Abstract
Background:
Heart failure with preserved ejection fraction (HFpEF) is the fastest growing form of HF and is associated with high morbidity and mortality. The primary chronic symptom in HFpEF is exercise intolerance, associated with reduced quality of life (QoL). Emerging evidence implicates left atrial (LA) dysfunction as an important pathophysiologic mechanism. Here we extend prior observations by relating LA dysfunction to peak oxygen uptake (peak VO2), physical function (distance walked in six minutes, 6MWD) and QoL (Kansas City Cardiomyopathy Questionnaire, KCCQ).
Methods:
We compared 75 older, obese, HFpEF patients to 53 healthy age-matched controls. LA strain was assessed by magnetic resonance cine imaging using feature tracking. LA function was defined according to its three distinct phases, with the LA serving as a reservoir during systole, as a conduit during early diastole, and as a booster pump at the end of diastole. LA stiffness index was calculated as the ratio of early mitral inflow velocity-to-early annular tissue velocity (E/e’, by Doppler ultrasound) and LA reservoir strain.
Results:
HFpEF had decreased reservoir strain (16.4±4.4% vs. 18.2±3.5%, p=0.018), lower conduit strain (7.7±3.3% vs. 9.1±3.4%, p=0.028), and increased stiffness index (0.86±0.39 vs. 0.53±0.18, p<0.001), as well as decreased peak VO2, 6MWD, and lower QoL. Increased LA stiffness was independently associated with impaired peak VO2 (β=9.0±1.6, p<0.001), 6MWD (β=117±22, p=0.003), and KCCQ score (β=−23±5, p=0.001), even after adjusting for clinical covariates.
Conclusion:
LA stiffness is independently associated with impaired exercise tolerance and QoL and may be an important therapeutic target in obese HFpEF.
Registration:
Keywords: Left atrial strain, heart failure with preserved ejection fraction, left atrial stiffness, functional capacity, exercise intolerance
INTRODUCTION
Heart failure (HF) with preserved ejection fraction (HFpEF) is the fastest growing form of HF and is associated with high morbidity and mortality.1, 2 The primary chronic symptom in HFpEF is exercise intolerance, manifested as severe exertional dyspnea and fatigue, and measured objectively as decreased peak oxygen uptake (peak VO2).3–8 However, the physiological mechanisms underpinning the decreased peak VO2 in HFpEF patients remain incompletely understood.
Emerging evidence implicates left atrial (LA) dysfunction as an important pathophysiologic mechanism driving exercise intolerance in HFpEF, with impaired LA reservoir and pump function, and increased LA stiffness, associated with abnormal exercise hemodynamics and peak oxygen uptake.9–12 While highly informative, prior investigations did not examine associations between LA function and measures of physical function (i.e. six minute walk distance, [6MWD]) or quality of life (QOL), and focused on patients who had modestly elevated body mass index (BMI). Excess adipose tissue is, however, a key driver of HFpEF, with many deleterious consequences that adversely impact cardiac, vascular, and skeletal musclefunction.13–18 Indeed, >80% of HFpEF patients are overweight/obese.19, 20
Accordingly, we conducted an analysis leveraging an existing database of cardiac magnetic resonance (CMRI) images and transthoracic echocardiography, collected in a well-phenotyped cohort of older, obese, HFpEF patients and healthy age-matched controls, to assess LA function. We hypothesized that measures of LA mechanical function (strain and stiffness) would be impaired in older, obese HFpEF compared to healthy age-match controls, and related to peak VO2, 6MWD, and quality of life.
METHODS
Study population
The design and conduct of the Study of the effect of Caloric Restriction and Exercise Training in Patients with Heart Failure and a Normal Ejection Fraction (SECRET) has been previously described (NCT00959660).3 Briefly, inclusion criteria were: age ≥60 years, left ventricular (LV) ejection fraction ≥50%, obesity as defined by a body mass index ≥30 kg/m2, and signs and symptoms of HF as assessed by an HF clinical score ≥3 on the National Health and Nutrition Examination Survey.21 Exclusion criteria were: contraindications to CMRI, creatinine ≥2.5 mg/dL, and other significant disease that could explain the patients’ symptoms, including significant ischemic or valvular heart disease, uncontrolled hypertension, and significant anemia.6, 22, 23 Healthy age-matched controls had no medical complaints or chronic medical conditions, took no medications, and had normal screening tests, including electrocardiogram, echocardiogram (with normal LV filling pattern), and cardiopulmonary exercise testing.6, 22, 24 All study participants provided written informed consent at the time of enrollment. The study protocol was approved by the Wake Forest University Health Sciences Institutional Review Board.
Echocardiography
Transthoracic echocardiography was performed with all participants resting in a semi-recumbent position for at least 15 minutes. Doppler ultrasound was used to assess LV filling patterns, mitral septal annular velocity, and pulse-wave velocity in accordance with the American Society of Echocardiography recommendations,24 as previously described.3 All Doppler values represent the average of three cardiac cycles. The ratio of early mitral filling velocity-to-early diastolic annular tissue velocity (E/e’) was calculated as a surrogate measure of LV filling pressures.24 LA diameter was measured at the widest region of the LA in the 4-chamber view in end-diastole.
