Abstract
Purpose:
It is unclear whether time from radiation (RT) completion to durvalumab influences the outcomes of stage III non-small-cell lung cancer (NSCLC) treated with definitive chemoradiation and adjuvant durvalumab.
Methods and Materials:
Using the United States Veterans Health Administration database, we retrospectively identified 728 patients with stage III NSCLC treated with definitive chemoradiation who started durvalumab within 120 days of radiation completion. Time between the last radiation treatment and first durvalumab infusion was analyzed in multivariable Cox regression models for the primary outcomes of progression-free survival (PFS) and overall survival (OS), adjusting for baseline patient and disease characteristics. The primary analysis used a 120-day landmark, measuring OS and PFS from 120 days after radiation completion.
Results:
Among 728 patients, the median time from RT completion to durvalumab start was 41 days (interquartile range [IQR] 30–58). In multivariable Cox regression, time from RT completion to durvalumab start showed no association with PFS (adjusted hazard ratio [aHR] 1.01 per week, 95% confidence interval [CI] 0.98–1.04, p=0.4) or OS (aHR 1.02 per week, 95% CI 0.98–1.06, p=0.3). Starting durvalumab ≤ 14 days after RT was also not associated with improved PFS or OS. Results were robust in sensitivity analyses varying analytical technique.
Conclusion:
Timing of durvalumab initiation up to 120 days after RT completion is not associated with PFS or OS in this real-world patient cohort.
Keywords: NSCLC, durvalumab, immunotherapy, chemoradiation
Introduction
One year of adjuvant durvalumab became the standard-of-care for most patients with stage III non-small-cell lung cancer (NSCLC) treated with definitive chemoradiation after the publication of the PACIFIC results in 2017.1,2 Post-hoc subgroup analyses of PACIFIC suggested that patients randomized within 14 days of radiation (RT) completion may achieve a better response to durvalumab compared to patients randomized greater than 14 days after completion of RT.1–3 These results may cause some to favor earlier initiation of durvalumab after chemoradiation completion. Further, PACIFIC required randomization to durvalumab or placebo within 42 days of RT completion, and it is unclear whether delaying initiation beyond 42 days compromises outcomes. We sought to evaluate the effect of time from RT completion to durvalumab start in a large real-world sample of patients with stage III NSCLC treated with definitive chemoradiation.
Methods and Materials
Data source.
We identified lung cancer patients using the Department of Veterans Affairs Informatics and Computing Infrastructure (VINCI). VINCI is an informatics platform that facilitates access to patient-level electronic health record information and administrative data housed in the corporate data warehouse (CDW) for all veterans within the VA healthcare system. VINCI also incorporates tumor registry data uploaded from individual VA sites; these data are gathered by trained registrars. This study was approved by the local institutional review board.
Patient selection.
We included patients with histologically confirmed stage III NSCLC (AJCC 7th–8th edition) treated with definitive chemoradiation, who received at least one dose of adjuvant durvalumab between November 2017 to April 2021 (n=1025). We then included only patients with known radiation completion date (n=790) and who initiated durvalumab within 120 days of radiation completion (n=756). Adjuvant durvalumab infusion dates were identified by intravenous infusion records and manually confirmed by chart review. Radiation completion dates were determined through chart review and represent the day of the final radiation treatment. Staging and treatment data were supplemented with tumor registry data where available.
Outcomes.
The primary outcome measures were progression-free survival (PFS) and overall survival (OS). Date of radiographic progression was determined by manual review of radiological reports by a licensed physician (KS and MDG). Date of death was obtained from the VA Vital Status File (drawn from Medicare, Social Security Administration, and the internal VA death registry) and supplemented with the VA Master Patient Index for more recent deaths.
Baseline patient characteristics.
