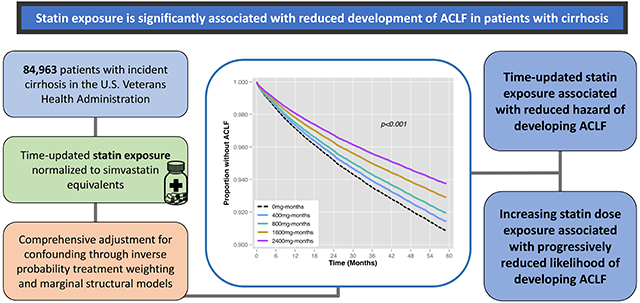
Abstract
Backgrounds & Aims:
There is a need to identify therapies that may prevent development of acute-on-chronic liver failure (ACLF) in patients with cirrhosis. This study sought to evaluate the association between statin exposure and the risk of developing ACLF in a large national cohort of patients with cirrhosis.
Methods:
We performed a retrospective cohort study of patients diagnosed with cirrhosis within the Veterans Health Administration from 2008 and 2018. Patients were stratified into three groups based on statin exposure (statin naïve, existing statin user, and new statin initiator). Cox proportional hazards regression models with inverse probability treatment weighting and marginal structural models were utilized to comprehensively address potential confounding in estimating the association between time-updated statin exposure and first occurrence of high-grade ACLF.
Results:
The cohort included 84,963 patients, of which 26.9% were on a statin at baseline. A total of 8,558 (10.1%) patients with cirrhosis were hospitalized with high-grade ACLF over median follow-up time of 51.6 months (IQR 27.5, 81.4). Time-updated statin use was associated with a significant reduction in the hazard of developing ACLF (HR 0.62, 95% CI 0.59-0.65, p<0.001). Increasing doses of statin were associated with progressively reduced hazard of developing ACLF (HR 0.75, 95% CI 0.66-0.86, p<0.001 for <20mg vs. 0mg of time-updated statin exposure, in simvastatin equivalents; HR 0.61, 95%, CI 0.58-0.64, p<0.001 for >20mg vs. 0mg statin exposure). Furthermore, every additional 5 months of statin exposure was associated with a 9% reduced hazard of high-grade ACLF (HR 0.91, 95% CI 0.90-0.92, p<0.001).
Conclusions:
In this large, retrospective cohort study in patients with cirrhosis, statin use was significantly associated with reduced development of high-grade ACLF.
Keywords: Acute-on-Chronic Liver Failure, Veterans Health Administration, Statins, Marginal Structural Models, Infection
Lay Summary
Statin therapy has been shown to have numerous beneficial effects in patients with chronic liver disease. This study demonstrated a strong association between statin therapy and a reduced risk for the development of acute-on-chronic liver failure in patients with cirrhosis. The results of this study support the promising role that statins may play in future prevention of acute-on-chronic liver failure in patients with cirrhosis.
Graphical Abstract
Introduction
Acute-on-chronic liver failure (ACLF) is a life-threatening syndrome in patients with cirrhosis or chronic liver disease characterized by an acute decompensation (AD) event, severe systemic inflammatory response, and one or more organ system failures (OFs).[1] Patients with ACLF have extremely poor short-term outcomes, with 90-day mortality typically exceeding 50%.[1, 2] Given its poor prognosis, there is great interest in identifying therapies to prevent or reduce the risk of developing ACLF in patients with cirrhosis.
Statin medications have garnered recent attention as one potential therapy due to their observed favorable effects in patients with chronic liver disease. Statins, or 3-Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, inhibit the endogenous production of cholesterol in the liver. In addition to their lipid lowering effects, statins have known anti-inflammatory, anti-oxidant, and vasoprotective properties, which may be key to their beneficial effects on liver disease.[3–5] Observational studies have demonstrated a reduction in the risk of hepatic decompensation, incidence of hepatocellular carcinoma, and risk of death in patients with cirrhosis receiving statins.[3, 6, 7] In a randomized controlled trial, simvastatin use was associated with improved survival in Child-Turcotte-Pugh (CTP) class A and B patients, though this was not the primary endpoint of the study.[8] Given these findings it is therefore plausible that statin therapy may similarly reduce the likelihood of developing future ACLF events in patients with cirrhosis, however this issue has not been explored in any large study to date.
To address this knowledge gap, we utilized a well-established national cohort of patients with cirrhosis to evaluate the effect of statin exposure on risk of developing high-grade ACLF. In particular, we aimed to comprehensively control for potential baseline and temporal confounders that might cloud this association, and to additionally investigate the impact of cumulative statin exposure on ACLF development.
Methods
Study Design and Cohort Creation
This was a retrospective cohort study using data from the Veterans Outcomes and Costs Associated with Liver Disease (VOCAL) cohort. The derivation of the VOCAL cohort has been detailed in prior publications[9] and has been used extensively in natural history studies of patients with cirrhosis,[10–12] including for the study of ACLF.[13–16] In brief it contains detailed longitudinal data for approximately 130,000 patients with cirrhosis in the Veterans Health Administration (VHA) identified between 1/1/2008 and 12/31/2018. Cirrhosis was classified using a validated algorithm of one inpatient or two outpatient ICD-9/10 codes for cirrhosis (571.2, 571.5, K74.6x, K70.3x).[17] We included patients age ≥18 years with incident cirrhosis as identified through a well-validated algorithm; the date of cirrhosis diagnosis was the index date in this study. To minimize the possibility of cirrhosis misclassification, we excluded patients with a baseline Fib-4 score <1.45.[18] Patients were also excluded if they received liver transplant prior to the index date, developed hepatocellular carcinoma within 6 months of the index date, had less than 6 months of follow-up time, had fewer than two outpatients visit in the index year, or did not have baseline lipid panel data.
Exposures Variables
For each patient we obtained data on demographics (age, sex, race), body mass index (BMI), comorbidities (diabetes mellitus, coronary artery disease [CAD], heart failure, atrial fibrillation),[19, 20] and history of transjugular portosystemic shunt (TIPS). Prior cirrhosis decompensation was determined using a validated algorithm.[21] Etiology of liver disease was ascertained using laboratory data, ICD-9/10 codes, and BMI data following a validated VHA algorithm,[22] and classified as hepatitis C virus (HCV), hepatitis B virus (HBV), alcohol-related liver disease (ALD), HCV+ALD, non-alcoholic fatty liver disease (NAFLD), and other. Baseline laboratory data included sodium, creatinine, albumin, total bilirubin, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, platelet count, and international normalized ratio (INR). Baseline model for end-stage liver disease-sodium (MELD-Na) was calculated from these data, and CTP class was determined using a validated VHA algorithm.[9] VHA pharmacy tables were then queried for all dispensed statin prescriptions and non-statin lipid lowering medications (e.g., fibrates, ezetimibe, niacin), including medication name, strength, and duration. Baseline exposure to these medications was determined in the 90 days prior to cirrhosis diagnosis. Statin doses were converted to simvastatin equivalents and categorized as 0mg, <20mg, and ≥20mg, similar to prior methods.[7] Finally, from the index date, the following variables were time-updated at 30-day intervals through 5 years of follow-up: statin exposure and dose, non-statin lipid lowering medications, low-density lipoprotein (LDL), total cholesterol, diabetes mellitus, and coronary artery disease.