Cardiac magnetic resonance imaging
For assessment of cardiac morphology and function, cine steady-state free precession images were acquired using a 1.5T Siemens Avanto scanner, using electrocardiogram-gating and a phased array surface coil (Siemens Healthineers). Typical imaging parameters included: flip angle 76°, repetition/echo time: 40–50/1.1–1.2 ms, slice thickness: 7–8 mm, with 25 cardiac phases. LV mass and volumes were assessed from a series of multi-slice, multi-phase gradient-echo sequences positioned perpendicular to the LV long axis, spanning the apex to base, as previously described.3, 25 LV longitudinal strain and LA strain were assessed from a single long axis 4-chamber image, using commercially available feature tracking software (CVI42 V5.3.0, Circle Cardiovacsular Imaging Inc.; Calgary, AB, Canada), as previously described by our group.25, 26 Briefly, the endocardial and epicardial borders of the LV and LA were manually delineated at LV end-diastole and end-systole, respectively. The feature tracking algorithm was then applied across the remainder of the cardiac phases. For LA strain, the cardiac cycle was divided into three distinct phases: (1) the reservoir phase, when the LA is passively filled by pulmonary venous flux; (2) the conduit phase, when there is passive filling of the LV along the transmitral pressure gradient; and (3) the booster phase, which represents LA systole, during which the LV is actively filled. LA strain rates were calculated as the time derivative of LA deformation across the cardiac cycle. Single-point LA stiffness index was defined by the ratio between Doppler-derived E/e’ and peak LA reservoir strain, such that higher LA stiffness index reflects a higher E/e’-to-LA reservoir strain ratio.10, 27–30
Measures of physical function and quality of life
Cardiopulmonary exercise testing was performed on a treadmill using either the Naughton protocol or the modified Bruce protocol, depending on the participants’ self-reported exercise tolerance, as previously described.3, 31 Participants were instructed to exercise to volitional fatigue during a symptom-limited exhaustive test with continuous metabolic gas exchange monitoring (Medgraphics Ultima, Medical Graphics Corp., St. Paul, Minnesota).3, 22, 31 Peak aerobic oxygen consumption (VO2) was determined from the average oxygen consumption during the last thirty seconds of peak exercise. Six-minute walk distance (6MWD) was measured according to guideline recommendations.32 Quality of life was assessed using the Kansas City Cardiomyopathy Questionnaire (KCCQ).33
Statistical analyses
Characteristics of study participants were compared between those with HFpEF and the healthy controls. Continuous variables are reported as mean ± standard deviation and compared using two-sided t-tests. Categorical variables are reported as frequency and percentages and compared using chi-square tests. All variables were tested for normality with histograms and quantile-quantile plots. LA measures were compared using analysis of covariance with age, sex, BMI, and race as covariates due to differences between HFpEF and healthy control groups. Bivariate associations between LA measures and functional status were initially explored in an unadjusted general linear regression. To control for potential confounders, subsequent multivariate models adjusted for age, sex, BMI, and race. Finally, separate multivariate linear regressions were used to identify LA measures that were significant predictors of physical function and quality of life outcomes, all including conventional factors of age, sex, BMI, and LV mass. Two-sided p-values below 0.05 were considered statistically significant. All statistical analyses were conducted at Wake Forest School of Medicine using SAS version 9.4 (Cary, NC).
RESULTS
Patient characteristics
Of the 100 participants with HFpEF included in the parent trial,3 nineteen did not undergo cardiac MRI due claustrophobia (n=11), stent/pacemaker contraindication (n=3), or scanner related weight/circumference limitations (n=5). Of the 81 MRI’s that were completed, data from 6 participants were excluded due to poor image/data quality. Compared to controls, study participants with HFpEF were predominantly female, less frequently of white race, and had higher weight and BMI (Table 1). Heart failure symptoms were consistent with New York Heart Association functional class II and III. Participants with HFpEF also had higher LV mass, relative wall thickness, diastolic dysfunction on echocardiogram, and larger LA diameter. Finally, peak VO2, 6MWD, and quality of life were significantly reduced in HFpEF compared to controls.
Table 1:
Baseline Characteristics of HFpEF and Healthy Control Groups
| Characteristic | HFpEF (n=75) | Healthy Controls (n=53) | p-value |
|---|---|---|---|
| Participant Characteristics | |||
| Age (years) | 67± 5 | 69 ± 7 | 0.042 |
| Women n (%) | 65 (87%) | 32 (60%) | 0.003 |
| White n (%) | 40 (53%) | 50 (94%) | <0.001 |
| Body Weight (kg) | 101.5 ± 15.1 | 74.1 ± 14.8 | <0.001 |
| BSA (m2) | 2.0 ± 0.2 | 1.8 ± 0.2 | <0.001 |
| BMI (kg/m2) | 38.5 ± 5.0 | 25.9 ± 4.6 | <0.001 |
| NYHA class II | 48 (65%) | n/a | |
| NYHA class III | 26 (35%) | n/a | |
| Systolic BP (mmHg) | 134 ± 14 | 124 ± 11 | <0.001 |
| Diastolic BP (mmHg) | 77 ± 8 | 75 ± 6 | 0.14 |
| Echocardiogram Measures | |||
| Ejection fraction (%) | 61 ± 6 | 59 ± 5 | 0.041 |
| Relative wall thickness (mm) | 0.56 ± 0.11 | 0.39 ± 0.05 | <0.001 |
| Diastolic filling pattern | |||
| Normal n (%) | 0 (0%) | 53 (100%) | <0.001 |
| Impaired Relaxation n (%) | 67 (89%) | 0 (0%) | <0.001 |
| Pseudonormal n (%) | 8 (13%) | 0 (0%) | 0.018 |
| Restrictive n (%) | 0 (0%) | 0 (0%) | n/a |
| E/e’ ratio | 12.9 ± 3.7 | 9.2 ± 1.9 | <0.001 |
| LA diameter (cm) | 4.0 ± 0.5 | 3.4 ± 0.5 | <0.001 |
| CMRI Measures | |||
| LV EDV (mL) | 117.3±31.3 | 113.9±25.4 | 0.70 |
| LV EDV index (mL/m2) | 57.3±14.1 | 61.7±11.6 | 0.044 |
| LV Mass (g) | 96.3±20.3 | 93.8±18.7 | 0.51 |
| LV Mass index (g/m2) | 43.2±9.8 | 44.0±7.4 | 0.53 |
| LV Mass/Volume (g/mL) | 0.78±0.17 | 0.74±0.19 | 0.25 |
| Medical History | |||
| History of atrial fibrillation n (%) | 1 (<1%) | n/a | --- |
| History of diabetes mellitus n (%) | 26 (35%) | n/a | --- |
| History of hypertension n (%) | 71 (96%) | n/a | --- |
| Current medications | |||
| ACE-inhibitors n (%) | 26 (35%) | n/a | --- |
| Diuretics n (%) | 54 (73%) | n/a | --- |
| Beta-blockers n (%) | 30 (41%) | n/a | --- |
| Calcium Antagonists n (%) | 24 (32%) | n/a | --- |
| Nitrates n (%) | 6 (8%) | n/a | --- |
| ARBs n (%) | 25 (34%) | n/a | --- |
| Physical Function | |||
| Peak VO2 (ml/kg/min) | 14.7 ± 2.5 | 25.3 ± 7.1 | <0.001 |
| Peak VO2 (ml/min) | 1479 ± 309 | 1865 ± 605 | <0.001 |
| Exercise Workload (METs) | 4.9 ± 1.1 | 11.1 ± 3.1 | <0.001 |
| 6-minute walk distance (meters) | 420 ± 64 | 563 ± 71 | <0.001 |
| Quality of Life | |||
| KCCQ Score | 63 ± 15 | 98 ± 2 | <0.001 |
Values shown as means ± standard deviation or frequency (%). Abbreviations: ESA, body surface area; body mass index; NYHA, New York Heart Association; LV, left ventricle; E, E-wave velocity; e’, early mitral annulus velocity (septal); EP, blood pressure; ACE, angiotensin-converting enzyme; ARE angiotensin receptor blocker; n/a, not applicable.
