Abstract
Objective:
Elevated carotid intima-media thickness (cIMT) and carotid plaque are markers of arterial injury and may be linked to structural brain injury. We hypothesized cIMT or presence of carotid plaque at midlife are associated with presence of infarcts and cerebral microbleeds, greater white matter hyperintensity (WMH) volume, and smaller regional brain volumes in late-life.
Methods:
We included 1,795 Atherosclerosis Risk in Communities (ARIC) Study participants (aged 57±6 years, 57% female, 23% Black) with carotid ultrasounds in 1990–1992 and brain MRI scans in 2011–2013. Weighted linear regression was used for brain volume outcomes, while logistic regression was used for infarcts and cerebral microbleeds.
Results:
After multivariable adjustments, the highest cIMT quintile was associated with smaller deep gray matter (β [95% CI]: −0.11 [−0.22, −0.01]) and cortical volume in a temporal-parietal meta region of interest (ROI) (β [95% CI]: −0.10 [−0.20, −0.01]) in late-life. Similarly, those with carotid plaque had smaller regional brain volumes than those without (βs [95% CIs]: −0.05 [−0.12, 0.03] and −0.06 [−0.13, 0.01] for deep gray matter and temporal-parietal meta ROI). No significant relations were observed with WMH volume, infarcts, or cerebral microbleeds.
Conclusion:
Over a median follow-up of 21 years, greater midlife cIMT and presence of carotid plaque were associated with smaller deep gray matter volume and cortical volume in a meta ROI involving temporal and parietal lobe regions typically involved in neurodegeneration, including Alzheimer’s disease, in later life. Contrary to our hypothesis, associations between measures of arterial injury and markers of vascular brain injury were null.
Keywords: brain imaging, carotid intima-media thickness, carotid plaque, epidemiology
INTRODUCTION
Elevated carotid intima-media thickness (cIMT) is a marker of arterial injury and can be quantified through a noninvasive ultrasound procedure.1 cIMT has been prospectively associated with an increased risk for dementia,2,3 including in the Atherosclerosis Risk in Communities (ARIC) study,4 and this association may be mediated by either clinical or silent cerebrovascular disease.5,6 There are several pathways through which these carotid markers may be linked to markers of neuropathology. Carotid atherosclerosis may reduce cerebral blood flow7 and result in insufficient delivery of oxygen to the brain.8 This increases the risk of cerebral hypoperfusion,9 which may contribute to white matter hyperintensities (WMH) and ultimately lead to brain atrophy and/or dementia.10,11 As the carotid arteries provide the majority of the brain’s blood supply,6 there is a potential link between elevated cIMT or presence of carotid plaque and MRI markers of neuropathology, either vascular or neurodegenerative. Alternatively, cIMT and carotid plaque may simply be a marker of cumulative exposure to vascular risk factors throughout the lifecourse.12 Midlife vascular risk factors such as hypertension, diabetes, and smoking have been linked to dementia, and even imaging biomarkers of dementia in nondemented adults.13,14
Prior research has suggested a relationship may exist between elevated cIMT and MRI markers of neuropathology; however, these studies have produced inconsistent results, and few examined the longitudinal association. Utilizing data from the community-based Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS), we tested the hypotheses that greater cIMT and presence of carotid plaque are associated with more imaging findings of vascular brain injury (infarcts, microbleeds, and greater WMH volume) and smaller regional brain volumes.
METHODS
Study Population and Design
The ARIC study is a population-based cohort of 15,792 participants aged 45 to 64 at baseline (1987–1989) who were recruited from four US communities: Washington County, Maryland; Forsyth County, North Carolina; selected suburbs of Minneapolis, Minnesota; and Jackson, Mississippi.15 Since the baseline exam, participants have attended several additional follow-up visits; for the present manuscript visit 2 (1990–1992) serves as baseline since this was the first visit in which cognitive data were collected and cIMT was measured at this visit, and brain imaging was conducted at visit 5 (2011–2013). Additionally, participants were regularly contacted by telephone (annually before 2012 and twice-yearly since) and there is continuous surveillance of catchment areas for hospitalizations. Participants provided written informed consent at each visit, and all study protocols were approved by Institutional Review Boards at the study sites.
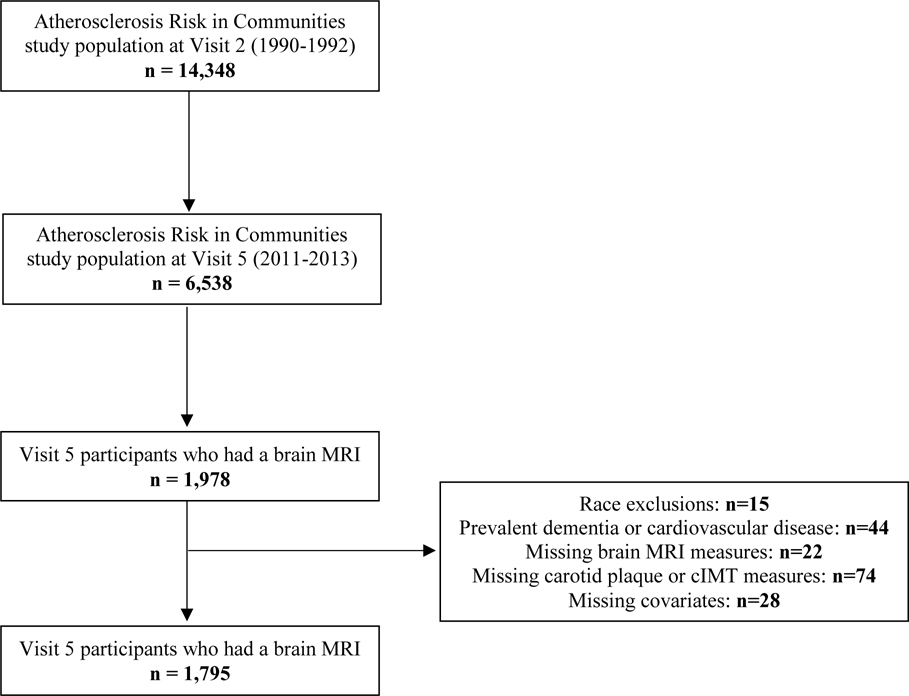
A total of 14,348 participants attended visit 2, and 6,538 attended visit 5, of whom 1,978 took part in brain imaging. From this subset, we excluded participants with missing brain MRI measures (n=22), races other than Black or white and non-whites from the Minneapolis and Washington County centers due to low numbers (n=15), participants with prevalent dementia or cardiovascular events (heart failure, stroke, coronary heart disease) at visit 2 (n=44), missing carotid plaque or cIMT measurements at visit 2 (n=74), and missing covariate information (n=28). After exclusions, 1,795 participants were included in this analysis (Figure 1).