Classification of ACLF
The primary outcome of high-grade ACLF was classified according to the European Association for the Study of the Liver- Chronic Liver Failure (EASL-CLIF) criteria.[1] Classification of ACLF using the VHA dataset has been detailed previously.[13, 23, 24] However, in brief, all patient hospitalizations during follow-up were identified, and acute decompensations (ADs) of infection, gastrointestinal bleed, ascites, or hepatic encephalopathy were ascertained using ICD-9/10 codes, CPT codes, and medication administration data (Supplemental Table 1). Organ failures (OFs) occurring within 28 days including kidney, liver, coagulation, respiratory, brain, and circulatory were defined precisely using granular laboratory, administrative coding, and medication administration data. Details are provided in Supplemental Tables 2–5. ACLF severity grades were then categorized from 0 (no ACLF) to 3 (severe ACLF), and grades 2 or 3 ACLF were considered to be “high-grade” for the purposes of the binary primary outcome classification. The time to high-grade ACLF was computed from the index date of cirrhosis diagnosis.
Primary Statistical Analysis
Descriptive statistics were reported as medians and interquartile ranges (IQRs) for continuous variables and as percentages for categorical variables. Cohort statistical comparisons were made as stratified by statin exposure: (1) no statin exposure (baseline or follow-up), (2) baseline statin exposure, (3) statin new initiator (no exposure at baseline, but initiated during follow-up). ADs and OFs for all ACLF hospitalizations were similarly summarized and compared among these strata, as were short-term mortality at 28 and 90 days.
We used inverse probability treatment weighting (IPTW) to create a pseudopopulation balanced across covariates of interest to simulate a randomized controlled trial. This entailed creation of a propensity score (PS) for any statin exposure (baseline or during follow-up) in a logistic regression model, where the following covariates were selected: age, sex, race, BMI, etiology of liver disease, diabetes, CAD, heart failure, atrial fibrillation, prior cirrhosis decompensation, TIPS, CTP class, MELD-Na, LDL, and total cholesterol. Inverse probability weights were then computed as 1/PS for patients who received statin therapy at baseline and as 1/(1-PS) for patients who did not received statin therapy at baseline.[25] To demonstrate the degree of matching achieved through IPTW, we plotted the standardized mean difference (SMD) in unweighted and IPTW-weighted fashion for each variable, where an SMD between −0.1 and 0.1 was regarded to represent adequate balance.[26] Next, IPTW-adjusted Cox proportional hazards regression was used to estimate the association between statin exposure and subsequent development of high-grade ACLF. The primary outcome was first occurrence of high-grade ACLF, and patients were censored at non-ACLF death, transplant, or maximum follow-up. Three primary models were created: (1) assessing time-updating statin exposure (binary, updated every 30 days), (2) assessing time-updated statin exposure relative to non-statin lipid-lowering medications or no lipid-lowering medications (multilevel categorical exposure, updated every 30 days), and (3) time-updated statin dose exposure, expressed in simvastatin equivalents (0mg, <20mg, ≥20mg,[27] updated every 30 days). To explore the impact of cumulative statin exposure over time, we then created IPTW-adjusted Cox regression models assessing (1) cumulative months of statin exposure (per 5 months) and (2) cumulative statin dose exposure (per 400mg-months, e.g. 80mg dose over 5 months), expressed in simvastatin equivalents. Each of these models was adjusted for LDL, total cholesterol, diabetes mellitus, and CAD as time-updating covariates. Hazard ratios (HRs) with 95% confidence intervals (CIs) were presented, as well as plots of Cox-adjusted survival curves. An alpha threshold of 5% was used to determine statistical significance.
Sensitivity Analysis
To account for the possibility of time-dependent confounding not addressed through IPTW-adjusted Cox regression, we estimated several marginal structural models. Selected covariates of interest could impact the decision to initiate statin therapy during follow-up and could in turn be modified by statin therapy as well as influence the primary outcome. These time-updated a priori variables included LDL, total cholesterol, diabetes mellitus, and coronary artery disease. IPTW methods were used to create stabilized and censored weights for statin treatment, in accordance with the methods outlined by Robins et al.[28] Stabilized weights generated in this fashion minimize bias in treatment estimates and variance relative to unstabilized weights. Five pooled logistic regression models weighted by the product of the stabilized and censored weights were created to separately estimate the association between (1) time-updated binary statin exposure, (2) time-updated categorical lipid lowering medication class exposure, (3) time-updated statin dose exposure, (4) cumulative months of statin exposure, and (5) cumulative statin dose exposure on the outcome of development of high-grade ACLF. Note that odds ratios produced using this method are near-equivalent to Cox-adjusted hazard ratios,[29] and were therefore presented as such. To approximate an intention-to-treat analysis (and thereby provide conservative estimates of treatment effect), patients were assumed to have continued statin therapy for all timepoints subsequent to initiation in marginal structural models.
Subgroup Analyses
First, to isolate the impact of statin exposure on ACLF specifically in statin-naïve patients, we repeated the IPTW-adjusted Cox regression and marginal structural modeling procedures excluding patients with baseline statin exposure. As above, HRs and 95% CIs were presented, and an alpha threshold of 5% was used to determine statistical significance. Second, given the possibility that statin use could co-associate with reductions in alcohol abuse and thereby reduce the likelihood of ACLF, we performed subgroup analyses of the above models where we excluded patients with ALD or ALD+HCV as an etiology of liver disease, and separately where we evaluated models only among patients with ALD or ALD+HCV.
All data management and analyses were performed using structured query language and STATA 17.0/BE (College Station, TX). Institutional Review Board approval was obtained from the Michael J. Crescenz Philadelphia Veterans Affairs Medical Center.
Results
Baseline Cohort and ACLF Characteristics
After application of selection criteria (Figure 1), a total 84,963 patients were included in the analytic cohort. Patients on a statin at baseline (N=22,876, 26.9%) were older (median 66 years vs. 61 with no statin exposure, p<0.001), had higher BMI (median 30.7 vs. 27.9 with no statin exposure, p<0.001), and had higher proportions of diabetes, CAD, heart failure, and atrial fibrillation (each p<0.001; Table 1). Baseline LDL and total cholesterol levels were lowest among patients on a statin at baseline, and were highest among patients who later initiated a statin during the course of follow-up (e.g. median LDL 73mg/dL baseline statin vs. 94mg/dL statin new initiator, p<0.001). A total 2,362 (2.8%) of patients were taking a non-statin lipid lowering medication at baseline.