Left Atrial Function
As illustrated in Figure 1, compared to controls, HFpEF participants had significantly lower LA reservoir strain and strain rate, lower LA conduit strain and strain rate, and higher LA stiffness index (0.86±0.39 vs. 0.53±0.18, P<0.001); all of which, except for LA reservoir strain rate (P = 0.09), remained significant after controlling for age, sex, BMI and ethnicity (Table 2). Differences also remained after adjusting LA reservoir and conduit strain for additional LV variables known to influence LA function, such as LV longitudinal strain (Table 2). In contrast, neither LA booster strain nor LA booster strain rate were different between HFpEF and controls.
FIGURE 1.
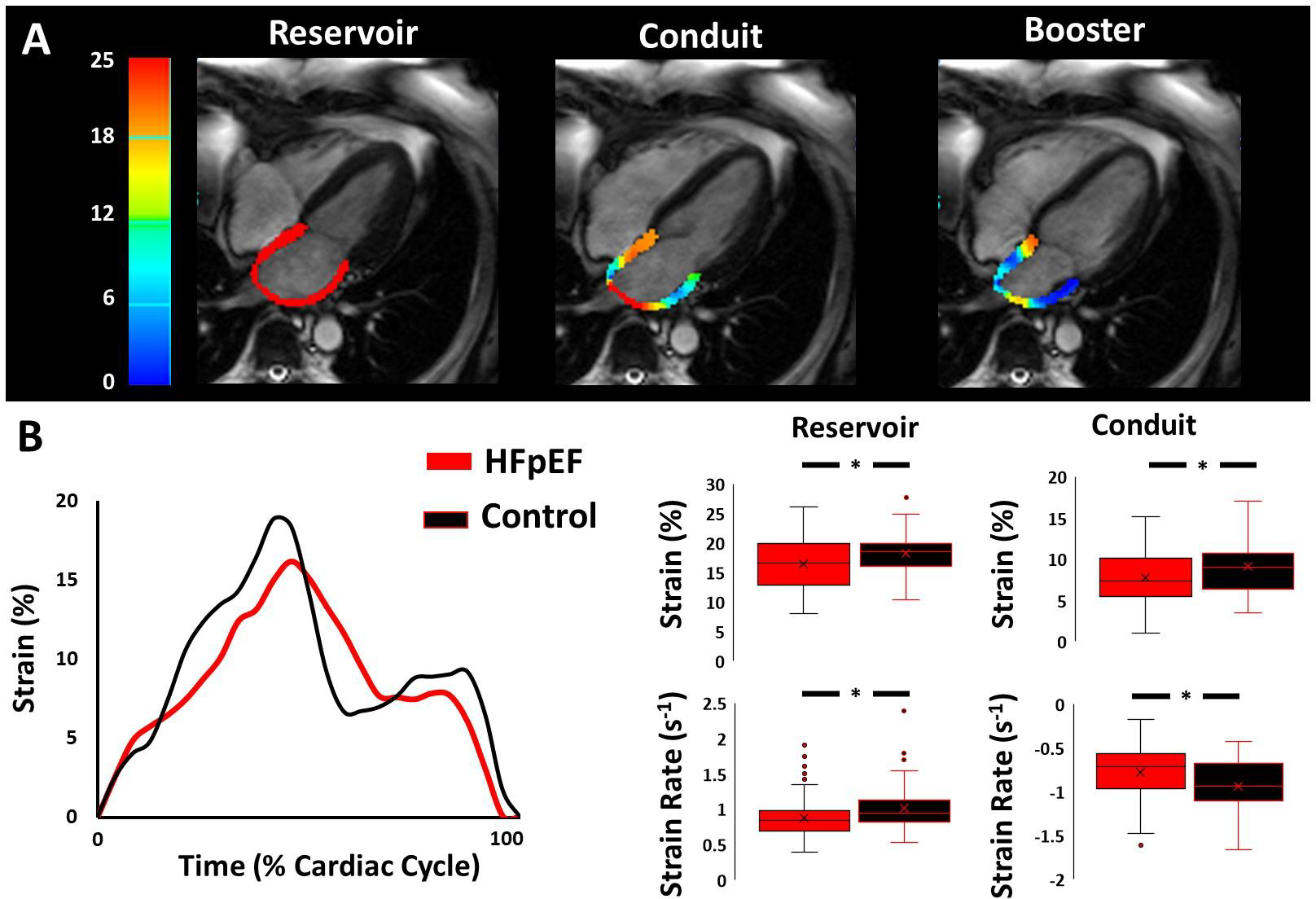
Impaired left atrial function in HFpEF compared to controls. (A) High resolution long-axis magnetic resonance cine images illustrating left atrial strain color maps across each phase of the cardiac cycle. (B) Strain curves from a representative control participant (black line) and HFpEF participant (red line). Note that peak reservoir strain is reduced in the HFpEF participant compared to the control participant, together with reduced reservoir and conduit strain rates. (C) Summary data (unadjusted) showing key group differences in left atrial mechanics between HFpEF (red bars) and controls (black bars).
