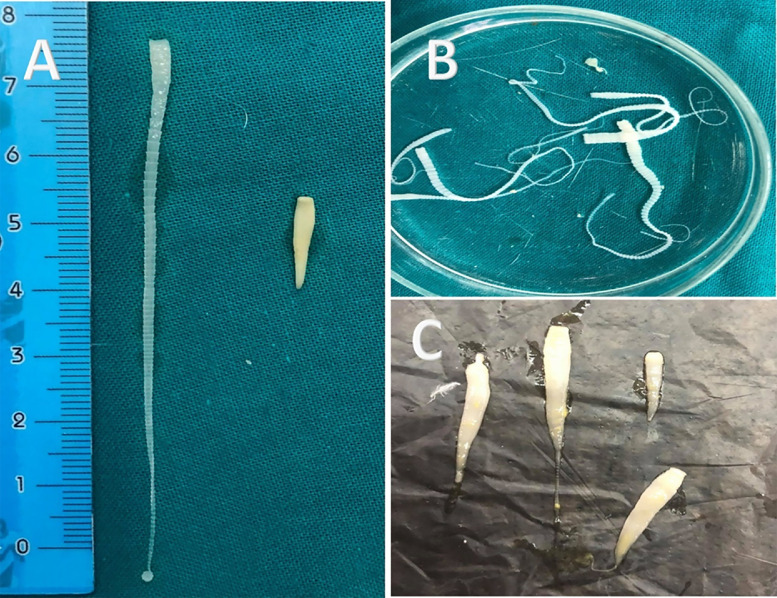
The helminthic and protozoan infections were recorded with different degrees of intensity and severity of the lesion. Both single and mixed parasitic infections were recorded. In this study, gut helminths (nematodes and cestodes) were reported in adults and squabs. In contrast, the detected three types of nematodes were identified as C. columbae with a total incidence of (12.5%), A. columbae (83.3.%) and H. gallinarum (18.7%). In this study, five different cestodes were detected (Figure 7) and identified as R. cesticillus, R. echinobothrida, C. infundibulum, D. proglottina, and C. proglottina with a total incidence of 29, 7.5, 22.9, 26.6, and 14.5%, respectively.
Figure 7.
(A) Macroscopic appearance of Raillietina and Davainea worms obtained from pigeon intestine. (B) Raillietina worms. (C) Davainea worms.
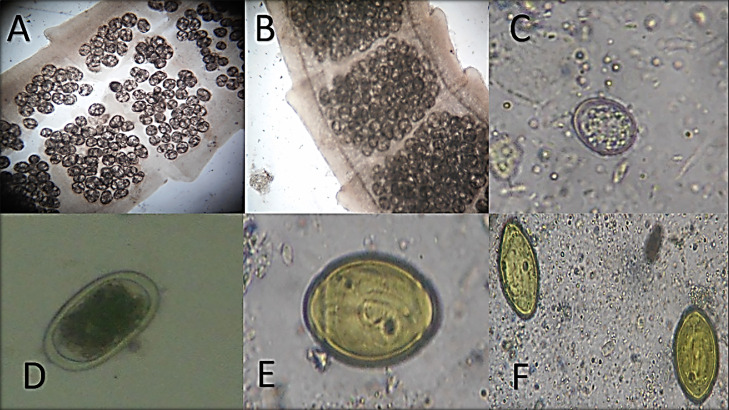
This study detected different parasitic eggs, oocyst, and gravid segments during direct microscopic fecal smears examination, as shown in Figure 8. The identified protozoan parasites were T. gallinae and E. columbae, with a total incidence of 25 and 79%, respectively. Helminths and Eimeria infections were higher in adults than squabs, while T. gallinae infection was reported with a higher incidence in squabs (62.5%) than adults (6.2%), as presented in Table 2.
Figure 8.
Fecal examination of pigeon infected with enteric parasites; (A and B) Raillietina spp. gravid segment; (C) Eimeria columbae un-sporulated oocyst; (D) Ascaridia columbae egg; (E and F) Capillaria columbae eggs.
Table 2.
Incidence of gastrointestinal parasites either and intensity of helminths in domestic pigeons.
| Parasites | Parasitic species | Total No. of positive cases out of 240 birds (Incidence%) | Intensity of parasites (Average number) | Adult total No. of positive cases out of 160 birds (Incidence%) | Squabs total No. of positive cases out of 80 birds (Incidence%) |
|---|---|---|---|---|---|
| Nematodes | Capillaria columbae | 30 (12.5%) | 10–35 (20) | 15 (9.3%) | 5 (6.2%) |
| Ascaridia columbae | 200 (83.3%) | 10–90 (45) | 155 (96.8%) | 45 (56.2%) | |
| Heterakis gallinarum | 45 (18.7%) | 16–35 (22) | 35 (21.8%) | 10 (12.5%) | |
| Cestodes | Raillietina cesticillus | 70 (29%) | 3–15 (9) | 62 (38.7%) | 8 (10%) |
| Raillietina echinobothrida | 18 (7.5%) | 1–5 (3) | 12 (7.5%) | 6 (7.5%) | |
| Choanotaenia infundibulum | 55 (22.9%) | 1–3 (2) | 38 (23.7%) | 17 (21.2%) | |
| Davainea proglottina | 64 (26.6%) | 1–10 (6) | 58 (36.2%) | 6 (7.5%) | |
| Cotugnia proglottina | 35 (14.5%) | 1–3 (2) | 22 (13.7%) | 13 (%) | |
| Enteric protozoa | Trichomonas gallinae | 60 (25%) | - | 10 (6.2%) | 50 (62.5%) |
| Eimeria columbae | 190 (79%) | - | 152 (95%) | 38 (47.5%) |
Single parasitic infection with A. columbae was recorded in 50 adult birds and 20 squabs showed a single infection with T. gallinae. In contrast, the rest number of examined birds revealed mixed infections. From observations, mixed infections with helminths were recorded in some of the examined pigeons (Table 3) and were detected as follow:
Mixed Nematoda: (C. columbae and A. columbae), (C. columbae and H. gallinarum), and (C. columbae, A. columbae and H. gallinarum)
Mixed cestode: (R. cesticillus and R. echinobothrida) and (R. cesticillus, R. echinobothrida and C. infundibulum)
Mixed helminths: (A. columbae and R. echinobothrida)
Mixed helminths and protozoa: (C. columbae and E. columbae) and (A. columbae, C. columbae and E. columbae).