Figure 1.
Study sample exclusion flowchart.
cIMT and Carotid Plaque Measurements
As previously described, ARIC ultrasound measurements were conducted at ARIC visit 2 by trained technicians and scans were read centrally at the ARIC Ultrasound Reading Center.16 In brief, Biosound 2000 II duplex scanners were used to acquire images. cIMT was assessed in three 1-cm segments of the extracranial carotid arteries: the internal carotid, the carotid bifurcation, and the common carotid.17 A total of 11 measurements of the far wall were attempted at each segment in 1-mm increments and the mean of these measurements was calculated. Measurements were made regardless of the presence of plaque. The site-specific reliability coefficients for the mean far-wall thickness at the internal carotid, carotid bifurcation, and common carotid were 0.73, 0.77, and 0.70, respectively.17 Carotid plaque was recorded as present if two of the following three criteria were met: abnormal wall thickness (>1.5 mm), abnormal shape (protrusion into the lumen, loss of alignment with adjacent arterial wall boundary), or abnormal wall texture (brighter echoes than adjacent boundaries). The intrareader agreement had a κ statistic of 0.76 and an inter-reader agreement of 0.56.18
Brain MRI Measurements
ARIC brain MRI imaging protocol has been previously described.19 Briefly, a subset of visit 5 participants who had no brain MRI contraindications and met any of the following criteria were selected to undergo a brain MRI: 1) participated in a prior ARIC brain MRI scan in 2004–2006, 2) had evidence of cognitive impairment and/or declines on longitudinally-administered tests, and 3) selected from an age-stratified random sample of cognitively normal participants to approximate the age distribution of cognitively impaired participants. Sampling weights were assigned based on inverse sampling fractions and the probability of completing the exam.19
3-T Siemens scanners were used at each study site using standardized protocols. Scans were read centrally at the ARIC MRI Reading Center at the Mayo Clinic (Rochester, MN). Total intracranial volume and distinct regional volumes were measured using FreeSurfer version 5.1. Deep gray matter was calculated as the combined volume of the thalamus, caudate, putamen, and pallidum. The cortical volume in a temporal-parietal meta region of interest (ROI) was calculated as the combined volume of the parahippocampal, entorhinal, inferior parietal lobules, hippocampus, and precuneus,19 based on prior studies demonstrating the relevance of these regions in individuals with Alzheimer’s disease.20,21 WMH burden was measured using an algorithm developed at Mayo Clinic, Rochester.22 Briefly, WMH volume was derived from a semiautomated segmentation of fluid-attenuated inversion recovery (FLAIR) images. To reduce the number of false positive segmentations of WMH from FLAIR, the magnetization-prepared rapid acquisition gradient echo (MPRAGE) image was resampled in the FLAIR space. A white matter mask was then generated by MPRAGE segmentation. WMH was segmented using an automated slice-based seed initialization and region-growing method.22
Lacunar infarcts were defined as subcortical T2 fluid-attenuated inversion recovery lesions (FLAIR) with central hypointensity>3 mm and hyperintensity ≤20 mm in maximum diameter located in the caudate, lenticular nucleus, internal capsule, thalamus, brainstem, deep cerebral white matter, centum semiovale, or corona radiata.23 Cortical infarcts were defined as lesions with a minimum extent >10 mm on T2 FLAIR.24 Cerebral microbleeds were identified as lesions of ≤10 mm in maximum diameter on gradient-echo T2-weighted (T2*GRE) imaging sequences and were divided into lobar and subcortical microbleeds depending on the location.25,26
Covariate Measurements
Covariates assessed from visit 2 included age, sex, race, ARIC field center, APOEɛ4 genotype (≥ 1 allele, 0 alleles), body mass index (BMI), systolic blood pressure, antihypertensive medications (yes, no), smoking status (current, former, never), pack-years of smoking, and diabetes status (yes, no). Education level (less than high school education, high school graduate or high school equivalent or vocational school, college or above) was obtained from visit 1. Race category, education level, smoking status, and amount smoked were self-reported by participants, while pack-years of smoking was calculated. Technicians recorded current medication use through review of medication bottles, which participants brought to the clinic visit. APOE ɛ4 genotyping was done using the TaqMan assay (Applied Biosystems, Foster City, CA), as previously described.27 Height and weight were measured by technicians to derive BMI. Sitting blood pressure was measured three times via a random-zero sphygmomanometer after a 5-minutue rest and the final two blood pressure measures were averaged. Diabetes was defined as a fasting serum glucose of ≥126 mg/dl, non-fasting serum glucose of ≥200 mg/dl, a self-reported physician diagnosis of diabetes, or use of antidiabetic medication in the past 2 weeks.
Statistical Analysis
Participant characteristics, stratified by cIMT quintiles, were described using frequencies and percentages for categorical variables and means and standard deviations (SD) for continuous variables. cIMT was categorized in quintiles for this analysis. To assess the relationship between cIMT and carotid plaque with brain MRI markers of cerebrovascular disease and neurodegeneration, linear regression was used for brain volume outcomes and logistic regression was used for dichotomous outcomes (infarcts and microbleeds). Infarcts and microbleeds were both assessed in three ways. For infarcts, we assessed the presence of 1) any infarcts, 2) lacunar infarcts, and 3) cortical infarcts. Microbleeds were analyzed as the presence of 1) any microbleeds, 2) subcortical microbleeds, and 3) lobar microbleeds. To compare the magnitude of association across brain regions, brain volumes were scaled based on their standard deviations. WMH volume was highly skewed; therefore, log base 2 transformation was performed for normality.