Table 2:
Left atrial strain and strain rate with adjustments for left ventricular function
| Mean ± SD | Adjusted Mean ± SE | |||||
|---|---|---|---|---|---|---|
| HFpEF | Healthy Controls | p-value | HFpEF | Healthy Controls | p-value | |
| LA reservoir strain (%) | 16.4±4.4 | 18.2±3.5 | 0.018 | 16.0±0.6 | 18.8±0.8 | 0.025 |
| LA reservoir strain rate (s−1) | 0.88±0.30 | 1.01±0.35 | 0.022 | 0.86±0.05 | 1.03±0.06 | 0.09 |
| LA conduit strain (%) | 7.7±3.3 | 9.1±3.4 | 0.028 | 7.4±0.5 | 9.5±0.6 | 0.024 |
| LA conduit strain rate (s−1) | −0.77±0.32 | −0.92±0.30 | 0.010 | −0.75±0.05 | −0.95±0.06 | 0.031 |
| LA booster strain (%) | 8.7±3.2 | 9.1±3.3 | 0.50 | 8.6±0.5 | 9.3±0.6 | 0.52 |
| LA booster strain rate (s−1) | −1.15±0.46 | −1.14±0.50 | 0.88 | −1.12±0.07 | −1.19±0.10 | 0.67 |
| LA Stiffness Index (a.u.) | 0.86±0.39 | 0.53±0.18 | <0.001 | 0.86±0.05 | 0.53±0.07 | 0.001 |
| Select LA Strain Metrics Indexed to LV function | ||||||
| LA reservoir Strain/ LV Longitudinal strain | −0.84±0.25 | −1.02±0.31 | <0.001 | −0.84±0.04 | −1.02±0.06 | 0.034 |
| Peak LA reservoir strain rate/Peak LV longitudinal strain rate | −0.05±0.02 | −0.06±0.03 | 0.009 | −0.05±0.01 | 0.06±0.01 | 0.13 |
| LA reservoir strain/E/e’ | 1.37±0.52 | 2.08±0.66 | <0.001 | 1.42±0.08 | 2.01±0.12 | <0.001 |
| LA Stiffness Index/LV Longitudinal Strain | −0.05±0.03 | −0.03±0.01 | <0.001 | −0.05±0.01 | 0.03±0.01 | 0.018 |
| LA conduit strain/E/e’ | 0.65±0.33 | 1.02±0.41 | <0.001 | 0.66±0.05 | 1.00±0.07 | 0.001 |
| Peak LA conduit strain rate/E/e’ | −0.07±0.03 | −0.10±0.04 | <0.001 | −0.07±0.01 | −0.10±0.01 | 0.002 |
| LA conduit strain/eCSRd | 8.22±3.97 | 8.19±2.98 | 0.96 | 7.96±0.56 | 8.59±0.77 | 0.59 |
| Peak LA Conduit Strain Rate/eCRSd | −0.83±0.36 | −0.86±0.38 | 0.71 | −0.83±0.06 | −0.88±0.08 | 0.68 |
| LA conduit strain/eLSRd | 9.53±6.65 | 10.19±3.40 | 0.53 | 9.32±0.85 | 10.53±1.21 | 0.50 |
| Peak LA Conduit Strain Rate/eLRSd | −0.94±0.70 | −1.04±0.29 | 0.35 | −0.92±0.09 | −1.09±0.12 | 0.35 |
Values shown as mean±SD or LSmeans±SE adjusted for age, sex, BMI, and race as indicated. Abbreviations: LV: left ventricle; LA: left atrium. LA Stiffness index calculated as E/e’/LA reservoir strain;. eCSRd, early diastolic circumferential strain rate; eLSRd, early diastolic longitudinal strain rate.
Relationships Between Left Atrial Function, Functional Capacity and Quality of Life
Our group has previously reported on the significant relationship between peak VO2 and E/e’ in this patient cohort.25 Here, we extend these prior observations, by also showing significant relationships between peak VO2 and LA reservoir strain and strain rate, LA conduit strain rate, and LA stiffness index (Table 3). In addition, lower 6MWD was associated with higher E/e’, lower LA reservoir strain and strain rate, lower LA conduit strain and strain rate, and higher LA stiffness index. Moreover, a lower KCCQ score was associated with higher E/e’ and LA stiffness index. Importantly, many of these relationships remained after adjusting for age, sex, BMI, and race (Table 3). Further adjustment for LA size did not make a major difference.