Demographics including race, sex, and age were obtained through the Master Patient Index. Charlson Comorbidity Index (CCI)4,5 was calculated from inpatient and outpatient ICD-10 diagnosis codes in the year before the radiation completion date. Smoking status was obtained through Health Factors data. Concurrent chemotherapy regimen was obtained through intravenous infusion records and supplemented with tumor registry data where available. Reasons for durvalumab discontinuation (classified as completion of planned therapy, progression, immune-related adverse event [irAE], or other) were obtained through manual review of physician notes. Patients were categorized as having durvalumab-related toxicity if the toxicity was possibly, probably, or definitely related to durvalumab in the judgement of the management outpatient oncologist or inpatient physician.
Statistical analysis.
Differences in baseline characteristics were assessed with the chi-square test for categorical variables and one-way ANOVA for continuous variables. OS and PFS estimates were generated with the Kaplan-Meier method and were compared between groups with the log-rank test in univariable analyses. Multivariable survival analyses were performed with Cox regression adjusting for time from RT completion to durvalumab start (continuous, per week), age (continuous, per 10 years), sex (male vs. female), race (African American, Caucasian, or other/unknown), smoking status (current, former, never, or unknown), CCI (0–2, 3–5, 6–8, or 9+), AJCC summary stage (IIIA, IIIB, IIIC, or III not otherwise specified), concurrent chemotherapy regimen (carboplatin-paclitaxel vs. other) and histology (adenocarcinoma, squamous cell carcinoma, or other).
As all durvalumab patients were required to have started durvalumab within 120 days of radiation completion, they were subject to up to 120 days of immortal time after the end of radiation. As such, the primary survival analyses used a 120-day landmark approach in which PFS and OS were measured from the date of radiation completion plus 120 days, and patients who died before this time point were excluded (n=28 of 756). Survival time was measured from the landmark time point to the date of death from any cause (for OS) or to disease progression or death from any cause (for PFS). Patients with missing progression data or who progressed before the landmark were excluded from the PFS analyses (n=46). Median follow-up was measured from the 120-day landmark. In sensitivity analyses, we performed Cox regression on the full cohort (n=756), treating time from RT completion to durvalumab start as a time-dependent covariate and measuring survival from the time of RT completion. In further sensitivity analyses we repeated the primary analysis using a grouping of early (1–14 days), standard (15–42 days), or delayed (43–120 days) durvalumab initiation. In all analyses, patients were censored at the date of last known follow-up, defined as the most recent encounter with a VA provider. Patients with ongoing follow-up past April 15, 2021 were administratively censored on that date.
Results
The primary cohort included 728 patients with stage III NSCLC who started durvalumab within 120 days of RT completion and did not die before the 120-day landmark. The median time from RT completion to durvalumab start was 41 days (interquartile range [IQR] 30–58; Supplemental Figure 1). Patients who started durvalumab 0–29 days, 30–59 days, 60–89 days, and 90–120 days from RT completion were broadly similar in baseline comorbidity and disease characteristics (Table 1). Baseline clinical characteristics and treatment tolerance were similar among patients who started durvalumab 1–14 days, 15–42 days, and 43–120 days after RT completion (Supplemental Table 1).
Table 1.
Characteristics of the sample.