Figure 1 –
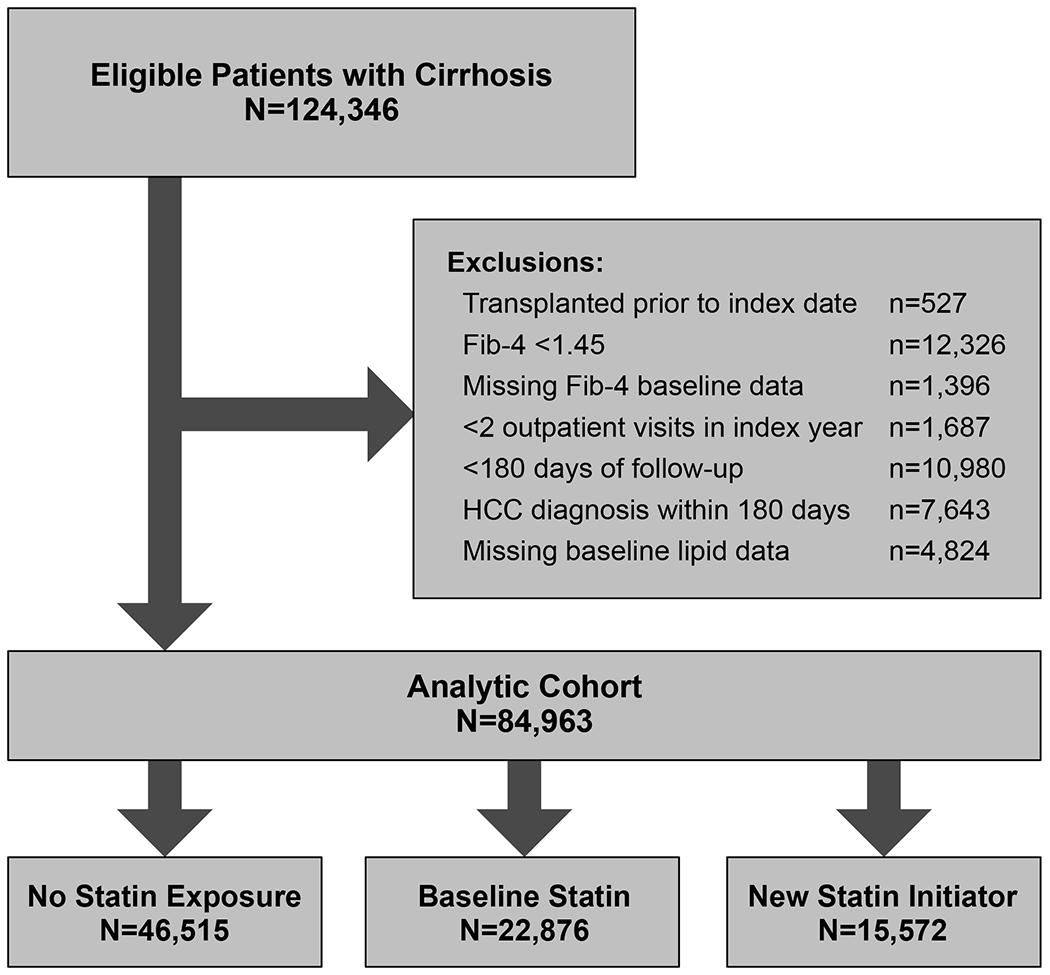
Patient Flow Diagram
Table 1 –
Baseline Cohort Characteristics, Stratified by Statin Exposure During Follow-up
| Factor | No Statin Exposure (N=46515) | Statin at Baseline (N=22876) | New Statin Initiator (N=15572) | p-value |
|---|---|---|---|---|
| Age, median (IQR) | 61 (56, 66) | 66 (62, 71) | 63 (58, 67) | <0.001 |
| Male Sex | 44989 (96.7%) | 22363 (97.8%) | 15202 (97.6%) | <0.001 |
| Race | <0.001 | |||
| White | 28998 (62.3%) | 15209 (66.5%) | 9053 (58.1%) | |
| Black | 8035 (17.3%) | 3636 (15.9%) | 3791 (24.3%) | |
| Hispanic | 3760 (8.1%) | 1740 (7.6%) | 1227 (7.9%) | |
| Asian | 557 (1.2%) | 308 (1.3%) | 192 (1.2%) | |
| Other | 5165 (11.1%) | 1983 (8.7%) | 1309 (8.4%) | |
| Body Mass Index, median (IQR) | 27.9 (24.4, 31.9) | 30.7 (26.9, 35.2) | 28.8 (25.2, 33.0) | <0.001 |
| Etiology of Liver Disease | <0.001 | |||
| Hepatitis C Virus (HCV) | 9879 (21.3%) | 3396 (14.8%) | 3984 (25.6%) | |
| Hepatitis B Virus | 369 (0.8%) | 252 (1.1%) | 169 (1.1%) | |
| Alcohol-related Liver Disease (ALD) | 15445 (33.2%) | 7049 (30.8%) | 4748 (30.5%) | |
| HCV + ALD | 11419 (24.6%) | 2438 (10.7%) | 3087 (19.8%) | |
| Non-alcoholic Fatty Liver Disease | 7440 (16.0%) | 9378 (41.0%) | 3158 (20.3%) | |
| Other | 1902 (4.1%) | 363 (1.6%) | 420 (2.7%) | |
| Diabetes Mellitus | 18206 (39.1%) | 17440 (76.2%) | 9039 (58.0%) | <0.001 |
| Coronary Artery Disease | 5835 (12.5%) | 11621 (50.8%) | 3915 (25.1%) | <0.001 |
| Heart Failure | 3828 (8.2%) | 6703 (29.3%) | 2108 (13.5%) | <0.001 |
| Atrial Fibrillation | 3160 (6.8%) | 4727 (20.7%) | 1401 (9.0%) | <0.001 |
| Child-Turcotte-Pugh Class | <0.001 | |||
| A | 27327 (58.7%) | 16231 (71.0%) | 11664 (74.9%) | |
| B | 15859 (34.1%) | 6239 (27.3%) | 3510 (22.5%) | |
| C | 3329 (7.2%) | 406 (1.8%) | 398 (2.6%) | |
| Prior Cirrhosis Decompensation | 7519 (16.2%) | 3259 (14.2%) | 1605 (10.3%) | <0.001 |
| TIPS | 46 (0.1%) | 11 (<1%) | 1 (<1%) | <0.001 |
| Sodium, median (IQR) | 138 (135, 140) | 138 (136, 140) | 138 (136, 140) | <0.001 |
| Creatinine, median (IQR) | 0.9 (0.7, 1.1) | 1.0 (0.8, 1.3) | 0.9 (0.8, 1.1) | <0.001 |
| Albumin, median (IQR) | 3.4 (2.9, 3.9) | 3.6 (3.2, 4.0) | 3.7 (3.2, 4.0) | <0.001 |
| Total Bilirubin, median (IQR) | 1.1 (0.8, 2.0) | 0.8 (0.6, 1.3) | 0.9 (0.6, 1.4) | <0.001 |
| Alkaline Phosphatase, median (IQR) | 106 (79, 148) | 95 (71, 133) | 96 (73, 132) | <0.001 |
| Aspartate Aminotransferase, median (IQR) | 64 (40, 106) | 42 (28, 64) | 54 (33, 90) | <0.001 |
| Alanine Aminotransferase, median (IQR) | 43 (26, 75) | 35 (22, 56) | 44 (26, 78) | <0.001 |
| Platelet Count, median (IQR) | 122 (86, 167) | 141 (105, 181) | 142 (104, 185) | <0.001 |
| INR, median (IQR) | 1.2 (1.1, 1.4) | 1.1 (1.1, 1.3) | 1.1 (1.0, 1.3) | <0.001 |
| MELD-Na, median (IQR) | 10 (6, 15) | 9 (6, 14) | 9 (6, 13) | <0.001 |
| HDL, median (IQR) | 42 (31, 56) | 40 (32, 51) | 41 (32, 54) | <0.001 |
| Triglycerides, median (IQR) | 97 (71, 138) | 116 (82, 173) | 118 (83, 174) | <0.001 |
| LDL, median (IQR) | 83 (63, 105) | 73 (55, 95) | 94 (72, 119) | <0.001 |
| Total Cholesterol, median (IQR) | 150 (125, 178) | 141 (117, 169) | 165 (137, 195) | <0.001 |
Over median follow-up time 51.6 months (IQR 27.5, 81.4), a total 8,558 (10.1%) patients were hospitalized with high-grade ACLF. Among patients with any statin exposure during follow-up, the trigger for ACLF was more often infection as compared to those without statin exposure (63.4% versus 58.5%, p<0.001; Table 2). By contrast, ADs of ascites and hepatic encephalopathy were less frequent in statin-exposed patients (e.g. for ascites, 33.6% vs. 42.1% no statin exposure, p<0.001). Kidney organ failure was present in the majority of ACLF hospitalizations (74.9% overall) and liver organ failure was least common (22.8% overall). As compared to patients with no statin exposure, patients with any statin exposure were less likely to have liver OF (11.9% vs. 29.6%, p<0.001) and brain OF (25.1% vs. 42.4%, p<0.001), but more likely to have kidney OF (82.6% vs. 70.1%, p<0.001) and circulatory OF (47.1% vs. 40.7%, p<0.001). Finally, statin-exposed patients with ACLF had lower 28- and 90-day mortality as compared to those with no statin exposure (27.0% vs. 37.6% 28-day, 40.1% vs. 53.7% 90-day, each p<0.001).