For all analyses, multivariable models were adjusted as follows: model 1 adjusted for age, sex, race/center (5 levels), education, and APOEɛ4 genotype; model 2 additionally adjusted for BMI, systolic blood pressure, smoking status, pack-years of smoking, antihypertensive medications, and diabetes status. Analyses for brain volume outcomes also adjusted for total intracranial volume in all models. To account for potential selection bias, inverse probability weighting was used in all analyses to account for inclusion in the brain MRI sample, attrition due to death or visit non-attendance.28,29 Logistic models were used to model the estimated probabilities of being alive at visit 5, attending visit 5 (conditional on being alive at the time of visit 5), and being selected for the brain MRI (conditional on being alive at and participating in visit 5). For each participant, their weights were the inverse of the product of the estimated probabilities. Multiplicative interactions by age, sex, and racewere analyzed by including cross-product terms in the model. P for trend values were obtained using quintile numbers. SAS software was used for all analyses (version 9.4; SAS Institute Inc., Cary, NC).
RESULTS
Demographic and clinical characteristics of the study participants, stratified by cIMT quintiles, are provided in Table 1. At baseline, participants had a mean (SD) age of 57(6) years, 57% were female, and 23% were Black. Those in the highest cIMT quintile were more likely to be older, male, heavier smokers, use antihypertensive medications, and have lower educational attainment. Additionally, participants in the highest cIMT quintile had lacunar infarcts present more often, greater WMH volume, and lower cortical volume in the temporal-parietal meta ROI when compared to those in the lower cIMT quintiles.
Table 1.
Baseline characteristics according to carotid intima-media thickness (cIMT) quintiles: the Atherosclerosis Risk in Communities (ARIC) study, 1990–1992a
| cIMT quintiles | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
|
|
|||||
| N | 359 | 359 | 359 | 359 | 359 |
| cIMT range, mm | <0.59 | 0.59 – 0.65 | 0.66 – 0.71 | 0.72 – 0.80 | >0.80 |
| Carotid plaque | 32 (8.9) | 55 (15.3) | 67 (18.7) | 108 (30.1) | 211 (58.8) |
| Demographics | |||||
| Age, years | 54.1 ± 4.8 | 54.8 ± 5.2 | 55.7 ± 5.2 | 56.0 ± 5.0 | 57.5 ± 5.1 |
| Male sex | 96 (26.7) | 114 (31.8) | 140 (39.0) | 164 (45.7) | 192 (53.5) |
| Black race | 76 (21.2) | 105 (29.2) | 89 (24.8) | 93 (25.9) | 105 (29.2) |
| Less than high school education | 43 (12.0) | 42 (11.7) | 45 (12.5) | 53 (14.8) | 61 (17.0) |
| Physiologic Indicators | |||||
| Body mass index, kg/m2 | 26.5 ± 4.9 | 27.3 ± 4.9 | 27.3 ± 5.0 | 28.0 ± 4.8 | 27.5 ± 4.5 |
| Systolic blood pressure, mmHg | 113.2 ± 14.5 | 114.9 ± 14.3 | 117.4 ± 16.7 | 119.4 ± 16.0 | 120.2 ± 16.7 |
| Use of antihypertensive medication | 58 (16.2) | 71 (19.8) | 86 (24.0) | 80 (22.3) | 90 (25.1) |
| Diabetes | 21 (5.8) | 21 (5.8) | 29 (8.1) | 41 (11.4) | 38 (10.6) |
| ≥1 APOE ε4 allele | 88 (24.5) | 101 (28.1) | 115 (32.0) | 106 (29.5) | 102 (28.4) |
| Behavioral Characteristics | |||||
| Smoking status | |||||
| Current smoker | 58 (16.2) | 53 (14.8) | 51 (14.2) | 54 (15.0) | 55 (15.3) |
| Former smoker | 120 (33.4) | 119 (33.1) | 128 (35.7) | 139 (38.7) | 157 (43.7) |
| Never smoker | 181 (50.4) | 187 (52.1) | 180 (50.1) | 166 (46.2) | 147 (40.9) |
| Pack-years smoking | 16.2 ± 32.5 | 16.7 ± 34.3 | 19.5 ± 37.5 | 16.3 ± 33.4 | 21.7 ± 36.2 |
| Brain MRI Measurements | |||||
| Cortical infarcts | 25 (7.0) | 41 (11.4) | 35 (9.7) | 46 (12.8) | 41 (11.4) |
| Lacunar infarcts | 53 (14.8) | 67 (18.7) | 61 (17.0) | 62 (17.3) | 76 (21.2) |
| Subcortical microbleeds | 52 (14.5) | 76 (21.2) | 73 (20.3) | 77 (21.4) | 72 (20.1) |
| Lobar microbleeds | 27 (7.5) | 33 (9.2) | 30 (8.4) | 34 (9.5) | 32 (8.9) |
| White matter hyperintensity volume, cm3 | 14.8 ± 14.4 | 16.9 ± 15.8 | 17.4 ± 16.1 | 17.2 ± 17.2 | 20.9 ± 20.5 |
| Deep gray matter volume, cm3 | 42.3 ± 4.2 | 42.4 ± 4.2 | 42.9 ± 4.4 | 42.6 ± 4.3 | 42.8 ± 4.3 |
| Temporal-parietal meta ROI cortical volume, cm3 | 59.1 ± 6.8 | 58.9 ± 7.0 | 59.2 ± 7.2 | 58.9 ± 7.1 | 58.7 ± 6.9 |
Restricted to the analytic sample, which required having brain imaging measure data at visit 5 (2011–2013). Continuous variables are expressed as mean ± SD, while categorical variables are n (%).
Abbreviations: APOE = apolipoprotein E; ROI = region of interest
cIMT and brain MRI markers
Table 2 presents the associations between cIMT and brain MRI volumetric markers. Compared to those in the lowest quintile, participants in the highest cIMT quintile had smallerdeep gray matter volume (β [95% CI]: −0.11 [−0.22, −0.01]) and cortical volume in the temporal-parietal meta ROI(β [95% CI]: −0.10 [−0.20, −0.01]) after multivariable adjustments.
Table 2.