Table 3.
Type of the observed mixed gastrointestinal parasites infections in domestic pigeons.
| Type of mixed infections | Detected parasites |
|---|---|
| Mixed Nematoda | (C. columbae and A. columbae), (C. columbae and H. gallinarum), and (C. columbae, A. columbae and H. gallinarum). |
| Mixed cestode | (R. cesticillus and R. echinobothrida) and (R. cesticillus, R. echinobothrida and C. infundibulum). |
| Mixed helminths | (A. columbae and R. echinobothrida). |
| Mixed helminths and protozoa | (C. columbae and E. columbae) and (A. columbae, C. columbae and E. columbae). |
The results revealed that A. columbae was the most detected gastrointestinal infection between the examined birds with an incidence of 83.3% (200/240); therefore, it was selected for evaluation of the nematocidal activity of chitosan nanoparticles.
The in vitro trial showed a direct relationship between the tested chitosan nanoparticles concentration and the mortality rate of Ascaridia worms, as shown in Table 4 and Figure 9. The in vivo study revealed that chitosan nanoparticles treated birds revealed improved clinical scores in Figure 10. Meanwhile, control positive infected untreated birds showed deterioration in general health with increased intensity of clinical signs (off food, increased water consumption. Meanwhile, control positive infected untreated birds showed deterioration in general health with increased intensity of clinical signs (off food, increased water consumption, weight loss and general depression).
Table 4.
The in vitro efficacy of chitosan nanoparticles (the preliminary investigation).
| Concentration |
A. columbae adult |
||
|---|---|---|---|
|
⁎M.M. % ± S. E | |||
| 1 h | 2 h | 4 h | |
| 25 ppm | 35.5 ± 0.57 | 60.9 ± 0.55 | 3.6 ± 0.00 |
| 12.5 ppm | 31.0 ± 0.8 | 65.45 ± 0.59 | 3.55 ± 0.00 |
| 6.25 ppm | 17.6 ± 0.55 | 50 ± 0.49 | 32.4 ± 0.57 |
| 3 ppm | 00 ± 0.00 | 00 ± 0.00 | 40 ± 0.84 |
| 2 ppm | 00 ± 0.00 | 00 ± 0.00 | 10.5 ± 0.98 |
M.M % ± S.E = Mean mortality ± Standard deviation.
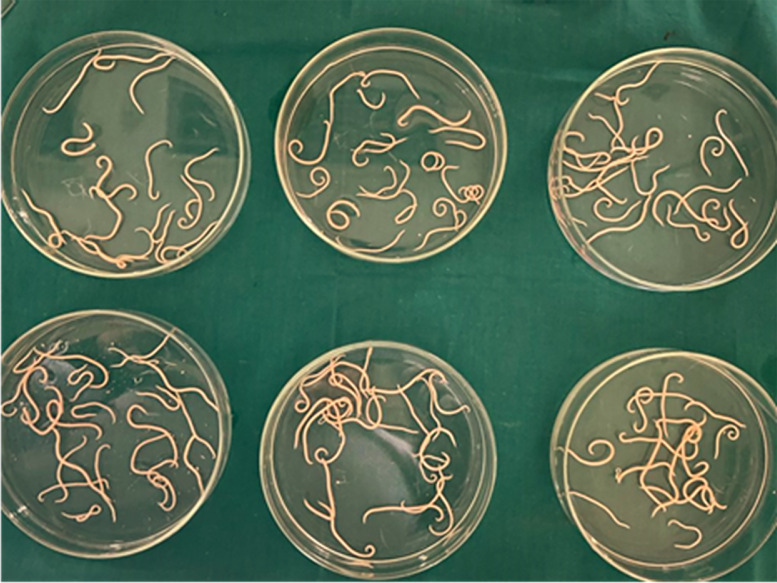
Figure 9.
The in vitro study to a total of 120 adult A. columbae worms sub-divided into six replicates with twenty worms each treated with one concentration of chitosan nanoparticles.
Figure 10.
The in vivo study showing one replicate including two birds were kept in wooden cage with wire net.
For 2 weeks observation periods, 40% (4/10) mortalities were reported only in control positive untreated birds while treated birds and control negative ones revealed no mortalities.
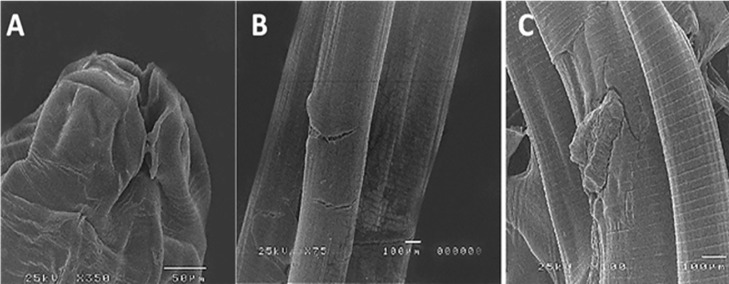
Dealing with the change of the morphological change after treatment with chitosan nanoparticles. Dealing with the genetic assessment of the treatment trials using analysis of the cell-mediated immune response initiated after infection with A. columbae and after treatment trials with chitosan nanoparticles. The examined nematodes were moribund and showed shrinkage of lips with destruction and rupture of the cuticle, as shown in Figure 11.