Table 3:
Associations of Cardiac Measures with Functional Capacity and QoL
| Peak VO2 | 6 Minute Walk Distance | KCCQ Score | ||||
|---|---|---|---|---|---|---|
| Parameter Estimate | p-value | Parameter Estimate | p-value | Parameter Estimate | p-value | |
| E/e’ | −1.0±0.2 | <0.001 | −13±2 | <0.001 | −3±0.5 | <0.001 |
| LA reservoir strain | 0.4±0.2 | 0.015 | 5±2 | 0.015 | 1±0.5 | 0.13 |
| LA reservoir strain rate | 6.3±1.9 | 0.001 | 86±26 | 0.001 | 7±6.5 | 0.28 |
| LA conduit strain | 0.3±0.2 | 0.09 | 6±2 | 0.016 | 0.7±0.6 | 0.28 |
| LA conduit strain rate | −4.2±2.0 | 0.034 | 89±26 | 0.001 | −11.3±6.2 | 0.07 |
| LA booster strain | 0.3±0.2 | 0.20 | 2±3 | 0.58 | 0.6±0.7 | 0.38 |
| LA booster strain rate | 0.2±1.4 | 0.89 | 14±19 | 0.44 | −1.1±4.7 | 0.82 |
| LA Stiffness index (E/e’/LA reservoir strain) | −9.0±1.6 | <0.001 | −117±22 | <0.001 | −23.2±5.0 | <0.001 |
| Adjusted for age, sex, BMI, and race | ||||||
| E/e’ | −0.3±0.1 | 0.002 | −4±2 | 0.012 | −1.4±0.4 | 0.002 |
| LA reservoir strain | 0.2±0.1 | 0.028 | 3±1 | 0.05 | 0.8±0.4 | 0.05 |
| LA reservoir strain rate | 2.5±1.1 | 0.022 | 39±17 | 0.022 | 2.8±5.3 | 0.59 |
| LA conduit strain | 0.1±0.1 | 0.48 | 2±2 | 0.28 | 0.6±0.5 | 0.23 |
| LA conduit strain rate | −1.0±1.2 | 0.42 | −40±18 | 0.025 | −8.9±5.0 | 0.08 |
| LA booster strain | 0.2±0.1 | 0.030 | 3±2 | 0.15 | 0.7±0.5 | 0.19 |
| LA booster strain rate | −0.6±0.8 | 0.40 | −1±12 | 0.93 | −5.6±3.7 | 0.13 |
| LA Stiffness index (E/e’/LA reservoir strain) | −3.5±1.0 | <0.001 | −49±16 | 0.003 | −14.1±4.2 | 0.001 |
Data presented as parameter estimate ± SE. Abbreviations: LV: left ventricle; LA: left atrium.
The predictive utility of measures of LA strain for the three functional measures of interest are provided in Table 4. Age, sex, and BMI were strong predictors of peak VO2 and 6MWD, while only BMI was predictive for quality of life. LA reservoir strain was not a significant predictor for any outcome; however, E/e’ and LA stiffness index (a composite of both LA reservoir strain and E/e’) were significant independent predictors of peak VO2, 6MWD, and quality of life.
Table 4.
Predictors of Outcomes
| Peak VO2 | 6 Minute Walk Distance | KCCQ Score | ||||
|---|---|---|---|---|---|---|
| Parameter Estimate | p-value | Parameter Estimate | p-value | Parameter Estimate | p-value | |
| E/e’ | −0.4±0.1 | 0.002 | −5±2 | 0.011 | −1.3±0.5 | 0.005 |
| Reservoir Strain (%) | 0.2±0.1 | 0.07 | 2±1 | 0.11 | 0.5±0.4 | 0.21 |
| LA Stiffness Index | −3.6±1.0 | <0.001 | −50±16 | 0.002 | 13.5±4.3 | 0.002 |
Data presented as parameter estimate ± SE. LV mass estimates are presented per 10 unit change in LV mass. Results of 3 different linear regression models each adjusted for conventional predictors (age, sex, BMI, and left ventricular mass).
DISCUSSION
The major novel finding of this investigation is that LA stiffness index is independently predictive of peak VO2, 6MWD and quality of life. Together, the data suggest that impaired LA function, particularly increased LA stiffness, may be an important pathophysiologic contributor to exercise intolerance in HFpEF, representing a potential therapeutic target to improve quality of life.
Left atrial structure and function are increasingly recognized as important pathophysiologic markers of disease severity. Strain analysis provides direct insight into the deformation patterns of myocardial tissue, with increased reproducibility and reduced variability compared with other imaging metrics.34, 35 LA function has a distinct tri-phasic pattern, beginning with passive filling of the atrium (reservoir phase, mitral valve closed), followed by passive emptying of the atrium into the LV (conduit phase, mitral valve open), and concluding with active emptying of the atrium (booster phase, subsequent to atrial depolarization). Consistent with prior observations,9, 10 summarized in a recent meta-analysis,36 LA reservoir and conduit strain were reduced in patients with HFpEF compared with controls. This is important, given that LA reservoir strain is predictive of both all-cause mortality and major adverse cardiovascular events; namely hospitalization for HF.37, 38 LA strain, in particular, has strong prognostic value in patients with HFpEF, and outperforms LV and right ventricular longitudinal strain in this regard.11 The data herein extend prior observations, by showing for the first time that LA reservoir and conduit strain are related to measures of physical function and quality of life, with LA stiffness index being independently predictive of these primary outcomes. While others have found LA function to be related to peak VO29, 11 and 6MWD39 among individuals without HF, to our knowledge, this is the first study to relate LA function with measures of physical function (i.e. 6MWD) in older, obese individuals with HF; providing novel insight into the potential role of LA function during activities of daily living.
Like other indices of cardiac function, however, LA strain is susceptible to hemodynamic loading conditions, and therefore needs to the considered in the context of cardiac filling pressures. That E/e’— a well-established surrogate measure40 of LV filling pressure— was markedly elevated in our HFpEF participants, compared to controls, underscores the importance of this variable. Indeed, not only was LA reservoir strain significantly reduced in our obese HFpEF participants, but was so despite markedly higher driving pressures (i.e. increased LA stiffness). A similar observation was also made when considering LA conduit strain in the context of elevated atrial driving pressure. Indeed, the LA and LV are intimately related, with shared anatomy (i.e basal annulus) and interdependence (i.e. “in series”). As such, LA function is inherently influenced by LV systole, creating a tethering effect to augment/facilitate LA passive filling (i.e. reservoir phase), and LV diastole, which ultimately contributes to transmitral filling during the passive ‘conduit’ phase of the cardiac cycle. That neither LV global longitudinal strain, nor early diastolic LV relaxation rate (i.e. strain rate), significantly influenced our interpretation of results, further highlights the independent contribution of LA dysfunction.