| Time from RT end to durvalumab start (days) | |||||
|---|---|---|---|---|---|
| Characteristic | 0–29, N = 1821 | 30–59, N = 3691 | 60–89, N = 1151 | 90–120, N = 621 | p-value2 |
| Age (years) | 69 (63, 72) | 69 (64, 72) | 71 (66, 74) | 68 (61, 72) | 0.042 |
| Time from RT end to durva start (days) | 22 (16, 27) | 41 (35, 49) | 72 (68, 80) | 102 (96, 106) | <0.001 |
| Median follow-up time (months) | 16 (9, 23) | 18 (11, 25) | 18 (10, 28) | 21 (11, 29) | 0.042 |
| Race | 0.7 | ||||
| Black | 44 (24%) | 81 (22%) | 29 (25%) | 13 (21%) | |
| Other/Unknown | 12 (6.6%) | 13 (3.5%) | 5 (4.3%) | 2 (3.2%) | |
| White | 126 (69%) | 275 (75%) | 81 (70%) | 47 (76%) | |
| Male | 171 (94%) | 354 (96%) | 111 (97%) | 60 (97%) | 0.6 |
| CCI | 0.075 | ||||
| 0–2 | 29 (16%) | 66 (18%) | 23 (20%) | 20 (32%) | |
| 3–5 | 54 (30%) | 120 (33%) | 33 (29%) | 24 (39%) | |
| 6–8 | 28 (15%) | 48 (13%) | 17 (15%) | 3 (4.8%) | |
| 9+ | 71 (39%) | 135 (37%) | 42 (37%) | 15 (24%) | |
| Smoking | 0.018 | ||||
| Current | 86 (47%) | 169 (46%) | 41 (36%) | 30 (48%) | |
| Former | 66 (36%) | 135 (37%) | 43 (37%) | 16 (26%) | |
| Never | 15 (8.2%) | 27 (7.3%) | 18 (16%) | 3 (4.8%) | |
| Unknown | 15 (8.2%) | 38 (10%) | 13 (11%) | 13 (21%) | |
| Summary stage | 0.5 | ||||
| 3A | 109 (60%) | 198 (54%) | 58 (50%) | 38 (61%) | |
| 3B | 57 (31%) | 135 (37%) | 49 (43%) | 20 (32%) | |
| 3C | 13 (7.1%) | 30 (8.1%) | 5 (4.3%) | 4 (6.5%) | |
| 3 NOS | 3 (1.6%) | 6 (1.6%) | 3 (2.6%) | 0 (0%) | |
| Carboplatin/paclitaxel chemotherapy | 129 (71%) | 270 (73%) | 76 (66%) | 44 (71%) | 0.5 |
| Histology | 0.7 | ||||
| Adenocarcinoma | 86 (47%) | 178 (48%) | 49 (43%) | 31 (50%) | |
| Other | 4 (2.2%) | 10 (2.7%) | 6 (5.2%) | 1 (1.6%) | |
| Squamous cell carcinoma | 92 (51%) | 181 (49%) | 60 (52%) | 30 (48%) | |
| Reason for durvalumab discontinuation | 0.039 | ||||
| Completed | 57 (31%) | 110 (30%) | 30 (26%) | 18 (29%) | |
| IrAE | 38 (21%) | 53 (14%) | 18 (16%) | 5 (8.1%) | |
| Ongoing | 29 (16%) | 50 (14%) | 14 (12%) | 3 (4.8%) | |
| Other | 26 (14%) | 72 (20%) | 19 (17%) | 17 (27%) | |
| Progression | 32 (18%) | 84 (23%) | 34 (30%) | 19 (31%) | |
| IrAE causing durvalumab discontinuation | 0.7 | ||||
| Arthritis | 2 (5.3%) | 1 (1.9%) | 2 (11%) | 0 (0%) | |
| Colitis | 1 (2.6%) | 5 (9.4%) | 2 (11%) | 0 (0%) | |
| Other | 4 (11%) | 8 (15%) | 2 (11%) | 1 (20%) | |
| Pneumonitis | 31 (82%) | 39 (74%) | 12 (67%) | 4 (80%) | |
Median (IQR); n (%)
One-way ANOVA; Pearson’s Chi-squared test
CCI: Charlson comorbidity index; NOS: not otherwise specified; IrAE: Immune-related adverse event; RT: radiation therapy; durva: durvalumab
Median follow-up for the sample was 17.6 months. Unadjusted survival analyses showed no significant differences in PFS (p=0.26) or OS (p=0.29) among patients who started durvalumab 0–29, 30–59, 60–89, and 90–120 days after RT (Figure 1). In multivariable Cox regression, time from RT completion to durvalumab start showed no association with PFS (adjusted hazard ratio [aHR] 1.01 per week, 95% confidence interval [CI] 0.98–1.04, p=0.4) or OS (aHR 1.02 per week, 95% CI 0.98–1.06, p=0.3; Table 2). Similar results were found when treating time from RT completion to durvalumab start as a categorical variable (Supplemental Table 2) and in the time-dependent Cox regressions for PFS (aHR 1.00 per week, 95% CI 0.97–1.03, p>0.9) and OS (aHR 1.00 per week, 95% CI 0.96–1.03, p=0.8; Supplemental Table 3). Starting durvalumab ≤ 14 days or 43–120 days after RT showed no association with PFS or OS relative to 15–42 days (Supplementary Table 4).