Weighteda estimates (95% confidence intervals) of brain MRI volume measurementsb (2011–2013) by carotid intima-media thickness (cIMT) quintiles (1990–1992), ARIC-NCS
| cIMT quintiles (mm) | ||||||
|---|---|---|---|---|---|---|
| <0.59 | 0.59 – 0.65 | 0.66 – 0.71 | 0.72 – 0.80 | >0.80 | p for trend | |
| N | 359 | 359 | 359 | 359 | 359 | |
|
|
||||||
| Log2(WMH Volume) | ||||||
| Model 1 | Reference | 0.10 (−0.03, 0.24) | 0.06 (−0.09, 0.21) | 0.03 (−0.11, 0.16) | 0.12 (−0.01, 0.27) | 0.27 |
| Model 2 | Reference | 0.09 (−0.04, 0.22) | 0.01 (−0.13, 0.15) | −0.03 (−0.17, 0.10) | 0.06 (−0.08, 0.20) | 0.99 |
| Deep Gray | ||||||
| Matter Volume | ||||||
| Model 1 | Reference | −0.02 (−0.12, 0.08) | −0.01 (−0.13, 0.12) | −0.07 (−0.17, 0.03) | −0.14 (−0.24, −0.03) | 0.01 |
| Model 2 | Reference | −0.02 (−0.12, 0.07) | 0.01 (−0.10, 0.12) | −0.05 (−0.15, 0.05) | −0.11 (−0.22, −0.01) | 0.03 |
| Temporal-Parietal Meta | ||||||
| ROI Cortical Volume c | ||||||
| Model 1 | Reference | −0.03 (−0.12, 0.05) | −0.06 (−0.16, 0.04) | −0.10 (−0.20, −0.01) | −0.14 (−0.24, −0.05) | 0.001 |
| Model 2 | Reference | −0.02 (−0.11, 0.06) | −0.02 (−0.12, 0.07) | −0.07 (−0.16, 0.02) | −0.10 (−0.20, −0.01) | 0.02 |
Inverse probability weighting was used.
Brain volume data is in SD units. Definition of 1-SD: log2(WMH) 1.28, deep gray matter 4.29 cm3, temporal-parietal meta ROI cortical volume 7.01 cm3.
Calculated as the combined volume of the parahippocampal, entorhinal, inferior parietal lobules, hippocampus, and precuneus regions.
Model 1: adjusted for age, sex, race/center, education, APOE ε4, total intracranial volume
Model 2: adjusted for model 1 plus body mass index, systolic blood pressure, smoking status, pack-years of smoking, antihypertensive medications, diabetes
Abbreviations: WMH = white matter hyperintensities; ROI = region of interest
No consistent associations between cIMT and markers of vascular brain injury were observed. Those in the highest cIMT quintile had greater WMH volume when compared to participants in the lowest cIMT quintile, though this association was not statistically significant (Table 2; β [95% CI]: 0.06 [−0.08, 0.20]). The association of cIMT with infarcts and microbleedsare presented in Table 3. Although more infarcts and microbleeds were observed among participants in the highest cIMT quintile, no consistent associations between cIMT quintiles and any of the infarcts or microbleeds categories were noted. When assessing cIMT continuously (per 1-SD) and lacunar infarcts, an interaction with age at baseline (split at the median) was present(p=0.02). For each 1-SD greater cIMT, older individuals at baseline had an increased odds of lacunar infarcts, while younger individuals had a reduced odds(ORs [95% CIs] per 1-SD cIMT:≤55 years: 0.85 [0.68, 1.06]; >55 years: 1.14 [0.99, 1.31]). No other interactions were observed.
Table 3.
Weighteda odds ratios (95% confidence intervals) of brain MRI measures (2011–2013) by carotid intima-media thickness (cIMT) quintiles (1990–1992), ARIC-NCS
| cIMT quintiles (mm) | p for trend | |||||
|---|---|---|---|---|---|---|
| <0.59 | 0.59 – 0.65 | 0.66 – 0.71 | 0.72 – 0.80 | >0.80 | ||
| N | 359 | 359 | 359 | 359 | 359 | |
|
|
||||||
| Any Infarcts | ||||||
| N events | 74 | 88 | 83 | 91 | 109 | |
| Model 1 | Reference | 1.28 (0.89, 1.84) | 0.96 (0.66, 1.40) | 1.24 (0.87, 1.78) | 1.37 (0.96, 1.97) | 0.12 |
| Model 2 | Reference | 1.23 (0.85, 1.78) | 0.82 (0.56, 1.20) | 1.08 (0.75, 1.57) | 1.16 (0.80, 1.68) | 0.60 |
| Lacunar Infarcts | ||||||
| N events | 53 | 67 | 61 | 62 | 76 | |
| Model 1 | Reference | 1.34 (0.89, 2.02) | 1.03 (0.68, 1.57) | 1.15 (0.76, 1.73) | 1.19 (0.79, 1.80) | 0.71 |
| Model 2 | Reference | 1.29 (0.85, 1.95) | 0.90 (0.59, 1.38) | 1.01 (0.66, 1.53) | 1.03 (0.67, 1.56) | 0.67 |
| Cortical Infarcts | ||||||
| N events | 25 | 41 | 35 | 46 | 41 | |
| Model 1 | Reference | 1.62 (0.96, 2.72) | 1.10 (0.64, 1.89) | 1.64 (0.98, 2.74) | 1.36 (0.80, 2.30) | 0.34 |
| Model 2 | Reference | 1.56 (0.92, 2.65) | 0.94 (0.54, 1.65) | 1.45 (0.86, 2.44) | 1.15 (0.67, 1.97) | 0.82 |
| Any Microbleeds | ||||||
| N events | 74 | 91 | 85 | 88 | 94 | |
| Model 1 | Reference | 1.25 (0.87, 1.81) | 1.03 (0.71, 1.49) | 1.14 (0.79, 1.64) | 1.08 (0.74, 1.56) | 0.95 |
| Model 2 | Reference | 1.22 (0.84, 1.77) | 0.97 (0.66, 1.41) | 1.11 (0.77, 1.62) | 1.02 (0.70, 1.49) | 0.87 |
| Subcortical Microbleeds | ||||||
| N events | 52 | 76 | 77 | 72 | ||
| Model 1 | Reference | 1.54 (1.03, 2.32) | 1.35 (0.89, 2.03) | 1.54 (1.02, 2.31) | 1.17 (0.77, 1.78) | 0.66 |
| Model 2 | Reference | 1.51 (1.01, 2.28) | 1.29 (0.85, 1.95) | 1.51 (1.01, 2.28) | 1.12 (0.73, 1.71) | 0.79 |
| Lobar Microbleeds | ||||||
| N events | 27 | 33 | 30 | 34 | 32 | |
| Model 1 | Reference | 1.20 (J.68, 2.11) | 0.90 (0.50, 1.60) | 1.16 (0.67, 2.03) | 0.92 (0.52, 1.64) | 0.75 |
| Model 2 | Reference | 1.17 (0.67, 2.07) | 0.85 (0.47, 1.53) | 1.16 (0.66, 2.05) | 0.88 (0.49, 1.58) | 0.67 |
Inverse probability weighting was used.