Figure 11.
Scanning electron microscopy study of the in vivo effects of chitosan nanoparticles showing: (A) Shrinkage of lips. (B and C) Destruction of the cuticle.
Interleukin1-β; control positive pigeon non treated significantly showed upregulation by 25-fold than that of control noninfected pigeon (normal), which showed 4-fold upregulation of the immunological genes; after 5, 8, 11, and 15-days post-treatment, the slaughtered birds showed downregulation in the immunological tested genes as 20; 18; 10 and 8 respectively.
The TNF-α control positive pigeon significantly showed upregulation by 20-fold than that of control noninfected pigeon (normal), which showed 3-fold upregulation of the immunological genes; after 5, 8, 11, and 15 d post-treatment the slaughtered birds showed down-regulation as 17, 14, 9, 5-fold of the tested genes.
Dealing with the analysis of the stress factor which released from the tissues because of parasites infection; examined pigeon showed significantly higher in Nitric oxide 40.67 ± 10.98 in control positive infected untreated birds; while the Nitric oxide level was 35.78 ± 7.45; 27.54 ± 9.82; 20.86 ± 7.45; 12.46 ± 0.50; respectively in the groups slaughtered from 5, 8, 11, and 15 d post-treatment in compared with the control noninfected pigeon 10.98 ± 5.40.
Dealing with MDA examined pigeons showed significantly higher in MDA 56.75 ± 3.65 in positive non treated birds, while the MDA level was 47.88 ± 6.47; 37.94 ± 8.85; 29.95 ± 3.60; 18.69 ± 2.49; respectively in the birds slaughtered from 5, 8, 11, and 15 d post-treatment when compared with control noninfected pigeon 8.75 ± 4.85.
DISCUSSION
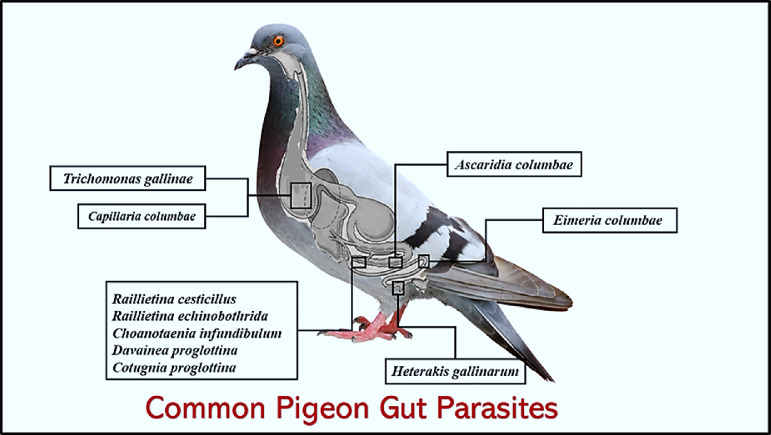
Pigeons are a point of anxiety since they can spread zoonoses to humans and reservoirs numerous parasite diseases affecting poultry (Sari et al., 2008; El-Dakhly et al., 2018). In this study, the detected gastrointestinal parasites between examined domesticated pigeons in Giza, Egypt, during the investigated period were presented in Figure 12.
Figure 12.
Most common pigeon gut parasites in Giza governorate, Egypt during the observation period.
This study revealed that most of the examined pigeons showed signs of ruffled feathers, severe emaciation, weakness, vomiting and diarrhea of varying colors. Abd El-Rahman et al. (2008), Mohamed et al. (2009) and Abdel Rahman et al. (2019) reported similar signs in pigeons suffering from gastrointestinal parasitic infestations.
From our observation, the most detected postmortem lesions were crop impaction with mucosal thickening of pigeons infected with trichomoniasis. At the same time, ballooning of the intestine with gases mixed with variable color contents is usually seen in cases infected with coccidia. Also, adult worms in the intestinal lumen are usually associated with worms’ infestations. Eman (2005), Hebat-Allah and Abd-El-Motelib (2007), Adang et al. (2008) recorded the same findings in similar parasitic infestation in pigeons.
The severe emaciation associated with gastrointestinal parasitism may contribute to the worm burrowing deep in the intestinal mucosa, resulting in epithelial necrosis, inflammation, and hemorrhage (Qamar et al., 2017). Also, endoparasites dilate the intestine; produce nodules and severe enteritis, thus impairing the absorbing power of the intestine for nutrients and vitamins from the host (Anwar et al., 2000).
The continuous exposure of poultry to different insects that serve as intermediate hosts for helminth parasites increases the severity of parasitic disease dangers (Ashenafi and Eshetu 2004; Salem et al., 2022a).
From our finding, pigeons harbor 3 different types of nematodes with different prevalence as follow: C. columbae (12.5%) with intensity 10 to 35 with an average number (20), A. columbae (83.3%) with intensity 10 to 90 with an average number (45) and H. gallinarum (18.7%) with intensity 16 to 35 with an average number (22). As well as five types of cestodes; R. cesticillus (29%) with intensity 3 to 15 with an average number (9); R. echinobothrida (7.5%) with intensity 1 to 5 with an average number (3); C. infundibulum (22.9%) with intensity 1 to three 15 with an average number (2); D. proglottina (26.6%) with intensity 1 to 10 with an average number (6) and C. proglottina (14.5%) with intensity 1 to 3 with an average number (2). In other studies in Gharbia, Egypt, Nagwa et al. (2013) reported all-over helminths prevalence rate of 51.7%.
Furthermore, 23.18% in Iran (Khezerpour and Naem, 2013) and 74% in Turkey (Senlik et al., 2005) Alkharigy et al. (2018). recorded the overall prevalence of helminths in investigated pigeons was 56%. They divided them into three species of Nematoda (4% Capillaria spp., 22% Ascaridia galli and18% Heterakis gallinarum) and three species of cestoda (2% Raillietina tetragona, 32% R. echinobothrida and 4% R. cesticillus) in Tripoli, Libya.
