To the Editor,
Loglio and colleagues[1], reported a unique hepatitis D virus (HDV) RNA kinetic case study under entry-inhibitor Bulevirtide (BLV) monotherapy in 3 patients. Historically, mathematical modeling of viral hepatitis kinetics predicts a monophasic viral decline under antiviral treatment that blocks virus infection. Modeling suggests that the monophasic decline is driven by the rate of virus productive-infected cells loss/death(parameter δ, Fig.1a). Indeed, assuming that BLV’s only mode of action(MOA) is blocking HDV entry/infection(assuming η~100%, Fig.1a)[2], the model(Fig.1a) fits well the measured HDV data in patients 2 and 3(Fig.S1), but not in patient 1 in whom a transient viral increase was seen during the first 4 weeks of treatment, consisting of a 0.4 log increase from pretreatment HDV-RNA level at week 2, followed by a monophasic HDV decline onwards(Fig.1b). Such a transient viral increase can also be noticed in several hepatitis B virus (HBV) mono-infected patients treated with BLV 10 mg/day[3], suggesting that this transient viral increase may occur in some patients treated with BLV for both HBV and HDV. The nature of this early transient viral increase phenomenon under BLV treatment is not known.
Fig. 1.
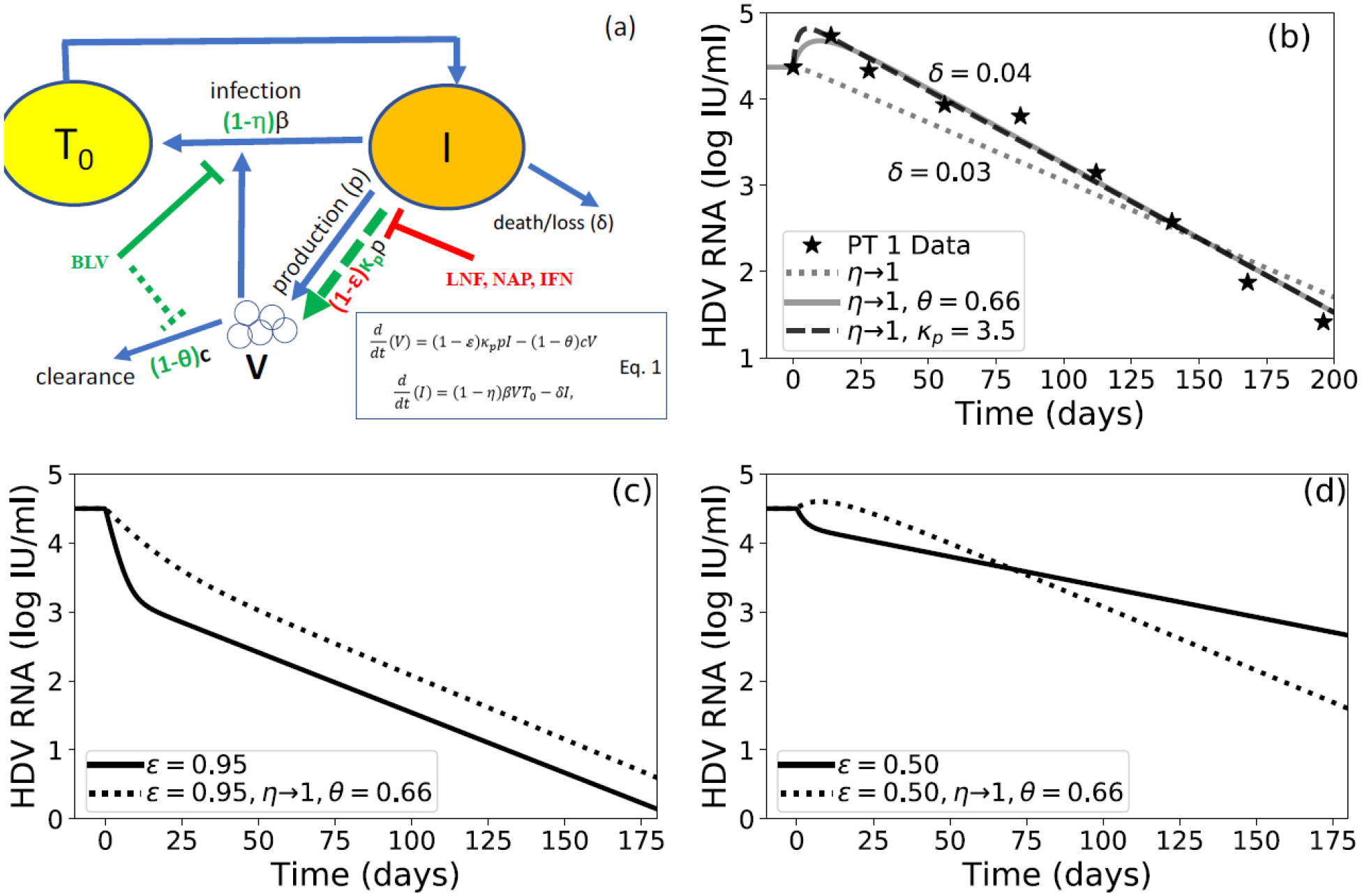
(a) Schematic and model equations (Eq. 1) of HDV infection and treatment. Target cells, T0, become HDV-infected cells, I, at rate β. Infected cells are then lost or die at rate δ. Infected cells also produce virions, V, at rate p. Virions in circulation are cleared at rate c. Bulevirtide (BLV) known mode of action (MOA) in blocking entry/infection is shown using solid green line (parameter η) and theoretical MOA are shown using dashed green lines (parameters θ representing reduced viral clearance and κp representing increase of viral production). Blocking HDV production by interferon-α (IFN), lonafarnib (LNF) or nucleic acid polymer (NAP) is shown using red symbols (parameter ε). (b) Model fitting, using Python version 3.7.4 and Scipy version 1.3.1, with measured HDV kinetics from patient 1 in [1] (symbols) assuming BLV MOA is blocking only viral infection (η~100%) does not fit the data well (dotted line). Assuming that in additional to BLV η~100%, viral clearance decreases (θ=66%, dashed line) or viral production increases (κp=3.5, solid line) fits the data well. Fixed parameters were c=0.42 day−1, p=10 