Model 1: adjusted for age, sex, race/center, education, APOE ε4
Model 2: adjusted for model 1 plus body mass index, systolic blood pressure, smoking status, pack-years of smoking, antihypertensive medications, diabetes
Carotid plaque and brain MRI markers
Of the 1,795 participants included in this analysis, 473 (26%) participants had carotid plaque present on their carotid ultrasound. Associations between presence of carotid plaque and any of the brain MRI measures were similar to that of cIMT. Participants with carotid plaque present had smaller regional brain volumes than those without carotid plaque (Table 4; βs [95% CIs]: −0.05 [−0.12, 0.03] and −0.06 [−0.13, 0.01] for deep gray matter and temporal-parietal meta ROI volumes, respectively). No association between carotid plaque with measures of vascular brain injury (infarcts, microbleeds, and WMH volume) was noted (Tables 4 and 5). An interaction with age at baseline (split at the median) was noted (p=0.02) when lacunar infarcts was the outcome. Older participants with carotid plaque present had greater odds of lacunar infarcts, but younger participants had a reduced odds (ORs [95% CIs]:≤55 years: 0.63[0.39, 1.01]; >55 years: 1.42 [0.99, 2.04]). No other interactions were noted.
Table 4.
Weighteda estimates (95% confidence intervals) of brain MRI markersb (2011–2013) by carotid plaque status (1990–1992), ARIC-NCS
| Plaque Absent | Plaque Present | |
|---|---|---|
| (n=1322) | (n=473) | |
|
|
||
| Log2(WMH Volume) | ||
| Model 1 | Reference | 0.02 (−0.08, 0.12) |
| Model 2 | Reference | −0.01 (−0.10, 0.09) |
| Gray Matter Volume | ||
| Model 1 | Reference | −0.06 (−0.13, 0.02) |
| Model 2 | Reference | −0.05 (−0.12, 0.03) |
| Temporal-Parietal Meta ROI Cortical Volume c | ||
| Model 1 | Reference | −0.07 (−0.14, −0.003) |
| Model 2 | Reference | −0.06 (−0.13, 0.01) |
Inverse probability weighting was used.
Brain volume data is in SD units. Definition of 1-SD: log2(WMH) 1.28, deep gray matter 4.29 cm3, temporal-parietal meta ROI cortical volume 7.01 cm3.
Calculated as the combined volume of the parahippocampal, entorhinal, inferior parietal lobules, hippocampus, and precuneus regions.
Model 1: adjusted for age, sex, race/center, education, APOE ε4, total intracranial volume
Model 2: adjusted for model 1 plus body mass index, systolic blood pressure, smoking status, pack-years of smoking, antihypertensive medications, diabetes
Abbreviations: WMH = white matter hyperintensities; ROI = region of interest
Table 5.
Weighteda odds ratios (95% confidence intervals) of brain MRI markers (2011–2013) by carotid plaque status (1990–1992), ARIC-NCS
| Plaque Absent | Plaque Present | |
|---|---|---|
| (n=1322) | (n=473) | |
|
|
||
| Any Infarcts | ||
| N events | 309 | 136 |
| Model 1 | Reference | 1.20 (0.95, 1.53) |
| Model 2 | Reference | 1.14 (0.89, 1.45) |
| Lacunar Infarcts | ||
| N events | 225 | 94 |
| Model 1 | Reference | 1.07 (0.82, 1.40) |
| Model 2 | Reference | 1.03 (0.78, 1.36) |
| Cortical Infarcts | ||
| N events | 130 | 58 |
| Model 1 | Reference | 1.18 (0.86, 1.64) |
| Model 2 | Reference | 1.11 (0.80, 1.55) |
| Any Microbleeds | ||
| N events | 316 | 116 |
| Model 1 | Reference | 1.03 (0.80, 1.32) |
| Model 2 | Reference | 0.99 (0.77, 1.28) |
| Subcortical Microbleeds | ||
| N events | 258 | 92 |
| Model 1 | Reference | 0.97 (0.74, 1.27) |
| Model 2 | Reference | 0.94 (0.72, 1.24) |
| Lobar Microbleeds | ||
| N events | 110 | 46 |
| Model 1 | Reference | 1.24 (0.86, 1.79) |
| Model 2 | Reference | 1.15 (0.79, 1.67) |
Inverse probability weighting was used.
Model 1: adjusted for age, sex, race/center, education, APOE ε4
Model 2: adjusted for model 1 plus body mass index, systolic blood pressure, smoking status, pack-years of smoking, antihypertensive medications, diabetes
DISCUSSION
In this population-based cohort followed for a median of 21 years, midlife elevated cIMT and presence of carotid plaque were associated with smaller regional brain volumes (deep gray matter and the temporal-parietal meta ROI) in laterlife. Counter to our hypotheses, midlife cIMT or carotid plaque were not related to laterlife markers of cerebrovascular disease (e.g. WMH volume). Additionally, an age interaction between both cIMT and carotid plaque with lacunar infarcts was present. Older participants with greater cIMT or carotid plaque had increased odds of lacunar infarcts, while younger participants had reduced odds.
In the present analysis, elevated cIMT was associated with smaller regional brain volumes. A prior study of symptomatic atherosclerotic participants reported elevated cIMT was cross-sectionally associated with decreased cortical gray matter volume; however, when assessed prospectively, no significant association with progression of brain atrophy was present over 4-years of follow-up.30 Community-based cohorts have reported contrasting results: CARDIA reported no relationship between cIMT and gray matter volume,8 while AGES–Reykjavik found that elevated cIMT was associated with a steeper decline in gray matter volume over 5 years of follow-up among older adults (mean age: 75 years).31 It has been suggested that long term exposure to cardiovascular risk factors results in an increased risk of brain atrophy.32 As atherosclerosis may represent a lifetime exposure to cardiovascular risk factors,8 it is plausible that atherosclerosis leads to greater brain atrophy over an extended time-frame of cardiovascular risk factor exposure. This concept is supported by our results indicating that elevated cIMT in midlife is prospectively associated withsmaller deep gray matter volume and cortical volume in the temporal-parietal meta ROI. Furthermore, this set of regions is likely particularly relevant for age-related neurodegenerative disease, including but not limited to typical amnestic Alzheimer’s disease. Although the involvement of this particular composite set of regions is interesting and may suggest a more specific Alzheimer’s-type mechanism, the lack of specificity of this set of regions makes a definitive connection impossible.