Table 2 –
Characteristics of High-Grade ACLF (2 or 3), Stratified by Statin Exposure
| Factor | Overall (N=8558) | No Statin Exposure (N=5268) | Any Statin Exposure (N=3290) | p-value |
|---|---|---|---|---|
| Acute Decompensation | ||||
| Infection | 5168(60.4%) | 3081 (58.5%) | 2087 (63.4%) | <0.001 |
| Gastrointestinal Bleed | 2285 (26.7%) | 1427 (27.1%) | 858 (26.1%) | 0.30 |
| Ascites | 3326 (38.9%) | 2220 (42.1%) | 1106 (33.6%) | <0.001 |
| Hepatic Encephalopathy | 3057 (35.7%) | 2232 (42.4%) | 825 (25.1%) | <0.001 |
| Organ Failures | ||||
| Kidney | 6412 (74.9%) | 3695 (70.1%) | 2717 (82.6%) | <0.001 |
| Liver | 1949 (22.8%) | 1558 (29.6%) | 391 (11.9%) | <0.001 |
| Coagulation | 4026 (47.0%) | 2537 (48.2%) | 1489 (45.3%) | 0.009 |
| Brain | 3057 (35.7%) | 2232 (42.4%) | 825 (25.1%) | <0.001 |
| Respiratory | 2745 (32.1%) | 1695 (32.2%) | 1050 (31.9%) | 0.80 |
| Circulatory | 3692 (43.1%) | 2143 (40.7%) | 1549 (47.1%) | <0.001 |
| Short-Term Mortality | ||||
| 28-day Mortality | 2868 (33.5%) | 1980 (37.6%) | 888 (27.0%) | <0.001 |
| 90-day Mortality | 4146 (48.5%) | 2828 (53.7%) | 1318 (40.1%) | <0.001 |
Association between Statin Use and ACLF
After generation of propensity scores for statin exposure and inverse probability weighting, excellent covariate balance was achieved for all variables as demonstrated by reduction in SMD to within +/− 0.1 (Figure 2). In IPTW-adjusted Cox regression models, time-updated statin use was associated with a significant reduction in the hazard of developing ACLF (HR 0.62, 95% CI 0.59-0.65, p<0.001; Table 3, Figure 3A). When lipid lowering medication classes were treated as a time-updated categorical variable, patients with statin exposure had a 38% reduced hazard of ACLF relative to patients on no lipid lowering medications (HR 0.62, 95% CI 0.59-0.65, p<0.001), however there was no significant association observed with exposure to non-statin lipid lowering medications (HR 1.08, 95% CI 0.97-1.21, p=0.16; Figure 3B). Increasing doses of statin exposure were also associated with progressively reduced hazard of developing ACLF. For example, relative to patients on no statin treatment (0mg), patients with <20mg exposure had an HR 0.75(95% CI 0.66-0.86) and those with ≥20mg of statin exposure had an HR 0.61 (95% CI 0.58-0.64, p<0.001; Figure 3C). When cumulative time on statin therapy was considered, every additional 5 months of statin exposure reduced the hazard of ACLF by 9% (HR 0.91, 95% CI 0.90-0.92, p<0.001; Figure 4A). Finally, for every additional 400mg-months of statin exposure, the hazard of ACLF was reduced by 6% (HR 0.94, 95% CI 0.93-0.95, p<0.001; Figure 4B).
Figure 2 – Covariate Balance in Unadjusted and IPTW-Adjusted Cohorts.
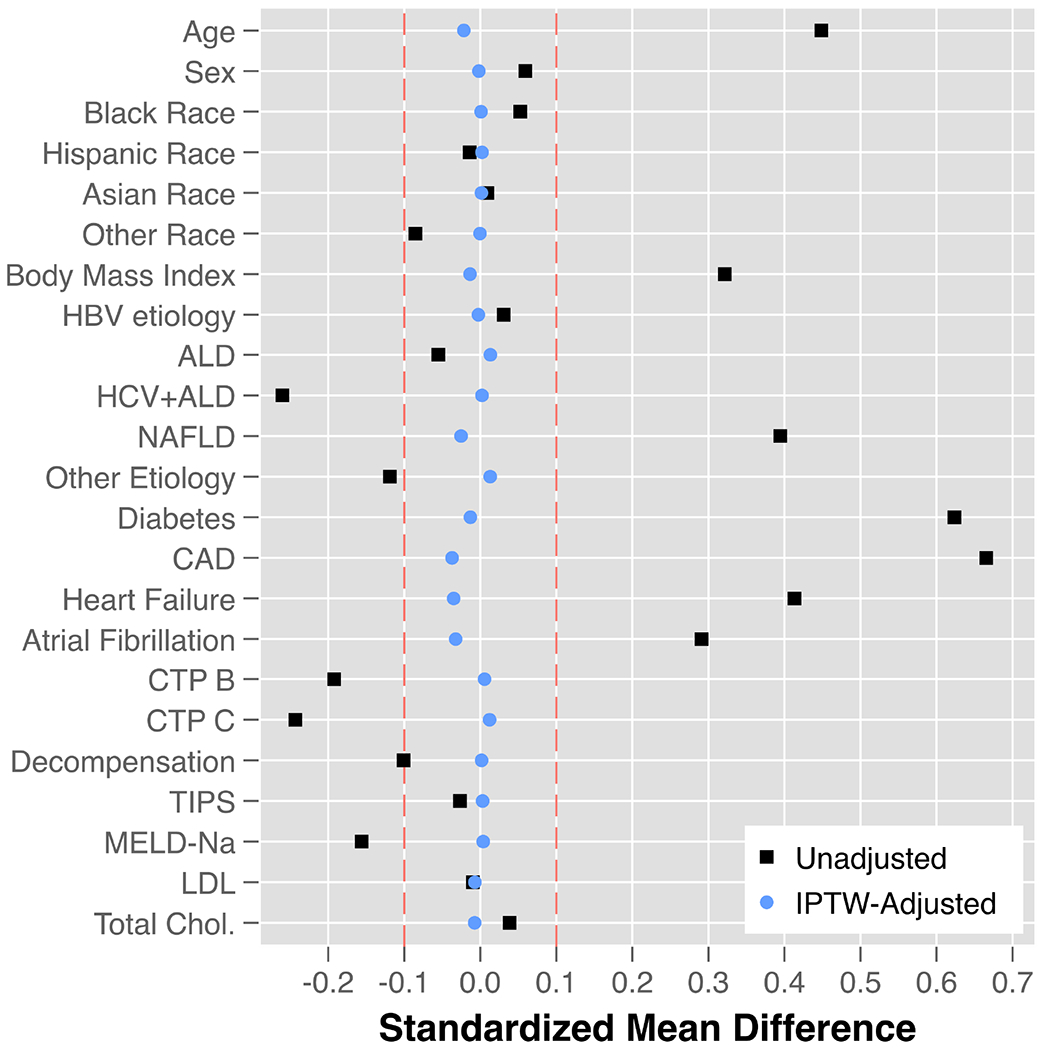