The exact mechanism causing LA dysfunction/stiffness in older patients with obese HFpEF remains incompletely understood, but is likely multifactorial. HFpEF is indeed associated with a clustering of cardiovascular risk factors, including diabetes mellitus, obesity and hypertension, each of which being important drivers of oxidative stress and inflammation,41–43 key constituents leading to myocardial fibrosis44–47— perhaps disproportionately so in the thin walled LA. HFpEF is also associated with chronic neurohormonal activation, another key constituent driving adverse cardiac remodeling.48–50 Finally, HFpEF predominately affects older individuals, with cardiovascular aging being associated with mitochondrial dysfunction, increased production of reactive oxygen species, decreased adrenergic signal sensitivity, reduced intracellular calcium reuptake, direct DNA toxicity, maladaptive gene expression, genomic instability and epigenetic changes that can adversely affect LA morphology and function.51–56 We therefore interpret the association between LA stiffness and exercise intolerance to reflect a complex interaction between a rise in cardiac output (needed to support oxygen delivery during activities of daily living), and the ensuing disproportionate rise in cardiac filling pressures that often accompanies HFpEF. In this way, the adverse hemodynamic response to everyday activities of daily living may also be directly associated with reduced quality of life.
The finding that LA dysfunction and LA stiffness are independent predictors of exercise intolerance and reduced quality of life in HFpEF has several important implications. First, it suggests that LA strain and the single point LA stiffness index, may be able to differentiate between cardiac and non-cardiac dyspnea. Second, when considered together with the growing body of evidence from others, LA strain/stiffness may serve as a potential HFpEF-clinical biomarker. In this regard, it is interesting to consider whether LA strain/stiffness can serve as an early predictor of future heart failure progression, independent of changes in LA size.57, 58 Prior data from our group in women with ischemic syndrome would indeed support this. 25 However, it should be acknowledged that E/e’ alone is strongly associated related to peak VO2, and is likely the primary driver of the relationship between LA stiffness and peak VO2. Whether elevated cardiac filling pressures is the result or cause of LA dysfunction remains unknown. Nevertheless, these data suggest LA stiffness and its derivatives contribute to exercise intolerance and thus represent potential therapeutic targets.
The strengths of our study include formal assessment of peak VO2, 6MWD, and quality of life in a comparatively-large and rigorously-phenotyped study population that included both patients with obese HFpEF and healthy age-matched controls. Nevertheless, our findings should be interpreted in the context of their limitations. Some of the between-group differences in measures of LA function may be partly related to demographic differences, including a higher proportion of the HFpEF patients being women, a lower proportion being of white race, and higher weight and BMI. However, differences in LA function between HFpEF patients and healthy controls remained, even after controlling for demographic covariates. Several of the between-group differences in measures of LA function did not reach statistical significance—this may be related to an inadequate sample size. The moderate sample size also prevented sex-specific interaction from being assessed Moreover, the poor acoustic windows associated with an obese HFpEF phenotype drove our decision to assess LA strain by MRI, providing excellent spatial resolution and confidence in the data. That LA strain and E/e’ were not collected during the same study visit is, however, a limitation. Finally, the cross-sectional nature of the study does not allow for assessment of causality, nor the longitudinal relationship between temporal changes in LA function, exercise tolerance, and quality of life.
CONCLUSIONS
In conclusion, older, obese patients with HFpEF have impaired LA function with increased LA stiffness. These differences independently predict decreased peak VO2, functional capacity, and quality of life. Together, these data highlight the importance of assessing LA function and contribute to a growing body of evidence identifying LA stiffness as a potential therapeutic target in HFpEF.
Supplementary Material
Funding Support:
This study was supported in part by the following research grants from the National Institutes of Health: R01AG045551; R01AG18915; P30AG021332; P30AG028716; P01HL137630. Also supported in part by the Kermit Glenn Phillips II Chair in Cardiovascular Medicine (DW Kitzman), the American Heart Association (TJ Samuel: 18PRE33960358), and the Potratz Family Endowment at the University of Texas at Arlington (MD Nelson).
Disclosures:
Dr. Kitzman reported receiving honoraria outside the present study as a consultant for Bayer, Merck, Medtronic, Relypsa, Merck, DCRI, Corvia Medical, Boehringer-Ingelheim, NovoNordisk, Astra Zeneca, and Novartis, and grant funding outside the present study from Novartis, Bayer, NovoNordisk, and Astra Zeneca, and stock ownership in Gilead Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Borlaug BA and Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gladden JD, Chaanine AH and Redfield MM. Heart Failure with Preserved Ejection Fraction. Annu Rev Med. 2018;69:65–79. [DOI] [PubMed] [Google Scholar]
- 3.Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J and Nicklas BJ. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA. 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J and Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM and Haykowsky M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM and Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeder MT, Thompson BR, Brunner-La Rocca HP and Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855–63. [DOI] [PubMed] [Google Scholar]
- 8.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC and Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–47. [DOI] [PubMed] [Google Scholar]