Figure 1.
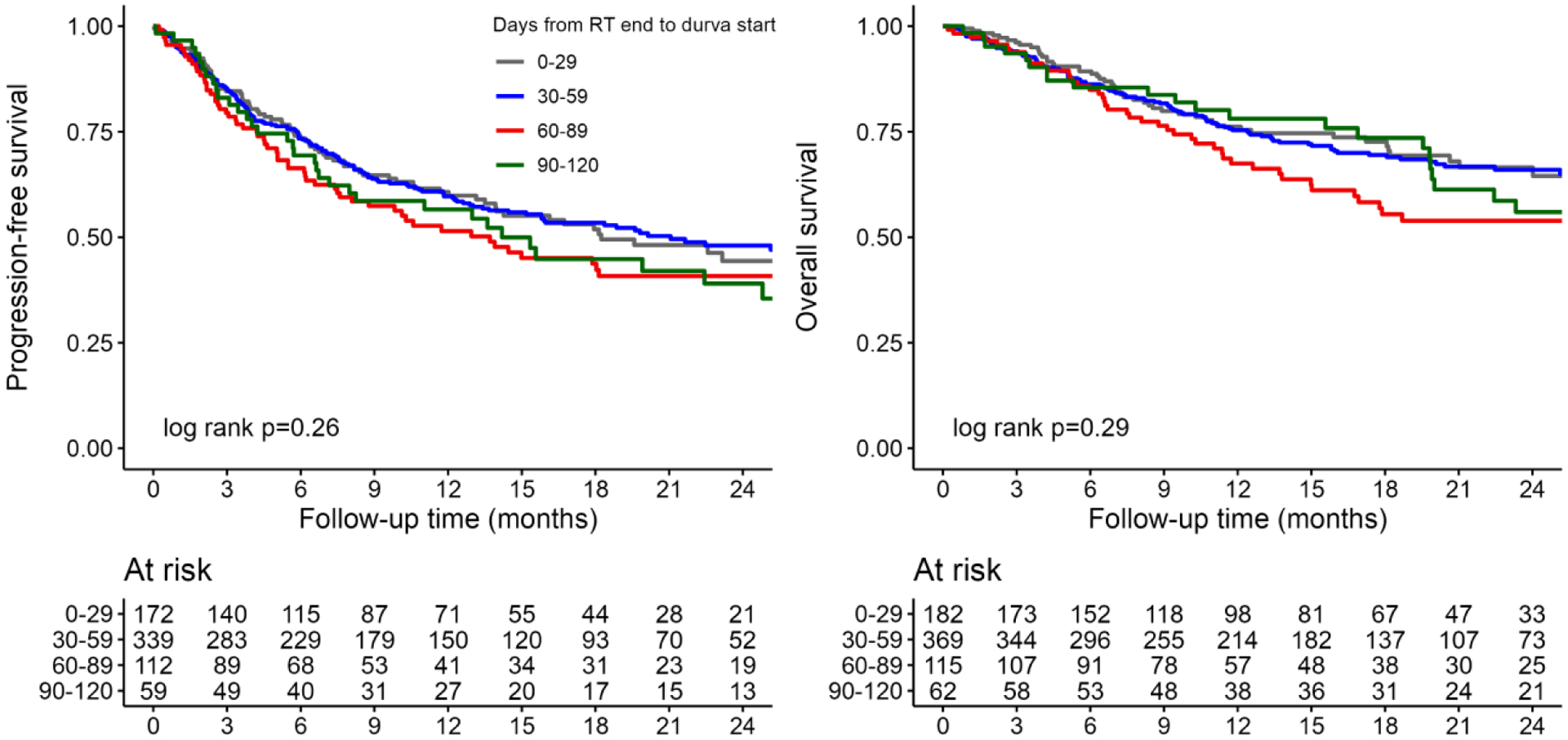
Kaplan-Meier estimates of progression-free (A) and overall survival (B). Time is measured from the 120-day landmark after radiation completion and is reported in months. Durva: durvalumab; RT: radiation therapy.