Abbreviations: IPTW = inverse probability treatment weighted; HBV = hepatitis B virus; ALD = alcohol-related liver disease; HCV = hepatitis C virus; NAFLD = non-alcoholic fatty liver disease; CAD = coronary artery disease; CTP = Child-Turcotte-Pugh; TIPS = transjugular intrahepatic portosystemic shunt; MELD-Na = model for end-stage liver disease-sodium; LDL = low density lipoprotein
Table 3 –
Association between Statin Exposure and High-Grade ACLF in IPTW-Adjusted Cox Regression and Marginal Structural Models
| Model | IPTW-Adjusted Cox Regression† HR (95% CI) | Marginal Structural Models‡ HR (95% CI) |
|---|---|---|
|
| ||
| Primary Models | ||
|
| ||
| (1) Binary Statin Exposure, Time-Updated | 0.62 (0.59 – 0.65)* | 0.73 (0.66 – 0.80)* |
|
| ||
| (2) Lipid Lowering Medication Exposure, Time-Updated | ||
| None | (ref) | (ref) |
| Statin Alone | 0.62 (0.59 – 0.65)* | 0.74 (0.67 – 0.81)* |
| Non-Statin Lipid Lowering Medication Alone | 1.08 (0.97 – 1.21) | 1.62 (1.28 – 2.05)* |
|
| ||
| (3) Statin Dose Exposure, Time-Updated | > | |
| 0mg (simvastatin equivalents) | (ref) | (ref) |
| <20mg (simvastatin equivalents) | 0.75 (0.66 – 0.86)* | 0.81 (0.63 – 1.05) |
| ≥20mg (simvastatin equivalents) | 0.61 (0.58 – 0.64)* | 0.72 (0.65 – 0.79)* |
|
| ||
| Cumulative Exposure Models | ||
|
| ||
| (1) Statin Time Exposure (per 5 months) | 0.91 (0.90 – 0.92)* | 0.95 (0.93 – 0.98)* |
|
| ||
| (2) Statin Dose-Time Exposure (per 400mg-months) | 0.94 (0.93 – 0.95)* | 0.95 (0.93 – 0.98)* |
Statistically significant at the alpha = 5% level
Each model adjusts for time-updating LDL, total cholesterol, diabetes mellitus, coronary artery disease, and baseline statin exposure.
Each model adjusts for age, sex, race, baseline diabetes, coronary artery disease, heart failure, atrial fibrillation, CTP class, prior cirrhosis decompensation, TIPS, MELD-Na, LDL, total cholesterol, and time-updating diabetes, coronary artery disease, LDL, and total cholesterol.
Figure 3 – Cox-adjusted Association between (A) Binary Statin Exposure, (B) Categorical Lipid Lowering Medications, and (C) Increasing Statin Dose Exposure* on Development of ACLF.
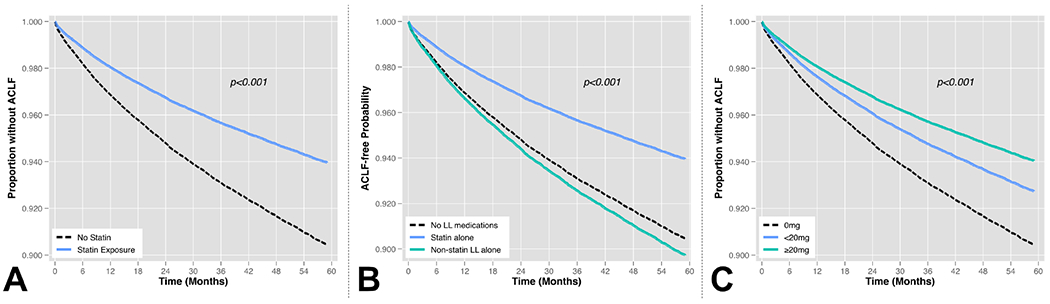
Abbreviations: LL = lipid lowering
* Time-updated dose exposures expressed in simvastatin equivalents
Figure 4 – Cox-adjusted Association between (A) Months of Cumulative Statin Exposure and (B) Dose-Months* of Statin Exposure on Development of ACLF.
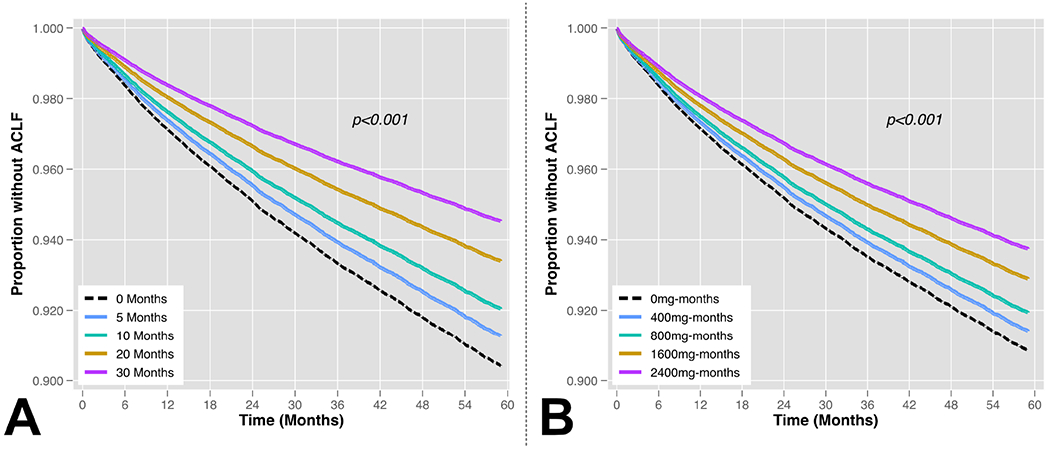
* Doses expressed in simvastatin equivalents. Dose-months were obtained by accumulating months at which a patient received a given statin dose. For example, a patient receiving 80mg of daily statin for five months would have 400mg-months of cumulative exposure.