Owing to our results, the incidence of C. columbae was (12.5%). Our result was higher than those recorded by Baris et al. (2008) in Turkey (4.3%); Khezerpour and Naem (2013) in Iran (0.72%) but lower than that reported by Ghosh et al. (2014) in Bangladesh (22%) and Eljadar et al. (2012) in Libya (20%).
A. columbae is one of the common nematodes of pigeon's worldwide (Mushi et al., 2000; Senlik et al., 2005; Msoffe et al., 2010). From our results, the prevalence of A. columbae was (83.3%). This result is higher than that recorded by Alkharigy et al. (2018) how found Ascaridia galli with prevalence (22%) in Libya, Radfar et al. (2012) in Iran, 16.66% for A. columbae, Nagwa et al. (2013) in Egypt for A. columbae, (12%), Natala et al. (2009) in Nigeria 1.2% for A. columbae, Djelmoudi et al. (2014) in Algeria 4.2% for A. columbae, Permin et al. (2002) in Zimbabwe 69%, Sam-Wobo and Mafiana (2003) in Nigeria 73.4% and Abed et al. (2014) in Al-Diwaniya city, Iraq 38.94%.
Heterakis gallinarum is a nonpathogenic worm, but it serves as a vector for a protozoon parasite (Histomonas meleagridis), a highly pathogenic worm that causes Black-head disease in chickens, turkeys, pheasants, and other fowls. From our results, the incidence of H. gallinarum was (18.7%) with worm intensity 16 to 35. This result was nearly similar was that recorded by Alkharigy et al. (2018) in pigeons in Libya (18%) and higher than Eljadar et al. (2012) in domestic pigeons in Green Mountain Region, Libya (10%); Borji et al. (2012) in Mashhad Iran (1.85%) and Baris et al., (2008) in Turkey (3.7%) but lower than that recorded by Ashenafi and Eshutu (2004) in Central Ethiopia (32.6%) in chickens. The variations in the prevalence rate in the different studies are predictable for many factors that affect the disease's occurrence, such as the host immunity, type of feed, climatic conditions, geographical distribution, availability of intermediate host, and managemental and hygienic conditions and housing type.
Most of the cestodes in our study belonged to the genus Raillietina. This agrees with similar studies in different locations, reporting the most common tapeworms in pigeons of the genus Raillietina (Dede and Richards, 1998; Dehlawi, 2006 and Alkharigy et al., 2018). In other studies, the prevalence of cestode infestations in pigeons was 65.3% in Central Ethiopia (Ashenafi and Eshutu, 2004), 35.9 in Nigeria (Sam-Wobo and Mafiana, 2003), 32.35 in South Khorasan, Iran (Radfar et al., 2011), 28.13% in Iran (Ashrafihelan et al., 2010), 17.7% in Gharbia, Egypt (Nagwa et al., 2013), and 7.6% in Nigeria (Natala et al., 2009).
In this study, the helminths intensity (burdens) in pigeons (worms/bird) was as follow; C. columba 10 to 35; A. columba 10 to 90; H. gallinarum 16 to 35; R. cesticillus 3 to 15, R. echinobothrida 1 to 5; C. infundibulum 1 to 3 15; D. proglottina 1 to 10 and C. proglottina 1 to 3 Alkharigy et al. (2018). recorded helminths intensity as follow: (1–119) for Ascaridia galli, (1–42) for H. gallinarum, (1–2) for Capillaria spp., (1) for Raillietina tetragona (1–14) for Raillietina echnobothrida, (1–30) for R. cesticillus. Also, Msoffe et al. (2010) found that worm's intensity was (2–160) for Ascaridia galli, (1–6) for Raillietina tetragona and (1–18) for Raillietina echnobothrida.
In another study, El-Dakhly et al. (2016) recorded (1–170) for Ascaridia spp., (1–5) for Raillietina tetragona, (2–41) for Raillietina echnobothrida, (2–7) for Raillietina cesticillus, and (1) for Capillaria spp. The infective dosage and the availability of intermediary hosts and host immunity may indicate helminths intensity variations (Alkharigy et al., 2018).
From our results, pigeons were infected with T. gallinae with the incidence of (25%). Alkharigy et al. (2018) recorded higher prevalence (55%) in Tripoli, Libya, Villanua et al. (2006) (34.2%) in Spain, Abd El-Rahman et al. (2008) (61%) in Qualiobia governorate Egypt. Also, Al- Said and Hamodi (2011) found that trichomonad infection is common in free-living pigeons in Mosul, Iraq. Squabs were infected with trichomoniasis in high prevalence (62.5%), followed by adults (6.2%). This finding may be attributed to those adult pigeons act as chronic carriers for trichomoniasis without clinical signs. They transmitted it through crop milk to the susceptible squabs, so adults are a constant source of infection for their squabs (Soulsby, 1986).
Avian coccidiosis is adevastinting problem facing avian spp. (Abolhadid et al., 2021; El-Shall et al., 2022; Salem et al., 2022b). With this study, the all-over prevalence of E. columbae in pigeons was (79%) and the coccidia highest incidence was recorded in adults (95%) then squabs (47.5%). A lower prevalence 59.6%, of the domestic pigeons (81/136) was recorded in Turkey by Sari et al. (2008).
In this study, mixed infections between different gut parasites have been recorded. The observation of multiple parasitic infections in pigeons is agreed with that recorded by Alkharigy et al. (2018) in Libya; El-Dakhly et al. (2016) in Beni-Suef, Egypt. Msoffe et al. (2010) in Tanzania and Abed et al. (2014) in Iraq recorded mixed parasitic infections in pigeons. The pigeons maintained in the free-range system are susceptible to a wide range of parasitic diseases due to their feeding behavior presence of the different infective parasitic stages t in addition to feeding on the snails that act as an intermediate host to different parasites in the surrounding agriculture environment.