Table 2.
Multivariable Cox regression for the effect of durvalumab timing on progression-free and overall survival (120 day landmark).
| Progression-free survival | Overall survival | |||||
|---|---|---|---|---|---|---|
| Characteristic | HR1 | 95% CI1 | p-value | HR1 | 95% CI1 | p-value |
| Time from RT end to durvalumab start (per week) | 1.01 | 0.98, 1.04 | 0.4 | 1.02 | 0.98, 1.06 | 0.3 |
| Age (per 10 years) | 1.14 | 0.96, 1.35 | 0.15 | 1.38 | 1.12, 1.70 | 0.002 |
| Histology | ||||||
| Adenocarcinoma | — | — | — | — | ||
| Other | 0.57 | 0.25, 1.27 | 0.2 | 0.58 | 0.22, 1.53 | 0.3 |
| Squamous cell carcinoma | 0.70 | 0.55, 0.88 | 0.002 | 0.77 | 0.59, 1.01 | 0.059 |
| CCI | ||||||
| 0–2 | — | — | — | — | ||
| 3–5 | 0.79 | 0.57, 1.11 | 0.2 | 0.76 | 0.52, 1.12 | 0.2 |
| 6–8 | 1.11 | 0.75, 1.63 | 0.6 | 1.02 | 0.65, 1.61 | >0.9 |
| 9+ | 0.72 | 0.51, 1.00 | 0.050 | 0.71 | 0.49, 1.05 | 0.085 |
| Summary stage | ||||||
| 3A | — | — | — | — | ||
| 3B | 1.27 | 1.00, 1.60 | 0.049 | 1.55 | 1.18, 2.04 | 0.002 |
| 3C | 1.67 | 1.10, 2.54 | 0.016 | 1.50 | 0.91, 2.47 | 0.11 |
| 3 NOS | 0.72 | 0.22, 2.39 | 0.6 | 1.32 | 0.38, 4.51 | 0.7 |
| Male | 1.55 | 0.79, 3.06 | 0.2 | 1.33 | 0.61, 2.86 | 0.5 |
| Smoking | ||||||
| Current | — | — | — | — | ||
| Former | 0.89 | 0.69, 1.16 | 0.4 | 0.89 | 0.66, 1.21 | 0.5 |
| Never | 1.31 | 0.88, 1.94 | 0.2 | 1.19 | 0.77, 1.84 | 0.4 |
| Unknown | 0.97 | 0.65, 1.45 | 0.9 | 0.82 | 0.51, 1.31 | 0.4 |
| Race | ||||||
| Black | — | — | — | — | ||
| Other/Unknown | 1.42 | 0.83, 2.43 | 0.2 | 1.62 | 0.87, 3.02 | 0.13 |
| White | 1.09 | 0.83, 1.44 | 0.5 | 1.15 | 0.83, 1.60 | 0.4 |
| Carboplatin/paclitaxel chemotherapy | 1.21 | 0.93, 1.57 | 0.2 | 1.06 | 0.78, 1.43 | 0.7 |
HR = Hazard Ratio, CI = Confidence Interval
CCI: Charlson comorbidity index; NOS: not otherwise specified
Discussion
In this study of over 700 patients with stage III NSCLC treated with definitive chemoradiation and adjuvant durvalumab, we found no evidence of improved oncologic outcomes with earlier initiation of durvalumab after RT completion, and delay of durvalumab start up to 120 days after RT completion did not appear to compromise outcomes. These results were robust to multiple analytic approaches.