virions/infected cell/day, and β=1e-7 mL ·virions−1·day−1. Initial conditions were set such that V0 equaled its value at the first data point, and I0 and T0 were set by steady state conditions as done in [6]. (c) Model simulations of HDV decline under antivirals that block HDV production (ε=95%, solid line) alone or in combination with BLV (ε=95%, η~100%) assuming a BLV-induced decrease in viral clearance of θ=66% (dashed line). We fixed δ=0.042 day−1 with other model parameters fixed as described in (b). (d) Same as (c) but with ε=50%.
Conceivably, the transient viral increase can be explained if one assumes that in addition to blocking of virus infection, BLV also enhances viral production(parameter κp, Fig.1a) or reduces viral clearance from circulation(parameter θ, Fig.1a). The latter two theoretical BLV MOAs can fit data from patient 1 well, with BLV enhancing viral production by κp~3.5-fold(Fig.1b) or reducing viral clearance by θ~66%(Fig.1b). It is unlikely that BLV enhances viral production since BLV blocks the binding site of the human sodium taurocholate co-transporting polypeptide on the HBV envelope, thereby inhibiting the entry of the virus into hepatocytes[2]. However, reducing viral clearance by BLV may be a more plausible MOA if virus clearance from the circulation is interrupted by BLV blocking virus entry into hepatocytes. In that case, it is possible that there was a modest effect on patients 2 and 3 that was not recognized due to infrequent sampling(Fig.S2). We recently showed for hepatitis C virus (HCV) that in some patients the liver not only produces virus but also clears virus from circulation[4], supporting the notion that blocking viral entry could reduce viral clearance by the liver in patient 1. In patients 2 and 3 other mechanism of viral clearance (e.g., adaptive-immune response) may play a major role in viral clearance that were not affected by BLV.
Reminiscent of the notion of predicting the duration of anti-HCV treatment needed to reach <1 virus copy in a patient’s total extracellular-body fluid (BF)[5], modeling predicts <1 HDV copy per BF after 75, 50 and 90 weeks of 10 mg/day BLV in patients 1, 2 and 3, respectively(Fig.S3). Thus, modeling may explain, retrospectively, why patient 1 had viral rebound after 52 weeks of BLV and suggests that patients 2 and 3 who were treated for 144 weeks already reached HDV clearance in BF.