Based on our results, a higher prevalence of nematodes and cestodes was detected in adults than in squabs. This finding may be because the maternal antibodies can protect the squabs from parasites at the first age stage. On the other hand, squabs were more susceptible to T. gallinae infection than adults which could be due to those young pigeons being more susceptible to parasitic infection as compared to the above 2 years old birds as well as crop feeding permit the transmission of the infective stage from chronic carrier adult pigeon to the young susceptible squabs so, adults are a continual source of infection for their nestling (Soulsby, 1986; Bahrami et al., 2013).
Many studies shed light on nanotechnology as a novel gold solution to overcome the anthelmintics resistance problem (Abd El-Ghany et al., 2021; Salem et al., 2021a). Based on the in vitro and in vivo evaluation of the nematocidal activity of chitosan nanoparticles, our results revealed that chitosan nanoparticles succeeded in ameliorating the severity of clinical signs, stopping mortalities, induced intestinal tissue repair. On the other hand, chitosan nanoparticles revealed shrinkage in the worm moth part and induced destructive damage to its body. Our finding concurs with Attia et al. (2021), who evaluated the antiparasitic activity of chitosan silver nanoparticles against the external parasite of pigeons and concluded that the used treatment has a potent antiparasitic effect and enhances the antiparasitic affect wound healing. Another parallel study conducted by Abu-Elala et al. (2018) confirmed that chitosan-silver nanocomposite was potent in controlling Lernaea cyprinacea infection in goldfish aquaria. The specific mechanisms of chitosan and its derivatives' nematicidal action are presently unclear however, it is known that when positively charged chitosan molecules (charge on the C-2 of the glucosamine monomer) interact with negatively charged microbial cell membranes, proteinaceous and other intracellular contents seep out (Badawy and Rabea, 2011).
From our result, we concluded that A. columba infested pigeons revealed increased gene expression for IL-1β, and TNFα activity as well as, the oxidative stress markers including MDA, Nitric oxide levels were markedly elevated in infested pigeons and chitosan nanoparticles treated birds revealed improvement in these parameters in comparison with control positive infected ones. These results concur with Salem et al. (2022) who confirmed that A. columba infestation in pigeons revealed a similar effect. Also, Attia et al. (2021) noticed that Gene expression for IL-1β and TNFα in experimentally infested pigeons with Pseudolynchia canariensis was significantly elevated (24.33 ± 5.71 and 19.00 ± 5.51). Pseudolynchia canariensis induced a case of oxidative stress expressed by elevated nitric oxide and MDA levels with a reduced antioxidant activity, which is expressed as lowered zinc serum levels of experimentally infested pigeon then lowered after treatment with chitosan silver nanocomposaites.
CONCLUSIONS
Based on the results, it could be concluded that gastrointestinal parasites of pigeons are widely spread among pigeons, threaten pigeons’ health, cause economic losses to the producers and need periodical monitoring with the application of strict control and prevention measures. Chitosan nanoparticles have a potent nematocidal activity and are recommended to control A. columba infestation in pigeons.
Acknowledgments
ACKNOWLEDGMENTS
The authors extend their appreciation to the Researchers Supporting Project number (RSP2022R439), King Saud University, Riyadh, Saudi Arabia for funding this research.
DISCLOSURES
Authors declare no conflict of interests.
REFERENCES
- Abd El-Ghany W.A., Shaalan M., Salem H.M. Nanoparticles applications in poultry production: an updated review. Worlds Poult. Sci. J. 2021;77:1001–1025. [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shehata A.M., Arif M., Paswan V.K., Batiha G.E.-S., Khafaga A.F., Elbestawy A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: a review. Environ. Sci. Pollut. Res. 2021;28:4989–5004. doi: 10.1007/s11356-020-11747-3. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Saad A.M., Salem H.M., Ashry N.M., Abo Ghanima M.M., Shukry M., Swelum A.A., Taha A.E., El-Tahan A.M., AbuQamar S.F., El Tarabily K.A. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition: a comprehensive review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Salem H.M., El-Tahan A.M., Soliman M.M., Youssef G.B.A., Taha A.E., Soliman S.M., Ahmed A.E., El-kott A.F., Al Syaad K.M., Swelum A.A. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird's health and production. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Rahman M.A.M., Seddiek Sh.A., Soliman A.S. Some studies on trichomoniasis of pigeons at Qualiobia governorate. Egyptian J. Comp. Pathol. Clin. Pathol. 2008;21:123–141. [Google Scholar]
- Abdel Rahman M.M.I.A., Tolba H.M.N., Abdel-Ghany H.M. Ultrastructure, morphological differentiation, and pathological changes of Ascaridia species in pigeons. Adv. Anim. Vet. Sci. 2019;7:66–72. [Google Scholar]
- AbdElKader N.A., Sheta E., AbuBakr H.O., El-Shamy O.A.A, Oryan A., Attia M.M. Effects of chitosan nanoparticles, ivermectin and their combination in the treatment of Gasterophilus intestinalis (Diptera: Gasterophilidae) larvae in donkeys (Equus asinus) Int. J. Trop. Insect. Sci. 2021;41:43–54. [Google Scholar]
- Abdelsalam M., Attia M.M., Mahmoud M.A. Comparative morphomolecular identification and pathological changes associated with Anisakis simplex larvae (Nematoda: Anisakidae) infecting native and imported chub mackerel (Scomber japonicus) in Egypt. Reg. Stud. Mar. Sci. 2020;39 [Google Scholar]
- Abed A.A., Naji H.A., Rhyaf A.G. Investigation study of some parasites infected domestic pigeon (Columba livia domestica) in Al-Dewaniya city. IOSR- JPBS. 2014;9:13–20. [Google Scholar]