- 9.von Roeder M, Rommel KP, Kowallick JT, Blazek S, Besler C, Fengler K, Lotz J, Hasenfuβ G, Lücke C, Gutberlet M, Schuler G, Schuster A and Lurz P. Influence of Left Atrial Function on Exercise Capacity and Left Ventricular Function in Patients With Heart Failure and Preserved Ejection Fraction. Circ Cardiovasc Imaging. 2017;10. [DOI] [PubMed] [Google Scholar]
- 10.Telles F, Nanayakkara S, Evans S, Patel HC, Mariani JA, Vizi D, William J, Marwick TH and Kaye DM. Impaired left atrial strain predicts abnormal exercise haemodynamics in heart failure with preserved ejection fraction. Eur J Heart Fail. 2019;21:495–505. [DOI] [PubMed] [Google Scholar]
- 11.Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, Klein DA, Dixon D, Baldridge A, Rasmussen-Torvik LJ, Maganti K and Shah SJ. Prognostic Utility and Clinical Significance of Cardiac Mechanics in Heart Failure With Preserved Ejection Fraction: Importance of Left Atrial Strain. Circ Cardiovasc Imaging. 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kusunose K, Motoki H, Popovic ZB, Thomas JD, Klein AL and Marwick TH. Independent association of left atrial function with exercise capacity in patients with preserved ejection fraction. Heart. 2012;98:1311–7. [DOI] [PubMed] [Google Scholar]
- 13.Katzel LI, Bleecker ER, Colman EG, Rogus EM, Sorkin JD and Goldberg AP. Effects of weight loss vs aerobic exercise training on risk factors for coronary disease in healthy, obese, middle-aged and older men. A randomized controlled trial. Jama. 1995;274:1915–21. [DOI] [PubMed] [Google Scholar]
- 14.Lai YH, Liu ME, Su CH, Yun CH, Liu CY, Hou CJ, Hu KC, Hung CL, Yeh HI and Lam CSP. Obesity-Related Changes in Cardiac Structure and Function Among Asian Men and Women. J Am Coll Cardiol. 2017;69:2876–2878. [DOI] [PubMed] [Google Scholar]
- 15.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V and Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure With Preserved Ejection Fraction. Circulation. 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koepp KE, Obokata M, Reddy YNV, Olson TP and Borlaug BA. Hemodynamic and Functional Impact of Epicardial Adipose Tissue in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2020;8:657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng ACT, Delgado V, Borlaug BA and Bax JJ. Diabesity: the combined burden of obesity and diabetes on heart disease and the role of imaging. Nat Rev Cardiol. 2021;18:291–304. [DOI] [PubMed] [Google Scholar]
- 18.Sorimachi H, Obokata M, Takahashi N, Reddy YNV, Jain CC, Verbrugge FH, Koepp KE, Khosla S, Jensen MD and Borlaug BA. Pathophysiologic importance of visceral adipose tissue in women with heart failure and preserved ejection fraction. Eur Heart J. 2021;42:1595–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM and Carson PE. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circulation Heart failure. 2011;4:324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitzman DW and Shah SJ. The HFpEF Obesity Phenotype: The Elephant in the Room. J Am Coll Cardiol. 2016;68:200–3. [DOI] [PubMed] [Google Scholar]
- 21.Schocken DD, Arrieta MI, Leaverton PE and Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–6. [DOI] [PubMed] [Google Scholar]
- 22.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM and Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–50. [DOI] [PubMed] [Google Scholar]
- 23.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP and Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3:659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B and Waggoner AD. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European heart journal cardiovascular Imaging. 2016;17:1321–1360. [DOI] [PubMed] [Google Scholar]
- 25.Samuel TJ, Kitzman DW, Haykowsky MJ, Upadhya B, Brubaker P, Nelson MB, Hundley WG and Nelson MD. Left Ventricular Diastolic Dysfunction and Exercise Intolerance in Obese Heart Failure with Preserved Ejection Fraction. Am J Physiol Heart Circ Physiol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zamani SK, Samuel TJ, Wei J, Thomson LEJ, Tamarappoo B, Sharif B, Bairey Merz CN and Nelson MD. Left atrial stiffness in women with ischemia and no obstructive coronary artery disease: Novel insight from left atrial feature tracking. Clin Cardiol. 2020;43:986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alsaied T, Niss O, Tretter JT, Powell AW, Chin C, Fleck RJ, Cnota JF, Malik P, Quinn CT, Nagueh SF, Taylor MD and Mazur WM. Left atrial dysfunction in sickle cell anemia is associated with diffuse myocardial fibrosis, increased right ventricular pressure and reduced exercise capacity. Sci Rep. 2020;10:1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurt M, Wang J, Torre-Amione G and Nagueh SF. Left atrial function in diastolic heart failure. Circulation Cardiovascular imaging. 2009;2:10–5. [DOI] [PubMed] [Google Scholar]
- 29.Meyhofer S, Schmid SM, Hohl M and Reil JC. Disturbed ventricular-arterial coupling and increased left atrial stiffness in a patient with heart failure with preserved ejection fraction and hyperaldosteronism: a case report. European heart journal Case reports. 2019;3:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moon I, Lee SY, Lee E, Lee SR, Cha MJ, Choi EK and Oh S. Extensive left atrial ablation was associated with exacerbation of left atrial stiffness and dyspnea. Journal of cardiovascular electrophysiology. 2019;30:2782–2789. [DOI] [PubMed] [Google Scholar]
- 31.Scott JM, Haykowsky MJ, Eggebeen J, Morgan TM, Brubaker PH and Kitzman DW. Reliability of peak exercise testing in patients with heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:1809–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH and van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 33.Green CP, Porter CB, Bresnahan DR and Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. Journal of the American College of Cardiology. 2000;35:1245–1255. [DOI] [PubMed] [Google Scholar]
- 34.Stokke TM, Hasselberg NE, Smedsrud MK, Sarvari SI, Haugaa KH, Smiseth OA, Edvardsen T and Remme EW. Geometry as a Confounder When Assessing Ventricular Systolic Function. Journal of the American College of Cardiology. 2017;70:942–954. [DOI] [PubMed] [Google Scholar]
- 35.Halliday BP, Senior R and Pennell DJ. Assessing left ventricular systolic function: from ejection fraction to strain analysis. Eur Heart J. 2020. [DOI] [PubMed] [Google Scholar]