When employing marginal structural models, causal estimates for a protective effect of statin use on ACLF development were globally similar to IPTW-adjusted Cox regression models, though somewhat attenuated. Binary statin exposure was associated with a 27% reduced hazard of ACLF (HR 0.73, 95% CI 0.66-0.80, p<0.001); high-dose statin exposure was associated with a 28% reduced hazard of ACLF, relative to no statin exposure (HR 0.72, 95% CI 0.65-0.79, p<0.001; Table 3). However, non-statin lipid lowering medications were associated with a significantly increased hazard of ACLF relative to patients with no exposure to lipid lowering medications (HR 1.62, 95% 1.28-2.05, p<0.001). Cumulative statin exposure, as measured in months or mg-months, was also found to be associated with reduced development in ACLF in marginal structural models, with generally similar estimates to those observed in adjusted Cox regression models (Table 3)
Subgroup Analyses
In models excluding patients with baseline statin exposure, the associations between statin exposure and development of ACLF were overall similar to the primary analyses in IPTW-adjusted Cox regression (Supplemental Table 6). In marginal structural models, however, the association was generally strengthened in new statin initiators across all models. For example, binary statin exposure had an HR 0.62 (95% CI 0.52-0.73, p<0.001) for development of ACLF. In cumulative exposure models, each additional 5 months of statin exposure conferred an 11% reduced hazard of ACLF (HR 0.89, 95% CI 0.84-0.95, p<0.001), and each additional 400mg-months or exposure reduced the hazard of ACLF by 14% (HR 0.86, 95% CI 0.80-0.93, p<0.001). Finally, when patients with alcohol-related liver disease were excluded, the protective association between statin exposure and reduction in ACLF development persisted in all models, though point estimates were slightly attenuated (Supplemental Table 7). For example, binary statin exposure was associated with a 34% reduction in ACLF development in IPTW-adjusted Cox regression models (HR 0.66, 95% CI 0.62-0.72, p<0.001) and a 21% reduction in marginal structural models (HR 0.79, 95% CI 0.68-0.92, p<0.001). Model results were again similar in the subgroup analysis limited only to patients with ALD or ALD+HCV (Supplemental Table 8). For example, in IPTW-adjusted Cox regression models, binary statin exposure was associated with an HR 0.64 (95% CI 0.60-0.68, p<0.001) for ACLF development, and in marginal structural models an HR 0.70 (95% CI 0.63-0.79, p<0.001).
Discussion
In this large study of diverse patients with cirrhosis, exposure to statin medications was associated with a significantly reduced risk of future development of ACLF. These findings were consistent across multiple measurements of exposure, including binary statin exposure, comparison to non-statin lipid lowering medications, and variations in cumulative exposure to statins across dosing and time. The results were also consistent across multiple methods of modeling (IPTW-adjusted Cox regression and marginal structural models) which were designed to comprehensively account for complex confounders.
The primary novel finding in this study is that statin use is significantly associated with reduced development of high-grade ACLF, and that increasing and cumulative statin exposure intensifies this association. Several retrospective and prospective studies have demonstrated that statins may have a beneficial effect in reducing the likelihood of cirrhosis decompensation and mortality. There is biological plausibility from chronic liver disease models to support this association, including that statins are known to reduce hepatic inflammation,[30, 31] block hepatic stellate cell activation,[32] and reduce fibrogenesis.[33, 34] Statins also improve endothelial dysfunction,[35] reduce intrahepatic resistance, and thereby lower portal pressures.[36] In one study by Tripathi et al, simvastatin was found to reduce hepatic inflammation, reduce portal pressures, and improve survival in a rat cirrhosis model specifically in the context of ACLF.[37] These mechanisms of action may mitigate or prevent ADs that reflect worsened intrinsic liver function, but would not necessarily reduce the likelihood of infection. Indeed, we found that patients on statin therapy who developed ACLF were significantly less likely to have ADs of ascites or hepatic encephalopathy, and somewhat more likely to have infection as an inciting event. Furthermore, ACLF patients on statin therapy were significantly less likely to have liver OF than ACLF patients not on statin therapy, potentially mediated by the pleiotropic hepatic effects noted above. While baseline differences in severity of liver disease between statin and non-statin users could potentially explain some of this difference, we attempted to comprehensively account for this through IPTW-based propensity scores that included MELD-Na, CTP class, and history of prior cirrhosis decompensation. Our findings therefore expand on prior literature and further the hypothesis that statin exposure may have salutary effects in patients with cirrhosis with respect to a range of liver-related adverse outcomes.
In addition to biological plausibility, other features of our analysis would argue for a potential causal relationship between statin exposure and reduction in ACLF. First, the observed association was unique to statin medications, and not observed with non-statin lipid lowering medications. This suggests a class effect from statins rather than reduction in ACLF being related to lipid lowering more broadly. Second, our findings suggest that a dose-response exists between statin exposure and reduced likelihood of ACLF. We found that increased cumulative exposure to statins in terms of duration of use (months) or dose plus duration (mg-months) both conferred increased protection against ACLF events, and the effect was especially strong in new statin initiators. This is consistent with recent data similarly identifying increased statin exposure, expressed in simvastatin equivalents, as being inversely associated with cirrhosis decompensation and death.[7] Both specificity of effect and dose-response are key elements of establishing causality,[38] however we must emphasize that findings presented herein must be interpreted as hypothesis generating. Randomized-controlled trials evaluating the impact of statins on liver-related outcomes which are ongoing may additionally aim to study ACLF as a patient-important outcome to further explore this association.
Our findings have significant clinical relevance. First, given the high short-term mortality associated with ACLF, there is substantial interest in identifying widely available and effective therapies that may help to prevent such events. Second, our findings that statin-treated patients who develop ACLF tend to have a different ACLF phenotype may help to direct anticipatory clinical management. For example, these patients are more likely to have an infectious precipitant of ACLF and to manifest circulatory and kidney OFs. Early antibiotics and escalation of care may therefore be especially important in statin-exposed patients with ACLF. Here it is also important to note that in an unadjusted analysis, statin-exposed patients with ACLF had a lower short-term mortality as compared to ACLF patients without statin exposure. This suggests that reductions in ACLF development may also translate to reductions in ACLF-related mortality, though this prospect requires additional study. Moreover, despite prior literature demonstrating poor prognosis in ACLF characterized by infection,[39] there may in fact be heterogeneity in outcomes that vary by type of infection and mechanism of OFs. Statin exposure could also modify the course of infections in the context of ACLF and reduce infection-related mortality, a concept that is well-supported by historic sepsis literature[40, 41] though data remain controversial.[42] Third, while there has been increasing interest in the role of statins in preventing cirrhosis decompensation and death, this marks the first real-world study to address statins specifically in the context of ACLF. Finally, demonstration of a protective association between statins and ACLF events lays the foundation for future prospective studies including clinical trials to better characterize the potential impact of statins on ACLF. This must of course include consideration of potential statin-associated adverse events in patients with advanced liver disease, especially in light of recent randomized-controlled trial data demonstrating an increased risk of rhabdomyolysis in patients with decompensated cirrhosis receiving a dose of simvastatin 40mg daily (but not 20mg daily).[43]
There are important limitations to acknowledge in this study. First, as with any large retrospective study, there is possible misclassification of exposures and outcomes. To minimize this, we used validated algorithms wherever possible, and only included patients in the cohort who actively followed in the VHA system. Second, although we made substantial efforts to control for all relevant confounders including accounting for time-dependent confounding, there of course remains the possibility of bias due to residual confounding. In particular, the observation that statin-treated patients with ACLF were more likely to have circulatory and kidney OFs could potentially result from residual confounding by indication, as these patients are presumably more likely to have vascular disease that could create susceptibility to these OFs. Conversely, patients taking statins may have been “healthier” in ways not captured in this analysis, and may in part explain the observed association with reduced ACLF. Indeed, exclusion of patients with ALD did attenuate the observed effect size somewhat, again illustrating the potential impact of residual confounding and the importance of prospective studies to confirm our findings. Third, there are potential external validity limitations given that the VHA is primarily male and relatively enriched in psychosocial comorbidities. However, it is not clear that these features would substantially alter the observed associations between statin use and ACLF. Fourth, we only studied the impact of statins on ACLF as defined by EASL-CLIF criteria. This definition best reflects ACLF with etiologies of liver disease that are well-represented in European and North American cohorts. It is therefore unclear if the observed associations with statin therapy would apply in other ACLF definitions derived from cohorts with predominantly HBV-related cirrhosis. Fifth, there are additional medications such beta blockers that may impact the risk of ACLF development that are not specifically addressed in this study but mark important areas for future research. Finally, we do not address impact of statins on ACLF-related mortality in detail, though we did note a lower crude mortality rate at 28 and 90 days in statin-exposed patients with ACLF. Given the complexity of the underlying analysis presented in the current study, the potential association between statin use and ACLF trajectory will be addressed in forthcoming work.