Prior studies have reported varying results when assessing the relationship of cIMT or carotid plaque with brain MRI markers of vascular brain injury. Elevated IMT of the common carotid artery was cross-sectionally associated with MRI infarcts in the population-based Cardiovascular Health Study (all aged ≥65 years),6 while a previous ARIC study reported a cross-sectional association between midlife elevated IMT (defined as >0.9 mm) and silent brain infarcts in African Americans, but not whites.33 However, other studies in middle-aged adults (mean age: 51 and 58 years) found no association present,34,35 similar to results from our prospective study. Additionally, we found no significant association between elevated cIMT and WMH volume, consistent with results from other population-based cohort studies.8,34,36 In contrast, the Northern Manhattan and Cardiovascular Health Study reported cross-sectional associations of cIMT with WMH in older populations (mean age: 71 and 75 years old, respectively).6,37 Prior studies reporting a significant association with infarcts and WMH were not only cross-sectional, but also measured cIMT in older adults. Our study differed in that we assessed cIMT at midlife and looked at markers of brain neuropathology at later life. Atherosclerosis and cIMT progresses over time and may represent the cumulative effect of a combination of vascular risk factors,38 such as diabetes or hypertension. Therefore, assessing atherosclerosis or cIMT at different points in one’s life may reflect different constructs of atherosclerosis severity and progression.
Relatively few prior studies have evaluated the association between cIMT and cerebral bleeding outcomes. A study in Taiwan found the highest quartile of cIMT is cross-sectionally associated with cerebral microbleeds among community-dwelling adults over 50 years old when compared to the lower three quartiles combined.39 When separated by location, deep/infratentorial microbleeds remained associated with greater cIMT, while lobar microbleeds did not.39 The Framingham Offspring study reported no significant association between cIMT and cerebral microbleeds overall.38 We observed no consistent associations between cIMT and microbleeds.
Of the few studies assessing carotid plaque and brain MRI measures, contrasting results were present. Carotid plaque was cross-sectionally associated with silent cerebral infarcts in a Japanese study,35 but no relationship with white matter disease or MRI infarcts was noted in the Cardiovascular Health Study.6 The Rotterdam study reported an association between carotid plaques and lacunar infarcts; however, precision was relatively poor in this study.40 In our analysis, participants with carotid plaque present had smaller regional brain volumes than those without carotid plaque, which are similar results to that of cIMT. However, no association between carotid plaque and infarcts, microbleeds, or WMH volume were observed. Given that the reliability coefficient in defining presence of carotid plaque was lower than that for cIMT measurements in ARIC,17,18 measurement error may have contributed to why we failed to find plaque to be a predictive factor for vascular brain injury.
Although no association between cIMT or carotid plaque with lacunar infarcts was found, an age interaction was present. Older participants with greater cIMT or carotid plaque present had an increased odds of lacunar infarcts, while younger participants had a reduced odds of lacunar infarcts. There are few studies assessing an age interaction in the relationship between cIMT and brain MRI markers. In the Northern Manhattan study, a similar age interaction was noted, in which cIMT was cross-sectionally associated with WMH in those ≥70 years, but not among those <70 years old.37 Younger participants in our analysis had lower systolic blood pressure and were less likely to have hypertension compared to older participants. In addition, atherosclerotic changes in the extracranial arteries are age dependent.41 Therefore, it may be that older participants had an increased odds of lacunar infarcts given that elevated cIMT is often due to aging and hypertension.42 Prior research has indicated that the rate of cIMT progression may be reduced by anti-hypertensive medications;43 this suggests the importance of screening for atherosclerosis markers early in the disease progression. Additional research is warranted to further clarify the association between cIMT and brain MRI markers among younger populations.
An important strength of our study, compared to most conducted previously, is the prospective design. In our study, carotid ultrasounds were performed approximately 20 years prior to brain MRIs, allowing us to explore the likely temporality of the relationship, though unfortunately midlife brain MRI data were not available in this subset, so we could not directly evaluate change in brain MRI markers over time. Other strengths include that the cohort with brain MRI measures is relatively large and consists of Black and white men and women. However, limitations also exist. We are unable to assess whether participants had carotid or arterial stenosis at the time of carotid ultrasound. It is possible that those with more severe stenosis at baseline may be more unlikely to attend and undergo brain MRI scans at the later visit, which may lead to selection bias. In addition, selection bias may have also occurred as it is likely that participants who returned for a brain MRI were a healthier subset of the baseline cohort. Therefore, we utilized inverse probability weighting in our analyses to account for attrition due to visit non-attendance, death, or selection for the brain MRI study, but it is still possible selection bias remains. Measurement error is another limitation; the inter-reader agreement for presence of carotid plaque was consideredjust fair, which may have resulted in a lack of association. Furthermore, similar to other observational studies, causal inference is limited as unmeasured confounding may exist.
CONCLUSION
In this community-based cohort, elevated cIMT and presence of carotid plaque, markers for arterial injury, were associated with smaller deep gray matter and temporal-parietal meta ROI volumes over 20 years later, independent of traditional vascular risk factors. Counter to our expectation, neither cIMT nor carotid plaque were not associated with markers of vascular brain injury over 20 years later. Although assessing the role of cIMT and brain pathology over a long timeframe is challenging due to issues of selection bias and measurement error, our findings suggest markers of arterial injury may contribute to brain atrophy in late-life, highlighting the importance of optimal control of modifiable vascular risk factors in midlife.
ACKNOWLEDGMENTS
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Neurocognitive data is collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, NINDS, NIA and NIDCD), and with previous brain MRI examinations funded by R01-HL70825 from the NHLBI. The authors thank the staff and participants of the ARIC study for their important contributions. This work was also supported by grants from the National Institute of General Medical Sciences [T32GM132063 (WW)] and the National Heart Lung and Blood Institute [K24HL159246 (PLL), K24HL148521 (AA)].