- Abolhadid S.M., Araf W.M., Abdelaty A.S, Moawad U.K., El-Ashram S., Gad S.M. Remarks of Eimeria labbeana infection in Egyptian pigeons. J. Parasit. Dis. 2021;45:1145–1151. doi: 10.1007/s12639-021-01411-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Elala N.M., Attia M.M., Abd-Elsalam R.M. Chitosan-silver nanocomposites in goldfish aquaria: a new perspective in Lernae cyprinacea control. Int. J. Biol. Macromol. 2018;111:614–622. doi: 10.1016/j.ijbiomac.2017.12.133. [DOI] [PubMed] [Google Scholar]
- Adang K.L., Oniye S.J., Ajanusi O.J., Ezealor A.U., Abdu P.A. Gastrointestinal helminths of the domestic pigeons (Columba livia domestica Gmelin, 1789 Aves: Columbidae) in Zaria, Northern Nigeria. Sci. World J. 2008;3:33–37. [Google Scholar]
- Al- Said H.I., Hamodi A.Z. Prevalence and pathology of trichomoniasis in free – living urban pigeons in the city of Mosul, Iraq. Vet. World. 2011;4:12–14. [Google Scholar]
- Alfy H., Ghareeb R.Y., Soltan E., Farag D.A. Impact of chitosan nanoparticles as insecticide and nematicide against Spodoptera littoralis, Locusta migratoria, and Meloidogyne incognita. Plant Cell Biotechnol. Mol. Biol. 2020;21:126–140. [Google Scholar]
- Alkharigy F.A., El Naas A.S., Maghrbi A.A.EL. Survey of parasites in domestic pigeons (Columba livia) in Tripoli, Libya. Open Vet. J. 2018;8:360–366. doi: 10.4314/ovj.v8i4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar A.H., Rana S.H., Shah A.H. Pathology of cestodes infection in indigenous and exotic layers. Path. J. Agri. Sci. 2000;37:1–2. [Google Scholar]
- Ashenafi H., Eshutu Y. Study on gastrointestinal helminths of local chickens in central Ethiopia. Revue. Med. Vet. 2004;155:504–507. [Google Scholar]
- Ashrafihelan J., Noroozi R., Hosseini SH., Nematollahi A.R., Mahpaekar H.A., Arefi A. Identification and infection rate of alimentary tract to helminth parasites in domestic pigeons (Columba livia domestica) of Tabriz. Sci.-Res. Iranian Vet. J. 2010;6:52–58. [Google Scholar]
- Attia M.M., Ismael E., Saleh N.M.K. A sensitive serodiagnostic tool for the detection of active infection of zoonotic visceral and nasopharyngeal linguatulosis. Vet. World. 2019;12:883–889. doi: 10.14202/vetworld.2019.883-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia M.M., Yehia N., Soliman M.M., Shukry M., El-Saadony M.T., Salem H.M. Evaluation of the antiparasitic activity of the chitosan-silver nanocomposites in the treatment of experimentally infested pigeons with Pseudolynchia canariensis. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia M.M., Salaeh N.M.K. Ultrastructure of adult Gasterophilus intestinalis (Diptera: Gasterophilidae) and its puparium. Int. J. Trop. Insect. Sci. 2020;40:327–335. [Google Scholar]
- Attia M.M., Salem H.M. Morphological and molecular characterization of Pseudolynchia canariensis (Diptera: Hippoboscidae) infesting domestic pigeons. Int. J. Trop. Insect Sci. 2022;42:733–740. [Google Scholar]
- Attia M.M., Soliman S.M., Salaeh N.M.K., Salem H.M., Mohamed Alkafafy M., Saad A.M., El-Saadony M.T., El-Gameel S.M. Evaluation of immune responses and oxidative stress in donkeys: immunological studies provoked by Parascaris equorum infection. Saudi J. Biol. Sci. 2022;29:2173–2179. doi: 10.1016/j.sjbs.2021.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy M.E.I., Rabea E.I. A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection. Int. J. Carbohydr. Chem. 2011;2011 [Google Scholar]
- Bahrami A.M., Hosseini E.S., Razmjo M. Important parasite in pigeon, its hematological parameter and pathology of intestine. World Appl. Sci. J. 2013;21:1361–1365. [Google Scholar]
- Baris S., Karatepe B., Karatepe M., Murat K. Parasites of domestic (Columba livia domestica) and wild (Columba livia livia) pigeons in Niğde, Turkey. Bull. Vet. Inst. Pulawy. 2008;52:551–554. [Google Scholar]
- Borji H., Moghaddas E., Razmi G.R., Azad M. A survey of ecto- and endo-parasites of domestic pigeons (Columba livia) in Mashhad, Iran. Iranian J. Vet. Sci. Technol. 2012;4:37–42. [Google Scholar]
- Dede P.M., Richards W.S. Prevalence of helminthiasis in wild and domestic pigeons from North-east zone of Nigeria. Bull. Anim. Health Prod. Afr. 1998;46:193–195. [Google Scholar]
- Dehlawi M.S. New records of cestodes from birds in Saudi Arabia. Saudi J. Biol. Sci. 2006;13:13–16. [Google Scholar]
- Divya K., Jisha MS. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018;16:101–112. [Google Scholar]
- Djelmoudi Y.A., Daoudi-Hacini M.S., Doumandji S. Common endoparasites of wild rock Pigeon (Columba livia livia) and wood Pigeon (Columba palumbus) in the Algiers Sahel, Algeria. Int. J. Zoo. 2014;4:99–106. [Google Scholar]
- El-Dakhly K.M., Mahrous L.N., Mabrouk G.A. Distribution pattern of intestinal helminths in domestic pigeons (Columba livia domestica) and turkeys (Meleagris gallopavo) in Beni-Suef province, Egypt. J. Vet. Med. Res. 2016;23:112–120. [Google Scholar]
- El-Dakhly K.M., El-Seify M.A., Mohammed E.S., Elshahawy I.S., Fawy S.A, Omar M.A. Prevalence and distribution pattern of intestinal helminths in chicken and pigeons in Aswan, Upper Egypt. Trop. Anim. Health Prod. 2018;51:713–718. doi: 10.1007/s11250-018-1725-1. [DOI] [PubMed] [Google Scholar]
- Eljadar M., Saad W., Elfadel G. A study on the prevalence of Endoparasites of domestic Pigeons (Columba livia domestica) inhabiting in the Green Mountain Region of Libya. J. Am. Sci. 2012;8:191–193. [Google Scholar]
- El-Saadony M.T., Salem H.M., El-Tahan A.M., Abd El-Mageed T.A., Soliman S.M., Khafaga A.F., Swelum A.A., Ahmed A.E., Alshammari F.A., Abd El-Hack M.E. The control of poultry salmonellosis using organic agents: an updated overview. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shall N.A., Abd El-Hack M.E., Albaqami N.M., Khafaga A.F., Taha A.E., Swelum A.A., El-Saadony M.T., Salem H.M., El-Tahan A.M., AbuQamar S.F., El-Tarabily K.A., Elbestawy A.R. Phytochemical control of poultry coccidiosis: a review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eman, A. 2005. Some studies on trichomoniasis in pigeons in Sharkia Province. M.V.Sc. Thesis, Avian and Rabbit Disease Dept., Fac. Vet. Med., Zagazig Univ. Egypt.