In conclusion, statin exposure is associated with reduced development of ACLF in a large cohort of patients with incident cirrhosis. This association was not observed with non-statin lipid lowering medications, and increasing doses and cumulative duration of statin therapy intensified the protective association between statins and development of ACLF. Though prospective studies are needed to validate these findings and better delineate the safety of statin therapy in patients with decompensated cirrhosis, they support a hypothesis that statins may mitigate high-mortality ACLF events over time.
Supplementary Material
Highlights.
Of 84,963 U.S. Veterans with cirrhosis, 8,558 (10.1%) were hospitalized with ACLF
Binary statin exposure was associated with a 38% reduced hazard of developing ACLF
Increasing dose exposure was associated with a progressively reduced hazard of ACLF
Statin-exposed ACLF patients had more kidney and less liver or brain organ failure
Funding Source:
Nadim Mahmud is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K08-DK124577) and by an American College of Gastroenterology Junior Faculty Development Award (ACG-JR-010-2020). David S. Goldberg has received support from Gilead, Merck, and AbbVie unrelated to the topic of this manuscript. He is also supported by a National Institutes of Health R01 (DK120561). David E. Kaplan has received support from Gilead, Glycotest and Bayer unrelated to the topic of this manuscript. He is also supported by VA Merit Grants (I01-CX-001933, I01-CX-002010). Tamar H. Taddei is supported by a VA Merit Grant (I01-CX-002010) and by the National Cancer Institute R01 (CA206465).
Abbreviations
- AD
Acute Decompensation
- ACLF
Acute-on-Chronic Liver Failure
- ALD
Alcohol-related Liver Disease
- BMI
Body Mass Index
- CAD
Coronary Artery Disease
- CIs
Confidence Intervals
- CTP
Child-Turcotte-Pugh
- EASL-CLIF
European Association for the Study of the Liver- Chronic Liver Failure
- HRs
Hazard Ratios
- HBV
Hepatitis B Virus
- HCV
Hepatitis C Virus
- HMG-CoA
3-Hydroxy-3-methylglutaryl Coenzyme A
- INR
International Normalized Ratio
- IQRs
Interquartile Ranges
- IPTW
Inverse Probability Treatment Weighting
- LDL
Low-density Lipoprotein
- MELD-Na
Model for End-stage Liver Disease-Sodium
- NAFLD
Non-alcoholic Fatty Liver Disease
- OFs
Organ Failures
- PS
Propensity Score
- SMD
Standardized Mean Difference
- TIPS
Transjugular Portosystemic Shunt
- VHA
Veterans Health Administration
- VOCAL
Veterans Outcomes and Costs Associated with Liver Disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no additional disclosures or conflicts as relevant to this manuscript.
Data Availability Statement
The data in this study were obtained with permission from the Veterans Health Administration. They are not publicly available, but may be made available upon reasonable request, and with approval from the Veterans Health Administration.
References
- [1].Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426–1437. e1429. [DOI] [PubMed] [Google Scholar]
- [2].Piano S, Tonon M, Vettore E, Stanco M, Pilutti C, Romano A, et al. Incidence, predictors and outcomes of acute-on-chronic liver failure in outpatients with cirrhosis. Journal of hepatology 2017;67:1177–1184. [DOI] [PubMed] [Google Scholar]
- [3].Bosch J, Gracia-Sancho J, Abraldes JG. Cirrhosis as new indication for statins. Gut 2020;69:953–962. [DOI] [PubMed] [Google Scholar]
- [4].Simon TG, Bonilla H, Yan P, Chung RT, Butt AA. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: Results from ERCHIVES. Hepatology 2016;64:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pose E, Trebicka J, Mookerjee RP, Angeli P, Ginès P. Statins: old drugs as new therapy for liver diseases? Journal of hepatology 2019;70:194–202. [DOI] [PubMed] [Google Scholar]
- [6].Kim RG, Loomba R, Prokop LJ, Singh S. Statin use and risk of cirrhosis and related complications in patients with chronic liver diseases: a systematic review and meta-analysis. Clinical Gastroenterology and Hepatology 2017;15:1521–1530. e1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kaplan DE, Serper MA, Mehta R, Fox R, John B, Aytaman A, et al. Effects of hypercholesterolemia and statin exposure on survival in a large national cohort of patients with cirrhosis. Gastroenterology 2019;156:1693–1706. e1612. [DOI] [PubMed] [Google Scholar]
- [8].Abraldes JG, Villanueva C, Aracil C, Turnes J, Hernandez-Guerra M, Genesca J, et al. Addition of simvastatin to standard therapy for the prevention of variceal rebleeding does not reduce rebleeding but increases survival in patients with cirrhosis. Gastroenterology 2016;150:1160–1170. e1163. [DOI] [PubMed] [Google Scholar]
- [9].Kaplan DE, Dai F, Aytaman A, Baytarian M, Fox R, Hunt K, et al. Development and performance of an algorithm to estimate the Child-Turcotte-Pugh score from a national electronic healthcare database. Clinical Gastroenterology and Hepatology 2015;13:2333–2341. e2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].John BV, Aitcheson G, Schwartz KB, Khakoo NS, Dahman B, Deng Y, et al. Male Sex Is Associated With Higher Rates of Liver-Related Mortality in Primary Biliary Cholangitis and Cirrhosis. Hepatology 2021. [DOI] [PubMed] [Google Scholar]
- [11].Mahmud N, Fricker Z, Hubbard RA, loannou GN, Lewis JD, Taddei TH, et al. Risk Prediction Models for Post-Operative Mortality in Patients With Cirrhosis. Hepatology 2021;73:204–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kanneganti M, Mahmud N, Kaplan DE, Taddei TH, Goldberg DS. Survival benefit of liver transplantation for hepatocellular carcinoma. Transplantation 2020;104:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mahmud N, Kaplan DE, Taddei TH, Goldberg DS. Incidence and mortality of acute-on-chronic liver failure using two definitions in patients with compensated cirrhosis. Hepatology 2019;69:2150–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mahmud N, Sundaram V, Kaplan DE, Taddei TH, Goldberg DS. Grade 1 acute on chronic liver failure is a predictor for subsequent grade 3 failure. Hepatology 2020;72:230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xiao KY, Hubbard RA, Kaplan DE, Taddei TH, Goldberg DS, Mahmud N. Models for acute on chronic liver failure development and mortality in a veterans affairs cohort. Hepatology International 2020;14:587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shah S, Goldberg DS, Kaplan DE, Sundaram V, Taddei TH, Mahmud N. Patient frailty is independently associated with the risk of hospitalization for acute-on-chronic liver failure. Liver Transplantation 2021;27:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kramer J, Davila J, Miller E, Richardson P, Giordano T, El-Serag H. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Alimentary pharmacology & therapeutics 2008;27:274–282. [DOI] [PubMed] [Google Scholar]