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gardener H, Caunca MR, Dong C, Cheung YK, Elkind MSV, Sacco RL, Rundek T, Wright CB. Ultrasound Markers of Carotid Atherosclerosis and Cognition. Stroke. 2017;48:1855–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Oijen M, de Jong FJ, Witteman JCM, Hofman A, Koudstaal PJ, Breteler MMB. Atherosclerosis and risk for dementia. Annals of Neurology. 2007;61:403–410. [DOI] [PubMed] [Google Scholar]
- 3.Wendell CR, Waldstein SR, Ferrucci L, O’Brien RJ, Strait JB, Zonderman AB. Carotid atherosclerosis and prospective risk of dementia. Stroke. 2012;43:3319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W, Norby FL, George KM, Alonso A, Mosley TH, Gottesman RF, Meyer ML, Lutsey PL. Association of Carotid Intima-Media Thickness and Other Carotid Ultrasound Features With Incident Dementia in the ARIC-NCS. J Am Heart Assoc. 2021;10:e020489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MMB. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. [DOI] [PubMed] [Google Scholar]
- 6.Manolio TA, Burke GL, O’Leary DH, Evans G, Beauchamp N, Knepper L, Ward B. Relationships of Cerebral MRI Findings to Ultrasonographic Carotid Atherosclerosis in Older Adults: The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1999;19:356–365. [DOI] [PubMed] [Google Scholar]
- 7.Moroni F, Ammirati E, Magnoni M, D’Ascenzo F, Anselmino M, Anzalone N, Rocca MA, Falini A, Filippi M, Camici PG. Carotid atherosclerosis, silent ischemic brain damage and brain atrophy: A systematic review and meta-analysis. International Journal of Cardiology. 2016;223:681–687. [DOI] [PubMed] [Google Scholar]
- 8.Cermakova P, Ding J, Meirelles O, Reis J, Religa D, Schreiner PJ, Jacobs DR, Bryan RN, Launer LJ. Carotid Intima–Media Thickness and Markers of Brain Health in a Biracial Middle-Aged Cohort: CARDIA Brain MRI Sub-study. J Gerontol A Biol Sci Med Sci. 2020;75:380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bokkers RPH, van der Worp HB, Mali WPTM, Hendrikse J. Noninvasive MR imaging of cerebral perfusion in patients with a carotid artery stenosis. Neurology. 2009;73:869–875. [DOI] [PubMed] [Google Scholar]
- 10.de la Torre JC. Critically attained threshold of cerebral hypoperfusion: the CATCH hypothesis of Alzheimer’s pathogenesis. Neurobiology of Aging. 2000;21:331–342. [DOI] [PubMed] [Google Scholar]
- 11.Appelman AP, van der Graaf Y, Vincken KL, Tiehuis AM, Witkamp TD, Mali WP, Geerlings MI. Total Cerebral Blood Flow, White Matter Lesions and Brain Atrophy: The SMART-MR Study. J Cereb Blood Flow Metab. 2008;28:633–639. [DOI] [PubMed] [Google Scholar]
- 12.Martinsson A, Östling G, Persson M, Sundquist K, Andersson C, Melander O, Engström G, Hedblad B, Smith JG. Carotid Plaque, Intima-Media Thickness, and Incident Aortic Stenosis: A Prospective Cohort Study. Arterioscler Thromb Vasc Biol. 2014;34:2343–2348. [DOI] [PubMed] [Google Scholar]
- 13.Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, Deal JA, McKhann GM, Mosley TH, Sharrett AR, Schneider ALC, Windham BG, Wruck LM, Knopman DS. Associations Between Midlife Vascular Risk Factors and 25-Year Incident Dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol. 2017;74:1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottesman RF, Schneider ALC, Zhou Y, Coresh J, Green E, Gupta N, Knopman DS, Mintz A, Rahmim A, Sharrett AR, Wagenknecht LE, Wong DF, Mosley TH. Association Between Midlife Vascular Risk Factors and Estimated Brain Amyloid Deposition. JAMA. 2017;317:1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 16.Riley WA, Barnes RW, Bond MG, Evans G, Chambless LE, Heiss G. High-Resolution B-Mode Ultrasound Reading Methods in the Atherosclerosis Risk in Communities (ARIC) Cohort. Journal of Neuroimaging. 1991;1:168–172. [PubMed] [Google Scholar]
- 17.Chambless LE, Zhong MM, Arnett D, Folsom AR, Riley WA, Heiss G. Variability in B-mode ultrasound measurements in the Atherosclerosis Risk in Communities (ARIC) study. Ultrasound in Medicine & Biology. 1996;22:545–554. [DOI] [PubMed] [Google Scholar]
- 18.Li R, Duncan BB, Metcalf PA, Crouse JR, Sharrett AR, Tyroler HA, Barnes R, Heiss G. B-mode-detected carotid artery plaque in a general population. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25:2377–2383. [DOI] [PubMed] [Google Scholar]
- 19.Knopman DS, Griswold ME, Lirette ST, Gottesman RF, Kantarci K, Sharrett AR, Jack CR, Graff-Radford J, Schneider ALC, Windham BG, Coker LH, Albert MS, Mosley TH, ARIC Neurocognitive Investigators. Vascular Imaging Abnormalities and Cognition: Mediation by Cortical Volume in Nondemented Individuals: Atherosclerosis Risk in Communities-Neurocognitive Study. Stroke. 2015;46:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jack CR, Wiste HJ, Weigand SD, Therneau TM, Knopman DS, Lowe V, Vemuri P, Mielke MM, Roberts RO, Machulda MM, Senjem ML, Gunter JL, Rocca WA, Petersen RC. Age-specific and sex-specific prevalence of cerebral β-amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50–95 years: a cross-sectional study. Lancet Neurol. 2017;16:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarz CG, Gunter JL, Wiste HJ, Przybelski SA, Weigand SD, Ward CP, Senjem ML, Vemuri P, Murray ME, Dickson DW, Parisi JE, Kantarci K, Weiner MW, Petersen RC, Jack CR, Alzheimer’s Disease Neuroimaging Initiative. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer’s disease severity. Neuroimage Clin. 2016;11:802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raz L, Jayachandran M, Tosakulwong N, Lesnick TG, Wille SM, Murphy MC, Senjem ML, Gunter JL, Vemuri P, Jack CR, Miller VM, Kantarci K. Thrombogenic microvesicles and white matter hyperintensities in postmenopausal women. Neurology. 