- Finney D.J. 3rd ed. Cambridge University Press; New York, NY: 1971. Probit Analysis. [Google Scholar]
- Ghosh K.K., Islam M.S., Sikder S., Das S., Chowdhury S., Alim M.A. Prevalence of ecto and gastrointestinal parasitic infections of pigeon at chittagong metropolitan area, Bangladesh. J. Adv. Parasitol. 2014;1:9–11. [Google Scholar]
- Hebat-Allah M., Abd El-Motelib T.Y. Studies on gram-positive organisms in sick pigeons. Assiut Vet. Med. J. 2007;53:280–290. [Google Scholar]
- Hegazy M.I., Hegazy A.M., Saad A.M., Salem H.M., El-Tahan A.M., El-Saadony M.T., Soliman S.M., Taha A.E., Alshehri M.A., Ahmed A.E., Swelum A.A. Biologically active some microorganisms have the potential to suppress mosquito larvae (Culex pipiens, Diptera: Culicidae) Saudi J. Biol. Sci. 2022;29:1998–2006. doi: 10.1016/j.sjbs.2021.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khezerpour A., Naem S. Investigation on parasitic helminthes of gastrointestinal, liver and lung of domestic pigeons (Columba livia) in Urmia, Iran. Int. J. Livest. Res. 2013;3:35–41. [Google Scholar]
- Krautwald-Junghanns M., Zebisch R., Schmidt V. Relevance and treatment of coccidiosis in domestic pigeons (Columba livia forma domestica) with particular emphasis on toltrazuril. J. Avian Med. Surg. 2009;23:1–5. doi: 10.1647/2007-049R.1. [DOI] [PubMed] [Google Scholar]
- Lawson B., Cunningham A., Chantrey J., Hughes L., Kirkwood J., Pennycott T., Simpson V. Epidemic finch mortality. Vet. Rec. 2006;159:367. doi: 10.1136/vr.159.11.367-a. [DOI] [PubMed] [Google Scholar]
- Liu C., Li M., Cao Y., Qu J.P., Zhang Z.W., Xu S.W., Li S. Effects of avermectin on immune function and oxidative stress in the pigeon spleen. Chem. Biol. Interact. 2014;5:43–50. doi: 10.1016/j.cbi.2013.12.015. [DOI] [PubMed] [Google Scholar]
- McDougald L.R. In: Diseases of Poultry. 11th ed. Saif Y.M., Barnes H.G., Glisson J.R., Fadly A.M., McDonald L.R., Swayne D.E., editors. Iowa State Press; Ames, IA: 2003. Coccidiosis; pp. 974–990. [Google Scholar]
- Mohamed I.E., El-Sakkar G.H., Moursi M.M.M. Pathological studies on pigeon trichomoniasis with reference to the associated bacteria. Egypt. J. Comp. Path. Clinic. Path. Vol. 2009;22:67–87. [Google Scholar]
- Msoffe P.L.M., Muhairwa A.P., Chiwanga G.H., Kassuku A.A. A study of ecto- and endo-parasites of domestic pigeons in Morogoro unicipality. Tanzania. African. J. Agric. Res. 2010;5:264–2672. [Google Scholar]
- Mushi E.Z., Binta M.G., Chabo R.G., Ndebele R., Panzirah R. Parasites of domestic pigeons (Columba livia domestica) in Sebele, Gaborone, Botswana. J. S. Afr. Vet. Assoc. 2000;71:249–250. [PubMed] [Google Scholar]
- Nagwa E.A., Loubna M.A., El-Madawy R.S., Toulan E.I. Studies on helminths of poultry in Gharbia governorate. Benha Vet. Med. J. 2013;25:139–144. [Google Scholar]
- Natala A.J., Asemadahun N.D., Okubanjo O.O., Ulayi B.M., Owolabi Y.H., Jato I.D., Yusuf K.H. A survey of parasites of domesticatedpigeon (Columba livia domestica) in Zaria, Nigeria. Int. J. Soft Comput. 2009;4:148–150. [Google Scholar]
- Permin A., Esmann J.B., Hoj C.H., Hove T., Mukaratirwa S. Ecto-, endo- and haemoparasites in free-range chickens in Goromonzi District in Zimbabwe. Prev. Vet. Med. 2002;54:213–224. doi: 10.1016/s0167-5877(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Qamar M.F., Butt A., Ehtisham-ul-Haque S., Zaman M.A. Attributable risk of Capillaria species in domestic pigeons (Columba livia domestica) Arq. Bras. Med. Vet. Zootec. 2017;69:1172–1180. [Google Scholar]
- Radfar M.H., Asl E.N., Seghinsara H.R., Dehaghi M.M., Faith S. Biodiversity and prevalence of parasites of domestic pigeons (Columba livia domestica) in a selected semiarid zone of South Khorasan, Iran. Trop. Anim. Health Prod. 2012;44:225–229. doi: 10.1007/s11250-011-0002-3. [DOI] [PubMed] [Google Scholar]
- Radfar M.H., Fathi S., Asl E.N., Dehaghi M.M., Seghinsara H.R. A survey of parasites of domestic pigeons (Columba livia domestica) in South Khorasan, Iran. Vet. Res. 2011;4:18–23. doi: 10.1007/s11250-011-0002-3. [DOI] [PubMed] [Google Scholar]