- [18].Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–1325. [DOI] [PubMed] [Google Scholar]
- [19].Davis LA, Mann A, Cannon GW, Mikuls TR, Reimold AM, Caplan L. Validation of diagnostic and procedural codes for identification of acute cardiovascular events in US veterans with rheumatoid arthritis. EGEMS 2013;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Niesner K, Murff HJ, Griffin MR, Wasserman B, Greevy R, Grijalva CG, et al. Validation of VA administrative data algorithms for identifying cardiovascular disease hospitalization. Epidemiology 2013;24:334–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Re III VL, Lim JK, Goetz MB, Tate J, Bathulapalli H, Klein MB, et al. Validity of diagnostic codes and liver-related laboratory abnormalities to identify hepatic decompensation events in the Veterans Aging Cohort Study. Pharmacoepidemiology and drug safety 2011;20:689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, loannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology 2015;149:1471–1482. e1475. [DOI] [PubMed] [Google Scholar]
- [23].Mahmud N, Hubbard RA, Kaplan DE, Taddei TH, Goldberg DS. Risk prediction scores for acute on chronic liver failure development and mortality. Liver International 2020;40:1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hernaez R, Kramer JR, Liu Y, Tansel A, Natarajan Y, Hussain KB, et al. Prevalence and short-term mortality of acute-on-chronic liver failure: a national cohort study from the USA. Journal of hepatology 2019;70:639–647. [DOI] [PubMed] [Google Scholar]
- [25].Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Statistics in medicine 2015;34:3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statistics in medicine 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Karlson BW, Palmer MK, Nicholls SJ, Lundman P, Barter PJ. A VOYAGER meta-analysis of the impact of statin therapy on low-density lipoprotein cholesterol and triglyceride levels in patients with hypertriglyceridemia. The American journal of cardiology 2016;117:1444–1448. [DOI] [PubMed] [Google Scholar]
- [28].Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Lww; 2000. [DOI] [PubMed] [Google Scholar]
- [29].D’Agostino RB, Lee ML, Belanger AJ, Cupples LA, Anderson K, Kannel WB. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Statistics in medicine 1990;9:1501–1515. [DOI] [PubMed] [Google Scholar]
- [30].Schierwagen R, Maybüchen L, Hittatiya K, Klein S, Uschner FE, Braga TT, et al. Statins improve NASH via inhibition of RhoA and Ras. American Journal of Physiology-Gastrointestinal and Liver Physiology 2016;311:G724–G733. [DOI] [PubMed] [Google Scholar]
- [31].Moreno M, Ramalho LN, Sancho-Bru P, Ruiz-Ortega M, Ramalho F, Abraldes JG, et al. Atorvastatin attenuates angiotensin II-induced inflammatory actions in the liver. American Journal of Physiology-Gastrointestinal and Liver Physiology 2009;296:G147–G156. [DOI] [PubMed] [Google Scholar]
- [32].Chong L-W, Hsu Y-C, Lee T-F, Lin Y, Chiu Y-T, Yang K-C, et al. Fluvastatin attenuates hepatic steatosis-induced fibrogenesis in rats through inhibiting paracrine effect of hepatocyte on hepatic stellate cells. BMC gastroenterology 2015;15:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Marrone G, Russo L, Rosado E, Hide D, García-Cardeña G, García-Pagán JC, et al. The transcription factor KLF2 mediates hepatic endothelial protection and paracrine endothelial-stellate cell deactivation induced by statins. Journal of hepatology 2013;58:98–103. [DOI] [PubMed] [Google Scholar]
- [34].Trebicka J, Hennenberg M, Odenthal M, Shir K, Klein S, Granzow M, et al. Atorvastatin attenuates hepatic fibrosis in rats after bile duct ligation via decreased turnover of hepatic stellate cells. Journal of hepatology 2010;53:702–712. [DOI] [PubMed] [Google Scholar]
- [35].Abraldes JG, Rodríguez-Vilarrupla A, Graupera M, Zafra C, García-Calderó H, García-Pagaán JC, et al. Simvastatin treatment improves liver sinusoidal endothelial dysfunction in CCl4 cirrhotic rats. Journal of hepatology 2007;46:1040–1046. [DOI] [PubMed] [Google Scholar]
- [36].Trebicka J, Schierwagen R. Statins, Rho GTPases and KLF2: new mechanistic insight into liver fibrosis and portal hypertension. Gut 2015;64:1349–1350. [DOI] [PubMed] [Google Scholar]
- [37].Tripathi DM, Vilaseca M, Lafoz E, Garcia-Caldero H, Haute GV, Fernandez-Iglesias A, et al. Simvastatin prevents progression of acute on chronic liver failure in rats with cirrhosis and portal hypertension. Gastroenterology 2018;155:1564–1577. [DOI] [PubMed] [Google Scholar]
- [38].Hill AB. The environment and disease: association or causation? : Sage Publications; 1965. [Google Scholar]
- [39].Fernánandez J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C, et al. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut 2018;67:1870–1880. [DOI] [PubMed] [Google Scholar]
- [40].Ou S-Y, Chu H, Chao P-W, Ou S-M, Lee Y-J, Kuo S-C, et al. Effect of the use of low and high potency statins and sepsis outcomes. Intensive care medicine 2014;40:1509–1517. [DOI] [PubMed] [Google Scholar]
- [41].Janda S, Young A, FitzGerald JM, Etminan M, Swiston J. The effect of statins on mortality from severe infections and sepsis: a systematic review and meta-analysis. Journal of critical care 2010;25:656.e657–656.e622. [DOI] [PubMed] [Google Scholar]
- [42].Deshpande A, Pasupuleti V, Rothberg MB. Statin therapy and mortality from sepsis: a meta-analysis of randomized trials. The American journal of medicine 2015;128:410–417. e411. [DOI] [PubMed] [Google Scholar]
- [43].Pose E, Napoleone L, Amin A, Campion D, Jimenez C, Piano S, et al. Safety of two different doses of simvastatin plus rifaximin in decompensated cirrhosis (LIVERHOPE-SAFETY): a randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet Gastroenterology & Hepatology 2020;5:31–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data in this study were obtained with permission from the Veterans Health Administration. They are not publicly available, but may be made available upon reasonable request, and with approval from the Veterans Health Administration.