PACIFIC initially required randomization to durvalumab or placebo within 14 days after the last radiation treatment, but this was later amended to within 42 days. Exploratory post-hoc analyses suggested that durvalumab may be more effective if started within 14 days of radiation completion as reflected in differing estimates of durvalumab efficacy by time from RT completion to randomization for PFS (<14 days: HR 0.39, 95% CI 0.26–0.58; ≥ 14 days: HR 0.63, 95% CI 0.49–0.80)3 and OS (<14 days: HR 0.42, 95% CI 0.27–0.67; ≥ 14 days: HR 0.81, 95% CI 0.62–1.06).2 These findings may lead clinicians to favor earlier initiation of durvalumab if possible, though concerns remain regarding tolerability of earlier immunotherapy initiation.
In contrast, we found no evidence of improved oncologic outcomes with earlier initiation of durvalumab, suggesting the PACIFIC subgroup results do not replicate in this real-world cohort. Several factors are relevant to this discrepancy. First is the well-documented risk of false-positive findings from post-hoc subgroup analyses.6,7 The subgroup difference in OS in PACIFIC appeared to be driven both by a higher crude event rate in the placebo arm if randomized within <14 days (57% with event vs. 46% for ≥14 days) as well as a somewhat lower event rate in the durvalumab arm (33% vs. 40%);2 the difference in crude event rates in the placebo arm is difficult to explain biologically and raises the possibility of a chance false-positive finding. Further, unmeasured differences in baseline characteristics between the PACIFIC and Veteran populations may have introduced additional confounding that obscured a link between earlier initiation and improved survival. Our data suggest that clinicians may delay durvalumab initiation beyond the 42-day window in PACIFIC without concern for compromised cancer control. This may be particularly important for patients with significant toxicities from concurrent chemoradiation who may need additional recovery time before durvalumab initiation. Limitations of the present study include the retrospective design, lack of a randomized control group, and limited number of patients starting durvalumab ≤ 14 days after RT completion which constrains statistical power in this subgroup. Compared to PACIFIC, our cohort has limited follow-up to assess long-term differences in OS. Additional follow-up will be needed to ensure no differences in OS emerge.
In summary, in this large, nationwide retrospective cohort study we identified no association between time from RT completion to durvalumab start and PFS or OS up to 120 days after RT completion. This suggests that clinicians may safely allow for prolonged recovery after chemoradiation before starting durvalumab.
Supplementary Material
Funding statement:
Lung Precision Oncology Program, VA Ann Arbor Healthcare System.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data sharing statement: Research data are not available at this time.
Conflicts of interest statement: G.W.S. serves an uncompensated position on the Board of Directors for the Optimal Cancer Care Alliance. A.K.B, K.S., L.Z., D.E., M.D.G. and N.R. do not have any conflicts of interest.
References
- 1.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377(20):1919–29. [DOI] [PubMed] [Google Scholar]
- 2.Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379(24):2342–50. [DOI] [PubMed] [Google Scholar]
- 3.Faivre-Finn C, Spigel DR, Senan S, et al. Efficacy and safety evaluation based on time from completion of radiotherapy to randomization with durvalumab or placebo in pts from PACIFIC. Ann Oncol 2018;29 Suppl 8(suppl_8):viii488. [Google Scholar]
- 4.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43(11):1130–9. [DOI] [PubMed] [Google Scholar]
- 5.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 6.Zhang S, Liang F, Li W, Hu X. Subgroup Analyses in Reporting of Phase III Clinical Trials in Solid Tumors. J Clin Oncol 2015;33(15):1697–702. [DOI] [PubMed] [Google Scholar]
- 7.Burke JF, Sussman JB, Kent DM, Hayward RA. Three simple rules to ensure reasonably credible subgroup analyses. BMJ 2015;351:h5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