- Rahman S., Khatun R., Nahar L., Khanum T. Chemotherapy of gastrointestinal parasiticdiseases in domestic pigeons (Columba livia) in Rajshahi division of Bangladesh. Res. Agric. Livest. Fish. 2019;6:323–328. [Google Scholar]
- Saad A.M., Salem H.M., El-Tahan A.M., El-Saadony M.T., Alotaibi S.S., El-Shehawi A.M., Abd El-Mageed T.A., Taha A.E., Alkahtani M.A., Ahmed A.E., Swelum A.A. Biological control: an effective approach against nematodes using black pep-per plants (Piper nigrum L.) Saudi J. Biol. Sci. 2022;29:2047–2055. doi: 10.1016/j.sjbs.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad M.F., Attia M.M. Milk as a new diagnostic tool for rapid detection of Fascioliasis in dairy goats using excretory/secretory antigen. Acta Parasitologica. 2021;66:336–345. doi: 10.1007/s11686-020-00286-z. [DOI] [PubMed] [Google Scholar]
- Salem H.M., Khattab M.S., Yehia N., Abd El-Hack M.E., El-Saadony M.T., Alhimaidi A.R., Swelum A.A., Attia M.M. Morphological and molecular characterization of Ascaridia columbae in the domestic pigeon (Columba livia domestica) and the assessment of its immunological responses. Poult. Sci. Volume. 2022;101 doi: 10.1016/j.psj.2021.101596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Ismael E., Shaalan M. Evaluation of the effects of silver nanoparticles against experimentally induced necrotic enteritis in broiler chickens. Int. J. Nanomed. 2021;16:6783–6796. doi: 10.2147/IJN.S319708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Salem M.A., Soliman M.M., Althobaiti S.A., Khafaga A.K., El-Tahan A.M., El-Saadony M.T., Attia M.M. Parasitological and histopathological examination of Cocktail love birds infected with Eimeria aratinga (Apicomplexa: Eimeriidae) Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Attia M.M. Accidental intestinal myiasis caused by Musca domestica L. (Diptera: Muscidae) larvae in broiler chickens: a field study. Int. J. Trop. Insect Sci. 2021;41:2549–2554. [Google Scholar]
- Salem H.M., Yehia N., Al-Otaibi S., El-Shehawi A.M., Elrys A.A.M.E., El-Saadony M.T., Attia M.M. The prevalence and intensity of external parasites in domestic pigeons (Columba livia domestica) in Egypt with special reference to the role of deltamethrin as insecticidal agent. Saudi J. Biol. Sci. 2022;29:1825–1831. doi: 10.1016/j.sjbs.2021.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam-Wobo S.O., Mafiana C.F. Prevalence and identification of helminth parasites of local chickens of Abeokuta, Nigeria. Asset. Series. 2003;2:141–147. [Google Scholar]
- Sari B., Karatepe B., Karatepe M., Kara M. parasites of domestic (Columba livia domestica) and wild (Columba livia livia) pigeons in niğde, Turkey. Bull. Vet. Inst. Pulawy. 2008;52:551–554. [Google Scholar]
- Senlik B., Gulegen E., Akyol V. Effect of age, sex and season on the prevalence and intensity of helminth infections in domestic pigeons (Columba livia) from Bursa Province, Turkey. Acta Vet. Hung. 2005;53:449–456. doi: 10.1556/AVet.53.2005.4.5. [DOI] [PubMed] [Google Scholar]
- Simon M.S., Emeritus . 2nd edition. American Soybean Association; St. Louis, MO: 2005. Enteric Diseases: ASA Handbook on Poultry Diseases; pp. 133–143. [Google Scholar]
- Soliman S.M., Attia M.M., Al-Harbi M.S., Saad A.M., El-Saadony M.T., Salem H.M. Low host specificity of Hippobosca equina infestation in different domestic animals and pigeon. Saudi J. Biol. Sci. 2022;29:2112–2120. doi: 10.1016/j.sjbs.2021.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulsby E.J.L. 7th ed. Bailliere Tindall; London, UK: 1986. Helminths, Arthropods and Protozoa of Domesticated Animals; pp. p167–p174. [Google Scholar]
- Swayne D.E. 14th Edition. Vol. 2020. Wiley-Blackwell; Hoboken, NJ: 2020. (Diseases of Poultry). [Google Scholar]
- Tanveer M.K., Kamran A., Abbas M., Umer N.C., Azhar M.A., Munir M. Prevalence and chemo-therapeutical investigations of gastrointestinal nematodes in domestic pigeons in Lahore, Pakistan. Trop. Biomed. 2011;28:102–110. [PubMed] [Google Scholar]
- Villanúa D., Höfle U., Pérez-Rodríguez L., Gortázar C. Trichomonas gallinae in wintering common wood pigeons Columba palumbus in Spain. Ibis. 2006;148:641–648. [Google Scholar]