2013;80:911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, DeCarli C, de Leeuw F-E, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge R van, Pantoni L, Speck O, Stephan BCM, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantarci K, Weigand SD, Przybelski SA, Shiung MM, Whitwell JL, Negash S, Knopman DS, Boeve BF, O’Brien PC, Petersen RC, Jack CR. Risk of dementia in MCI: Combined effect of cerebrovascular disease, volumetric MRI, and 1H MRS. Neurology. 2009;72:1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graff-Radford J, Simino J, Kantarci K, Mosley TH, Griswold ME, Windham BG, Sharrett AR, Albert MS, Gottesman RF, Jack CR, Vemuri P, Knopman DS. Neuroimaging Correlates of Cerebral Microbleeds: The ARIC Study (Atherosclerosis Risk in Communities). Stroke. 2017;48:2964–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kantarci K, Gunter JL, Tosakulwong N, Weigand SD, Senjem MS, Petersen RC, Aisen PS, Jagust WJ, Weiner MW, Jack CR, Alzheimer’s Disease Neuroimaging Initiative. Focal hemosiderin deposits and β-amyloid load in the ADNI cohort. Alzheimers Dement. 2013;9:S116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volcik KA, Barkley RA, Hutchinson RG, Mosley TH, Heiss G, Sharrett AR, Ballantyne CM, Boerwinkle E. Apolipoprotein E polymorphisms predict low density lipoprotein cholesterol levels and carotid artery wall thickness but not incident coronary heart disease in 12,491 ARIC study participants. Am J Epidemiol. 2006;164:342–8. [DOI] [PubMed] [Google Scholar]
- 28.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, Beck TL, Aggarwal NT, Wilson RS, Evans DA, Mendes de Leon CF. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottesman RF, Rawlings AM, Sharrett AR, Albert M, Alonso A, Bandeen-Roche K, Coker LH, Coresh J, Couper DJ, Griswold ME, Heiss G, Knopman DS, Patel MD, Penman AD, Power MC, Selnes OA, Schneider ALC, Wagenknecht LE, Windham BG, Wruck LM, Mosley TH. Impact of Differential Attrition on the Association of Education With Cognitive Change Over 20 Years of Follow-up. Am J Epidemiol. 2014;179:956–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller M, Graaf Y van der, Algra A, Hendrikse J, Mali WP, Geerlings MI. Carotid atherosclerosis and progression of brain atrophy: The SMART-MR Study. Annals of Neurology. 2011;70:237–244. [DOI] [PubMed] [Google Scholar]
- 31.Sabayan B, van Buchem MA, Sigurdsson S, Zhang Q, Meirelles O, Harris TB, Gudnason V, Arai AE, Launer LJ. Cardiac and carotid markers link with accelerated brain atrophy: The AGES–Reykjavik Study. Arterioscler Thromb Vasc Biol. 2016;36:2246–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caughey MC, Qiao Y, Windham BG, Gottesman RF, Mosley TH, Wasserman BA. Carotid Intima-Media Thickness and Silent Brain Infarctions in a Biracial Cohort: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Hypertens. 2018;31:869–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero JR, Beiser A, Seshadri S, Benjamin EJ, Polak JF, Vasan RS, Au R, DeCarli C, Wolf PA. Carotid Artery Atherosclerosis, MRI Indices of Brain Ischemia, Aging, and Cognitive Impairment: The Framingham Study. Stroke. 2009;40:1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue K, Matsumoto M, Shono T, Toyokawa S, Moriki A. Increased Intima Media Thickness and Atherosclerotic Plaques in the Carotid Artery as Risk Factors for Silent Brain Infarcts. Journal of Stroke and Cerebrovascular Diseases. 2007;16:14–20. [DOI] [PubMed] [Google Scholar]
- 36.Pico F, Dufouil C, Lévy C, Besançon V, de Kersaint-Gilly A, Bonithon-Kopp C, Ducimetière P, Tzourio C, Alpérovitch A. Longitudinal study of carotid atherosclerosis and white matter hyperintensities: the EVA-MRI cohort. Cerebrovasc Dis. 2002;14:109–115. [DOI] [PubMed] [Google Scholar]
- 37.Della-Morte D, Dong C, Markert MS, Elkind MSV, Sacco RL, Wright CB, Rundek T. Carotid Intima-Media Thickness is Associated with White Matter Hyperintensities: The Northern Manhattan Study. Stroke. 2018;49:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero JR, Preis SR, Beiser A, DeCarli C, D’Agostino RB, Wolf PA, Vasan RS, Polak JF, Seshadri S. Carotid Atherosclerosis and Cerebral Microbleeds: The Framingham Heart Study. J Am Heart Assoc. 2016;5:e002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung C-P, Chou K-H, Chen W-T, Liu L-K, Lee W-J, Huang A-C, Chen L-K, Lin C-P, Wang P-N. Location of Cerebral Microbleeds And Their Association with Carotid Intima-media Thickness: A Community-based Study. Sci Rep. 2017;7:12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hollander M, Bots ML, Iglesias del Sol A., Koudstaal PJ, Witteman JCM, Grobbee DE, Hofman A, Breteler MMB Carotid Plaques Increase the Risk of Stroke and Subtypes of Cerebral Infarction in Asymptomatic Elderly. Circulation. 2002;105:2872–2877. [DOI] [PubMed] [Google Scholar]
- 41.D’Armiento FP, Bianchi A, de Nigris F, Capuzzi DM, D’Armiento MR, Crimi G, Abete P, Palinski W, Condorelli M, Napoli C. Age-related effects on atherogenesis and scavenger enzymes of intracranial and extracranial arteries in men without classic risk factors for atherosclerosis. Stroke. 2001;32:2472–2479. [DOI] [PubMed] [Google Scholar]
- 42.Finn AV, Kolodgie FD, Virmani R. Correlation between carotid intimal/medial thickness and atherosclerosis: a point of view from pathology. Arterioscler Thromb Vasc Biol. 2010;30:177–181. [DOI] [PubMed] [Google Scholar]
- 43.Wang J-G, Staessen JA, Li Y, Van Bortel LM, Nawrot T, Fagard R, Messerli FH, Safar M. Carotid Intima-Media Thickness and Antihypertensive Treatment: A Meta-Analysis of Randomized Controlled Trials. Stroke. 2006;37:1933–1940. [DOI] [PubMed] [Google Scholar]