- 36.Khan MS, Memon MM, Murad MH, Vaduganathan M, Greene SJ, Hall M, Triposkiadis F, Lam CSP, Shah AM, Butler J and Shah SJ. Left atrial function in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Heart Fail. 2020;22:472–485. [DOI] [PubMed] [Google Scholar]
- 37.Santos AB, Roca GQ, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Fang JC, Zile MR, Pitt B, Solomon SD and Shah AM. Prognostic Relevance of Left Atrial Dysfunction in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2016;9:e002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Fang F, Wai-Kwok Yip G, Sanderson JE, Feng W, Xie JM, Luo XX, Lee AP and Lam YY. Left ventricular long-axis performance during exercise is an important prognosticator in patients with heart failure and preserved ejection fraction. Int J Cardiol. 2015;178:131–5. [DOI] [PubMed] [Google Scholar]
- 39.Patel RB, Freed BH, Beussink-Nelson L, Allen NB, Konety SH, Post WS, Yeboah J, Kitzman DW, Bertoni AG and Shah SJ. Associations of Cardiac Mechanics with Exercise Capacity: The Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andersen OS, Smiseth OA, Dokainish H, Abudiab MM, Schutt RC, Kumar A, Sato K, Harb S, Gude E, Remme EW, Andreassen AK, Ha JW, Xu J, Klein AL and Nagueh SF. Estimating Left Ventricular Filling Pressure by Echocardiography. J Am Coll Cardiol. 2017;69:1937–1948. [DOI] [PubMed] [Google Scholar]
- 41.Franssen C, Chen S, Hamdani N and Paulus WJ. From comorbidities to heart failure with preserved ejection fraction: a story of oxidative stress. Heart. 2016;102:320–330. [DOI] [PubMed] [Google Scholar]
- 42.Lindman BR, Dávila-Román VG, Mann DL, McNulty S, Semigran MJ, Lewis GD, De Las Fuentes L, Joseph SM, Vader J and Hernandez AF. Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. Journal of the American College of Cardiology. 2014;64:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aimo A, Castiglione V, Borrelli C, Saccaro LF, Franzini M, Masi S, Emdin M and Giannoni A. Oxidative stress and inflammation in the evolution of heart failure: from pathophysiology to therapeutic strategies. European journal of preventive cardiology. 2020;27:494–510. [DOI] [PubMed] [Google Scholar]
- 44.Su M-YM, Lin L-Y, Tseng Y-HE, Chang C-C, Wu C-K, Lin J-L and Tseng W-YI. CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. JACC: Cardiovascular Imaging. 2014;7:991–997. [DOI] [PubMed] [Google Scholar]
- 45.Schelbert EB, Fridman Y, Wong TC, Abu Daya H, Piehler KM, Kadakkal A, Miller CA, Ugander M, Maanja M, Kellman P, Shah DJ, Abebe KZ, Simon MA, Quarta G, Senni M, Butler J, Diez J, Redfield MM and Gheorghiade M. Temporal Relation Between Myocardial Fibrosis and Heart Failure With Preserved Ejection Fraction: Association With Baseline Disease Severity and Subsequent Outcome. JAMA Cardiol. 2017;2:995–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roy C, Slimani A, de Meester C, Amzulescu M, Pasquet A, Vancraeynest D, Beauloye C, Vanoverschelde J-L, Gerber BL and Pouleur A-C. Associations and prognostic significance of diffuse myocardial fibrosis by cardiovascular magnetic resonance in heart failure with preserved ejection fraction. Journal of cardiovascular magnetic resonance. 2018;20:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanagala P, Cheng AS, Singh A, Khan JN, Gulsin GS, Patel P, Gupta P, Arnold JR, Squire IB and Ng LL. Relationship between focal and diffuse fibrosis assessed by CMR and clinical outcomes in heart failure with preserved ejection fraction. JACC: Cardiovascular Imaging. 2019;12:2291–2301. [DOI] [PubMed] [Google Scholar]
- 48.Packer M Derangements in adrenergic–adipokine signalling establish a neurohormonal basis for obesity-related heart failure with a preserved ejection fraction. European journal of heart failure. 2018;20:873–878. [DOI] [PubMed] [Google Scholar]
- 49.Jimenez-Marrero S, Moliner P, Rodríguez-Costoya I, Enjuanes C, Alcoberro L, Yun S, Gonzalez-Costello J, Garay A, Tajes M and Calero E. Sympathetic activation and outcomes in chronic heart failure: Does the neurohormonal hypothesis apply to mid-range and preserved ejection fraction patients? European Journal of Internal Medicine. 2020;81:60–66. [DOI] [PubMed] [Google Scholar]
- 50.Lim GB. Neurohormonal activation in HFpEF. Nature Reviews Cardiology. 2019;16:700–700. [DOI] [PubMed] [Google Scholar]
- 51.Kumar AA, Kelly DP and Chirinos JA. Mitochondrial dysfunction in heart failure with preserved ejection fraction. Circulation. 2019;139:1435–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hahn VS, Knutsdottir H, Luo X, Bedi K, Margulies KB, Haldar SM, Stolina M, Yin J, Khakoo AY and Vaishnav J. Myocardial gene expression signatures in human heart failure with preserved ejection fraction. Circulation. 2021;143:120–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y-T, Wong LL, Liew OW and Richards AM. Heart failure with reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF): the diagnostic value of circulating microRNAs. Cells. 2019;8:1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paneni F, Diaz Cañestro C, Libby P, Lüscher TF and Camici GG. The aging cardiovascular system: understanding it at the cellular and clinical levels. Journal of the American College of Cardiology. 2017;69:1952–1967. [DOI] [PubMed] [Google Scholar]
- 55.Gevaert AB, Boen JR, Segers VF and Van Craenenbroeck EM. Heart failure with preserved ejection fraction: a review of cardiac and noncardiac pathophysiology. Frontiers in physiology. 2019;10:638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Melenovsky V, Hwang S-J, Redfield MM, Zakeri R, Lin G and Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circulation: Heart Failure. 2015;8:295–303. [DOI] [PubMed] [Google Scholar]
- 57.Douglas PS. The left atrium: a biomarker of chronic diastolic dysfunction and cardiovascular disease risk. J Am Coll Cardiol. 2003;42:1206–7. [DOI] [PubMed] [Google Scholar]
- 58.Tsang TS, Barnes ME, Gersh BJ, Takemoto Y, Rosales AG, Bailey KR and Seward JB. Prediction of risk for first age-related cardiovascular events in an elderly population: the incremental value of echocardiography. J Am Coll Cardiol. 2003;42:1199–205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