We further investigated in-silico the predicted effect of BLV slowing HDV viral clearance from circulation in combination with other drugs that are predicted to block HDV viral production, i.e, parameter ε in Fig.1a (e.g., interferon-α, lonafarnib, and nucleic-acid polymers, Fig.1c) that we have previously shown to cause a biphasic HDV decline[6–8]. Mathematical modeling suggests that the first phase is driven by the rate of HDV clearance from circulation (parameter c, Fig.1b), which based on prior work we set to have t1/2=1.6 days[6–8]. The second phase is driven by parameter δ[6–8]. Modeling predicts that in patients in whom BLV will slow HDV clearance (θ~66%), BLV combined with drugs that block viral production (ε≥95%[6–8]) will lead to a slower viral decline (Fig.1c) compared to BLV monotherapy (Fig.1c). However, BLV plus drugs with low efficacy (ε=50%) will first lead to a slower viral decline during the first phase compared to BLV monotherapy(Fig.1d), but later (~11 weeks, Fig.1d) BLV-based therapy will cause a higher suppression of HDV compared to therapy that blocks viral production without BLV. In patients in whom BLV will not or moderately slow HDV clearance(θ~20%), modeling predicts an enhancement in viral decline under combination therapy compared to BLV monotherapy(Fig.S4), indicating the importance of including BLV in future anti-HDV regimens.
Studies in experimentally tractable in-vivo systems might be able to dissect the mechanism underlying the apparent increases in HDV and HBV viremia under BLV and to ultimately gain an in-depth understanding of BLV’s MOA[9]. Previously, BLV efficiently limited HDV spread in serially-transplanted humanized mice[10]. Future BLV perturbation experiments and theoretical modeling during HBV/HDV chronic infection are needed.
In conclusion, the monophasic HDV decline observed in two patients is consistent with the known MOA of BLV as an entry inhibitor. The transient increase in HDV in a third patient with initiation of BLV raises the possibility that blocking HDV entry into the liver has a secondary effect of reducing viral clearance by the liver.
Supplementary Material
Financial support:
NIH grants R01AI144112, R01AI138797, R01AI107301, R01AI146917, and R01AI153236
Footnotes
Conflicts of interest:
The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Data availability statement:
The authors declare that all data supporting the findings of this study are available in the article or supplementary information file.
References
- 1.Loglio A, Ferenci P, Uceda Renteria SC, Tham CYL, Scholtes C, Holzmann H, et al. Safety and effectiveness of up to 3 years’ bulevirtide monotherapy in patients with HDV-related cirrhosis. Journal of Hepatology. 2021. in press. [DOI] [PubMed] [Google Scholar]
- 2.Urban S, Bartenschlager R, Kubitz R, Zoulim F. Strategies to inhibit entry of HBV and HDV into hepatocytes. Gastroenterology. 2014;147(1):48–64. [DOI] [PubMed] [Google Scholar]
- 3.Goyal A Modeling HBV DNA and ALT responses observed under an entry inhibitor in HBV monoinfected individuals supports heterogeneity in the infected cell population. 5th Workshop on Virus Dynamics. https://www.fredhutch.org/en/events/workshop-on-virus-dynamics/agenda.html2021.
- 4.Shekhtman L, Navasa M, Sansone N, Crespo G, Subramanya G, Chung TL, et al. Modeling hepatitis C virus kinetics during liver transplantation reveals the role of the liver in virus clearance. Elife. 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Etzion O, Dahari H, Yardeni D, Issachar A, Nevo-Shor A, Cohen-Naftaly M, et al. Response guided therapy for reducing duration of direct acting antivirals in chronic hepatitis C infected patients: a Pilot study. Sci Rep. 2020;10(1):17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh C, Canini L, Dahari H, Zhao X, Uprichard SL, Haynes-Williams V, et al. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. Lancet Infect Dis. 2015;15(10):1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shekhtman L, Cotler SJ, Hershkovich L, Uprichard SL, Bazinet M, Pantea V, et al. Modelling hepatitis D virus RNA and HBsAg dynamics during nucleic acid polymer monotherapy suggest rapid turnover of HBsAg. Sci Rep. 2020;10(1):7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guedj J, Rotman Y, Cotler SJ, Koh C, Schmid P, Albrecht J, et al. Understanding early serum hepatitis D virus and hepatitis B surface antigen kinetics during pegylated interferon-alpha therapy via mathematical modeling. Hepatology. 2014;60(6):1902–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douam F, Ploss A. The use of humanized mice for studies of viral pathogenesis and immunity. Curr Opin Virol. 2018;29:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giersch K, Bhadra OD, Volz T, Allweiss L, Riecken K, Fehse B, et al. Hepatitis delta virus persists during liver regeneration and is amplified through cell division both in vitro and in vivo. Gut. 2019;68(1):150–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data supporting the findings of this study are available in the article or supplementary information file.


